Significance
Oxygen-starved coastal waters are rapidly increasing in prevalence worldwide. However, little is known about the impacts of these “dead zones” in tropical ecosystems or their potential threat to coral reefs. We document the deleterious effects of such an anoxic event on coral habitat and biodiversity, and show that the risk of dead-zone events to reefs worldwide likely has been seriously underestimated. Awareness of, and research on, reef hypoxia is needed to address the threat posed by dead zones to coral reefs.
Keywords: biodiversity, coral bleaching, dissolved oxygen, hypoxia, water quality
Abstract
Degradation of coastal water quality in the form of low dissolved oxygen levels (hypoxia) can harm biodiversity, ecosystem function, and human wellbeing. Extreme hypoxic conditions along the coast, leading to what are often referred to as “dead zones,” are known primarily from temperate regions. However, little is known about the potential threat of hypoxia in the tropics, even though the known risk factors, including eutrophication and elevated temperatures, are common. Here we document an unprecedented hypoxic event on the Caribbean coast of Panama and assess the risk of dead zones to coral reefs worldwide. The event caused coral bleaching and massive mortality of corals and other reef-associated organisms, but observed shifts in community structure combined with laboratory experiments revealed that not all coral species are equally sensitive to hypoxia. Analyses of global databases showed that coral reefs are associated with more than half of the known tropical dead zones worldwide, with >10% of all coral reefs at elevated risk for hypoxia based on local and global risk factors. Hypoxic events in the tropics and associated mortality events have likely been underreported, perhaps by an order of magnitude, because of the lack of local scientific capacity for their detection. Monitoring and management plans for coral reef resilience should incorporate the growing threat of coastal hypoxia and include support for increased detection and research capacity.
The decades-long decline of coral reefs and the roles of overfishing, pollution, and climate change in this decline have been documented extensively (1–6). Although hypoxia and the associated phenomenon of “dead zones” have gained increasing attention of late (7, 8), the threat posed by hypoxia to coral reefs is rarely mentioned. For example, hypoxia is not discussed in several of the most influential reviews of threats to coral (4, 9, 10) and was mentioned in only 0.2% of the abstracts from the 2016 International Coral Reef Symposium (Table S1). Here we describe a massive coral-mortality event caused by hypoxia and document how such events may be underreported globally because of the lack of scientific capacity in regions where coral reefs are found.
Table S1.
Threats to coral reefs ranked by frequency of appearance in abstracts from the 2016 International Coral Reef Symposium (2,101 abstracts total)
| Type of threat | No. of abstracts | % of abstracts |
| Climate/thermal stress | 610 | 29.0 |
| Fishing | 423 | 20.1 |
| Disease/invasive species | 216 | 10.3 |
| Sedimentation/land use | 214 | 10.2 |
| Ocean acidification | 180 | 8.6 |
| Eutrophication | 163 | 7.8 |
| Hypoxia | 5 | 0.2 |
Bahiá Almirante in the Bocas del Toro region of Panama is a large, semienclosed basin of 450 km2. It displays many characteristics common to temperate estuaries that suffer from episodic or seasonal anthropogenic hypoxia, including restricted exchange with the open ocean, seasonal periods of low winds and high temperatures, and a watershed delivering increasing amounts of nutrients from agricultural run-off and untreated sewage (11, 12). Although the reefs in the bay showed some signs of human impacts before the hypoxic event, they were relatively healthy for the region, averaging 25–30% cover of live coral (6, 13). The dominant corals were Porites spp. in shallow water and a mixture of Agaricia spp. and mounding corals at greater depths (13).
In late September 2010, reefs within Bahia Almirante exhibited widespread coral bleaching and some mortality (Fig. 1 A–D). Some of those reefs showed additional signs of severe stress not typically associated with coral bleaching: Sponges exhibited extensive necrosis; recruitment tiles retrieved after 1 y had an atypical lack of successful recruitment by fouling organisms; mobile organisms displayed unusual behaviors such as migration out of crevices; dead bodies of crustaceans, gastropods, and echinoderms littered the bottom (Fig. 1E); and thick mats of Beggiatoa bacteria covered the surface of sediments. The presence of dead but intact bodies of sponges, crustaceans, and echinoderms suggested that the extreme stress leading to mortality had occurred relatively recently and was possibly ongoing, and that hypoxia likely excluded consumers that otherwise would have targeted dead and moribund prey (14). Most notably on these reefs, signs of mortality and stress occurred below a clearly demarcated depth horizon. This line was sufficiently distinct that sponges and mounding coral colonies that straddled the line initially suffered mortality at the bottom but not at the tops of their structures (Fig. 1F). Together these observations were highly suggestive of hypoxic stress caused by benthic microbial activity whose effects were isolated from shallower waters by stratification of the water column (15). Initial measurements confirmed this hypothesis, with extremely low oxygen levels (<0.5 mg/L) near the bottom at some sites, a phenomenon that had not been recorded previously in the lagoon (16).
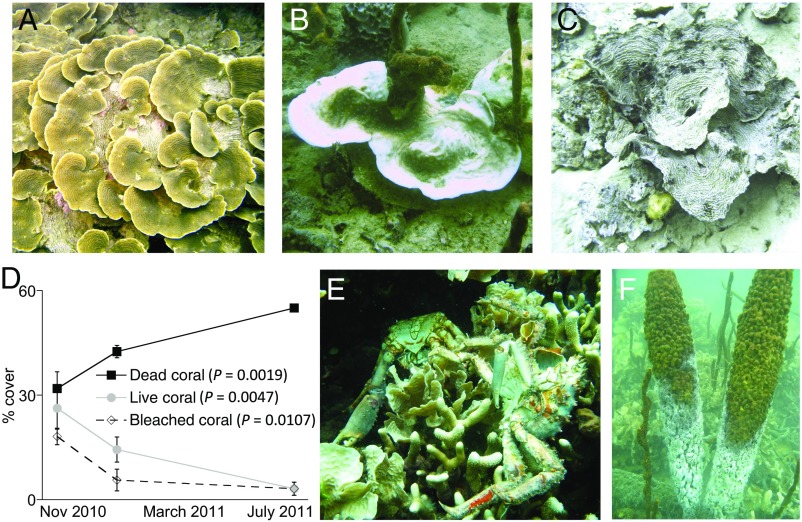
Fig. 1.
Immediate effects of the hypoxic event. (A–C) Photographs of the dominant reef-building coral A. lamarcki found alive in normoxic sites (A), bleached during a hypoxic event (B), and dead following hypoxia (C). (D) Sampling initiated in the hypoxic area tracked initially high coral bleaching that declined in association with an increase in the prevalence of dead coral and a reduction in cover by living coral. There was no change in cover of living, dead, or bleached coral during the same period at a nearby site without hypoxia (P > 0.38 all analyses). Total values for a given date are <100% because of noncoral habitat within the survey area. Data are the mean (±SE) of the percent cover of coral category and were analyzed by ANOVA (n = 4 transects with 40 points per time point per site) where P values indicate change through time within a given coral category. (E and F) As is typical of hypoxic events, mortality affected a broad taxonomic cross-section of the community including reef crabs (E) and sponges (F), the latter showing gray, dead portions below the distinct depth horizon of the oxycline. A–C courtesy of Cindy T. Gonzalez (Universidad de los Andes, Bogotá, Colombia). E–F courtesy of Arcadio Castillo (Smithsonian Tropical Research Institute, Balboa, Panama).
We therefore initiated a widespread survey of water-column conditions across Bahia Almirante on October 1, 2010. The 19 survey sites spanned from the more impacted inner bay, where biological evidence of stressful conditions was most marked, to the outer bay near passages that promote exchange with the open ocean. We found that oxygen concentrations varied widely along this spatial gradient, with very low concentrations (<0.5 mg/L) of dissolved oxygen near the bottom (at a depth >10 m) at several sites (Fig. 2A). Consistent with biological observations, dissolved oxygen levels were always high at shallow depths, with concentrations >4.8 mg/L at 3–4 m at all sites. Notably, the most hypoxic deeper waters were those closest to the mainland, where terrestrial inputs are expected to be greatest and exchange with the open ocean lowest, and near settlements that discharge untreated sewage.
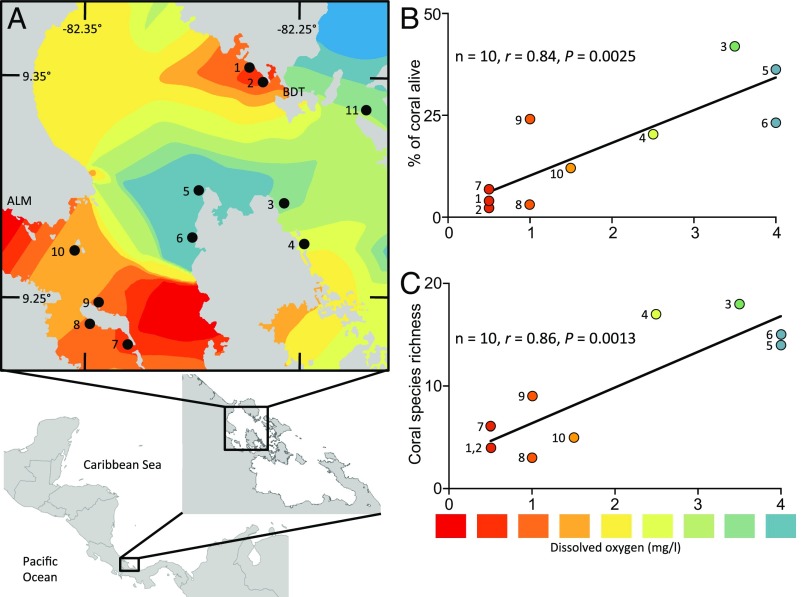
Fig. 2.
Spatial distribution of hypoxia at 10–12 m depth (October 2010) and its consequences. (A) There were two distinct pockets of hypoxia, one near the mainland with terrestrial inputs [lower left, near Almirante town (ALM)], and the other near dense settlements of Bocas del Toro (BDT) where the Bocas del Toro Research Station is located. Land is shown in gray. Color indicates the intensity of hypoxia as shown in the scale in the figure. Sites 1 and 11 were initial reef-assessment sites (2010–2011); sites 1–10 were 10 subsequent coral-survey sites (2012). (B and C) Dissolved oxygen during the hypoxic period in 2010 was correlated with both the percent of coral cover at a site that was alive (B) and species richness of coral remaining alive in 2012 (C). Data were analyzed by Pearson’s correlation. Note that dissolved oxygen levels were always >4.8 mg/L at the shallow depth (3–4 m) (Fig. S1A).
To document the effect of this hypoxia on corals further, we quantified coral bleaching and mortality at a pair of sites near the Smithsonian’s Bocas del Toro Research Station: one located within, and the other located outside of, the hypoxic area (Fig. 2A). At a depth of 10–12 m, both sites had >25% cover of live coral in November 2010. However, 76 ± 11% (mean ± SE) of the corals were bleached at the hypoxic site, whereas only 3 ± 2% of corals were bleached at the normoxic site. By July 2011, many of the bleached corals at the hypoxic site had died, and the cover of live coral was reduced by an order of magnitude, whereas the normoxic site saw no change in the cover of live coral (Fig. 1D). These field patterns were in agreement with subsequent observations in our laboratory experiment (described below) in which hypoxic stress alone was sufficient to induce bleaching followed by death at nonstressful temperatures.
We returned to Bahia Almirante in April 2012 to conduct a more thorough and widespread survey of 10 sites for the imprint of hypoxic stress on patterns of coral community structure (Fig. 2A). At depths of 10–12 m, all sites had >45% of space occupied by coral skeletons (indicative of their potential for coral occupancy), but the proportion of coral that remained alive varied widely (2–42%). That variation was correlated with dissolved oxygen levels surveyed during the hypoxic episode (Fig. 2B). Notably, the site where widespread bleaching was documented during our earlier assessments suffered severe coral mortality, with only 4% cover of live coral remaining. There was also a spatial relationship between previous oxygen levels and live coral diversity, with the lowest diversity at the most hypoxic sites (Fig. 2C).
We detected no evidence for strong differences in water quality among the sites that could explain patterns in coral cover and richness at depth apart from the differences in observed oxygen concentration. Temperature varied by 1.5 °C, and salinity varied by one part per thousand (ppt) across our study area at the depth of the coral mortality (Fig. S1); however, we detected no influence of temperature or salinity variation on patterns of coral cover and richness among sites when considering their effects separately or their potential interactions with dissolved oxygen (P > 0.12 all analyses). Nevertheless, overall elevated temperatures in the region during the hypoxic period may have increased background stress levels and the susceptibility of the coral community to hypoxia (17, 18), as is known to occur in other taxa (19). Lowered pH, which can co-occur with hypoxic conditions (20), is unlikely to have played a primary role in the mortality event we observed because coral survivorship is relatively unaffected by acidified conditions (21, 22), and coral reefs commonly tolerate wide fluctuations in pH (23).
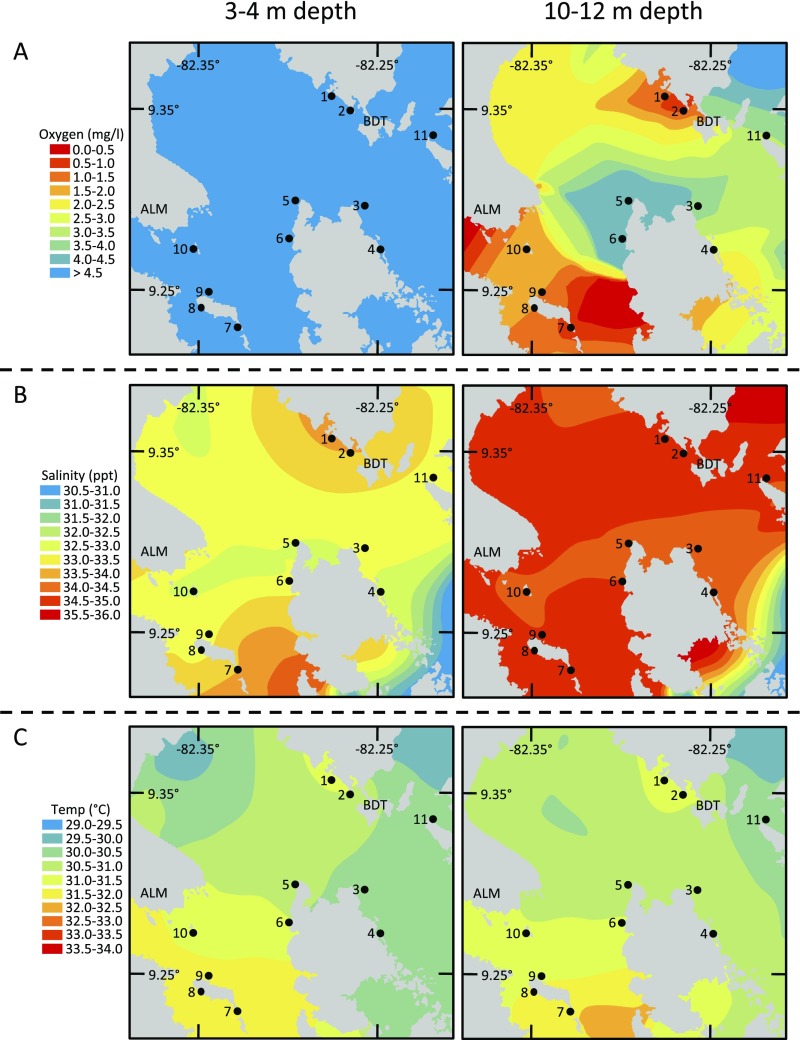
Fig. S1.
Maps of water quality in shallow (Left) and deep (Right) water during the hypoxic period in October 2010. Data are shown for (A) oxygen, (B) salinity, and (C) temperature. Consistent differences between depths in oxygen and salinity indicate the stratification of the water column. The map of oxygen at 10–12 m is the same as in Fig. 2.
We collected analogous community and water-quality data at shallower depths that substantiated our initial observations that the mortality event primarily impacted deeper portions of reefs and was driven by hypoxia. We found no change in cover of live coral at either of the initially surveyed sites at the 3–4 m depth (F < 4.20, P > 0.05 for all analyses) spanning the period in which we documented decline at deeper depths at the hypoxic site. At the two sites that were found in the later survey to be most impacted by hypoxia (sites 1 and 2), the percent of living coral was substantially greater in shallow water than in deep water, by a factor of three at one site and by more than an order of magnitude at the other. Thus, mortality patterns were decoupled across depths, with overall higher survivorship at shallow depths. Stratification of the water column explains this pattern; dissolved oxygen concentrations at a given site were consistently higher at a depth of 3–4 m than at a depth of 10–12 m (t1,18 = 7.56, P < 0.0001) and were normoxic at 3–4 m at all sites (>4.8 mg/L), including sites that were hypoxic at deeper depths (Fig. S1A). Stratification was driven primarily by variation in salinity, which was slightly (1.55 ± 0.13 ppt) but consistently (t1,18 = 11.54, P < 0.0001) higher at a depth of 10–12 m than at a depth of 3–4 m (Fig. S1B). Temperature did not differ between shallow and deep depths across our study area (t1,18 = 0.69, P = 0.50) and so cannot explain the difference in coral mortality across depths or the stratification of the water column (Fig. S1C).
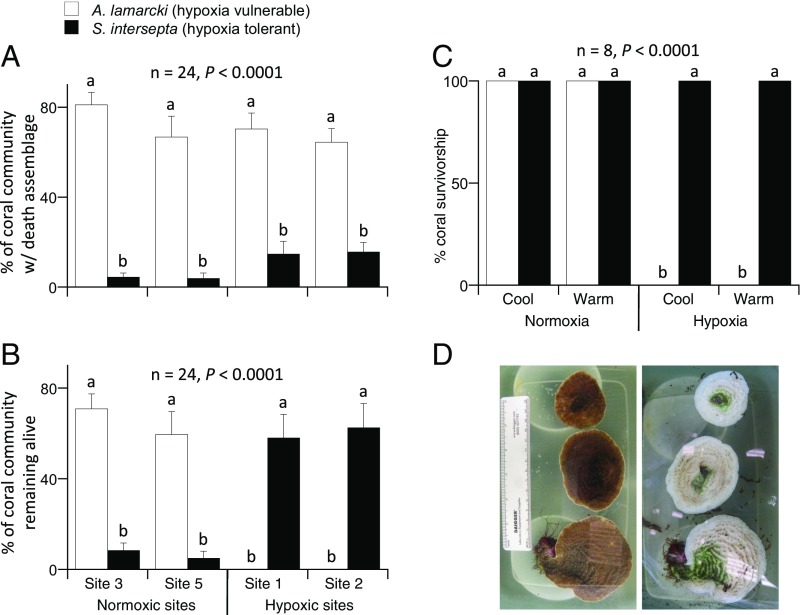
Although the overall effects on coral cover were strong, not all species were equally affected by the hypoxic event. In particular, the relative abundance of live corals at the most hypoxic sites shifted from Agaricia spp. to Stephanocoenia intersepta (Fig. 3 A and B), suggesting that selective mortality was caused by variation in hypoxia tolerance. We tested this hypothesis (and the alternative that thermal stress was the cause of mortality) by conducting multifactorial laboratory experiments in which we challenged Agaricia lamarcki and S. intersepta with the oxygen and temperature conditions that occurred during the hypoxic event. We found that the two species were similarly able to tolerate thermal stress but varied greatly in their hypoxic tolerance, with S. intersepta exhibiting a threefold greater hypoxia tolerance than A. lamarcki (Fig. 3 C and D).
Fig. 3.
Shifts in community structure toward hypoxia-tolerant coral species. (A) Before the hypoxic event, using the record of all corals both alive and dead as a proxy for relative abundance, the cover of Agaricia spp. was consistently higher than S. intersepta across all four sites, and there was no difference among sites in the cover of either taxon. (B) Following the hypoxic event, S. intersepta emerged as the dominant species among the corals remaining alive at the two sites that experienced hypoxia, whereas Agaricia spp. persisted as the dominant living coral at the two sites that did not experience hypoxia. (C) Subsequent laboratory tolerance experiments suggested that hypoxia, not thermal stress, was the primary driver of the mortality patterns in the field because S. intersepta had high survivorship across all treatments, and A. lamarcki (92% of Agaricia spp. at study sites) survivorship was affected by hypoxia but not by temperature treatment. Cool = 28 °C; warm = 32 °C; normoxic = >5 mg/L dissolved oxygen; hypoxic = 0.5 mg/L dissolved oxygen. (D) Photos of A. lamarcki on day 0 (Left) and day 7 (Right) of the cool, hypoxic treatment. Note green algae colonizing dead colonies on day 7. Data in A and B are mean (±SE) percent cover analyzed by ANOVA. Data in C are the proportion of replicate containers with surviving corals analyzed by Kaplan–Meier survivorship analysis. Different letters above bars indicate significant differences in percent cover and survivorship, respectively.
To examine whether comparable hypoxic events had occurred in the recent past in Bocas del Toro, we compared the death assemblage at impacted sites with the living assemblage at unimpacted sites by measuring the size of the largest colonies of A. lamarcki (living or dead) in randomly selected plots at the sites that were most (sites 1 and 2) and least (sites 3 and 5) impacted by hypoxic mortality. We assumed that large colony size is indicative of colony longevity and that previous hypoxic events that caused coral morality would have truncated colony size. We found no difference in average colony size across sites (F3,77 = 0.64, P = 0.59), suggesting that other major hypoxia-associated mortality events had not occurred recently.
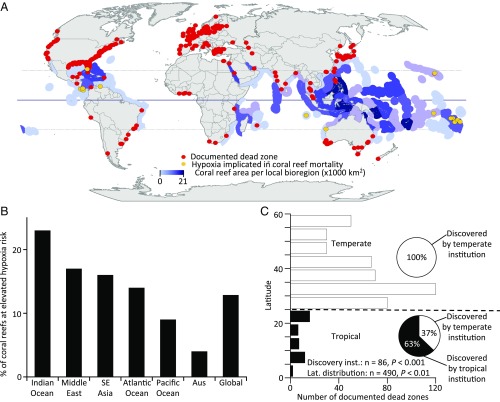
To determine the likelihood of similar hypoxic events occurring on coral reefs elsewhere, we compiled and analyzed several global databases. First, we assessed a global database of all known dead zones and found that coral reefs are located in approximately half (22/43) of the tropical bays or estuaries where dead zones have been reported (Fig. 4A). Second, we randomly selected 100 reefs from each of the six global coral regions and found that an average of 13% of coral reefs worldwide are at an increased risk of hypoxia, both because of their elevated score on an index of exposure to anthropogenic impacts that contribute to low oxygen conditions (e.g., eutrophication and climate change) and because of their occurrence in semienclosed bays that can support the formation of dead zones (Fig. 4B). Third, we conducted a thorough review of the literature and identified 20 instances in which hypoxia was implicated in the mass mortality of coral reef organisms (Fig. 4A and Table S2).
Fig. 4.
Worldwide distribution of dead zones and coral reefs. (A) Global map with all known dead zones (red dots) and coral reefs where hypoxia has been implicated in mass mortality of reef organisms (gold dots). Documented dead zones are notably concentrated in temperate regions in areas with relatively greater research capacity, whereas coral reefs are found primarily in the tropics. The solid horizontal line represents the equator, and the upper and lower dashed lines represent the Tropics of Cancer and Capricorn respectively. Intensity of purple color indicates densities of coral reef area per ecoregion, from lightest to darkest: 0, 1–1,000, 1,001–2,500, 2,501–5,000, 5,001–10,000, and 10,001–21,000 km2. (B) The proportion of coral reefs at elevated risk of hypoxia, for each major coral region and a global average weighted by relative coral area in each region, based on coastal geomorphology and anthropogenic drivers of low oxygen. (C) The distribution of dead zones by latitude. Dead zones are relatively underrepresented in tropical regions and are overrepresented in temperate areas given the length of the coastline in each region (analysis by Kolmogorov–Smirnov test). Degrees of latitude are absolute values, so northern and southern hemisphere latitudes are pooled. (Insets) Circle graphs show that tropical dead zones were likely to be first identified by research teams from temperate institutions, whereas temperate dead zones were never identified by teams from tropical institutions (analysis by χ2 test).
Table S2.
Events in which hypoxia was implicated in mortality of coral reef organisms
| System | Country | Latitude | Longitude | Reference | Mortality quantified? | Dissolved oxygen quantified? | ||
| Fish | Invertebrates | Corals | ||||||
| Coral Bay | Australia | −23.13° | 113.75° | (41) | Yes | Yes | Yes | No |
| Cocos (Keeling) Islands | Australia | −12.17° | 96.83° | (42) | Yes | No | No | Yes |
| Cocos (Keeling) Islands | Australia | −12.17° | 96.83° | (43) | Yes | No | Yes | No |
| Manihiki Atoll | Cook Islands | −10.42° | −160.98° | (44) | No | Yes | No | No |
| Cano Island | Costa Rica | 8.71° | −83.87° | (45) | Yes | Yes | Yes | No |
| Hikueru Atoll | French Polynesia | −17.57° | −142.62° | (44) | Yes | Yes | No | No |
| Manihi Atoll | French Polynesia | −14.43° | −145.97° | (44) | No | Yes | No | No |
| Manihi Atoll | French Polynesia | −14.43° | −145.97° | (44) | No | Yes | No | No |
| Manihi Atoll | French Polynesia | −14.43° | −145.97° | (44) | No | Yes | No | No |
| Takapoto Atoll | French Polynesia | −14.57° | −145.17° | (44) | No | Yes | No | No |
| Takaroa Atoll | French Polynesia | −14.42° | −144.95° | (44) | No | Yes | No | No |
| Takaroa Atoll | French Polynesia | −14.42° | −144.95° | (44) | No | Yes | No | No |
| Fangatau Atoll | French Polynesia | −15.82° | −140.85° | (44) | No | Yes | No | No |
| Tatakoto Atoll | French Polynesia | −17.33° | −138.37° | (44) | No | Yes | No | No |
| Ahe Atoll | French Polynesia | −14.27° | −146.27° | (44) | No | Yes | No | No |
| Uva Island | Panama | 7.82° | −81.77° | (45) | Yes | Yes | Yes | No |
| Bahia Almirante | Panama | 9.33° | −82.25° | This study | No | Yes | Yes | Yes |
| Kaneohe Bay | United States | 21.45° | −157.80° | (46) | Yes | No | No | Yes |
| Florida Keys | United States | 24.62° | −81.40° | (47) | No | No | No | Yes |
| Morrocoy National Park | Venezuela | 10.87° | −68.27° | (40) | Yes | Yes | Yes | Yes |
These analyses also suggest that dead zones on coral reefs are likely severely underreported, perhaps by an order or magnitude. The number of documented dead zones is higher in temperate regions than in the tropics by a factor of 10 (Fig. 4 A and C), a trend that persists even after correcting for the length of coastline in each region and even though higher temperatures increase the severity of dead zones and the sensitivity of organisms to hypoxia (24). If the density of dead zones scales from temperate to tropical regions as a function of shoreline length, then at least 370 dead zones are yet to be described, half of which could be expected to affect coral reefs based on the assessment above. Although the historically greater intensity of agricultural fertilizer use in developed countries may have contributed to the relatively higher number of documented hypoxic ecosystems in temperate regions (7), the widespread evidence for eutrophication effects on coral reefs associated with run-off from intensifying agricultural practices, poorly regulated sewage discharge, and rapidly growing coastal populations suggests that tropical ecosystems are highly susceptible to the localized effects of anthropogenic nutrient inputs (25).
Many factors likely contribute to the underreporting of dead zones affecting tropical ecosystems and the lack of recognition of dead zones as a threat to coral reefs (4, 9, 26). In the case of the Bocas del Toro event, documentation of hypoxia and associated coral reef mortality in the present study was largely a serendipitous result of having an active international research station near one of the most impacted reefs (Fig. 2A); had observers not been on hand, attributing the cause of mortality to low oxygen even a few months later would have been extremely difficult. In addition, as is generally the case, scientific research on dead zones is dominated by investigators from temperate zone countries or other countries with strong research investment (Fig. 4C); 37% of the 43 known tropical dead zones were first described by research teams led by principal investigators based in the United States or Europe, and another 28% were described by teams with principal investigators from Brazil, India, or China–countries with advanced research infrastructure. These findings suggest that the skewed distribution of research capacity contributes to the apparent deficit of documented dead zones in tropical ecosystems, and that further economic development may increase the detection of those hypoxic ecosystems.
Other aspects of coral reef research traditions probably contribute to the lack of recognition of hypoxia on coral reefs as well. Monitoring protocols for coral reefs typically do not call for measurements of oxygen concentrations (27), making it difficult to identify hypoxia-driven mortality after it has occurred. In fact, of the 20 instances that we uncovered in which coral reef mortality was attributed to hypoxia, only four studies in addition to ours collected both biological and oxygen data, and only one included data on corals (Table S2). Moreover, investigators often focus their studies on relatively healthy coral communities and avoid those near shoreline development and terrestrial inputs (28). Hence, hypoxia may cause a shift toward tolerant species or the elimination of entire reefs before scientific documentation, resulting in a shifted baseline (29).
How coral reefs recover from hypoxia is even less understood. In the case of reefs in Bocas del Toro, it remains to be seen what factors will control recovery, whether phase shifts will occur, and how ecosystem functions will be affected. Changes in coral community structure were still evident nearly 4 y after the hypoxic event, with no apparent recovery in the cover of live coral (P > 0.10). Moreover, these changes occurred in an ecosystem where the coral community had already shifted toward more stress-tolerant species in recent centuries in association with human impacts, including fisheries exploitation and land-use change (30–32). The long-term effects of hypoxia are potentially different from, and more substantial than, those of other disturbances on coral reefs because hypoxia affects a broad range of taxa including consumers, habitat formers, and pathogens. However, at least some functional groups show high resilience, because several years after the event the diversity and abundance of mobile invertebrates on previously hypoxic reefs was found to be the same as, or higher than, the diversity and abundance on unaffected reefs (33), and grazing pressure has been sufficient to preempt overgrowth of dead coral by algae (34).
Although death by hypoxia can be widespread and swift, local management of terrestrial inputs (e.g., nutrients and organic carbon loading from run-off and sewage) within a watershed can be effective in reducing the probability that hypoxic events will occur (7). Indeed 494 coastal dead zones are currently listed, but 55 are recorded as having improved water quality (35). Thus, enhanced research capacity, particularly in developing countries, and awareness of hypoxia’s potential impacts on coral reefs could form the basis for targeted efforts to manage tropical shorelines to protect them from the threat of coral reef mortality associated with dead zones.
Materials and Methods
To rank the research attention that hypoxia receives relative to other threats to coral reefs, we quantified the number of ICRS 2016 abstracts that examined the effects of climate/thermal stress, fishing, disease/invasive species, sedimentation/land use, ocean acidification, and hypoxia. We assessed dissolved oxygen, salinity, and temperature conditions at shallow (3–4 m) and deep (10–12 m) depths at 19 sites in Bahia Almirante in October 2010, and maps of variation in each parameter across the study area (at 10–12 m depth) were created in ArcGIS (Fig. 2 and Fig. S1). To assess the initial response of coral reefs to hypoxia, we sampled the cover of live, dead, and bleached coral from representative reefs inside (site 1) and outside (site 11) the hypoxic area (Fig. 2A) on November 2010, January 2011, and July 2011. We then conducted a more detailed survey of coral cover and species richness at both shallow and deep depths at 10 sites that spanned the hypoxia gradient in April 2012 (Fig. 2) and again in April 2014. To test for evidence of prior mass-mortality events that would truncate the size distribution of corals, we quantified colony size at two dead zone sites and two unimpacted sites. We conducted a laboratory experiment to examine the relative tolerance of A. lamarcki and S. intersepta to temperature and hypoxia conditions associated with the dead-zone event at the Bocas del Toro Research Station and to test whether levels of those factors during the event were sufficient to cause mortality in either species (Fig. 3). We updated the global database of hypoxic sites (7, 35) by conducting a literature search and then examined geographic trends in the risk of dead zones to coral reefs in three ways (Table S3). First, we quantified the proportion of coral reefs worldwide that are at elevated risk of exposure to hypoxia based on geomorphological setting and an index of exposure to anthropogenic factors (Fig. 4 A and B) (27). Second, we examined the proportion of known tropical hypoxic ecosystems in our global hypoxia database that contain coral reefs according to reef locations documented in the databases (e.g., ref. 27) and the literature. Third, we searched the literature for all studies that associated hypoxia with the death of corals or other reef organisms (Table S2). We tested whether the distribution of known dead zones was disproportionately skewed away from the tropics and toward temperate regions by comparing the distribution of dead zones in our database with the distribution of relative coastline length across 5° latitude bins (Fig. 4C). To examine whether research capacity could explain this skewness, we tested whether dead zones in tropical and temperate regions were more likely to have been first described by investigators from the other region (Fig. 4C). Extended materials and methods that detail procedures and analyses are available in SI Materials and Methods.
Table S3.
Ecosystems with newly identified occurrence of hypoxia
| System | Country | Latitude | Longitude | Decade of first report | Citation | Reference |
| Yarra River Estuary | Australia | −38.24° | 145.43° | 2000 | Roberts et al., 2012 | (48) |
| Baltic Sea | Baltic Sea countries | 60.00° | 20.00° | 1990–2000 | Conley et al., 2001* | (49) |
| Aby Lagoon | Cote d’Ivoire | 5.17° | −3.20° | 1970 | Chantraine 1980 | (50) |
| Cienfuegos | Cuba | 22.12° | −80.43° | 1990 | Seisdedo 2006 | (51) |
| Gironde Estuary | France | 45.43° | −0.83° | 2000 | Lanoux et al., 2013 | (52) |
| Aitoliko | Greece | 38.45° | 21.33° | 1950 | Gianni et al., 2011 | (53) |
| Amvrikikos | Greece | 39.00° | 21.00° | 2000 | Ferentinos et al., 2010 | (54) |
| Cuvum River | India | 13.07° | 80.28° | 2000 | Shanmugam et al., 2007 | (55) |
| Malad Creek | India | 19.15° | 72.80° | 2000 | Vijay et al., 2010 | (56) |
| Sabarmati Estuary | India | 22.13° | 72.33° | 2000 | Deshkar et al., 2012 | (57) |
| Siak River Estuary | Indonesia | 1.38° | 102.17° | 2000 | Rixen et al., 2008 | (58) |
| Ardbear Salt Lake | Ireland | 53.47° | ‒10.00° | 1990 | Henry et al., 2008 | (59) |
| Ago Bay | Japan | 34.27° | 136.80° | 2000 | Haraguchi et al., 2010 | (60) |
| Lake Nakaumi | Japan | 35.47° | 133.18° | 1990 | Ichikawa 2007 | (61) |
| Ohfunato Bay | Japan | 39.03° | 141.72° | 1990 | Okada and Yakanama 2007 | (62) |
| Hangan Bay | Korea | 35.13° | 128.68° | 2000 | Jang et al., 2011 | (63) |
| Haoyfjord | Norway | 59.02° | 9.75° | 2000 | Bouchet et al., 2012 | (64) |
| Bahia Almirante | Panama | 9.33° | ‒82.25° | 2010 | This study | |
| Bolinao | Philippines | 16.38° | 119.92° | 2000 | San Diego-McGlone et al., 2008 | (65) |
| Amur Bay | Russia | 43.12° | 131.77° | 2000 | Mikhailik et al., 2011 | (66) |
| Goukamma Estuary | South Africa | −34.07° | 22.95° | 2010 | Kaselowski and Adames 2013 | (67) |
| Remolar Lagoon | Spain | 41.28° | 2.07° | 2000 | Canedo-Arguelles et al., 2012 | (68) |
| Dapeng Bay | Taiwan | 22.45° | 120.47° | 2000 | Hsieh et al., 2012 | (69) |
| Oyster grounds | United Kingdom | 54.50° | 4.50° | 2000 | Weston et al., 2008 | (70) |
| Pepper Creek | United States | 38.57° | −75.18° | 2000 | Tyler et al., 2009 | (71) |
Conley et al., 2001 represents >100 sites within the Baltic Sea.
SI Materials and Methods
Rank of Threats in Abstracts of the International Coral Reef Symposium.
The International Coral Reef Symposium is the premier event of coral reef science, occurring once every 4 y and thus represents an excellent snapshot of the activities of coral reef researchers around the globe. To quantify the relative attention given to various threats to coral reefs by attendees and their collaborators, we counted the number of abstracts that referenced each threat. We used the following search terms for each topic to identify candidate abstracts and then examined each abstract for relevance to eliminate false positives: climate/thermal stress (thermal OR temperature OR warm* OR climate); fishing (fishing OR fisher OR fished OR harvest OR exploitation OR aquarium trade OR aquarium industry); disease/invasive species (disease OR pathogen OR epidemic OR infection OR invasive OR exotic species OR introduced species OR species introduction OR nonnative OR non-native); sedimentation/land use (sediment* OR runoff OR run-off OR turbid OR land use OR land-use OR land cover OR land-cover OR shoreline OR coastline OR development); ocean acidification (acidifi* OR pH OR OA); eutrophication (eutrophic* OR nutrient* OR nutrification OR sewage OR fertilizer); Hypoxia (anoxi* OR hypoxi* OR dead zone OR oxygen OR DO). Abstracts were counted for each topic independently, so each abstract may have been counted toward more than one topic.
Water-Quality Survey.
We assessed water-quality conditions at 19 sites on 1 October 2010. Sites were selected for coverage across Bahia Almirante that represented varying distances from land and isolation from channels with the open ocean. At each site, a YSI Pro2030 sonde was used to measure dissolved oxygen, salinity, temperature, and depth. We characterized conditions across the bay using data collected from the same shallow (3–4 m) and deep (10–12 m) depths as subsequent coral surveys. Maps of variation in each parameter across the study area (at 10–12 m depth) were created using the Gaussian kernel interpolation function with barriers in ArcGIS v10.2 with Geostatistical Analyst extension.
Initial Coral Assessment.
To assess the initial response of coral reefs to hypoxia, we sampled representative reefs inside (site 1) and outside (site 11) the hypoxic area (Fig. 2A). The reef at each site was surveyed in November 2010, January 2011, and July 2011 using the Reef Check protocol (36). At each site and time, four replicate 20-m transects were laid shallow (3–4 m depth) and deep (10–12 m depth), and cover was recorded at 0.5-m intervals using the following categories: live coral, live bleached coral, dead coral, or other. For each site and depth, the effect of time on the percent of cover by live coral and bleached coral was analyzed with one-way ANOVAs. No data were collected at the shallow depth of the hypoxic site at the middle time point January 2011, so that depth and site were analyzed by comparing the two end points of November 2010 and July 2011.
Detailed Coral Survey.
We quantified the coral community at 10 sites that spanned the hypoxia gradient in Bahia Almirante in April 2012 (Fig. 2A). Surveys were conducted at shallow (3–4 m) and deep (10–12 m) depths. At each site and depth, we assessed the percent of coral cover by recording the identity and status (live or dead) of coral under 25 points in each of 24 randomly placed 50 × 50 cm quadrats. We examined the relationship of the average proportion of living coral [live coral cover/(live coral cover + dead coral cover)] and coral species richness at each site with the dissolved oxygen, temperature, and salinity values at each site derived from the interpolated maps of each physical variable using linear least squares regression analysis. Because of the potential interaction of temperature and hypoxia on organismal survivorship (19), we also considered the combined and interactive effects of dissolved oxygen and temperature on the percent of live coral cover and species richness with multiple linear regression using forward substitution model selection and found that the addition of a temperature term alone or the addition of temperature and temperature × dissolved oxygen interaction terms did not significantly improve the overall model fit (P ≥ 0.12 for all analyses). Similarly, we found that salinity, which varied by <1 ppt across the study sites, did not contribute to patterns of coral cover or richness (P ≥ 0.12 for all analyses). We did not conduct similar analyses of the relationship between coral cover or coral diversity and physical parameters at shallow depths because some sites did not have coral reefs at shallow depths and thus the sample size was insufficient for rigorous analysis. We compared temperature, salinity, and dissolved oxygen between shallow (3–4 m) and deep (10–12 m) depths across our study area with a paired t test that compared the two depths at each of the 19 sites.
We returned to our 10 survey sites in the spring of 2014 to examine if there was any trend of change in coral cover since our initial surveys. We used the same survey methods and then tested for a change through time using a paired t test with average percent cover in 2012 and 2014 at each site as the response variable.
To test for evidence of prior mass-mortality events that would truncate the size distribution of corals, we conducted a detailed examination of colony size at two dead-zone sites and two unimpacted sites. We measured the length of the largest Agaricia lamarcki colony (living or dead) in each of 19–23 randomly placed 50 × 50 cm quadrats. We tested for differences among sites in the size of the largest colonies with a one-way ANOVA.
Stress Tolerance Experiment.
We conducted a laboratory experiment to examine the relative tolerance of A. lamarcki and Stephanocoenia intersepta to temperature and hypoxia conditions associated with the dead-zone event at the Bocas del Toro Research Station and to test whether levels of those factors during the event were sufficient to cause mortality in either species. The experiment focused on those two factors of interest and was not meant to mimic the full suite of conditions associated with the event. Coral colonies (5–8 cm in diameter) were collected from a common site without previous evidence of hypoxic stress in April 2013 and then were acclimated to laboratory conditions in flowing seawater tanks at the Bocas del Toro Research Station for 1 wk before the start of experimental conditions. We crossed two temperature levels (low: 28 °C; high: 32 °C) by two dissolved oxygen treatments (normoxic: >5 mg/L; hypoxic: 0.5 mg/L, the minimum level observed in water-quality surveys) in a fully factorial design for each species, with eight replicate containers (volume: 3.7 L) per treatment. Each replicate container contained three coral colonies of a given species. The temperature of the replicate containers was maintained by heating a water bath surrounding each container with an Eheim Jager 300-W heater. Oxygen levels were maintained by bubbling each container with air for the normoxic treatment or with nitrogen for the hypoxic treatment. The water in each container was replaced with preconditioned water daily. Survivorship was analyzed by Kaplan–Meier χ2 analysis as the proportion of containers with colonies alive after 7 d.
Global Hypoxia Database.
We examined geographic trends in the occurrence of dead zones and research efforts in the global database first established by Diaz and Rosenberg (7). That database was updated through 2011 by the World Resources Institute (35). We further updated that database through 2013 by conducting two searches of publications in the Web of Science for the years 2007–2013. The first was an examination of all papers that have cited the 2008 Diaz and Rosenberg paper. The second used the search string “Topic=(suboxic OR sub-oxic OR hypoxi* OR anoxi* OR dead zone OR anaerobic OR oxygen) AND (eutrophic* OR anthropogenic OR nutrient* OR pollut*) AND (coast* OR ocean OR estuar* OR marine OR sea).” Each of the papers yielded by the searches was examined for evidence of hypoxia (dissolved oxygen < 2.8 mg/L) and was cross-checked with the existing database to exclude redundant references. Our addendum with coordinates of newly identified dead zones is presented in Table S3. We plotted the locations of known dead zones from this updated database on a global map and included a data layer of known coral reef densities by ecoregions from the following database accessed on 3 March 2015: www.arcgis.com/home/item.html?id=304e87e8b3154027935b226ecd2691aa.
Assessment of the Risk of Coral Reef Hypoxia.
We estimated the global potential incidence of hypoxia on coral reefs in three ways. First, we quantified the proportion of coral reefs worldwide that are at elevated risk of exposure to hypoxia based on two classes of factors that contribute to the establishment of low oxygen. The first was the geomorphology of coral reef setting, because hypoxia is known to establish in semienclosed bays, estuaries, lagoons, and other coastal features with limited circulation; the second was exposure to anthropogenic factors such as eutrophication and warming that contribute to low oxygen levels (7, 37–39). For each of the six major coral regions of the world we randomly sampled 100 reefs (n = 600 reefs total) and determined the proportion of reefs that are located in a semienclosed coastal setting and are also at a high, very high, or critical level of risk of integrated anthropogenic impacts using the World Resources Institute year 2050 projections (27). We also determined a weighted global average given the relative distribution of coral reef area across the six regions.
Second, we estimated the relative risk of hypoxia to coral reefs by examining the proportion of known tropical hypoxic ecosystems in our global hypoxia database that contain coral reefs according to documented reef locations in the databases of the World Resources Institute (27), ReefBase (www.ReefBase.org/main.aspx, accessed 21 April 2015), and our search of the literature.
Third, we searched the literature for all studies that associated hypoxia with the death of corals or other reef organisms using the search term: “Topic=(suboxic OR sub-oxic OR hypoxi* OR anoxi* OR dead zone OR anaerobic OR oxygen) AND (coral*).” Of the 20 instances in which hypoxia was implicated in the mortality of coral reef organisms, only one (40) had both oxygen and coral data; however, they attributed mortality primarily to co-occurring salinity and thermal stress.
Global Distribution and Estimation of Dead Zones.
We examined the distribution of dead zones by latitude for temperate regions (−60° to −24° and 24° to 60° latitude) and tropical regions (−24° to 24° latitude) by plotting the number of dead zones per degree of latitude. To simplify comparisons between temperate and tropical areas, southern and northern data were plotted together as absolute degree of latitude. Four dead zones known to occur at polar latitudes (>60°) were not included in the analyses.
We tested whether the distribution of known dead zones was disproportionately skewed away from the tropics and toward temperate regions by dividing the world into bins of five degrees of latitude and comparing the distribution of dead zones in our database with the distribution of relative coastline length across latitude bins. For this analysis, we tested the null hypothesis that the likelihood of dead zone occurrence is proportional the amount of coastline. Coastline length was determined by measuring the length of coastline on all continents and islands >100,000 km2 in Google Earth (v7.1.2.2041, accessed December 2014) using the latitude and longitude grid overlay and ruler function. Measurements were made at a resolution that allowed a one degree of latitude division on the grid. We tested whether the distributions of dead zones and coastline across latitude differed with a Kolmogorov–Smirnov two sample test. Because this difference was found to be significant (D = 0.39, P < 0.01), we then conducted a post hoc test to examine whether there was a consistent difference in the distribution of known dead zones and coastline in tropical or temperate regions by conducting a paired t test for both the tropical and temperate regions using the difference between the proportion of dead zones and coastline length in each latitude bin. We also estimated the approximate number of dead zones yet to be described in the tropics, assuming that the number of dead zones scales with the length of shoreline. Because our database found 447 dead zones along 120,153 km of temperate shoreline (−60° to −24.1° and 24.1°to 60° latitude), we estimated that 414 dead zones should be found along 111,161 km of tropical shorelines (−24° to 24° latitude). This estimate is nearly an order of magnitude greater than the 43 known tropical dead zones.
We tested whether dead zones in tropical and temperate regions were more likely to have been first described by investigators from the other region. We examined the publications that first described each of the tropical dead zones and examined a randomly selected subsample of temperate dead zones for the same sample size (n = 43 per region). For each publication we identified whether the lead author’s home institution was in a tropical or temperate region. We then conducted a χ2 analysis to determine if the proportion of dead zones first identified by investigators from the other region differed in temperate and tropical regions.
Acknowledgments
We thank B. Silliman for comments on earlier versions of this paper; P. Gondola, C. Gonzalez, S. Loranger, and A. Castillo for assistance in the field; and M. Solano for assistance with Geographical Information System maps. This research was supported by the Smithsonian Tropical Research Institute, a grant from Deutscher Akademischer Austauschdienst-The German Academic Exchange Service and the Pawel-Rammingen Foundation, the Sant Chair for Marine Science, and the Hunterdon and Johnson Oceanographic Research Endowment. Research permits were provided by the Autoridad de Recursos Acuático de Panamá and the Autoridad Nacional del Ambiente de Panamá. This paper is contribution number 8 from the Marine Global Earth Observatory (MarineGEO) and the Smithsonian’s Tennenbaum Marine Observatories Network.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1621517114/-/DCSupplemental.
References
- 1.Hoegh-Guldberg O, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318(5857):1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 2.Pandolfi JM, et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301(5635):955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- 3.Bruno JF, et al. Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol. 2007;5(6):e124. doi: 10.1371/journal.pbio.0050124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mumby PJ, Steneck RS. Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol Evol. 2008;23(10):555–563. doi: 10.1016/j.tree.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 5.McCauley DJ, et al. Marine defaunation: Animal loss in the global ocean. Science. 2015;347(6219):1255641. doi: 10.1126/science.1255641. [DOI] [PubMed] [Google Scholar]
- 6.Jackson JBC, Donovan MK, Cramer KL, Lam W. Status and Trends of Caribbean Coral Reefs: 1970-2012. Global Coral Reef Monitoring Network. International Union for the Conservation of Nature; Gland, Switzerland: 2014. [Google Scholar]
- 7.Diaz RJ, Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321(5891):926–929. doi: 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- 8.Vaquer-Sunyer R, Duarte CM. Thresholds of hypoxia for marine biodiversity. Proc Natl Acad Sci USA. 2008;105(40):15452–15457. doi: 10.1073/pnas.0803833105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Côté IM, Knowlton N. Coral reef ecosystems: A decade of discoveries. In: Bertness MD, Bruno JF, Silliman BR, Stachowicz JJ, editors. Marine Community Ecology and Conservation. Sinauer Associates; Sunderland, MA: 2013. pp. 299–314. [Google Scholar]
- 10.Jackson JBC, et al. Ecological effects of a major oil spill on Panamanian coastal marine communities. Science. 1989;243(4887):37–44. doi: 10.1126/science.243.4887.37. [DOI] [PubMed] [Google Scholar]
- 11.Cramer KL. History of human occupation and environmental change in western and central Caribbean Panama. Bull Mar Sci. 2013;89(4):955–982. [Google Scholar]
- 12.Guzmán HM, Barnes PAG, Lovelock CE, Feller IC. A site description of the CARICOMP mangrove, seagrass and coral reef sites in Bocas del Toro, Panama. Caribb J Sci. 2005;41(3):430–440. [Google Scholar]
- 13.Guzmán HM. Caribbean coral reefs of Panama: Present status and future perspectives. In: Cortés J, editor. Latin American Coral Reefs. Elsevier Science; Amsterdam: 2003. pp. 241–274. [Google Scholar]
- 14.Altieri AH. Dead zones enhance key fisheries species by providing predation refuge. Ecology. 2008;89(10):2808–2818. doi: 10.1890/07-0994.1. [DOI] [PubMed] [Google Scholar]
- 15.Diaz RJ, Rosenberg R. Marine benthic hypoxia: A review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr Mar Biol Annu Rev. 1995;33:245–303. [Google Scholar]
- 16.Collin R, D’Croz L, Gondola P, Del Rosario JB. Climate and hydrological factors affecting variation in chlorophyll concentration and water clarity in the Bahia Almirante, Panama. Smithson Contrib Mar Sci. 2009;38:323–334. [Google Scholar]
- 17.Neal BP, et al. When depth is no refuge: Cumulative thermal stress increases with depth in Bocas del Toro, Panama. Coral Reefs. 2014;33(1):193–205. [Google Scholar]
- 18.Levitan DR, Boudreau W, Jara J, Knowlton N. Long-term reduced spawning in Orbicella coral species due to temperature stress. Mar Ecol Prog Ser. 2014;515:1–10. [Google Scholar]
- 19.Vaquer-Sunyer R, Duarte CM. Temperature effects on oxygen thresholds for hypoxia in marine benthic organisms. Glob Change Biol. 2011;17(5):1788–1797. [Google Scholar]
- 20.Wallace RB, Baumann H, Grear JS, Aller RC, Gobler CJ. Coastal ocean acidification: The other eutrophication problem. Estuar Coast Shelf Sci. 2014;148:1–13. [Google Scholar]
- 21.Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc Natl Acad Sci USA. 2008;105(45):17442–17446. doi: 10.1073/pnas.0804478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stubler AD, Furman BT, Peterson BJ. Sponge erosion under acidification and warming scenarios: Differential impacts on living and dead coral. Glob Change Biol. 2015;21(11):4006–4020. doi: 10.1111/gcb.13002. [DOI] [PubMed] [Google Scholar]
- 23.Duarte CM, et al. Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuaries Coasts. 2013;36(2):221–236. [Google Scholar]
- 24.Altieri AH, Gedan KB. Climate change and dead zones. Glob Change Biol. 2015;21(4):1395–1406. doi: 10.1111/gcb.12754. [DOI] [PubMed] [Google Scholar]
- 25.Fabricius KE. Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar Pollut Bull. 2005;50(2):125–146. doi: 10.1016/j.marpolbul.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 26.Pandolfi JM, et al. Ecology. Are U.S. coral reefs on the slippery slope to slime? Science. 2005;307(5716):1725–1726. doi: 10.1126/science.1104258. [DOI] [PubMed] [Google Scholar]
- 27.Burke L, Reytar K, Spalding M, Perry A. Reefs at Risk Revisited. World Resources Institute; Washington, DC: 2011. p. 130. [Google Scholar]
- 28.Sandin SA, et al. Baselines and degradation of coral reefs in the Northern Line Islands. PLoS One. 2008;3(2):e1548. doi: 10.1371/journal.pone.0001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knowlton N, Jackson JBC. Shifting baselines, local impacts, and global change on coral reefs. PLoS Biol. 2008;6(2):e54. doi: 10.1371/journal.pbio.0060054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aronson RB, MacIntyre IG, Wapnick CM, O’Neill MW. Phase shifts, alternative states, and the unprecedented convergence of two reef systems. Ecology. 2004;85(7):1876–1891. [Google Scholar]
- 31.Cramer KL, Jackson JBC, Angioletti CV, Leonard-Pingel J, Guilderson TP. Anthropogenic mortality on coral reefs in Caribbean Panama predates coral disease and bleaching. Ecol Lett. 2012;15(6):561–567. doi: 10.1111/j.1461-0248.2012.01768.x. [DOI] [PubMed] [Google Scholar]
- 32.Aronson RB, Hilbun NL, Bianchi TS, Filley TR, McKee BA. Land use, water quality, and the history of coral assemblages at Bocas del Toro, Panama. Mar Ecol Prog Ser. 2014;504:159–170. [Google Scholar]
- 33.Nelson HR, Kuempel CD, Altieri AH. The resilience of reef invertebrate biodiversity to coral mortality. Ecosphere. 2016;7(7):e1399. [Google Scholar]
- 34.Kuempel CD, Altieri AH. The emergent role of small-bodied herbivores in pre-empting phase shifts on degraded coral reefs. Sci Rep. 2017;7:39670. doi: 10.1038/srep39670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz RJ, Selman M, Chique C. Global Eutrophic and Hypoxic Coastal Systems. World Resources Institute; Washington, DC: 2011. [Google Scholar]
- 36.Hodgson G, Maun L, Shuman C. Reef Check Survey Manual. Reef Check. Institute of the Environment, University of California; Los Angeles, CA: 2004. [Google Scholar]
- 37.Diaz RJ. Overview of hypoxia around the world. J Environ Qual. 2001;30(2):275–281. doi: 10.2134/jeq2001.302275x. [DOI] [PubMed] [Google Scholar]
- 38.Levin LA, et al. Effects of natural and human-induced hypoxia on coastal benthos. Biogeosciences. 2009;6(10):2063–2098. [Google Scholar]
- 39.Rabalais NN, et al. Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences. 2010;7(2):585–619. [Google Scholar]
- 40.Laboy-Nieves EN, et al. Mass mortality of tropical marine communities in Morrocoy, Venezuela. Bull Mar Sci. 2001;68(2):163–179. [Google Scholar]
- 41.Simpson CJ, Cary JL, Masini RJ. Destruction of corals and other reef animals by coral spawn slicks on Ningaloo Reef, Western Australia. Coral Reefs. 1993;12(3-4):185–191. [Google Scholar]
- 42.Hobbs J-PA, McDonald CA. Increased seawater temperature and decreased dissolved oxygen triggers fish kill at the Cocos (Keeling) Islands, Indian Ocean. J Fish Biol. 2010;77(6):1219–1229. doi: 10.1111/j.1095-8649.2010.02726.x. [DOI] [PubMed] [Google Scholar]
- 43.Hobbs J-PA, Macrae H. Unusual weather and trapped coral spawn lead to fish kill at a remote coral atoll. Coral Reefs. 2012;31(4):961. [Google Scholar]
- 44.Andréfouët S, Dutheil C, Menkes CE, Bador M, Lengaigne M. Mass mortality events in atoll lagoons: Environmental control and increased future vulnerability. Glob Change Biol. 2015;21(1):195–205. doi: 10.1111/gcb.12699. [DOI] [PubMed] [Google Scholar]
- 45.Guzman HM, Cortes J, Glynn PW, Richmond RH. Coral mortality associated with dinoflagellate blooms in the eastern Pacific (Costa Rica and Panama) Mar Ecol Prog Ser. 1990;60(3):299–303. [Google Scholar]
- 46.Jokiel PL, Hunter CL, Taguchi S, Watarai L. Ecological impact of a fresh-water reef kill in Kaneohe Bay, Oahu, Hawaii. Coral Reefs. 1993;12(3-4):177–184. [Google Scholar]
- 47.Lapointe BE, Matzie WR. Effects of stormwater nutrient discharges on eutrophication processes in nearshore waters of the Florida Keys. Estuaries. 1996;19(2B):422–435. [Google Scholar]
- 48.Roberts KL, Eate VM, Eyre BD, Holland DP, Cook PLM. Hypoxic events stimulate nitrogen recycling in a shallow salt-wedge estuary: The Yarra River estuary, Australia. Limnol Oceanogr. 2012;57(5):1427–1442. [Google Scholar]
- 49.Conley DJ, et al. Hypoxia is increasing in the coastal zone of the Baltic Sea. Environ Sci Technol. 2011;45(16):6777–6783. doi: 10.1021/es201212r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chantraine J-M. La Lagune Abe (Cote D'Ivoire) morphologie, hydrologie, parametres physico-chimiques. Documents Scientifique de Centre de recherches oceanographiques Abidjan. 1980;11:39–77. [Google Scholar]
- 51.Seisdedo Losa M. Variaciones espaciales y temporales en indicadores de la calidad ambiental de las aguas de la Bahia de Cienfuegos, Cuba. Rev Investig Mar. 2006;27(2):159–164. [Google Scholar]
- 52.Lanoux A, et al. Factors contributing to hypoxia in a highly turbid, macrotidal estuary (the Gironde, France) Environ Sci Process Impacts. 2013;15(3):585–595. doi: 10.1039/c2em30874f. [DOI] [PubMed] [Google Scholar]
- 53.Gianni A, Kehayias G, Zacharias I. Geomorphology modification and its impact to anoxic lagoons. Ecol Eng. 2011;37(11):1869–1877. [Google Scholar]
- 54.Ferentinos G, et al. Fjord water circulation patterns and dysoxic/anoxic conditions in a Mediterranean semi-enclosed embayment in the Amvrakikos Gulf, Greece. Estuar Coast Shelf Sci. 2010;88(4):473–481. [Google Scholar]
- 55.Shanmugam P, Neelamani S, Ahn YH, Philip L, Hong GH. Assessment of the levels of coastal marine pollution of Chennai city, Southern India. Water Resour Manage. 2007;21(7):1187–1206. [Google Scholar]
- 56.Vijay R, Sardar VK, Dhage SS, Kelkar PS, Gupta A. Hydrodynamic assessment of sewage impact on water quality of Malad Creek, Mumbai, India. Environ Monit Assess. 2010;165(1-4):559–571. doi: 10.1007/s10661-009-0967-9. [DOI] [PubMed] [Google Scholar]
- 57.Deshkar S, Lakhmapurkar J, Gavali D. State of three estuaries of Gulf of Khambhat. Indian J Mar Sci. 2012;41(1):70–75. [Google Scholar]
- 58.Rixen T, et al. The Siak, a tropical black water river in central Sumatra on the verge of anoxia. Biogeochemistry. 2008;90(2):129–140. [Google Scholar]
- 59.Henry LM, Kennedy R, Keegan BF. An investigation of periodic hypoxia at Ardbear Salt Lake. J Mar Biol Assoc U K. 2008;88(7):1297–1307. [Google Scholar]
- 60.Haraguchi K, Yamamoto T, Chiba S, Shimizu Y, Nagao M. Effects of phytoplankton vertical migration on the formation of oxygen depleted water in a shallow coastal sea. Estuar Coast Shelf Sci. 2010;86(3):441–449. [Google Scholar]
- 61.Ichikawa T, Aizaki M, Takeshita M. Numerical study on amelioration of water quality in Lakes Shinji and Nakaumi: A coastal brackish lagoon system. Limnology. 2007;8(3):281–294. [Google Scholar]
- 62.Okada T, Nakayama K. Density intrusion and variation in dissolved oxygen concentrations in a bay with a sill at its mouth. J Environ Eng. 2007;133(4):447–453. [Google Scholar]
- 63.Jang PG, Shin K, Chang M, Kim D. Spatial and temporal trends in water quality in response to sewage discharge in Masan and Hangam Bays, Korea. J Coast Res. 2011;27(6A):144–155. [Google Scholar]
- 64.Bouchet VMP, Alve E, Rygg B, Telford RJ. Benthic foraminifera provide a promising tool for ecological quality assessment of marine waters. Ecol Indic. 2012;23:66–75. [Google Scholar]
- 65.San Diego-McGlone ML, Azanza RV, Villanoy CL, Jacinto GS. Eutrophic waters, algal bloom and fish kill in fish farming areas in Bolinao, Pangasinan, Philippines. Mar Pollut Bull. 2008;57(6-12):295–301. doi: 10.1016/j.marpolbul.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 66.Mikhailik TA, Tishchenko PY, Koltunov AM, Tishchenko PP, Shvetsova MG. The effect of Razdol’naya River on the environmental state of Amur Bay (The Sea of Japan) Water Resour. 2011;38(4):512–521. [Google Scholar]
- 67.Kaselowski T, Adams JB. Not so pristine - characterising the physico-chemical conditions of an undescribed temporarily open/closed estuary. Water SA. 2013;39(5):627–635. [Google Scholar]
- 68.Canedo-Arguelles M, Rieradevall M, Farres-Corell R, Newton A. Annual characterisation of four Mediterranean coastal lagoons subjected to intense human activity. Estuar Coast Shelf Sci. 2012;114:59–69. [Google Scholar]
- 69.Hsieh WC, et al. Community metabolism in a tropical lagoon: Carbon cycling and autotrophic ecosystem induced by a natural nutrient pulse. Environ Eng Sci. 2012;29(8):776–782. [Google Scholar]
- 70.Weston K, et al. Sedimentary and water column processes in the Oyster Grounds: A potentially hypoxic region of the North Sea. Mar Environ Res. 2008;65(3):235–249. doi: 10.1016/j.marenvres.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 71.Tyler RM, Brady DC, Targett TE. Temporal and spatial dynamics of diel-cycling hypoxia in estuarine tributaries. Estuaries Coasts. 2009;32(1):123–145. [Google Scholar]