Abstract
Exposure of human monocytes to Chlamydia pneumoniae resulted in a significant enhancement of matrix metalloproteinase (MMP) 1 and 9 production following stimulation with tumor necrosis factor alpha and granulocyte monocyte-colony stimulating factor. The effect of C. pneumoniae on monocyte MMPs was mediated through the induction of prostaglandin E2. These findings may have implications for atherosclerotic plaque rupture.
The role of Chlamydia pneumoniae in atherosclerosis is still uncertain. Evidence implicating this organism in atherosclerosis is based on the presence of C. pneumoniae in the atheroma (9-11) and seroepidemiology studies that link immunoglobulin A (IgA) to C. pneumoniae and coronary artery disease (13, 14). However, recent prospective controlled trials have shown that there is no correlation between the seropositivity to C. pneumoniae and coronary events (2, 15). Thus, there is still debate as to the role of C. pneumoniae in coronary artery disease, and present findings are not strong enough to support or dispute whether C. pneumoniae plays an active role in the development of atherosclerosis or if this organism contributes to plaque rupture.
Present understanding of the mechanism of C. pneumoniae infection of vessels is that the organism is transported by the monocytes. Because the pathogen is a common cause of respiratory infection, the alveolar monocytes exposed to or infected with the organism may travel in the bloodstream and enter the atherosclerotic plaque, where they undergo differentiation into macrophages and cause active C. pneumoniae infection. This explanation has been supported by a study involving experimental infection of mice with C. pneumoniae that showed that the bacteria were spread by peripheral blood monocytes (12).
Monocytes may play an important role in acute coronary syndrome through their production of matrix metalloproteinases (MMP) 1 and 9. MMP-1 is an interstitial collagenase that can cleave fibrillar collagens (types I, II, and III), and MMP-9 is a gelatinase that can degrade denatured or cleaved fibrillar collagens and basement membrane components, including type IV collagen. These enzymes have been colocalized in atherosclerotic plaques (4) and are capable of degrading the extracellular matrix in the thin fibrous cap of a vulnerable plaque, leading to the rupture of the atherosclerotic plaque. Production of MMP-1 and -9 by monocytes is stimulated by endogenous inflammatory stimulants, tumor necrosis factor alpha (TNF-α), and granulocyte monocyte-colony stimulating factor (GM-CSF) (17). Thus, monocytes exposed to C. pneumoniae may produce larger amounts of MMPs in the presence of TNF-α and GM-CSF. Murine monocytes in the presence of C. pneumoniae have been shown to produce MMP-9 (8); similarly, human monocyte-derived macrophages exposed to C. pneumoniae had increased levels of MMP-9 but failed to show an increase in MMP-1, MMP-3, and a tissue inhibitor of MMP-1 (16).
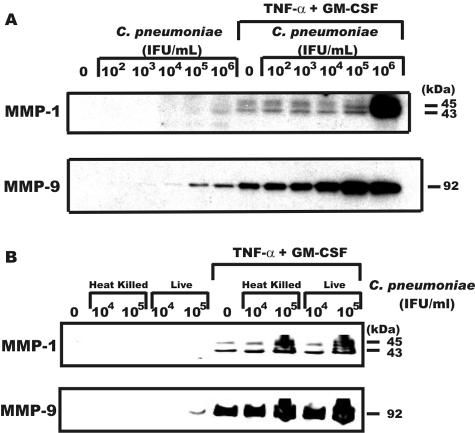
To explore the effect of C. pneumoniae on monocyte MMP production, we compared the production of MMP-1 and MMP-9 by monocytes that were exposed to live or heat-killed C. pneumoniae followed by stimulation with TNF-α and GM-CSF, cytokines present in atheroma. Purified human peripheral blood cells were obtained by elutriation as previously described (17). Purified monocytes were plated at 5 × 106/ml of serum-free Dulbecco's modified Eagle medium (Bio Whittaker) in 12-well plates (Costar) and were adhered for at least 30 min prior to the addition of reagents. Some of the monocyte cultures were treated with live or heat-killed C. pneumoniae (strain AR39) as previously described (5). Six hours later the cultures were washed, and TNF-α (50 ng/ml; PeproTech) and GM-CSF (50 ng/ml; PeproTech) were added to some of the cultures. Proteins in the medium conditioned for 48 h were analyzed by Western blotting for MMP-1 with peptide-specific antibodies (generously provided by Henning Birkedal-Hansen, National Institute of Dental and Craniofacial Research, Bethesda, Md.) and for MMP-9 (Chemicon International, Temecula, Calif.) as previously described (17). All experiments were repeated at least three times with different donors. Monocytes exposed to live C. pneumoniae induced detectable MMP-1 at 106 infectious units (IFU)/ml, whereas MMP-9 was detected at 105 to 106 IFU (Fig. 1A). However, when C. pneumoniae-treated monocytes were stimulated with TNF-α and GM-CSF, a slight enhancement of MMP-1 production by C. pneumoniae occurred at 102 to 104 IFU, with a greater increase at 105 IFU and a substantial increase at 106 IFU. Monocyte MMP-9 levels were also affected by C. pneumoniae, with a slight increase detected at 105 and 106 IFU and substantial increases occurring at 102 to 106 IFU when challenged with cytokines (Fig. 1A). We next compared heat-killed versus live C. pneumoniae to determine if contact and/or infection mediated the increase in MMPs. As shown in Fig. 1B, heat-killed and live C. pneumoniae caused a similar synergistic increase in cytokine-induced MMP production, indicating that contact with C. pneumoniae was sufficient to prime monocytes for enhanced MMP production.
FIG. 1.
C. pneumoniae increases the sensitivity of monocytes to MMP-1 and MMP-9 induction by TNF-α and GM-CSF. Monocytes were incubated with the IFU for 6 h, washed, and cultured in the presence or absence of TNF-α (50 ng/ml) and GM-CSF (50 ng/ml) for an additional 48 h. Supernatants were harvested at 48 h and were assayed for MMP-1 and MMP-9 by Western blotting. Panel A represents a dose response of IFU of live C. pneumoniae, whereas panel B compares IFU of live C. pneumoniae to the equivalent IFU of C. pneumoniae following heating at 90°C for 15 min.
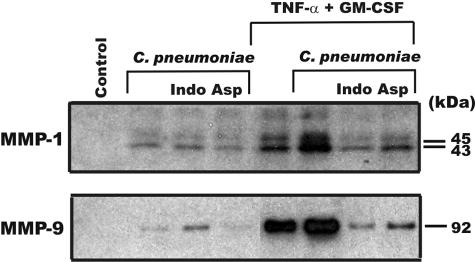
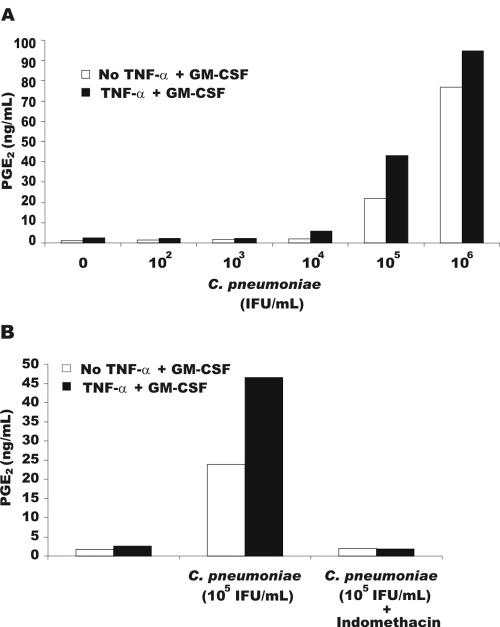
Stimulation of monocyte MMPs by cytokines involves, at least in part, a prostaglandin E2 (PGE2)-dependent mechanism (17). To examine if C. pneumoniae increased MMP production in a PGE2-dependent manner, the cyclooxygenase inhibitors indomethacin and aspirin (Sigma) were added to monocytes. Both cyclooxygenase inhibitors suppressed the synergistic increase in MMP-1 and MMP-9 production by the addition of cytokines to C. pneumoniae-treated monocytes (Fig. 2). To determine the PGE2 levels in the cultures, the media conditioned for 24 h were analyzed for PGE2 production by enzyme-linked immunosorbent assay (ELISA) (Cayman Chemicals, Ann Arbor, Mich., or Peninsula Laboratories, Inc., San Carlos, Calif.) according to the manufacturer's suggestions. Measurement of PGE2 levels in the media revealed that C. pneumoniae alone caused a substantial increase in PGE2 at 105 and 106 IFU, which was further enhanced when monocytes were stimulated with cytokines (Fig. 3A). The increase in the production of PGE2 following exposure of monocytes to C. pneumoniae was inhibited by indomethacin (Fig. 3B). These findings demonstrate that the interaction of monocytes with C. pneumoniae results in a substantial increase in the production of PGE2 that, when coupled with additional signaling components induced by cytokines, results in a synergistic increase in MMP production.
FIG. 2.
Indomethacin and aspirin inhibit C. pneumonia enhancement of MMP-1 and MMP-9. Monocytes were cultured for 6 h with C. pneumoniae (105/ml), washed, and cultured in the presence or absence of TNF-α plus GM-CSF and indomethacin (Indo) (1 μM) or aspirin (Asp) (1 μM) for 48 h. The levels of MMP-1 and -9 in the 48-h supernatants were determined by Western blotting.
FIG. 3.
Induction of PGE2 by C. pneumoniae. Monocytes were incubated with different concentrations of C. pneumoniae IFU for 6 h, washed, and cultured with or without TNF-α plus GM-CSF for 24 h. The 24-h supernatants were assayed for PGE2 by ELISA (A) and from cultures treated with 1 μM indomethacin (B). The data are representative of three separate experiments from different donors.
The striking increase in MMP production by C. pneumoniae-primed monocytes by TNF-α and GM-CSF indicates that C. pneumoniae may play a role in the development of acute coronary syndrome by increasing the sensitivity of monocytes to cytokine stimulation when they enter a vulnerable plaque. These findings support results of a clinical trial that demonstrated that individuals taking macrolide antibiotics had significantly smaller numbers of ruptured plaque than the placebo group (6). Thus, treatment leading to C. pneumoniae clearance may be important in decreasing the events leading to acute coronary syndrome.
Monocytes may also contribute to the levels of C. pneumoniae, because infected monocytes express C. pneumoniae RNA for 3 days after infection (1) and are also resistant to the development of infectious progeny of the bacteria (1), which prevents apoptosis of monocytes (3). Moreover, the monocytes containing C. pneumoniae have altered functions, such as increased adherence to human aortic endothelial cells (7). These observations provide evidence for a role of C. pneumoniae in acute coronary syndrome and possible approaches that include the combination of antibiotics and cyclooxygenase inhibitors for treatment of acute coronary syndrome.
Acknowledgments
We thank Nancy McCartney-Francis and Gang Peng for critical reviews of the manuscript.
Editor: J. B. Bliska
REFERENCES
- 1.Airenne, S., H. M. Surcel, H. Alakarppa, K. Laitinen, J. Paavonen, P. Saikku, and A. Laurila. 1999. Chlamydia pneumoniae infection in human monocytes. Infect. Immun. 67:1445-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danesh, J., P. Whincup, S. Lewington, M. Walker, L. Lennon, A. Thomson, Y. K. Wong, X. Zhou, and M. Ward. 2002. Chlamydia pneumoniae IgA titres and coronary heart disease; prospective study and meta-analysis. Eur. Heart J. 23:371-375. [DOI] [PubMed] [Google Scholar]
- 3.Fan, T., H. Lu, H. Hu, L. Shi, G. A. McClarty, D. M. Nance, A. H. Greenberg, and G. Zhong. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J. Exp. Med. 187:487-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galis, Z. S., G. K. Sukhova, M. W. Lark, and P. Libby. 1994. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Investig. 94:2493-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaydos, C. A., J. T. Summersgill, N. N. Sahney, J. A. Ramirez, and T. C. Quinn. 1996. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect. Immun. 64:1614-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurfinkel, E. 1998. Link between intracellular pathogens and cardiovascular diseases. Clin. Microbiol. Infect. 4(Suppl. 4):S33-S36. [PubMed] [Google Scholar]
- 7.Kalayoglu, M. V., B. N. Perkins, and G. I. Byrne. 2001. Chlamydia pneumoniae-infected monocytes exhibit increased adherence to human aortic endothelial cells. Microbes Infect. 3:963-969. [DOI] [PubMed] [Google Scholar]
- 8.Kol, A., G. K. Sukhova, A. H. Lichtman, and P. Libby. 1998. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-alpha and matrix metalloproteinase expression. Circulation 98:300-307. [DOI] [PubMed] [Google Scholar]
- 9.Kuo, C. C., A. M. Gown, E. P. Benditt, and J. T. Grayston. 1993. Detection of Chlamydia pneumoniae in aortic lesions of atherosclerosis by immunocytochemical stain. Arterioscler. Thromb. 13:1501-1504. [DOI] [PubMed] [Google Scholar]
- 10.Kuo, C. C., J. T. Grayston, L. A. Campbell, Y. A. Goo, R. W. Wissler, and E. P. Benditt. 1995. Chlamydia pneumoniae (TWAR) in coronary arteries of young adults (15-34 years old). Proc. Natl. Acad. Sci. USA 92:6911-6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo, C. C., A. Shor, L. A. Campbell, H. Fukushi, D. L. Patton, and J. T. Grayston. 1993. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J. Infect. Dis. 167:841-849. [DOI] [PubMed] [Google Scholar]
- 12.Moazed, T. C., C. C. Kuo, J. T. Grayston, and L. A. Campbell. 1998. Evidence of systemic dissemination of Chlamydia pneumoniae via macrophages in the mouse. J. Infect. Dis. 177:1322-1325. [DOI] [PubMed] [Google Scholar]
- 13.Saikku, P., M. Leinonen, K. Mattila, M. R. Ekman, M. S. Nieminen, P. H. Makela, J. K. Huttunen, and V. Valtonen. 1988. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet ii:983-986. [DOI] [PubMed] [Google Scholar]
- 14.Saikku, P., M. Leinonen, L. Tenkanen, E. Linnanmaki, M. R. Ekman, V. Manninen, M. Manttari, M. H. Frick, and J. K. Huttunen. 1992. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki Heart Study. Ann. Intern. Med. 116:273-278. [DOI] [PubMed] [Google Scholar]
- 15.Tavendale, R., D. Parratt, S. D. Pringle, R. A'Brook, and H. Tunstall-Pedoe. 2002. Serological markers of Chlamydia pneumoniae infection in men and women and subsequent coronary events; the Scottish Heart Health Study Cohort. Eur. Heart J. 23:301-307. [DOI] [PubMed] [Google Scholar]
- 16.Vehmaan-Kreula, P., M. Puolakkainen, M. Sarvas, H. G. Welgus, and P. T. Kovanen. 2001. Chlamydia pneumoniae proteins induce secretion of the 92-kDa gelatinase by human monocyte-derived macrophages. Arterioscler. Thromb. Vasc. Biol. 21:E1-E8. [DOI] [PubMed] [Google Scholar]
- 17.Zhang, Y., K. McCluskey, K. Fujii, and L. M. Wahl. 1998. Differential regulation of monocyte matrix metalloproteinase and TIMP-1 production by TNF-alpha, granulocyte-macrophage CSF, and IL-1 beta through prostaglandin-dependent and -independent mechanisms. J. Immunol. 161:3071-3076. [PubMed] [Google Scholar]