Significance
Viruses are important causes of human disease and provide tools for understanding host immune defense mechanisms. Poxviruses are good models for probing the immune system because many replicate well in human cells, some cause severe disease, and nearly half of the 200 viral genes are devoted to host interactions. The virus/host equilibrium can be perturbed by mutating a viral gene and restored by suppressing the opposing host genes. To find relevant host genes for one such poxvirus mutation, we used a high-throughput human genome-wide RNAi screen and monitored virus spread. Three proteins were identified: Two participate in processive DNA replication and another activates transcription of one of the DNA replication proteins to form a putative antiviral network.
Keywords: poxviruses, host range, DNA replication factors, interferon regulatory factor, RNAi screen
Abstract
Viruses and their hosts can reach balanced states of evolution ensuring mutual survival, which makes it difficult to appreciate the underlying dynamics. To uncover hidden interactions, virus mutants that have lost defense genes may be used. Deletion of the gene that encodes serine protease inhibitor 1 (SPI-1) of rabbitpox virus and vaccinia virus, two closely related orthopoxviruses, prevents their efficient replication in human cells, whereas certain other mammalian cells remain fully permissive. Our high-throughput genome-wide siRNA screen identified host factors that prevent reproduction and spread of the mutant viruses in human cells. More than 20,000 genes were interrogated with individual siRNAs and those that prominently increased replication of the SPI-1 deletion mutant were subjected to a secondary screen. The top hits based on the combined data—replication factor C3 (RFC3), FAM111A, and interferon regulatory factor 2 (IRF2)—were confirmed by custom assays. The siRNAs to RFC1, RFC2, RFC4, and RFC5 mRNAs also enhanced spread of the mutant virus, strengthening the biological significance of the RFC complex as a host restriction factor for poxviruses. Whereas association with proliferating cell nuclear antigen and participation in processive genome replication are common features of FAM111A and RFC, IRF2 is a transcriptional regulator. Microarray analysis, quantitative RT-PCR, and immunoblotting revealed that IRF2 regulated the basal level expression of FAM111A, suggesting that the enhancing effect of depleting IRF2 on replication of the SPI-1 mutant was indirect. Thus, the viral SPI-1 protein and the host IRF2, FAM111A, and RFC complex likely form an interaction network that influences the ability of poxviruses to replicate in human cells.
Prokaryotes and eukaryotes use diverse mechanisms to recognize and survive virus infections. For example, triggering the type I IFN response leads to expression of hundreds of proteins with antiviral effector functions (1). To persist in nature, viruses evolved a variety of subterfuges to hide from their hosts or actively counter defense mechanisms. The numerous viral genes devoted to host interactions provide a living record of the natural selection that has occurred over millennia. Indeed, such genes constitute approximately half of the large DNA genomes of poxviruses (2, 3). In general, the defense genes can be recognized by their location near the ends of the genome and their variability compared with the ∼100 genes highly conserved in all vertebrate poxviruses that are needed for replication and dissemination (4). The diversity of defense genes likely reflects their acquisition at different times during evolution and in different hosts. Among the members of the orthopoxvirus genus, more narrow host adaptations have led to the inactivation of many genes. Thus, cowpox virus retains the full set of orthopoxvirus defense genes (5) and can replicate in rodents, felines, and humans, whereas many defense genes are interrupted or truncated in the viruses that cause smallpox (6) and camelpox (7, 8), which specifically infect humans and camels, respectively. In addition to species variation, spontaneous and targeted mutations lead to host-range restrictions. Because the latter genetic alterations are well defined, such mutants are ideal for probing the molecular basis of virus–host interactions, many of which are incompletely understood.
Large-scale screens in which expression of individual cellular genes is reduced or prevented are useful for identifying virus–host interactions. Several such screens have been carried out with vaccinia virus (VACV), the prototype member of the poxvirus family, and have identified cell proteins that the virus uses for entry, uncoating, DNA replication, and assembly (9–13). In principle, such screens should also identify host antiviral pathways. However, inactivation of host antiviral genes may not enhance virus replication if the virus already has an adequate defense. This potential roadblock to discovery could be overcome by screening mutant viruses that are lacking one or more defense genes and consequently have lost the ability to replicate in certain nonpermissive cells (14). An appealing feature of this approach is that depletion of a relevant mRNA in nonpermissive cells should enable replication of the mutant virus providing a positive response. One caveat is that screens with individual siRNAs might fail if redundant antiviral host genes exist. Nevertheless, this host-range strategy was used successfully to identify the cellular genes encoding SAMD9 and WDR6; small interfering RNAs (siRNAs) to mRNA of each gene alleviated the restriction of a VACV K1L/C7L deletion mutant in human cells (14). Here we further demonstrate the usefulness of this strategy by identifying additional human genes with an antiviral function.
The serpins are a superfamily of serine protease inhibitors present in animals and plants that regulate numerous biological processes. Serine protease inhibitor 1 (SPI-1) is conserved in all orthopoxviruses and is expressed early in infection as an intracellular, nonglycosylated 40-kDa protein (15–17). In vitro studies indicate that SPI-1 can inhibit cathepsin G, a serine protease with chymotrypsin- and trypsin-like activities, although this is unlikely to be a significant substrate in cultured cells (18). SPI-1 has a 44% amino acid identity to another VACV protein called SPI-2, also known as crmA but with a different predicted active center. SPI-2 inhibits the cysteine protease caspase 1 and has antiinflammatory properties attributed to blocking IL-1β and IL-18 (19, 20). A third VACV protein, SPI-3, is more distantly related and functions as an inhibitor of virus-mediated cell fusion and virus superinfection (21–23). Deletion of the SPI-1 gene but not SPI-2 or SPI-3 from VACV or the closely related rabbitpox virus (RPXV) causes an inability to efficiently propagate in human A549 and pig kidney 15 cells but not in several tested avian or monkey cells (24–26). The host-range defect is correlated with a severe block in formation of infectious virions, some decrease in postreplicative gene expression, and alterations in nuclear morphology. Remarkably, passage of a RPXV SPI-1 mutant in nonpermissive cells led to suppressor mutations that mapped to viral proteins essential for viral genome replication, even though the mutant had displayed no obvious defect in DNA synthesis (27). The functions of those extragenic suppressors suggest that some cellular proteins may have a subtle involvement in the regulation of poxvirus DNA synthesis, which occurs in discrete regions of the cytoplasm, called factories. Barrier-to-autointegration factor is an example of a host DNA-binding protein that inhibits VACV replication unless inactivated by the poxvirus B1 kinase (28). No cellular proteins essential for poxvirus DNA replication have been identified, although DNA ligase 1 can substitute for the VACV DNA ligase (29) and topoisomerase II is recruited to sites of viral DNA replication (30).
In the present study, we performed a high-throughput human genome-wide RNAi screen in which the endpoint was enhanced spread of a RPXV SPI-1 deletion mutant in human A549 cells. The primary and secondary screens yielded three strongly positive hits: interferon regulatory factor 2 (IRF2), family with sequence similarity 111 member A (FAM111A), and replication factor C3 (RFC3), which were confirmed by additional experiments. IRF2 is a regulatory factor that competitively inhibits IRF1-mediated transcriptional activation of type 1 interferons and activates transcription of vascular cell adhesion molecule 1 (31) and H4 histone (32). FAM111A is a chromatin-associated protein that has homology with trypsin-like peptidases, interacts with proliferating cell nuclear antigen (PCNA) at replication sites (33), binds to SV40 large T antigen, and acts as host restriction factor for SV40 (34). RFC3 is a component of the five-subunit RFC, which loads PCNA onto DNA at template primer junctions (35). The finding that two of the three best hits were DNA replication proteins and one of them is a predicted serine protease correlated with previous data regarding the properties of SPI-1 and the extragenic suppressors of the host-range defect. Our discovery that IRF2 is a transcriptional activator of FAM111A ties the proteins together into a putative antiviral network.
Results
Host-Range Restriction of SPI-1 Deletion Mutants Expressing Enhanced GFP.
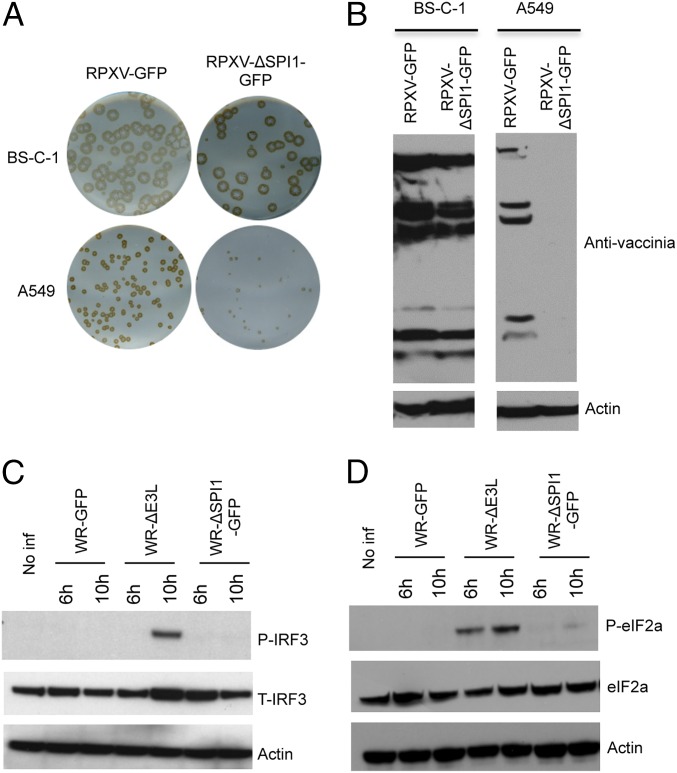
Previous studies demonstrated that deletion of the gene encoding SPI-1 from the genome of RPXV or VACV significantly diminished replication of the mutant viruses in human cells but did not impair replication in monkey and some other mammalian and avian cells. To construct a candidate virus suitable for an RNAi screen and confirm the replication defect, we replaced the SPI-1 gene of RPXV and VACV with one encoding GFP under the control of the viral late p11 promoter to produce RPXV-ΔSPI1-GFP and VACV-ΔSPI1-GFP. As a control, we also inserted the GFP gene between two ORFs of RPXV and left the SPI-1 gene intact to produce RPXV-GFP. The plaques formed by the RPXV (Fig. 1A) and VACV (Fig. S1A) SPI-1 deletion mutants were similar in size to the control viruses in monkey BS-C-1 cells but much smaller in human A549 cells. Furthermore, robust viral protein synthesis was detected in BS-C-1 cells but not in A549 cells following a low multiplicity infection with RPXV-ΔSPI1-GFP (Fig. 1B) and VACV-ΔSPI1-GFP (Fig. S1B). However, the host-range effect of the SPI-1 deletion mutant was consistently greater in RPXV than VACV, apparently because of the different virus backgrounds. In contrast to a VACV mutant with a deletion of the gene encoding the E3 dsRNA binding protein (36), the host-range defects of VACV-ΔSPI1-GFP (Fig. 1C) and RPXV-ΔSPI1-GFP (Fig. S1C) did not involve phosphorylation of IRF3, a component of the IFN signaling pathway or the translation initiation factor eIF2α (Fig. 1D and Fig. S1C), suggesting involvement of novel restriction factors and prompting a genome-wide RNAi screen to identify them.
Fig. 1.
Host-range restriction of SPI-1 mutants. (A) Plaque formation. BS-C-1 and A549 cells were infected with control RPXV-GFP or SPI-1 deletion mutant RPXV-ΔSPI1-GFP. Plaques formed in 72 h were detected by immunostaining with rabbit polyclonal anti-VACV antibody. (B) Immunoblots of viral proteins. Proteins from BS-C-1 and A549 cells infected for 28 h with RPXV-GFP or RPXV-ΔSPI1-GFP were resolved by polyacrylamide gel electrophoresis, transferred to a membrane, and probed with polyclonal antibody to VACV and actin as a loading control. (C) Immunoblot of IRF3. Proteins from A549 cells that were noninfected (No Inf) or infected with wild-type VACV strain WR (WR), a VACV E3 deletion mutant (ΔE3L), or VACV-ΔSPI1-GFP (ΔSPI1-GFP) for 6 or 10 h, as indicated, were analyzed as in B and probed with antibody to phosphorylated IRF3 (P-IRF3), total IRF3 (T-IRF3), or actin. (D) Immunoblot of eIF2α. Same as C except that blots were probed with antibody to phosphorylated eIF2α (P-eIF2α) or total eIF2α protein (eIF2α).
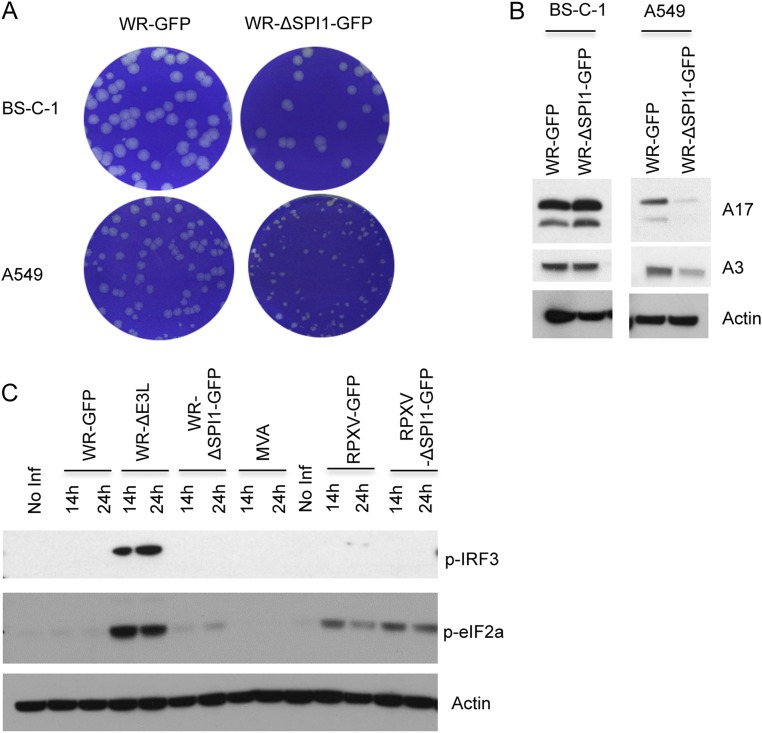
Fig. S1.
Host-range restriction of VACV and RPXV SPI-1 mutants. (A) Plaque formation. BS-C-1 and A549 cells were infected with control vaccinia virus WR-GFP or SPI-1 deletion mutant WR-ΔSPI1-GFP. After 72 h, plaques were detected by staining with Crystal violet. (B) Immunoblot of viral proteins. Proteins from BS-C-1 and A549 cells infected for 28 h with WR-GFP or WR-ΔSPI1-GFP were resolved by SDS/PAGE, transferred to a membrane, and probed with polyclonal antibodies to viral late A17 and A3 proteins and to actin as a loading control. (C) Immunoblots of IRF3 and eIF2α. Proteins from A549 cells that were noninfected (No Inf) or infected with WR-GFP, an E3 deletion mutant WR ΔE3L, WR-ΔSPI1-GFP, modified vaccinia virus Ankara (MVA), RPXV-GFP, or RPXV-ΔSPI1-GFP for the indicated hours were analyzed as in panel B and probed with antibody to phosphorylated IRF3 (P-IRF3) or to phosphorylated eIF2α (P-eIF2α) or actin.
Genome-Wide RNAi Screen with the RPXV SPI-1 Deletion Mutant.
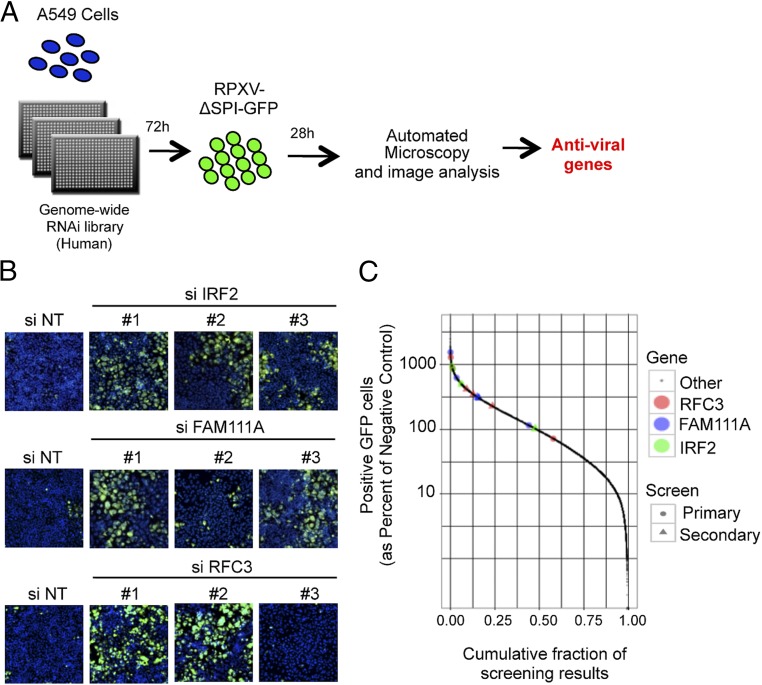
The scheme used for the RNAi screen is depicted in Fig. 2A. A549 cells were reverse-transfected with the Silencer Select siRNA library from Ambion, which consists of three different siRNAs for each of 21,584 human genes in individual wells of a 384-well plate. After 72 h, the cells were infected with RPXV-ΔSPI1-GFP at a low multiplicity chosen to allow virus spread to ∼50% of the permissive RK-13 cells by 28 h but only to 1–2% of nonpermissive A549 cells. Automated microscopy was used to count the number of cells determined by Hoechst-stained nuclei and the percentage of those exhibiting GFP fluorescence. Genes were selected for follow-up based both on the effects observed by siRNAs designed to target them and based on imperfect, seed-based pairing of other siRNAs with the 3′UTRs of these genes, as revealed by Haystack analysis (37). As a result, some genes (notably FAM111A and IRF2) were elevated in priority because of the high statistical significance of these seed-based effects. Confirmation of FAM111A and IRF2 with nonoverlapping siRNAs in the secondary screen demonstrated the effectiveness of this strategy. Indeed, based on the combined primary and secondary screens the three most significant hits were RFC3, FAM111A, and IRF2 (Dataset S1). The complete dataset for the primary screen and the Haystack analysis are in Dataset S2.
Fig. 2.
Genome-wide siRNA screen. (A) Schematic of the human genome-wide screen. A549 cells in a 384-well plate were reverse transfected for 72 h with the Silencer Select siRNA library from Ambion, infected with 0.01 PFU per cell of RPXV-ΔSPI1-GFP for 28 h, fixed, and screened for cells that stained with Hoechst and exhibited GFP fluorescence. Antiviral genes were determined by increased number of cells with GFP fluorescence compared with median. (B) Images of the siRNA-transfected and virus-infected cells from the primary screen. NT stands for nontargeting siRNA. Hoechst stain, blue; GFP, green. (Magnification: 10×.) (C) The percentages of fluorescent cells from the primary and secondary screens for individual IRF2, FAM111A, and RFC3 siRNAs (divided by the percentages of fluorescent cells for negative controls) compared with siRNAs for all other genes. Color and symbol keys for siRNAs on right.
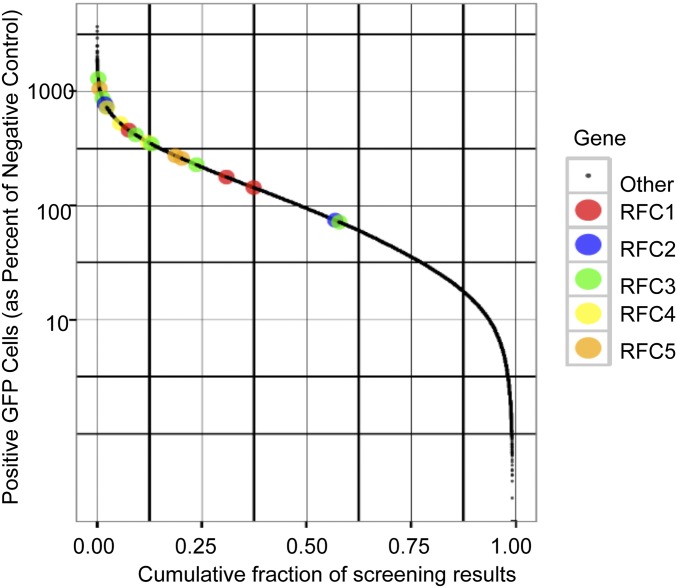
Images taken directly from the primary screen are shown for IRF2, FAM111A, and RFC3 (Fig. 2B), which were determined to be the most significant hits following the secondary screen. The number of GFP+ cells for IRF2, FAM111A, and RFC3 siRNAs compared with siRNAs for all other genes from both the primary and secondary screens are depicted in Fig. 2C. RFC3 exists in a complex with four other RFC subunits (RFC1, RFC2, RFC4, and RFC5). The finding that 5 of 6 siRNAs targeting RFC3 and 11 of 12 siRNAs targeting the other four RFC subunits increased GFP fluorescence above the median assay response (Fig. S2) suggested that the entire RFC is a host-range factor.
Fig. S2.
Effects of depleting RFC components on mutant virus rescue. Data were obtained from the primary genome-wide screen. The percentages of fluorescent cells for RFC1, RFC2, RFC3, RFC4, and RFC5 siRNAs (divided by the percentages of fluorescent cells for negative controls) compared with siRNAs for all other genes are depicted. Color key on right.
IRF2, FAM111A, and RFC3 Depletions Rescue Replication of RPXV and VACV SPI-1 Deletion Mutants.
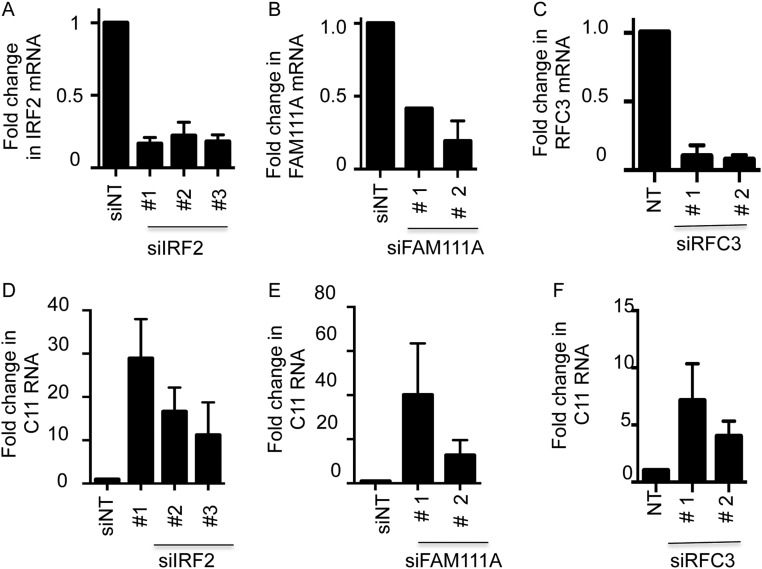
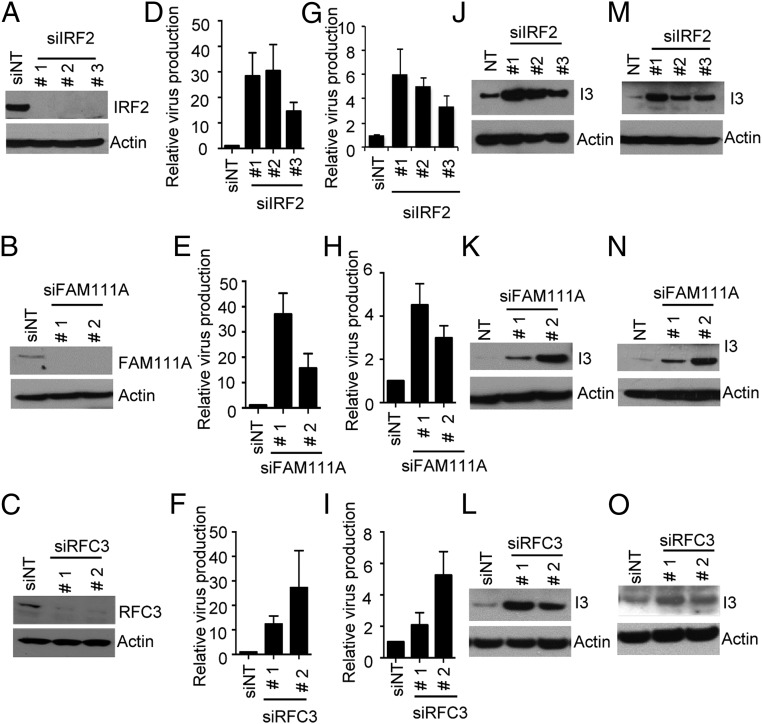
In the high-throughput screens we found that siRNAs to IRF2, FAM111A, or RFC3 enhanced spread of RPXV-ΔSPI1-GFP based on fluorescent detection of GFP. The major host-range defect of the RPXV SPI-1 deletion mutant is a decrease in infectious virus production. We confirmed the results of the screen using additional siRNAs to directly assess infectious virus production and extended the results to the VACV SPI-1 deletion mutant, as detailed below. Depletion of the cognate mRNAs and proteins by siRNAs for IRF2, FAM111A, and RFC3 were determined by quantitative RT-PCR (RT-qPCR) (Fig. S3 A–C) and Western blotting (Fig. 3 A–C), respectively. The siRNAs enhanced production of RPXV-ΔSPI1-GFP virus by 10- to 30-fold (Fig. 3 D–F) and VACV-ΔSPI1-GFP virus by two- to fivefold (Fig. 3 G–I), depending on the siRNA. This difference for the two viruses reflects the greater restriction of RPXV-ΔSPI1-GFP compared with VACV-ΔSPI1-GFP in A549 cells, and was the reason we used the RPXV mutant in the high-throughput screen. We also confirmed the results by measuring spread of RPXV-ΔSPI1-GFP (Fig. 3 J–L) and VACV-ΔSPI1-GFP (Fig. 3 M–O) by an immunoblot probed with antibody to the I3 protein and by measuring viral C11 gene expression by RT-qPCR (Fig. S3 D–F).
Fig. S3.
Effects of siRNAs to IRF2, FAM111A and RFC3 on replication of a RPXV SPI-1 deletion mutant. (A–C) Uninfected A549 cells were transfected with control (NT) siRNA or nonoverlapping siRNAs targeted to IRF2, FAM111A or RFC3. After 72 h, gene expression was determined by RT-qPCR. Data represents normalized fold change compared with actin mRNA. (D–F) A549 cells were transfected with control (NT) siRNA or nonoverlapping siRNAs targeted to IRF2, FAM111A, or RFC3 and infected with RPXV-ΔSPI1-GFP at a multiplicity of 0.001 PFU per cell. At 28 h of infection the cells were harvested to determine virus-encoded C11 gene expression by RT-qPCR.
Fig. 3.
Effects of siRNAs to IRF2, FAM111A and RFC3 on replication of RPXV and VACV SPI-1 deletion mutants. (A–C) A549 cells were transfected with control nontarget (NT) siRNA or nonoverlapping siRNAs targeted to IRF2, FAM111A, or RFC3, which are numbered consistently in all panels. After 72 h, expression of the three proteins was determined by immunoblotting with specific antibodies to IRF2, FAM111A, and RFC3. Antibody to actin was used as a loading control. (D–F) A549 cells were transfected with control or IRF2, FAM111A, or RFC3 siRNAs for 72 h and infected with RPXV-ΔSPI1-GFP at a multiplicity of 0.001 PFU per cell. At 28 h of infection, the cells were harvested to determine infectious virus production by plaque assay on permissive BS-C-1 cells. Ratios of virus from cells transfected with specific siRNAs to control siRNA were plotted as bar graphs. SEMs from triplicate infections are shown. (G–I) Same as D–F except that cells were infected with VACV-ΔSPI1-GFP. (J–L) A549 cells were transfected with control or IRF2, FAM111A, or RFC3 siRNAs for 72 h and infected with RPXV-ΔSPI1-GFP at a multiplicity of 0.001 PFU per cell. At 28 h of infection the cells were harvested and lysates were analyzed by immunoblotting with antibody to the VACV I3 protein or actin. (M–O) Same as J–L except that cells were infected with VACV-ΔSPI1-GFP.
IRF2 Regulates Expression of a Subset of Human Genes Including FAM111A.
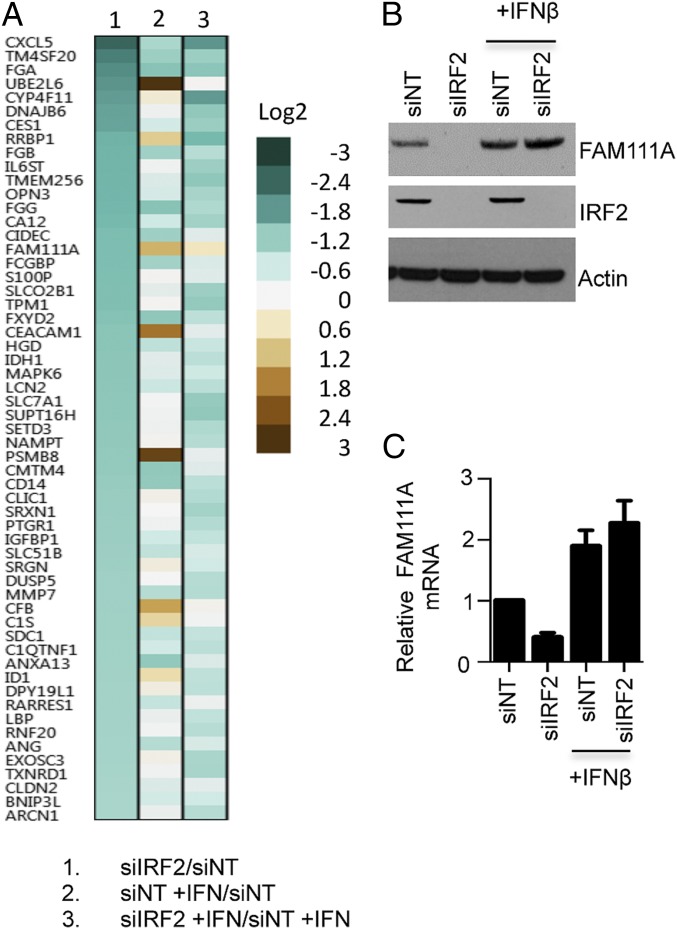
Our finding that depletion of three different host proteins restored replication of the SPI-1 mutants suggested that they might be acting in a common pathway or network. Although IRF2 is a negative regulator of IRF1 gene expression (38), there are reports of activation of some other genes (31, 32, 39). Therefore, we considered that IRF2 could be acting indirectly to inhibit SPI-1 deletion mutants by attenuating or inducing expression of some cellular genes. Because we could not find a report describing a comprehensive screen of gene regulation by IRF2, we carried out a microarray analysis of control cells and cells depleted of IRF2 and parallel analyses in which the cells were treated with IFN-β and a nontargeting or targeting siRNA to IRF2. Expression of 57 genes was reduced twofold or more by siRNA to IRF2, suggesting that IRF2 positively regulates their basal level of expression (Fig. 4A and Dataset S3). When we cross-checked the genes affected by IRF2 depletion with the significant hits from our RNAi screen, a match to FAM111A but to no other gene was found. This result suggested that the host-range role of IRF2 might be activation of FAM111A expression. Interestingly none of the canonical IFN-stimulated genes (1) were regulated by IRF2. In Fig. 4A, we also analyzed the genes modulated by addition of IFN-β with those affected by depletion of IRF2. Overall, the patterns of gene regulation were very different. However, a subset of the genes regulated by IRF2, which included FAM111A, was also activated by IFN-β (Fig. 4A and Dataset S3). Moreover, IFN-β increased expression of FAM111A even in cells depleted of IRF2.
Fig. 4.
Expression profiling of IRF2-dependent genes. (A) Microarray heat map. A549 cells were transfected with control (NT) siRNA or IRF2-specific siRNA for 72 h and treated or not treated with IFN-β for the last 16 h. Total RNA was extracted and fluorescently labeled cDNAs were hybridized to Illumina HumanHT-12 V4.0 Expression BeadChip. Column 1, ratio of signal from siIRF2 transfected cells divided by signal from NT siRNA transfected cells; column 2, ratio of signal from NT siRNA transfected cells treated with IFNβ divided by signal from NT siRNA transfected cells that were untreated; column 3, ratio of siIRF2 transfected cells treated with IFNβ to NT siRNA transfected cells treated with IFN-β. Genes that show a twofold or more changes in expression with siRNA to IRF2 are listed. Color bars indicate the degrees of change. (B) Basal level of FAM111A is regulated by IRF2 and IFN-β. A549 cells were transfected with IRF2 siRNA or control NT siRNA for 72 h and either treated with IFN-β or left untreated for the last 16 h. FAM111A, IRF2, and actin were analyzed by immunoblotting with specific antibodies. (C) Regulation of FAM111A by IRF2 and IFNβ occurs at transcriptional level. Transfections, infections and IFN-β treatment were as in B. The relative amounts of FAM111A mRNA were determined by RT-qPCR. Fold-changes were normalized to NT siRNA of IFN-β untreated sample. P values are less than 0.05 in t test for depletion of FAM111A compared with NT (without INF-β) and with each of other conditions in one-way ANOVA.
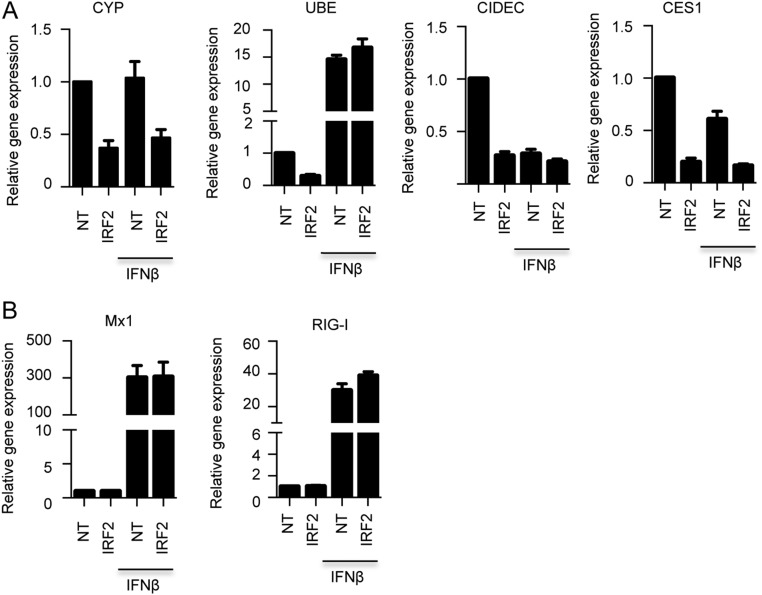
Immunoblotting confirmed that depletion of IRF2 effectively depleted FAM111A in A549 cells but not if the cells were pretreated with IFN-β (Fig. 4B). Depletion of FAM111A by siRNA and activation by IFN-β both occurred at the transcriptional level (Fig. 4C). We also validated the regulation of several other genes by IRF2 using RT-qPCR, although they were not involved in the host restriction. Thus, depletion of IRF2 by siRNAs decreased RNAs encoded by CYP4F11, UBE2L6, CIDEC, and CES1 (Fig. S4). We also verified that the basal expression of the known IFN stimulatory genes MX1 and RIG-I was stimulated by IFN-β but unaffected by depletion of IRF2 (Fig. S4).
Fig. S4.
IRF2-dependent gene expression. (A) Validation of representative microarray data by RT-qPCR for genes indicated. The relative amounts of mRNA for each of the indicated genes were determined by RT-qPCR. Fold-changes were normalized to NT siRNA of IFN-β untreated sample. Data are mean ± SEM of four independent experiments. (B) RT-qPCR examination of the known IFN stimulated genes Mx1 and RIG-I.
Discussion
We chose to investigate the SPI-1 protein because the cognate gene is intact in all orthopoxviruses and a related gene is present in the genera of some other chordopoxviruses, suggesting an important but poorly understood function (24–26). Our preliminary experiments showed that neither IRF3 nor eIF2α phosphorylation, which can occur as a result of activation of the IFN pathway, was increased under nonpermissive conditions. Because a novel pathway might be involved, a genome-wide RNAi approach was used to discover the identity of the host genes that restrict replication of the SPI-1 mutants. Because the molecular basis of the replication defect was unknown, a virus cell-to-cell spread assay using a recombinant RPXV SPI-1 deletion mutant expressing GFP was optimized for the high-throughput gain-of-function screen.
Of the more than 20,000 genes interrogated, the siRNAs targeted to IRF2, FAM111A, and RFC3 provided the most notable enhancement of virus spread. As a group, IFN regulatory factors are involved in antiviral defense but less is known about IRF2 than others (40). IRF2 is a stable nuclear protein that is constitutively expressed in many species and cell types. Although IRF2 and IRF1 bind to the same promoter elements of IFN and IFN-inducible genes, IRF2 is only a weak activator (39) and competitively inhibits the strong activation by IRF1 (41). In IRF2 knockout mice IFN-inducible genes are overexpressed (42) and type 1 interferons are up-regulated following New Castle disease virus infection (43). In addition, IRF2 confers some protection of mice to Venezuelan equine encephalitis virus (44). Because IRF1 inhibits the replication of diverse viruses and IRF2 negatively regulates IRF1 functions, one might expect depletion of IRF2 would restrict poxvirus replication rather than enhance it, as shown here. To understand the possible role of IRF2 in mediating restriction of the RPXV SPI-1 mutant, we compared gene expression in IRF2-depleted and control A549 cells. Microarray analysis revealed that when IRF2 was depleted, expression of 57 genes, notably including FAM111A, was reduced and 21 genes were increased by twofold or more. Furthermore, immunoblotting and RT-qPCR demonstrated that IRF2 positively regulated basal expression of FAM111A in A549 cells. Comparison of gene expression after IRF2 depletion and after IFN-β addition, indicated distinct though partially overlapping patterns of gene regulation. Expression of FAM111A was up-regulated by IFN-β even when IRF2 was depleted. However, IRF2 itself was not up-regulated by IFN.
The role of IRF2 in establishing a basal level of FAM111A in A549 cells suggested that IRF2 has an indirect role in restricting replication of the SPI-1 mutant, whereas FAM111A may have a more direct role. FAM111A is a chromatin-associated protein that has a trypsin-like peptidase site and interacts with PCNA at replication sites (33). Because FAM111A exhibits cell cycle-dependent expression (45), and as we have shown here is induced by IFN, IRF2 might not be needed for induction under some conditions. Mutations of FAM111A cause Kenny-Caffey syndrome (46, 47), hypoparathyriodism, and impaired skeletal development (48). Interestingly, FAM111A is a host restriction factor for an SV40 mutant (34). SV40 with C-terminal mutations of large T antigen is unable to reproduce or provide helper function to adenovirus in certain African green monkey cells (49). The defect appears to be in late gene expression and virion production rather than DNA replication (50, 51). The C-terminal segment of SV40 large T antigen binds FAM111A and depletion of FAM111A overcomes the host restriction in nonpermissive cells (34). Nevertheless, the role of FAM111A in mediating the host-range restriction of SV40 is uncertain because both permissive and nonpermissive African green monkey cells express FAM111A that can bind large T antigen. It would be interesting to determine whether depletion of RFC3 would also overcome the host-range restriction of SV40 mutants, as occurs with poxvirus SPI-1 mutants.
RFC3 is a third host-restriction factor for poxvirus SPI-1 mutants found in our RNAi screen. Enhanced virus spread was also found with siRNAs for the other four subunits of the RFC complex, strengthening the biological importance of this hit. RFC loads PCNA onto DNA at template primer junctions by an ATP-dependent process (35). It seems relevant for their common host-range function that both FAM111A and RFC interact physically and functionally with PCNA. PCNA is a ring-like homotrimer that encircles double-stranded DNA. PCNA mediates the localization of many proteins, including FAM111A and RFC, to replication sites and organizes proteins involved in DNA replication, repair, and modification.
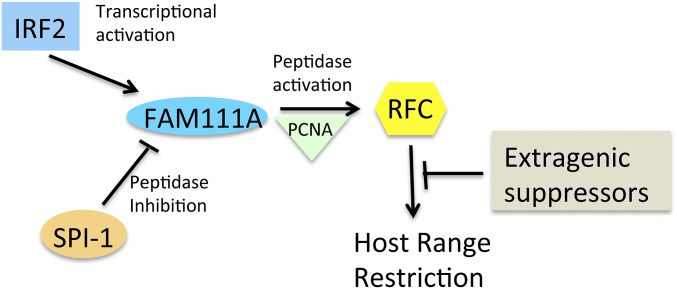
The finding that mutations in viral DNA replication proteins, including the DNA polymerase and primase helicase, suppress the host-range defect of the RPXV SPI-1 mutant was unanticipated because the mutant did not display an obvious defect in viral DNA replication (27). Nevertheless, that result relates to our current finding that the cellular DNA replication proteins FAM111A and RFC are host factors that restrict replication of the SPI-1 mutant. Thus far, investigations of poxvirus DNA replication have focused on viral proteins, except to show that cellular topoisomerase II is recruited by the viral DNA ligase to sites of viral DNA replication (30) and that cellular DNA ligase 1 can substitute for the viral ligase (29). At this time we can only suggest a speculative model for the roles of SPI-1, IRF2, FAM111A, and RFC3 in host restriction of poxviruses that may be useful for designing further studies (Fig. 5). The first step, transcriptional activation of FAM111A by IRF2, was demonstrated in this study. The interaction of FAM111A and RFC with PCNA has been previously shown. We suggest that the putative peptidase activity of FAM111A activates RFC either directly or indirectly and that RFC in an unknown way interferes with poxvirus replication. According to our model, the antiviral network is interrupted when SPI-1 inhibits the peptidase activity of FAM111A or if there are suppressor mutations of viral DNA replication proteins. In the absence of SPI-1 or suppressors, siRNAs to either IRF2, FAM111A, or RFC3 interrupt the network. Future studies aimed at identifying the target of SPI-1, the substrate of FAM111A peptidase and viral and cellular proteins associated with the DNA of wild-type and mutant poxviruses should prove enlightening.
Fig. 5.
Model of SPI-1 host-range restriction. During infection with an SPI-1 mutant: (i) IRF2 transcriptionally activates the basal expression of FAM111A; (ii) the putative peptidase activity of FAM111A activates RFC directly or indirectly while both are associated with PCNA; (iii) RFC interferes with virus replication in a yet to be determined way. The antiviral network can be interrupted by SPI-1 inactivation of the FAM111A peptidase during infection by wild-type virus or by extragenic suppressor mutations during infection with a SPI-1 mutant.
Materials and Methods
The primary genome-wide siRNA screen was conducted using the Ambion Silencer Select Human Genome siRNA Library v4, which consists of three unique, nonoverlapping, nonpooled siRNAs for ∼21,584 gene targets. The secondary screen was conducted using three independent siRNAs from the Ambion Silencer library. The siRNAs were reverse transfected using the Lipofectamine RNAiMAX Transfection Reagent. The wells were seeded with A549 cells and after 72 h were infected with 0.01 PFU of RPXV-ΔSPI1-GFP and incubated for 28 h. After fixation and Hoechst staining the plates were imaged with Molecular Devices ImageXpress Micro XL High-Content Screening System and the percent of GFP+ cells in each well was determined. Additional information regarding the screen analysis, custom siRNA assays, and other methods and reagents is provided in SI Materials and Methods.
SI Materials and Methods
Cells and Reagents.
BS-C-1 (ATCC CCL-26) and A549 (ATCC CCL-185) cells were grown in minimum essential medium with Earle’s salt and F-12 medium, respectively, supplemented with 10% (vol/vol) FBS, 100 U of penicillin, and 100 µg of streptomycin per milliliter (Quality Biologicals). RK-13 cells were grown in Dulbecco minimum essential medium supplemented with 5% FBS and penicillin and streptomycin. Antibodies to IRF2 (Sc-498), actin (Sc-8432), and GFP (Sc-9996) were from Santa Cruz Biotechnology; antibodies to phospho-IRF3 (4947), IRF3 (4302), phosphor-eIF2α (5324S), eIF2α (9722), and OAS1 (14498) were from Cell Signaling Technology; antibodies to FAM111A (Ab-184572) and RFC3 (Ab154899) were from Abcam, and IFN-β was purchased from PBL Assay Science.
Construction of Recombinant Poxviruses.
RPXV-ΔSPI1-GFP and VACV-ΔSPI1-GFP recombinant viruses were generated as follows. Briefly, ∼500 bp of SPI-1 flanking regions were attached to the ends of the GFP ORF regulated by the VACV p11 promoter. The resultant 1,720-bp PCR product was transfected into BS-C-1 cells, which were then infected with RPXV strain Utrecht or VACV strain WR virus. Recombinant virus expressing GFP was clonally purified by repeated plaque isolation. The loss of the SPI-1 gene was confirmed by PCR.
siRNA Screening.
The primary RNAi screen was conducted using the Ambion Silencer Select Human Genome siRNA Library Version 4, which consists of three unique, nonoverlapping, nonpooled siRNAs per 21,584 gene targets. siRNA reagents (2 µL, 400 nM) were stamped into Greiner Bio-One 384 Well Black Clear Flat-Bottom Microplates using a Velocity11 VPrep liquid handling system (Agilent) integrated into a BioCel robotic platform (Agilent) in columns 1–22, leaving columns 23–24 empty for negative (Ambion Silencer Select Negative Control #2) and positive (Qiagen Allstars Hs Cell Death) controls, respectively. The median value of each plate’s negative control column was used to normalize sample wells, and the positive control was used to assess transfection efficiency and assay performance.
Lipofectamine RNAiMAX Transfection Reagent (0.12 µL; Invitrogen) was added in 20 µL serum-free, antibiotic-free media to plate wells using a Thermo Scientific Matrix WellMate and Microplate Stacker. Plates were incubated for 45 min at room temperature to allow for the sufficient formation of siRNA-lipid complexes. Cells were seeded at a density of 2,000 cells per well in 20 µL media containing 20% (vol/vol) FBS without antibiotics. The final concentration of siRNA in each well was 20 nM. Cells were cultured for 72 h at 37 °C in 5% CO2 before addition of VACV. After 72 h, medium from each well was aspirated using the 8-channel HandEvac handheld Aspirator (Sigma-Aldrich) and virus was dispensed in 10 µL using the Biotek MultiFlo Dispenser and incubated for 1 h at 37 °C in 5% CO2 and then 30 µL of medium was added to each plate well and incubated for 28 h at 37 °C in 5% CO2 before fixation.
Paraformaldehyde in PBS was added to plate wells to a final 2% solution and incubated at room temperature for 20 min before aspiration of supernatant and washing with PBS (2 × 40 µL). Nuclei were stained using Hoechst 33342 (1 µg/mL) in PBS for 20 min and subsequently washed twice with PBS before a final addition of 20 µL PBS and plate sealing using the Agilent PlateLoc Thermal Microplate Sealer.
Image Acquisition and Processing.
Plates were imaged with a Molecular Devices ImageXpress Micro XL High-Content Screening System integrated into a BioCel robotic platform (Agilent). The ImageXpress Micro uses a 1.4-megapixel cooled CCD camera and image acquisition was performed with a 10× Nikon lens objective and Semrock DAPI (catalog no. DAPI-5060B) and FITC (catalog no. FITC-3540B) filter sets/dichroic mirrors for detection of Hoechst stain and GFP, respectively. Acquired data were transferred to the Molecular Devices MDCStore data management system database and indexed by plate barcode.
High-throughput image analysis was performed on the MetaXpress PowerCore server software using the “multiwavelength cell scoring” module to determine the percentage of GFP+ cells in each well. The following parameters were used for nuclei segmentation (Hoechst stain): approximate minimum width, 6 µm; approximate maximum width, 25 µm; intensity above local background, 100 gray levels. The following parameters were used for GFP+ cell segmentation: approximate minimum width, 20 µm; approximate maximum width, 80 µm; intensity above local background, 220 gray levels; minimum stained area, 20 µm2.
Primary Screen Analysis and Hit Selection.
The percent cells positive for virus was provided for each well by the MetaXpress software. We then divided these values by the median negative control percent cells positive for virus and multiplied by 100 to achieve a negative control normalized metric for each well/siRNA. This normalized value was then used to generate a robust z-score by first taking the log of each value (because the results were log-normally distributed, as is commonly observed for rescue assays) and then by subtracting the median and dividing by the mean absolute deviation.
To correct for seed-based off-target effects (52) in the data, the following heuristic was used to calculate a seed-corrected z-score. For each siRNA z-score (z), the median z-score (c) of all siRNAs having the same seed sequence (bases 2–7 of the guide strand) was calculated. Then:
-
i)
If sign of c is not equal to sign of z, z remains unchanged;
-
ii)
If sign of c equals sign of z and abs(z) < abs(c) (effect is less than median for that seed), set z to zero;
-
iii)
If sign of c equals sign of z and abs(z) > abs(c), set z = z − c.
The median seed-corrected z-score was then used to rank genes for follow-up. In addition, Haystack analysis (37, 52) was used to find the gene transcripts whose off-targeting was most responsible for the results of the screen as a whole.
Secondary Screen.
The secondary screen was conducted using the Ambion Silencer library. In a few cases, the siRNA sequence in the Ambion Silencer library was the same as in the primary screening library, in which case it was eliminated from the data set as it would not be an independent data point. Data in the secondary screen was analyzed as it was in the primary, including negative control normalization, z-score, and seed-corrected z-score calculation. Ranking the follow-up genes by the median seed-corrected z-score for all independent siRNAs tested against the gene (usually six) provided the basis for selecting genes for further validation.
Microarray Hybridization.
RNA quality was verified by Agilent Bioanalyzer, with RNA Integrity Number (RIN) greater than 8.0 for all samples except for one (RIN 7.6), which otherwise showed no deviation in downstream quality control metrics. Amplification and labeling of the RNA samples were performed using the Illumina TotalPrep RNA Amplification (Applied Biosystems) and an input of 500 ng of total RNA per sample. Biotinylated RNA was hybridized to the Illumina HumanHT-12 V4.0 Expression BeadChip (GEO accession no. GPL10558) having more than 47,000 unique probes, using reagents provided, and imaged using the Illumina HiScan-SQ.
Microarray Expression Analysis.
Signal data were extracted from the image files with the Gene Expression module (v1.9.0) of the GenomeStudio software (v2011.1) from Illumina. Signal intensities were converted to log2 scale. Calculation of detection P values is described in the GenomeStudio Gene Expression Module User Guide. Data for array probes with insufficient signal (detection P < 0.1 in at least two arrays) were removed from the dataset. After dropping nonperforming probes, quantile normalization was applied across all arrays. ANOVA was performed on the normalized log2 expression estimates to test for mRNA expression differences by treatment (IFN or no treatment) and siRNA (IRF2 or control). A P value of 0.05 was used for the statistical significance cut-off, after adjusting for the familywise error rate using Benjamini–Hochberg method (53) to account for multiple testing. Statistical analysis was performed using JMP/Genomics software v6.0 (SAS Institute Inc.).
Virus Replication Assay.
A549 cells were infected with 0.01 PFU per cell of virus and lysed after 28 h by freezing and thawing. Dilutions of the lysate were plated on BSC-1 cells. Plaques were allowed to form under a methylcellulose overlay for 2–3 d. Cells were fixed and stained with Crystal violet to enumerate plaques.
Immunoblotting.
Cells were lysed in RIPA buffer and total protein was determined by the Bradford protein assay. Equal amounts of protein were separated on 10% SDS polyacrylamide gel and transferred to a nitrocellulose or PVDF membrane. Membranes were incubated with primary antibodies at 4 °C overnight followed by washing and incubating with appropriate secondary antibodies conjugated with horseradish peroxidase and analyzed by enhanced chemiluminescence.
RT-qPCR.
RNA was isolated with RNAeasy mini columns and reverse transcribed using Moloney murine leukemia virus reverse transcriptase (28025-013; Life Technologies). cDNA was subjected to PCR using SYBR green (4367659; Life Technologies) and analyzed by the ΔΔCT method, where CT is threshold cycle, and normalized to actin mRNA. Data are represented as levels of mRNA relative to that of the control samples and are displayed as the means ± SEM of results from at least three independent experiments.
Supplementary Material
Acknowledgments
We thank Timothy Myers for help with the microarray screen. Research support was provided by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700678114/-/DCSupplemental.
References
- 1.Schoggins JW, Rice CM. Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol. 2011;1(6):519–525. doi: 10.1016/j.coviro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bratke KA, McLysaght A, Rothenburg S. A survey of host range genes in poxvirus genomes. Infect Genet Evol. 2013;14:406–425. doi: 10.1016/j.meegid.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haller SL, Peng C, McFadden G, Rothenburg S. Poxviruses and the evolution of host range and virulence. Infect Genet Evol. 2014;21:15–40. doi: 10.1016/j.meegid.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upton C, Slack S, Hunter AL, Ehlers A, Roper RL. Poxvirus orthologous clusters: Toward defining the minimum essential poxvirus genome. J Virol. 2003;77(13):7590–7600. doi: 10.1128/JVI.77.13.7590-7600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shchelkunov SN, et al. The genomic sequence analysis of the left and right species-specific terminal region of a cowpox virus strain reveals unique sequences and a cluster of intact ORFs for immunomodulatory and host range proteins. Virology. 1998;243(2):432–460. doi: 10.1006/viro.1998.9039. [DOI] [PubMed] [Google Scholar]
- 6.Esposito JJ, et al. Genome sequence diversity and clues to the evolution of variola (smallpox) virus. Science. 2006;313(5788):807–812. doi: 10.1126/science.1125134. [DOI] [PubMed] [Google Scholar]
- 7.Gubser C, Smith GL. The sequence of camelpox virus shows it is most closely related to variola virus, the cause of smallpox. J Gen Virol. 2002;83(Pt 4):855–872. doi: 10.1099/0022-1317-83-4-855. [DOI] [PubMed] [Google Scholar]
- 8.Afonso CL, et al. The genome of camelpox virus. Virology. 2002;295(1):1–9. doi: 10.1006/viro.2001.1343. [DOI] [PubMed] [Google Scholar]
- 9.Moser TS, Jones RG, Thompson CB, Coyne CB, Cherry S. A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics. PLoS Pathog. 2010;6(6):e1000954. doi: 10.1371/journal.ppat.1000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mercer J, et al. RNAi screening reveals proteasome- and Cullin3-dependent stages in vaccinia virus infection. Cell Reports. 2012;2(4):1036–1047. doi: 10.1016/j.celrep.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Sivan G, et al. Human genome-wide RNAi screen reveals a role for nuclear pore proteins in poxvirus morphogenesis. Proc Natl Acad Sci USA. 2013;110(9):3519–3524. doi: 10.1073/pnas.1300708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sivan G, Weisberg AS, Americo JL, Moss B. Retrograde transport from early endosomes to the trans-Golgi network enables membrane wrapping and egress of vaccinia virions. J Virol. 2016;90(19):8891–8905. doi: 10.1128/JVI.01114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison K, et al. Vaccinia virus uses retromer-independent cellular retrograde transport pathways to facilitate the wrapping of intracellular mature virions during viral morphogenesis. J Virol. 2016;90(22):10120–10132. doi: 10.1128/JVI.01464-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sivan G, Ormanoglu P, Buehler EC, Martin SE, Moss B. Identification of restriction factors by human genome-wide RNA interference screening of viral host range mutants exemplified by discovery of SAMD9 and WDR6 as inhibitors of the vaccinia virus K1L-C7L- mutant. MBio. 2015;6(4):e01122. doi: 10.1128/mBio.01122-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotwal GJ, Moss B. Vaccinia virus encodes two proteins that are structurally related to members of the plasma serine protease inhibitor superfamily. J Virol. 1989;63(2):600–606. doi: 10.1128/jvi.63.2.600-606.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith GL, Howard ST, Chan YS. Vaccinia virus encodes a family of genes with homology to serine proteinase inhibitors. J Gen Virol. 1989;70(Pt 9):2333–2343. doi: 10.1099/0022-1317-70-9-2333. [DOI] [PubMed] [Google Scholar]
- 17.Kettle S, Blake NW, Law KM, Smith GL. Vaccinia virus serpins B13R (SPI-2) and B22R (SPI-1) encode Mr 38.5 and 40K, intracellular polypeptides that do not affect virus virulence in a murine intranasal model. Virology. 1995;206(1):136–147. doi: 10.1016/s0042-6822(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 18.Moon KB, Turner PC, Moyer RW. SPI-1-dependent host range of rabbitpox virus and complex formation with cathepsin G is associated with serpin motifs. J Virol. 1999;73(11):8999–9010. doi: 10.1128/jvi.73.11.8999-9010.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray CA, et al. Viral inhibition of inflammation: Cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992;69(4):597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- 20.Kettle S, et al. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1beta-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1beta-induced fever. J Gen Virol. 1997;78(Pt 3):677–685. doi: 10.1099/0022-1317-78-3-677. [DOI] [PubMed] [Google Scholar]
- 21.Turner PC, Moyer RW. An orthopoxvirus serpinlike gene controls the ability of infected cells to fuse. J Virol. 1992;66(4):2076–2085. doi: 10.1128/jvi.66.4.2076-2085.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Law KM, Smith GL. A vaccinia serine protease inhibitor which prevents virus-induced cell fusion. J Gen Virol. 1992;73(Pt 3):549–557. doi: 10.1099/0022-1317-73-3-549. [DOI] [PubMed] [Google Scholar]
- 23.Wagenaar TR, Moss B. Expression of the A56 and K2 proteins is sufficient to inhibit vaccinia virus entry and cell fusion. J Virol. 2009;83(4):1546–1554. doi: 10.1128/JVI.01684-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali AN, Turner PC, Brooks MA, Moyer RW. The SPI-1 gene of rabbitpox virus determines host range and is required for hemorrhagic pock formation. Virology. 1994;202(1):305–314. doi: 10.1006/viro.1994.1347. [DOI] [PubMed] [Google Scholar]
- 25.Brooks MA, Ali AN, Turner PC, Moyer RW. A rabbitpox virus serpin gene controls host range by inhibiting apoptosis in restrictive cells. J Virol. 1995;69(12):7688–7698. doi: 10.1128/jvi.69.12.7688-7698.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shisler JL, Isaacs SN, Moss B. Vaccinia virus serpin-1 deletion mutant exhibits a host range defect characterized by low levels of intermediate and late mRNAs. Virology. 1999;262(2):298–311. doi: 10.1006/viro.1999.9884. [DOI] [PubMed] [Google Scholar]
- 27.Luttge BG, Moyer RW. Suppressors of a host range mutation in the rabbitpox virus serpin SPI-1 map to proteins essential for viral DNA replication. J Virol. 2005;79(14):9168–9179. doi: 10.1128/JVI.79.14.9168-9179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiebe MS, Traktman P. Poxviral B1 kinase overcomes barrier to autointegration factor, a host defense against virus replication. Cell Host Microbe. 2007;1(3):187–197. doi: 10.1016/j.chom.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paran N, De Silva FS, Senkevich TG, Moss B. Cellular DNA ligase I is recruited to cytoplasmic vaccinia virus factories and masks the role of the vaccinia ligase in viral DNA replication. Cell Host Microbe. 2009;6(6):563–569. doi: 10.1016/j.chom.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin YC, et al. Vaccinia virus DNA ligase recruits cellular topoisomerase II to sites of viral replication and assembly. J Virol. 2008;82(12):5922–5932. doi: 10.1128/JVI.02723-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jesse TL, LaChance R, Iademarco MF, Dean DC. Interferon regulatory factor-2 is a transcriptional activator in muscle where It regulates expression of vascular cell adhesion molecule-1. J Cell Biol. 1998;140(5):1265–1276. doi: 10.1083/jcb.140.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaughan PS, et al. Cell cycle regulation of histone H4 gene transcription requires the oncogenic factor IRF-2. J Biol Chem. 1998;273(1):194–199. doi: 10.1074/jbc.273.1.194. [DOI] [PubMed] [Google Scholar]
- 33.Alabert C, et al. Nascent chromatin capture proteomics determines chromatin dynamics during DNA replication and identifies unknown fork components. Nat Cell Biol. 2014;16(3):281–293. doi: 10.1038/ncb2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fine DA, et al. Identification of FAM111A as an SV40 host range restriction and adenovirus helper factor. PLoS Pathog. 2012;8(10):e1002949. doi: 10.1371/journal.ppat.1002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majka J, Burgers PM. The PCNA-RFC families of DNA clamps and clamp loaders. Prog Nucleic Acid Res Mol Biol. 2004;78:227–260. doi: 10.1016/S0079-6603(04)78006-X. [DOI] [PubMed] [Google Scholar]
- 36.Chang HW, Watson JC, Jacobs BL. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc Natl Acad Sci USA. 1992;89(11):4825–4829. doi: 10.1073/pnas.89.11.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buehler E, et al. siRNA off-target effects in genome-wide screens identify signaling pathway members. Sci Rep. 2012;2:428. doi: 10.1038/srep00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harada H, et al. Absence of the type I IFN system in EC cells: Transcriptional activator (IRF-1) and repressor (IRF-2) genes are developmentally regulated. Cell. 1990;63(2):303–312. doi: 10.1016/0092-8674(90)90163-9. [DOI] [PubMed] [Google Scholar]
- 39.Ren G, Cui K, Zhang Z, Zhao K. Division of labor between IRF1 and IRF2 in regulating different stages of transcriptional activation in cellular antiviral activities. Cell Biosci. 2015;5:17. doi: 10.1186/s13578-015-0007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao GN, Jiang DS, Li H. Interferon regulatory factors: At the crossroads of immunity, metabolism, and disease. Biochim Biophys Acta. 2015;1852(2):365–378. doi: 10.1016/j.bbadis.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 41.Harada H, et al. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989;58(4):729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- 42.Hida S, et al. CD8(+) T cell-mediated skin disease in mice lacking IRF-2, the transcriptional attenuator of interferon-alpha/beta signaling. Immunity. 2000;13(5):643–655. doi: 10.1016/s1074-7613(00)00064-9. [DOI] [PubMed] [Google Scholar]
- 43.Matsuyama T, et al. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell. 1993;75(1):83–97. [PubMed] [Google Scholar]
- 44.Grieder FB, Vogel SN. Role of interferon and interferon regulatory factors in early protection against Venezuelan equine encephalitis virus infection. Virology. 1999;257(1):106–118. doi: 10.1006/viro.1999.9662. [DOI] [PubMed] [Google Scholar]
- 45.Litovchick L, et al. Evolutionarily conserved multisubunit RBL2/p130 and E2F4 protein complex represses human cell cycle-dependent genes in quiescence. Mol Cell. 2007;26(4):539–551. doi: 10.1016/j.molcel.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 46.Nikkel SM, et al. Mother-to-daughter transmission of Kenny-Caffey syndrome associated with the recurrent, dominant FAM111A mutation p.Arg569His. Clin Genet. 2014;86(4):394–395. doi: 10.1111/cge.12290. [DOI] [PubMed] [Google Scholar]
- 47.Isojima T, et al. A recurrent de novo FAM111A mutation causes Kenny-Caffey syndrome type 2. J Bone Miner Res. 2014;29(4):992–998. doi: 10.1002/jbmr.2091. [DOI] [PubMed] [Google Scholar]
- 48.Unger S, et al. FAM111A mutations result in hypoparathyroidism and impaired skeletal development. Am J Hum Genet. 2013;92(6):990–995. doi: 10.1016/j.ajhg.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pipas JM. Mutations near the carboxyl terminus of the simian virus 40 large tumor antigen alter viral host range. J Virol. 1985;54(2):569–575. doi: 10.1128/jvi.54.2.569-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khalili K, et al. Carboxyl-terminal mutants of the large tumor antigen of simian virus 40: A role for the early protein late in the lytic cycle. Proc Natl Acad Sci USA. 1988;85(2):354–358. doi: 10.1073/pnas.85.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stacy T, Chamberlain M, Cole CN. Simian virus 40 host range/helper function mutations cause multiple defects in viral late gene expression. J Virol. 1989;63(12):5208–5215. doi: 10.1128/jvi.63.12.5208-5215.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marine S, Bahl A, Ferrer M, Buehler E. Common seed analysis to identify off-target effects in siRNA screens. J Biomol Screen. 2012;17(3):370–378. doi: 10.1177/1087057111427348. [DOI] [PubMed] [Google Scholar]
- 53.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.