Abstract
Background:
A deep level of sedation is required for magnetic resonance imaging (MRI) in children to ensure optimum image quality. The present study was conducted to evaluate the efficacy and safety of dexmedetomidine versus propofol for sedation in children undergoing MRI.
Materials and Methods:
A total of sixty children aged 2–10 years, having physical status 1 or 2 according to the American Society of Anesthesiologists, undergoing MRI were included in the study. Group D: (n = 30) received injection dexmedetomidine 2 μg/kg for 10 min followed by continuous infusion of 1.0 μg/kg/h. Group P (n = 30) received injection propofol 1 mg/kg bolus followed by continuous infusion of 100 μg/kg/min.
Results:
The mean time for onset of sedation in Group D was much longer than in Group P (P = 0.000). Mean duration of sedation was comparable in the two groups. The number of patients requiring increased infusion of study drug was significantly higher in Group D (30%) as compared to Group P (16.7%) (P < 0.05). The average recovery time in Group D was much longer than in Group P (P < 0.001).
Conclusion:
Propofol had an advantage of providing rapid onset of sedation and quicker recovery time. Dexmedetomidine resulted in a better preservation of respiratory rate and oxygen saturation, so it may be more suitable in children who are prone to respiratory depression. Hence, both the drugs could achieve required sedation in children posted for MRI.
Keywords: Dexmedetomidine, magnetic resonance imaging, propofol
Introduction
The frequency of magnetic resonance imaging (MRI) has increased in recent years in children; however, it is very sensitive to motion artifacts. This investigation requires children to stay still for a variable time of up to an hour in a magnetic, closed, claustrophobic, and noisy environment; hence, a deep level of sedation is required during MRI.[1,2]
The success of sedation for MRI is measured by two factors: The safety of the sedation procedure (lack of adverse events) and the effectiveness of the procedure (successful completion of the diagnostic investigation). Therefore, appropriate drugs need to be selected, administered, and titrated to achieve these objectives.
In the past, chloral hydrate and pentobarbital had been the drugs of choice for pediatric sedation in radiological investigations, but the complications associated with them limit their use.[3] With time, more drugs such as midazolam and ketamine became popular for sedation in children for diagnostic procedures. Midazolam may cause paradoxical excitation and agitation with higher doses while adverse effects such as hypertonicity and hypertension are commonly seen with ketamine.[4,5]
Propofol by continuous infusion provides the ability to titrate a desired level of sedation and provides a rapid recovery after infusion is terminated. However, propofol can cause hypotension, respiratory depression, bradycardia, and loss of protective airway reflexes.[6]
Dexmedetomidine, a potent and highly selective α2- receptor agonist, provides profound levels of sedation without affecting cardiovascular and respiratory stability. However, it causes dose-dependent decrease in heart rate (HR) and mean arterial blood pressure.[7]
There are limited studies comparing propofol with dexmedetomidine for procedural sedation in children. Hence, we conducted the present study to evaluate dexmedetomidine versus propofol for sedation in children undergoing MRI.
Materials and Methods
This prospective randomized study was conducted in the Department of Anaesthesiology and Critical Care in collaboration with Department of Radiology, Pt. B.D. Sharma Postgraduate Institute of Medical Sciences, Rohtak (India). After local institutional research and Ethical Committee approval and written parental consent, a total of sixty children aged 2–10 years, having physical status 1 or 2 according to the American Society of Anesthesiologists, undergoing MRI were included in the study.
Exclusion criteria
Any known allergies to the study drugs
Episodes of vomiting, apnea, and active respiratory illness
Unstable cardiac status
Anticipated difficult airway
Children on digoxin, beta-blockers, or calcium channel blockers.
Baseline HR, systolic blood pressure (SBP), respiratory rate (RR), and oxygen saturation (SpO2) were recorded on arrival to the preparation room.
Children were allocated according to a random number table to receive either dexmedetomidine (Group D) or propofol (Group P).
Group D (n = 30) received injection dexmedetomidine 2 μg/kg for 10 min followed by continuous infusion of 1.0 μg/kg/h.
Group P (n = 30) received injection propofol 1 mg/kg bolus followed by continuous infusion of 100 μg/kg/min.
The sedation level of the children was measured using the Ramsay sedation scale every 1 min till a score of 5 was achieved.[8] Children were positioned on the scanning table after a Ramsay sedation score (RSS) of 5 was achieved and hemodynamic as well as respiratory stability was ensured. Thereafter, RSS was measured every 5 min till the imaging was over.
If RSS of 5 was not achieved after infusion of the study drug for 25 min, the infusion rate of the study drugs was increased to 1.5 μg/kg/h in Group D and to 150 μg/kg/min in Group P for 5 min.
Patients were allowed to breathe spontaneously without an artificial airway throughout the procedure. Ventilatory function was assessed by the observation of respiratory activity. If the SpO2 level decreased below 93% for 30 s, the imaging process was interrupted, and the patient was taken out of the MRI tunnel. After assessing airway, the neck was extended and oxygen administered via facemask, and the study drug infusion was discontinued temporarily. The imaging process was started again once the SpO2 returned to normal. At the end of the MRI, the drug infusion was discontinued, and the children were then transferred to the recovery room.
The quality of the MRI was evaluated using a three-point scale (1 = no motion; 2 = minor movement; and 3 = major movement necessitating another scan). Point scale 1 and 2 were considered satisfactory for imaging.
Site and duration of MRI, onset of sedation (RSS = 5), duration of sedation (time in minutes from beginning of infusion of the drug to the point at which infusion was stopped), incremental infusion requirement, and recovery time (time in minutes from the last dose of sedation to the point at which patient was discharged) were recorded. Hemodynamic and respiratory parameters such as HR, SBP, SpO2, and RR were recorded at 5 min interval up to 50 min. Complications such as nausea, vomiting, hypotension, bradycardia, respiratory depression, desaturation, and allergic reaction if any were noted. Criteria for bradycardia and hypotension were taken as >20% decrease in HR and SBP from baseline values.[9,10] Respiratory depression was taken as RR <10/min.[11]
Statistical analysis
At the end of the study, the data were collected and analyzed statistically. Intergroup statistical analysis was performed using Student's t-test and nonparametric data was analyzed using Chi-square test. Comparison of continuous data between groups was done using ANOVA. Comparison of categorical data between groups was done using Fisher's exact test. P < 0.05 was considered as statistically significant, and P < 0.001 was considered highly significant.
Results
Mean age, weight, and sex ratio among the two groups were comparable. The distribution of patients according to site, duration, and quality of MRI were comparable in both the groups [Table 1].
Table 1.
Demographic profile, site, duration, and quality of magnetic resonance imaging
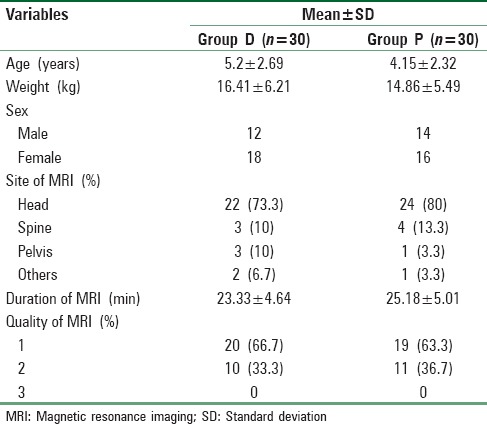
The mean time for onset of sedation in Group D was much longer than in Group P, the difference being highly significant (P = 0.000). Mean duration of sedation was comparable in the two groups. The number of patients requiring increased infusion of study drug was significantly higher in Group D (30%) as compared to Group P (16.7%) (P < 0.05). The average recovery time in Group D was much longer than in Group P, and the difference was statistically highly significant (P < 0.001) [Table 2].
Table 2.
Onset and duration of sedation, increased infusion requirement, and recovery time
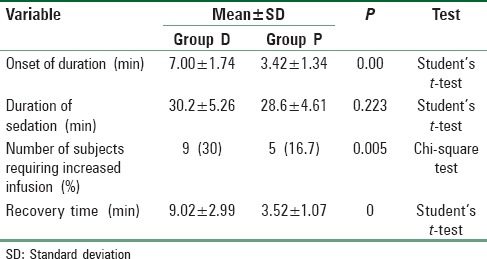
In both the groups, a highly significant increase in RSS was observed during the entire monitoring period of 50 min when compared to the baseline score (P < 0.001). On comparing between the two groups, initially, at 5 and 10 min intervals, there was a highly significant increase in RSS in Group P than Group D (P < 0.001) while it was similar in the two groups at 15, 20, and 25 min intervals (P > 0.05). Thereafter, patients in Group D showed a significantly higher RSS at 35, 40, and 45 min intervals (P < 0.05) [Table 3].
Table 3.
Ramsay sedation score in patients
At the beginning of the study, HR in both the groups was comparable. Patients in Group D showed a highly significant downtrend in HR from the baseline up to 35 min interval (P < 0.001). Thereafter, there was an increase in HR up to 50 min interval, but it was still significantly lower than the baseline (P < 0.05). Similarly, in Group P, there was a highly significant decrease in HR from the baseline up to 30 min interval (P < 0.001) and a significant decrease thereafter, up to 40 min (P < 0.05). Comparing the mean HR between the groups, it was observed that patients in Group D exhibited consistently lower HR than Group P throughout the duration of the study, the difference being statistically significant up to 25 min interval (P < 0.05).
There was a highly significant decrease in the mean SBP from the baseline in patients belonging to Group D up to 35 min interval (P < 0.001). Although there was an increase in SBP after that, it remained significantly lower than the baseline at 40 and 45 min intervals (P < 0.05). Similarly, in Group P, SBP was observed to decrease considerably from the baseline value up to 30 min, which was found to be highly significant (P < 0.001). Thereafter, SBP in Group P showed an increasing trend at 35 and 40 min intervals but were still significantly lower than the baseline values (P < 0.05). At 45 and 50 min intervals, SBP was found to be comparable to the baseline value (P > 0.05). When the mean SBP was compared between the two groups, it was observed that the patients in Group D exhibited consistently lower SBP throughout the duration of the study than Group P. However, the difference between the mean SBP was found to be statistically insignificant (P > 0.05).
Patients in Group D showed a significant decrease in their RR from the baseline during the 15, 20, and 25 min intervals (P < 0.05). After that, the RR increased progressively and it was found to be statistically comparable to the baseline (P > 0.05). However, the patients in Group P experienced a considerable decrease in RR from the baseline at 5, 10, 15, 20, 25, 30, 35, 40, and 45 min intervals, and the difference was found to be highly significant (P < 0.001). When the two groups were compared together, it was found that the patients in Group P exhibited consistently lower RR throughout with the difference being highly significant at 5, 10, 15, 20, 25, 30, 35, and 40 min intervals (P < 0.001).
In Group D, no statistical difference with regard to the baseline value of SpO2 was seen throughout the study (P > 0.05). On the other hand, patients in Group P showed a consistently decreasing trend in SpO2 levels up to 40 min which was found to be significantly lower when compared to the baseline (P < 0.05). Thereafter, SpO2 was found to be comparable to the baseline (P > 0.05). When the two groups were compared, it was observed that patients in Group P exhibited consistently lower SpO2 with the difference being significant at 20 and 25 min intervals (P < 0.05).
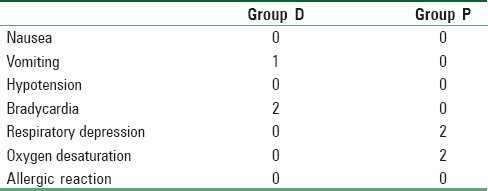
One patient in Group D experienced an episode of vomiting in the recovery room. While bradycardia was seen in 2 patients in Group D, there was no bradycardia in Group P. Respiratory depression was observed in 2 patients in Group P. There was no oxygen desaturation or hemodynamic instability in both the patients. SpO2 was noted in 2 patients in Group P. No such episode was observed in any patient in Group D [Table 4].
Table 4.
Incidence of complications in patients
Discussion
The frequency of MRI scan in children has increased in recent years making its role crucial in the diagnosis of various diseases. Depending on the need, an MRI scan takes about 10–30 min. For optimum image quality enabling precise diagnosis, patients have to remain motionless, which is difficult for children. Consequently, a deep level of sedation is required during MRI.[1,2] In the present study, interpretable MRI scans were obtained for all subjects, whether they were sedated with dexmedetomidine or propofol. This is in contrast with the results of a study conducted by Koroglu et al., where adequate sedation was obtained in 83% of the children who received dexmedetomidine and 90% of the children who received propofol.[9] The high failure rates seen in their dexmedetomidine group may be attributable to the lower dose of dexmedetomidine used by them, i.e., a loading dose of 1 μg/kg over 10 min followed by infusion of 0.5 μg/kg/h. In another study by Koroglu et al., where dexmedetomidine was compared with midazolam for sedation during MRI in children, 80% of the children administered dexmedetomidine achieved adequate sedation.[10] However, the results of the present study are in consensus with a study conducted by Mahmoud et al., where successful MRI sleep studies were recorded in 98% of the children in dexmedetomidine group and 100% in the propofol group.[12]
The mean onset of sedation in the present study was 7.00 ± 1.74 min in Group D while it was 3.42 ± 1.34 min in Group P, the difference being highly significant statistically (P < 0.001). This was found to be in contrast with the study conducted by Koroglu et al., where the average onset of sedation was found to be 19 min in patients who received dexmedetomidine.[10] The considerably longer onset of sedation could be attributable to the difference in the end-point of accepted level of adequate sedation taken as RSS score of 6 in their study as opposed to an RSS of 5 in the present study. However, in another study by the same authors comparing dexmedetomidine and propofol for MRI sedation in children, the mean onset of sedation was 9 min in dexmedetomidine group while for propofol, it was observed to be 4 min, similar to our results.[9]
Recovery time after dexmedetomidine was more than double (P < 0.001) than that after propofol, i.e. 9.02 ± 2.99 min for dexmedetomidine while 3.52 ± 1.07 min for propofol. These findings are similar to the study of Arain and Ebert.[13] In another study, Heard et al. observed that the time to recovery of full responsiveness after dexmedetomidine-midazolam infusion for MRI sedation was significantly greater than that after propofol by 50% (P < 0.05).[14] However, these authors used midazolam along with dexmedetomidine.
Initially, up to the 10 min interval, the RSS scores were higher in Group P than in Group D and the difference was found to be highly significant (P < 0.001). This difference is probably due to the rapid onset of sedation with propofol than dexmedetomidine. After the drug infusion was stopped, the RSS scores in both the groups decreased. RSS scores in Group P were significantly lower as compared to Group D over the 35, 40, and 45 min intervals, which may be explained by the slower recovery time seen with dexmedetomidine as compared to propofol.
The mean HR was found to be lower in Group D as compared to Group P, the difference being significant up to 25 min interval (P < 0.05). Our results are consistent with the findings of Koroglu et al., who found a highly significant decrease in HR from the baseline during sedation with dexmedetomidine as well as propofol (P < 0.001). HR at 10, 20, and 25 min was significantly higher in propofol group than in dexmedetomidine group. They did not report bradycardia in any patient.[9] Our findings are in accordance with the study of Heard et al., who found that the HR throughout the study in the dexmedetomidine group was significantly less than the baseline (P < 0.001). HR in the dexmedetomidine group was significantly less than that in the propofol group (P < 0.05). No episode of bradycardia was noted in any patient in either group.[14]
The mean SBP was found to decrease in both the groups from the baseline, the difference being highly significant up to 35 min in Group D and up to 30 min in Group P. This can be correlated with the mean duration of infusion of dexmedetomidine and propofol in Group D and Group P, respectively. The SBP at 50 min interval was comparable to the baseline value in both the groups (P > 0.05). Mahmoud et al., in their study, found a significant decrease in SBP with propofol (P < 0.05), with no significant decrease in SBP with dexmedetomidine (P > 0.05). The maintenance of SBP in the dexmedetomidine group can be attributed to the lower doses of dexmedetomidine used in their study as compared to the doses used by us.[12]
When the two groups were compared with each other, there was a highly significant decrease in RR in Group P as compared to Group D up to 40 min interval (P < 0.001). Our findings were consistent with the observations of Koroglu et al., where the mean RR was significantly lower in patients who received propofol as compared to patients who received dexmedetomidine (P < 0.05).[10] However, in the study by Heard et al., a significant decrease in the RR was seen in patients who received dexmedetomidine-midazolam as well as propofol (P < 0.05). The decrease in RR in dexmedetomidine-midazolam may be due to the respiratory depressant action of midazolam.[14]
Clinically, significant decrease in SpO2 was not seen in any patient who received dexmedetomidine. Our findings are consistent with those published by Mason et al., where out of the 120 children who received dexmedetomidine for MRI sedation, none of them had any episode of SpO2 (SpO2 <93%).[15]
No patient in the propofol group experienced vomiting. The lack of nausea and vomiting after receiving propofol is consistent with its antiemetic action. Only one patient in the dexmedetomidine group had an episode of vomiting which was managed by giving injection Ondansetron 0.1/mg/kg intravenously. This episode of vomiting may be attributable to the recent history of meningitis in that child. These findings were found to be consistent with those of Koroglu et al. and Heard et al., who did not report any similar episode of nausea or vomiting in any patient who received dexmedetomidine or propofol.[10,14]
Conclusion
Propofol has an advantage of providing rapid onset of sedation and quicker recovery time. Dexmedetomidine results in a better preservation of RR and SpO2, so it may be more suitable in children who are prone to respiratory depression. Hence, both dexmedetomidine and propofol are suitable agents for sedation in children for MRI.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lawson GR. Controversy: Sedation of children for magnetic resonance imaging. Arch Dis Child. 2000;82:150–3. doi: 10.1136/adc.82.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasan RA, Shayevitz JR, Patel V. Deep sedation with propofol for children undergoing ambulatory magnetic resonance imaging of the brain: Experience from a pediatric intensive care unit. Pediatr Crit Care Med. 2003;4:454–8. doi: 10.1097/01.PCC.0000090013.66899.33. [DOI] [PubMed] [Google Scholar]
- 3.Mason KP, Sanborn P, Zurakowski D, Karian VE, Connor L, Fontaine PJ, et al. Superiority of pentobarbital versus chloral hydrate for sedation in infants during imaging. Radiology. 2004;230:537–42. doi: 10.1148/radiol.2302030107. [DOI] [PubMed] [Google Scholar]
- 4.Sanborn PA, Michna E, Zurakowski D, Burrows PE, Fontaine PJ, Connor L, et al. Adverse cardiovascular and respiratory events during sedation of pediatric patients for imaging examinations. Radiology. 2005;237:288–94. doi: 10.1148/radiol.2371041415. [DOI] [PubMed] [Google Scholar]
- 5.Vardy JM, Dignon N, Mukherjee N, Sami DM, Balachandran G, Taylor S. Audit of the safety and effectiveness of ketamine for procedural sedation in the emergency department. Emerg Med J. 2008;25:579–82. doi: 10.1136/emj.2007.056200. [DOI] [PubMed] [Google Scholar]
- 6.Wilson E, Mackenzie N, Grant IS. A comparison of propofol and midazolam by infusion to provide sedation in patients who receive spinal anaesthesia. Anaesthesia. 1988;43(Suppl 1):91–4. doi: 10.1111/j.1365-2044.1988.tb09084.x. [DOI] [PubMed] [Google Scholar]
- 7.Ramsay MA, Luterman DL. Dexmedetomidine as a total intravenous anesthetic agent. Anesthesiology. 2004;101:787–90. doi: 10.1097/00000542-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 8.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koroglu A, Teksan H, Sagir O, Yucel A, Toprak HI, Ersoy OM. A comparison of the sedative, hemodynamic, and respiratory effects of dexmedetomidine and propofol in children undergoing magnetic resonance imaging. Anesth Analg. 2006;103:63–7. doi: 10.1213/01.ANE.0000219592.82598.AA. [DOI] [PubMed] [Google Scholar]
- 10.Koroglu A, Demirbilek S, Teksan H, Sagir O, But AK, Ersoy MO. Sedative, haemodynamic and respiratory effects of dexmedetomidine in children undergoing magnetic resonance imaging examination: Preliminary results. Br J Anaesth. 2005;94:821–4. doi: 10.1093/bja/aei119. [DOI] [PubMed] [Google Scholar]
- 11.Krane EJ, Jacobson LE, Lynn AM, Parrot C, Tyler DC. Caudal morphine for postoperative analgesia in children: A comparison with caudal bupivacaine and intravenous morphine. Anesth Analg. 1987;66:647–53. [PubMed] [Google Scholar]
- 12.Mahmoud M, Gunter J, Donnelly LF, Wang Y, Nick TG, Sadhasivam S. A comparison of dexmedetomidine with propofol for magnetic resonance imaging sleep studies in children. Anesth Analg. 2009;109:745–53. doi: 10.1213/ane.0b013e3181adc506. [DOI] [PubMed] [Google Scholar]
- 13.Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg. 2002;95:461–6. doi: 10.1097/00000539-200208000-00042. [DOI] [PubMed] [Google Scholar]
- 14.Heard C, Burrows F, Johnson K, Joshi P, Houck J, Lerman J. A comparison of dexmedetomidine-midazolam with propofol for maintenance of anesthesia in children undergoing magnetic resonance imaging. Anesth Analg. 2008;107:1832–9. doi: 10.1213/ane.0b013e31818874ee. [DOI] [PubMed] [Google Scholar]
- 15.Mason KP, Zurakowski D, Zgleszewski SE, Robson CD, Carrier M, Hickey PR, et al. High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr Anaesth. 2008;18:403–11. doi: 10.1111/j.1460-9592.2008.02468.x. [DOI] [PubMed] [Google Scholar]


