Significance
Bacteria regulate their intracellular environment through two ubiquitous posttranscriptional regulatory mechanisms: enzymatic synthesis of small signaling molecules that allosterically regulate protein targets, and complex formation of RNA-binding proteins with target RNAs. We show that these two mechanisms can be combined within a single protein. The small alarmone synthetase RelQ from the Gram-positive pathogen Enterococcus faecalis enzymatically regulates the levels of alarmone nucleotide (p)ppGpp, a key regulator of stress adaptation, pathogenicity, and antibiotic tolerance. In its other role as an RNA-binding protein, RelQ interacts with single-stranded RNA in a sequence-specific manner. Because (p)ppGpp synthesis and pppGpp binding are mutually incompatible with RelQ:RNA complex formation, the RelQ:RNA interaction acts as a regulatory switch between inactive and active forms of the enzyme.
Keywords: stringent response, (p)ppGpp, RNA–protein interaction, allosteric regulation, nucleotide signaling
Abstract
The alarmone nucleotides guanosine pentaphosphate (pppGpp) and tetraphosphate (ppGpp), collectively referred to as (p)ppGpp, are key regulators of bacterial growth, stress adaptation, pathogenicity, and antibiotic tolerance. We show that the tetrameric small alarmone synthetase (SAS) RelQ from the Gram-positive pathogen Enterococcus faecalis is a sequence-specific RNA-binding protein. RelQ’s enzymatic and RNA binding activities are subject to intricate allosteric regulation. (p)ppGpp synthesis is potently inhibited by the binding of single-stranded RNA. Conversely, RelQ’s enzymatic activity destabilizes the RelQ:RNA complex. pppGpp, an allosteric activator of the enzyme, counteracts the effect of RNA. Tetramerization of RelQ is essential for this regulatory mechanism, because both RNA binding and enzymatic activity are abolished by deletion of the SAS-specific C-terminal helix 5α. The interplay of pppGpp binding, (p)ppGpp synthesis, and RNA binding unites two archetypal regulatory paradigms within a single protein. The mechanism is likely a prevalent but previously unappreciated regulatory switch used by the widely distributed bacterial SAS enzymes.
The alarmone nucleotides guanosine pentaphosphate and tetraphosphate, collectively referred to as (p)ppGpp, are key regulators of bacterial growth, stress adaptation, pathogenicity, and antibiotic tolerance (reviewed in refs. 1–3). In Escherichia coli, (p)ppGpp signaling is orchestrated by two large multidomain proteins, RelA and SpoT, the namesakes of the RelA/SpoT homolog (RSH) protein family (4). Both RelA (5) and SpoT (6) synthesize (p)ppGpp using either GDP or GTP as substrates and ATP as a donor of the pyrophosphate moiety. SpoT, but not RelA, also can hydrolyze pppGpp and ppGpp, yielding GTP and GDP, respectively (7). The enzymatic activities of the two E. coli RSH enzymes are regulated allosterically. Synthesis of (p)ppGpp by RelA is strongly induced on amino acid limitation by so-called “starved” ribosomal complexes loaded with cognate deacylated tRNA in the A-site (8), and RelA activation is further potentiated by the product of the reaction, ppGpp (9). SpoT has both (p)ppGpp synthesis and hydrolysis activities and is regulated by numerous stress signals, including fatty acid (10), iron (11), and carbon source (6) limitations.
In the last decade, the repertoire of RSH enzymes has been expanded by the discovery of small, single-domain, monofunctional enzymes that either synthesize [small alarmone synthetases (SASs)] (12–14) or hydrolyze [small alarmone hydrolases (SAHs)] (15, 16) (p)ppGpp. Bacterial SAHs are largely uncharted territory, with our knowledge of these enzymes limited to mapping their phylogenetic distribution across the tree of life (16). The biological role and regulation of SAS enzymes are better understood. In contrast to allosterically regulated RelA and SpoT, induction of (p)ppGpp production by SASs in response to cell wall stress stimuli, such as alkaline shock or treatment with cell wall-active antibiotics, is believed to be effectuated chiefly via transcriptional up-regulation, leading to an increase in the enzyme’s abundance (13, 17). The consequent increase in the (p)ppGpp level in turn renders bacteria more resilient to the signal that is inducing stress, e.g., tolerance to antibiotics targeting the cell wall (14, 17). Crystallographic analysis of the Bacillus subtilis RelQ (SAS1) revealed that it forms a tetramer that binds two pppGpp molecules at the interface between subunits, leading to an allosteric activation of the enzyme’s catalytic activity (18). Activation by both ppGpp and pppGpp has been reported for RelQ from Enterococcus faecalis (19).
Using biochemical assays with E. faecalis SAS RelQ, we have discovered an unexpected regulatory interplay among (p)ppGpp binding, (p)ppGpp synthesis, and inhibition of the enzymatic activity by single-stranded RNA. This constitutes an example of two archetypical regulatory paradigms combined within a single protein—namely, an RNA-binding activity and a switch in catalytic activity in response to a second messenger. This provides insight into a previously unknown function of RelQ that is likely to be relevant for many other bacterial SAS enzymes.
Results
Enzymatic Activity of E. faecalis RelQ Is Inhibited by mRNA, and ppGpp Counteracts the Inhibition.
As we have shown previously (19), the enzymatic activity of E. faecalis RelQ is insensitive to the addition of E. coli 70S ribosomes (Fig. 1A). This is unsurprising given that, unlike E. coli RelA (9), SAS RSH enzymes are not expected to interact with—or to be regulated by—ribosomes, because they lack the C-terminal domains mediating this interaction in long RSHs (20–22).
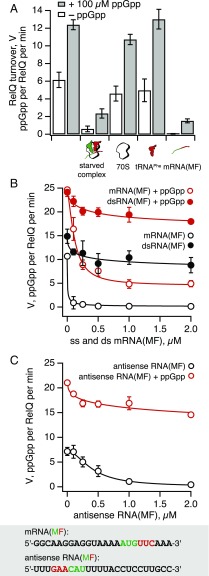
Fig. 1.
mRNA is a potent inhibitor of ppGpp synthesis by E. faecalis RelQ. (A) 3H ppGpp synthesis activity of 250 nM E. faecalis RelQ (62.5 nM tetrameric RelQ) in the presence (gray bars) and absence (empty bars) of 100 μM ppGpp, as well as starved ribosomal complexes or individual components thereof. Note that ppGpp is a strong activator of RelQ’s enzymatic activity and mitigates the inhibition by starved ribosomal complexes or mRNA(MF). (B and C) Single-stranded mRNA inhibits RelQ’s activity in a sequence-specific manner, and this inhibition is countered by ppGpp. Titrations were performed with increasing concentrations of either single-stranded (empty circles) or double-stranded (filled circles) RNA, in the absence (black circles) or presence (red circles) of 100 μM ppGpp. All reaction mixtures contained 250 nM (62.5 nM tetramer) E. faecalis RelQ, 300 μM 3H GDP, and 1 mM ATP. Titration data were fitted with the 4PL model. Error bars represent SDs of the turnover estimates determined by linear regression. Each experiment was performed at least three times.
Unexpectedly, when we added the ultimate activator of E. coli RelA—namely “starved” ribosomal complexes assembled from 70S ribosomes, model mRNA(MF) coding for MF dipeptide, deacylated tRNAMet and tRNAPhe—to RelQ, its synthetic activity was almost abolished (Fig. 1A). Because long ribosome-dependent RSHs interact directly with deacylated tRNA (21, 22), we tested whether the deacylated tRNAPhe is responsible for this inhibition. We found that tRNAPhe had little effect on RelQ in either the presence or absence of ppGpp. Because neither 70S ribosomes nor deacylated tRNA inhibit RelQ, via a process of elimination we concluded that the mRNA(MF) is responsible for RelQ inhibition by starved ribosomal complexes. Further experiments supported this conclusion. In the absence of externally added ppGpp, addition of 1 μM model mRNA(MF) abolished ppGpp synthesis by RelQ, whereas in the presence of 100 μM ppGpp, mRNA(MF) decreased the turnover rate of RelQ by only approximately sixfold, from 12.4 ± 0.6 to 1.5 ± 0.2 ppGpp molecules per RelQ per minute.
One possible explanation for this finding is that the mRNA nonspecifically binds and inhibits RSH enzymes. However, the addition of up to 10 μM mRNA(MF) had no effect on E. coli RelA activated by ribosomal complexes (Fig. S1), demonstrating that the inhibitory effect of mRNA(MF) is specific to SAS RelQ to the exclusion of the ribosome-associated RSH RelA.
Fig. S1.
Synthetic activity of 100 nM E. coli RelA activated by starved ribosomal complexes is unaffected by up to 10 μM mRNA(MF). Starved ribosomal complexes were assembled from 0.5 μM E. coli 70S, 2 μM deacylated tRNAPhe and tRNAMeti, and 2 μM model mRNA encoding the Met-Phe (MF) dipeptide (5′-GGCAAGGAGGUAAAAAUGUUCAAA-3′) in Hepes:Polymix buffer at 37 °C; 300 μM 3H GDP and 1 mM ATP served as substrates for the 3H ppGpp synthesis. Error bars represent SDs of the turnover estimates determined by linear regression. Each experiment was performed at least three times.
Single-Stranded RNA Potently Inhibits RelQ in a Sequence-Specific Manner.
We next investigated the specificity of RelQ inhibition by nucleic acids (Fig. 1 B and C). We characterized the effects of single-stranded (empty circles) and double-stranded (filled circles) RNA as well as the corresponding DNA in both the presence and absence of externally added 100 μM ppGpp (red and black traces, respectively). The oligonucleotides had a sequence identical to that of either model mRNA(MF) or its antisense. Single-stranded mRNA(MF) was found to be a very potent inhibitor of RelQ in the absence of externally added ppGpp; 150 nM mRNA virtually abolished ppGpp synthesis by 250 nM RelQ (i.e., 62.5 nM tetrameric RelQ) (Fig. 1B, empty black circles). The addition of 100 μM ppGpp mitigated this inhibition, but did not relieve it completely; at 1 μM, mRNA(MF) still inhibited RelQ activity by approximately fivefold (Fig. 1B, empty red circles). The inhibitory effect exhibited a pronounced sequence-specificity; single-stranded RNA with a complementary sequence—the antisense mRNA—was a considerably weaker inhibitor, despite having the same GC content (Fig. 1C). Double-stranded RNA was virtually inactive in both the presence and absence of ppGpp (Fig. 1B, filled red and black circles, respectively). Likewise, single- and double-stranded DNA were poor inhibitors; in the presence of 100 μM ppGpp, 2 μM single-stranded DNA had virtually no inhibitory effect on RelQ, and in the absence of ppGpp, it inhibited RelQ activity by approximately fivefold (Fig. S2A). Long-chain polyphosphate demonstrated no inhibitory effect when added in concentrations of up to 2 mM (Fig. S3).
Fig. S2.
Double-stranded DNA ineffectively inhibits E. faecilis RelQ. (A) ssDNA(MF) is a poor inhibitor of RelQ. Titrations were performed with increasing concentrations of either single-stranded (empty circles) or double-stranded (filled circles) DNA(MF), in the absence (black circles) or presence (red circles) of 100 μM ppGpp. All reaction mixtures contained 250 nM (62.5 nM tetramer) E. faecalis RelQ, 300 μM 3H GDP, and 1 mM ATP. Experimental data were fitted using the 4PL model (Hill equation). (B) EMSA analysis detected a negligible amount of complex formation between 0.15 μM dsDNA(MF) or dsRNA(MF) and increasing concentrations of RelQ.
Fig. S3.
Enzymatic activity of RelQ is unaffected by inorganic polyphosphate. Experiments were performed with 0.5 mg/mL BSA, 0.15 µM RelQ (37.5 nM tetramer), 300 µM 3H GDP, 100 µM ppGpp, 1 mM ATP, and increasing concentrations of long-chain (p700) polyphosphate. Error bars represent SDs of the turnover estimates by linear regression. The experiment was performed twice.
Using the 24-nt-long inhibitory mRNA(MF) and its ineffective complementary antisense RNA as a starting point, we set out to define the sequence specificity for RelQ inhibition. By swapping the 5′ and 3′ halves of the two RNA molecules, we identified the 5′ half of the mRNA(MF) spanning the Shine–Dalgarno sequence AGGAGG as an essential element for the inhibitory activity (Fig. 2A). We then tested a series of 5′ and 3′ truncations of mRNA(MF) (Fig. 2B). The absence of six or nine 3′ terminal nucleotides (RNA 5 and 6) did not affect the inhibitory activity of the RNA, but the lack of an additional three nucleotides—which shortens the mRNA to its 5′ half (RNA 8)—significantly reduced the inhibitory effect. Because replacement of the 3′ AAA by UUU did not abrogate inhibition (RNA 7), we conclude that the loss of activity of RNA 8 is not due to the loss of a specific sequence element, but rather indicates the existence of a minimum length requirement between 12 and 15 nucleotides. Similar to the 5′ half (RNA 8), the 3′ half was also inactive (RNA 11); however, the addition of an extra six nucleotides at the 5′ (GAGGUA) nearly restored the activity (RNA 10).
Fig. 2.
Sequence specificity of RelQ inhibition by RNA. Here 24-nt-long mRNA(MF) (red) and its complementary antisense RNA (blue) were used as a positive and negative controls, respectively. Based on the two RNAs, we generated chimeras (A), cut-backs (B), and point mutants (C). We also reconstituted the inhibitory activity by adding two GG elements to otherwise inactive poly(A) RNA (D). To calculate the RelQ activity, the turnover rate (3H ppGpp synthesized per RelQ per minute) in the presence of RNA was divided by that in the absence of RNA. All reaction mixtures contained 100 nM RNA, 250 nM (62.5 nM tetramer) E. faecalis RelQ, 300 μM 3H GDP, and 1 mM ATP. Error bars represent SDs of the turnover estimates determined by linear regression. Each experiment was performed at least three times.
Given our results suggesting that the activity is localized to the 5′ half of the mRNA(MF), we performed mutational studies (Fig. 2C) on a fully active RNA lacking the six 3′ nucleotides of mRNA(MF) (RNA 5). This RNA retains the three GG motifs, which are reminiscent of the GGA motifs that are essential for RNA binding by the bacterial global regulator Csr/Rsm (23). Although substitution of any one of the three GG repeats by CC did not affect the activity (RNAs 12–14), simultaneous mutation of two or three GG motifs significantly decreased the potency of RNAs 15 and 16 as a RelQ inhibitor, suggesting a possible consensus sequence.
To test this hypothesis, we introduced either one or two GG motifs in a poly(A) RNA (Fig. 2D). Although none of the four homopolymeric RNAs [poly(G), poly(C), poly(U), or poly(A)] significantly inhibited RelQ, the addition of two GG motifs, resulting in a GGAGG cluster (RNA 22), turned poly(A) into a potent inhibitor that completely abolished RelQ activity.
We conclude that RelQ inhibition by nucleic acids displays the following traits: (i) RNA is more efficient than DNA; (ii) single-stranded nucleic acids are more efficient than double-stranded nucleic acids; (iii) inhibition by single-stranded RNA is sequence-specific, with a tentative consensus of GGAGG; and (iv) ppGpp has a universal protective effect.
mRNA and pppGpp Reciprocally Destabilize Each Other’s Binding to RelQ.
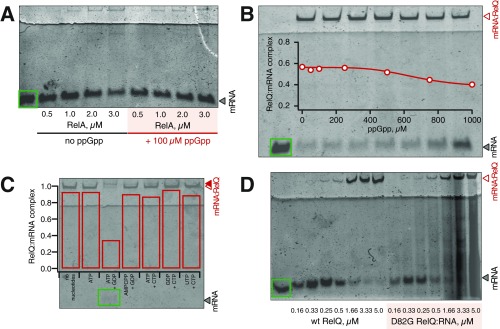
Inhibition of RelQ’s enzymatic activity by mRNA(MF) is indicative of complex formation between the two. We documented this interaction using an electrophoretic mobility shift assay (EMSA) (Fig. 3A). Unlike E. faecalis RelQ, E. coli RelA did not form a complex with mRNA (Fig. S4A), as was expected given the absence of RelA inhibition by mRNA(MF) (Fig. 1B). Similarly, neither double-stranded RNA nor DNA formed complexes with RelQ (Fig. S2B).
Fig. 3.
mRNA and pppGpp have a destabilizing effect on each other’s binding to RelQ. (A) Complex formation between 0.15 μM mRNA(MF) and increasing concentrations of E. faecalis RelQ was monitored by EMSA in the absence (empty circles) and presence (filled circles) of 100 μM ppGpp. (B) EMSA analysis of complex between 0.15 μM mRNA(MF) and 2 μM RelQ in the presence of increasing pppGpp concentrations. (C) Complex formation of increasing concentrations of RelQ with 50 nM 32P-labeled ATP, ppGpp, or pppGpp monitored by DRaCALA. (D) 32P pppGpp is displaced from 20 μM RelQ by increasing concentrations of mRNA(MF), as monitored by DRaCALA. Error bars represent SDs of the mean. Each experiment was performed at least three times.
Fig. S4.
EMSA analysis of E. coli RelA (A) and E. faecilis RelQ (C and D) interactions with mRNA(MF). (A) E. coli RelA does not bind mRNA(MF) in either the presence or absence of 100 μM ppGpp. (B) Increasing ppGpp concentrations up to 1 mM moderately destabilizes complex formation between 0.15 μM mRNA(MF) and 2 μM RelQ. Experimental data were fitted using the 4PL model (Hill equation). (C) The addition of ATP and GDP, but not of any other combinations of nucleotides, disrupts RelQ:mRNA(MF) complex formation. All tested nucleotides were added to a final concentration of 1 mM. (D) Omission of the additional 1 M KCl during purification of catalytically inactive D82G RelQ results in a smearing pattern. EMSA analysis was performed in the presence of 0.15 μM mRNA(MF). The positions of RelQ:mRNA and its supershifted complex in the presence of substrates are indicated with empty and filled red triangles, respectively.
Because the addition of 100 μM ppGpp has a dramatic effect in enzymatic assays (Fig. 1), we tested whether ppGpp destabilizes the RelQ:mRNA(MF) complex. The addition of 100 μM ppGpp had a very mild destabilizing effect (Fig. 3A), which increased somewhat as ppGpp was titrated up to 1 mM into the system (Fig. S4B). Given that experiments with B. subtilis RelQ suggest that guanosine pentaphosphate pppGpp is a dramatically more potent effector of SASs (18), we titrated pppGpp in the EMSA assay (Fig. 3B). The binding of mRNA(MF) to RelQ was potently abrogated by pppGpp with an IC50 of 35 ± 6 μM.
According to the detailed balance argument (24), destabilization of the RelQ:mRNA(MF) complex by (p)ppGpp should be reciprocated by destabilization of (p)ppGpp binding in the presence of mRNA. We tested this prediction with a differential radial capillary action of ligand assay (DRaCALA) (25). In good agreement with results reported for B. subtilis RelQ (18), E. faecalis RelQ efficiently bound pppGpp (EC50pppGpp of 2.1 ± 0.1 μM), whereas ppGpp was a significantly poorer binder. Even in the presence of 20 μM RelQ, only 10% of ppGpp was associated with the protein, precluding quantitative analysis of the complex formation (Fig. 3C). 32P-labeled pppGpp was displaced by mRNA(MF) with an IC50 of 2.8 ± 0.1 μM (Fig. 3D). A similar effect was observed with 32P-labeled ppGpp (IC50 of 5.2 ± 1.9 μM), but not with 32P-labeled ATP (Fig. S5A).
Fig. S5.
DRaCALA analysis of RelQ interactions with 32P ATP, 32P ppGpp, and 32P pppGpp. (A) ppGpp, but not ATP, is displaced from RelQ by increasing concentrations of mRNA(MF). (B) Catalytically inactive RelQ mutant D82G (EF2671 locus numbering) efficiently binds pppGpp, but not ATP. Complex formation is monitored using 50 nM 32P-labeled ATP, ppGpp, or pppGpp and increasing concentrations of RelQ. IC50, EC50, and the Hill coefficient (nH) were calculated using the 4PL model (Hill equation). Error bars represent SDs of the mean. Each experiment was performed at least three times.
RelQ’s Association with RNA Is Mutually Exclusive with ppGpp Synthesis.
The moderate effects of ppGpp on the RelQ:mRNA(MF) interaction are in stark contrast to the nucleotide’s dramatic effect on RelQ’s enzymatic activity in the presence of mRNA. However, in enzymatic assays, ppGpp is always tested in the presence of RelQ substrates ATP and GDP. Therefore, we tested the effects of the simultaneous addition of both RelQ enzymatic substrates in an EMSA assay.
The simultaneous addition of ATP and GDP significantly destabilized the RelQ:mRNA(MF) complex, resulting in a supershift of the RelQ:mRNA(MF), suggesting a structural rearrangement (Fig. 4A). The addition of nucleotide combinations that are not accepted by the enzyme, such as CTP combined with GDP or substitution of ATP for nonhydrolyzable analog AMPCPP, did not destabilize the RelQ:mRNA(MF) complex (Fig. S4C). The effect of substrates on the RelQ:mRNA(MF) complex was indistinguishable in the presence or absence of ppGpp (Fig. 4B). This finding seemingly contradicts the very pronounced effect of externally added ppGpp observed in enzymatic assays (Fig. 1); however, the negated effect of ppGpp in the EMSA assays is due to efficient formation of the alarmone nucleotide in situ because of the excess of RelQ over mRNA [2 μM RelQ vs. 150 nM mRNA(MF)] (Fig. S6).
Fig. 4.
RelQ binding to mRNA and ppGpp synthesis are mutually exclusive. (A) Although 1 mM ppGpp, ATP, or GDP alone does not affect the stability of the RelQ:mRNA(MF) complex, a combination of ATP and GDP has a strong destabilizing effect in both the presence and absence of 100 μM ppGpp. The positions of RelQ:mRNA (open red triangles) and supershifted complex in the presence of substrates (filled red triangles) are indicated to the left. (B) Increasing GDP substrate concentration in the presence of 1 mM ATP progressively destabilizes the RelQ:mRNA(MF) complex. (C) Addition of 100 μM ppGpp to RelQ both increases its catalytic efficiency (Vmax) and relaxes the positive substrate cooperativity, as shown by a decrease in the Hill constant, nH. (D) Enzymatically inactive RelQ mutant D82G (EF2671 locus numbering) forms the complex with mRNA(MF) as efficiently as the WT protein, whereas the addition of 1 mM ATP and GDP does not destabilize the complex in either the presence or absence of 100 μM ppGpp. Error bars represent SDs of the mean. Each experiment was performed at least three times.
Fig. S6.
Excess of RelQ over mRNA masks the inhibitory effect of mRNA on 3H ppGpp. In contrast to enzymatic assays in which mRNA(MF) is added in excess over RelQ [0.2 μM RelQ vs. 2 μM mRNA(MF)] (A), in EMSA experiments RelQ is added to the reaction mixture in excess over mRNA(MF) [2 μM RelQ vs. 0.15 μM mRNA(MF)] (B). This results in inefficient inhibition of RelQ’s enzymatic activity under EMSA conditions and accumulation of in situ-produced ppGpp.
The addition of increasing concentrations of RelQ substrate GDP in the presence of ATP at a constant 1 mM concentration led to gradual destabilization of the RelQ:mRNA complex (Fig. 4B). Fitting the EMSA data to the 4-parameter logistic (4PL) model (Hill equation) (26) yielded a Hill coefficient, nH, of 1.3 ± 0.4, which is in good agreement with the Michaelis–Menten-like behavior that we observed in previous enzymatic assays (19) but seemingly contradicts the strongly cooperative sigmoidal responses documented by Steinchen et al. (18). The likely cause of this difference is the absence (18) or presence (19) of externally added 100 μM ppGpp in enzymatic assays. The addition of ppGpp increased the enzyme’s efficiency (Vmax–ppGpp of 19 ± 1 vs. Vmax+ppGpp of 51 ± 4 ppGpp per RelQ per minute) and rendered the response curve more Michaelis–Menten-like (nH–ppGpp of 3.6 ± 0.8 vs. nH+ppGpp of 1.8 ± 06) (Fig. 4C).
The absence of RelQ:RNA(MF) destabilization in the presence of AMPCPP and GDP (Fig. S4C) suggests that it is the very act of ppGpp synthesis, rather than binding of the substrates per se, that dislodges the mRNA from RelQ. To test this hypothesis, we used an enzymatically inactivated RelQ mutant in which a conserved aspartic acid residue in position 82 (EF2671 locus numbering) is substituted with glycine; a similar mutant of B. subtilis RelQ has been described previously (18, 27). The D82G RelQ protein exhibited no detectable ppGpp synthesis activity, and although it formed a complex with mRNA as efficiently as the wild type (WT), this complex was insensitive to the addition of ATP, GDP, and ppGpp (Fig. 4D).
Allosteric Regulator pppGpp and Substrate GDP Synergize in Protecting RelQ from RNA.
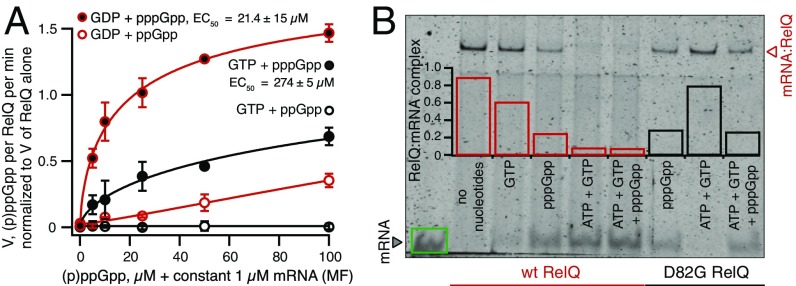
Because pppGpp binds to RelQ considerably better than ppGpp and is more efficient in dislodging the mRNA(MF) (Fig. 3 and Fig. S4A), we tested pppGpp’s protective effect against mRNA(MF) in enzymatic assays. Using either GDP or GTP as a substrate, we titrated ppGpp or pppGpp in the presence of mRNA(MF) (Fig. 5A). The RNA was added at a constant concentration of 1 μM to ensure complete inhibition of RelQ’s enzymatic activity in the absence of allosteric nucleotide regulators. When GDP was used as a substrate, pppGpp had a dramatic protective effect, completely rescuing the inhibition by mRNA(MF) with an EC50 of 21.4 ± 15 μM. ppGpp had a considerably weaker effect; at 100 μM, it restored RelQ’s enzymatic activity to only 35%. In the case of GTP, the protective effect of pppGpp was significantly less pronounced (EC50 of 274 ± 5 μM), reflecting the lower catalytic efficiency of GTP utilization as a substrate (Fig. S7). Finally, when GTP was used as a substrate, ppGpp failed to rescue any enzymatic activity.
Fig. 5.
The combination of GDP as a RelQ substrate and pppGpp as an allosteric regulator provides the best protective effect against RelQ inhibition by mRNA(MF). (A) The combination of the preferred substrate (GDP) and the best binding allosteric regulator (pppGpp) provides the strongest protective effect against mRNA(MF). All reaction mixtures contained 250 nM (62.5 nM tetramer) E. faecalis RelQ, 1 mM ATP, 1 μM mRNA(MF), 300 μM 3H GDP/GTP, and increasing concentrations of ppGpp/pppGpp. Error bars represent SDs of the mean. Each experiment was performed at least three times. (B) EMSA analysis of complex formation between the WT and enzymatically inactive D82G mutant RelQ and 0.15 μM mRNA(MF) in the presence of 1 mM substrates GTP and ATP and 100 μM allosteric regulator pppGpp.
Fig. S7.
Addition of 100 μM pppGpp to RelQ moderately increases its affinity to GTP substrate. All reaction mixtures contained 250 nM (62.5 nM tetramer) E. faecalis RelQ, 1 mM ATP, and increasing concentrations of 3H GTP in the presence or absence of 100 μM pppGpp. Maximum velocity (Vmax), the substrate concentration at which enzyme achieves 0.5 Vmax (K0.5), and the Hill coefficient (nH) were calculated using the 4PL model (Hill equation). Error bars represent SDs of the mean. Each experiment was performed at least three times.
EMSA assays performed with WT and D82G RelQ variants in the presence of GTP and ATP as substrates and pppGpp as an allosteric activator showed that, similarly to the case of GDP, the catalytic activity of RelQ led to destabilization of the RelQ:mRNA(MF) complex (Fig. 5B). However, because pppGpp by itself has a dramatic destabilizing effect, it is impossible to discriminate between the effects of RelQ-mediated catalysis per se and that of pppGpp generated in situ in the EMSA reaction mixture.
Deletion of SAS-Specific C-Terminal Helix 5α, Which Is Essential for RelQ Tetramerization, Abrogates Both ppGpp Synthesis and RNA Binding.
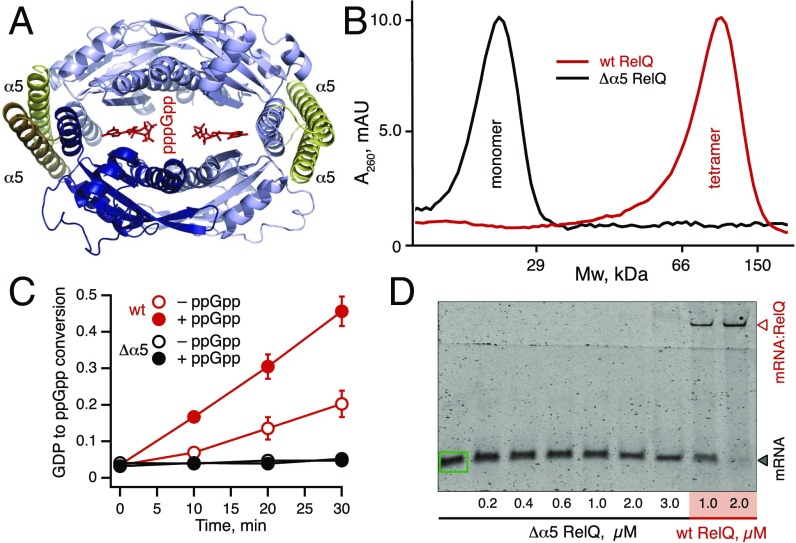
We next set out to test the connection between RelQ tetramerization and allosteric regulation by mRNA and ppGpp. Tetramerization has been proposed to play an important role in RelQ’s enzymatic activity; the allosteric regulator (p)ppGpp binds at the interfaces between subunits, and the catalytic sites of monomers have been suggested to operate in a cooperative mode (18). We tested the role of tetramerization and subunit cross-talk in the regulation of RelQ’s enzymatic activity using two types of perturbations: (i) formation of heterotetramers containing both WT and enzymatically inactive D82G subunits and (ii) complete abrogation of tetramerization via disruption of RelQ:RelQ contacts within the tetramer via deletion of the C-terminal helix 5α (amino acids 174–234; EF2671 locus numbering) (18) (Fig. 6 A and B).
Fig. 6.
An intact tetrameric structure is essential for ppGpp synthesis by RelQ. (A) C-terminal helix 5α (amino acids 174–234 in E. faecalis RelQ; EF2671 locus numbering) is highlighted in yellow in this homology model of E. faecalis RelQ based on the SAS1 tetramer of B. subtilis (18). Helix 5α, which forms contacts in tetrameric RelQ, is SAS-specific, i.e., absent in ribosome-associated RSHs such as RelA. Sequence alignment is shown in Fig. S10. Two allosteric pppGpp molecules are intercalated in the central cleft. (B–D) Deletion of the C-terminal α5 helix results in Δα5 RelQ, which is monomeric as shown by analytical gel filtration (B), enzymatically inactive in the presence or absence of 100 μM ppGpp (C), and unable to bind mRNA (D). Error bars represent SDs of the mean. Each experiment was performed at least three times.
Monomeric Δ5α RelQ was found to be enzymatically inactive (Fig. 6C), in good agreement with an earlier report of inactivation of Mycobacterium smegmatis MS_RHII-RSD on destabilization of oligomerization by 0.2% SDS (28). The truncated RelQ protein did not bind mRNA(MF) (Fig. 6D), suggesting that native complex formation is essential for protein function, and that disrupting it could serve as an off-switch exploited by an allosteric regulator. At the same time, formation of a heterotetramer of WT and D82G RelQ mutant did not affect the protein activity, even when D82G RelQ was added at fourfold excess over WT protein (Fig. S8), indicating that active sites of individual subunits are not strictly cooperative. Both WT RelQ and its D82G derivative formed stable tetramers that were not dissociated on the addition of substrates, mRNA, or a combination thereof (Fig. S9). We suggest that inhibition of RelQ by mRNA is mediated by rearrangement of the tetrameric structure, which is responsible for the observed supershift of the RelQ:mRNA complex migration in EMSA assays in the presence of GDP substrate (Fig. 4).
Fig. S8.
Enzymatically inactive D82G RelQ does not affect the synthetic activity of WT RelQ. (A) When the D82G (EF2671 locus numbering) mutant is purified following the standard protocol for purification of WT RelQ (19), the resulting protein is contaminated with RNA and exerts a strong inhibitory activity when added in excess over 0.5 μM WT RelQ. (B) Addition of an extra 1 M KCl to buffers used for purification mitigates the RNA contamination. RNA-free D82G RelQ does not inhibit the synthetic activity of 0.5 μM WT RelQ in either the presence or absence of externally added 100 μM ppGpp. Error bars represent SDs of the turnover estimates by linear regression. The experiment was performed twice.
Fig. S9.
Tetrameric structure of RelQ is not affected by substrates and/or mRNA. Here 10-μL samples of WT (black and brown traces) or D82G RelQ (EF2671 locus numbering, red traces) at a concentration of 18.5 μM were analyzed on a Superdex 200 Increase 5/150 GL analytical gel filtration column, either alone (A) or supplemented with 1 mM ATP and GDP (B), or with 60 μM mRNA(MF) along with 1 mM ATP and GDP (C).
Discussion
Here we report that tetrameric RelQ is an example of an oligomeric bacterial RNA-binding protein. In contrast to well-studied RNA-binding proteins, such as hexameric Hfq (29) and dimeric Csr/Rsm (23), RelQ has the additional ability to synthesize and allosterically respond to the second messenger (p)ppGpp, thus combining two regulatory paradigms within a single protein. Through biochemical experimentation, we have demonstrated the mutual exclusivity of RelQ’s activities as an RNA-binding protein and a signaling enzyme synthesizing and responding to the alarmone nucleotide messenger. RelQ’s enzymatic activity is potently inhibited by association with single-stranded RNA, and we have identified GGAGG as a putative consensus sequence for inhibition. Association of the primary allosteric regulator pppGpp or, to a lesser extent, the secondary allosteric regulator ppGpp strongly counteracts the inhibition by RNA and destabilizes the RelQ:RNA complex. The protective effect is especially strong when the primary allosteric regulator pppGpp synergizes with the preferred substrate, GDP. Tetramerization of RelQ is apparently essential for this regulatory mechanism, given that both mRNA binding and enzymatic activity are abolished by deletion of the SAS-specific C-terminal helix 5α (Fig. S10).
Fig. S10.
The 3D structure of E. faecalis RelQ and alignment with the ppGpp synthesis domain of E. coli RelA. (A) Secondary structure elements (α-helixes and β-sheets) of B. subtilis SAS1 (9) are indicated as spirals and arrows, respectively. Insertions in RelQ are highlighted in green, deletions are highlighted in red, and the C-terminal helix α5 is in yellow. Amino acid D82 (EF2671 locus numbering) is indicated with an orange asterisk. The first 17 characters are unaligned. The figure was generated using ESPript (34). (B) A homology model of the RelQ monomer based on B. subtilis SAS1 (18). Green indicates insertions, and red indicates the boundary of the deletion relative to RelA. Catalytically important amino acid D82 (EF2671 locus numbering) is highlighted in orange.
We propose a model for RelQ:RNA interaction as a regulatory switch between catalytically inactive and active forms of the enzyme (Fig. S11). Such a switch would mediate the cross-talk among cellular RSH enzymes by sensing the intracellular alarmone concentration. An increase in pppGpp (the primary nucleotide effector) and ppGpp (the secondary nucleotide effector) levels would allosterically stimulate RelQ’s synthetase activity and drive the protein’s dissociation from the RNA target. In principle, both the (p)ppGpp synthetic activity of RelQ and RNA binding can act as effectors in a cellular context; regulation of RelQ’s enzymatic activity would result in modulation of intracellular alarmone levels, whereas regulation of RNA binding would directly affect a target RNA. The similarity of the putative consensus for efficient RelQ inhibition, GGAGG, identified by our mutational analysis and the Shine–Dalgarno sequence AGGAGG suggests the possibility that the RelQ:RNA interaction exerts its regulatory function via sequestration or occlusion of a ribosome-binding site of RelQ’s mRNA target(s). Further investigations are needed to reveal the cellular RNA targets of RelQ and structural aspects of the interplay between mRNA binding and enzymatic activity. Given the broad evolutionary distribution of SAS enzymes, the allosteric regulatory interplay uncovered here for E. faecalis RelQ provides an example of a likely widespread regulatory mechanism.
Fig. S11.
RelQ:mRNA interaction as a regulatory switch. RelQ’s enzymatic activity is potently inhibited by association with the single-stranded RNA in a sequence-specific manner with a putative consensus of GGAGG. Association of the primary allosteric regulator pppGpp or, to a lesser extent, the secondary allosteric regulator ppGpp strongly counteracts the inhibition by RNA and destabilizes the RNA:RelQ complex. The protective effect is especially strong when the primary allosteric regulator pppGpp synergizes with the preferred substrate, GDP. Both the enzymatic activity and mRNA binding can serve as cellular effectors, acting via intracellular concentration of the alarmone and direct interaction with mRNA, respectively.
Materials and Methods
Biochemical in vitro translation system from purified E. coli components and TLC measurements of 3H ppGpp synthesis have been described previously (9). Enzymatic assays with E. faecalis RelQ were performed following the method of Gaca et al. (19). DRaCALA assays were performed as described by Roelofs et al. (25). Titration data were fitted with the 4PL model, or the Hill equation, Y = (a - d)/(1 + (X/c)b) + d, following Sebaugh (26). In this equation, b is the slope factor or Hill coefficient, nH; c is the half-response concentration of the titrant (IC50/EC50 for binding studies, K0.5 for enzymatic assays); and a and d are the lower and higher plateaus, respectively (d = Vmax in enzymatic assays). The experimental procedures are described in detail in SI Materials and Methods.
SI Materials and Methods
Preparation of Biochemical Reagents.
Construction of the expression construct for 6×His-tagged WT E. faecalis RelQ has been described previously (19). RelQ ORF was PCR-amplified from the EF2671 E. faecalis genomic locus and cloned into a pET21a expression vector (Novagen) using NheI and XhoI restriction sites. This resulted in the addition of three extra N-terminal amino acids (MAS) and a C-terminal 6×His tag preceded by a Leu-Glu linker. The D82G substitution (EF2671 locus numbering) was introduced into WT E. faecalis RelQ using the Agilent QuikChange Kit and forward primer 5′-CGGATTGAAGAAGAAATGCAAGGCATTGCTGGTTTGCGG-3′ and reverse primer 5′-CCGCAAACCAGCAATGCCTTGCATTTCTTCTTCAATCCG-3′. C-terminally truncated version lacking amino acids 174–234 (EF2671 locus numbering) of RelQ was cloned into a pET24d (Novogen) vector at Umeå University’s Protein Expertise Platform. Compared with the EF2671 E. faecalis genomic locus, the resulting protein possessed N-terminal amino acids (MAS) and C-terminal 6×His preceded by Leu-Glu linker.
WT and mutant RelQs were purified as described previously (19), with the following modifications: (i) during the affinity purification step, ionic strength was increased to 1 M KCl; and (ii) after elution from NiNTA, protein preparations were also run on a gel filtration column (HiLoad 16/600 Superdex 200 pg; GE Healthcare) in 20 mM Tris⋅HCl pH 8.0, 500 mM NaCl, 1 mM EDTA, and 5% glycerol. The increase in ionic strength during the affinity capture step is crucial in the case of D82G RelQ; lower ionic strength results in RNA-contaminated protein preparations that inhibit the activity of WT RelQ (Fig. S8A) and show a smearing pattern in EMSA assays (Fig. S4D).
E. coli 70S ribosomes and E. coli RelA were purified as described previously (9). The mRNA(MF) 5′-GGCAAGGAGGUAAAAAUGUUCAAA-3′ and antisense mRNA 5′-UUUGAACAUUUUUACCUCCUUGCC-3′ were purchased from Sigma-Aldrich, RNA 3–22 (Fig. 2) were purchased from Metabion, and deacylated tRNAfMeti and tRNAPhe were purchased from Chemical Block. Long-chain (p700) polyphosphate was purchased from Kerafest. The secondary structures of RNAs 1–22 (Fig. 2) were analyzed in silico using the Mfold web server (30). With the exception of poly(G) (RNA 17), which forms a G-quadruplex, mRNA(MF) has the lowest folding Gibson free energy of the set (ΔG of −1.40 kcal/mol). This suggests that RNAs 2–16 and 18–22 are less structured than mRNA(MF). Importantly, although RNA 22 (5′-AAAAAGGAGGAAAAA-3′) potently inhibits RelQ (Fig. 2D), it does not form a predictable secondary structure.
AMPCPP was purchased from Jena Bioscience, and ppGpp was prepared using GDP and ATP as described previously (9). To prepare 32P-labeled ppGpp, γ32P ATP (3.125 mCi/mL) was incubated with 2 μM purified RelQ, 1 mM nonradioactive ATP, and 0.5 mM GDP for 1 h at 37 °C (buffer: 30 mM Tris⋅HCl pH 8.0, 10 mM MgCl2 and 100 mM NaCl), followed by phenol extraction to remove RelQ. The resultant mixture was loaded on strong anion-exchange column (MonoQ 5/50 GL; GE Healthcare), and nucleotides were resolved by a 0.5–1,000 mM LiCl gradient. Peak fractions for [32P]ATP and 32P-ppGpp were desalted using Sephadex G-10 columns (PD MidiTrap G-10; GE Healthcare). 32P-pppGpp was prepared following a similar approach, using γ32P GTP (3.125 mCi/mL, 3.7 μM) and 1 mM ATP as substrates for RelQ.
All experiments were performed in Hepes:Polymix buffer (31) with 5 mM Mg2+ at 37 °C.
Enzymatic Assays with E. coli RelQ and RelA.
Reaction mixtures containing starved ribosomal complexes or components thereof [0.5 μM E. coli 70S, 2 μM each of deacylated tRNAPhe and tRNAMet, 2 μM model mRNA encoding the Met-Phe (MF) dipeptide, 5′-GGCAAGGAGGUAAAAAUGUUCAAA-3′] were mixed with either 250 nM (62.5 nM tetrametic RelQ) E. faecalis RelQ or 100 nM E. coli RelA, supplemented by 300 μM 3H GDP. Reactions were initiated by the addition of 1 mM ATP. The 5-μL aliquots taken throughout the course of the reaction were quenched with 4 μL of 70% formic acid supplemented with a cold nucleotide standard (10 mM GDP and 10 mM GTP) used for UV shadowing after resolution on PEI-TLC plates (Macherey-Nagel). TLC analysis was performed as described previously (9), with several modifications. Nucleotides were resolved in 0.5 KH2PO4 pH 3.5 buffer, after which the plates dried and then cut into sections as guided by UV shadowing. 3H radioactivity was quantified by scintillation counting in Optisafe-3 scintillation mixture (PerkinElmer). Conversion of substrate to product was quantified as described previously (9).
EMSA of RelQ:mRNA Complexes.
Here 10 μL of total reactions were performed in Hepes:Polymix buffer (31) with 5 mM Mg2+ at 37 °C. Before the reaction mixtures were assembled, stock mRNA was incubated for 2 min at 65 °C to melt possible secondary structures. Reaction mixtures were assembled by adding combinations of nucleotides (final concentrations: 1 mM ATP, 1 mM GDP, 1 mM CTP, 1 mM UTP, 1 mM AMPCPP, and 100 μM ppGpp) to the mRNA (0.15 μM final concentration), followed by the addition of RelQ and 4 U/μL of RiboLock RNase Inhibitor (Thermo Fisher Scientific). After incubation for 5 min at 37 °C, 5 μL of loading dye (40% sucrose supplemented with bromophenol blue) was added per 10 μL (i.e., 1.5 pmol of mRNA), and the samples were resolved on a 12–15% Tris:Borate:EDTA gel electrophoresed at 4 °C (120–140 V) for 1.5–2 h. Gels were stained with SYBR Gold nucleic acid stain (Life Technologies) for 20 min, followed by visualization using a Typhoon Trio Variable Mode Imager (Amersham Biosciences). Bands were quantified using ImageQuant TL (Amersham Biosciences). The Hill coefficient (nH) and inhibition efficiency (IC50)/effective concentration (EC50) were calculated using the 4PL model (Hill equation) as described by Sebaugh (26).
Analytical Gel Filtration Analysis of RelQ Tetramerization.
A Superdex 200 Increase 5/150 GL (GE Healthcare) column was pre-equilibrated with 12 mL (4 column volumes) of running buffer (20 mM Tris⋅HCl pH 8.0, 500 mM NaCl, 1mM EDTA, and 5% glycerol) on a Micro-ÄKTA system (GE Healthcare) at 4 °C. Then 10-μL samples, prepared in running buffer and containing 18.5 μM RelQ supplemented with combinations of 60 μM mRNA(MF), 100 μM ppGpp, 1 mM ATP, and 1 mM GDP, were applied and resolved.
DRaCALA of RelQ:ppGpp, RelQ:pppGpp, and RelQ:ATP Complex Formation.
DRaCALA binding reactions containing 50 nM 32P-labeled nucleotide (ATP, ppGpp, or pppGpp) and either 0–20 µM RelQ or 20 µM RelQ and 0–37 µM mRNA(MF) were preincubated at 37 °C for 10 min. Then 2 μL of the reaction mixtures were spotted on a 0.45-µm nitrocellulose blotting membrane (Amersham Protran; Sigma-Aldrich) and allowed to dry, followed by phosphoimaging (Typhoon 9500; GE Healthcare) and quantification as described by Roelofs et al. (25) using using ImageQuant TL image analysis software (GE Healthcare Life Sciences). All experiments were performed in Hepes:Polymix buffer (5 mM Mg2+) at pH 7.5 (31).
Sequence Alignment and Generation of a Homology Structural Model for E. faecalis RelQ.
A sequence alignment of RelQ and RelA was generated with MAFFT (32). The homology model of E. faecalis RelQ was constructed using Swiss-Model (33), with B. subtilis SAS1 (Protein Data Bank ID codes 5F2V and 5DED) (34) as the template.
Acknowledgments
We thank Andreas Carlström for creating the expression construct for the D82G RelQ derivative, Jose Lemos for introducing us to E. faecalis RelQ, and Elena Sineva for her valuable advice on the DRaCALA assay. This work was supported by the Estonian Research Council (Grant IUT2-22, to T.T.), the European Regional Development Fund through the Centre of Excellence for Molecular Cell Technology (V.H. and T.T.), the Estonian Science Foundation (Grant PUT37, to V.H.), the Kempe Foundation (V.H.), the Ragnar Söderberg Foundation (V.H.), and the Swedish Research Council (Grants 2013-4680, to V.H., and 2011-4791, to V.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1617868114/-/DCSupplemental.
References
- 1.Dalebroux ZD, Svensson SL, Gaynor EC, Swanson MS. ppGpp conjures bacterial virulence. Microbiol Mol Biol Rev. 2010;74(2):171–199. doi: 10.1128/MMBR.00046-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, Gerdes K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat Rev Microbiol. 2015;13(5):298–309. doi: 10.1038/nrmicro3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinchen W, Bange G. The magic dance of the alarmones (p)ppGpp. Mol Microbiol. 2016;101(4):531–544. doi: 10.1111/mmi.13412. [DOI] [PubMed] [Google Scholar]
- 4.Mittenhuber G. Comparative genomics and evolution of genes encoding bacterial (p)ppGpp synthetases/hydrolases (the Rel, RelA, and SpoT proteins) J Mol Microbiol Biotechnol. 2001;3(4):585–600. [PubMed] [Google Scholar]
- 5.Haseltine WA, Block R, Gilbert W, Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- 6.Xiao H, et al. Residual guanosine 3′,5′-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J Biol Chem. 1991;266(9):5980–5990. [PubMed] [Google Scholar]
- 7.Sy J. In vitro degradation of guanosine 5′-diphosphate, 3′-diphosphate. Proc Natl Acad Sci USA. 1977;74(12):5529–5533. doi: 10.1073/pnas.74.12.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haseltine WA, Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci USA. 1973;70(5):1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shyp V, et al. Positive allosteric feedback regulation of the stringent response enzyme RelA by its product. EMBO Rep. 2012;13(9):835–839. doi: 10.1038/embor.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seyfzadeh M, Keener J, Nomura M. spoT-dependent accumulation of guanosine tetraphosphate in response to fatty acid starvation in Escherichia coli. Proc Natl Acad Sci USA. 1993;90(23):11004–11008. doi: 10.1073/pnas.90.23.11004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinella D, Albrecht C, Cashel M, D’Ari R. Iron limitation induces SpoT-dependent accumulation of ppGpp in Escherichia coli. Mol Microbiol. 2005;56(4):958–970. doi: 10.1111/j.1365-2958.2005.04601.x. [DOI] [PubMed] [Google Scholar]
- 12.Lemos JA, Lin VK, Nascimento MM, Abranches J, Burne RA. Three gene products govern (p)ppGpp production by Streptococcus mutans. Mol Microbiol. 2007;65(6):1568–1581. doi: 10.1111/j.1365-2958.2007.05897.x. [DOI] [PubMed] [Google Scholar]
- 13.Nanamiya H, et al. Identification and functional analysis of novel (p)ppGpp synthetase genes in Bacillus subtilis. Mol Microbiol. 2008;67(2):291–304. doi: 10.1111/j.1365-2958.2007.06018.x. [DOI] [PubMed] [Google Scholar]
- 14.Abranches J, et al. The molecular alarmone (p)ppGpp mediates stress responses, vancomycin tolerance, and virulence in Enterococcus faecalis. J Bacteriol. 2009;191(7):2248–2256. doi: 10.1128/JB.01726-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun D, et al. A metazoan ortholog of SpoT hydrolyzes ppGpp and functions in starvation responses. Nat Struct Mol Biol. 2010;17(10):1188–1194. doi: 10.1038/nsmb.1906. [DOI] [PubMed] [Google Scholar]
- 16.Atkinson GC, Tenson T, Hauryliuk V. The RelA/SpoT homolog (RSH) superfamily: Distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS One. 2011;6(8):e23479. doi: 10.1371/journal.pone.0023479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geiger T, Kästle B, Gratani FL, Goerke C, Wolz C. Two small (p)ppGpp synthases in Staphylococcus aureus mediate tolerance against cell envelope stress conditions. J Bacteriol. 2014;196(4):894–902. doi: 10.1128/JB.01201-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinchen W, et al. Catalytic mechanism and allosteric regulation of an oligomeric (p)ppGpp synthetase by an alarmone. Proc Natl Acad Sci USA. 2015;112(43):13348–13353. doi: 10.1073/pnas.1505271112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaca AO, et al. From (p)ppGpp to (pp)pGpp: Characterization of regulatory effects of pGpp synthesized by the small alarmone synthetase of Enterococcus faecalis. J Bacteriol. 2015;197(18):2908–2919. doi: 10.1128/JB.00324-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiber G, et al. Overexpression of the relA gene in Escherichia coli. J Biol Chem. 1991;266(6):3760–3767. [PubMed] [Google Scholar]
- 21.Arenz S, et al. The stringent factor RelA adopts an open conformation on the ribosome to stimulate ppGpp synthesis. Nucleic Acids Res. 2016;44(13):6471–6481. doi: 10.1093/nar/gkw470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown A, Fernández IS, Gordiyenko Y, Ramakrishnan V. Ribosome-dependent activation of stringent control. Nature. 2016;534(7606):277–280. doi: 10.1038/nature17675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vakulskas CA, Potts AH, Babitzke P, Ahmer BM, Romeo T. Regulation of bacterial virulence by Csr (Rsm) systems. Microbiol Mol Biol Rev. 2015;79(2):193–224. doi: 10.1128/MMBR.00052-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollard TD. A guide to simple and informative binding assays. Mol Biol Cell. 2010;21(23):4061–4067. doi: 10.1091/mbc.E10-08-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roelofs KG, Wang J, Sintim HO, Lee VT. Differential radial capillary action of ligand assay for high-throughput detection of protein-metabolite interactions. Proc Natl Acad Sci USA. 2011;108(37):15528–15533. doi: 10.1073/pnas.1018949108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sebaugh JL. Guidelines for accurate EC50/IC50 estimation. Pharm Stat. 2011;10(2):128–134. doi: 10.1002/pst.426. [DOI] [PubMed] [Google Scholar]
- 27.Kriel A, et al. GTP dysregulation in Bacillus subtilis cells lacking (p)ppGpp results in phenotypic amino acid auxotrophy and failure to adapt to nutrient downshift and regulate biosynthesis genes. J Bacteriol. 2014;196(1):189–201. doi: 10.1128/JB.00918-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnan S, Petchiappan A, Singh A, Bhatt A, Chatterji D. R-loop induced stress response by second (p)ppGpp synthetase in Mycobacterium smegmatis: Functional and domain interdependence. Mol Microbiol. 2016;102(1):168–182. doi: 10.1111/mmi.13453. [DOI] [PubMed] [Google Scholar]
- 29.Vogel J, Luisi BF. Hfq and its constellation of RNA. Nat Rev Microbiol. 2011;9(8):578–589. doi: 10.1038/nrmicro2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31(13):3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jelenc PC, Kurland CG. Nucleoside triphosphate regeneration decreases the frequency of translation errors. Proc Natl Acad Sci USA. 1979;76(7):3174–3178. doi: 10.1073/pnas.76.7.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katoh K, Kuma K, Toh H, Miyata T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005;33(2):511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biasini M, et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42(Web Server issue):W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gouet P, Robert X, Courcelle E. ESPript/ENDscript: Extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res. 2003;31(13):3320–3323. doi: 10.1093/nar/gkg556. [DOI] [PMC free article] [PubMed] [Google Scholar]