Abstract
Aspergillus fumigatus causes invasive pulmonary aspergillosis (IPA). This disease is one of the most life-threatening opportunistic infections in immunocompromised patients. The type of immunosuppressive regimen under which IPA occurs has rarely been investigated. In this study, we evaluated various parameters of the innate immune response during the progression of murine IPA induced by the intratracheal administration of A. fumigatus conidia as a function of two immunosuppressive treatments: a corticosteroid and a chemotherapeutic agent. We compared host responses various times after infection in terms of survival, pulmonary production of pro- and anti-inflammatory cytokines, cellular trafficking in the airways, lung injury, respiratory distress, and fungal development. We found that IPA pathogenesis involved predominantly fungal development in mice treated by chemotherapy and an adverse host response in mice treated with a corticosteroid. These previously unrecognized differences should be taken into account in evaluations of the pathogenesis of IPA in animal models.
Aspergillus fumigatus belongs to a family of saprophytic filamentous fungi found in most environments. It sporulates abundantly, leading to the release into the atmosphere of large quantities of conidia. A. fumigatus spreads within the environment by means of airborne conidia, and the small diameter of these spores (2 to 3 μm) enables them to reach the lung alveoli (19). Pulmonary host defenses against A. fumigatus are mediated by phagocytic cells of the innate immune system. Resident alveolar macrophages are thought to eliminate conidia by phagocytosis, whereas recruited polymorphonuclear neutrophils (PMN) destroy hyphae by producing reactive oxygen species (30, 34).
If the immune defense system of the lung is weakened, then conidia germinate and produce hyphae that invade the surrounding lung tissues (39), leading to the development of invasive pulmonary aspergillosis (IPA). This disease is one of the most life-threatening opportunistic infections in immunocompromised patients (19), and its incidence has increased in recent decades (14, 18). This increase in incidence has been associated with an increase in the number of people at risk due to solid organ transplantation, hematopoeitic stem cell transplantation, and intensive chemotherapeutic or immunosuppressive regimens (24). Furthermore, the difficulty of diagnosing IPA early and the low efficacy of treatments result in a high rate of mortality due to this disease in immunocompromised patients (10, 23).
Despite the increase in the number of IPA patients, the pathogenesis of this mycosis remains poorly understood. Experimental animal models of IPA have been developed for investigation of the role of innate immunity in the progression of the disease. Various infection protocols have been used and have generated conflicting data. More importantly, the type of immunosuppressive regimen in which IPA develops has received very little attention. In this study, we investigated various parameters of the innate defense system during IPA progression as a function of the immunological status of the host. We aimed to compare host pulmonary responses and IPA development in mice infected with the same inoculum and in which innate immunity status was modified by treatment with either a corticosteroid or a chemotherapeutic agent. We chose to study these two types of immunosuppressive treatments because patients receiving corticosteroids for the prevention or treatment of rejection after allogeneic transplantation and those receiving myelotoxic chemotherapy for cancer are at the highest risk of IPA (37).
After establishing the optimal conditions for lethal infection following the intratracheal administration of a given concentration of conidia, we compared host responses in terms of survival, production of pro- and anti-inflammatory cytokines, cellular trafficking, lung injury, respiratory distress, and fungal development. We also investigated therapeutic treatment with an antifungal drug.
Our results clearly indicate that the pathogenesis of IPA varies according to the type of immunosuppression, with fungal development or an adverse host response playing the predominant role. These previously unrecognized differences should be taken into account in evaluations of the pathogenesis of IPA in animal models.
MATERIALS AND METHODS
Reagents.
Cortisone acetate, O-dianisidine dihydrochloride, Tween 20, hydrogen peroxide (H2O2), and acetyl acetone were obtained from Sigma-Aldrich (St. Louis, Mo.). Vinblastine was purchased from Lilly (St. Cloud, France). Ketamine was obtained from Merial (Lyon, France), and xylazine was obtained from Bayer (Puteau, France). Pentobarbital was obtained from Sanofi (Libourne, France), and Diff-Quick products were supplied by Dade-Behring (Paris, France). Kits for immunoenzymatic detection of the galactomannan antigen of Aspergillus were obtained from Bio-Rad (Marnes la Coquette, France). d-Glucosamine hydrochloride 4-(dimethylamino)-benzaldehyde (DMAB) was obtained from Merck (Darmstadt, Germany). Amphotericin B was obtained from Bristol-Myers Squibb (Paris, France).
Animals.
Seven-week-old male C57BL/6 mice (Janvier, Le Genest St. Isle, France) were used in all experiments. All mice were cared for in accordance with standard guidelines.
Preparation of A. fumigatus conidia.
A. fumigatus strain CBS 144.89 was maintained on 2% malt agar for 1 week at 27°C. The strain was a clinical isolate that has been used by one of us (J.-P.L.) to induce experimental murine IPA (see references 16 and 29). It was verified that >99% of the harvested condidia germinated after 10 h of incubation at 37°C on 2% glucose-1% peptone medium. Conidia were harvested from the slant into 0.1% Tween 20 in phosphate-buffered saline (PBS). The resulting suspension of conidia was centrifuged for 2 min at 13,000 × g, the supernatant was discarded, and the number of conidia per milliliter was determined with a hemocytometer. The concentration was adjusted so that the conidial load was administered in a total volume of 50 μl of sterile PBS-0.1% Tween 20.
Immunosuppressive treatments and infection.
Corticosteroid-induced immunosuppression was achieved by the intraperitoneal administration of 10 mg of cortisone acetate in saline. Mice were treated 3 days before infection, on the day of infection, and on days 2 and 4 after infection (16). Chemotherapy-induced granulocytopenia was achieved by the intravenous administration of 5 mg of the antineoplastic drug vinblastine/kg 66 h before infection. Under these conditions, animals displayed total PMN depletion from day 0 (the day of infection) until day 2 (21; unpublished data). In a preliminary work dealing with A. fumigatus infection (data not shown), we observed that under the vinblastine regimen mouse survival and cytokine production were similar to those obtained when neutropenia was induced with antigranulocyte monoclonal antibody RB6-8C5 as described previously (1).
Animals were infected intratracheally under general anesthesia achieved with a mixture of ketamine (40 mg/kg) and xylazine (8 mg/kg) administered via the intramuscular route. A catheter (diameter, 0.86 mm) was inserted into the trachea via the oropharynx. Proper insertion was verified by checking the formation of mist due to expiration on a mirror placed in front of the external end. A 50-μl conidial suspension was placed at the internal end of the catheter by introducing a micropipette with a sterile disposable tip for gel loading into the catheter. Mice then were immediately held upright in order to facilitate inhalation of conidia and until normal breathing resumed. This protocol allows highly reproducible infection of the lungs, and 10 times more inoculum reaches the lungs via this route than via the intranasal route.
Antifungal treatments.
In some experiments, immunosuppressed mice were treated with an antifungal drug. Animals were treated 24 h after infection and every 24 h over the next 5 days with intravenously injected amphotericin B (1 mg/kg).
Collection of BALF and alveolar cells.
Mice were killed by the administration of a lethal dose of pentobarbital at various times, and bronchoalveolar lavage fluid (BALF) was collected. The lungs were washed out eight times with 0.5 ml of PBS. Total cell counts in BALF were determined with a Coulter Counter (Coulter Electronics, Margency, France), and differential cell counts were determined after cytospin centrifugation and staining with Diff-Quick products. We centrifuged 2 ml of BALF for 15 min at 300 × g and immediately processed 100 μl of the supernatant for myeloperoxidase (MPO) activity determinations. The remaining supernatant was frozen at −20°C for subsequent use for the quantification of immunoreactive tumor necrosis factor alpha (TNF-α) and interleukin 10 (IL-10) as previously described (33). Cell pellets were used for hemoglobin determinations.
MPO and hemoglobin determinations.
As described by Hirano (15), we incubated 100 μl of BALF at room temperature for 15 min with 100 μl of O-dianisidine dihydrochloride (1.25 mg/ml in Hank's medium) supplemented with 0.004% H2O2. The reaction was stopped by the addition of 10 μl of NaN3 (1% [wt/vol]). MPO activity was determined as the change in the absorbance at 450 nm. Cell pellets obtained from BALF were lysed in 1 ml of distilled water. The lysate was centrifuged at 15,000 × g, and the amount of hemoglobin was determined by measuring the absorbance at 414 nm.
Histological analyses.
Whole lungs were fixed in 3.7% neutral buffered formaldehyde, embedded in paraffin, and cut into 5-μm sections. The sections were stained with hematoxylin-eosin for tissue examination and with methenamine silver stain for fungus detection (36).
Respiratory distress measurement.
Respiratory function in freely moving mice was assessed by barometric plethysmography with a whole-body plethysmograph (Buxco Electronic, Sharon, Conn.) according to the manufacturer's instructions as previously described (6). Each animal was placed in the main chamber; the difference in pressure between this chamber and a reference chamber was determined with a differential pressure transducer connected to an amplifier, and the data were recorded with BioSystem XA analyzer software (Buxco Electronic). The enhanced pause (Penh) correlated very closely and positively with the pulmonary resistance and was calculated as follows: Penh = [(Te − Tr)/Tr](PEP/PIP), where Te is the expiratory time (seconds), Tr is the relaxation time (time to pressure decay to 36% the total box pressure at expiration), PEP is the peak expiratory pressure (milliliters per second), and PIP is the peak inspiratory pressure (milliliters per second).
Chitin determination in lungs, kidneys, and brains.
The lungs, kidneys and brains of mice were homogenized in 5 ml of 0.05% Tween 20 in distilled water, frozen, and freeze-dried. Preparations were hydrolyzed in 1 ml of 8 N HCl and heated at 100°C for 4 h. The reaction mixture was neutralized by the addition of 1 ml of 8 N NaOH and centrifuged (1,500 × g, 10 min, 20°C). We added 200 μl of glucosamine (50 to 200 μg/ml of H2O) for the standards or 200 μl of supernatant from a tissue preparation for the test samples to 200 μl of a 25:1 mixture of Na2CO3 (1.5 M) and acetyl acetone (4%); the mixture was heated at 100°C for 20 min and cooled in water. We added 1.4 ml of 95% ethanol to this mixture. A fresh solution of DMAB (1.6 g in 60 ml of HCl and 95% ethanol [vol/vol]) was prepared, and 200 μl of this solution was added to each tube. We measured the absorbance at 520 nm after 45 min. Chitin content was expressed in glucosamine equivalents (17).
Detection of the galactomannan antigen of A. fumigatus in lungs, kidneys, and brains.
Aliquots (300 μl) of organ homogenates prepared for the chitin assay were centrifuged at 1,500 × g for 10 min at 4°C. Galactomannan antigen (35, 38) was detected in supernatants by means of a commercially available kit as recommended by the manufacturer. We calculated the ratio of the absorbance in a sample to the absorbance in threshold serum to determine whether the results should be classified as negative (ratio of <1) or positive (ratio of ≥1).
Statistical analysis.
Survival data were analyzed by means of log-rank comparisons of Kaplan-Meier survival curves, followed by the Wilcoxon test with JMP 5.0 software. All other data were expressed as means and standard errors of the means (SEMs) and were compared by use of the Mann-Whitney U test. Data were considered statistically significant when P values were <0.05.
RESULTS
Survival.
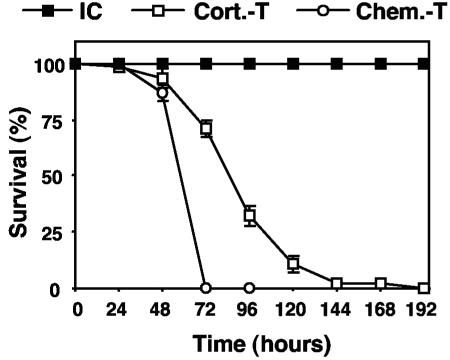
The survival curves (Fig. 1) were established in the various models with the same concentration of inoculum (107 conidia/mouse). Infected immunocompetent mice survived, whereas 100% of corticosteroid-treated mice died between days 2 and 6. Infection of chemotherapy-treated mice resulted in 100% mortality between days 2 and 3.
FIG. 1.
Survival of immunocompetent and immunosuppressed mice after intratracheal infection with conidia of A. fumigatus. Corticosteroid-treated (Cort.-T) mice were subjected to immunosuppression by administration of cortisone acetate (10 mg/mouse) 3 days before infection, on the day of infection, and on days 2 and 4 after infection. Chemotherapy-treated (Chem.-T) mice were subjected to immunosuppression by administration of vinblastine (5 mg/kg) 66 h before infection. Control immunocompetent (IC) mice were left untreated. Ten animals per group were infected with 107 conidia in four independent experiments. The values presented are the means and SEMs for 40 mice. The difference between the survival of corticosteroid-treated mice and the survival of chemotherapy-treated mice was statistically significant (P < 0.001), with median survival times of 4.13 ± 0.16 and 2.88 ± 0.05 days, respectively.
Cellular trafficking in BALF during infection.
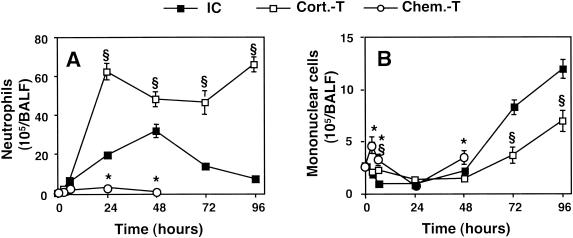
PMN (Fig. 2A) were recovered in BALF from immunocompetent mice as soon as 3 h after infection. These cells peaked in number at 48 h [(31.5 ± 3.4) × 105 cells/BALF sample] and decreased thereafter. The mononuclear cell concentration (Fig. 2B) started to increase at 72 h. In corticosteroid-treated mice, the PMN concentration increased rapidly (within 24 h) and extensively [(62.4 ± 4.8) × 105 cells/BALF sample] and remained constant thereafter until the death of the animals. The kinetics of mononuclear cell recruitment were similar to those in immunocompetent mice, but the values obtained were lower [(11.8 ± 2.1) × 105 and (6.9 ± 1.1) × 105 cells/BALF sample (n = 5; P < 0.05) at 96 h for immunocompetent and corticosteroid-treated mice, respectively]. As expected, almost no PMN recruitment was observed in chemotherapy-treated mice. As the animals died soon after infection, the influx of mononuclear cells, which generally occurs later, was quite low.
FIG. 2.
Leukocyte counts in BALF of immunocompetent and immunosuppressed mice infected with A. fumigatus. Mice were killed, and their lungs were washed out at 3, 6, 24, 48, 72, and 96 h after infection with 107 conidia. Neutrophils (A) and mononuclear cells (B) were counted as described in Materials and Methods. Abbreviations are as defined in the legend to Fig. 1. The values presented are the means and SEMs for at least five mice. Section signs and asterisks indicate statistically significant differences (P < 0.05) between corticosteroid- and chemotherapy-treated mice and immunocompetent mice, respectively.
TNF-α and IL-10 concentration profiles in BALF.
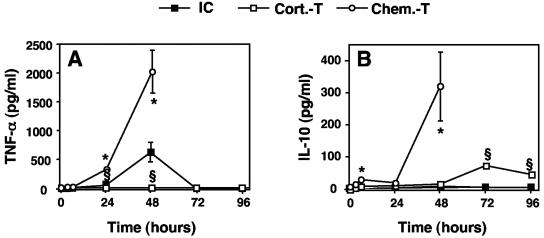
In immunocompetent mice, the TNF-α concentration increased by 48 h after infection and decreased to undetectable levels thereafter (Fig. 3A). No IL-10 production was detected (Fig. 3B). TNF-α was not detected in corticosteroid-treated mice (Fig. 3A), whereas IL-10 production peaked (73 ± 11 pg/ml) at 72 h (Fig. 3B). It was hypothesized that the lack of TNF-α production could be accounted for by the known inhibitory effect of corticosteroids on the NF-κB-dependent synthesis of proinflammatory cytokines (41). In chemotherapy-treated mice, both TNF-α and IL-10 (Fig. 3) were detected at very high concentrations at 48 h (2,008 ± 393 and 313 ± 111 pg/ml [n = 5], respectively).
FIG. 3.
TNF-α and IL-10 concentrations in BALF of immunocompetent and immunosuppressed mice infected with A. fumigatus. TNF-α (A) and IL-10 (B) concentrations in BALF were determined at 3, 6, 24, 48, 72, and 96 h after infection with 107 conidia as described in Materials and Methods. Abbreviations are as defined in the legend to Fig. 1. The values presented are the means and SEMs for at least five mice. Section signs and asterisks indicate statistically significant differences (P < 0.05) between corticosteroid- and chemotherapy-treated mice and immunocompetent mice, respectively.
Of note is that immunocompetent and immunocompromised mice that received sham infections with the conidium vehicle did not show significant PMN influx or TNF-α production. Thus, upon PBS-0.1% Tween 20 administration, the highest PMN concentration was observed in corticosteroid-treated mice at 48 h, i.e., (0.45 ± 0.45) × 105 cells/BALF sample (n = 3), while the highest TNF-α concentration was detected in chemotherapy-treated mice at 24 h, i.e., 41.7 ± 14.8 pg/ml (n = 3). These two values represent 1 and 2% of the corresponding values obtained upon administration of conidia, respectively.
MPO and hemoglobin levels in BALF.
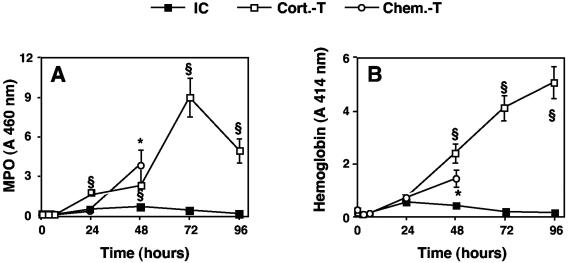
MPO activity was used as an index of the release of the granular content of PMN, and hemoglobin concentration was used as an index of alveolocapillary injury. MPO activity (Fig. 4A) and hemoglobin (Fig. 4B) were not detected in BALF from immunocompetent mice, demonstrating low levels of PMN degranulation and the absence of pulmonary tissue injury. In corticosteroid-treated mice, MPO activity was high, but the kinetics of this enzyme did not parallel the influx of PMN. MPO activity was low until 48 h after infection and then began to increase rapidly and substantially (by a factor of four). The hemoglobin concentration increased gradually until the death of the animals, indicating the presence of major alveolocapillary lesions. In chemotherapy-treated mice, MPO activity was relatively high, whereas there were very few PMN. This finding might be accounted for by the influx of monocytes (Fig. 2B) shown to contain MPO (28). The hemoglobin concentration 24 h before death was lower than that in corticosteroid-treated mice, indicating the presence of fewer or less severe alveolocapillary lesions (1.4 ± 0.4 versus 5.1 ± 0.7 [n = 5]) (P < 0.05).
FIG. 4.
MPO and hemoglobin levels in BALF of immunocompetent and immunosuppressed mice infected with A. fumigatus. MPO (A) and hemoglobin (B) levels in BALF were determined at 3, 6, 24, 48, 72, and 96 h after infection with 107 conidia as described in Materials and Methods. Abbreviations are as defined in the legend to Fig. 1. The values presented are the means and SEMs for at least five mice. Section signs and asterisks indicate statistically significant differences (P < 0.05) between corticosteroid- and chemotherapy-treated mice and immunocompetent mice, respectively.
Pulmonary tissue lesions and fungal development.
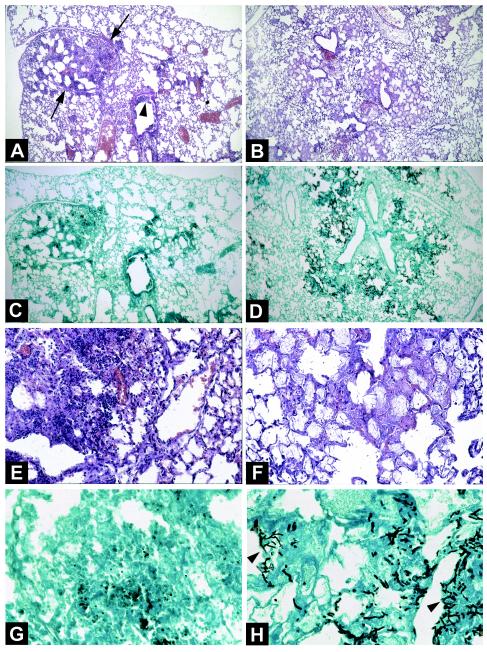
The differences in alveolocapillary injury deduced on the basis of hemoglobin determinations were confirmed by histological studies (Fig. 5) performed on lung samples collected 72 and 48 h after infection for corticosteroid-treated and chemotherapy-treated animals, respectively. The rationale was to analyze IPA at a time when most of the mice had survived the infection but were nonetheless at a terminal stage of illness; i.e., most of them would die within the next 24 h. As the survival of corticosteroid-treated mice is prolonged compared to that of vinblastine-treated mice, the chosen time points were 72 and 48 h, respectively. The lungs of corticosteroid-treated mice showed large foci of pneumonia (Fig. 5A) and exudative bronchiolitis (Fig. 5A) with the destruction of bronchi and alveolae. Hemorrhagic necrosis with neutrophil infiltration was also observed (Fig. 5E). A. fumigatus was observed in small numbers, mainly in the form of conidia (Fig. 5G). In contrast, for chemotherapy-treated mice, we observed only a few bronchiolitis lesions and diffuse pneumonia with edema and congestion within alveolae (Fig. 5B) but with no inflammatory exudate involving PMN or other cells (Fig. 5F). Of note is that alveolae and parenchyma were invaded by numerous hyphae of A. fumigatus (Fig. 5D and H).
FIG. 5.
Lung histological features of corticosteroid- and chemotherapy-treated mice infected with A. fumigatus. (A, C, E, and G) Lung sections from a corticosteroid-treated mouse 72 h after intratracheal inoculation with 107 conidia. (A) Hematoxylin-eosin stain; magnification, ×60. (E) Hematoxylin-eosin stain; magnification, ×250. A large focus of pneumonia (A, arrows) and exudative bronchiolitis (A, arrowhead) with the destruction of bronchi and alveolae can be seen. Hemorrhagic necrosis and numerous inflammatory cells, especially PMN, also can be seen (E). (C) Methenamine silver stain; magnification, ×60. (G) Methenamine silver stain; magnification, ×400. A few conidia and very few hyphae within the lesions of necrosis can be seen. (B, D, F, and H) Lung sections from a chemotherapy-treated mouse 48 h after intratracheal inoculation with 107 conidia. (B) Hematoxylin-eosin stain; magnification, ×60. (F) Hematoxylin-eosin stain; magnification, ×250. Lesions of bronchiolitis and diffuse pneumonia with edema and congestion within alveolae (B) but without an inflammatory exudate of any cell type, including PMN, can be seen. (D) Methenamine silver stain; magnification, ×60. (H) Methenamine silver stain; magnification, ×400. Several foci of bronchocentric necrosis containing numerous hyphae (H, arrowheads) can be seen. Invasion of alveolae by numerous hyphae of A. fumigatus also can be seen. Note the absence of an inflammatory exudate.
Measurement of respiratory distress.
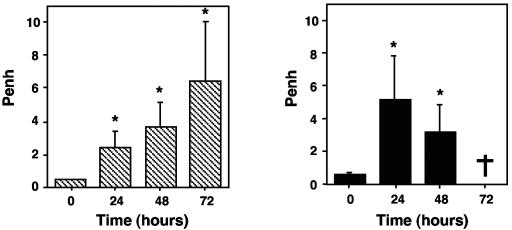
We investigated whether the pulmonary lesions observed in corticosteroid-treated mice and the fungal development observed in chemotherapy-treated mice were responsible for a deleterious effect on lung function by calculating a basal respiratory index reflecting respiratory waveforms, Penh, in conscious immunosuppressed mice at various times during infection (Fig. 6). Penh gradually increased in corticosteroid-treated mice until the death of the animals. In chemotherapy-treated mice, Penh increased by 24 h after infection (5.1 ± 2.8 [n = 10]) but decreased by 48 h after infection (3.1 ± 1.8 [n = 10]). These results suggest that the respiratory system was more severely affected in corticosteroid-treated mice than in chemotherapy-treated mice at the time of death.
FIG. 6.
Measurement of respiratory distress in corticosteroid-treated (left) or chemotherapy-treated (right) mice infected with A. fumigatus. Penh was determined in conscious mice just before infection (time zero) and every day after infection with 107 conidia until the death of the animals. The values presented are the means and SEMs for at least five mice. A dagger indicates that no chemotherapy-treated mice survived to day 3. Asterisks indicate statistically significant differences (P < 0.05) between various time points and time zero.
Dissemination of A. fumigatus.
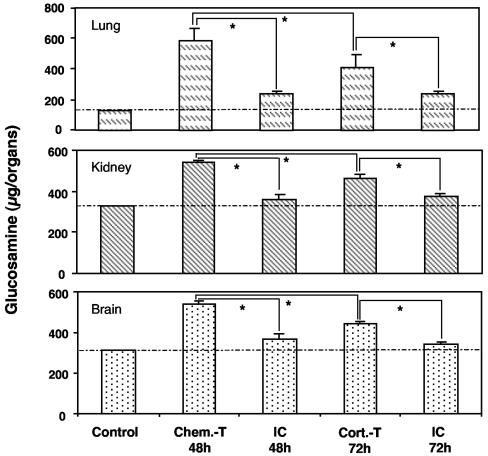
Chitin is a component of the hyphal wall and is absent from mammalian cells. We assayed chitin to estimate the amounts of hyphal fungi in lungs, kidneys, and brains (Fig. 7). Chitin determinations confirmed histological results, as chitin concentrations were significantly lower in lungs of corticosteroid-treated mice than in those of chemotherapy-treated mice. This difference was also observed in brains and kidneys, indicating that the level of fungal dissemination was higher in chemotherapy-treated mice than in corticosteroid-treated mice. Experiments were performed to check the viability of the invading A. fumigatus. Brains and kidneys from chemotherapy-treated animals were collected 48 h after infection and homogenized for 30 s (Potter-Elvehjem glass homogenizer) in 1 ml of 0.1% Tween in water. Homogenates were plated on 2% glucose-1% peptone medium and incubated at 37°C for 48 h. Growing A. fumigatus was observed for both organs, indicating the viability of the invading fungus (data not shown).
FIG. 7.
Chitin determination in lungs, kidneys, and brains of immunocompetent and immunosuppressed mice infected with A. fumigatus. Organs of corticosteroid- and chemotherapy-treated mice were collected 72 and 48 h, respectively, after infection with 107 conidia. Chitin content is expressed in glucosamine equivalents. Abbreviations are as defined in the legend to Fig. 1. The values presented are the means and SEMs for at least five mice. Asterisks indicate statistically significant differences (P < 0.05) between the various values. The dotted line indicates the mean of the background values obtained with uninfected control mice.
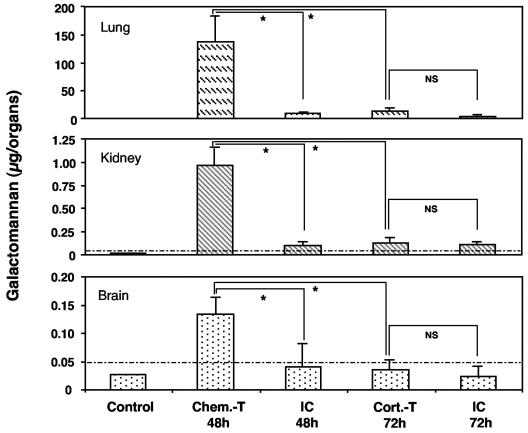
We determined galactomannan concentrations in these organs to confirm the results obtained for chitin (Fig. 8), as galactomannan is released by growing A. fumigatus. In corticosteroid-treated mice, galactomannan concentrations were very low, close to the detection threshold. In chemotherapy-treated mice, galactomannan was detected at high concentrations. These data suggest that fungal development was extensive and systemic in chemotherapy-treated mice, whereas it was restricted in terms of both magnitude and location in corticosteroid-treated mice.
FIG. 8.
Galactomannan determination in the lungs, kidneys, and brains of immunocompetent and immunosuppressed mice infected with A. fumigatus. Organs of corticosteroid- and chemotherapy-treated mice were collected 72 and 48 h, respectively, after infection with 107 conidia. Abbreviations are as defined in the legend to Fig. 1. The values presented are the means and SEMs for at least five mice. Asterisks indicate statistically significant differences (P < 0.05) between the various values; NS, not significant. The dotted line indicates the background value (see Materials and Methods) below which the various means were considered negative.
Effect of fungicidal treatment on IPA development in immunosuppressed mice.
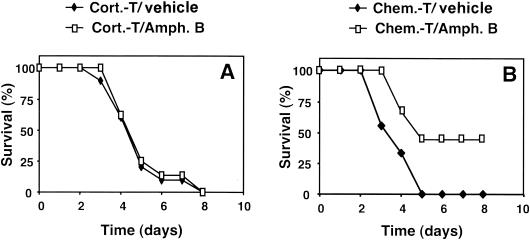
We investigated the extent to which fungal development was responsible for death in the two types of immunosuppressed mice by administering a fungicidal treatment: amphotericin B. In infections with 107 conidia/mouse, the level of the inoculum was too high for any major effect of the antifungal treatment to be detected. Therefore, in subsequent experiments, we infected mice with 3 × 106 conidia/mouse. The efficacy of the antifungal treatment was estimated by assessing survival in the immunosuppressed animals (Fig. 9). Chemotherapy-treated mice were sensitive to amphotericin B treatment, with 44% of mice still alive on day 8 after infection compared with 0% of untreated mice. In contrast, antifungal treatment had no effect in corticosteroid-treated mice, which were as likely to die from infection as untreated mice.
FIG. 9.
Effect of antifungal treatment on the survival of immunosuppressed mice infected with A. fumigatus. Corticosteroid-treated (A) and chemotherapy-treated (B) mice were treated 24 h after infection and every 24 h for 5 days with intravenous amphotericin B (1 mg/kg) (Amph. B). The survival of 10 animals per group was monitored after infection with 3 × 106 conidia. Abbreviations are as defined in the legend to Fig. 1. One experiment representative of two is shown. The effect of the antifungal agent was statistically significant (P < 0.02) only for the chemotherapy-treated group.
DISCUSSION
This study demonstrates that host pulmonary responses to A. fumigatus differ according to the immunosuppressive regimen used.
The immune response of immunocompetent mice is characterized by moderate, transient inflammation leading to the complete elimination of the fungus without the formation of pulmonary lesions. The features of this inflammatory response are a transient influx of PMN and the production of the proinflammatory cytokine TNF-α. These data are consistent with other studies showing that PMN (25, 34) and TNF-α are beneficial for the defense against A. fumigatus (4, 5, 26, 27).
In corticosteroid-treated mice, IPA is characterized by the production of the anti-inflammatory cytokine IL-10 and by major PMN recruitment. In chemotherapy-treated mice, we observed both pro- and anti-inflammatory cytokine responses, with the simultaneous presence of TNF-α and IL-10. In both immunosuppression models, the presence of IL-10 was thus associated with death. In chemotherapy-treated mice, the results were consistent with previous data suggesting that high IL-10 concentrations contribute to the pathogenesis of IPA (7, 9, 32). In contrast, in corticosteroid-treated mice, the IL-10 concentrations might not have been sufficient (Fig. 3), as these animals seemed to die of tissue damage due to an excessive host response (see below). TNF-α has been reported to be essential for the induction of protective immunity against A. fumigatus, as its presence is associated with resistance to IPA in mice (5, 26, 27). Our present data are in agreement, as we found that this cytokine was present in immunocompetent mice but absent in corticosteroid-treated mice. However, the presence of large amounts of TNF-α in BALF from chemotherapy-treated mice did not prevent the development of the disease. This finding is most likely explained by the absence of circulating PMN, as the major mode of TNF-α action is precisely the recruitment of this cell population (40).
The differences between these two models of immunosuppression were even more marked with regard to fungal development. As in humans (12), chemotherapy-treated mice were more sensitive to A. fumigatus than corticosteroid-treated mice. These data confirm the essential role of PMN because, in the absence of this cell population, the fungus rapidly invaded the lungs. However, we found neither severe pulmonary lesions nor major respiratory distress in PMN-depleted mice. Furthermore, the presence of chitin in various vital organs, in association with galactomannan, which is released by the fungus and can be used as a viability index, suggests that fungal invasion of the lungs led to substantial dissemination outside the pulmonary sphere. This suggestion is supported by the recovery of growing A. fumigatus in brains and kidneys 48 h postinfection. Conversely, the low levels of chitin and galactomannan found in the organs of corticosteroid-treated mice indicate that the innate pulmonary response of these mice was still functional and prevented fungal development by limiting the germination of conidia in the lung parenchyma and further fungal dissemination. These results suggest that the inhibitory effects of corticosteroids on the antifungal activities of phagocytes observed in vitro (31) may be less efficient in vivo. Our results are consistent with two previous studies reporting extensive infiltration of the lungs and blood by hyphae in granulocytopenic rabbits (3) and by fragmented and damaged hyphae in the lungs of corticosteroid-treated mice (11).
Corticosteroids are known to have anti-inflammatory properties due to their inhibitory effects on the activation of various transcription factors, in particular, NF-κB (41). However, some studies have shown that they do not inhibit the synthesis of IL-8, a chemokine inducing PMN recruitment (8, 22). Furthermore, a recent study showed that the inhibition of NF-κB activation during the resolution of inflammation protracts the inflammatory response and prevents apoptosis (20). These observations may account for the persistent high concentration of PMN in BALF from corticosteroid-treated mice. Duong et al. (11) also observed a major influx of PMN and the release of large amounts of MPO. They concluded that the development of A. fumigatus in lung tissue resulted in irreversible tissue injury. In contrast, our comparison of host responses under two types of immunosuppression suggests that the lung tissue injury observed in corticosteroid-treated mice is not due to fungal invasion but instead is due to PMN influx, as neutropenic mice displayed low levels of lung injury despite extensive fungal development. This hypothesis is in agreement with data reported by Graybill et al. (13). Although they observed an influx of only a small number of PMN, a finding which could be explained by differences in mouse strains, A. fumigatus strains, or corticosteroid regimens, they reported that granulocyte colony-stimulating factor treatment of corticosteroid-treated mice was harmful, resulting in an increase in the leukocyte count and the development of large pulmonary abscesses. They concluded that the pneumonia caused by A. fumigatus might actually expand as a large number of PMN migrates to the site of infection.
In a previous study (29), alveolar macrophages alone were shown to control A. fumigatus infection in neutropenic mice only when the concentration of conidia instilled was low, i.e., ≤105 conidia/mouse. Very low levels of PMN recruitment were observed upon instillation of such an inoculum in immunocompetent and corticosteroid-treated mice. In contrast, at a high concentration of inoculum (107 conidia/mouse), PMN were essential for the clearance of A. fumigatus. Thus, in the present study, the lack of PMN recruitment in chemotherapy-treated mice was associated with extensive pulmonary invasion by the fungus. The death of animals can be explained by the dissemination of the fungus and the colonization of other vital organs. In corticosteroid-treated mice, paradoxically, the inflammatory response was exacerbated, with high levels of PMN recruitment. We suggest that this situation may lead to pulmonary lesions, resulting in respiratory distress. Thus, although the inflammatory response can prevent fungal invasion, its exacerbation leads to death. This hypothesis is supported by the findings obtained for antifungal treatment. Amphotericin B increased the survival of chemotherapy-treated mice. This result, similar to that already reported for neutropenic rats (2), indicates that fungal development was responsible for the death of these animals. In contrast, the lack of efficacy of this treatment in corticosteroid-treated mice suggests that these mice died from other causes.
This study demonstrates that IPA development varies according to the immunosuppressive treatment administered. Although A. fumigatus is obviously a contributory factor, our data suggest that the host response may also play a part in the pathogenesis of IPA. Of note is that the experimental identification of virulence factors in A. fumigatus is based on an analysis of the infectivity of mutants in animal models in which IPA is systematically induced with corticosteroids (with or without prior cyclophosphamide treatment). The difference in the patterns of infection observed in the two models presented here suggests that corticosteroid-treated animals may not be the best screening system for the identification of virulence determinants associated with the mycelial growth of this opportunistic pathogen in the lungs. It would therefore be of value to revisit the virulence of mutants in both models.
Editor: T. R. Kozel
REFERENCES
- 1.Balloy, V., J. M. Sallenave, B. Crestani, M. Dehoux, and M. Chignard. 2003. Neutrophil DNA contributes to the antielastase barrier during acute lung inflammation. Am. J. Respir. Cell Mol. Biol. 28:746-753. [DOI] [PubMed] [Google Scholar]
- 2.Becker, M. J., S. de Marie, M. H. Fens, H. A. Verbrugh, and I. A. Bakker-Woudenberg. 2003. Effect of amphotericin B treatment on kinetics of cytokines and parameters of fungal load in neutropenic rats with invasive pulmonary aspergillosis. J. Antimicrob. Chemother. 52:428-434. [DOI] [PubMed] [Google Scholar]
- 3.Berenguer, J., M. C. Allende, J. W. Lee, K. Garrett, C. Lyman, N. M. Ali, J. Bacher, P. A. Pizzo, and T. J. Walsh. 1995. Pathogenesis of pulmonary aspergillosis. Granulocytopenia versus cyclosporin and methylprednisolone-induced immunosuppression. Am. J. Respir. Crit. Care Med. 152:1079-1086. [DOI] [PubMed] [Google Scholar]
- 4.Brieland, J. K., C. Jackson, F. Menzel, D. Loebenberg, A. Cacciapuoti, J. Halpern, S. Hurst, T. Muchamuel, R. Debets, R. Kastelein, T. Churakova, J. Abram, R. Hare, and A. O'Garra. 2001. Cytokine networking in lungs of immunocompetent mice in response to inhaled Aspergillus fumigatus. Infect. Immun. 69:1554-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cenci, E., A. Mencacci, C. F. d'Ostiani, G. Del Sero, P. Mosci, C. Montagnoli, A. Bacci, and L. Romani. 1998. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J. Infect. Dis. 178:1750-1760. [DOI] [PubMed] [Google Scholar]
- 6.Chabot, S., K. Koumanov, G. Lambeau, M. H. Gelb, V. Balloy, M. Chignard, J. A. Whitsett, and L. Touqui. 2003. Inhibitory effects of surfactant protein A on surfactant phospholipid hydrolysis by secreted phospholipases A2. J. Immunol. 171:995-1000. [DOI] [PubMed] [Google Scholar]
- 7.Clemons, K. V., G. Grunig, R. A. Sobel, L. F. Mirels, D. M. Rennick, and D. A. Stevens. 2000. Role of IL-10 in invasive aspergillosis: increased resistance of IL-10 gene knockout mice to lethal systemic aspergillosis. Clin. Exp. Immunol. 122:186-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culpitt, S. V., D. F. Rogers, P. Shah, C. De Matos, R. E. Russell, L. E. Donnelly, and P. J. Barnes. 2003. Impaired inhibition by dexamethasone of cytokine release by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 167:24-31. [DOI] [PubMed] [Google Scholar]
- 9.Del Sero, G., A. Mencacci, E. Cenci, C. F. d'Ostiani, C. Montagnoli, A. Bacci, P. Mosci, M. Kopf, and L. Romani. 1999. Antifungal type 1 responses are upregulated in IL-10-deficient mice. Microbes Infect. 1:1169-1180. [DOI] [PubMed] [Google Scholar]
- 10.Denning, D. W. 1996. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. 23:608-615. [DOI] [PubMed] [Google Scholar]
- 11.Duong, M., N. Ouellet, M. Simard, Y. Bergeron, M. Olivier, and M. G. Bergeron. 1998. Kinetic study of host defense and inflammatory response to Aspergillus fumigatus in steroid-induced immunosuppressed mice. J. Infect. Dis. 178:1472-1482. [DOI] [PubMed] [Google Scholar]
- 12.Gerson, S. L., G. H. Talbot, S. Hurwitz, B. L. Strom, E. J. Lusk, and P. A. Cassileth. 1984. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann. Intern. Med. 100:345-351. [DOI] [PubMed] [Google Scholar]
- 13.Graybill, J. R., R. Bocanegra, L. K. Najvar, D. Loebenberg, and M. F. Luther. 1998. Granulocyte colony-stimulating factor and azole antifungal therapy in murine aspergillosis: role of immune suppression. Antimicrob. Agents Chemother. 42:2467-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groll, A. H., P. M. Shah, C. Mentzel, M. Schneider, G. Just-Nuebling, and K. Huebner. 1996. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J. Infect. 33:23-32. [DOI] [PubMed] [Google Scholar]
- 15.Hirano, S. 1996. Migratory responses of PMN after intraperitoneal and intratracheal administration of lipopolysaccharide. Am. J. Physiol. Lung Cell Mol. Physiol. 270:L836-L845. [DOI] [PubMed] [Google Scholar]
- 16.Jaton-Ogay, K., S. Paris, M. Huerre, M. Quadroni, R. Falchetto, G. Togni, J. P. Latgé, and M. Monod. 1994. Cloning and disruption of the gene encoding an extracellular metalloprotease of Aspergillus fumigatus. Mol. Microbiol. 14:917-928. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, A. R. 1971. Improved method of hexosamine determination. Anal. Biochem. 44:628-635. [DOI] [PubMed] [Google Scholar]
- 18.Kontoyiannis, D. P., and G. P. Bodey. 2002. Invasive aspergillosis in 2002: an update. Eur. J. Clin. Microbiol. Infect. Dis. 21:161-172. [DOI] [PubMed] [Google Scholar]
- 19.Latgé, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrence, T., D. W. Gilroy, P. R. Colville-Nash, and D. A. Willoughby. 2001. Possible new role for NF-kappaB in the resolution of inflammation. Nat. Med. 7:1291-1297. [DOI] [PubMed] [Google Scholar]
- 21.Lefort, J., M. Singer, D. Leduc, P. Renesto, M. A. Nahori, M. Huerre, C. Creminon, M. Chignard, and B. B. Vargaftig. 1998. Systemic administration of endotoxin induces bronchopulmonary hyperreactivity dissociated from TNF-alpha formation and neutrophil sequestration into the murine lungs. J. Immunol. 161:474-480. [PubMed] [Google Scholar]
- 22.Levine, S. J., P. Larivee, C. Logun, C. W. Angus, and J. H. Shelhamer. 1993. Corticosteroids differentially regulate secretion of IL-6, IL-8, and G-CSF by a human bronchial epithelial cell line. Am. J. Physiol. 265:L360-L368. [DOI] [PubMed] [Google Scholar]
- 23.Lin, S. J., J. Schranz, and S. M. Teutsch. 2001. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 32:358-366. [DOI] [PubMed] [Google Scholar]
- 24.Marr, K. A., T. Patterson, and D. Denning. 2002. Aspergillosis. Pathogenesis, clinical manifestations, and therapy. Infect. Dis. Clin. N. Am. 16:875-894. [DOI] [PubMed] [Google Scholar]
- 25.Mehrad, B., R. M. Strieter, T. A. Moore, W. C. Tsai, S. A. Lira, and T. J. Standiford. 1999. CXC chemokine receptor-2 ligands are necessary components of neutrophil-mediated host defense in invasive pulmonary aspergillosis. J. Immunol. 163:6086-6094. [PubMed] [Google Scholar]
- 26.Mehrad, B., R. M. Strieter, and T. J. Standiford. 1999. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J. Immunol. 162:1633-1640. [PubMed] [Google Scholar]
- 27.Nagai, H., J. Guo, H. Choi, and V. Kurup. 1995. Interferon-gamma and tumor necrosis factor-alpha protect mice from invasive aspergillosis. J. Infect. Dis. 172:1554-1560. [DOI] [PubMed] [Google Scholar]
- 28.Neill, M. A., W. R. Henderson, and S. J. Klebanoff. 1985. Oxidative degradation of leukotriene C4 by human monocytes and monocyte-derived macrophages. J. Exp. Med. 162:1634-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Philippe, B., O. Ibrahim-Granet, M. C. Prevost, M. A. Gougerot-Pocidalo, M. Sanchez Perez, A. Van der Meeren, and J. P. Latgé. 2003. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect. Immun. 71:3034-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rex, J. H., J. E. Bennett, J. I. Gallin, H. L. Malech, and D. A. Melnick. 1990. Normal and deficient neutrophils can cooperate to damage Aspergillus fumigatus hyphae. J. Infect. Dis. 162:523-528. [DOI] [PubMed] [Google Scholar]
- 31.Roilides, E., K. Uhlig, D. Venzon, P. A. Pizzo, and T. J. Walsh. 1993. Prevention of corticosteroid-induced suppression of human polymorphonuclear leukocyte-induced damage of Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect. Immun. 61:4870-4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roilides, E., A. Dimitriadou, I. Kadiltsoglou, T. Sein, J. Karpouzas, P. A. Pizzo, and T. J. Walsh. 1997. IL-10 exerts suppressive and enhancing effects on antifungal activity of mononuclear phagocytes against Aspergillus fumigatus. J. Immunol. 158:322-329. [PubMed] [Google Scholar]
- 33.Salez, L., M. Singer, V. Balloy, C. Creminon, and M. Chignard. 2000. Lack of IL-10 synthesis by murine alveolar macrophages upon lipopolysaccharide exposure. Comparison with peritoneal macrophages. J. Leukoc. Biol. 67:545-552. [DOI] [PubMed] [Google Scholar]
- 34.Schaffner, A., H. Douglas, and A. Braude. 1982. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J. Clin. Investig. 69:617-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibuya, K., M. Takaoka, K. Uchida, M. Wakayama, H. Yamaguchi, K. Takahashi, S. Paris, J. P. Latgé, and S. Naoe. 1999. Histopathology of experimental invasive pulmonary aspergillosis in rats: pathological comparison of pulmonary lesions induced by specific virulent factor deficient mutants. Microb. Pathog. 27:123-131. [DOI] [PubMed] [Google Scholar]
- 36.Sinha, B. K., D. P. Monga, and S. Prasad. 1988. A combination of Gomori-Grocott methenamine silver nitrate and hematoxylene and eosin staining technique for the demonstration of Candida albicans in tissue. Quad. Sclavo Diagn. 24:129-132. [PubMed] [Google Scholar]
- 37.Stevens, D. A., V. L. Kan, M. A. Judson, V. A. Morrison, S. Dummer, D. W. Denning, J. E. Bennett, T. J. Walsh, T. F. Patterson, G. A. Pankey, et al. 2000. Practice guidelines for diseases caused by Aspergillus. Clin. Infect. Dis. 30:696-709. [DOI] [PubMed] [Google Scholar]
- 38.Stynen, D., A. Goris, J. Sarfati, and J. P. Latgé. 1995. A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J. Clin. Microbiol. 33:497-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomee, J. F., and T. S. van der Werf. 2001. Pulmonary aspergillosis. Neth. J. Med. 59:244-258. [DOI] [PubMed] [Google Scholar]
- 40.Ulich, T. R., L. R. Watson, S. M. Yin, K. Z. Guo, P. Wang, H. Thang, and J. del Castillo. 1991. The intratracheal administration of endotoxin and cytokines. I. Characterization of LPS-induced IL-1 and TNF mRNA expression and the LPS-, IL-1-, and TNF-induced inflammatory infiltrate. Am. J. Pathol. 138:1485-1496. [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto, Y., and R. B. Gaynor. 2001. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J. Clin. Investig. 107:135-142. [DOI] [PMC free article] [PubMed] [Google Scholar]