Abstract
The vaccination of C3H/HeJ mice with Escherichia coli Dr fimbrial antigen reduced mortality associated with an experimental urinary tract infection due to a homologous strain bearing Dr adhesin. Immune sera with high titers of anti-Dr antibody inhibited bacterial binding to bladders and kidneys but did not affect the rate of renal colonization.
Escherichia coli expressing Dr fimbriae and related adhesins are associated with urinary tract infection (UTI), including cystitis and/or pyelonephritis and diarrhea (12). Children and pregnant women are prone to recurrent or persistent infections caused by these organisms (1). E. coli strains bearing Dr fimbriae or the related fimbriae F1845, afimbrial adhesin I (AFA-I), and AFA-III display similar patterns of binding to the natural receptor, a decay-accelerating factor (CD55) (10). Decay-accelerating factor is a complement-regulatory protein expressed on most mammalian cells that protects cells from autologous complement-mediated damage by preventing the formation of C3 convertases (8).
Lipopolysaccharide (LPS) nonresponder C3H/HeJ mice are a useful tool in studying chronic UTI (3). A lack of response to LPS has been attributed to a mutated Toll-like receptor 4 gene (6). The capability of uropathogenic E. coli to produce Dr fimbriae contributes to establishing chronic kidney infection in C3H/HeJ mice (3). Clinical strain IH11128, bearing the Dr adhesin, persistently colonized the renal interstitia of 50% of the tested animals for 1 year, with histologic evidence of chronic pyelonephritis, whereas its isogenic Dr-negative (Dr−) mutant DR14 was gradually eliminated. Interestingly, Dr-positive (Dr+) E. coli was associated with a 10 to 20% mortality rate, which has also been observed recently in pregnant rats (9). In contrast to the well-documented role of Dr fimbriae in experimental chronic kidney infection, it is unknown whether the ability to produce Dr or related adhesins is associated with chronic bladder infection in mice. It is believed that antibodies generated by immunization with fimbrial antigens protect against UTI by interfering with bacterial binding to urothelial receptors. In this report, we assess the impact of the vaccination of C3H/HeJ mice with purified Dr fimbrial antigen on the course of ascending UTI due to a homologous clinical E. coli strain bearing Dr adhesin and particularly on the mortality and rates of colonization in the bladder and kidney.
For vaccination, Dr fimbrial protein was purified from a recombinant E. coli strain, TP406.3, as previously described (14). A fimbrial preparation resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and stained with silver showed a single band with a molecular mass of 15.6 kDa. A nondiluted preparation displayed hemagglutination, with human erythrocytes being inhibited in the presence of chloramphenicol. We used 80 female, 10-week-old C3H/HeJ mice (Jackson Laboratories, Bar Harbor, Maine) divided into two groups of 40 mice to evaluate the effectiveness of immunization with purified fimbrial antigen against infection with a clinical strain carrying Dr fimbriae. Forty C3H/HeJ mice were immunized on day 0 and given a booster injection 4 weeks later. For the first immunization, the mice were given 10 μg of Dr fimbrial antigen emulsified in complete Freund adjuvant (H37Ra; Difco Laboratories, Franklin Lakes, N.J.). Forty control animals received emulsion prepared from equal volumes of complete Freund adjuvant and phosphate-buffered saline (PBS). A fimbrial preparation emulsified in incomplete Freund adjuvant was used in the second immunization. All mice were bled 1 week prior to immunization and 1 week after the second immunization to assess the titer of the anti-Dr antibody. Two weeks after the second immunization, animals under intraperitoneal anesthesia with nembutanol (50 μg/g of body weight) were challenged by instillation of Dr+ E. coli IH11128 into the bladder (50 μl; optical density at 600 nm, 2.0). The expression of Dr fimbriae was confirmed by the hemagglutination of a 2% suspension of human erythrocytes in the presence of α-methyl mannoside and the inhibition of hemagglutination by chloramphenicol (11). The average numbers of CFU per bladder and kidney for groups of eight animals each from the immunized and control groups were evaluated 2 days after infection and again 2, 4, 8, and 12 weeks after infection. The bladders and kidneys were aseptically removed, weighed, and homogenized in 1 ml of PBS. We plated 20 μl of homogenates on Luria-Bertani agar plates and MacConkey agar to enumerate bacterial colonies. In addition, the 5-μm-thick kidney sections of randomly selected animals (eight in each group) were subjected to standard hematoxylin and eosin staining to evaluate histopathologic changes. Immune responses (total immunoglobulin G) in sera and urine samples were assessed by a standard enzyme-linked immunosorbent assay (ELISA) with purified Dr fimbriae as the capture antigen. Pooled sera or urine samples collected prior to immunization were used as negative controls. Each sample was tested in duplicate. Absorbance was read at 405 nm in an ELISA microplate reader (Bio-Rad Laboratories, Hercules, Calif.), and the results were processed with Microplate Manager software, version 4.0 (Bio-Rad Laboratories). A mean value greater than two times the value for the negative control was considered to indicate the presence of a specific antibody.
We assessed the distribution of colonization in the bladders and kidneys of 39 mice immunized with purified Dr antigen and in 32 control animals during a 12-week experiment. Overall, we found a higher average number of CFU per gram of bladder in the group of nonvaccinated animals than in the vaccinated animals. The bladder colonization tended to decrease in the group of immunized animals at the 12th week of the experiment; however, differences between the groups did not reach statistical significance (Student's t test, P > 0.05) (Fig. 1). Colonization densities in the kidneys of nonimmunized animals higher than those in vaccinated mice were noticed 4 weeks after infection and persisted until the end of the experiment (Fig. 1). Similar to bladder colonization, the differences did not reach statistical significance (P > 0.05). Therefore, we found no obvious differences in kidney histopathology between vaccinated and nonvaccinated animals. The grades of renal injury and the types of infiltrating cells (plasma cells, neutrophils, and fibroblasts) were similar in the two groups. It is worth noting that colonization displayed remarkable variation among the animals, ranging from negative cultures to heavy colonization exceeding 106 CFU per gram of renal tissue. The anti-Dr antibody in the mouse sera prior to experimental infection with Dr+ E. coli was detected in dilutions ranging from 1:32,000 to 1:500,000. However, we could not find any association between the titers of antibody and the densities of bladder and kidney colonization. None of the control mice had measurable levels of anti-Dr antibody. The titers of anti-Dr antibody in 21 urine samples collected from vaccinated animals were at low levels or at levels below the sensitivity of the ELISA. Anti-Dr antibody in sera collected from nonvaccinated and infected animals was detected in dilutions from 1:500 to 1:8,000. We evaluated the mortality rates of the groups of vaccinated and nonvaccinated mice. One nonvaccinated mouse died at the second week after being challenged with Dr+ E. coli. Four nonvaccinated animals were reported dead in the 4th week after challenge, and three more were reported dead in the 11th week. Overall, in the first week after infection, eight mice (20%) in the group of nonvaccinated animals died, in contrast to one mouse (2%) in the group of animals immunized with Dr fimbrial antigen. The Fisher exact test was used to determine the differences, which were statistically significant (P < 0.05).
FIG. 1.
Quantitative bacterial counts in bladders and kidneys of mice immunized and not immunized with purified Dr fimbriae and challenged with Dr+ E. coli IH11128. Bars indicate mean values for the tested groups. Statistical analysis was performed with Student's t test.
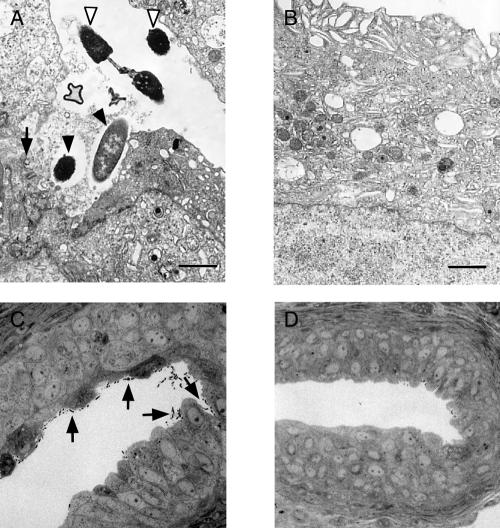
In vivo colonization of the bladder was assessed by light and electron microscopy techniques. We used four 10-week-old C3H/HeJ female mice for the study. The mice were anesthetized and then infected by bladder catheterization and the instillation of 50 μl of bacterial suspension (optical density at 600 nm, 2.0) of either Dr+ E. coli strain IH11128 or DR14 (3). After 48 h, the mice were sacrificed and their bladders were aseptically removed, sliced in half, and either snap-frozen for hematoxylin and eosin staining or fixed and processed for transmission electron microscopy. Microscopic studies demonstrated that the expression of Dr fimbriae promotes binding to mouse uroepithelium. After 48 h, bacterial cells of clinical Dr+ E. coli IH11128 covered the surface layer of the bladder urothelia, which displayed signs of damage, such as partial detachment or altered appearance (Fig. 2A and C). The inconsistencies between the results of bladder culture and electron microscopy may have been brought about by the different means of processing bladders for culture and for electron microscopy. The bladders for electron microscopy were removed and immediately immersed in fixative. For culture, the bladders were washed with sterile PBS prior to homogenization to eliminate bacteria not associated with bladder epithelium. This procedure may decrease the number of detected bacteria and may indicate that within 48 h, Dr+ E. coli cells remain loosely associated with bladder epithelium. Bacteria were not seen in bladders infected with the Dr− mutant DR14 (Fig. 2B and D). We also tested whether selected immune sera from the mice with the highest titers of anti-Dr antibody were able to inhibit the binding of Dr+ E. coli IH11128 to surface receptors. Inhibitory activity was assessed in a 3-h binding assay with acetone-fixed sections of mouse bladders and kidneys. Bacterial suspensions were preincubated for 1 h with immune sera diluted 1:1,000, followed by a 3-h incubation with tissue sections. Immune serum at a dilution from 1:10 to 1:1,000 did not cause noticeable agglutination of Dr+ E. coli cells. Bacterial suspensions in PBS or bacteria preincubated with preimmune mouse serum were used as positive controls. A bacterial suspension of E. coli DR14, a Dr− isogenic mutant of IH11128, was included as a negative control. Staining with Sytox Green (Molecular Probes, Inc., Eugene, Oreg.) was used to visualize bacteria associated with tissue structures. Goat anti-mouse antibody conjugated with Texas Red (Molecular Probes, Inc.) was used to visualize bacterial Dr antigen. After binding, controls were incubated with mouse immune sera prior to being stained with conjugated antibody. Slides were examined with a fluorescence microscope. Binding was evaluated by counting bacterial cells associated with tissue sections in 10 microscopic fields in two sets of slides. Incubation of Dr+ E. coli IH11128 with immune sera resulted in a 75 to 85% reduction in bacterial adherence to sections of mouse bladders and kidneys, respectively (Table 1). Sera of preimmune animals were not effective. In addition, we tested the reactivity of mouse immune sera with fimbrial antigens of prototype E. coli strains bearing Dr, Dr-II, F1845, AFA-I, or AFA-III adhesins. Bacterial smears on microscopic slides were fixed with 4% formaldehyde, incubated with mouse immune sera, and then stained with anti-mouse goat antibody conjugated with Texas Red. Immune sera recognized homologous Dr antigen and AFA-III fimbrial antigen but did not react with fimbrial antigens of Dr-II, F1845, and AFA-I.
FIG. 2.
Electron and light microscopy showing sections of mouse urinary bladder 48 h after infection with Dr+ E. coli 11128 or with the Dr− mutant DR14. (A) Electron microscopy shows that Dr+ bacteria adhere to the bladder epithelium (open arrowhead), invade the superficial layer of epithelium (filled arrowheads), and cause its focal damage (arrow); bar, 1 μm. (B) Normal appearance of the bladder epithelium in a mouse infected with a Dr− E. coli strain. (C) Light microscopy shows multiple bacterial cells associated with the surface of the bladder epithelium. (D) Lack of bacterial cells in the bladder of a mouse infected with a Dr− E. coli strain.
TABLE 1.
Reduction in Dr+ E. coli IH11128 binding to bladder and kidney sections after incubation with immune mouse serum
| Section used for E. coli binding | Scorea with:
|
|||
|---|---|---|---|---|
| IH11128 (Dr+) | IH11128 (Dr+) immune serum | IH11128 (Dr+) preimmune serum | DR14 (Dr−) | |
| Mouse kidney | +++ | + | +++ | ± |
| Mouse bladder | +++ | + | +++ | ± |
+++, ≥100 bacterial cells per microscopic field; +, ≤25 bacterial cells per microscopic field; ±, ≤10 bacterial cells per microscopic field.
Our earlier attempts to establish chronic experimental UTI in healthy, nonimmunocompromised C3H/HeN mice were unsuccessful due to effective innate immunity mechanisms leading to the clearance of a challenging strain of Dr+ E. coli in a few days. Consequently, in this project, we did not attempt the vaccination of immunologically intact animals with purified Dr antigen to study the effect of this approach in preventing UTI. We found earlier that experimental UTI in C3H/HeJ mice challenged with uropathogenic Dr+ E. coli is characterized by a fluctuation in colonization density in the course of infection (3). Mean values for kidney colonization in mice challenged with Dr+ E. coli were higher at the 2nd through 4th weeks of infection than at the 6th through 8th weeks of infection and tended to increase at the 12th through 16th weeks. In this report, we demonstrate that immunization of LPS-hyporesponsive animals with purified Dr antigen elicits an excellent humoral response that may prevent increased mortality associated with experimental UTI. The number of dead animals reported for the group of nonimmunized animals significantly outnumbered that for immunized animals. Surprisingly, however, the bladders and kidneys of vaccinated mice remained colonized despite the high titers of anti-Dr antibody in sera, with a trend of decreasing average numbers of bacteria seen at weeks 4, 8, and 12. These findings are consistent with the lack of antibinding activity in the urine of immunized mice. On the contrary, reduction in mortality appears to be consistent with the presence of high titers of anti-Dr antibody in the serum. Interestingly, we have noticed a low level of immune response to Dr antigen in the sera of nonvaccinated animals infected with Dr+ E. coli. This finding suggests that in some animals, infection might spread beyond the urinary tract and thus elicit a stronger humoral immune response. Preincubation of Dr+ E. coli with immune sera resulted in a noticeable reduction in bacterial binding to the structures of mouse bladders and kidneys. Collagen type IV appears to be a significant binding site in mouse kidneys for E. coli expressing Dr adhesin (15). The receptor for Dr fimbriae on murine epithelial cells remains to be determined and might hypothetically involve integrins (4) or carcinoembryonic-like antigens (5). We hypothesized that the colonization of the mouse urinary tract occurs in a two-step process, initiated by binding and entry into uroepithelial cells and followed by a translocation to the interstitial compartment and attachment to collagen fibers (15). Binding to collagen may enhance the colonization and further contribute to dissemination of the infection. Immune mouse sera recognized fimbrial antigen expressed by prototype clinical strains bearing Dr and AFA-III adhesins, which is most likely due to high levels of homology between the DraE and the corresponding protein, Afa-III, from the afa operon (2). Due to low homology, there was no reactivity with prototype strains representing adhesins Dr-II, F1845, and AFA-I. Although vaccination of animals with fimbrial antigen was found to effectively protect them from bladder or kidney infection due to E. coli strains producing type 1 or P fimbriae, the antigenic variability of pilus fibers remains the major drawback of immunization with whole fimbriae (7, 13). As a result, effective protection might be directed against only a limited number of antigenically closely related strains. It remains to be investigated whether a change in the route of immunization with Dr fimbriae would decrease bladder and kidney colonization.
Acknowledgments
This work was supported by Public Health Service grant DK-420209 from the National Institute of Diabetes and Digestive and Kidney Diseases.
For editorial and graphic assistance, we thank the Ob/Gyn Publication, Grant and Media Support director and staff.
Editor: V. J. DiRita
REFERENCES
- 1.Arthur, M., C. E. Johnson, R. H. Rubin, R. D. Arbeit, C. Campanelli, C. Kim, S. Steinbach, M. Agarwal, R. Wilkinson, and R. Goldstein. 1989. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect. Immun. 57:303-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia, M. I., P. Gounon, P. Courcoux, A. Labigne, and C. Le Bouguenec. 1996. The afimbrial adhesive sheath encoded by the afa-3 gene cluster of pathogenic Escherichia coli is composed of two adhesins. Mol. Microbiol. 19:683-693. [DOI] [PubMed] [Google Scholar]
- 3.Goluszko, P., S. L. Moseley, L. D. Truong, A. Kaul, J. R. Williford, R. Selvarangan, S. Nowicki, and B. Nowicki. 1997. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J. Clin. Investig. 99:1662-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guignot, J., M.-F. Bernet-Camard, C. Poüs, L. Plançon, C. Le Bouguenec, and A. L. Servin. 2001. Polarized entry of uropathogenic Afa/Dr diffusely adhering Escherichia coli strain IH11128 into human epithelial cells: evidence for α5β1 integrin recognition and subsequent internalization through a pathway involving caveolae and dynamic unstable microtubules. Infect. Immun. 69:1856-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guignot, J., I. Peiffer, M.-F. Bernet-Camard, D. M. Lublin, C. Carnoy, S. L. Moseley, and A. L. Servin. 2000. Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering family of Escherichia coli that infect the human polarized intestinal Caco-2/TC7 cells. Infect. Immun. 68:3554-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 7.Langermann, S., S. Palaszynski, M. Barnhart, G. Auguste, J. S. Pinkner, J. Burlein, P. Barren, S. Koenig, S. Leath, C. H. Jones, and S. J. Hultgren. 1997. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 276:607-611. [DOI] [PubMed] [Google Scholar]
- 8.Lublin, D. M., and K. E. Coyne. 1991. Phospholipid-anchored and transmembrane versions of either decay-accelerating factor or membrane cofactor protein show equal efficiency in protection from complement-mediated cell damage. J. Exp. Med. 174:35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowicki, B., L. Fang, J. Singhal, S. Nowicki, and C. Yallampalli. 1997. Lethal outcome of uterine infection in pregnant but not in nonpregnant rats and increased death rate with inhibition of nitric oxide. Am. J. Reprod. Immunol. 38:309-312. [DOI] [PubMed] [Google Scholar]
- 10.Nowicki, B., A. Labigne, S. Moseley, R. Hull, S. Hull, and J. Moulds. 1990. The Dr hemagglutinin, afimbrial adhesins AFA-I and AFA-III, and F1845 fimbriae of uropathogenic and diarrhea-associated Escherichia coli belong to a family of hemagglutinins with Dr receptor recognition. Infect. Immun. 58:279-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowicki, B., J. Moulds, R. Hull, and S. Hull. 1988. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect. Immun. 56:1057-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nowicki, B., R. Selvarangan, and S. Nowicki. 2001. Family of Escherichia coli Dr adhesins: decay-accelerating factor receptor recognition and invasiveness. J. Infect. Dis. 183(Suppl. 1):S24-S27. [DOI] [PubMed] [Google Scholar]
- 13.Pecha, B., D. Low, and P. O'Hanley. 1989. Gal-Gal pili vaccines prevent pyelonephritis by piliated Escherichia coli in a murine model. Single-component Gal-Gal pili vaccines prevent pyelonephritis by homologous and heterologous piliated E. coli strains. J. Clin. Investig. 83:2102-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pham, T. Q., P. Goluszko, V. Popov, S. Nowicki, and B. J. Nowicki. 1997. Molecular cloning and characterization of Dr-II, a nonfimbrial adhesin-I-like adhesin isolated from gestational pyelonephritis-associated Escherichia coli that binds to decay-accelerating factor. Infect. Immun. 65:4309-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selvarangan, R., P. Goluszko, J. Singhal, C. Carnoy, S. Moseley, B. Hudson, S. Nowicki, and B. Nowicki. 2004. Interaction of Dr adhesin with collagen type IV is a critical step in Escherichia coli renal persistence. Infect. Immun. 72:4827-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]