Significance
As obligate photosynthetic and sessile organisms, plants are particularly exposed to the damaging effects of excess light and UV wavelengths, which can impact genome integrity by inducing DNA sequence alterations. As a response, plants have evolved efficient genome surveillance processes, some of which appear to also overlap with mechanisms of gene expression control. Our study extends this emerging notion by uncovering complex interconnections linking DNA repair and RNA silencing in Arabidopsis, illustrating the ever-expanding array of biological functions mediated by silencing small RNAs in plants.
Keywords: DNA repair, small RNA, DNA photolesions, Arabidopsis
Abstract
As photosynthetic organisms, plants need to prevent irreversible UV-induced DNA lesions. Through an unbiased, genome-wide approach, we have uncovered a previously unrecognized interplay between Global Genome Repair and small interfering RNAs (siRNAs) in the recognition of DNA photoproducts, prevalently in intergenic regions. Genetic and biochemical approaches indicate that, upon UV irradiation, the DNA DAMAGE-BINDING PROTEIN 2 (DDB2) and ARGONAUTE 1 (AGO1) of Arabidopsis thaliana form a chromatin-bound complex together with 21-nt siRNAs, which likely facilitates recognition of DNA damages in an RNA/DNA complementary strand-specific manner. The biogenesis of photoproduct-associated siRNAs involves the noncanonical, concerted action of RNA POLYMERASE IV, RNA-DEPENDENT RNA POLYMERASE-2, and DICER-LIKE-4. Furthermore, the chromatin association/dissociation of the DDB2-AGO1 complex is under the control of siRNA abundance and DNA damage signaling. These findings reveal unexpected nuclear functions for DCL4 and AGO1, and shed light on the interplay between small RNAs and DNA repair recognition factors at damaged sites.
Ultraviolets (UVs), as sunlight components, produce DNA damage potentially detrimental to cell/genome integrity. The major forms of UV-induced DNA lesions are cyclobutane pyrimidines dimers (CPDs) and (6-4)-photoproducts (6-4 PP) (1), which promote DNA helix distortion altering replication and transcription. Being photosynthetic and sessile, plants need to prevent UV-induced irreversible DNA damage at their growing points, potentially also transmissible to their progeny. UV-induced DNA lesions are preferentially repaired by direct repair (DR), via photolyases, converting CPDs and 6-4 PP to monomers using UV-A/blue light (2). Nucleotide excision repair (NER) also promotes repair of UV-induced lesions in a light-independent manner via two subpathways, global genome repair (GGR) and transcription-coupled repair (TCR), processing bulky DNA lesions throughout the genome or along actively transcribed DNA strands, respectively (3). By binding both photolesions and “compound” lesions, DNA DAMAGE-BINDING PROTEIN 2 (DDB2) is a key factor for damage recognition during GGR (4) and, accordingly, human DDB2 (XPE: XERODERMA PIGMENTOSUM complementation group E) mutations cause sunlight hypersensitivity and skin cancer predisposition (5).
Maintenance of genome integrity is also a major function of RNA silencing, a pan-eukaryotic process enabling sequence-specific gene expression control, with crucial roles in defense against transposable elements (TEs) and viruses, as well as developmental patterning/growth. Plant RNA silencing operates at the RNA level via mRNA cleavage or translational repression [post-transcriptional gene silencing (PTGS)], whereas RNA silencing at the DNA level involves DNA/histone methylation and heterochromatin formation often leading to transcriptional gene silencing (TGS) (6). Plant RNA silencing is invariably triggered by double-stranded RNA (dsRNA) processed into 21- to 24-nt small RNA (sRNA) duplexes by III-like DICER enzymes, of which Arabidopsis encodes four DICER-LIKE (DCL) paralogs with specialized sRNA products and biological functions (6). DCL1 produces mainly micro (mi)RNAs from noncoding, imperfect stem-loop RNAs, whereas populations of 21-, 22-, and 24-nt small-interfering (si)RNAs are synthesized from long, perfectly/near-perfectly base-paired dsRNA via DCL4, DCL2, and DCL3 activity, respectively. The miRNAs loaded into mostly ARGONAUTE 1 (AGO1) guide PTGS of complementary mRNAs (7, 8), whereas DCL3-dependent 24-nt siRNAs act in cis upon loading into mostly AGO4, to mediate RNA-directed DNA methylation (RdDM) and chromatin compaction at TE-enriched and DNA repeat-enriched loci (9). The concerted action of the plant-specific RNA POLYMERASE (POL) IV and RNA-DEPENDENT RNA POLYMERASE 2 (RDR2) provides dsRNA substrates to DCL3 for synthesis of nearly all 24-nt siRNAs (10), whereas the POL IV-related RNA POL V targets de novo cytosine methylation via specific DNA methyltransferases (11–14). Alternative RdDM mechanisms also rely on RNA POL II, RDR6, and 21- or 24-nt siRNAs (15–18).
Little is known about the possible interconnection between RNA silencing and DNA repair pathways. DNA damage alters expression of specific miRNAs (19, 20) whose targets encode proteins involved in cell cycle checkpoint, DNA repair, and signal transduction. Upon double-strand breaks (DSBs) induction, miRNA-unrelated 21-nt sRNAs coined “diRNAs” are also produced from damaged sites’ flanking regions and recruited by AGO2 to enable DSB repair in Arabidopsis and human cells (21). In mouse, Drosophila, and zebra fish, the sRNA-processing enzymes DICER and DROSHA activate the DNA damage response upon exposure to genotoxic stress (22, 23). Although these studies strongly suggest interconnections between RNA silencing and DNA repair, they have relied exclusively on studies of transgenic reporter loci (21, 22) or linearized plasmids (23) uninformative about the natural mechanisms of sRNA-mediated repair at a genome-wide scale, and whose artificial nature might have influenced the processes involved. Indeed, recent findings show that, in Drosophila, DSB-derived siRNAs may have roles in RNA quality control rather than in DNA repair per se (24).
Combining genome-wide mapping of CPDs, total sRNAs sequencing, genetics, and biochemistry, we document, here, a previously unknown role for sRNAs in GGR by showing how AGO1 forms a chromatin-bound complex with the GGR factor DDB2 to initiate repair predominantly at intergenic UV-damaged loci. Abundance of RNA POL IV-, RDR2-, and DCL4-dependent 21-nt siRNAs is enhanced at these UV-damaged loci, and they are loaded onto the DDB2-AGO1 complex, which, we propose, they guide in a sequence-specific RNA/DNA complementary strand manner to facilitate DNA damage recognition.
Results
Deficiency in RNA Silencing Pathways Leads to UV Hypersensitivity.
To assess the influence of sRNAs on DNA repair efficiency, the UV-C sensitivity of RdDM- and PTGS-defective Arabidopsis was measured by the root growth inhibition assay (25, 26); these assays were conducted in nrpd1, rdr2, dcl3, and ago4 plants impaired in RdDM, and in rdr6, dcl4, ago1, and ago2 plants impaired in PTGS, respectively. Root growth was significantly reduced in irradiated rdr6, dcl4, nrpd1, rdr2, ago2, and ago4 plants compared with WT plants, unlike in DCL3- and AGO1-deficient plants (Fig. 1 A and B, Upper). We were cautious in interpreting the apparent UV-C insensitivity of ago1-27, which, unlike the other mutations tested, is hypomorphic (27). Moreover, AGO1 is the prevalent effector of miRNAs controlling, notably, root development, which, when perturbed, might confound light-dependent DNA repair deficiency. UV-C sensitivity assayed upon a dark recovery period indeed showed that ago1-27 is significantly more sensitive to UV-C than WT plants (Fig. 1C), suggesting that AGO1 deficiency causes a more specific impairment in the dark repair of UV-induced DNA lesions. Complementary to the root growth inhibition assay (25, 26), the CPD removal assay was conducted under normal light conditions. It also showed that all RdDM and PTGS mutants tested, including ago1-27 but still excluding dcl3, exhibit significantly reduced/delayed CPD removal capacities (Fig. 1 A and B, Lower). Therefore, of the PTGS and RdDM mutants tested, all but dcl3 are defective in repair of UV-induced DNA lesions, suggesting that sRNAs participate in this process.
Fig. 1.
UV sensitivity of RdDM and PTGS loss of function Arabidopsis plants. (A) (Top) Root growth assay. Seven-day-old WT, RdDM mutant plants (nrpd1, rdr2, dcl3, and ago4) were exposed to UV-C. Root growth was calculated relative to the corresponding untreated plants (±SD). Eight plants per replicate were used, and three independent biological replicates were performed; ddb2-3 was used as control as DNA repair deficient plants. (Bottom) CPDs removal assay. Histogram represents the amounts of CPDs 1 h after UV-C treatment (± SD). Intensity of each dot was quantified and normalized to that of CPDs at time 0 to calculate the remaining CPDs content after 1 h. Twenty plants per replicate were used, and experiments were duplicated; ddb2-3 was used as control as DNA repair deficient plants; t test *P < 0.01; **P < 0.05; ns, nonsignificant. (B) Same as A for PTGS-deficient plants (rdr6, dcl4, ago1, and ago2). (C) Genetic interactions between ddb2 and ago1. Seven-day-old single (ago1 and ddb2) and double (ddb2-ago1) mutant plants were exposed to UV-C and grown for 24 h either under light or in the dark. Because ddb2-2 is in the No ecotype, the control for double mutant plants is No/Col. Eight plants per replicate were used, and three independent biological replicates were performed; t test *P < 0.01; ns, nonsignificant compared with the corresponding single mutants for double mutant. (D) Genetic interactions between GGR and RdDM (nrpd1, rdr2, dcl3, ago4, and ddb2) and double mutant plants (ddb2-nrpd1, ddb2-rdr2, ddb2-dcl3, and ddb2-ago4). Because ddb2-2 is in the No ecotype, the control for double mutant plants is No/Col; t test *P < 0.01; **P < 0.05 compared with the corresponding WT plants. Eight plants per replicate were used, and three independent biological replicates were performed; ns, nonsignificant compared with the corresponding single mutants for double mutant. (E) Same as D for GGR and PTGS. Seven-day-old single WT (rdr6, dcl4, ago1, ago2, and ddb2) and double mutant plants (ddb2-rdr6, ddb2-dcl4, ddb2-ago1, and ddb2-ago2) were exposed to UV-C. Eight plants per replicate were used, and three independent biological replicates were performed; t test *P < 0.01; ns, nonsignificant compared with the corresponding single mutants for double mutants.
PTGS and RdDM Factors Act in the GGR Pathway.
To assess which UV-induced DNA lesion repair pathway cooperated with PTGS and RdDM, we conducted genetic interaction studies. Plants defective in PHOTOLYASE I (PHRI, phrI), acting in the DR pathway, COCKAYNE SYNDROME complementation group A (CSA, csa-a1), acting in the TCR pathway, or DDB2 (ddb2-2), acting in the GGR pathway, were crossed to RdDM mutants (collectively referred to here as rddm: nrpd1, rdr2, dcl3, ago4) and PTGS mutants (collectively referred to here as ptgs: rdr6, dcl4, ago1, ago2). Double-homozygous mutants were characterized for UV-C sensitivity using leaf growth and photobleaching as the readout for DR (26), or root growth inhibition as the readout for TCR and GGR (25). None of the ddb2-rddm double mutants exhibited significant additive effects on root growth inhibition compared with the respective single mutants (Fig. 1D), and this was also observed for all ddb2-ptgs double mutants (Fig. 1 C and E), except for ddb2-rdr6 and ddb2-ago2 (Fig. 1E). Epistasis thus strongly suggests that most RdDM and PTGS factors tested, RDR6 and AGO2 excepted, act in the GGR pathway; phrI ago1 and phrI ago4 double mutants, by contrast, exhibited increased UV sensitivity compared with the corresponding single mutants (Fig. S1 A and B); double mutants with a csa background showed a similar additive/synergistic phenotype (Fig. S1C), suggesting, therefore, that most RdDM and PTGS factors tested act in pathways distinct from DR and TCR. These results thus consolidate the idea that RNA silencing contributes to GGR during UV-induced DNA damage repair.
Fig. S1.
Genetic interactions of RdDM and PTGS with DR and TCR. (A) Genetic interactions between phrI, ago1, and ago4. Ten-day-old WT, single (ago1, ago4, and phrI) and double mutant plants (phrI-ago1 and phrI-ago4) were irradiated with UV-C (1,500 J/m2, 3,000 J/m2) three times in a row every 2 d. (Left) Histogram representing the percentage of bleached plants. At least 20 plants were used per replicate, and this experiment was duplicated; t test *P < 0.01 compared with single mutant plants; ns, nonsignificant. (Right) Histogram representing the average number of leaves (±SD); t test *P < 0.01 compared with untreated plants; ns, nonsignificant. (B) Pictures showing the phenotype of Col, phrI, ago1, ago4, phrI-ago1, and phrI-ago4 plants 1 wk after the last UV-C exposure (3,000 J/m2; scale bar, 2 cm). (C) Genetic interactions between csa, nrpd1, rdr2, ago1, and ago4. Seven-day-old WT, single (csa, nrpd1, rdr2, ago1, and ago4) and double mutant plants (csa-nrpd1, csa-rdr2, csa-ago1, and csa-ago4) were exposed to 600 J/m2 of UV-C and grown for 24 h either under light or in the dark. Eight plants per replicate were used, and three independent biological replicates were performed; t test *P < 0.01; ns, nonsignificant.
Genome-Wide Mapping of CPDs Identifies Hot Spots of UV-Induced DNA Damage.
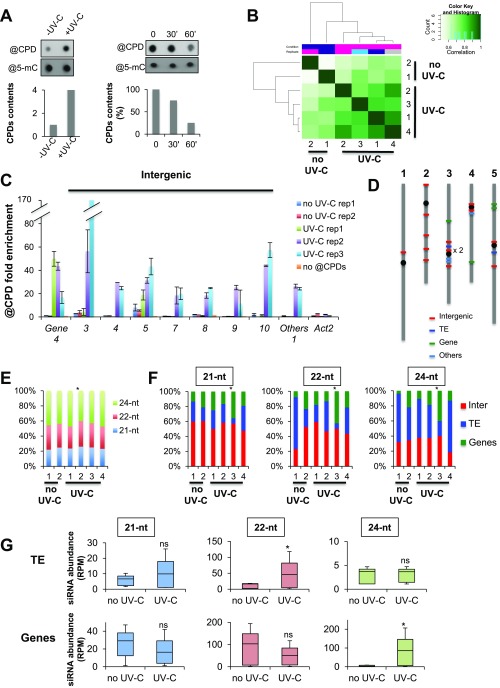
Given the impairments of RdDM- and PTGS-deficient plants in repair of UV-induced DNA lesions, and their epistatic interaction with GGR pathway mutants, endogenous sRNAs were likely to play some role in DNA repair. To comprehensibly address this issue at a genome-wide level, we developed a large-scale genomic approach to map the UV-C-induced DNA photolesions via immunoprecipitation (IP) coupled to DNA sequencing, referred to as Immunoprecipitation of UV-induced DNA (IPOUD) lesions (Fig. 2A). The DNA of untreated and UV-C-treated plants was immunoprecipitated with a monoclonal antibody raised against CPDs at time points chosen according to the repair kinetics under our experimental conditions (Fig. S2A) and was subsequently processed for deep sequencing. Unique sequences were mapped onto the reference, nuclear Arabidopsis genome, and CPD-enriched sequences (IP vs. input) were determined (Materials and Methods for details). For statistical analysis, two biological replicates of untreated plants and four biological replicates of UV-C-treated plants were subjected to IPOUD (Fig. 2A).
Fig. 2.
CPD mapping and overlap with canonical, 21-, 22-, and 24-nt siRNAs. (A) Design of the experiment. Three-week-old plants (n = 12/replicate), grown in soil, were used in two independent biological replicates for untreated plants and four independent biological replicates for UV-C-treated plants. The sRNA and DNA were prepared from the pool of tissue for each replicate and subsequently used for sRNA sequencing and IPOUD + DNA sequencing. CPDs are displayed in yellow triangles. (B) Visualization of genome-wide CPDs distribution (black bar) on Arabidopsis chromosomes using Circos representation. The outermost circle displays the five Arabidopsis chromosomes. The inner circles represent the genome-wide CPDs distribution for each replicate of untreated and UV-C-treated samples (rainbow colors). The inner circle represents the siRNAs (21, 22, and 24 nt) overlapping CPD-damaged loci. The height of the histogram bins indicates siRNA abundance. (C) Histogram representing the origins (intergenic, TE, and protein-coding genes) of CPD-containing loci for each replicate of untreated (no UV-C) and UV-C-treated samples. The Arabidopsis thaliana genome (At) was used as a reference. Chi2 test: *P < 0.05. (D) Independent confirmation of hot spots using IPOUD-qPCR. Histogram (±SD) representing the enrichment of CPDs (IP/input) at hot spots overlapping with intergenic regions, protein-coding genes, and other types of regions excluding TEs (Others). Two biological replicates of untreated and three biological replicates of UV-C-treated in vitro-grown plants were used. Numbers indicate the hot spot sequence name. Actin 2 was used as negative control. (E) Pie chart representing the origins of hot spots of CPDs containing loci. (F) Histogram representing the origins of CPD containing loci mapping with 21-, 22-, and 24-nt siRNAs for each replicate of untreated (no UV-C) and UV-C-treated samples. Chi2 test: *P < 0.05 compared with untreated samples. (G) Box plots representing the abundance of 21-, 22-, and 24-nt siRNAs mapping to intergenic regions enriched in CPDs. Mann−Whitney U test *P < 0.05, ns, nonsignificant.
Fig. S2.
CPDs mapping and siRNAs. (A) (Left) Dot blot detecting CPDs content in untreated (−UV-C) and UV-C-treated (+UV-C) plants grown in soil (see experimental procedures for details); @5-mC were used as loading control. Histogram represents the amounts of CPDs (±SD). (Right) Dot blot detecting CPD content in a time course upon UV-C treatment; @5-mC were used as loading control. Histogram represents the amounts of CPDs (±SD) immediately after UV-C treatment (time point 0), and at 30 and 60 min upon irradiation. (B) Heat maps of CPDs enrichment all over the genome. Biological replicates are clustered. (C) Histogram representing the enrichment (IP/input ± SD) of CPDs at hot spots overlapping with intergenic regions, protein-coding genes, and other types of regions (others) using IPOUD-qPCR. Two biological replicates of untreated and three biological replicates of UV-C-treated in vitro-grown plants were used. Numbers indicate the hot spot sequence name. Actin 2 was used as negative control. (D) Schematic representation of hot spot locations on the five Arabidopsis chromosomes. (E) Repartition of 21-, 22-, and 24-nt siRNA at CPD-damaged sites. Chi2 test *P < 0.01. (F) Repartition of intergenic, TE, and genic regions enriched in CPDs overlapping with 21-, 22-, and 24-nt siRNA. Chi2 test: *P < 0.01 compared with untreated plants. (G) Box plots representing the abundance of 21-, 22-, and 24-nt siRNAs mapping to TE and genic regions enriched in CPDs. Mann−Whitney U test *P < 0.05; ns, nonsignificant.
Using stringent statistical parameters (P < 10−6 and q < 10−2), the multiple IPOUD experiments allowed recovery of >2,000 high-confidence CPD-enriched sequences (228 up to 677 loci per sample analyzed) distributed genome-wide (Fig. 2B). The average length of these CPD-enriched regions ranges from 130 bp to 187 bp for all samples analyzed (Table S1). Untreated plants also exhibited CPD-damaged loci (Fig. 2B), which was expected because Arabidopsis growth and development require the full spectrum of sunlight containing a proportion of UV-B as possible source of CPDs. CPD content was confirmed by dot blot analyses conducted under our growth conditions (Materials and Methods), revealing that a basal CPD level in untreated plants was increased upon UV-C exposure (Fig. S2A). Accordingly, untreated and treated samples clustered separately in correlation analyses (Fig. S2B). Detailed CPD analysis among samples showed that UV-C treatment induces damages at mostly random genomic positions (Fig. 2B) despite slight variations between samples (Fig. 2C). Interestingly, 23 loci were CPD-enriched (i.e., statistically higher than expected by chance according to Poisson Distribution) in common in at least three out of six independent IPOUD experiments, suggesting that these loci are generally more prone to UV damage and/or display reduced DNA repair abilities (Dataset S1). Using an additional set of independent biological replicates (two untreated and three UV-C-treated batches) further confirmed that IPOUD allows identification of CPD-containing loci and that several loci reproducibly exhibit a higher chance to contain CPDs (Fig. 2D and Fig. S2C). Interestingly, among these 23 loci, 60% were located in intergenic regions (Fig. 2E and Fig. S2D) and are referred to, hereinafter, as “hot spots” of UV-induced DNA damage, which, we anticipated, could be possibly used as endogenous reporter loci to decipher the possible connections between RNA silencing and GGR.
Table S1.
NGS summary
| Sample | Type of experiment | Total number of reads | Reads with inserts, % | Size of mapped DNA/RNA |
| Input | IPOUD | 9,928,905 | 99.90 | nd |
| WT no UV rep 1 | IPOUD | 10,071,017 | 96.13 | 136 bp ± 1.38* |
| WT no UV rep 2 | IPOUD | 10,529,991 | 98.37 | 130.8 bp ± 1.59* |
| WT UV rep 1 | IPOUD | 11,518,309 | 99.66 | 187.2 bp ± 12* |
| WT UV rep 2 | IPOUD | 11,901,736 | 98.89 | 150.7 bp ± 2.97* |
| WT UV rep 3 | IPOUD | 11,026,009 | 98.37 | 153.6 bp ± 1.55* |
| WT UV rep 4 | IPOUD | 13,338,184 | 99.59 | 138.4 bp ± 2.21* |
| WT no UV rep 1 | Small RNA seq | 12,145,652 | 99.71 | 15 nt to 45 nt |
| WT no UV rep 2 | Small RNA seq | 11,705,035 | 99.83 | 15 nt to 45 nt |
| WT UV rep 1 | Small RNA seq | 15,395,135 | 99.80 | 15 nt to 45 nt |
| WT UV rep 2 | Small RNA seq | 11,545,623 | 99.84 | 15 nt to 45 nt |
| WT UV rep 3 | Small RNA seq | 14,805,788 | 99.79 | 15 nt to 45 nt |
| WT UV rep 4 | Small RNA seq | 12,812,206 | 99.51 | 15 nt to 45 nt |
| nrpd1 no UV rep 1 | Small RNA seq | 11,731,877 | 99.34 | 15 nt to 45 nt |
| nrpd1 no UV rep 2 | Small RNA seq | 15,298,205 | 99.80 | 15 nt to 45 nt |
| nrpd1 UV rep 1 | Small RNA seq | 14,239,678 | 99.83 | 15 nt to 45 nt |
| nrpd1 UV rep 2 | Small RNA seq | 15,605,926 | 99.68 | 15 nt to 45 nt |
| Input no UV | Small RNA seq | 30,401,033 | 99.92 | 15 nt to 45 nt |
| IP DDB2 no UV | RIP and small RNA seq | 25,591,663 | 99.34 | 15 nt to 45 nt |
| IP AGO1 no UV | RIP and small RNA seq | 22,389,349 | 96.52 | 15 nt to 45 nt |
| Input UV | Small RNA seq | 22,932,956 | 99.67 | 15 nt to 45 nt |
| IP DDB2 UV | RIP and small RNA seq | 23,363,408 | 99.50 | 15 nt to 45 nt |
| IP AGO1 UV | RIP and small RNA seq | 31,510,294 | 99.92 | 15 nt to 45nt |
Average size in base pair ± SD.
sRNAs and CPDs.
To determine the potential overlap between sRNA populations and CPDs, we deep-sequenced the 15- to 45-nt fraction of total RNA extracted from the same tissues as those used for IPOUD (Fig. 2A). Genomic mapping of sRNA at the CPD-enriched regions (Materials and Methods for details) showed that ∼30% of the CPD-containing loci and ∼80% of the hot spots overlapped with 21-, 22-, and 24-nt siRNAs. The distribution of the genomic origins of siRNA-associated CPD-enriched loci and of the 21-, 22-, and 24-nt siRNAs varied only slightly between untreated and treated samples (Fig. 2F and Fig. S2E). The 21-, 22-, and 24-nt siRNAs predominantly overlapped with damaged DNA of TE-enriched and intergenic regions (Fig. S2F). This high proportion (>40% in our experiments vs. 10% in the untreated Arabidopsis genome) of intergenic regions overlapping with 21-nt siRNAs suggested their possible role in DNA repair (Fig. 2F), especially because GGR predominantly repairs untranscribed/poorly transcribed DNA such as that of intergenic regions (3). Accordingly, 21-nt siRNA levels overlapping with damaged intergenic regions increased in UV-C-treated, compared with untreated, plants (Fig. 2G). By contrast, 22-nt siRNA levels remained unchanged, and 24-nt siRNA levels decreased (Fig. 2G). In TE-enriched regions, 22-nt siRNA levels increased upon UV-C irradiation, whereas 21- and 24-nt siRNA levels remained unchanged (Fig. S2G). In genic-enriched regions, only 24-nt siRNA levels increased upon UV-C irradiation (Fig. S2G). Importantly, basal 21-, 22-, and 24-nt siRNAs levels were detectable in all these CPD-enriched regions before UV-C irradiation, suggesting that the treatment induces de novo siRNA biogenesis and/or stabilizes preexisting precursors or mature siRNAs (Fig. 2G and Fig. S2G), which we refer to as “uviRNAs,” for UV-Induced siRNAs, hereinafter. The results of these genome-wide analyses are thus consistent with the genetic interactions between GGR and RNA silencing (Fig. 1), leading us to speculate that uviRNAs may facilitate GGR, particularly in intergenic regions.
The 21-nt uviRNAs Overlap with Intergenic Loci Enriched in Di-Pyrimidines.
Given the high proportion of intergenic regions overlapping with uviRNAs (Fig. 2 F and G) and that UVs target di-pyrimidines (TT, CC, TC, and CT) to form CPDs, we calculated the di-pyrimidines frequencies of intergenic damaged regions, in a DNA strand-specific manner. CT and TC frequencies were statistically higher at siRNA-containing damaged loci compared with the whole set of Arabidopsis intergenic regions overlapping with siRNAs, and this was more pronounced for the minus DNA strand (Fig. 3A). We also identified a positive correlation between CT and TC frequencies for both DNA strands (Fig. S3A). By contrast, no significant correlation was found for CC and TT di-pyrimidines (Fig. S3A). For TEs and genic regions, no significant correlations between any specific siRNA species, photodamages, and di-pyrimidines frequencies were found.
Fig. 3.
Di-pyrimidines, CPDs, and siRNAs strand specificity. (A) Box plots representing the di-pyrimidines frequencies (CC, TT, TC, and CT) for each DNA strand (+ and – strand) in Arabidopsis intergenic regions (At) and in CPD-damaged intergenic regions (Inter) identified in IPOUD experiments. Mann−Whitney U test *P < 0.05; ns, nonsignificant. (B) Box plots representing the abundance of sense and antisense 21-nt uviRNAs at CPD-damaged intergenic regions. *P < 0.05 calculated according to Wilcoxon matched-pairs signed rank test; ns, nonsignificant. (C) Graphical representation of consensus ribonucleotide sequences of sense (RNA+) and antisense (RNA-) 21-nt uviRNAs mapping at intergenic CPD-damaged regions.
Fig. S3.
Di-pyrimidines frequencies and uviRNAs. (A) Heat map representing the coefficient of correlation between each di-pyrimidines (CC, TT, TC, and CT) at CPD-damaged intergenic regions in a DNA strand-specific manner (+ and – strand). (B) (Left) Box plots representing the abundance of sense (RNA+) and antisense (RNA−) 22-nt siRNAs at CPD-damaged intergenic regions; t test: nonsignificant (ns). (Right) Graphical representation (seq logo) of consensus ribonucleotide sequences of sense (RNA+) and antisense (RNA−) 22-nt siRNAs mapping at intergenic CPD-damaged regions. (C) Same as B for 24-nt siRNAs; t test, ns, nonsignificant.
Next, we measured the levels of sense and antisense uviRNAs overlapping these particular intergenic regions in treated vs. untreated plants. Sense 21-nt uviRNA levels, unlike those of antisense species, were statistically higher in UV-treated, compared with untreated, plants (Fig. 3B), consistent with the high CT and TC frequencies on the minus DNA strand of damaged intergenic loci. No significant difference was observed for the 22- and 24-nt siRNA species (Fig. S3 B and C). Strikingly, all size classes of sense uviRNAs matching intergenic, damaged loci were strongly enriched in A and G (Fig. 3C and Fig. S3 B and C) as expected from complementary to all di-pyrimidine forms (TT, CC, TC and CT), and supporting the observed minus DNA strand bias. The ribonucleotide sequences of 21-, 22-, and 24-nt uviRNAs were also strikingly similar, suggesting their common origins from the same set of dsRNA precursors (Fig. 3C and Fig. S3 B and C, Right). Together, these results suggest that 21-nt uviRNAs may contribute to UV-induced DNA repair at intergenic loci, possibly in a cDNA strand-specific manner.
The 21-nt uviRNA Biogenesis Requires the Action of POL IV, RDR2, and DCL4.
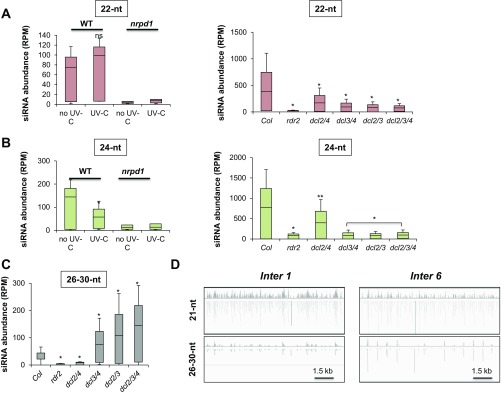
To identify which factors enable intergenic 21-nt uviRNA biogenesis, we first analyzed sRNA sequencing libraries from nrpd1 mutants treated in our experiments with or without UV-C (as in Fig. 2A). In a complementary analysis, we used publicly available libraries for untreated WT, rdr2, dcl2/4, dcl3/4, dcl2/3, or dcl2/3/4 mutant plants (28) to determine the genetic requirements for basal intergenic 21-nt uviRNA biogenesis. The basal albeit measurable 21-nt uviRNA levels found at intergenic loci under normal growth were dramatically decreased in nrpd1 loss-of-function plants (Fig. 4A), as were those of the 22- and 24-nt species (Fig. S4 A and B), underscoring, again, that these siRNAs likely originate from the same precursors. Basal 21-nt uviRNA levels also decreased in rdr2, dcl2/4, dcl3/4, or dcl2/3/4, but not in dcl2/3 mutants (Fig. 4B); the 22- and 24-nt species were, again, similarly affected (Fig. S4 A and B). Collectively, these results suggest that intergenic 21-nt uviRNA basal production occurs via a noncanonical RNA POL IV-, RDR2-, and DCL4-dependent RNA silencing pathway.
Fig. 4.
Intergenic 21-nt uviRNAs biogenesis. (A) Box plots representing the abundance of 21-nt uviRNAs at intergenic CPD-damaged regions in WT plants and in RNA POL IV-deficient plants (nrpd1) ± UV-C. *P < 0.01 calculated according to Wilcoxon matched-pairs signed rank test. (B) Box plots representing the abundance of 21-nt uviRNAs at intergenic CPD-damaged regions in WT plants (Col) and in rdr2, dcl2/4, dcl3/4, dcl2/3, and dcl2/3/4 plants. *P < 0.01 calculated according to Wilcoxon matched-pairs signed rank test; ns, nonsignificant. (C) Genetic interactions between RdDM and PTGS loss of function. Seven-day-old WT, single (ndpr1, rdr2, rdr6, dcl3, and dcl4) and double mutant plants (ndpr1-rdr6, ndpr1-dcl4, rdr2-dcl4, and rdr6-dcl3) were exposed to UV-C; t test *P < 0.01; **P < 0.05; ns, nonsignificant. (D) Genetic interactions between DCLs loss of function. Seven-day-old WT, single (dcl2, dcl3, and dcl4), double (dcl2/3, dcl2/4, and dcl3/4), and triple mutant plants (dcl2/3/4) were exposed to UV-C; t test *P < 0.01; ns, nonsignificant. (E) Box plots representing the abundance of 26- to 30-nt RNAs at intergenic CPD-damaged regions in WT plants and in RNA POL IV-deficient plants (nrpd1) ± UV-C. *P < 0.01 calculated according to Wilcoxon matched-pairs signed rank test. (F) Same as E for 26-nt RNAs. Shown is graphical representation of consensus ribonucleotide sequences of 26-nt RNAs mapping at intergenic CPD-damaged regions. *P = 0.0164 calculated according to Wilcoxon matched-pairs signed rank test. (G) Same as F for 27-nt RNAs **P < 0.01.
Fig. S4.
The siRNA biogenesis. (A) Box plots representing the abundance of 22-nt siRNA at intergenic CPD-damaged regions in WT plants and in RNA POL IV-deficient plants (nrpd1) ± UV-C and in WT plants (Col), rdr2, dcl2/4, dcl3/4, dcl2/3, and dcl2/3/4 plants. *P < 0.01 calculated according to Wilcoxon matched-pairs signed rank test. (B) Same as A for 24-nt siRNA. (C) Box plots representing the abundance of 26- to 30-nt siRNAs at intergenic CPD-damaged regions in WT plants (Col) and in rdr2, dcl2/4, dcl3/4, dcl2/3, and dcl2/3/4 plants. *P < 0.01 calculated according to Wilcoxon matched-pairs signed rank test. (D) Browser view of (Top) 21-nt siRNA and (Bottom) 26- to 30-nt RNA abundances at two intergenic hot spots loci.
To further ascertain the above results, UV-C sensitivities of single (nrpd1, rdr2, rdr6, dcl3, and dcl4) and double mutants (nrpd1-dcl4, nrpd1-rdr6, rdr2-dcl4, and rdr6-dcl3) were monitored via root growth inhibition assays. Although nrpd1, rdr2, and dcl4 exhibited epistatic genetic interactions (Fig. 4C), nrpd1, rdr6, and dcl3 displayed enhanced UV sensitivity, suggesting that NRPD1, RDR6, and DCL3 act in parallel pathways (Fig. 4C). Consistent with these observations, the double dcl3/4 and triple dcl2/3/4 mutants also had increased UV sensitivity in contrast to dcl2/3 and dcl2/4 double mutants, compared with corresponding single mutants (Fig. 4D). These data support the idea that 21-nt uviRNA biogenesis entails the noncanonical, concerted action of RNA POL IV, RDR2, and DCL4.
POL IV-dependent 26- to 40-nt RNAs serve as RDR2 templates to produce the dsRNA precursors of 24-nt siRNAs in the RdDM pathway [referred as P4R2 precursors (28, 29)]. We thus tested if such precursors were enriched upon UV exposure. Analyses of our deep-sequencing libraries showed that accumulation of 26- to-30-nt RNAs overlapping with 21-nt uviRNA-enriched loci strongly increased in UV-treated WT plants and, conversely, dramatically decreased in nrpd1 mutants (Fig. 4E and Fig. S4 C and D). A refined analysis showed that the levels of RNA POL IV-dependent 26- and 27-nt RNAs were specifically increased upon UV irradiation (Fig. 4 F and G). The 5′-terminal nucleotide of 26- and 27-nt RNAs was statistically more frequently an A or a G, a described feature of P4R2 precursors (28, 29) (Fig. 4G). These data suggest that RNA POL IV-dependent transcripts produced at intergenic regions are converted into dsRNA and 21-nt uviRNAs by RDR2 and DCL4, respectively, possibly to facilitate GGR-mediated repair of UV-induced DNA lesions in intergenic regions.
GGR Factor DDB2 Forms a Complex with AGO1.
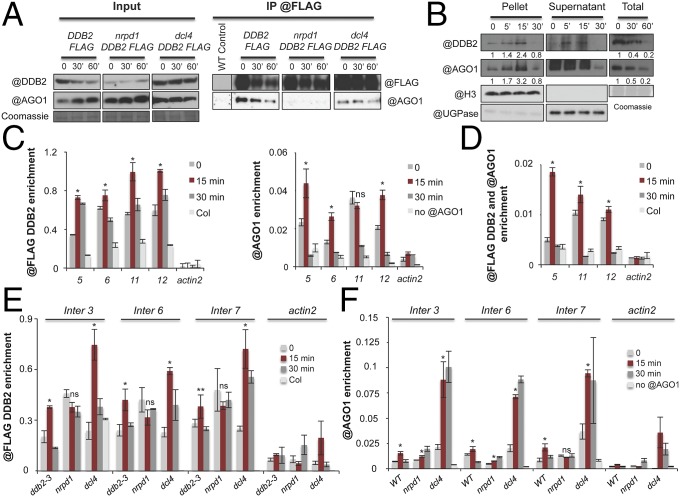
AGO1 is the major effector protein of endogenous 21-nt si/miRNAs in Arabidopsis. Because DDB2 is a key recognition factor of UV-induced DNA lesions during GGR, we thus tested if DDB2 could associate with AGOs and, more specifically, AGO1, using transgenic ddb2-3 mutant plants expressing a functional DDB2-FLAG version (Fig. S5A). DDB2-FLAG effectively coimmunoprecipitated with endogenous AGO1 in plant whole-cell extracts prepared before and after UV-C exposure (Fig. 5A). By contrast, a FLAG-tagged, DNA-binding-deficient point-mutant allele of DDB2 (DDB2K314E-FLAG) (30, 31), which failed to rescue the ddb2-3 UV hypersensitivity (Fig. S5A), coimmunoprecipitated with AGO1 significantly less than the WT DDB2-FLAG allele (Fig. S5B). Therefore, DDB2-FLAG likely assembles with AGO1 into a protein complex whose formation/stability requires DNA binding mediated by DDB2. Given the RNA POL IV and DCL4 dependency of 21-nt uviRNAs biogenesis, we tested if the corresponding loss-of-function mutants altered formation/stability of the DDB2-AGO1 complex. Indeed, AGO1-DDB2-FLAG coimmunoprecipitation was, respectively, strongly and moderately reduced in nrpd1 and dcl4 mutants (Fig. 5A); it was also strongly impaired compared with WT plants in mutants deficient for the ataxia telangiectasia and Rad3-related protein (ATR) kinase, which plays a crucial role in transmitting DNA damage signals to downstream repair factors (Fig. S5C). We conclude that formation/stability of a DDB2-FLAG-AGO1 complex strongly relies on DDB2-mediated DNA binding, RNA POL IV transcription, ATR-mediated signaling of DNA damage, and, to a lesser extent, DCL4 activity.
Fig. S5.
DDB2−AGO1 complex. (A) Root growth assay. Seven-day-old WT (Col), ddb2-3, ddb2-3/DDB2-FLAG, and ddb2-3/DDB2K314E-FLAG expressing plants were exposed to 900 J/m2 of UV-C; t test *P < 0.01; **P < 0.05. (B) In vivo pull-down of AGO1 with DDB2-FLAG protein upon UV-C exposure; ddb2-3/DDB2-FLAG and ddb2-3/DDB2K314E-FLAG expressing plants were used for IP assays using anti-FLAG antibody. WT (Col) plants were used as negative control. Coomassie blue staining of the blot is shown. (C) In vivo pull-down of AGO1 with DDB2-FLAG protein upon UV-C exposure; ddb2-2/DDB2-FLAG and atr ddb2-2/DDB2-FLAG expressing plants were used for IP assays using anti-FLAG antibody. WT (No) plants were used as negative control. Coomassie blue staining of the blot is shown. (D) Immunoblot analysis of DDB2 and AGO1 protein contents upon UV-C exposure in chromatin extracts from WT and atr plants. Anti-histone H3 and anti-UGPase antibodies were used as controls for insoluble (P, chromatin) and soluble (S) fractions, respectively. Signal intensity relative to H3 is indicated below each lane. (E) Same as D for AGO1 protein in ddb2 plants. (F) In vivo pull-down of AGO1 with DDB2 protein upon UV-C exposure in chromatin fraction; ddb2-2/DDB2-FLAG plants were used for IP assays using anti-FLAG antibody. WT plants were used as negative control. Anti-histone H3 and anti-UGPase antibodies were used as controls for insoluble (P, chromatin) and soluble (S) fractions, respectively. Coomassie blue staining of the blot is shown. (G) Tandem ChIP (Tandem-ChIP) of AGO1 and DDB2-FLAG, upon UV-C exposure, at two hot spots in ddb2-3/DDB2-FLAG expressing plants using anti-AGO1 followed by anti-FLAG antibody. As negative control for ChIP, WT plants were used as well as actin2 region. Data are presented as enrichment (±SD) of the IP signal and are representative of two independent biological replicates; t test *P < 0.01 compared with time point 0.
Fig. 5.
DDB2 AGO1 homeostasis and DDB2−AGO1 complex. (A) In vivo pull-down of AGO1 with DDB2-FLAG protein upon UV-C exposure; ddb2-2/DDB2-FLAG, nrpd1 ddb2-2/DDB2-FLAG, and dcl4 ddb2-2/DDB2-FLAG expressing plants were used for IP assays using anti-FLAG antibody. WT (No) plants were used as negative control. Coomassie blue staining of the blot is shown. (B) Immunoblot analysis of DDB2 and AGO1 protein contents upon UV-C exposure in chromatin (pellet), supernatant, and total extracts from WT plants. Anti-histone H3 and anti-UGPase antibodies were used as controls for insoluble (Pellet; chromatin) and soluble fractions (Supernatant), respectively. Signal intensity relative to H3 or Coomassie is indicated below each lane. Coomassie blue staining of the blot is shown. (C) ChIP of (Left) DDB2-FLAG and (Right) AGO1, upon UV-C exposure, at four hot spots in ddb2-3/DDB2-FLAG expressing plants using anti-FLAG and anti-AGO1 antibodies, respectively. As negative control for DDB2 ChIP, WT (Col) plants were used with anti-FLAG antibody as well as actin2 region. As negative control for AGO1 ChIP, WT (Col) plants were used with protein A magnetic beads as well as actin2 region. Data are presented as enrichment (±SD) of the IP signal and are representative of three independent biological replicates; t test *P < 0.01; ns, nonsignificant compared with time point 0. (D) Tandem ChIP (Tandem-ChIP) of DDB2-FLAG and AGO1, upon UV-C exposure, at three hot spots in ddb2-3/DDB2-FLAG expressing plants using anti-FLAG antibody followed by anti-AGO1 antibody. As negative control for ChIP, WT (Col) plants were used as well as actin2 region. Data are presented as enrichment (±SD) of the IP signal and are representative of two independent biological replicates; t test *P < 0.01 compared with time point 0. (E) ChIP of DDB2-FLAG upon UV-C exposure, at three hot spots in ddb2-3 DDB2-FLAG, nrdp1 DDB2-FLAG, and dcl4 DDB2-FLAG expressing plants using anti-FLAG antibody. As negative control for DDB2 ChIP, WT (Col) plants were used with anti-FLAG antibody as well as actin2 region. Data are presented as enrichment (±SD) of the IP signal and are representative of three independent biological replicates; t test *P < 0.01; **P < 0.05; ns, nonsignificant compared with time point 0. (F) ChIP of AGO1, upon UV-C exposure, at three hot spots in WT, dcl4, and nrpd1 plants using anti-AGO1 antibody. As negative control for AGO1 ChIP, WT (Col) plants were used with protein A magnetic beads as well as actin2 region. Data are presented as enrichment (±SD) of the IP signal and are representative of three independent biological replicates; t test *P < 0.01; ns, nonsignificant compared with time point 0.
DDB2 and AGO1 Exhibit Similar Nuclear Accumulation and Chromatin Recruitment Dynamics upon UV Exposure.
Because DDB2 senses photolesions on chromatin, a fraction of AGO1 was expected to accumulate in the nucleus upon UV-C treatment, as recently reported upon salt stress (32). Immunoblot analyses conducted over a 30-min time course showed that, upon UV-C irradiation, both DDB2 and AGO1 were indeed progressively enriched in chromatin extracts, reaching a peak at 15 min and decreasing subsequently at 30 min posttreatment (Fig. 5B). In addition to signaling DNA damage, ATR also negatively regulates DDB2 (26). Interestingly, DDB2 and AGO1 releases from chromatin were delayed in UV-C-irradiated atr loss-of-function, but not in WT, plants (Fig. S5D); by contrast, increased AGO1 association was abolished in ddb2 mutants (Fig. S5E). Finally, AGO1 was coimmunoprecipitated with DDB2-FLAG in the insoluble chromatin fraction 5 and 15 min after UV-C exposure, upon which AGO1 was at or below detection limit in the IP (Fig. S5F). Collectively, these results suggest that, upon UV-C exposure, the interacting DDB2 and AGO1 follow similar nuclear dynamics controlled by related mechanisms/factors.
The comparable nuclear dynamics of DDB2 and AGO1 might entail the rapid loading of both proteins onto chromatin upon UV irradiation and their subsequent release. ChIP quantitative PCR (qPCR) indeed showed enrichment of both DDB2-FLAG and AGO1 at various uviRNA hot spots 15 min after UV-C exposure, followed by a release of both proteins at 30 min (Fig. 5C). Moreover, tandem ChIP qPCR confirmed that DDB2-FLAG and AGO1 are coenriched at damaged sites 15 min after UV-C treatment, and released 30 min postirradiation (Fig. 5D and Fig. S5G). Consistent with the compromised DDB2-AGO1 complex formation/stability in nrpd1 mutants, ChIP analyses showed that, before UV irradiation, the DDB2-FLAG chromatin association was higher in POL IV-deficient plants compared with ddb2-3 plants complemented with DDB2-FLAG (Fig. 5E), suggesting the constitutive association of DDB2 with chromatin. In addition, release of DDB2-FLAG was no longer stimulated after UV-C exposure in nrpd1 mutants (Fig. 5E). Conversely, DDB2-FLAG remained efficiently chromatin-associated at damaged sites in dcl4 mutant plants 15 min postirradiation, and its release remained effective at 30 min (Fig. 5E). AGO1 chromatin association at damaged sites was, respectively, moderately compromised and unaffected in nrdp1 and dcl4 mutants 15 min postirradiation. AGO1 chromatin release was, by contrast, strongly compromised in both mutants 30 min postirradiation (Fig. 5F). Thus, DDB2 and AGO1 chromatin association/release depend on their ability to efficiently form a complex together and rely, as shown in Fig. 5 E and F, on siRNA abundance/biogenesis. However, it cannot be excluded that other factors are likely additionally involved. Nonetheless, the results collectively suggest that DDB2 and AGO1 assemble into a nuclear protein complex whose stability and chromatin dynamics rely on siRNA abundance and signaling of DNA damage.
The DDB2-AGO1 Complex Assembles with 21-nt uviRNAs.
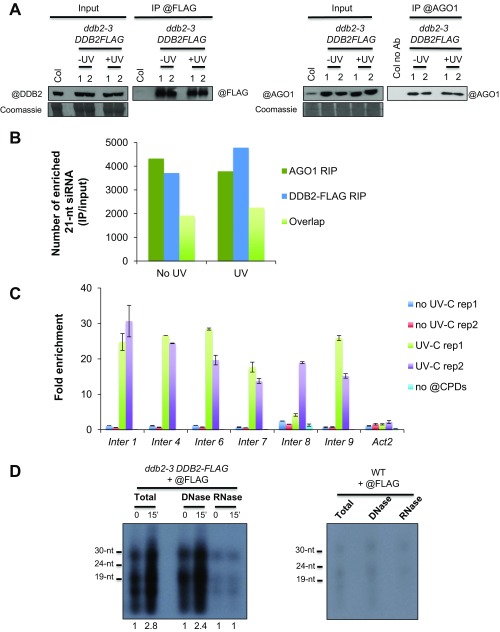
To test if DDB2 could be part of AGO1-uviRNAs complexes, DDB2-FLAG and AGO1 IPs followed by sRNA-seq [RNA immunoprecipitation (RIP)] were performed to determine the siRNA population associated with the complex before, and 15 min after, UV-C exposure. RIP experiments allowed the assembly of large libraries of DDB2- and AGO1-associated 21-nt siRNAs (enriched in IP/input; Fig. S6 A and B) mapping to intergenic regions covering the whole genome. These results agree (i) with the random damaging effect of UV-C and the putative role of 21-nt siRNAs in GGR, (ii) with the preferential loading of 21-nt siRNAs into AGO1, and (iii) with the characterization of a DDB2-AGO1 chromatin-associated complex. Moreover, ∼50% of DDB2-associated 21-nt siRNAs were also found associated with AGO1, further supporting the existence of this complex (Fig. S6B).
Fig. S6.
DDB2 and AGO1 RIP. (A) In vivo pull-down of AGO1 and DDB2-FLAG protein upon UV-C exposure during the RIP experiments; ddb2-3/DDB2-FLAG expressing plants were used with (Top) anti-FLAG or (Bottom) anti-AGO1 antibodies. WT plants were used as negative control. Coomassie blue staining of the blot is shown. The two independent biological replicates are shown. (B) Histograms representing the number of intergenic sequences exhibiting enrichment (IP/input) of 21-nt siRNAs in DDB2 RIP and AGO1 RIP ± UV-C. The number of overlapping sequences between DDB2 and AGO1 RIP are also shown. (C) Histograms (±SD) representing the enrichment (IP/input) of CPDs at six hot spots during the RIP experiments. (D) DDB2-FLAG coprecipitated nucleic acids from UV-C untreated and treated plants. (Left) The ddb2-3/DDB2-FLAG expressing plants were used with anti-FLAG antibody. Nucleic acids were isolated, 32P-radioactively labeled and treated with either A or DNase I (5 h incubation). Samples were fractionated onto a 15% urea PAGE. (Right) Untreated WT plants were used as control for IP with anti-FLAG antibody. Signal intensity relative to each time point 0 is indicated below each lane.
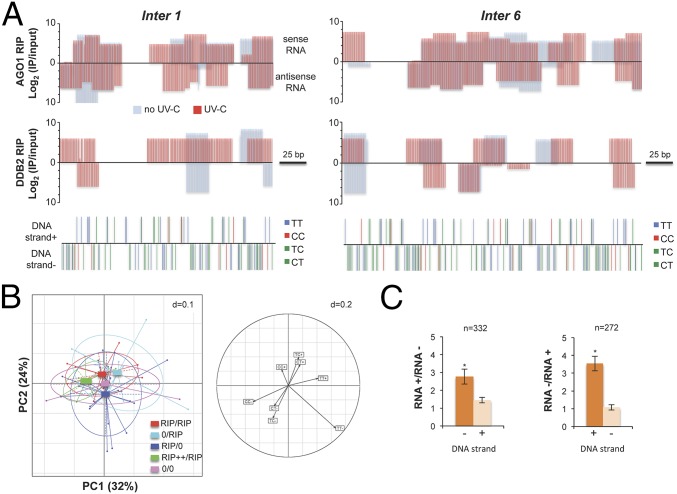
To further characterize the AGO1/DDB2-asssociated 21-nt uviRNAs at damaged sites, we performed DNA/RNA strand-specific mapping at six of the aforementioned (Fig. S6C), damaged intergenic hot spots, two examples of which are depicted in Fig. 6A. RIP data analysis showed that AGO1-associated 21-nt uviRNAs mapped to both DNA strands, consistent with AGO1 loading with a broad population of sense and antisense guide strands (Fig. 6A). By contrast, analysis of the DDB2 RIP data revealed that the DDB2-associated 21-nt uviRNAs mapped predominantly to the minus DNA strand (Fig. 6A). Moreover, DNA minus strands were generally more enriched in di-pyrimidines at the investigated damaged hot spots (Fig. 6A). Principal Component Analysis (PCA) including the six confirmed hot spots, containing CPDs, highlighted that a higher frequency of di-pyrimidines on the minus DNA strand correlates positively with an enhanced abundance of DDB2-associated 21-nt sense uviRNAs (Fig. 6B, Left, green rectangle) and that, conversely, a lower di-pyrimidines frequency correlates with a near absence of DDB2-associated 21-nt uviRNAs (Fig. 6B, Left, pink rectangle). This analysis indicates that CC and TT frequencies also influence strand-specific DDB2-associated 21-nt uviRNAs abundance with a prominent effect on the minus DNA strand (Fig. 6B, Right). Given that CT-TC richness positively correlates with abundance of complementary strand uviRNAs, we extended the analysis to all Arabidopsis intergenic regions, regardless of the presence of photolesions. We observed that CT-TC-enriched DNA strands significantly associate with an enhanced abundance of complementary 21-nt siRNAs, confirming, at the genome-wide scale, the trend observed at specific hot spots (Fig. 6C). These results led us to finally test the existence of a DDB2 DNA/RNA complex. DDB2-associated nucleic acids were sensitive to RNase A treatment, and the levels of DDB2-associated 15- to 30-nt RNAs increased 15 min upon UV exposure with a substantial peak around 21 nt (Fig. S6D), consistent with our total sRNA sequencing and RIP data. Moreover, and as expected, DDB2 interacted with DNA fragments in the size range of excised DNA fragments released during the NER process (3) (15 nt to 30 nt; Fig. S6D), supporting, overall, the existence of a DDB2-AGO1-uviRNAs-DNA complex.
Fig. 6.
DDB2 associated 21-nt uviRNAs at damaged sites. (A) Schematic representation of AGO1 and DDB2-FLAG RIP data, showing examples of two hot spots. Log2 (IP/Input) of 21-nt ± UV-C are plotted in a RNA strand-specific manner (top bars: sense RNA; bottom bars: antisense RNA), and di-pyrimidines are plotted in a DNA strand-specific manner for each locus (top bars: DNA strand +; bottom bars: DNA strand −). (B) PCA of DDB2 RIP 21-nt uviRNAs and di-pyrimidines at six confirmed damaged loci; + and – indicate the DNA or RNA strands. (Left) Representation of DDB2 RIP data with center of gravity and lines connected to each coordinate enriched in 21-nt uviRNAs in a RNA strand-specific manner. RIP/RIP, equal enrichment of 21-nt uviRNAs mapping with each DNA strand; 0/RIP, enrichment of 21-nt uviRNAs mapping only with + DNA strand; RIP/0, enrichment of 21-nt uviRNAs mapping only with − DNA strand; RIP++/RIP, stronger enrichment of 21-nt uviRNAs mapping with − DNA strand than with the + DNA strand; 0/0, no 21-nt uviRNAs. PC1 explains 32% of the variation, and PC2 explains 24%. (Right) Circles of correlations of the PC1 and PC2 of the PCA built using di-pyrimidines (CC, TT, CT, and TC) in + and − DNA strands. (C) Fold change abundance (±SD) of sense and antisense 21-nt siRNA in CT-TC-rich DNA strands (+ and −) of Arabidopsis intergenic regions; t test *P < 0.01.
Discussion
Based on a combination of functional genomics, genetics, and biochemistry, our study establishes, on a genome-wide scale, a strong correlation between locus-specific 21-nt uviRNA accumulation and GGR-mediated repair of UV-induced DNA damage at intergenic regions. Our results indicate that uviRNAs are produced noncanonically via RNA POL IV, RDR2, and DCL4 activities, and that their abundance is enhanced upon UV exposure. The uviRNAs are loaded into a nuclear pool of the PTGS effector AGO1, which forms a chromatin complex with DDB2 at UV-damaged sites, likely in an RNA/DNA complementary strand-specific manner. The results finally suggest that chromatin association/release of the DDB2-AGO1 complex rely, at least partly, on uviRNA accumulation and DNA damage signaling by ATR.
Nuclear Pools of PTGS-Related AGOs and Interactions of AGOs with Chromatin.
Although Arabidopsis AGO1 has been mainly implicated in cytoplasmic processes (33), AGO1 interaction with nuclear MIR161 and MIR173 primary transcripts under salt stress conditions causes transcriptional complex disassembly in a manner predicted to involve AGO1-chromatin association (32). Furthermore, AGO1 exhibit nucleolar and nucleoplasmic localization (34). Accordingly, our data demonstrate that a chromatin-associated AGO1 pool exists in Arabidopsis and that UV irradiation triggers and modulates its recruitment/dissociation at CPD-enriched, damaged DNA sites. A second main PTGS effector in Arabidopsis, AGO2, has been also implicated in diRNA biogenesis and DSB repair in the nucleus, where AGO2 accumulation is enhanced by genotoxic treatments and most likely also interacts with chromatin (21). In human cells, Ago1 and Ago2, originally characterized as cytoplasmic PTGS effectors, are now recognized to regulate transcriptional silencing/activation, alternative splicing, and DNA repair in the nucleus (35). Human Ago2, in particular, also interacts with Rad51 involved in homologous recombination (HR) (36). Thus, our findings support the emerging notion that plant and metazoan PTGS-related AGOs are more versatile than originally anticipated, and additionally contribute, via specific nuclear/chromatin protein pools, to various nuclear RNA- and DNA-related processes.
The 21-nt uviRNAs associated with the DDB2-AGO1 complex were generally complementary to the DNA strand enriched in di-pyrimidines, supporting the notion that DDB2 possibly forms a bona fide uviRNA/DNA duplex. The ensuing DDB2-AGO1-uviRNA complex could represent a dynamic and sequence-specific platform for CPD recognition and further DNA cleavage required for excision repair. Interactions between prokaryotic AGO orthologs and both sRNA and DNA fragments were recently documented (37), and, interestingly, the sizes of DDB2-associated DNA fragments are in the median size-range of excised DNA released during NER in prokaryotes (12 nt to 13 nt) and eukaryotes (38) (25 nt to 30 nt). Moreover, prokaryotic and eukaryotic AGOs are structurally similar to RNase H, possibly enabling DNA/RNA duplex formation for DNA processing (39) whereas, in Tetrahymena thermophile, AGO-related proteins loaded with Dicer-dependent sRNAs promote physical DNA elimination during macronucleus development (40). Nonetheless, nuclear Ago2 interacts with sRNAs but not DNA following ionizing radiation of human cells (41), suggesting that nuclear Ago-bound nucleic acids may vary depending on the organism and DNA repair process involved.
Upon CPD sensing, DDB2 must be released from the chromatin to allow the next GGR steps to proceed efficiently, a key process regulated at the protein stability level by ATR and the CUL4 ubiquitin E3-ligase (26, 42); interestingly, AGO1 homeostasis is also tightly controlled, including via ubiquitin-mediated proteolytic degradation (43). Although only AGO1 pools involved in miRNA action or antiviral defense (thus likely mostly cytoplasmic) have been studied in this respect thus far (6), the ATR and DDB2 influences on AGO1 chromatin association/release rates may rely on similar protein stability control processes in the nucleus, for instance upon protein complex dissociation.
A Model for DNA Repair Mediated by uviRNAs and Their Possible Connections to diRNAs.
The DDB2-AGO1-uviRNAs complex possibly facilitates recognition of UV-induced DNA lesions at intergenic regions during the first GGR steps (Fig. 7). We propose a model whereby, upon UV exposure, CPD sensing by DDB2 would be cooperatively aided by AGO1-loaded uviRNAs, enabling cDNA sequence recognition and, hence, stabilization of the complex at damaged sites. In this complex, DDB2 would bring about the photodamage recognition specificity, on the one hand, and AGO1-uviRNAs the nucleotide sequence recognition specificity, on the other, allowing efficient sensing of the CPDs. In addition to DNA damage signaling, ATR would subsequently promote chromatin release of the DDB2-AGO1-uviRNA complex to facilitate repair (Fig. 7).
Fig. 7.
Model for siRNA-mediated GGR of UV-induced DNA damage. (Left) In the absence of UV-induced DNA damage, some intergenic genomic regions are transcribed by the RNA POL IV to form precursors that are further processed by RDR2. The produced dsRNAs are diced by DCL4 into 21-nt siRNAs and subsequently loaded into an AGO1 nuclear pool that can form a complex with DDB2. (Right) Upon UV-C exposure, CPDs are formed on DNA (yellow triangle). The 21-nt uviRNAs abundance is increased either by enhanced stabilization of 21-nt uviRNAs or of their dsRNA precursors or by increased DCL4 activity. The DDB2-AGO1-uviRNAs complex is loaded on chromatin at damaged sites. DDB2 would allow recognition of CPDs, and AGO1-uviRNAs would allow stabilization of the complex in an RNA−DNA complementary sequence manner. Upon this recognition step, the DDB2−AGO1−uviRNAs complex is released in an ATR-dependent manner from the damaged sites, allowing the next steps of the GGR to efficiently occur.
Our data suggest that DDB2-AGO1 complex formation/stabilization depends on siRNA biogenesis and DNA damage signaling by ATR. Similarly, ATR was shown to play a role in regulating diRNA biogenesis and AGO2-dependent action during DSB repair, which depends, at least in part, on HR (21). Conceptually similarly, UV exposure induced a strong increase in abundance of AGO1-bound 21-nt siRNAs mapping to intergenic regions. Thus, both UV repair and DSB repair likely rely on a related core of regulatory processes involving sRNAs. Indeed, both UV treatments and photosynthetically active radiation induce somatic HR (44, 45), and DR- as well as GGR-deficient plants exhibit higher somatic HR (25, 46). One may therefore speculate that some discrete pools of AGO2-diRNAs may also act at, or in the vicinity of, UV-damaged sites in response to UV-induced DSBs.
uviRNA Biogenesis.
Biogenesis of 21-nt uviRNAs overlapping with intergenic UV-damaged regions is RNA POL IV-, RDR2-, and DCL4-dependent (Fig. 7), which differs from the canonical process involving RNA POL IV, RDR2, and DCL3 in RdDM, required for de novo and maintenance of DNA methylation (9). Nonetheless, alternative RdDM mechanisms relying on RNA POL II, RDR6, DCL4/DCL3, and 21-nt/24-nt siRNAs were also more recently characterized (15–18). In the context of DSB-induced DNA repair, ∼21-nt diRNA biogenesis occurs through a complex RNA POL IV/V-dependent process involving multiple DCLs and RDRs (21), but the underlying experimental design only enabled analysis of the global, steady-state DSB repair response, which involves several superimposed processes, including DSB sensing, processing, and chromatin reconstruction, as opposed to the single damage recognition step studied here.
Production of 26- and 27-nt RNAs overlapping with UV-damaged intergenic regions depends on RNA POL IV activity. Although these regions do not overlap with well-characterized RNA POL IV-interacting loci previously mapped genome-wide (47), the 26- to 27-nt RNAs exhibit the 5′-nucleotide bias typical of P4R2 precursors (28, 29). We note that only low basal levels of uviRNAs were sequenced before UV-C irradiation at the loci investigated here, suggesting that POL IV chromatin association might be too weak or too transient at these loci to be detected under normal laboratory growth conditions such as those previously used for the genome-wide mapping study (47). We further note that weak or transient POL IV association to chromatin may not be necessarily incompatible with production of biologically relevant sRNAs, which, like uviRNAs and diRNAs, are unrelated to RdDM, the main function ascribed so far to POL IV as part of the machinery producing conventional heterochromatic siRNAs. Upon UV irradiation, RNA POL II is usually removed from the lesions of transcribed genomic regions via ubiquitin-dependent proteolytic degradation allowing TCR (48), although lesion bypass may be also used alternatively (49). Photolesions might similarly interfere with RNA POL IV stability, possibly explaining its weak or transient interaction with chromatin at uviRNA-generating loci. We consider this scenario unlikely, however, because we showed that the TCR pathway is genetically unrelated to the uviRNA-mediated repair of UV-induced DNA lesions. Consequently, we speculate that a lesion bypass mechanism is involved and/or that a low level of preexisting P4R2 precursors is stabilized upon UV exposure and processed by DCL4 to enhance uviRNA abundance at damaged loci. Consistent with both ideas, the abundance of P4R2-like 26- to 27-nt RNAs is enhanced upon UV irradiation via mechanisms that await further characterization.
DCL4-dependent processing of POL IV-RDR2 dsRNA precursors into 21-nt uviRNAs agrees with the previously documented DCL4 colocalization with DCL3 in the nucleolar periphery of interphasic Arabidopsis nuclei (34). Moreover, a nuclear localization signal-containing nuclear isoform of Arabidopsis DCL4 produced at alternative transcription site was recently identified, which produces POL IV-RDR2-dependent 21-nt siRNAs prevalently from TE-associated genic regions and intergenic regions in seeds of unstressed Arabidopsis (50). It will thus be interesting to assess the role of this isoform in uviRNA biogenesis following UV irradiation. Regardless, our results confirm and extend the emerging notion that complex interconnections exist between core sRNA pathways in plants, thus considerably widening the scope of sRNAs repertoires and functions including in genome/epigenome surveillance.
Materials and Methods
Plant Material.
Arabidopsis thaliana mutant plants used in this study are in a Nossen ecotype (No) for ddb2-2 26 and in the Columbia ecotype (Col) for ddb2-3 31, atr-2 (SALK_032841), csa-a1 (SALK_024816), phrI (WiscDsLox_466C12), nrpd1 (SALK_583051), rdr2-1 (SAIL_1277H08), rdr6-12 (51), dcl3 (SALK_005512), dcl4-2 (GABI_160G05), dcl2 (SALK _064627), ago1-27 (27), and ago4-1 (52).
Generation of Transgenic Plants.
The cDNA of Arabidopsis DDB2 and DDB2K314E were amplified by PCR using primers described in ref. 31. Both cDNA were sequenced and cloned into the pOEX2 vector, between the NcoI and AvrII sites (25). The resulting plasmids pOEX2 DDB2 FLAG, pOEX2 DDB2K314E FLAG were mobilized into Agrobacterium tumefaciens and used to transform ddb2-2, ddb2-2 nrpd1, ddb2-2 dcl4, ddb2-2 atr, ddb2-3, nrpd1, and dcl4 Arabidopsis plants.
Plant Growth Conditions.
For in vitro conditions, plants were grown in a culture chamber under a 16-h light (light intensity ∼150 μmol⋅m−2⋅s−1; 21 °C and 8 h dark 19 °C) photoperiod. For soil conditions, plants were grown (one plant per pot) in a 16-h light (light intensity ∼150 μmol⋅m−2⋅s−1; 21 °C) and 8 h dark (19 °C) photoperiod (bulbs T5 cool day L49W/965HO; Osram; 70% humidity).
UV Sensitivity Assays.
To evaluate the UV-C (λ = 254 nm) sensitivity, 7-d-old in vitro germinated WT, homozygous mutant, and transgenic plants were transferred to square plates containing germination medium (GM) medium [MS salts (Duchefa), 1% sucrose, 0.8% Agar-agar ultrapure (Merck), pH 5.8] and grown vertically for 24 additional hours. Thereafter, roots were UV-C irradiated (600 J/m2 or 900 J/m2) using the Stratalinker 2400 (Stratagene). Root length was measured 24 h after a recovery period in the light or in the dark. The relative root growth was calculated: (root length treated/root length untreated) ×100 (± SD). Eight plants per replicate were used. Experiments were performed in triplicates. Because ddb2-2 is in the No ecotype, the control for double mutant plants is No/Col. These control F2 plants were generated from 24 individual F1 No X Col plants to minimize the bias due to an inappropriate selection of a single F2 plant. To evaluate the UV-C sensitivity on whole plants, 1-wk-old in vitro germinated WT and homozygous mutant plants were irradiated with UV-C (1,500 J/m2 or 3,000 J/m2) three times in a row every 2 d. Plants were immediately returned to the growth chamber. The total number of leaves and the number of bleached plants were evaluated 1 wk later as described in ref. 26. At least 20 plants per replicate were used, and the experiment was duplicated.
Determination of CPD Removal by Immuno-Dot Blot.
Fourteen-day-old in vitro grown seedlings were irradiated with UV-C (3,000 J/m2). Samples were harvested just after irradiation (time 0) or kept under normal light conditions and harvested 30 min and/or 1 h later. Genomic DNA was extracted using plant DNA extraction kit (Macherey-Nagel). DNA samples were processed as described in ref. 53. Repair efficiency was determined by quantifying the relative CPD amounts remaining after 30 min and/or 1 h, based on the initial CPD formation at time 0 for each specific genotype.
UV-C Treatment of Whole Arabidopsis Plants for Genomic Studies.
WT, Columbia, and nrpd1 Arabidopsis plants were germinated in vitro on solid GM medium for 10 d. Afterward, seedlings were transferred in soil (one plant per pot) and put in a growth chamber for 2 wk. For treated samples, plants (12 per biological replicate) were irradiated with UV-C (3,000 J/m2). Fifteen and 30 min upon UV-C exposure, leaves numbers 1, 2, 3, and 4 were harvested and pooled. Unirradiated leaves were harvested before UV-C exposure.
IPOUD and Mapping of CPDs.
Genomic DNA was extracted using the Plant DNA Extraction kit (Macherey-Nagel). Two micrograms were sonicated (Diagenode Bioruptor: 18 × 30 s) and denatured for 10 min at 95 °C in Buffer 1 (10 mM Tris HCl pH 7.5, 500 mM NaCl, 1 mM EDTA). IP was performed by adding 5 μg of anti-CPD mouse monoclonal antibody (CAC-NM-DND-001; Cosmio Bio) and incubated overnight at 4 °C. The suspension was incubated with M280 Dynabeads (Invitrogen) for 4 h at 4 °C. The pellet was washed four times with Buffer 1. DNA was eluted with Buffer 2 (30 mM Tris HCl pH: 8.0; 150 μg Proteinase K) for 1 h at 42 °C. DNA from the IP and input fractions was purified using the Nucleospin Gel and PCR clean-up kit (Macherey-Nagel). DNA from IP and input were used for library preparation and sequencing by Illumina Hi-Seq (Fasteris). Unique sequences (input and IPs) were mapped onto the Arabidopsis nuclear genome (TAIR10) using Bowtie 1.0.0: /bowtie-1.0.0/bowtie -q -v 2 -m 1 -S <index> <fastqfile> <output> option -q: input = fastq option -v 2: report end-to-end hits w/ <=v mismatches option -m 1: suppress all alignments if > <int> exist option -S: write hits in SAM format. CPD-enriched regions were determined using MACS2 (callpeak -t -c -n –g, P < 10−6 and q < 10−2). Numbers of reads and related statistics are reported in Table S1.
sRNA Sequencing and siRNA Mapping.
sRNAs were prepared from WT and nrpd1 untreated and treated plants using the Tri-Reagent (Sigma), used for library preparation and sequencing by Illumina Hi-Seq (Fasteris). For sRNAs mapping, reads were aligned onto the Arabidopsis genome (TAIR10) using Bowtie (version 1.0.0; parameters: -y -e 50 -n 0 -a–best–strata–nomaqround). Reads overlapping miR coordinates were suppressed with bedtools (intersectbed -f 0.9). Upon conversion with samtools (version 0.1.18), reads exactly overlapping with the CPD-damaged loci (50% overlap at the edge of the CPD-enriched sequences) were calculated with intersectBed (BEDtools version 2.16.2). Numbers of reads and related statistics are reported in Table S1.
UV-C Treatment of in Vitro-Grown Arabidopsis Plants for IPOUD Confirmation, Coimmunoprecipiation, and Immunoblotting.
Arabidopsis plants were germinated in vitro on solid GM medium for 7 d. Afterward, seedlings were transferred into large plates on GM medium (diameter: 14 cm; one plant per centimeter) and put in a growth chamber for 10 d. For treated samples, plants (40 to 50 per biological replicate) were irradiated with UV-C (3,000 J/m2). Five, 15, 30, and 60 min upon UV-C exposure, plants were harvested and incubated by rapid freezing into liquid nitrogen. Unirradiated control plants were harvested before UV-C exposure.
Protein Extraction and Immunoblotting.
Whole protein extracts were prepared from 2-wk-old in vitro grown plants before UV-C irradiation (time point 0), 15, 30, and/or 60 min upon UV-C exposure (3,000 J/m2) using a denaturing buffer (26). Twenty micrograms of total protein were separated by 8% SDS gel and blotted onto an Immobilon-P membrane (Millipore). Anti-peptidic anti-AtDDB2 antibody (26) was used as a 1:2,000 dilution (v:v); the anti-AGO1 (AS09 527, Agrisera) at a 1:30,000 dilution (v:v) in PBST [PBS (PBS X 1), nonfat dry milk (5%, w:v), and Tween-20 (0.1%, v:v; Sigma)].
Chromatin Preparation for Immunoblotting.
Fractions of soluble/insoluble proteins were extracted from 1 g of 14-d-old seedlings using Nonidet P-40 lysis buffer [25 mM Tris⋅HCl (pH 8.0), 0.3 M NaCl, 1 mM EDTA, 10% (vol/vol) glycerol, Nonidet P-40 1% (vol/vol), 0.2 mM phenylmethylsulfonyl fluoride, and EDTA-free protease inhibitor mixture (1 tablet/50 mL; Roche)] (54). After grinding, powder was incubated in 6 mL of Nonidet P-40 lysis buffer during 30 min on a rotating wheel at 4 °C (8 rpm), and the solution was Miracloth-filtered. Removal of extra cell debris was performed by centrifugation (2,000 × g, 5 min, 4 °C). Free chromatin-unbound proteins were recovered in the soluble fraction after centrifugation (13,000 × g, 10 min, 4 °C). The pellet containing insoluble and chromatin-bound proteins was resuspended in 75 µL of Nonidet P-40-containing resuspension buffer (26). Variable amounts (25 to 50%) of the insoluble fraction and 2% of the soluble fraction were separated by SDS/PAGE and analyzed by immunoblotting with the indicated antibodies. The polyclonal anti-H3, (06-755; Millipore) at a 1:10,000 dilution (v:v) and the anti-UGPase antibody (AS05 086, Agrisera) at a 1:10,000 dilution (v:v) were used as fractionation controls.
IP Assays.
Total soluble proteins were extracted from 0.5 g of 14-d-old seedlings using 3 mL of IP buffer (55). IP was performed using anti-FLAG gel affinity (A2220; Sigma) or anti-AGO1 antibody (Agrisera). The precipitate was washed four times in IP buffer, resuspended in 50 µL of SDS sample buffer, and heated 3 min at 100 °C before immunoblotting. The DDB2 FLAG protein was detected using the anti-FLAG HRP (A8592; Sigma) at a 1:10,000 (v:v) dilution in PBST, and the AGO1 protein was detected using the anti-AGO1 antibody. For RIP experiments, ddb2-3 DDB2-FLAG expressing plants were used. After four washes in IP buffer, RNAs were extracted from the immunoprecipitated samples by TRIzol (Sigma). Numbers of reads and related statistics for RIP experiments are reported in Table S1.
ChIP.
ChIP experiments were performed using 14-d-old in vitro grown seedlings (ddb2-3 DDB2-FLAG, nrdp1 DDB2-FLAG, and dcl4 DDB2-FLAG). Preparation of chromatin, sonication, and IP using anti-FLAG or anti-AGO1 antibodies were carried out as described in ref. 56. The immunoprecipitated DNA was analyzed by qPCR. Data analysis was done as described in ref. 57. Experiments were triplicated using independent biological samples. Three technical replicates were performed for each independent biological sample. Tandem ChIP experiments were performed in both directions (DDB2-FLAG followed by AGO1 and AGO1 followed by DDB2-FLAG) as described in ref. 58.
qPCR.
The qPCR was performed using a LightCycler 480 and LightCycler 480 SYBR green I Master mix (Roche) following manufacturer’s instructions. All primers are listed in Table S2.
Table S2.
List of primers used for qPCR
| Primer name | Target name | Sequence 5′ -> 3′ |
| Act2 fw | Act2 | CTTGCACCAAGCAGCATGAA |
| Act2 rv | CCGATCCAGACACTGTACTTC | |
| Inter 1 fw | Inter 1 | AAGCCTAAGTAGTGTATCCTTGTT |
| Inter 1 rv | ACCAACCTTCTTCTTGCTTCT | |
| Inter 3 fw | Inter 3 | TAGCGTGTTTGGTGCTCT |
| Inter 3 rv | TCTCTCTCTCTCTCTCTCTCTC | |
| Inter 4 fw | Inter 4 | ACCAACCTTCTTCTTGCTTCT |
| Inter 4 rv | AAACCGCAACCGGATCTT | |
| Inter 5 fw | Inter 5 | GTGTGTTAAGTATTTATACCCTCT |
| Inter 5 rv | CCTCAGGACCTCACTTTACAAT | |
| Inter 6 fw | Inter 6 | TGTACCATATCTAGAGATCAATTTAGCC |
| Inter 6 rv | CAGCTCGAAGCCCTATTCTATTT | |
| Inter 7 fw | Inter 7 | AAACCGCAACCCGATCTT |
| Inter 7 rv | ACCAACCATCTTCTTGCTTCT | |
| Inter 8 fw | Inter 8 | GCTAACCTAGAACCCACAAAGG |
| Inter 8 rv | GATTCGGCAGGTGAGTTGTTA | |
| Inter 9 fw | Inter 9 | GCCAAATGCCTCGTCATCTA |
| Inter 9 rv | CTGTGGTTTCGCTGGATAGTAG | |
| Inter 10 fw | Inter 10 | GACAAACCCATGCCAAGTAAAG |
| Inter 10 rv | ACGATTCCTCAGAACCGATTATT | |
| Inter 11 fw | Inter 11 | TCTCTCTCTCTCTCTCTCTCTCTC |
| Inter 11 rv | GCCATGGTCTTCCCAGTATT | |
| Inter 12 fw | Inter 12 | CACTCGTGCAAAGAGTTGTTATC |
| Inter 12 rv | CCTCAGGACCTCACTTTACAAT | |
| Inter 14 fw | Inter 14 | TCTCTCTCTCTCTCTCTCTCTCTC |
| Inter 14 rv | AAATGTGTGTGTTGTGTGTGTGTG | |
| Gene 1 fw | Gene 1 | TTCGAAATGCCAATGGATGTAT |
| Gene 1 rv | CAATGTGACGAACAAGAAGATTAAC | |
| Gene 2 fw | Gene 2 | GGGACATCTCGGTATTTCGTG |
| Gene 2 rv | GAAGAGAATGGGCGTGTCAT | |
| Gene 4 fw | Gene 4 | CGGATCTTGGATTCGACGATAG |
| Gene 4 rv | GTGTGTGTGTTACCTGGTAGTC | |
| Other 1 fw | Other 1 | CTCAGCAGTTCTCGGACAAA |
| Other 1 rv | CCTAACGCCTCGAAGAACTAAT | |
| Other 3 fw | Other 3 | GAAACTTACCAGGTCCAGACATA |
| Other 3 rv | TCGCTCCACCAACTAAGAAC |
PCA.
PCA was performed using DDB2-FLAG RIP 21-nt uviRNAs and di-pyrimidines frequencies at six confirmed damaged loci. Five different categories were defined for the analysis within these damaged loci: RIP/RIP, equal enrichment of 21-nt uviRNAs mapping with each DNA strand; 0/RIP, enrichment of 21-nt uviRNAs mapping only with + DNA strand; RIP/0, enrichment of 21-nt uviRNAs mapping only with − DNA strand; RIP++/RIP, higher enrichment of 21-nt uviRNAs mapping with − DNA strand than with the + DNA strand; and 0/0, no 21-nt uviRNAs mapping with each DNA strand. For each category, the di-pyrimidine frequency was calculated in overlapping windows with 21-nt uviRNAs and for each DNA strand. PCA results were displayed using the ade4 package-II (55).
Statistics.
The Shapiro test was performed to determine whether the distribution of the population was normal or not. A t test was used for normal distribution. Mann−Whitney U or Wilcoxon matched-pairs signed rank tests were used as nonparametric statistical hypothesis tests.
Accession Numbers.
The GEO accession number for the IPOUD, the sRNA deep sequencing, and the RIP data reported in this paper is GSE86403.
Supplementary Material
Acknowledgments
We thank Prof. Barbara Hohn for critical comments on the manuscript. This work was supported by grants from the European Research Council (Frontiers of RNAi Grant 210890; Frontiers of RNAi Grant 323071), the Prix Louis D. of the French Academy of Sciences, and the Bettencourt Schueller Foundation (all attributed to O.V.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE86403).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1618834114/-/DCSupplemental.
References
- 1.Schreier WJ, Gilch P, Zinth W. Early events of DNA photodamage. Annu Rev Phys Chem. 2015;66:497–519. doi: 10.1146/annurev-physchem-040214-121821. [DOI] [PubMed] [Google Scholar]
- 2.Sancar A. Photolyase and cryptochrome blue-light photoreceptors. Adv Protein Chem. 2004;69:73–100. doi: 10.1016/S0065-3233(04)69003-6. [DOI] [PubMed] [Google Scholar]
- 3.Schärer OD. Nucleotide excision repair in eukaryotes. Cold Spring Harb Perspect Biol. 2013;5:a012609. doi: 10.1101/cshperspect.a012609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu G, Chang E. Xeroderma pigmentosum group E cells lack a nuclear factor that binds to damaged DNA. Science. 1988;242:564–567. doi: 10.1126/science.3175673. [DOI] [PubMed] [Google Scholar]
- 5.Nichols AF, et al. Human damage-specific DNA-binding protein p48. Characterization of XPE mutations and regulation following UV irradiation. J Biol Chem. 2000;275:21422–21428. doi: 10.1074/jbc.M000960200. [DOI] [PubMed] [Google Scholar]
- 6.Bologna NG, Voinnet O. The diversity, biogenesis, and activities of endogenous silencing small RNAs in Arabidopsis. Annu Rev Plant Biol. 2014;65:473–503. doi: 10.1146/annurev-arplant-050213-035728. [DOI] [PubMed] [Google Scholar]
- 7.Brodersen P, Voinnet O. Revisiting the principles of microRNA target recognition and mode of action. Nat Rev Mol Cell Biol. 2009;10:141–148. doi: 10.1038/nrm2619. [DOI] [PubMed] [Google Scholar]
- 8.Mallory A, Vaucheret H. Form, function, and regulation of ARGONAUTE proteins. Plant Cell. 2010;22:3879–3889. doi: 10.1105/tpc.110.080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11:204–220. doi: 10.1038/nrg2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie Z, et al. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2004;2:E104. doi: 10.1371/journal.pbio.0020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanno T, et al. Atypical RNA polymerase subunits required for RNA-directed DNA methylation. Nat Genet. 2005;37:761–765. doi: 10.1038/ng1580. [DOI] [PubMed] [Google Scholar]
- 12.Pontier D, et al. Reinforcement of silencing at transposons and highly repeated sequences requires the concerted action of two distinct RNA polymerases IV in Arabidopsis. Genes Dev. 2005;19:2030–2040. doi: 10.1101/gad.348405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pontes O, et al. The Arabidopsis chromatin-modifying nuclear siRNA pathway involves a nucleolar RNA processing center. Cell. 2006;126:79–92. doi: 10.1016/j.cell.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 14.Mosher RA, Schwach F, Studholme D, Baulcombe DC. PolIVb influences RNA-directed DNA methylation independently of its role in siRNA biogenesis. Proc Natl Acad Sci USA. 2008;105:3145–3150. doi: 10.1073/pnas.0709632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pontier D, et al. NERD, a plant-specific GW protein, defines an additional RNAi-dependent chromatin-based pathway in Arabidopsis. Mol Cell. 2012;48:121–132. doi: 10.1016/j.molcel.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 16.Marí-Ordóñez A, et al. Reconstructing de novo silencing of an active plant retrotransposon. Nat Genet. 2013;45:1029–1039. doi: 10.1038/ng.2703. [DOI] [PubMed] [Google Scholar]
- 17.Nuthikattu S, et al. The initiation of epigenetic silencing of active transposable elements is triggered by RDR6 and 21-22 nucleotide small interfering RNAs. Plant Physiol. 2013;162:116–131. doi: 10.1104/pp.113.216481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bond DM, Baulcombe DC. Epigenetic transitions leading to heritable, RNA-mediated de novo silencing in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2015;112:917–922. doi: 10.1073/pnas.1413053112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu W, et al. Negative regulation of tumor suppressor p53 by microRNA miR-504. Mol Cell. 2010;38:689–699. doi: 10.1016/j.molcel.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu H, Gatti RA. MicroRNAs: New players in the DNA damage response. J Mol Cell Biol. 2011;3:151–158. doi: 10.1093/jmcb/mjq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei W, et al. A role for small RNAs in DNA double-strand break repair. Cell. 2012;149:101–112. doi: 10.1016/j.cell.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Francia S, et al. Site-specific DICER and DROSHA RNA products control the DNA-damage response. Nature. 2012;488:231–235. doi: 10.1038/nature11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michalik KM, Böttcher R, Förstemann K. A small RNA response at DNA ends in Drosophila. Nucleic Acids Res. 2012;40:9596–9603. doi: 10.1093/nar/gks711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidts I, Böttcher R, Mirkovic-Hösle M, Förstemann K. Homology directed repair is unaffected by the absence of siRNAs in Drosophila melanogaster. Nucleic Acids Res. 2016;44:8261–8271. doi: 10.1093/nar/gkw570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molinier J, Ramos C, Fritsch O, Hohn B. CENTRIN2 modulates homologous recombination and nucleotide excision repair in Arabidopsis. Plant Cell. 2004;16:1633–1643. doi: 10.1105/tpc.021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molinier J, Lechner E, Dumbliauskas E, Genschik P. Regulation and role of Arabidopsis CUL4-DDB1A-DDB2 in maintaining genome integrity upon UV stress. PLoS Genet. 2008;4:e1000093. doi: 10.1371/journal.pgen.1000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morel JB, et al. Fertile hypomorphic ARGONAUTE (ago1) mutants impaired in post-transcriptional gene silencing and virus resistance. Plant Cell. 2002;14:629–639. doi: 10.1105/tpc.010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhai J, et al. A one precursor one siRNA model for Pol IV-dependent siRNA biogenesis. Cell. 2015;163:445–455. doi: 10.1016/j.cell.2015.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blevins T, et al. Identification of Pol IV and RDR2-dependent precursors of 24 nt siRNAs guiding de novo DNA methylation in Arabidopsis. eLife. 2015;4:e09591. doi: 10.7554/eLife.09591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cleaver JE, Thompson LH, Richardson AS, States JC. A summary of mutations in the UV-sensitive disorders: Xeroderma pigmentosum, Cockayne syndrome, and trichothiodystrophy. Hum Mutat. 1999;14:9–22. doi: 10.1002/(SICI)1098-1004(1999)14:1<9::AID-HUMU2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Schalk C, et al. DNA DAMAGE BINDING PROTEIN 2 (DDB2) shapes the dna methylation landscape. Plant Cell. 2016;28:2043–2059. doi: 10.1105/tpc.16.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolata J, et al. Salt stress reveals a new role for ARGONAUTE 1 in miRNA biogenesis at the transcriptional and post-transcriptional levels. Plant Physiol. 2016;172:297–312. doi: 10.1104/pp.16.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poulsen C, Vaucheret H, Brodersen P. Lessons on RNA silencing mechanisms in plants from eukaryotic argonaute structures. Plant Cell. 2013;25:22–37. doi: 10.1105/tpc.112.105643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pontes O, et al. Intersection of small RNA pathways in Arabidopsis thaliana sub-nuclear domains. PLoS One. 2013;8:e65652. doi: 10.1371/journal.pone.0065652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schraivogel D, Meister G. Import routes and nuclear functions of Argonaute and other small RNA-silencing proteins. Trends Biochem Sci. 2014;39:420–431. doi: 10.1016/j.tibs.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Gao M, et al. Ago2 facilitates Rad51 recruitment and DNA double-strand break repair by homologous recombination. Cell Res. 2014;24:532–541. doi: 10.1038/cr.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olovnikov I, Chan K, Sachidanandam R, Newman DK, Aravin AA. Bacterial argonaute samples the transcriptome to identify foreign DNA. Mol Cell. 2013;51:594–605. doi: 10.1016/j.molcel.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canturk F, et al. Nucleotide excision repair by dual incisions in plants. Proc Natl Acad Sci USA. 2016;113:4706–4710. doi: 10.1073/pnas.1604097113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willkomm S, Zander A, Gust A, Grohmann D. A prokaryotic twist on argonaute function. Life (Basel) 2015;5:538–553. doi: 10.3390/life5010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mochizuki K, Gorovsky MA. Conjugation-specific small RNAs in Tetrahymena have predicted properties of scan (scn) RNAs involved in genome rearrangement. Genes Dev. 2004;18:2068–2073. doi: 10.1101/gad.1219904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gioia U, d’Adda di Fagagna F. Human nuclear ARGONAUTE 2 interacts in vivo only with small RNAs and not with DNA. Cell Cycle. 2015;1:2001–2002. doi: 10.1080/15384101.2015.1044171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, Zhang Y, Douglas L, Zhou P. UV-damaged DNA-binding proteins are targets of CUL-4A-mediated ubiquitination and degradation. J Biol Chem. 2001;276:48175–48182. doi: 10.1074/jbc.M106808200. [DOI] [PubMed] [Google Scholar]
- 43.Derrien B, Genschik P. When RNA and protein degradation pathways meet. Front Plant Sci. 2014;5:161. doi: 10.3389/fpls.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swoboda P, Gal S, Hohn B, Puchta H. Intrachromosomal homologous recombination in whole plants. EMBO J. 1994;13:484–489. doi: 10.1002/j.1460-2075.1994.tb06283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ries G, Buchholz G, Frohnmeyer H, Hohn B. UV-damage-mediated induction of homologous recombination in Arabidopsis is dependent on photosynthetically active radiation. Proc Natl Acad Sci USA. 2000a;97:13425–13429. doi: 10.1073/pnas.230251897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ries G, et al. Elevated UV-B radiation reduces genome stability in plants. Nature. 2000b;406:98–101. doi: 10.1038/35017595. [DOI] [PubMed] [Google Scholar]
- 47.Law JA, et al. Polymerase IV occupancy at RNA-directed DNA methylation sites requires SHH1. Nature. 2013;498:385–389. doi: 10.1038/nature12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bregman DB, et al. UV-induced ubiquitination of RNA polymerase II: a novel modification deficient in Cockayne syndrome cells. Proc Natl Acad Sci USA. 1996;93:11586–11590. doi: 10.1073/pnas.93.21.11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu L, et al. Molecular basis of transcriptional fidelity and DNA lesion-induced transcriptional mutagenesis. DNA Repair (Amst) 2014;19:71–83. doi: 10.1016/j.dnarep.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pumplin N, et al. DNA methylation influences the expression of DICER-LIKE4 isoforms, which encode proteins of alternative localization and function. Plant Cell. 2016;28:2786–2804. doi: 10.1105/tpc.16.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peragine A, Yoshikawa M, Wu G, Albrecht HL, Poethig RS. SGS3 and SGS2/SDE1/RDR6 are required for juvenile development and the production of trans-acting siRNAs in Arabidopsis. Genes Dev. 2004;18:2368–2379. doi: 10.1101/gad.1231804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zilberman D, Cao X, Jacobsen SE. ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science. 2003;299:716–719. doi: 10.1126/science.1079695. [DOI] [PubMed] [Google Scholar]
- 53.Castells E, et al. det1-1-induced UV-C hyposensitivity through UVR3 and PHR1 photolyase gene over-expression. Plant J. 2010;63:392–404. doi: 10.1111/j.1365-313X.2010.04249.x. [DOI] [PubMed] [Google Scholar]
- 54.Fei J, et al. Regulation of nucleotide excision repair by UV-DDB: prioritization of damage recognition to internucleosomal DNA. PLoS Biol. 2011;9:e1001183. doi: 10.1371/journal.pbio.1001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pazhouhandeh M, Molinier J, Berr A, Genschik P. MSI4/FVE interacts with CUL4-DDB1 and a PRC2-like complex to control epigenetic regulation of flowering time in Arabidopsis. Proc Natl Acad Sci USA. 2011;108:3430–3435. doi: 10.1073/pnas.1018242108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mutskov V, Felsenfeld G. Silencing of transgene transcription precedes methylation of promoter DNA and histone H3 lysine 9. EMBO J. 2004;23:138–149. doi: 10.1038/sj.emboj.7600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berr A, et al. Arabidopsis SET DOMAIN GROUP2 is required for H3K4 trimethylation and is crucial for both sporophyte and gametophyte development. Plant Cell. 2010;22:3232–3248. doi: 10.1105/tpc.110.079962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dray S, Dufour AB, Chessel D. The ade4 package-II: Two-table and K-table methods. R News. 2007;2:47–52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.