Significance
A diverse collection of peptides mediates cell–cell communication. Enzymes that cleave these peptides modulate their signals and thus play an important role in the physiology of multicellular organisms. Insulin-degrading enzyme (IDE) is one such enzyme that cleaves a number of bioactive peptides. IDE is activated by polyanions, but physiological activators remain unidentified. Here we show that inositol-containing molecules, known to modulate various cellular functions, activate IDE, identifying them as potential physiological regulators. Inositol phosphates are potent soluble activators of IDE. Phosphatidylinositol phosphates, lipid components of cell membranes, also activate but in addition facilitate the localization of IDE to intracellular compartments, where the enzyme gains access to substrates, such as insulin, internalized by cells.
Keywords: insulin-degrading enzyme, inositols, phosphatidylinositols, activation, subcellular localization
Abstract
Insulin-degrading enzyme (IDE) hydrolyzes bioactive peptides, including insulin, amylin, and the amyloid β peptides. Polyanions activate IDE toward some substrates, yet an endogenous polyanion activator has not yet been identified. Here we report that inositol phosphates (InsPs) and phosphatdidylinositol phosphates (PtdInsPs) serve as activators of IDE. InsPs and PtdInsPs interact with the polyanion-binding site located on an inner chamber wall of the enzyme. InsPs activate IDE by up to ∼95-fold, affecting primarily Vmax. The extent of activation and binding affinity correlate with the number of phosphate groups on the inositol ring, with phosphate positional effects observed. IDE binds PtdInsPs from solution, immobilized on membranes, or presented in liposomes. Interaction with PtdInsPs, likely PtdIns(3)P, plays a role in localizing IDE to endosomes, where the enzyme reportedly encounters physiological substrates. Thus, InsPs and PtdInsPs can serve as endogenous modulators of IDE activity, as well as regulators of its intracellular spatial distribution.
Insulin-degrading enzyme (IDE), also known as insulysin (EC 3.4.24.56), hydrolyzes a broad range of bioactive peptides in vitro, including angiotensin, glucagon, β-endorphin, amylin, and amyloid β peptides (Aβ), with the two most established in vivo substrates being insulin and Aβ. Evidence supporting a role for IDE in degrading insulin and Aβ in vivo includes associations of IDE gene variants with type 2 diabetes (1, 2) and Alzheimer’s disease (3, 4), decreased clearance of the two peptides in IDE-deficient mice (5, 6), and antidiabetic activity produced by IDE inhibitors (7, 8), although some reports have questioned its role in insulin degradation (9, 10). In addition, IDE has been reported to have noncatalytic functions, such as acting as a receptor for varicella-zoster virus (11) and serving as a heat shock protein in stressed cells (12). IDE also modulates the activity of the proteasome (13), reportedly in conjunction with the retinoblastoma tumor-suppressor protein (14).
We previously established that polyanions, such as free ATP and triphosphate, increase IDE activity by up to 100-fold toward a synthetic peptide substrate (15) and that ATP binds to a strongly electropositive inner surface of IDE that forms one-half of the substrate-binding chamber (16, 17). Significantly, mutations in IDE that reduce its activation by polyanions also decrease its ability to rescue production of a mature yeast mating factor, indicating that activation by polyanions plays an important physiological role in cells (16). Physiological polyanionic activators of IDE have not yet been identified, however.
Inositol phosphates (InsPs) are important intracellular second messengers generated by activation of many cell surface receptors (18, 19). Phosphatidylinositol phosphates (PtdInsPs; phosphoinositides) participate in signaling and help define the identity of subcellular compartments by enrichment of their membranes with one or more PtdInsPs (20–22). We report here that both InsPs and PtdInsPs activate IDE by interacting with the same polyanion-binding site that binds ATP and triphosphate.
Found mainly in the cytosol, IDE also has been reported to be associated with various subcellular compartments, including endosomes (23–25) and peroxisomes (26). It also is reported to be secreted from cells. Localization of IDE to different intracellular compartments determines access to substrates, although the mechanism whereby IDE traffics to and becomes associated with intracellular compartments remains largely unknown. Here we provide evidence that IDE binding to PtdInsPs facilitates its localization to endosomes. Thus, the interaction of IDE with InsPs and PtdInsPs can modulate the function of IDE by affecting both its activity and its spatial distribution.
Results
InsPs Activate IDE.
Because of their anionic nature and prominent role in signaling, we tested a series of InsPs for their ability to increase the activity of IDE toward the synthetic quenched fluorescent substrate Abz-Gly-Gly-Leu-Arg-Lys-His-Gly-Gln-EDDnp (Table 1). All of the tested InsPs increased IDE activity, exhibiting a hyperbolic response, with the extent of activation generally greater as the number of phosphates on the inositol was increased. For example inositol 3-monophosphate [Ins(3)P] produced a maximal sevenfold increase in activity, whereas inositol 1,3,4,5-tetrakisphosphate [Ins(1,3,4,5)P4] produced a maximal ∼60 fold increase in activity. The activation constant (KA) was decreased from ∼100 µM for Ins(3)P to ∼1 µM for inositols containing six or more phosphate moieties.
Table 1.
Activation of IDE by myo-InsPs
| Myo-inositol | Maximal fold activation, mean ± SD | KA, µM, mean ± SD |
| 3-monophosphate | 6.2 ± 0.8 | 94.5 ± 21.5 |
| 1,2-bisphosphate | 3.1 ± 0.4 | 56.8 ± 17.4 |
| 1,3-bisphosphate | 6.1 ± 0.5 | 27.2 ± 6.0 |
| 4,5-bisphosphate | 13.8 ± 3.4 | 83.3 ± 24.2 |
| 1,3,5-trisphosphate | 12.9 ± 0.7 | 7.8 ± 0.8 |
| 1,4,5-trisphosphate | 30.6 ± 1.4 | 39.9 ± 4.3 |
| 1,3,4,5-tetrakisphosphate | 58.6 ± 1.5 | 18.7 ± 1.5 |
| 1,3,4,5,6-pentakisphosphate | 83.3 ± 4.0 | 8.2 ± 1.5 |
| 1,2,3,4,5,6-hexakisphosphate (phytic acid) | 72.0 ± 4.0 | 0.7 ± 0.1 |
| 1-diphosphoinositol pentakisphosphate | 79.7 ± 3.1 | 0.9 ± 0.1 |
| 5-diphosphoinositol pentakisphosphate | 94.7 ± 4.1 | 1.7 ± 0.2 |
To test for positional effects regarding the placement of the phosphate group, we compared inositol bisphosphates with phosphate moieties at the 1 and 2, the 1 and 3, and the 4 and 5 positions. Positional effects were observed, with the 4,5-bisphosphate producing the highest level of activation, followed by the 1,2-bisphosphate and then the 1,3-bisphosphate. The KA did not correlate with the level of activation, being highest for the 4,5-bisphosphate, followed by the 1,3-bisphosphate and then the 1,2-bisphosphate. A comparison of two triphosphate isomers gave similar results, with Ins(1,4,5)P3 producing greater activation than Ins(1,3,5)P3, but with the latter having a lower affinity based on its KA value.
We examined in more detail the effects of Ins(1,4,5)P3 and Ins(1,2,3,4,5,6)P6 (phytic acid) on the kinetics of the IDE reaction with the fluorogenic Abz substrate. As shown in Fig. 1 and summarized in Table 2, Ins(1,4,5)P3 increased Vmax for the reaction by ∼40-fold, with little effect on the substrate KM or the extent of cooperativity (Hill coefficient). Phytic acid had the same effect at low substrate concentrations, producing an increase in Vmax in the substrate range of 1–25 µM, but decreased reaction rates at substrate concentrations >25 μM. Competition between phytic acid and substrate at high substrate concentrations may account for the observed biphasic effect on activity. We used phytic acid to test activation with respect to physiological substrates, because it has the lowest KA as well as high activation with the Abz fluorogenic substrate. With smaller peptides, namely angiotensin, bradykinin, and dynorphin B9, phytic acid stimulated hydrolysis by ∼6-fold to as much as 26-fold (Table 3). In contrast, there was no significant effect of phytic acid with the larger peptides insulin, β-endorphin, and glucagon, whereas an ∼twofold increase in activity was observed with β-amyloid peptide1–40 (Aβ1–40).
Fig. 1.
Activation of IDE by Ins(1,4,5)P3 and phytic acid [Ins(1,2,3,4,5,6)P6]. IDE activity was measured using the fluorogenic peptide Abz-GGFLRKHGQ-Eddnp at the indicated concentrations in 50 mM Tris buffer pH 7.4 with and without the addition of 50 µM Ins(1,4,5)P3 or 5 µM phytic acid.
Table 2.
Kinetic parameters for activation of IDE by two InsPs
| Addition | Vmax, nmol/min/µg, mean ± SD | Km, µM, mean ± SD | Hill coefficient, mean ± SD |
| None | 29.1 ± 2.8 | 11.1 ± 1.8 | 1.6 ± 0.4 |
| Ins(1,4,5)P3 | 1,276 ± 99 | 8.1 ± 1.3 | 1.9 ± 0.5 |
| Ins(1,2,3,4,5,6)P6 (phytic acid)* | 1,153 ± 119 | 8.5 ± 1.1 | 1.6 ± 0.2 |
Based on a substrate concentration range of 0–25 µM.
Table 3.
Effect of phytic acid on the hydrolysis of physiological peptides by IDE
| Substrate | Rate, nmol/min/mg IDE | |
| No addition | Plus phytic acid (fold increase) | |
| Angiotensin | 6.1 | 38.3 (6.3) |
| Bradykinin | 9.6 | 254 (26.4) |
| Dynorphin B9 | 35 | 340 (9.7) |
| Insulin | 22.4 | 22.4 (1.0) |
| β-endorphin | 1,400 | 1,200 (0.86) |
| Amyloid β peptide (1–40) | 1,500 | 2,720 (1.8) |
| Glucagon | 3,200 | 2,672 (0.8) |
The concentration of phytic acid, when present, was 5 μM.
PtdInsPs also Activate IDE.
The observation that InsPs activate IDE prompted us to examine the effects of phospholipids with inositol head groups. PtdIns and its phosphorylated derivatives share the configuration of the InsP activators, and the substrate-binding cavity of IDE could accommodate these larger lipid molecules (or portions of them when associated with membranes). We initially tested the binding of IDE to a panel of PtdInsPs immobilized on a membrane using an anti-IDE antibody. A strong signal was obtained for IDE binding to immobilized PtdIn3P, 4P, or 5P and to PtdIn(3,4)P2, PtdIn(3,5)P2, and PtdIn(4,5)P2 (Fig. 2). Weak signals were observed with PtdIns(3,4,5)P3, phosphatidic acid, phosphatidylserine (PS), lysophosphatidic acid, and phosphatidylethanolamine, and no signal was observed with PtdIns, phosphatidylcholine (PC), lysophosphatidylcholine, and sphingosine-1-phosphate. The strength of the observed binding signal was the reverse of the pattern seen with InsPs. In general, the more phosphates present on an InsP, the tighter the binding (i.e., lower observed KA). In contrast, the binding of IDE to immobilized PtdIn monophosphates in general gave a stronger signal than observed with immobilized PtdIn bisphosphates, which in turn exhibited a stronger signal than PtdIn(3,4,5)P3.
Fig. 2.
IDE binds to immobilized phosphatidylinositols. IDE was incubated with commercial lipid strips (100 pmol/spot) as described in Materials and Methods. Binding was determined using anti-IDE antibody in a Western blot-like procedure. LPA, lysophosphatidic acid; LPC, lysophosphocholine; PE, phosphatidylethanolamine; PC, phosphatidylcholine; S1P, sphingosine 1-phosphate; PA, lysophosphatidic acid; PS, phosphatidylserine; dye, blue marker dye. Numbers in parentheses indicate intensities of the spots relative to the intensity for PC.
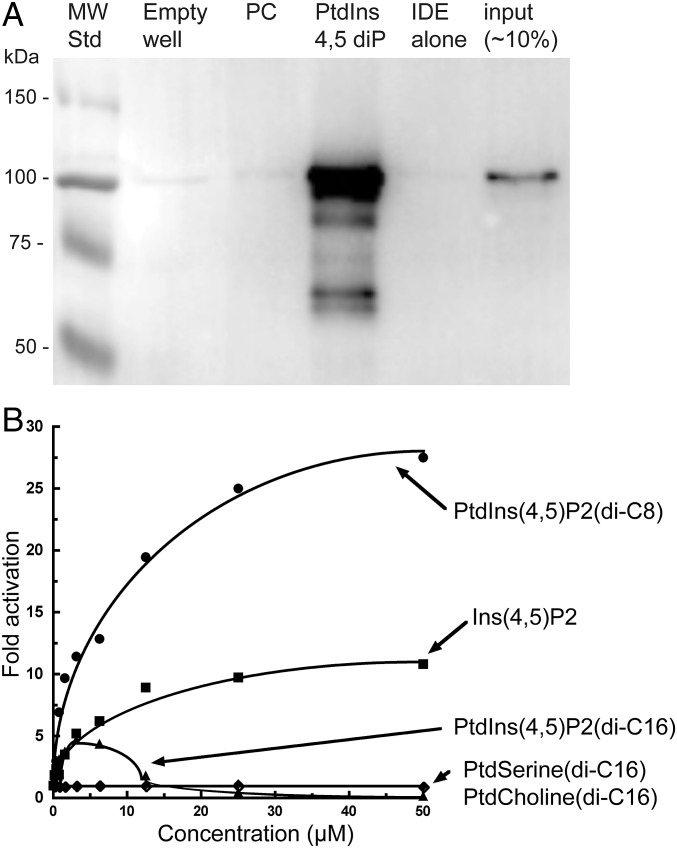
To determine whether IDE could interact with PtdInsPs in bilayer membranes, we tested the ability of IDE to bind to liposomes containing PtdIns(4,5)P2. IDE bound to PtdIns(4,5)P2-containing liposomes, but not to liposomes containing only PC (Fig. 3A), demonstrating an interaction with a PtdInsP present in a lipid bilayer.
Fig. 3.
IDE binds to phosphatidylinositol-containing liposomes and PtdIns(4,5)P2 activates the enzyme. (A) IDE binding to liposomes was tested using 260 μg of total lipid and 0.9 µg of IDE. Liposomes isolated after incubation were separated on a polyacrylamide gel and stained by Western blotting with anti-IDE antibody. Lane 1 (MW Std), molecular weight markers; lane 2 (empty well), no IDE or liposomes; lane 3 (PC), IDE plus liposomes containing only DOPC; lane 4 (PtdIns 4,5 diP), IDE plus DOPC liposomes with PtdIns(4,5)P2 at a ratio of 10:1; lane 5 (IDE alone), enzyme only; lane 6 (input), 0.1 µg of purified IDE without incubation. (B) Comparison of IDE activation by PtdIns(4,5)P2 (di-C16 or di-C8 acyl chains), Ins(4,5)P2, phosphatidylserine, and phosphatidylcholine. IDE activity was measured by the hydrolysis of Abz-GGFLRKHGQ-Eddnp substrate at varying concentrations of the indicated lipid or Ins(4,5)P2. The curves for activation by PtdIns(4,5)P2 (di-C8 acyl chains) and Ins(4,5)P2 are the fits to a hyperbolic function. Curves are drawn through the data points for activation by other compounds to show trends.
PtdInsPs also have the ability to increase the rate of the IDE reaction with the fluorogenic Abz substrate. Activation by PtdIns(4,5)P2 (di-C8) followed a hyperbolic curve, with a maximum 31-fold rate enhancement and a KA of 5.7 μM (Fig. 3B). The longer-acyl chain PtdIns(4,5)P2(di-C16) also activated IDE up to a concentration of ∼3 μM, after which the extent of activation decreased, leading to inhibition at the highest concentrations tested. The loss of activation began at the critical micelle concentration (CMC) of this longer-acyl chain lipid (Fig. S1), indicating that the decrease in free lipids as micelles form accounted for this effect. Although a limited series was studied, the more phosphates present, the tighter the KA of the PtdInsPs, as with the InsPs but in contrast to the immobilized lipids. The maximum level of activation also did not follow this trend. PtdIns(3)P(di-C8) had a KA of 30 μM with ∼15-fold activation, and PtdIns(3,4,5)P3(di-C8) had a KA of 0.5 μM with ∼20-fold activation. These values and those noted above for PtdIns(4,5)P2(di-C8) can be compared with the KA of ∼90 μM and ∼6-fold activation for Ins(3)P, KA of ∼83 μM and ∼14-fold activation for Ins(4,5)P2, and KA of 7.8 μM and ∼30-fold activation for Ins(3,4,5)P3. The tighter KA values for the lipids suggest that moieties other than the InsPs in their head groups contribute to binding and, at least in some cases, may make a modest contribution to activation. Neither PS nor PC had an effect over the same concentration range (Fig. 3), showing that the observed effects were specific to the PtdInsPs.
Fig. S1.
CMC determination for PtdIns(4,5)P2(di-C16). (A) The wavelength of the 1-pyrenecarboxaldehyde fluorescence maximum at varying PtdIns(4,5)P2(di-C16) concentrations was monitored under kinetic assay conditions (79) for excitation at 380 nm (89). The emission data were fit to fifth-order polynomials (curves shown) in GraphPad Prism to determine the position of the maxima. (B) The shift in emission wavelength with lipid concentration was fit to a Boltzmann sigmoid function in Prism. The inflection point, considered the CMC, occurs at 3.0 μM PtdIns(4,5)P2(di-C16).
Three small physiological IDE substrates—bradykinin, angiotensin, and dynorphin B9—as well as four large physiological substrates—insulin, β-endorphin, Aβ1–40 and glucagon—were tested with PtdIns(4,5)P2(di-C8) as an activator. At 25 µM PtdIns(4,5)P2, which is fivefold above its KA, both bradykinin and angiotensin hydrolysis were increased by ∼20-fold, whereas the hydrolysis of dynorphin B9 was increased by ∼60-fold. With insulin as the substrate, there was a small (1.4-fold) increase in the hydrolytic rate, whereas with β-endorphin and Aβ1–40, the rate was increased by 1.3-fold, and there was essentially no change in the rate of glucagon hydrolysis (1.1-fold increase).
IDE Interaction with InsPs and PtdInsPs Is Mediated by the Polyanion-Binding Site.
An IDE variant, IDEK898A,K899A,S901A, with mutations in the polyanion-binding site shows reduced activation by Ins(1,4,5)P3 and phytic acid, supporting the concept that these activators bind at the same site as ATP (Table 4). In addition to the polyanion-binding site, IDE contains another allosteric site, the distal binding site, which interacts with the carboxyl terminus of extended peptide substrates (27) or, in the case of shorter substrates, binds a second peptide (27, 28). Binding of a peptide at this distal site produces a threefold to fivefold increase in activity toward the Abz substrate (29).
Table 4.
Effect of polyanion-binding site and distal site mutations on activation by InsPs
| Activator | Fold activation | ||
| IDEwt | IDEK898A,K899A,S901A | IDEY609F | |
| Ins(4,5)P2 | 7.8 | 1.6 | 10 |
| PtdIns(4,5)P2 | 3.9* | 1.7 | 1.6† |
| Ins(1,4,5)P3 | 32.6 | 6.7 | 9.4 |
| Phytic acid | 68.6 | 6.4 | 29.4 |
Assays were conducted with 50 µM Ins(4,5)P2, 5 µM PtdIns(4,5)P2, 50 µM PtdIns(1,4,5)P3, and 5 µM phytic acid.
Based on a concentration range of 0–3 µM above which inhibition was observed (Fig. 3B).
Data could not be fit to a hyperbola, because an increase in rate was observed only at 1.5 and 3.0 µM. Reported increase is based on a single value at 3 µM.
Mutations at either the polyanion-binding or distal binding sites reduce activation at the other site, indicating they are coupled in some way (16). Thus, we tested the effect of a mutation in the distal site, IDEY609F, on activation by Ins(1,4,5)P3 and phytic acid. Activation by these InsPs was decreased in IDEY609F, further establishing the link between the two sites.
The polyanion-binding site and distal site mutations also affected the ability of IDE to bind to PtdIns(4,5)P2 immobilized on a membrane. By measuring the amount of IDE bound to increasing amounts of immobilized PtdIns(4,5)P2 (Fig. 4 A and B), we found that, relative to IDEwt, the polyanion-binding site mutant IDEK898A,K899A,S901A exhibited reproducibly decreased binding at all levels of PtdIns(4,5)P2. For example, using 150 pmol of immobilized PtdIns(4,5)P2 and 0.5 µg of IDE, a ratio of IDEK898A,K899A,S901A to IDEwt intensity of 0.5 was obtained. This loss of binding in IDEK898A,K899A,S901A suggests that the phosphatidylinositol is interacting with the polyanion-binding site. Somewhat surprisingly, the distal site mutant IDEY609F showed reproducibly increased binding; for example, a ratio of 3.0 in intensity relative to IDEwt for the concentrations noted above was obtained. Perhaps this region interacts with the fatty acid portion of bound lipid, and mutating to a more hydrophobic residue enhanced the interaction. (In fact, residue 609 is positioned to interact with one acyl chain of PtdIns in a binding model described below.)
Fig. 4.
Phosphoinositides interact with the polyanion-binding site. (A) Mutation of the polyanion-binding site, but not the distal site, reduces binding to PtdIns(4,5)P2. PtdIns(4,5)P2 was spotted at the indicated amounts on a hydrophobic membrane along with a phosphatidylcholine control. Membrane strips were incubated with IDEwt, the polyanion site mutant IDEK898A,K899A,S901A, or the distal site mutant IDEY609F at the amounts indicated. Bound enzyme was visualized by incubation with anti-IDE antibody. (B) Coomassie blue-stained gel indicating the same amount of the three IDE constructs used for membrane binding. Amounts of IDE for the trials were all within 15.5% of the mean. (C) Displacement of TNP-ATP from the IDE polyanion-binding site by PtdIns(4,5)P2. IDE (66 µg) was mixed with 10 µM TNP-ATP in 50 mM Tris buffer, pH 7.4, and the fluorescent spectra were recorded on an LS 55 Luminescence Spectrometer (PerkinElmer) over the indicated wavelengths. Also shown are the fluorescent spectra of IDE alone, TNP-ATP alone, and IDE with TNP-ATP in the presence of the indicated concentration of PtdIns(4,5)P2.
To obtain more direct evidence that IDE binds to PtdInsPs through the polyanion-binding site, we measured the ability of PtdIns(4,5)P2 to compete with the fluorescent ATP analog trinitrophenyl-ATP (TNP-ATP) for binding to IDE. It was previously established that the fluorescence of TNP-ATP increases when bound to IDE at the polyanion-binding site (30). When PtdIns(4,5)P2 was added to IDE in the presence of TNP-ATP, there was a decrease in fluorescence consistent with decreased TNP-ATP binding (Fig. 4C). Phytic acid and PtdIns(3)P also competed with TNP-ATP for binding to IDE. Free 40 µM PtdIns(4,5)P2 has no effect on the fluorescence of free TNP-ATP.
IDE Localization to Endosomes Involves the Polyanion-Binding Site.
Given that IDE has been reported to be present in endosomes (23–25), we hypothesized that the enzyme initially might be recruited to endosomes by binding to PtdIns(3)P known to be present in the outer leaflet of the endosomal membrane (21, 22, 31). Discontinuous sucrose density centrifugation was used to enrich endosomes from COS-1 cells as described by Jang et al. (32). In COS-1 cells transfected with wild-type (WT) IDE, the enzyme was detectable in the endosomal fraction (Fig. 5A and Fig. S2), which showed the expected enrichment in the endosomal markers Rab-5 and early endosome antigen 1 (Fig. S3). To ensure that the endosomal fractions were free of any significant amount of plasma membrane, we subjected samples to Western blot analysis with antibodies against the Na+/K+ ATPase (Fig. S3). We were unable to detect Na-K ATPase in the endosomal fraction, estimating a detection limit of 0.01% contamination based on the Na-K ATPase staining intensity in the plasma membrane fraction. In contrast, the estimated endosomal content of IDE was 0.22% of the total IDE applied to the sucrose gradient. These data indicate that IDE found in the endosomal fraction is not an artifact due to contamination of endosomes with other cellular components.
Fig. 5.
Endosomal IDE content of COS-1 cells. (A) Western blot of the PNS and endosomal fractions isolated from COS-1 cells transfected with IDEwt. (B) Western blot of fractions isolated from COS-1 cells transfected with polyanion-binding site mutant IDEK898A,K899A,S901A. (C) Western blot of fractions isolated from COS-1 cells transfected with the distal site mutant IDEY609F. The total protein loaded in each lane is indicated. (D) Green channel indicating labeled endosomes for a representative micrograph (single plane from a 3D image stack), supporting the localization of IDE to endosomes. Endosomes in COS-1 cells were labeled by uptake of Alexa Fluor 488-dextran. A number of cells are visible in the image. (Scale bar: 5 μm.) (E) Red channel indicating IDE distribution for the same micrograph. Expressed IDEwt was labeled by immunostaining with Alexa Fluor 549-conjugated antibody. (F) Merged red and green channels from the same micrograph. In D–F, the Insets show magnified views of the areas indicated by the smaller rectangles to emphasize the degree of dye overlap. (G) Scatterplot showing distribution of red and green channel intensities in voxels from a single COS-1 cell masked in a 3D image stack. The distribution shows a strong spatial correlation between channel intensities consistent with localization of IDE with endosomes. Vertical and horizontal lines indicate threshold cutoffs for statistical analysis. Symbol colors represent the number of voxels in color intensity bins, progressing from blue (lowest number) to red (highest number).
Fig. S2.
Western blot analysis of three IDE variants showing approximately equal staining. A 10% SDS polyacrylamide gel was loaded with 0.25 µg of each IDE variant used in the study. Western blot analysis was performed, and the three bands were quantified as described in Materials and Methods. The IDEK898A,K899A,S901A mutant gave a value of 0.83 compared with WT IDE, and IDEY609F gave a value of 0.94. These values are within the limits of our experimental error and would not account for the differences found in subcellular fractionation studies.
Fig. S3.
Marker proteins for COS-1 cell fractionation. Subcellular compartment marker proteins were visualized by Western blot analysis for the COS-1 cell fractionation shown in Fig. 5 A–C. The markers are Na+/K+ ATPase, plasma membrane; Rab-5, endosome; and EEA1, endosome. Results are shown as indicated for cells transfected with IDEwt, IDEK898A,K899A,S901A, or IDEY609F.
Fluorescence microscopy confirmed colocalization in COS-1 cells of immunostained IDEwt, with endosomes marked by cellular uptake of dye-labeled dextran. Images showed visible fluorescent dye spatial overlap and characteristic pixel color intensity correlation scatterplots (Fig. 5 D–G). For 18 masked cells from six 3D image stacks, the average Pearson correlation coefficient of dye color values was 0.68 (+/− 0.13), indicating strong spatial correlation of the dye intensities consistent with colocalization.
To provide evidence that endosomal binding occurred through an interaction with the polyanion-binding site, we compared the amount of endosomal IDE in COS-1 cells transfected with IDEwt with that in COS-1 cells transfected with either of the IDE mutants, IDEK898A,K899A,S901A or IDEY609F. As noted above, IDEK898A,K899A,S901A contains mutations within the polyanion-binding site and shows decreased activation by polyanions, whereas IDEY609F has a mutation in the distal site that affects activation by polyanions, but not by directly affecting the polyanion-binding site. We assessed the distribution of these mutants in enriched endosomal fractions (Fig. 5 B and C). The IDEK898A,K899A,S901A mutant showed a decreased amount of IDE in the endosomal fraction relative to the WT enzyme, whereas the amount of IDEY609F present in the endosomal fraction was comparable to that of IDEwt. We quantified this by comparing the ratio of Western blot intensity of endosomal IDE relative to total input IDE. The distal site mutant yielded a ratio corresponding to 0.18%, close to that of WT IDE (0.22%). In contrast, a lower value (0.09%) was observed for the polyanion-binding site mutant. We also compared the ratio of Western blot intensity for endosomal IDE relative to Rab5, a known endosomal protein. Again, the WT and distal site mutants gave similar values (0.13% and 0.10%, respectively), whereas the polyanion site mutant exhibited a lower value (0.04%).
Wortmannin is a potent inhibitor of phosphoinositide 3-kinases (PI3Ks), lowering cellular PtdIns3P levels. Thus, if PtdIns3P were involved in IDE recruitment to endosomes, presumably through binding to the polyanion site, then wortmannin would be expected to decrease the amount of IDE bound to endosomes. That this is the case is demonstrated by our measurements of the amount of IDE in the endosomal fraction derived in COS-1 cells treated with 200 nM wortmannin (Table 5). There was a time-dependent decrease in endosomal IDE in wortmannin-treated cells.
Table 5.
Effect of wortmannin on the endosomal level of IDE in COS-1 cells
| Duration of treatment, h | Intensity of IDE band per milligram of endosomal protein |
| 0 | 290 |
| 0.5 | 214 |
| 4 | 119 |
Because the sucrose gradient system used in this study also generates a Golgi/ER fraction, we compared the IDE content in this fraction between WT IDE and the polyanion and distal site mutants. In this case, there was no discernible difference between WT IDE and the two mutants (Fig. S4). This suggests that localization to the Golgi/ER does not involve the polyanion-binding site and likely is not mediated by PtdInsPs. Because IDE is reported to be in peroxisomes, we determined the levels of two peroxisomal enzymes, catalase and thiolase, in the endosomal and Golgi/ER fractions. We found no detectable thiolase or catalase in the endosomal fraction, but detected catalase and thiolase in the Golgi/ER fraction, showing that it contained peroxisomes, which likely accounts for much of the IDE in this fraction. The lack of dependence of peroxisomal IDE on the polyanion-binding site is consistent with the report that IDE has a C-terminal peroxisome-targeting signal (33).
Fig. S4.
IDE in the Golgi fraction of transfected COS-1 cells. Western blot of the PNS and Golgi fractions isolated from COS-1 cells transfected with IDEwt, IDEK898A,K899A,S901A, or IDEY609F as indicated.
IDE Retains Activity at Endosomal pH in Some Buffer Systems.
The level of IDE activity in endosomes as they acidify is an important consideration in its role in degrading endosomal peptides, and IDE has been reported to lose substantial activity at low pH (34). Thus, we examined the effect of pH on IDE activity using the quenched fluorogenic peptide substrate, and found that IDE activity was dependent on the buffer as well as on the pH (Table S1). For example, we observed activity in MES buffer at a pH of close to 5, but no activity in acetate or citrate buffer at this pH. Similarly, at a pH of ∼5.5, we observed activity in citrate buffer, and much higher activity at this pH in MES buffer. We also observed high activity at pH 6 in MES buffer, ∼10-fold greater than the activity at pH 7.5 in Tris buffer. We found that MES serves as an activator of IDE, by demonstrating an increase in activity when MES was added to Tris buffer. Thus, IDE can exhibit significant activity at low pH, as is seen in endosomes, particularly when activators are present.
Table S1.
Buffer-dependent activity of IDE at low pH
| Buffer | pH | Activity, nmol/min/mg IDE |
| Na acetate | 5.06 | No activity |
| Na citrate | 5.05 | No activity |
| MES | 5.11 | 11.8 |
| Na acetate | 5.48 | No activity |
| Na citrate | 5.50 | 8.9 |
| MES | 5.62 | 71.1 |
| MES | 6.08 | 68.9 |
| Tris | 7.45 | 6.9 |
All buffers were at 50 mM. Assays were conducted with 10 µM Abz-GGFLRKHGQ-Eddnp. pH values were determined from dummy reaction mixtures containing 20% DMF in place of substrate. In a separate experiment, the Abz substrate was completely cleaved, and the total fluorescence change measured at the different pH values. There was no effect of pH on the total fluorescence change.
Discussion
IDE is known to be allosterically activated in vitro by both peptides and polyanions (15, 29). The results of this study now clearly establish InsPs as polyanion activators of IDE. As previously observed with nucleotide phosphates (15), the greater the number of phosphates present on inositol, the greater the extent of activation up to six phosphate moieties. There are also positional effects of phosphate placement, although these effects are smaller than the total charge effect. The dependence on the number of negative charges, the competitive binding with a fluorescent ATP analog, and the finding that a polyanion-binding site mutant binds less well to InsPs all indicate that InsPs bind to the previously identified polyanion-binding region of IDE. This site/region lies on one-half of the inner surface of the substrate-binding chamber formed by the C-terminal two domains of the enzyme (16). As noted previously, this appears to be not a single binding site in the classical sense, but rather a positively charged region of the enzyme that can accommodate polyanion binding in at least several different binding modes (16, 17).
Given that a series of peptides exhibits quite different rates of hydrolysis by IDE, the rate-determining step of the reaction must be dependent on the substrate or products. This could be cleavage of the peptide itself, conversion of the IDE-substrate complex from an open conformation to a closed conformation, or conversion of the IDE-product complex from a closed conformation to an open conformation, permitting product release (17). One of these steps must be the one increased by InsPs (or PtdInsPs).
InsPs increase the hydrolytic rate of the small peptides tested (9–12 aa), but have no effect or only a mild effect with larger peptides. An unexplained exception is Aβ1–40, the rate of which was increased by approximately twofold by phytic acid. In crystal structures of IDE with bound peptides (28, 35–39), larger substrates are seen to make contacts at both the active site and the distal site (which mediates activation by peptides). It is possible that activity is not stimulated for large peptides because they themselves activate in a manner similar to polyanions, particularly because their hydrolysis rates are often higher than those for small peptides. The basis for such activation is not clear from existing structures, however. Generally, only substrate residues at the active and distal site are ordered, and the larger peptides do not make contacts with the anion-binding surface. Intact insulin, with its disulfide cross-linking, is mostly well ordered in the binding chamber of the enzyme, but it also does not interact with the anion-binding surface. There is no obvious reason why the Aβ1–40 peptide should behave differently in terms of activation than, for example, glucagon, given that the number and conformation of ordered residues for the two peptides are similar when bound to IDE.
Ins(1,4,5)P3 has been established as a second messenger that mobilizes calcium pools, and recently more highly phosphorylated InsPs have been shown to be important effectors in a number of signaling pathways (18, 40). For example, activation of the insulin receptor stimulates, among other effects, generation of highly phosphorylated inositols, such as 5-diphosphoinositolpentakisphosphate (InsP7) (40–42). Our present finding that InsPs stimulate IDE catalysis suggests that they may serve as physiological activators of the cytosolic pool of the enzyme. Concentrations of various InsPs in mammalian cells range from submicromolar to >100 μM, and their levels can be modulated substantially by various events, such as receptor activation and progression through the cell cycle (43, 44). These InsP concentrations, including the potent activator phytic acid, are in ranges shown here to produce IDE activation, consistent with a possible physiological role.
The most well-documented role for cytosolic IDE is in degradation of the amyloid precursor protein intracellular domain (AICD) (6, 45), which serves as a transcriptional regulatory factor. Interestingly, AICD recently has been shown to activate the kinase PIKfyve (46), which increases the production of PtdIns(3,5)P2, a signaling lipid and a source of soluble Ins(5)P (47). This connection to PtdIns and InsP production suggests a possible regulatory system for control of AICD levels involving IDE activation. Other roles for cytosolic IDE are not well established, but given the broad range of substrates in vitro, it seems likely that additional peptides are substrates within cells. It also may be that interactions with InsPs affect noncatalytic IDE functions, such as regulating proteasome activity (13, 48, 49) and serving as a heat shock-like protein in stressed cells (12). The functions of cytosolic IDE and the role of allosteric modulation of its activity likely will be promising areas for future investigation.
Ever since IDE was identified as a cytosolic enzyme (50), the question of how it gains access to substrates that are degraded in other subcellular compartments has been of considerable interest (51). For example, insulin is metabolized primarily in early to late endosomes after internalization of the peptide bound to its receptor (23, 52, 53) and thus is inaccessible to cytosolic IDE. However, small pools of IDE have been found in endosomes (23–25) as confirmed here, as well as in peroxisomes (26, 54) and mitochondria (55). In addition, low levels of IDE are reportedly secreted from some cell types through an unconventional pathway (56) that may involve routing through multivesicular bodies and release in association with exosomes (25). As noted above, a targeting sequence at the C terminus that localizes some IDE to peroxisomes has been reported previously (26, 33, 54), and a potential alternative splice form with an extended N terminus was shown to direct IDE to mitochondria (55). Otherwise, determinants of IDE intracellular localization have not been defined conclusively. We found here that a portion of cellular IDE localizes to the endosomal system (Fig. 5 and Fig. S3), and our observation that IDE binds to PtdInsPs suggests a mechanism for intracellular localization of the enzyme. One role of PtdInsPs is to establish the identities of subcellular compartments by enrichment of their membranes with differently phosphorylated forms of the lipid (20, 22, 57). These markers direct a diverse set of protein domains to target membranes based on preferential binding to the enriched PtdInsP (21, 57). We postulate that in the same manner, PtdInsP binding can recruit IDE to subcellular locations through binding at the polyanion site. In particular, the binding to immobilized PtdIns(3)P is consistent with initial recruitment to early endosomes (22, 31, 57), and this possibility is supported by our finding that wortmannin treatment, which reduces PtdIns(3)P levels, decreases IDE localization to endosomes (Table 5). Binding to PtdIns(3,4)P2 also would cause early endosome localization, because this phospholipid is present in vesicles just after endocytosis.
Recruitment to endosomes and downstream compartments is particularly relevant to substrate access by IDE. As noted above, insulin degradation occurs largely in the endosomal system (23, 52, 53). Similarly, Aβ peptides are generated primarily in endosomes, and one mechanism for clearance of extracellular Aβ is uptake into endocytic compartments with degradation in endosomes, multivesicular bodies, and lysosomes (58, 59). Amylin, another IDE physiological substrate (49), also is taken up by receptor-mediated endocytosis (60).
Once localized to endosomes, IDE would still have to gain access to the interior of the compartment to encounter substrates. A possible internalization mechanism is the budding of vesicles into the endosome that occurs during normal endosome maturation to multivesicular bodies and late endosomes (31, 61, 62). This formation of intraluminal vesicles is a central aspect of the sorting process that determines whether endocytosed membrane proteins are targeted for lysosomal degradation or for recycling to other organelles, including the plasma membrane and Golgi (62, 63). Endosomal vesicle uptake (microautophagy) of cytosolic proteins bearing a targeting sequence or linked to ubiquitin occurs (64, 65). IDE could be internalized with these targeted proteins by virtue of its attachment to PtdIns(3)P, which is known to be present on intraluminal vesicles (61). Recently, cytosolic proteins taken up by this microautophagy process in late endosomes were observed in the endosome lumen (64), presumably through disruption of some luminal vesicles. The relatively weak interaction of IDE with PtdInsPs [Kd of ∼3 mM for PtdIns(4,5)P2-containing liposomes] found here would permit the enzyme to escape into the endosome lumen during this process.
As noted above, considerable evidence from genetic associations (1, 2), knockout mouse and inhibitor studies (6–8, 66, 67), as well as characterization of hydrolysis products (23, 68), supports the role of IDE in metabolism of insulin. However, Durham et al. (10) found that an IDE inhibitor did not increase plasma insulin levels or improve insulin sensitization, and concluded that IDE plays only a limited role in insulin clearance. Moreover, Steneberg et al. (9) reported that IDE knockout mice did not exhibit elevated fasting insulin levels, in contrast to other findings. Our results supporting the presence of IDE in endosomes and providing a mechanism for localization strengthen the case for its role in insulin degradation. The sensitivity of endosomal localization to mutations in the IDE anion-binding site should provide a basis for a strong test of this function.
IDE recruitment to endosomes also may be relevant to the mechanism underlying the reported secretion of IDE from certain cell types (25, 56, 69). Secreted IDE has been proposed to be involved in extracellular Aβ degradation (6, 70, 71) and potentially some insulin hydrolysis as well (72, 73). IDE does not contain any standard secretion signaling sequences, reportedly being secreted by unconventional pathways (25, 56, 69, 71). Enzyme localized to the endosomal system could contribute to this secreted pool, given that some multivesicular bodies fuse with the cell plasma membrane, releasing their contents, including the intraluminal vesicles (exosomes), into the extracellular medium (25, 74). Endosomal IDE also could be trafficked to the Golgi (or taken up directly into that organelle) and subsequently secreted, but this pathway does not appear to be significant in the cell types in which secretion of the enzyme was studied (25, 56, 69).
Because the anion-biding site is not accessible in the closed form of IDE, the question naturally arises as to how IDE uses this surface to bind PtdInsPs present in a lipid bilayer. Computational docking of Ins(1,3)P2, representing the head group of PtdIns(3)P, as well as several other InsPs to IDE shows a small number of preferred binding sites, two of which appear particularly relevant to PtdInsP binding (Fig. 6A). One site occurs at an edge of the anion-binding surface near the interface between the two halves of IDE. This interface would open first when IDE undergoes a hinge-like motion that must accompany substrate binding and product release (Fig. 6A), and docking of InsPs to this site persists in models of open forms of IDE generated by a clamshell-like motion of the two halves of the enzyme. Rotating the two halves of IDE by 6 degrees about the likely hinge point is the minimum amount to allow placement of a model PtdIns(3)P without steric clashes when the lipid head group is aligned with a high-scoring docked Ins(1,3)P2 (Fig. 6B). This model of PtdInsP binding provides a plausible mechanism for IDE interaction with membrane-bound lipids, closely resembling structural models for interactions between known PtdInsP-binding domains and membrane PtdInsPs (75). The hydrophobic acyl chains of the lipid would remain outside the IDE inner chamber, allowing them to remain embedded in the lipid bilayer. Of potential significance, we note that the molecular surface surrounding this proposed membrane lipid-binding site is the most extensively conserved portion of the outer IDE surface (Fig. 6C), possibly reflecting its role in interaction with the membrane.
Fig. 6.
Computational docking and models for lipid binding by IDE. (A) Binding site clusters for Ins(1,3)P2 computationally docked to IDE, with the ligands drawn as stick figures. The site that possibly mediates interaction with membrane-bound PtdIns head groups is indicated by the red circle. The site that may mediate the head group interaction with activating lipid bound within the substrate-binding chamber is circled in black. Side chains of active site residues are shown in a stick representation. (B) A model for the interaction with membrane-bound PtdIns(3)P with the lipid in a space-filling representation and the protein in a ribbon representation. The lipid is shown with 17:0 and 20:4 acyl chains and was placed manually so that its head group matches the binding of Ins(1,3)P2, indicated by the red circle in A. Side chains of active site residues are shown. (C) Space-filling view of the IDE surface that would interact with the membrane in the model shown in B. Residue atoms were colored based on conservation using the ConSurf server, with blue the least conserved and dark red the most conserved. The conservation of this surface is the highest for the any external surface of IDE. (D) A model for the internal binding of PtdIns(3)P with the lipid head group manually placed to match the binding of Ins(1,3)P2 in the docking cluster indicated by the black circle in A. The lipid is shown with 17:0 and 20:4 acyl chains. (E) Cutaway view of the IDE substrate-binding chamber showing another view of the bound PtdIns(3)P model to emphasize the ability of the inner chamber to accommodate the lipid. (F) Cutaway view of the substrate-binding chamber showing the surface that would interact with PtdIns acyl chains in the model shown in D and E. The surface is color-coded by electrostatic potential using the adaptive Poisson–Boltzmann solver server with cutoffs of ±10 kT. The surface proposed to interact with the acyl chains has a relatively low electrostatic potential, consistent with this interaction.
Whereas the anion-binding surface site near the half-molecule interface is a good candidate for mediating membrane lipid binding, and thus localization to endosomes, interaction with PtdInsPs in this manner likely would not account for their ability to activate IDE. The bound lipid would partially occlude the active site and, by holding the enzyme in an open conformation, displace residues in domain IV that contribute to catalysis. It also is unlikely that a free PtdInsP would bind in a manner that places its acyl tails in bulk solvent. However, a second high-scoring docking site on the anion-binding surface (Fig. 6A) allows placement of a PtdIns(3)P in the inner chamber of IDE, again aligning the Ins(3)P group of the lipid with a docked Ins(1,3)P molecule (Fig. 6 D and E). In this position, the acyl chains of the bound lipid extend toward the distal site in domain 2, interacting with a relatively nonpolar portion of the inner chamber surface (Fig. 6F). This model has the virtue of not overlapping with known substrate-binding surfaces in the active and distal sites and thus is compatible with activation of IDE. Mutations in the IDEK898A,K899A,S901A variant occur adjacent to both proposed lipid head group interaction sites, consistent with their effect on both types of PtdInsP-binding interaction observed in this work.
The finding that a number of different InsPs share some computational docking sites (Fig. S5) has relevance to the observed enhancement of interaction affinities and activation levels with an increasing number of phosphate groups found for the various InsPs tested (Table 1). If interactions occur primarily at the same site or sites, then enhancement of affinity by additional phosphates simply reflects an increased number of interactions with positively charged or hydrogen-bond donor groups on the anion-binding surface. With respect to activation, studies with adenine nucleoside phosphate ligands (15) have shown that increasing the number of phosphates increases the extent of activation, and the results here with InsPs indicate that this effect likely reflects a general dependence on the total charge or the charge density of the interacting ligand. On the other hand, there are subtle differences in computational docking preferences for different InsPs, suggesting that different binding modes may play roles in ligand affinity and the level of activation. Indeed, the finding of modest differences in affinity and activation level between isomers bearing the same number of phosphate groups, such as Ins(1,3,5)P3 and Ins(1,4,5)P3, supports this possibility.
Fig. S5.
Computational docking of InsPs to IDE. Three InsPs, in addition to the Ins(1,3)P2 shown in Fig. 5, were computationally docked to the IDE as described in Materials and Methods. The other InsPs are Ins(1,3,5)P3 (A), Ins(1,4,5)P3 (B), and Ins(1,2,3,4,5,6)P6 (phytic acid) (C). Docked ligands are shown in stick representation with green carbons. Bound ATP from a previously reported crystal structure (16) is shown in stick representation with yellow carbons. Circles indicate the sites of clustered, docked ligands used to model PtdIns(3)P binding to IDE. All three additional InsPs cluster at these sites in addition to Ins(1,3)P2, suggesting that these sites are general robust binding surfaces for InsPs.
Overall, the results presented here suggest InsPs and PtdInsPs as potential physiological modulators of IDE, with InsPs serving as activators with respect to small peptide substrates and PtdInsPs playing a role in the intracellular spatial regulation of IDE.
Materials and Methods
Materials.
InsPs were purchased from Cayman Chemical Company, and PtdInsPs were purchased from Echelon Bioscience. The PtdInsPs used in this study contained palmitic acid as the fatty acid in both the 1 and 2 positions (di-C16) or octanoic acid in both position (di-C8). Avanti Polar Lipids was the source for 1,2-dioleoyl-sn-glycero-3-phosphocholine; 1-diphosphoinositol pentakisphosphate and 5-diphosphoinositol pentakisphosphate were synthesized as described previously (76, 77) by A. Saiardi, MRC Laboratory for Molecular Cell Biology, University College London, London, UK. The anti-IDE antibody (rIDE4020) used in this study has been described previously (5). Western blot analysis established that this antibody exhibits the same avidity for IDE and the IDE mutants used in the present study (Fig. S2). Anti-His antibody was obtained from GE Healthcare Life Science. IDE and its mutant forms were expressed in Sf9 cells and purified to homogeneity as described previously (16, 29, 78). Dextran (molecular weight 10,000) labeled with Alexa Fluor 488 was obtained from Thermo Fisher Scientific.
Activity Measurements.
IDE was routinely assayed using the fluorogenic peptide Abz-GGFLRKHGQ-Eddnp as described previously (79). Kinetic data were fit to the Hill or Michaelis–Menten equation using GraphPad Prism software.
Peptide Hydrolysis.
The hydrolysis of unlabeled peptide substrates was followed by measuring the disappearance of the parent peptide by HPLC. Reactions of 80 µL containing 10 µM peptide, IDE, and InsP as indicated in 50 mM Tris⋅HCl pH 7.4 were incubated at 37 °C for 5–40 min. The reaction was terminated by the addition of 8 µL of 5% TFA, and 75 µL was injected onto a Vydac C18 column. Peptides were eluted with a linear gradient of 5–50% acetonitrile (15), and quantified by measuring peak areas.
Lipid and Western Blot Analyses.
Membranes containing 15 different biologically active lipids at 100 pmol/spot (Echelon Bioscience) were first blocked with 5% fatty acid-free BSA prepared in PBS at room temperature for 2 h, after which 4 mL of fresh blocking solution containing 0.49 µg/µL IDE was added. The membrane was incubated at room temperature for 1 h and then at 4 °C overnight. The membrane was washed three times for 30 min each with PBS containing 0.1% Tween 20 (PBST). After washing, the membrane was treated with either anti-IDE antibody or anti-His antibody in PBS containing 5% fatty acid-free BSA for 1 h. After three washes with PBST for 30 min each, the membrane was incubated with horseradish peroxidase-conjugated anti-mouse antibody or peroxidase-conjugated anti-rabbit antibody (Invitrogen) in PBS containing 5% fatty acid-free BSA for 1 h, and then rinsed three times for 30 min with PBST. The signal was detected using ECL Plus Western Blotting Reagent (Thermo Fisher Scientific).
Membranes containing varying amounts of PtdIns(4,5)P2 were prepared by spotting 2 µL of a lipid solution containing the indicated amount of lipid onto Hybond-C Extra membranes (Amersham). After drying, the membranes were incubated with IDE or its mutant forms and treated as above. Lipid solutions were prepared in 250 µL of a 1:2:1 chloroform:MeOH:water solution to which 2 µL of Ponceau S was added.
For Western blots, when feasible protein concentrations were measured using the Coomassie Plus Protein Assay Kit (Thermo Fisher Scientific). Samples were subjected to electrophoresis on 10% polyacrylamide gels. Bands were transferred to PVDF membranes (GE Healthcare), and membranes were probed with the appropriate primary and secondary antibodies and developed as described above for the lipid blots. In the case of subcellular fractionation studies, the blots for expression of different IDE variants were done on separate membranes but compared as ratios with either the total amount of IDE protein loaded or the amount of Rab5 as an endosomal marker.
Quantitation of lipid and Western blots was carried out on a Gel Doc XR+ gel documentation system (Bio-Rad) with Image Lab software (Bio-Rad). Either box (Western) or circle (lipid) integration areas with minor adjustments across lanes were used with the automatic background subtraction function. Linearity was determined by comparing results for at least three different exposure times.
Preparation of Liposomes.
Liposomes were prepared as described by Buser and McLaughlin (80). In brief, 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC; 6 μmol) with or without PtdIns(4,5)P2 (1 μmol) in chloroform were mixed and dried under vacuum in a rotary evaporator immersed in a 30 °C water bath. The crude liposomes were resuspended in a 6-mL sucrose solution (176 mM sucrose and 1 mM Mops, pH 7.0) and taken through five cycles of freezing in liquid N2 and thawing in a 30 °C water bath. Large unilamellar vesicles were prepared by subjecting the mixture to 10 cycles of extrusion through a stack of two polycarbonate filters (100-nm pore size) in a Lipex Biomembranes Extruder. The sucrose solution on the outside of the vesicles was removed by mixing the large unilamellar vesicles with a salt solution (100 mM KCI and 1 mM Mops, pH 7.0) and recovering the vesicles by ultracentrifugation at 100,000 × g for 1 h at 25 °C. The concentration of PtdIns(4,5)P2 in the liposomes was determined by measuring the phosphate content (81) and corrected for the number of phosphates in the inositol.
Liposome-Binding Assay.
IDE (0.9 μg) was incubated with DOPC liposomes with or without phosphatidylinositol 4,5-bisphosphate (total lipid concentration, 260 µM) in a volume of 280 µL for 1 h at room temperature with gentle rocking. After centrifugation at 112,000 × g for 1 h at 20 °C, the liposomes were pelleted and then resuspended in SDS/PAGE sample buffer. The pelleted liposomes were subjected to SDS/PAGE and Western blot analysis.
Sucrose Gradient Subcellular Fractionation.
Sucrose gradient subcellular fractions were prepared as described by de Araujo et al. (82). In brief, COS-1 cells were grown on 15-cm dishes, washed, and scraped with a rubber policeman in cold PBS. The cells were then pelleted, resuspended in homogenization buffer (250 mM sucrose and 3 mM imidazole, pH 7.4, containing protease and phosphatase inhibitors), and homogenized until ∼90% of the cells were broken without major breakage of the nucleus, as monitored by microscopy. The samples were centrifuged at 2,000 × g for 10 min at 4 °C, and the resulting supernatant was designated the postnuclear supernatant (PNS). The PNS samples were adjusted to 40.6% sucrose and then overlaid with 1.5 volumes of 35% sucrose. The remaining volume of the centrifuge tube was then filled with 8.6% sucrose. Sucrose gradients were centrifuged at 100,000 × g for 6 h at 4 °C, and the endosomal and Golgi/ER membranes were collected.
Wortmannin Treatment.
COS-1 cells were treated with 200 nM wortmannin for 0, 0.5, and 4 h, after which the endosomal fraction was isolated by sucrose density gradient centrifugation as described above. A constant amount of endosomal protein was subjected to Western blot analysis using anti-IDE antibody.
Immunofluorescence Staining and Analysis.
Cos-1 cells were grown on polylysine-coated coverslips in serum-free DMEM media and transformed with pCDNA-3.1 plasmid expressing IDEwt. For dextran uptake experiments, cells were pulse-labeled in serum-free DMEM with 2 mg/mL dextran-Alexa Fluor 488 (molecular weight 10,000, lysine fixable; Invitrogen) for 2 h at 37 °C in 5% CO2, followed by permeabilization with 0.05% saponin before cell fixation. Cells were then fixed with 4% paraformaldehyde (Electron Microscopy Sciences) for 10 min at room temperature and then blocked with 10% normal serum, 1% BSA, and 0.3 M glycine. For immunostaining, cells were incubated with the primary antibody for 90 min in PBS containing 1% BSA, rinsed, and incubated for 60 min with secondary Alexa Fluor 549-conjugated antibody (Jackson ImmunoResearch) in PBS with 1% BSA. Cells were rinsed and mounted for microscopy in Mowiol mounting medium.
All images were acquired using a Mariannas Imaging system consisting of a Zeiss inverted microscope equipped with a cooled CCD CoolSnap HQ (Roper), dual filter wheels, and a 175W xenon light source, all controlled by SlideBook software (Intelligent Imaging Innovations). The detection of Alexa Fluor 488 fluorescence was performed using an FITC filter channel, and the detection of Alexa 549 Fluor fluorescence was performed using a TRITC channel. Images were acquired in 2 × 2 binning mode. Image analysis was performed using SlideBook 6 software (Intelligent Imaging Innovations). Colocalization analysis was done with the interactive segmentation and colocalization modules in SlideBook 6. Statistics for colocalization are averages for 18 cells from six independent 3D images. Threshold levels were determined using the method developed by Costes et al. (83).
Computational Docking and Modeling of Phosphoinositide Binding.
Computational docking of InsPs to the WT unliganded IDE structure (Protein Data Bank ID code 3P7L) (27) was carried out with Autodock Vina 1.1.2 (84) as implemented in Yasara (85). The 3D structures for InsP ligands were generated from SMILES representations, and energy was minimized in Yasara using the NOVA force field and the supplied em_runclean macro. Either 25 or 100 docking runs were carried out with Ins(1,3)P2, Ins(1,4,5)P3, Ins(1,3,5)P3, or phytic acid using the Yasara dock_run macro with the choice of Vina docking. A model and molecular descriptors for PtdIns(3)P were generated from a SMILES descriptor using the eLBOW (86) module of PHENIX (87) and acyl chains were adjusted manually in Coot (88). The resulting model was then energy-minimized in Yasara as described for the InsPs. The PtdIns(3)P ligand was manually modeled into the binding site IDE, aligning its head group with a high-scoring docked Ins(1,3)P2. Open models of IDE were generated manually in Coot.
Acknowledgments
We thank Dr. A. Saiardi for providing the isomer of InsP7 used in this study and acknowledge the use of facilities at the University of Kentucky Center for Structural Biology and the Center for Molecular Medicine Protein Core (supported by National Institutes of Health Grant P20 GM103486). This work was supported by National Institutes of Health grants GM 11787 (to L.B.H.), NS38041 (to D.W.R.), GM113087 (to E.G.); American Cancer Society Grant RSG-14-172-01-CSM (to E.G.); American Heart Association Grant 15PRE25090207 (to H.J.); and National Science Foundation Grant IIA-1355438 (to D.W.R.). Part of this work was supported by the Fundação de Amparo à Pesquisado Estado de São Paulo (Project 12/50191-4R) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (Projects 443978-2014-0 and 467478-2014-7).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613447114/-/DCSupplemental.
References
- 1.Sladek R, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445(7130):881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 2.Zeggini E, et al. Wellcome Trust Case Control Consortium Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316(5829):1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim M, et al. Decreased catalytic activity of the insulin-degrading enzyme in chromosome 10-linked Alzheimer disease families. J Biol Chem. 2007;282(11):7825–7832. doi: 10.1074/jbc.M609168200. [DOI] [PubMed] [Google Scholar]
- 4.Wang S, He F, Wang Y. Association between polymorphisms of the insulin-degrading enzyme gene and late-onset Alzheimer disease. J Geriatr Psychiatry Neurol. 2015;28(2):94–98. doi: 10.1177/0891988714554707. [DOI] [PubMed] [Google Scholar]
- 5.Miller BC, et al. Amyloid-beta peptide levels in brain are inversely correlated with insulysin activity levels in vivo. Proc Natl Acad Sci USA. 2003;100(10):6221–6226. doi: 10.1073/pnas.1031520100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farris W, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA. 2003;100(7):4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maianti JP, et al. Anti-diabetic activity of insulin-degrading enzyme inhibitors mediated by multiple hormones. Nature. 2014;511(7507):94–98. doi: 10.1038/nature13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deprez-Poulain R, et al. Catalytic site inhibition of insulin-degrading enzyme by a small molecule induces glucose intolerance in mice. Nat Commun. 2015;6:8250. doi: 10.1038/ncomms9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steneberg P, et al. The type 2 diabetes-associated gene ide is required for insulin secretion and suppression of α-synuclein levels in β-cells. Diabetes. 2013;62(6):2004–2014. doi: 10.2337/db12-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durham TB, et al. Dual exosite-binding inhibitors of insulin-degrading enzyme challenge its role as the primary mediator of insulin clearance in vivo. J Biol Chem. 2015;290(33):20044–20059. doi: 10.1074/jbc.M115.638205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Ali MA, Cohen JI. Insulin-degrading enzyme is a cellular receptor mediating varicella-zoster virus infection and cell-to-cell spread. Cell. 2006;127(2):305–316. doi: 10.1016/j.cell.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tundo GR, et al. Insulin-degrading enzyme (IDE): A novel heat shock-like protein. J Biol Chem. 2013;288(4):2281–2289. doi: 10.1074/jbc.M112.393108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sbardella D, et al. Proteasome activity is affected by fluctuations in insulin-degrading enzyme distribution. PLoS One. 2015;10(7):e0132455. doi: 10.1371/journal.pone.0132455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radulescu RT, Duckworth WC, Levy JL, Fawcett J. Retinoblastoma protein co-purifies with proteasomal insulin-degrading enzyme: Implications for cell proliferation control. Biochem Biophys Res Commun. 2010;395(2):196–199. doi: 10.1016/j.bbrc.2010.03.157. [DOI] [PubMed] [Google Scholar]
- 15.Song ES, et al. ATP effects on insulin-degrading enzyme are mediated primarily through its triphosphate moiety. J Biol Chem. 2004;279(52):54216–54220. doi: 10.1074/jbc.M411177200. [DOI] [PubMed] [Google Scholar]
- 16.Noinaj N, et al. Anion activation site of insulin-degrading enzyme. J Biol Chem. 2012;287(1):48–57. doi: 10.1074/jbc.M111.264614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song ES, et al. An extended polyanion activation surface in insulin-degrading enzyme. PLoS One. 2015;10(7):e0133114. doi: 10.1371/journal.pone.0133114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsui MM, York JD. Roles of inositol phosphates and inositol pyrophosphates in development, cell signaling, and nuclear processes. Adv Enzyme Regul. 2010;50(1):324–337. doi: 10.1016/j.advenzreg.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson MS, Livermore TM, Saiardi A. Inositol pyrophosphates: Between signalling and metabolism. Biochem J. 2013;452(3):369–379. doi: 10.1042/BJ20130118. [DOI] [PubMed] [Google Scholar]
- 20.Behnia R, Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438(7068):597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- 21.Kutateladze TG. Translation of the phosphoinositide code by PI effectors. Nat Chem Biol. 2010;6(7):507–513. doi: 10.1038/nchembio.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balla T. Phosphoinositides: Tiny lipids with giant impact on cell regulation. Physiol Rev. 2013;93(3):1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamel FG, Mahoney MJ, Duckworth WC. Degradation of intraendosomal insulin by insulin-degrading enzyme without acidification. Diabetes. 1991;40(4):436–443. doi: 10.2337/diab.40.4.436. [DOI] [PubMed] [Google Scholar]
- 24.Fernández-Gamba A, Leal MC, Morelli L, Castaño EM. Insulin-degrading enzyme: Structure-function relationship and its possible roles in health and disease. Curr Pharm Des. 2009;15(31):3644–3655. doi: 10.2174/138161209789271799. [DOI] [PubMed] [Google Scholar]
- 25.Bulloj A, Leal MC, Xu H, Castaño EM, Morelli L. Insulin-degrading enzyme sorting in exosomes: A secretory pathway for a key brain amyloid-beta degrading protease. J Alzheimers Dis. 2010;19(1):79–95. [Google Scholar]
- 26.Morita M, et al. Insulin-degrading enzyme exists inside of rat liver peroxisomes and degrades oxidized proteins. Cell Struct Funct. 2000;25(5):309–315. doi: 10.1247/csf.25.309. [DOI] [PubMed] [Google Scholar]
- 27.Noinaj N, et al. Identification of the allosteric regulatory site of insulysin. PLoS One. 2011;6(6):e20864. doi: 10.1371/journal.pone.0020864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen Y, Joachimiak A, Rosner MR, Tang WJ. Structures of human insulin-degrading enzyme reveal a new substrate recognition mechanism. Nature. 2006;443(7113):870–874. doi: 10.1038/nature05143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song ES, Juliano MA, Juliano L, Hersh LB. Substrate activation of insulin-degrading enzyme (insulysin): A potential target for drug development. J Biol Chem. 2003;278(50):49789–49794. doi: 10.1074/jbc.M308983200. [DOI] [PubMed] [Google Scholar]
- 30.Yao H, Hersh LB. Characterization of the binding of the fluorescent ATP analog TNP-ATP to insulysin. Arch Biochem Biophys. 2006;451(2):175–181. doi: 10.1016/j.abb.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Bissig C, Gruenberg J. Lipid sorting and multivesicular endosome biogenesis. Cold Spring Harb Perspect Biol. 2013;5(10):a016816. doi: 10.1101/cshperspect.a016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jang ER, et al. Spatial control of Shoc2 scaffold-mediated ERK1/2 signaling requires remodeling activity of the ATPase PSMC5. J Cell Sci. 2015;128(23):4428–4441. doi: 10.1242/jcs.177543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo WL, Gehm BD, Rosner MR, Li W, Keller G. Inducible expression and cellular localization of insulin-degrading enzyme in a stably transfected cell line. J Biol Chem. 1994;269(36):22599–22606. [PubMed] [Google Scholar]
- 34.Grasso G, Satriano C, Milardi D. A neglected modulator of insulin-degrading enzyme activity and conformation: The pH. Biophys Chem. 2015;203-204:33–40. doi: 10.1016/j.bpc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 35.Guo Q, Manolopoulou M, Bian Y, Schilling AB, Tang WJ. Molecular basis for the recognition and cleavages of IGF-II, TGF-alpha, and amylin by human insulin-degrading enzyme. J Mol Biol. 2010;395(2):430–443. doi: 10.1016/j.jmb.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manolopoulou M, Guo Q, Malito E, Schilling AB, Tang WJ. Molecular basis of catalytic chamber-assisted unfolding and cleavage of human insulin by human insulin-degrading enzyme. J Biol Chem. 2009;284(21):14177–14188. doi: 10.1074/jbc.M900068200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ralat LA, et al. Insulin-degrading enzyme modulates the natriuretic peptide-mediated signaling response. J Biol Chem. 2011;286(6):4670–4679. doi: 10.1074/jbc.M110.173252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ralat LA, et al. Ubiquitin is a novel substrate for human insulin-degrading enzyme. J Mol Biol. 2011;406(3):454–466. doi: 10.1016/j.jmb.2010.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang WG, Ren M, Zhao F, Tang WJ. Structures of human CCL18, CCL3, and CCL4 reveal molecular determinants for quaternary structures and sensitivity to insulin-degrading enzyme. J Mol Biol. 2015;427(6 Pt B):1345–1358. doi: 10.1016/j.jmb.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manning BD. Insulin signaling: Inositol phosphates get into the Akt. Cell. 2010;143(6):861–863. doi: 10.1016/j.cell.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chakraborty A, et al. Inositol pyrophosphates inhibit Akt signaling, thereby regulating insulin sensitivity and weight gain. Cell. 2010;143(6):897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Illies C, et al. Requirement of inositol pyrophosphates for full exocytotic capacity in pancreatic beta cells. Science. 2007;318(5854):1299–1302. doi: 10.1126/science.1146824. [DOI] [PubMed] [Google Scholar]
- 43.Sasakawa N, Sharif M, Hanley MR. Metabolism and biological activities of inositol pentakisphosphate and inositol hexakisphosphate. Biochem Pharmacol. 1995;50(2):137–146. doi: 10.1016/0006-2952(95)00059-9. [DOI] [PubMed] [Google Scholar]
- 44.Barker CJ, Wright J, Hughes PJ, Kirk CJ, Michell RH. Complex changes in cellular inositol phosphate complement accompany transit through the cell cycle. Biochem J. 2004;380(Pt 2):465–473. doi: 10.1042/BJ20031872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edbauer D, Willem M, Lammich S, Steiner H, Haass C. Insulin-degrading enzyme rapidly removes the beta-amyloid precursor protein intracellular domain (AICD) J Biol Chem. 2002;277(16):13389–13393. doi: 10.1074/jbc.M111571200. [DOI] [PubMed] [Google Scholar]
- 46.Currinn H, Wassmer T. The amyloid precursor protein (APP) binds the PIKfyve complex and modulates its function. Biochem Soc Trans. 2016;44(1):185–190. doi: 10.1042/BST20150179. [DOI] [PubMed] [Google Scholar]
- 47.Zolov SN, et al. In vivo, Pikfyve generates PI(3,5)P2, which serves as both a signaling lipid and the major precursor for PI5P. Proc Natl Acad Sci USA. 2012;109(43):17472–17477. doi: 10.1073/pnas.1203106109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duckworth WC, Bennett RG, Hamel FG. A direct inhibitory effect of insulin on a cytosolic proteolytic complex containing insulin-degrading enzyme and multicatalytic proteinase. J Biol Chem. 1994;269(40):24575–24580. [PubMed] [Google Scholar]
- 49.Bennett RG, Fawcett J, Kruer MC, Duckworth WC, Hamel FG. Insulin inhibition of the proteasome is dependent on degradation of insulin by insulin-degrading enzyme. J Endocrinol. 2003;177(3):399–405. doi: 10.1677/joe.0.1770399. [DOI] [PubMed] [Google Scholar]
- 50.Kirschner RJ, Goldberg AL. A high molecular weight metalloendoprotease from the cytosol of mammalian cells. J Biol Chem. 1983;258(2):967–976. [PubMed] [Google Scholar]
- 51.Hersh LB. The insulysin (insulin-degrading enzyme) enigma. Cell Mol Life Sci. 2006;63(21):2432–2434. doi: 10.1007/s00018-006-6238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bergeron JJ, Cruz J, Khan MN, Posner BI. Uptake of insulin and other ligands into receptor-rich endocytic components of target cells: The endosomal apparatus. Annu Rev Physiol. 1985;47:383–403. doi: 10.1146/annurev.ph.47.030185.002123. [DOI] [PubMed] [Google Scholar]
- 53.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: Progress and potential. Endocr Rev. 1998;19(5):608–624. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 54.Authier F, et al. Degradation of the cleaved leader peptide of thiolase by a peroxisomal proteinase. Proc Natl Acad Sci USA. 1995;92(9):3859–3863. doi: 10.1073/pnas.92.9.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leissring MA, et al. Alternative translation initiation generates a novel isoform of insulin-degrading enzyme targeted to mitochondria. Biochem J. 2004;383(Pt. 3):439–446. doi: 10.1042/BJ20041081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao J, Li L, Leissring MA. Insulin-degrading enzyme is exported via an unconventional protein secretion pathway. Mol Neurodegener. 2009;4:4. doi: 10.1186/1750-1326-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shewan A, Eastburn DJ, Mostov K. Phosphoinositides in cell architecture. Cold Spring Harb Perspect Biol. 2011;3(8):a004796. doi: 10.1101/cshperspect.a004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tarasoff-Conway JM, et al. Clearance systems in the brain: Implications for Alzheimer disease. Nat Rev Neurol. 2015;11(8):457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nixon RA. Endosome function and dysfunction in Alzheimer’s disease and other neurodegenerative diseases. Neurobiol Aging. 2005;26(3):373–382. doi: 10.1016/j.neurobiolaging.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 60.Trikha S, Jeremic AM. Distinct internalization pathways of human amylin monomers and its cytotoxic oligomers in pancreatic cells. PLoS One. 2013;8(9):e73080. doi: 10.1371/journal.pone.0073080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Falguières T, Luyet PP, Gruenberg J. Molecular assemblies and membrane domains in multivesicular endosome dynamics. Exp Cell Res. 2009;315(9):1567–1573. doi: 10.1016/j.yexcr.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 62.Hu YB, Dammer EB, Ren RJ, Wang G. The endosomal-lysosomal system: From acidification and cargo sorting to neurodegeneration. Transl Neurodegener. 2015;4:18. doi: 10.1186/s40035-015-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burd C, Cullen PJ. Retromer: a master conductor of endosome sorting. Cold Spring Harb Perspect Biol. 2014;6(2):a016774. doi: 10.1101/cshperspect.a016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sahu R, et al. Microautophagy of cytosolic proteins by late endosomes. Dev Cell. 2011;20(1):131–139. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu XM, et al. ESCRTs cooperate with a selective autophagy receptor to mediate vacuolar targeting of soluble cargos. Mol Cell. 2015;59(6):1035–1042. doi: 10.1016/j.molcel.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 66.Leissring MA, et al. Designed inhibitors of insulin-degrading enzyme regulate the catabolism and activity of insulin. PLoS One. 2010;5(5):e10504. doi: 10.1371/journal.pone.0010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abdul-Hay SO, et al. Deletion of insulin-degrading enzyme elicits antipodal, age-dependent effects on glucose and insulin tolerance. PLoS One. 2011;6(6):e20818. doi: 10.1371/journal.pone.0020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hamel FG, Posner BI, Bergeron JJ, Frank BH, Duckworth WC. Isolation of insulin degradation products from endosomes derived from intact rat liver. J Biol Chem. 1988;263(14):6703–6708. [PubMed] [Google Scholar]
- 69.Glebov K, Schütze S, Walter J. Functional relevance of a novel SlyX motif in non-conventional secretion of insulin-degrading enzyme. J Biol Chem. 2011;286(26):22711–22715. doi: 10.1074/jbc.C110.217893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qiu WQ, Folstein MF. Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer’s disease: Review and hypothesis. Neurobiol Aging. 2006;27(2):190–198. doi: 10.1016/j.neurobiolaging.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 71.Tamboli IY, et al. Statins promote the degradation of extracellular amyloid beta-peptide by microglia via stimulation of exosome-associated insulin-degrading enzyme (IDE) secretion. J Biol Chem. 2010;285(48):37405–37414. doi: 10.1074/jbc.M110.149468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yokono K, Roth RA, Baba S. Identification of insulin-degrading enzyme on the surface of cultured human lymphocytes, rat hepatoma cells, and primary cultures of rat hepatocytes. Endocrinology. 1982;111(4):1102–1108. doi: 10.1210/endo-111-4-1102. [DOI] [PubMed] [Google Scholar]
- 73.Goldfine ID, et al. Degradation of insulin by isolated mouse pancreatic acini: Evidence for cell surface protease activity. Diabetes. 1984;33(1):64–72. doi: 10.2337/diab.33.1.64. [DOI] [PubMed] [Google Scholar]
- 74.Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 75.Kutateladze TG. Molecular analysis of protein-phosphoinositide interactions. Curr Top Microbiol Immunol. 2012;362:111–126. doi: 10.1007/978-94-007-5025-8_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Azevedo C, Burton A, Bennett M, Onnebo SM, Saiardi A. Synthesis of InsP7 by the inositol hexakisphosphate kinase 1 (IP6K1) Methods Mol Biol. 2010;645:73–85. doi: 10.1007/978-1-60327-175-2_5. [DOI] [PubMed] [Google Scholar]
- 77.Saiardi A, Bhandari R, Resnick AC, Snowman AM, Snyder SH. Phosphorylation of proteins by inositol pyrophosphates. Science. 2004;306(5704):2101–2105. doi: 10.1126/science.1103344. [DOI] [PubMed] [Google Scholar]
- 78.Song ES, et al. Analysis of the subsite specificity of rat insulysin using fluorogenic peptide substrates. J Biol Chem. 2001;276(2):1152–1155. doi: 10.1074/jbc.M008702200. [DOI] [PubMed] [Google Scholar]
- 79.Csuhai E, et al. New fluorogenic substrates for N-arginine dibasic convertase. Anal Biochem. 1999;269(1):149–154. doi: 10.1006/abio.1999.4033. [DOI] [PubMed] [Google Scholar]
- 80.Buser CA, McLaughlin S. Ultracentrifugation technique for measuring the binding of peptides and proteins to sucrose-loaded phospholipid vesicles. Methods Mol Biol. 1998;84:267–281. doi: 10.1385/0-89603-488-7:267. [DOI] [PubMed] [Google Scholar]
- 81.Barenholz Y, Amselem S. Quality control assays in the development and clinical use of liposome-based formulation. In: Gregoriadis G, editor. Liposome Technology. 2nd Ed. Vol I. CRC Press; Boca Raton, FL: 1993. p. 527. [Google Scholar]
- 82.de Araùjo ME, Huber LA, Stasyk T. Isolation of endocitic organelles by density gradient centrifugation. Methods Mol Biol. 2008;424:317–331. doi: 10.1007/978-1-60327-064-9_25. [DOI] [PubMed] [Google Scholar]
- 83.Costes SV, et al. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J. 2004;86(6):3993–4003. doi: 10.1529/biophysj.103.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trott O, Olson AJ. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krieger E, Vriend G. Models@Home: Distributed computing in bioinformatics using a screensaver-based approach. Bioinformatics. 2002;18(2):315–318. doi: 10.1093/bioinformatics/18.2.315. [DOI] [PubMed] [Google Scholar]
- 86.Moriarty NW, Grosse-Kunstleve RW, Adams PD. Electronic ligand builder and optimization workbench (eLBOW): A tool for ligand coordinate and restraint generation. Acta Crystallogr D Biol Crystallogr. 2009;65(Pt 10):1074–1080. doi: 10.1107/S0907444909029436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 89.Goddard ED, Turro NJ, Kuo PL, Ananthapadmanabhan KP. Fluorescence probes for critical micelle concentration determination. Langmuir. 1985;1(3):352–355. doi: 10.1021/la00063a015. [DOI] [PubMed] [Google Scholar]