Significance
Common single-amino acid variations of proteins are traditionally regarded as functionally neutral polymorphisms because these substitutions are mostly located outside functionally relevant surfaces. In this study, we present an example of a functionally relevant coding sequence variation, which, as we show here, confers risk for large artery atherosclerotic stroke. The single-residue variation M1(A213V) in serpin family A member 1 (SERPINA1) [encoding alpha-1 antitrypsin (AAT)] is situated outside the protease-reactive inhibitory loop and is found in a β-turn on the protein surface. We show that the Ala-to-Val exchange in the gate region of AAT alters its functional dynamics toward neutrophil elastase in the presence of complex lipid-containing plasma and also affects the overall structural flexibility of the protein.
Keywords: genetics, ischemic stroke, large artery stroke, antitrypsin, variation
Abstract
Large artery atherosclerotic stroke (LAS) shows substantial heritability not explained by previous genome-wide association studies. Here, we explore the role of coding variation in LAS by analyzing variants on the HumanExome BeadChip in a total of 3,127 cases and 9,778 controls from Europe, Australia, and South Asia. We report on a nonsynonymous single-nucleotide variant in serpin family A member 1 (SERPINA1) encoding alpha-1 antitrypsin [AAT; p.V213A; P = 5.99E-9, odds ratio (OR) = 1.22] and confirm histone deacetylase 9 (HDAC9) as a major risk gene for LAS with an association in the 3′-UTR (rs2023938; P = 7.76E-7, OR = 1.28). Using quantitative microscale thermophoresis, we show that M1 (A213) exhibits an almost twofold lower dissociation constant with its primary target human neutrophil elastase (NE) in lipoprotein-containing plasma, but not in lipid-free plasma. Hydrogen/deuterium exchange combined with mass spectrometry further revealed a significant difference in the global flexibility of the two variants. The observed stronger interaction with lipoproteins in plasma and reduced global flexibility of the Val-213 variant most likely improve its local availability and reduce the extent of proteolytic inactivation by other proteases in atherosclerotic plaques. Our results indicate that the interplay between AAT, NE, and lipoprotein particles is modulated by the gate region around position 213 in AAT, far away from the unaltered reactive center loop (357–360). Collectively, our findings point to a functionally relevant balance between lipoproteins, proteases, and AAT in atherosclerosis.
Stroke is the leading cause of long-term disability and the second most common cause of death worldwide (1, 2). About a quarter of ischemic stroke cases are caused by large artery atherosclerotic stroke (LAS) (3, 4). Atherosclerosis is a chronic inflammatory condition that involves a number of well-characterized steps. Initial stages include the deposition of lipids in vascular endothelial cells, whereas more advanced stages are characterized by fibrotic changes with formation of a fibrotic cap and, eventually, plaque rupture (5). LAS exhibits the highest heritability of all stroke subtypes, with estimates ranging from 40.3 to 66.6% (6, 7). This fact is reflected by recent genome-wide association studies that found common variants for LAS at multiple genomic loci (8–10). The lead SNPs from these regions all reside within intergenic (4, 7, 11) or intronic (12) regions, and most of them are situated within a regulatory sequence marked by DNase I hypersensitivity sites.
Whole-exome (13) and whole-genome (14) sequencing efforts have identified multiple common, low-frequency, and rare variants that have not yet been examined for association with LAS. Conceivably, these variants might account for some of the missing heritability of LAS. To search for novel variants and genes implicated in atherosclerotic stroke, we assembled the largest cohort of LAS cases to date (3,127 cases and 9,778 controls from Germany, the United Kingdom, Australia, and Pakistan).
In the current study, we found two exome-wide significant variants, one in the established LAS risk gene histone deacetylase 9 (HDAC9) and one in serpin family A member 1 (SERPINA1). The main target of the inhibitor alpha-1 antitrypsin (AAT), encoded by SERPINA1, is neutrophil elastase (NE). AAT and NE are both involved in inflammation, and an imbalance between AAT and NE has previously been discussed as a possible mechanism in atherosclerotic plaque and aneurysm formation (15–18).
The rate-determining step of the inhibitory reaction between ATT and NE is the reversible formation of a noncovalent docking intermediate (encounter complex), which subsequently progresses to the covalent tetrahedral complex (16, 19, 20). The common M1 variants of AAT, the minor A213 allele and the prevailing V213 allele (rs6647), have previously been characterized as normal, functionally equivalent plasma isoforms (20) with very similar plasma levels and association rate constants for the purified isoforms (16, 19, 20). However, adjacent to the loop containing residue 213 is a hydrophobic groove (16), which, together with the polymorphic side chain, may differentially interact with endogenous hydrophobic components of plasma. In this study, we functionally characterized the interaction between AAT and NE in human plasma using microscale thermophoresis, and provide evidence for differential behavior of the two major M1(V213) and M1(A213) alleles toward lipoproteins.
Results
Common Variants in SERPINA1 and HDAC9 Associate with LAS.
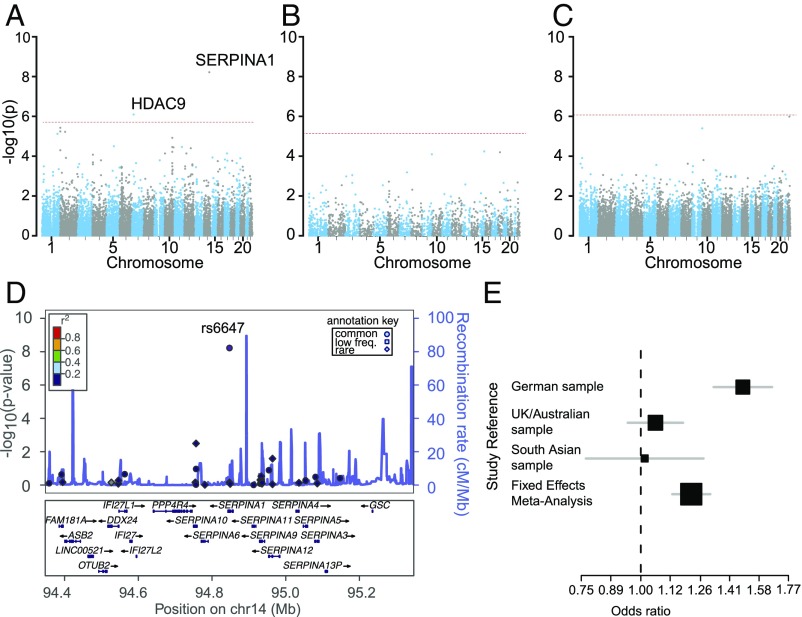
Characteristics of the case and control samples, including details on quality control (QC), are presented in Figs. S1 and S2, Table S1, and SI Materials and Methods. The overall strategy for the transethnic, exome-wide association study is shown in Fig. 1. We found two common variants to be associated with LAS on an exome-wide level (P < 1.88E-6 with Bonferroni correction for all common variants studied; Fig. 2 A–C and Table 1). The first variant, exm1124208 (rs6647) in SERPINA1, encoding M1(A213) in AAT showed a minor allele frequency (MAF) of 17.8% in Caucasian controls and 17.1% in South Asian controls [odds ratio (OR) 95% confidence interval [CI_95] = 1.22 [1.13–1.31], P = 5.99E-9 in the transethnic meta-analysis] (Fig. 2 D and E). PolyPhen2 (score = 0.0), PROVEAN (score = 1.11), and SIFT (score = 0.54) predict this variant to be likely benign and tolerated. The association between LAS and rs6647 remained significant (P = 7.01E-9) when removing carriers of the low-frequency Z and S alleles (98 cases and 262 controls) that have previously been shown to be associated with lower plasma levels of AAT. The second variant, rs2023938 in HDAC9 (MAF = 9.1% and 10.8% in Caucasians and South Asian controls, respectively; OR [CI_95] = 1.28 [1.16–1.40, P = 7.76E-7]), is in the 3′-UTR of HDAC9 and in high linkage disequilibrium (LD) with previously published risk variants for LAS in the 3′ region of HDAC9 [rs11984041 (11): r2 = 1; rs2107595 (4): r2 = 0.53].
Fig. S1.
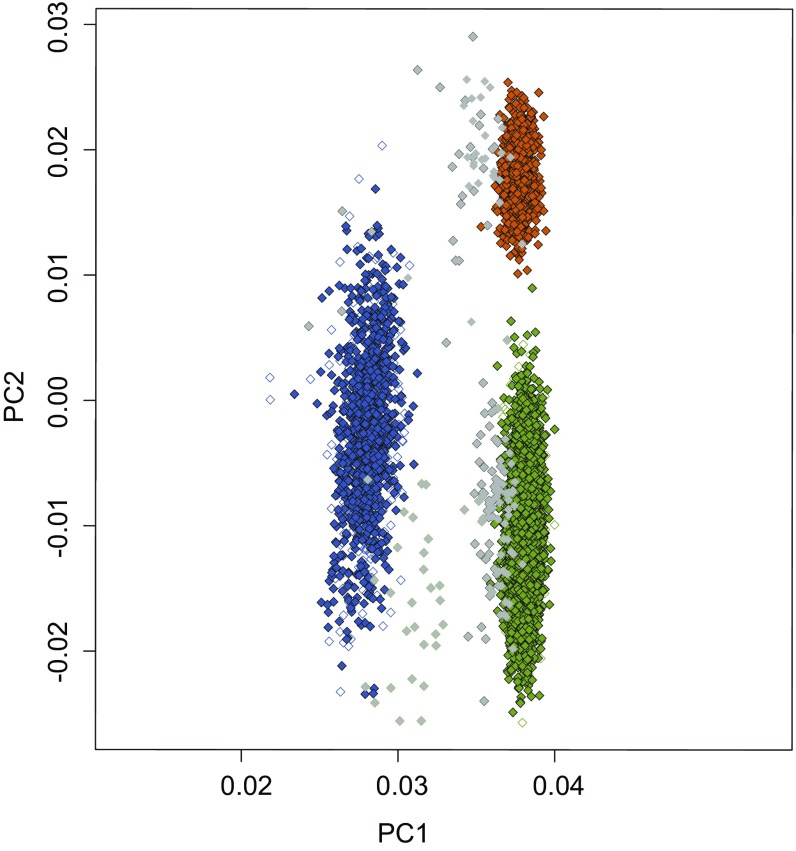
Outlier analysis by PC analysis. German, UK, Australian, and South Asian samples were checked to remove potential outliers with respect to genetic background. Green with black outline, German cases; white with green outline, Kooperative Gesundheitsforschung in der Region Augsburg (KORA) controls; blue with black outline, UK/Australian cases; white with blue outline, Wellcome Trust Case Control Consortium 2 controls; orange with black outline, Pakistan cases; white with orange outline, Pakistan controls. Samples in gray were removed from the analysis after visual inspection. PC1 and PC2 are displayed on the x axis and y axis, respectively.
Fig. S2.
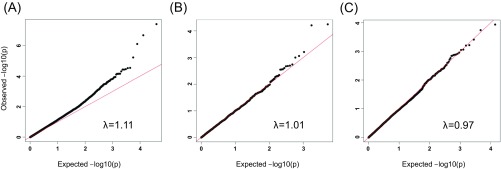
Quantile–quantile (QQ) plots for single-variant analysis. Shown are the observed versus expected −log10 P value distributions for common (A), low-frequency (B), and rare (C) variants, along with the genomic inflation factors (λ). The red line shows the expected (null) distribution of the statistic.
Table S1.
Overview of case and control samples
| Study | No. of subjects | Age, mean ± SD | Male, % | Ancestry |
| Cases | ||||
| Munich | 597 | 72.0 ± 9.4 | 54.0 | Caucasian |
| Muenster | 879 | 69.9 ± 6.4 | 67.1 | Caucasian |
| Immunochip London | 466 | 66.9 ± 8.3 | 67.5 | Caucasian |
| Edinburgh | 378 | 73.5 ± 9.7 | 57.4 | Caucasian |
| Oxford | 348 | 70.2 ± 6.5 | 57.4 | Caucasian |
| ASGC | 158 | 70.0 ± 13.0 | 69.6 | Caucasian |
| RACE | 376 | 48.2 ± 9.7 | 60.2 | South Asian |
| Controls | ||||
| 1958BC (WTCCC2) | 5,963 | NA | 56.1 | Caucasian |
| KORA | 2,921 | 55.6 ± 13.2 | 52.0 | Caucasian |
| PROMIS | 978 | NA | NA | South Asian |
Shown are the LAS case and stroke-free control studies used for the current analysis. ASGC, Australian Stroke Genetics Consortium; KORA, Kooperative Gesundheitsforschung in der Region Augsburg; NA, information not available; WTCCC2, Wellcome Trust Case Control Consortium 2.
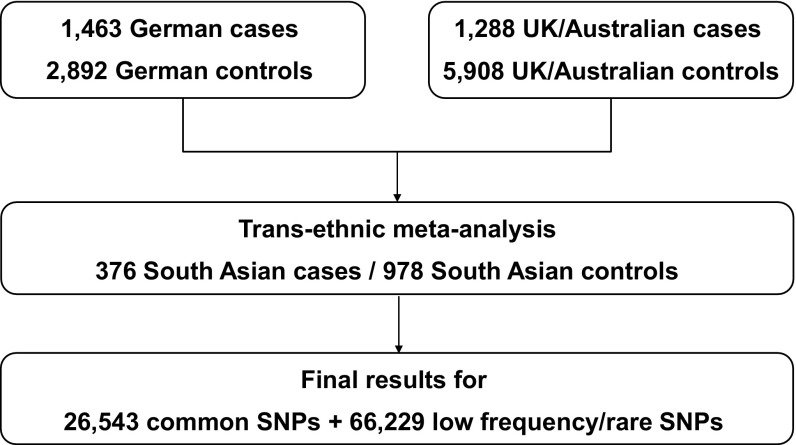
Fig. 1.
Flow chart of the exome chip analysis. Samples from Germany, the United Kingdom, and Australia were pooled with samples from South Asia for the transethnic metaanalysis.
Fig. 2.
Main association results. Shown are the results for common (A, MAF > 5%), low-frequency (B, 1% < MAF < 5%), and rare (C, MAF < 1%) variants. Genomic position is plotted on the x axis, and the −log10 of the P value is displayed on the y axis. The threshold for exome-wide significance after Bonferroni correction for the number of variants studied is displayed as a dashed red line. (D) LocusZoom plot [−log10 (p-value)] for the SERPINA1 region and LAS. Shown is the region for the top signal ± 500 kb. Common, low-frequency, and rare variants are displayed as filled circles (●), squares (■), and diamonds (♦), respectively. Blue peaks represent estimated recombination rates. (E) Forest plot of associations with rs6647 in individual samples and in the resulting fixed-effects metaanalysis. Sample sizes are reflected by the size of each square. Gray bars show the [CI_95] of the point estimate.
Table 1.
Top association signals from the exome chip analysis
| dbSNP ID no. | Chromosome:position | Minor/major allele | MAF | Functional annotation | Gene | P value | OR [CI_95] |
| Common variants | |||||||
| rs6647 | 14:94847415 | G/A | 0.18 | p.Val237Ala | SERPINA1 | 5.99E-9 | 1.22 [1.13–1.31] |
| rs2023938 | 7:19036775 | G/A | 0.09 | 3′-UTR | HDAC9 | 7.76E-7 | 1.28 [1.16–1.40] |
| rs11553746 | 2:272203 | T/C | 0.35 | p.Thr95Ile | ACP1 | 3.67E-6 | 1.14 [1.07–1.21] |
| rs11681377 | 2:65394714 | T/C | 0.47 | Intergenic | Intergenic | 5.91E-6 | 1.15 [1.08–1.22] |
| rs2290911 | 2:224919 | G/A | 0.34 | Synonymous | SH3YL1 | 5.65E-6 | 1.14 [1.07–1.21] |
| rs10863936 | 1:212237798 | G/A | 0.49 | Intronic | DTL | 7.33E-6 | 1.19 [1.12–1.26] |
| rs11191447 | 10:104652323 | T/C | 0.09 | Intronic | C10orf32-AS3MT | 1.18E-5 | 1.22 [1.10–1.36] |
| rs1004467 | 10:104594507 | C/T | 0.10 | Intronic | CYP17A1 | 3.22E-5 | 1.20 [1.08–1.32] |
| rs17368582 | 11:102738075 | C/T | 0.13 | Synonymous | MMP12 | 3.30E-5 | 1.20 [1.10–1.31] |
| rs1172822 | 19:55819845 | T/C | 0.36 | Intronic | BRSK1 | 3.69E-5 | 1.19 [1.10–1.28] |
| rs11191580 | 10:104906211 | C/T | 0.09 | 5′-UTR | NT5C2 | 5.70E-5 | 1.21 [1.09–1.35] |
| Low-frequency variants | |||||||
| rs62019510 | 15:85657120 | G/A | 0.03 | p.Asn329Ile | PDE8A | 5.60E-5 | 1.39 [1.18–1.63] |
| rs111986709 | 18:29054294 | T/C | 0.04 | p.Ser771Tyr | DSG3 | 6.08E-5 | 1.35 [1.17–1.57] |
| rs2231400 | 9:135753629 | G/A | 0.04 | p.Ile5Thr | AK8 | 7.77E-5 | 1.30 [1.12–1.50] |
| Rare variants | |||||||
| rs45439291 | 22:41077613 | A/G | 0.003 | p.Arg191Gln | MCHR1 | 9.99E-7 | 2.08 [1.34–3.24] |
| rs114895119 | 9:139264888 | A/T | 0.003 | p.Glu270Val | CARD9 | 3.81E-6 | 1.84 [1.41–2.40] |
Shown are the results from the single-variant analysis for common, low-frequency, and rare variants with a P value <1E-4. P values and ORs are displayed from the transethnic metaanalysis of the German, UK/Australian, and South Asian samples. SNP position and functional annotation are given for hg19. Exome-wide significant results are shown in bold. dbSNP, the single nucleotide polymorphism database.
Among 14 variants meeting the criterion of suggestive association (P < 1E-4), three are situated within known risk loci for atherosclerotic phenotypes or risk factors for atherosclerosis (Table 1). MMP12 (21) and CYP17A1 (22) are associated with LAS and coronary artery disease, respectively, whereas C10orf32-AS3MT (23, 24) is an established risk locus for hypertension. None of the low-frequency (MAF < 5%) and rare (MAF < 1%) variants reached exome-wide significance (Fig. 2 B and C). Gene-based tests revealed no exome-wide significant signals (Table S2).
Table S2.
Top 10 results of the gene-based test
| Gene name | No. of SNPs in test | P value of metaSKAT test |
| B4GALNT4 | 2 | 2.28E-05 |
| TTC29 | 3 | 4.08E-05 |
| KANK4 | 2 | 4.38E-05 |
| RSPO3 | 4 | 6.39E-05 |
| SLC25A17 | 2 | 7.43E-05 |
| CALM2 | 2 | 9.68E-05 |
| TRPM4 | 3 | 0.00010258 |
| SPTBN5 | 16 | 0.00014232 |
| GSTA5 | 2 | 0.00017318 |
| RPS6KL1 | 3 | 0.00020523 |
P values were derived from metaSKAT tests. The number of SNPs refers to the number of rare input SNPs into the individual SKAT tests before metaSKAT.
M1(A213) and Atherosclerotic Plaque Characteristics in Advanced Stages of Disease.
To explore associations between the M1(A213) variant and histological characteristics of atherosclerotic plaques, we analyzed 1,414 carotid endarterectomy samples from patients with advanced atherosclerotic lesions assembled through the Athero-Express study. The M1(A213) variant was nominally associated with a lower macrophage content (P = 0.03, beta = −0.19, SE = 0.09). However, when controlling for the assessment of multiple plaque characteristics, including plaque hemorrhage, collagen content, and smooth muscle cell number, the association did not reach statistical significance. The results did not materially change when correcting for antithrombotic medication, lipid-lowering drugs, and smoking status. Also, expression quantitative trait locus (eQTL) analysis revealed no association between M1(A213) and AAT levels in atherosclerotic plaques.
M1(V213) Has a Higher Dissociation Constant Toward NE than M1(A213).
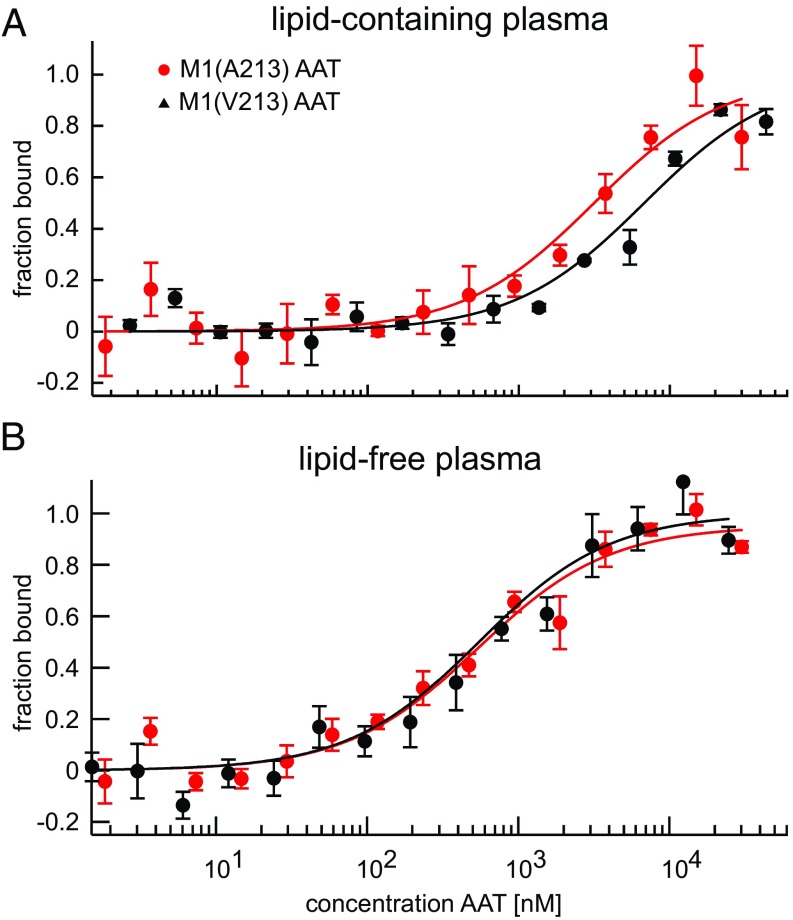
To explore potential functional differences between M1(A213) and M1(V213) with respect to their inhibitory behavior, we analyzed the initial interaction between AAT and NE. We characterized the formation of the encounter complex under equilibrium conditions using fluorescently labeled and catalytically inactive NE (S195A variant) in a microscale thermophoresis assay (25). AAT-deficient plasma [1:12 dilution in PBS (vol/vol)] was used as a matrix to include plasma-specific interactions that may influence the encounter complex formation between AAT and NE. Under this condition, the M1(A213) variant [dissociation constant (KD) = 3,200 ± 500 nM] exhibited an almost twofold lower dissociation constant than the M1(V213) variant (KD = 6,800 ± 1,000 nM) toward NE (Fig. 3A). These allele-specific differences disappeared when AAT-deficient plasma was freed from lipoproteins by ultracentrifugation and dialyzed against a phosphate buffer over a membrane with a 10-kDa cutoff (Fig. 3B).
Fig. 3.
Functional differences between M1 AAT variants found in lipid-containing, but not lipid-free, plasma. Shown are the fitted binding curves of the microscale thermophoresis assay under equilibrium conditions in lipid-containing (A) and lipid-free (B) plasma. A fixed concentration of fluorescently labeled and catalytically inactive NE was titrated with the M1(A213) or M1(V213) variant in AAT-deficient plasma. (A) In the presence of lipids, the KD (KD) observed with the M1(V213) variant [KD(A213) = 6,800 ± 1000 nM] was substantially higher than the KD observed with the M1(A213) variant [KD(V213) = 3,200 ± 500 nM]. (B) In the absence of lipids, there was no discernible difference between the two variants [KD(A213) = 540 ± 50 nM; KD(V213) = 550 ± 60 nM]. Fitted binding curves and KD values (mean ± SD) were derived from global fitting of three measurements (three independent protein expressions). The measurements were performed in 7.5% plasma and with 5 nM labeled NE.
M1(A213) Enhances the Structural Flexibility of AAT.
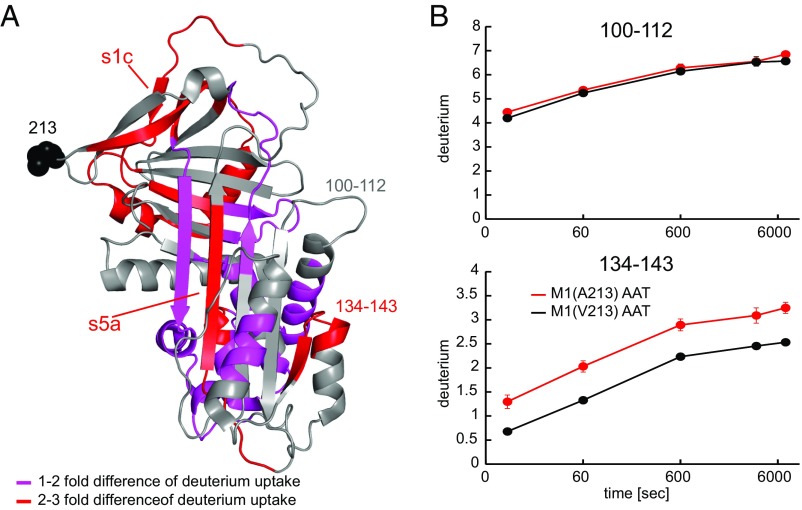
To determine the impact of the two variants on AAT structure, we further determined the hydrogen/deuterium exchange rates for M1(V213) and M1(A213). As illustrated in Fig. 4, the exchange rates between the two variants significantly differed for multiple pepsin-generated peptides. In several surface regions, the M1(A213) variant was more susceptible to deuterium uptake by one to three ions per fragment compared with M1(V213). Of note, significant differences in the deuterium uptake were also observed at a distance from residue 213. Specifically, the Ala-213 substitution led to a higher flexibility of the C-terminal end of the reactive center loop, including strand 1 of the C β-sheet (s1c) as well as strand 5 of the A-sheet in the shutter region (s5A) (26) (Fig. 4). Conceivably, the increased dynamics of the Ala-213 variant may reduce exosite interactions with other plasma components, and may thus account for the observed higher affinity for NE in the complex plasma environment.
Fig. 4.
Differences in hydrogen/deuterium exchange kinetics between M1(A213) and M1(V213) AAT. (A) Shown is the cumulative difference across five time points (10 s to 120 min) in deuterium uptake between M1(A213) and M1(V213) mapped onto the crystal structure of AAT (Protein Data Bank ID code 1QLP). Peptides displaying a significant increase in deuterium uptake in M1(A213) compared with M1(V213) are colored red (two- to threefold increase) and pink (one- to twofold increase). Otherwise, there was no significant difference in deuterium uptake between the two variants, such as the stretch between positions 100 and 112 (gray arrow). Substitution of Ala for Val at position 213 results in subtle but widespread destabilization of AAT. (B) Example kinetic curves for the deuterium uptake with two peptides. Peptide 134–143 (Lower) displays a significantly higher deuterium uptake in the M1(A213) AAT variant compared with M1(V213), whereas no significant difference is seen with peptide 100–112 (Upper). Values represent means from three different measurements.
SI Materials and Methods
Genotyping.
Study case samples were genotyped at the Helmholtz Zentrum München (Deutsches Forschungszentrum für Gesundheit und Umwelt, Institute of Human Genetics). Genotype calling was carried out according to best practices from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium (48). For the stroke-free controls, raw genotyping intensities were not available, thus prohibiting reclustering of variants. Genotypes were converted to PLINK (42) format, TOP-oriented. The strand orientation and the same coding for minor alleles were precisely controlled to match between cases and controls both within and between studies. Allele frequencies of all variants were compared with reported allele frequencies in the 1,000 Genomes phase I reference panel.
QC was performed separately for cases and controls according to best practices, merged, and aggregated into three files. Australian and UK cases were pooled because principal component (PC) analysis showed no significant difference in population structure. Samples with a call rate <95% per variant and one individual per pair of close relatives or duplicates (Pi-Hat > 0.5, calculated with PLINK on identity by descent markers) were excluded from analysis. Variants with a call rate <98%, Hardy Weinberg Equilibrium (HWE) P value < 10-6, MAF < 0.01%, mean heterozygosity > ±1 SD difference from mean in cases, and mean heterozygosity > ±3 SD in controls were excluded.
Ancestry-informative autosomal markers were LD-pruned (r2 > 0.5) and carried forward to PC analysis to check the population genetic structure and identify outliers in cases and controls using R. Genetic outliers were identified by visual inspection of the PC plots (Fig. S1).
QC Characteristics.
PC analysis partitioned the Caucasian samples into groups consistent with their geographical location. Thirteen German cases, 62 UK/Australian cases, 29 German controls, and 55 UK controls were removed during QC or after visual inspection of plotting the first two PCs (Fig. S1).
Association analyses were performed separately for the Caucasian and South Asian samples using an additive logistic model with the first two PCs as covariates to correct for population stratification. The results were then entered into a transethnic metaanalysis (Fig. 1). This strategy reduced the inflation factor to nominal levels without any sign of systemic inflation (Fig. S2). Inspection of the distribution of χ2 (score) tests showed an overdispersion of test statistics (genomic control λ estimate of ∼1.21). However, after removal of very rare SNPs (MAF < 0.1%, or fewer than three alleles in cases), Wald statistics for the remaining SNPs appeared to be sampled from an overall central χ2 distribution (λ = 1.06). For common (λ = 1.11 after removal of significant loci from this analysis), low-frequency (λ = 1.01), and rare (λ = 0.97) variants, we did not observe significant inflation of test statistics (Fig. S2).
The single-variant analysis covered 229,504 exonic variants, 2,262 splice site variants, 6,209 intronic variants, 78 5′-UTR variants, and 525 3′-UTR variants. A total of 92,772 variants passed QC procedures. The remaining variants were either monomorphic or had an allele frequency that was too low to yield meaningful test statistics (MAF < 0.1% or fewer than three minor alleles).
In the final transethnic metaanalysis, 66,229 SNPs had a MAF < 5% (Fig. 1). MAF distributions were similar across the German and UK/Australian controls and showed a correlation of R2 = 0.98 compared with the 1000 Genomes phase 1 reference panel.
Gene-based association tests were performed using SKAT and SKAT-O (49, 50). In total, 17,168 genes harboring at least two rare variants within ±1 kb of gene boundaries were analyzed for association. Studies were analyzed separately and metaanalyzed using the R package metaSKAT (51).
For the SKAT and SKAT-O analyses, the total number of genes having at least two polymorphic variants passing QC was 17,168.
Association with Carotid Plaque Characteristics in Athero-Express.
Human carotid atherosclerotic plaques (n = 1,414) were obtained from the Athero-Express study, an ongoing, longitudinal, multicenter study collecting carotid atherosclerotic plaques from patients with significant (>70%) stenosis who undergo carotid endarterectomy (52, 53). The medical ethics committee of the participating centers approved the study, and written informed consent was obtained from all patients.
Immunohistochemical plaque phenotyping was performed as described elsewhere (52, 53). In brief, carotid plaques were divided into segments of 5-mm thickness. The culprit lesion was defined and used for immunohistochemical staining. Calcification (hematoxylin and eosin) and collagen content (picrosirius red) were semiquantitatively defined as no or minor versus moderate/heavy staining as previously described (52, 53). Atheroma size was defined as less than or more than 10% fat content (hematoxylin and eosin and picrosirius red). We quantitatively scored macrophages (CD68) and smooth muscle cells (α-actin) as the percentage of plaque area. We also determined the presence of intraplaque hemorrhage (hematoxylin and eosin) and counted the number of vessels per three to four hotspots (CD34). Genotyping was done in two batches using Affymetrix arrays. Using phased data from HapMap 2 (release 22, b36) as a reference, rs6647 was imputed. QC was performed according to standard procedures. We adopted a significance level of P < 0.01 to account for testing multiple plaque phenotypes.
Expression and Purification of Recombinant Proteins.
The cDNA modification of the human M1(A213) variant was introduced by PCR from a cDNA encoding the human M1(V213) variant (54) using the forward primer DJ3689 (5′-GACTTCCACGTG-GACCAGGcGACCACCGTGAA-3′) and reverse primer DJ3613 (5′-GATGACCGGT TTTTTGGGTGGGATTCACCACTT-3′). Following digestion of the PCR product with PmlI and AgeI, the insert was cloned into the respective sites of the wild-type AAT pTT5 plasmid. Recombinant clones were identified by BstEII digestion because this site is lost by the V (GTG)-to-A (GCG) replacement. The cDNA constructs were expressed under identical conditions in HEK293 cells stably transfected with EBNA-1 cells (Yves Durocher, National Research Council Canada, Montreal) cultured in serum-free Free-Style 293 expression medium (Thermo Fisher Scientific, Inc.), 1% Pluronic, and G416 (25 μg/mL) at 37 °C and 8% CO2. Harvesting of supernatants and purification of recombinant AAT variants were performed as described (54).
As a binding partner for AAT, we used a catalytically inactive NE (S195A variant). We changed Ser195 to Ala by inserting an oligoduplex [DJ3532 (5′-GTGAACGTATGCACTCTGGTGCCACGTCGGCAGGCAGGCATCTGCTTCGGGGACGCT-3′) and DJ3533 (5′-CGTCCCCGAAGCAGATGCCTGCCTGCCGACGTGGCACCAGAGTGCATACGTTCACAC-3′)] into the AlfI site of the previously described wild-type mouse NE construct in pTT5 (55). A cysteine tag (DDDCDDD) was added by insertion of an oligoduplex DJ3632 (5′-CTAGCGACGACGATTGCGACGATGATC-3′) and DJ3633 (5′-CTAGGATCATCGTCGCAATCGTCGTCG-3′) into the AvrII site.
Labeling of NE.
To reduce all cysteine tags, we incubated recombinant NE in phosphate buffer [20 mM Na2HPO4, 300 mM NaCl (pH 7.4)] and 1 mM DTT for 2 h at room temperature. DTT was removed by precipitating NE with 75% ammonium sulfate. This reduction and precipitation step was repeated once. NE was dissolved in labeling buffer 1 [MO-L004 Monolith Protein Labeling Kit RED-MALEIMIDE (Cysteine Reactive); NanoTemper Technologies] and incubated with fivefold molar excess of dye [Alexa Fluor 647 NHS Ester (Succinimidyl Ester); Thermo Fisher Scientific] at room temperature for 1 h. The excess dye was removed with a PD MiniTrap G-10 column (GE Healthcare), used according to the manufacturer’s instructions.
Determination of the Concentration of Recombinant AAT.
Concentrations of active AAT were determined using active-site titrated human NE. A dilution series of AAT was incubated with 2.8 nM NE (Elastin Products) in 150 mM NaCl, 50 mM Tris, and 0.01% Triton X-100 (pH 7.4) at 4 °C for 1 h. Subsequently, the residual activity was determined by adding MCA-GEAIPSSIPPEVK(Dnp)-rr (EMC Microcollections) and measuring the fluorescence progression curve (excitation = 320 nm, emission = 405 nm). The initial cleavage rate was plotted against increasing AAT concentrations and decreased linearly with the amount of AAT added. By extrapolating this straight line, the molar concentration of functional AAT was determined at which the cleavage rate was zero. Each measurement was performed in duplicate, and the whole experiment was repeated twice.
Formula for Determination of Dissociation Constants.
where FB is fraction-bound, [A] is the concentration of AAT, [B] is the concentration of labeled catalytically inactive NE, and KD is the dissociation constant.
Description of Individual Study Samples.
Australian Stroke Genetics Collaborative.
Stroke cases comprised stroke patients of European ancestry who were admitted to four clinical centers across Australia (The Neurosciences Department at Gosford Hospital, Gosford; the Neurology Department at John Hunter Hospital, Newcastle; The Queen Elizabeth Hospital, Adelaide; and the Royal Perth Hospital, Perth) between 2003 and 2008. Stroke was defined by WHO criteria as a sudden focal neurological deficit of vascular origin lasting more than 24 h and confirmed by imaging, such as computerized tomography (CT) and/or magnetic resonance imaging (MRI) brain scan. Other investigative tests, such as electrocardiogram, carotid Doppler, and transesophageal echocardiogram, were conducted to define ischemic stroke (IS) mechanism as clinically appropriate. Cases were excluded from participation if patients were aged <18 y, diagnosed with hemorrhagic stroke or transient ischemic attack rather than IS, or were unable to undergo baseline brain imaging. IS subtypes were assigned using Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria, based on clinical, imaging, and risk factor data. All study participants provided informed consent for participation in genetic studies. Approval for the individual studies was obtained from relevant institutional ethics committees.
Wellcome Trust Case Control Consortium 2.
Stroke cases included samples recruited by investigators at St. George’s University London and the University of Oxford, the University of Edinburgh, and Ludwig Maximilians University Munich. The London collection comprised 1,224 IS samples from a hospital-based setting. All cases were of self-reported Caucasian ancestry. IS subtypes were determined according to TOAST criteria based on relevant clinical imaging and available information on cardiovascular risk factors. The University of Oxford collection comprised 896 IS cases, consecutively collected as part of the Oxford Vascular Study. Patients were of self-reported Caucasian ancestry, and IS subtypes were determined according to TOAST criteria based on relevant clinical imaging. The University of Edinburgh collection comprised 727 IS cases, consecutively collected as part of the Edinburgh Stroke Study. Patients were of self-reported Caucasian ancestry, with IS subtypes determined according to TOAST criteria based on relevant clinical and imaging data. The Munich samples included 1,383 IS cases. Patients were consecutive European Caucasians recruited from a single dedicated stroke unit at the Department of Neurology, Klinikum Grosshadern, Ludwig Maximilians University. IS subtypes were determined according to TOAST criteria based on relevant clinical and imaging data. The study was approved by the respective local ethics committees. All participants gave their informed consent.
Muenster (Westphalian stroke cases and controls from the Dortmund Health Study, Germany).
Cases were recruited through hospitals participating in the Westphalian Stroke Registry, located in the west of the country. For the current analysis, patients recruited during the period 2000–2005 were included. The register’s standardized patient documentation form included major stroke type and severity, comorbidities, and diagnostic and therapeutic details of the treatment process. IS was further subtyped according to the TOAST classification by the documenting physician. Patients who had experienced a transient ischemic attack or a hemorrhagic stroke were excluded from this analysis. The study was approved by the ethics committee of the University of Münster. All participants gave their informed consent.
Risk Assessment of Cerebrovascular Events Study, Pakistan.
The Risk Assessment of Cerebrovascular Events (RACE) is a retrospective case-control study designed to identify and evaluate genetic, lifestyle, and biomarker determinants of stroke and its subtypes in Pakistan. Samples were recruited from six hospital centers in Pakistan. Patients were eligible for study inclusion if they met the following criteria: (i) aged at least 18 y; (ii) presented with a sudden onset of neurological deficit affecting a vascular territory, with sustained deficit at 24 h verified by medical attention within 72 h after onset (onset is defined by when the patient was last seen normal and not when found with deficit); (iii) the diagnosis was supported by CT/MRI; and (iv) presented with a modified Rankin score of <2 before the stroke. TOAST and Oxfordshire classification systems were used to subphenotype all stroke cases. Control participants were individuals enrolled in the Pakistan Risk of Myocardial Infarction Study (PROMIS), a case-control study of acute myocardial infarction based in Pakistan. Controls in the PROMIS were recruited following procedures, and inclusion criteria were as adopted for RACE cases. To minimize any potential selection biases, PROMIS controls selected for this stroke study were frequency-matched to RACE cases based on age and gender, and were recruited in the following order of priority: (i) non–blood-related or blood-related visitors of patients of the outpatient department, (ii) non–blood-related visitors of stroke patients, and (iii) patients of the outpatient department presenting with minor complaints.
Control samples.
Controls for the UK samples were drawn from shared Wellcome Trust Case Control Consortium controls obtained from the 1958 Birth Cohort. This cohort is a prospectively collected cohort of individuals born in 1958 and ascertained as part of the national child development study (www.cls.ioe.ac.uk/ncds). Data from this cohort are available as a common control set for a number of genetic and epidemiological studies.
For the German samples, controls were Caucasians of German origin participating into the population Kooperative Gesundheitsforschung in der Region Augsburg (KORAgen) study (https://www.helmholtz-muenchen.de/en/kora/). This survey represents a gender- and age-stratified random sample of all German residents of the Augsburg area and consists of individuals 25–74 y of age, with about 300 subjects for each 10-y increment. All controls were free of a history of stroke or transient ischemic attack.
Discussion
Our study enabled us to detect the common variants with a moderate effect size that are associated with LAS (90% power to detect variants with >5% MAF and OR > 1.2), although the power to detect rare variants was limited. The results obtained for HDAC9, MMP12, and CYP17A1 are consistent with previous studies (4, 11, 21, 22) that have shown associations between LAS and common variants in these regions within noncoding DNA. The variant rs2023938, which reached exome-wide significance in the current study, is located in the 3′-UTR of HDAC9 and is in high LD with rs2107595 and other variants previously shown to be associated with LAS (4, 11). The variant rs2107595 resides in regulatory DNA, and the risk allele of this variant has previously been shown to associate with elevated expression levels of HDAC9 (27). Genetic ablation of HDAC9 attenuates atheroprogression in atherosclerosis-prone mice (27, 28). Hence, the effects of this locus seem to be mediated by altered HDAC9 expression levels.
The primary finding of this study is an association of the common M1(A213) variant of AAT with LAS. This variant has not previously been identified through regular genome-wide association study (GWAS) approaches, and it constitutes a potential novel risk factor for LAS. No other variant within or near AAT showed a significant association with LAS. Several factors might explain why this association was not identified by previous GWAS approaches: (i) variations in the accuracy of phenotyping across studies and, hence, reduced statistical power (29); (ii) variations in imputation accuracy; and (iii) differences in allele frequencies for M1(A213) within the European superpopulation (14) and different regional compositions of previous GWAS discovery cohorts.
A role of SERPINA1 in atherosclerosis is further supported by a recent study that found sixfold higher expression levels of SERPINA1 in human atherosclerotic lesions compared with healthy arteries (30). The target enzyme of AAT, NE, is expressed by macrophages in human atherosclerotic plaques (17), particularly within advanced atherosclerotic lesions (31). AAT might facilitate protection against matrix breakdown by NE and clearance of lipoprotein deposits. However, the issues of whether AAT is protective against atherosclerosis and whether AAT levels correlate with the progression of vascular disease are still debated (16, 32–34).
The observed association between rs6647 and LAS is unlikely to be mediated by differences in AAT levels. First, previous studies have shown similar concentrations of circulating total AAT among carriers of the M1(A213) and M1(V213) alleles (16, 19, 20). Second, we found no differences in the frequency of the Z and S alleles between cases and controls. Third, the association between rs6647 and LAS remained stable when removing carriers of a Z or S allele from our cohorts (35). Finally, eQTL analysis in the Athero-Express data showed no eQTL of M1(A213).
Previous functional studies found no difference in the inhibitory capacity and association rate constants between the two most frequent M1 AAT variants in Caucasians (20). However, these studies were performed with purified proteins in simple protein- and lipid-free buffer systems. In contrast, the microscale thermophoresis method applied here disclosed a clear difference between the two variants. This method accounts for all interactions of AAT with components of the plasma matrix, which can modify the strength of interactions between AAT and NE, as we recently showed for the AAT-Z variant (36). Under these conditions, the dissociation constant between the enzymatically inactive NE and AAT was found to be lower for M1(V213) than for M1(A213), indicating that components of the plasma matrix interfered more strongly with the former allele. The dimorphic residue at position 213 is located in the loop between strands 3 and 4 of the C sheet (s3C and s4C) and is exposed to the surface. The exposed position and hydrophobic nature of this turn make it suited for hydrophobic interactions with major plasma proteins, particularly lipoprotein particles. Compared with Val-213, the Ala-213 residue displays lower hydrophobicity and side-chain entropy, which reduces the extent of hydrophobic interactions by the s3C-s4C connecting turn of M1(A213).
Apart from the obvious single-residue substitution, further differences of the global structure were noticed in several surface regions at a distance from position 213. The subtle change in global structural flexibility between the two variants evidently does not influence the interaction between AAT and catalytically inactive NE in lipid-free plasma. This finding is consistent with earlier studies that used simple buffer systems (20). However, the lower hydrophobicity of the Ala-213 side chain and the higher surface entropy of the M1(A213) variant most likely reduce its interactions with plasma lipoproteins compared with the M1(V213) variant.
Recent studies have provided evidence for an interaction between AAT and lipoproteins, including both LDL (37) and HDL (38, 39). Binding of AAT to reconstituted HDL reduced the inhibitory capacity of AAT toward NE (39), and a similar effect was seen in our study when lipoproteins were removed from the plasma matrix. Moreover, we noticed that allele-specific differences of the dissociation constant disappeared, supporting our hypothesis of an altered interaction of M1(V213) AAT with lipoproteins in the complex plasma environment.
Better binding to lipoproteins could improve the local availability of the functional Val-213 inhibitor in atherosclerotic plaques. Moreover, we suggest that the more dynamic flexible state of the Ala-213 variant increases the likelihood of proteolytic inactivation by other proteases, particularly metalloproteases (MMP12), in atherosclerotic lesions. The precise molecular mechanism by which the M1(V213) variant reduces the risk of LAS, however, remains to be determined.
Primate sequences, including sequences of archaic hominids, indicate that the M1(V213) change, which does not occur in other mammals, arose in Africa before the divergence of modern humans (40, 41). In contemporary populations, M1(V213) is least frequent in Africa, more frequent in Europe, and almost fixed in East Asians and Native Americans. Whether this difference in allele frequencies relates to differences in LAS risk across different populations remains to be determined.
Materials and Methods
Study Participants, Genotyping, and QC.
We studied 3,202 participants with LAS involved in the following cross-sectional stroke studies: (i) German samples from Munich and the Westphalian Stroke Registry; (ii) UK samples from London, Edinburgh, and Oxford, including samples from the Wellcome Trust Case Control Consortium 2; (iii) Australian samples from the Australian Stroke Genetics Consortium study; and (iv) South Asian samples from the Risk Assessment of Cerebrovascular Events study (Pakistan). LAS was defined according to the Trial of Org 10172 in Acute Stroke Treatment classification, with radiological confirmation of stroke subtype. A total of 9,862 stroke-free control samples were selected from the Kooperative Gesundheitsforschung in der Region Augsburg S4/F4 study to match the German sample, from the 1958 Birth Cohort/Wellcome Trust Case Control Consortium 2 to match the UK and Australian sample, and from the Pakistan Risk of Myocardial Infarction study to match the South Asian sample (Table S1 and SI Materials and Methods). The relevant institutional review boards and ethics committees of participating institutions (medical faculty of Ludwig Maximilians University, John Hunter Hospital, Queen Elizabeth Hospital, Royal Perth Hospital, St. George’s Hospital, University of Oxford, University of Edinburgh, and University of Münster) approved these studies, and written or oral consent was obtained from all participants. Genotyping of cases and controls was done using the Illumina HumanExome-12v1 or HumanExome-12v2 Beadchip, holding information on more than 240,000 functional exonic variants. Further information on the genotyping, QC, and statistical analysis can be found in SI Materials and Methods. In total, 75 cases and 84 controls were removed after QC, resulting in 3,127 cases and 9,778 controls being included in the final analysis.
Statistical Analysis.
Single-variant association analysis was conducted using PLINK (42) by logistic regression adjusted for the first two principal components using genomic control to correct for population genetic substructure. Common variants (MAF > 5%; n = 26,543), low-frequency variants (1% < MAF < 5%; n = 7,042) and rare variants (MAF < 1%; n = 59,187) were studied separately, setting Bonferroni-corrected P-value cutoffs at P < 1.88E-6, P < 7.10E-6, and P < 8.45E-7, respectively. Metaanalysis was performed using fixed-effects models based on inverse variance-weighted effect size for the German and UK/Australian cohorts. Han and Eskin’s (43) random effects model optimized to detect associations under heterogeneity was used for the transethnic metaanalysis. Effects of nonsynonymous variants were checked by PolyPhen2 (44), SIFT (45), and PROVEAN (46). The significance level of gene-based association tests was set at P < 2.91E-6 (accounts for Bonferroni correction for the number of genes).
Determination of the Dissociation Constants.
Information about expression and purification of recombinant proteins, labeling of NE, and determination of the concentration of recombinant AAT is provided in SI Materials and Methods.
Before each experiment, all protein stocks and plasma samples were centrifuged for 5 min at 20,000 × g at 4 °C. Plasma and fluorescently labeled catalytically inactive elastase were mixed in buffer 1 [20 mM Na2HPO4, 300 mM NaCl (pH 7.4)] and 0.02% Tween-20. Antiphotobleaching enzyme and substrate components were added according to the manufacturer’s protocol (Monolith Anti Photobleach Kit; NanoTemper Technologies). A dilution series of both AAT variants in buffer 1 was prepared. The AAT dilutions were then mixed with buffer 1 at a volume ratio of 1:1, yielding a final concentration of 7.5% (vol/vol) AAT-deficient plasma and 4.5 nM elastase. The relatively high background of blood plasma necessitates a background subtraction. The background mixture includes everything but labeled elastase and AAT. The samples were incubated at 22 °C for 2 h. Samples were measured in NT.115 MST Premium-coated capillaries (NanoTemper Technologies) on a Monolith NT.115 Pico instrument (NanoTemper Technologies) at 22 °C using 10% light-emitting diode and 40% infrared (IR) laser powers with IR laser on/off times of 20/5 s. Each dilution point was measured in triplicate. For each plasma sample, the whole procedure was performed three times to yield independent triplicates. After subtracting the background, the relative fluorescence depletion values were normalized to the saturation value, at which value all molecules were in the bound state. Normalized depletion values corresponding to the fraction of bound elastase molecules of three technical replicates were plotted on a linear y axis against the concentration of the serially diluted AAT on the log10 x axis, resulting in binding curves. In Igor Pro-5.03, a global fit of at least three replicates to the quadratic solution of the mass action law was performed to yield the dissociation constant of each curve with a weighted error fit (further details are provided in SI Materials and Methods).
Hydrogen/Deuterium Exchange Measurements Using Mass Spectrometry.
Deuterium/hydrogen exchange and quantification were performed as previously described (47). In brief, exchange reactions were initiated by diluting 5 μL of 25 μM AAT with 45 μL of D2O containing buffer, and the reaction was stopped at different time points (10 s, 1 min, 10 min, 60 min, and 120 min) by further addition of 50 μL of quench buffer (100 mM phosphate buffer, pH 2.5). The quench reactions were immediately injected in a Waters HDX system with in-line pepsin digestion. The peptides were separated on a C18 column before analysis on a Waters Synapt G2 mass spectrometer. The back-exchange reaction of hydrogens was corrected using a fully deuterated sample that was obtained by incubating AAT in D2O containing high-concentration deuterated guanidine DCl before quenching. At all time points, undeuterated and fully deuterated controls were analyzed in triplicate. Peptide identification was performed with the ProteinLynx Global Server. Coverage maps and deuterium uptake for each peptide was obtained with DynamX 3.0.
Acknowledgments
Samples were processed in the Genetics Core Laboratory of the WT Clinical Research Facility, Edinburgh. Neuroimaging was performed at the Scottish Funding Council Brain Imaging Research Centre Division of Clinical Neurosciences, University of Edinburgh, a core area of the WT Clinical Research Facility and part of the Scottish Imaging Network, a Platform for Scientific Excellence collaboration. This study makes use of data generated by the Wellcome Trust Case Control Consortium. A full list of investigators who contributed to the generation of the data is available (https://www.wtccc.org.uk/). This work was supported by grants from the German Federal Ministry of Education and Research (e:Med program e:AtheroSysMed), the FP7 European Union (EU) project CVgenes{at}target(261123), the Deutsche Forschungsgemeinschaft (DFG) (CRC 1123, B3), the Corona Foundation, and the Fondation Leducq (Transatlantic Network of Excellence on the Pathogenesis of Small Vessel Disease of the Brain) (all to M.D.), as well as by grants from the Australian National and Medical Health Research Council (Grant 569257), Australian National Heart Foundation (NHF; Grant G 04S 1623), Gladys M. Brawn Fellowship scheme, and Vincent Fairfax Family Foundation in Australia. E.G.H. was supported by a Fellowship from the NHF and National Stroke Foundation of Australia (Grant 100071). The Wellcome Trust (WT) Case Control Consortium 2 (WTCCC2) was funded by the WT (WTCCC2 Projects 085475/B/08/Z, 085475/Z/08/Z, and WT084724MA). The Stroke Association provided support for collection of some of the St. George’s Hospital cases. H.S.M. is supported by a National Institute for Health Research (NIHR) Senior Investigator Award and by the Cambridge University Hospitals National Health Service Trust NIHR Comprehensive Biomedical Research Centre. The work of H.S.M. and S.B. is supported by the Evelyn Trust. The Oxford cases were collected as part of the Oxford Vascular Study (funded by the Medical Research Council, Stroke Association, Dunhill Medical Trust, NIHR, and Biomedical Research Centre, Oxford). The Edinburgh Stroke Study was supported by the WT (clinician scientist award to C.L.M.S.) and the Binks Trust. Additional funding for this study was provided by the WT (Awards 076113 and 090355), a joint DFG grant (JE194/4-1 and BR 2152/2-1), the EU Horizon 2020 research and innovation program [Grant Agreement 668036, relapses prevention (RELENT)], and the University of Maryland Baltimore, School of Pharmacy Mass Spectrometry Center (Grant SOP1841-IQB2014).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.I. is a Guest Editor invited by the Editorial Board.
4Members of the International Stroke Genetics Consortium: Daniel Woo, Stephanie Debette, Jane Maguire, John W. Cole, Jennifer Majersik, Jordi Jimenez-Conde, Jin-Moo Lee, Natalia Rost, Guillaume Pare, Christina Jern, Arne G. Lindgren, and Israel Fernandez Cardenas.
See Commentary on page 3555.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616301114/-/DCSupplemental.
Contributor Information
Collaborators: Daniel Woo, Stephanie Debette, Jane Maguire, John W. Cole, Jennifer Majersik, Steve Bevan, Jordi Jimenez-Conde, Jin-Moo Lee, Natalia Rost, Guillaume Pare, Christina Jern, Arne G Lindgren, and Israel Fernandez Cardenas
References
- 1.Mozaffarian D, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2015 update: A report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.WHO 2014 The top 10 causes of death. Available at www.who.int/mediacentre/factsheets/fs310/en/. Accessed December 7, 2015.
- 3.Schneider AT, et al. Ischemic stroke subtypes: A population-based study of incidence rates among blacks and whites. Stroke. 2004;35(7):1552–1556. doi: 10.1161/01.STR.0000129335.28301.f5. [DOI] [PubMed] [Google Scholar]
- 4.Traylor M, et al. Australian Stroke Genetics Collaborative; Wellcome Trust Case Control Consortium 2 (WTCCC2); International Stroke Genetics Consortium Genetic risk factors for ischaemic stroke and its subtypes (the METASTROKE collaboration): A meta-analysis of genome-wide association studies. Lancet Neurol. 2012;11(11):951–962. doi: 10.1016/S1474-4422(12)70234-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber C, Noels H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat Med. 2011;17(11):1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 6.Bevan S, et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke. 2012;43(12):3161–3167. doi: 10.1161/STROKEAHA.112.665760. [DOI] [PubMed] [Google Scholar]
- 7.Holliday EG, et al. Australian Stroke Genetics Collaborative; International Stroke Genetics Consortium; Wellcome Trust Case Control Consortium 2 Common variants at 6p21.1 are associated with large artery atherosclerotic stroke. Nat Genet. 2012;44(10):1147–1151. doi: 10.1038/ng.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falcone GJ, Malik R, Dichgans M, Rosand J. Current concepts and clinical applications of stroke genetics. Lancet Neurol. 2014;13(4):405–418. doi: 10.1016/S1474-4422(14)70029-8. [DOI] [PubMed] [Google Scholar]
- 9.Malik R, et al. ISGC Analysis Group; METASTROKE collaboration; Wellcome Trust Case Control Consortium 2 (WTCCC2); NINDS Stroke Genetics Network (SiGN) Low-frequency and common genetic variation in ischemic stroke: The METASTROKE collaboration. Neurology. 2016;86(13):1217–1226. doi: 10.1212/WNL.0000000000002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NINDS Stroke Genetics Network; International Stroke Genetics Consortium Loci associated with ischaemic stroke and its subtypes (SiGN): A genome-wide association study. Lancet Neurol. 2015;15(2):174–184. doi: 10.1016/S1474-4422(15)00338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellenguez C, et al. International Stroke Genetics Consortium (ISGC); Wellcome Trust Case Control Consortium 2 (WTCCC2) Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischemic stroke. Nat Genet. 2012;44(3):328–333. doi: 10.1038/ng.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gschwendtner A, et al. International Stroke Genetics Consortium Sequence variants on chromosome 9p21.3 confer risk for atherosclerotic stroke. Ann Neurol. 2009;65(5):531–539. doi: 10.1002/ana.21590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auer PL, et al. Imputation of exome sequence variants into population-based samples and blood-cell-trait-associated loci in African Americans: NHLBI GO Exome Sequencing Project. Am J Hum Genet. 2012;91(5):794–808. doi: 10.1016/j.ajhg.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.1000 Genomes Project Consortium et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henriksen PA, Sallenave JM. Human neutrophil elastase: Mediator and therapeutic target in atherosclerosis. Int J Biochem Cell Biol. 2008;40(6-7):1095–1100. doi: 10.1016/j.biocel.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Talmud PJ, et al. Diabetes Atherosclerosis Intervention Study Investigators Progression of atherosclerosis is associated with variation in the alpha1-antitrypsin gene. Arterioscler Thromb Vasc Biol. 2003;23(4):644–649. doi: 10.1161/01.ATV.0000065196.61663.8D. [DOI] [PubMed] [Google Scholar]
- 17.Dollery CM, et al. Neutrophil elastase in human atherosclerotic plaques: Production by macrophages. Circulation. 2003;107(22):2829–2836. doi: 10.1161/01.CIR.0000072792.65250.4A. [DOI] [PubMed] [Google Scholar]
- 18.Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res. 2012;110(6):875–888. doi: 10.1161/CIRCRESAHA.111.257535. [DOI] [PubMed] [Google Scholar]
- 19.Ferrarotti I, et al. Serum levels and genotype distribution of α1-antitrypsin in the general population. Thorax. 2012;67(8):669–674. doi: 10.1136/thoraxjnl-2011-201321. [DOI] [PubMed] [Google Scholar]
- 20.Nukiwa T, et al. Characterization of the M1(Ala213) type of alpha 1-antitrypsin, a newly recognized, common “normal” alpha 1-antitrypsin haplotype. Biochemistry. 1987;26(17):5259–5267. doi: 10.1021/bi00391a008. [DOI] [PubMed] [Google Scholar]
- 21.Traylor M, et al. METASTROKE, International Stroke Genetics Consortium; Wellcome Trust Case Consortium 2 (WTCCC2) A novel MMP12 locus is associated with large artery atherosclerotic stroke using a genome-wide age-at-onset informed approach. PLoS Genet. 2014;10(7):e1004469. doi: 10.1371/journal.pgen.1004469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schunkert H, et al. Cardiogenics; CARDIoGRAM Consortium Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43(4):333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simino J, et al. LifeLines Cohort Study Gene-age interactions in blood pressure regulation: A large-scale investigation with the CHARGE, Global BPgen, and ICBP Consortia. Am J Hum Genet. 2014;95(1):24–38. doi: 10.1016/j.ajhg.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newton-Cheh C, et al. Wellcome Trust Case Control Consortium Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41(6):666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lippok S, et al. Direct detection of antibody concentration and affinity in human serum using microscale thermophoresis. Anal Chem. 2012;84(8):3523–3530. doi: 10.1021/ac202923j. [DOI] [PubMed] [Google Scholar]
- 26.Tsutsui Y, Liu L, Gershenson A, Wintrode PL. The conformational dynamics of a metastable serpin studied by hydrogen exchange and mass spectrometry. Biochemistry. 2006;45(21):6561–6569. doi: 10.1021/bi060431f. [DOI] [PubMed] [Google Scholar]
- 27.Azghandi S, et al. Deficiency of the stroke relevant HDAC9 gene attenuates atherosclerosis in accord with allele-specific effects at 7p21.1. Stroke. 2015;46(1):197–202. doi: 10.1161/STROKEAHA.114.007213. [DOI] [PubMed] [Google Scholar]
- 28.Cao Q, et al. Histone deacetylase 9 represses cholesterol efflux and alternatively activated macrophages in atherosclerosis development. Arterioscler Thromb Vasc Biol. 2014;34(9):1871–1879. doi: 10.1161/ATVBAHA.114.303393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ioannidis JP, Thomas G, Daly MJ. Validating, augmenting and refining genome-wide association signals. Nat Rev Genet. 2009;10(5):318–329. doi: 10.1038/nrg2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inouye M, et al. Novel loci for metabolic networks and multi-tissue expression studies reveal genes for atherosclerosis. PLoS Genet. 2012;8(8):e1002907. doi: 10.1371/journal.pgen.1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larionov S, Dedeck O, Birkenmeier G, Thal DR. Expression of alpha2-macroglobulin, neutrophil elastase, and interleukin-1alpha differs in early-stage and late-stage atherosclerotic lesions in the arteries of the circle of Willis. Acta Neuropathol. 2007;113(1):33–43. doi: 10.1007/s00401-006-0134-0. [DOI] [PubMed] [Google Scholar]
- 32.Dahl M, et al. Blood pressure, risk of ischemic cerebrovascular and ischemic heart disease, and longevity in alpha(1)-antitrypsin deficiency: The Copenhagen City Heart Study. Circulation. 2003;107(5):747–752. doi: 10.1161/01.cir.0000049642.20484.5d. [DOI] [PubMed] [Google Scholar]
- 33.Dahl M, Tybjaerg-Hansen A, Nordestgaard BG. Risk of ischemic heart and ischemic cerebrovascular disease is not increased in S, Z, and 11478A alpha1-antitrypsin carriers of the Copenhagen City Heart Study. Arterioscler Thromb Vasc Biol. 2003;23(11):e55; author reply e55. doi: 10.1161/01.ATV.0000097980.11819.93. [DOI] [PubMed] [Google Scholar]
- 34.Elzouki AN, Ryden Ahlgren A, Lanne T, Sonesson B, Eriksson S. Is there a relationship between abdominal aortic aneurysms and alpha1-antitrypsin deficiency (PiZ)? Eur J Vasc Endovasc Surg. 1999;17(2):149–154. doi: 10.1053/ejvs.1998.0740. [DOI] [PubMed] [Google Scholar]
- 35.Thun GA, et al. Causal and synthetic associations of variants in the SERPINA gene cluster with alpha1-antitrypsin serum levels. PLoS Genet. 2013;9(8):e1003585. doi: 10.1371/journal.pgen.1003585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dau T, et al. Quantitative analysis of protease recognition by inhibitors in plasma using microscale thermophoresis. Sci Rep. 2016;6:35413. doi: 10.1038/srep35413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mashiba S, et al. In vivo complex formation of oxidized alpha(1)-antitrypsin and LDL. Arterioscler Thromb Vasc Biol. 2001;21(11):1801–1808. doi: 10.1161/hq1101.098232. [DOI] [PubMed] [Google Scholar]
- 38.Moreno JA, et al. High-density lipoproteins potentiate α1-antitrypsin therapy in elastase-induced pulmonary emphysema. Am J Respir Cell Mol Biol. 2014;51(4):536–549. doi: 10.1165/rcmb.2013-0103OC. [DOI] [PubMed] [Google Scholar]
- 39.Gordon SM, et al. Rosuvastatin alters the proteome of high density lipoproteins: Generation of alpha-1-antitrypsin enriched particles with anti-inflammatory properties. Mol Cell Proteomics. 2015;14(12):3247–57. doi: 10.1074/mcp.M115.054031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reich D, et al. Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature. 2010;468(7327):1053–1060. doi: 10.1038/nature09710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer M, et al. A high-coverage genome sequence from an archaic Denisovan individual. Science. 2012;338(6104):222–226. doi: 10.1126/science.1224344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purcell S, et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han B, Eskin E. Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. Am J Hum Genet. 2011;88(5):586–598. doi: 10.1016/j.ajhg.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adzhubei IA, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4(7):1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 46.Choi Y, Chan AP. PROVEAN web server: A tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics. 2015;31(16):2745–2747. doi: 10.1093/bioinformatics/btv195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hughes VA, Meklemburg R, Bottomley SP, Wintrode PL. The Z mutation alters the global structural dynamics of α1-antitrypsin. PLoS One. 2014;9(9):e102617. doi: 10.1371/journal.pone.0102617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grove ML, et al. Best practices and joint calling of the HumanExome BeadChip: The CHARGE Consortium. PLoS One. 2013;8(7):e68095. doi: 10.1371/journal.pone.0068095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ionita-Laza I, Lee S, Makarov V, Buxbaum JD, Lin X. Sequence kernel association tests for the combined effect of rare and common variants. Am J Hum Genet. 2013;92(6):841–853. doi: 10.1016/j.ajhg.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee S, et al. NHLBI GO Exome Sequencing Project—ESP Lung Project Team Optimal unified approach for rare-variant association testing with application to small-sample case-control whole-exome sequencing studies. Am J Hum Genet. 2012;91(2):224–237. doi: 10.1016/j.ajhg.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S, Teslovich TM, Boehnke M, Lin X. General framework for meta-analysis of rare variants in sequencing association studies. Am J Hum Genet. 2013;93(1):42–53. doi: 10.1016/j.ajhg.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verhoeven BA, et al. Athero-express: differential atherosclerotic plaque expression of mRNA and protein in relation to cardiovascular events and patient characteristics. Rationale and design. Eur J Epidemiol. 2004;19(12):1127–1133. doi: 10.1007/s10564-004-2304-6. [DOI] [PubMed] [Google Scholar]
- 53.van den Borne P, et al. Leukotriene B4 levels in human atherosclerotic plaques and abdominal aortic aneurysms. PLoS One. 2014;9(1):e86522. doi: 10.1371/journal.pone.0086522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perera NC, et al. NSP4 is stored in azurophil granules and released by activated neutrophils as active endoprotease with restricted specificity. J Immunol. 2013;191(5):2700–2707. doi: 10.4049/jimmunol.1301293. [DOI] [PubMed] [Google Scholar]
- 55.Dau T, Sarker RS, Yildirim AO, Eickelberg O, Jenne DE. Autoprocessing of neutrophil elastase near its active site reduces the efficiency of natural and synthetic elastase inhibitors. Nat Commun. 2015;6:6722. doi: 10.1038/ncomms7722. [DOI] [PubMed] [Google Scholar]