Significance
T-cell trafficking is essential for the function of the adaptive immune system, and regulation of T-cell entry into tissues can be an effective therapy in diseases such as autoimmunity. However, the mechanisms regulating T-cell migration and trafficking are poorly understood. We have identified a key role for Ena/VASP (vasodilator-stimulated phosphoprotein) family cytoskeletal effectors selectively in activated T-cell trafficking to secondary lymphoid organs and to peripheral sites of inflammation. Ena/VASP deficiency in T cells causes a defect in α4 integrin function, which impairs transendothelial migration. Our work suggests that further studies of the Ena/VASP pathway in T cells could identify therapeutically useful ways to more selectively modulate α4 integrin activity and activated T-cell trafficking.
Keywords: T cell, migration, cytoskeleton, extravasation
Abstract
Vasodilator-stimulated phosphoprotein (VASP) and Ena-VASP–like (EVL) are cytoskeletal effector proteins implicated in regulating cell morphology, adhesion, and migration in various cell types. However, the role of these proteins in T-cell motility, adhesion, and in vivo trafficking remains poorly understood. This study identifies a specific role for EVL and VASP in T-cell diapedesis and trafficking. We demonstrate that EVL and VASP are selectively required for activated T-cell trafficking but are not required for normal T-cell development or for naïve T-cell trafficking to lymph nodes and spleen. Using a model of multiple sclerosis, we show an impairment in trafficking of EVL/VASP-deficient activated T cells to the inflamed central nervous system of mice with experimental autoimmune encephalomyelitis. Additionally, we found a defect in trafficking of EVL/VASP double-knockout (dKO) T cells to the inflamed skin and secondary lymphoid organs. Deletion of EVL and VASP resulted in the impairment in α4 integrin (CD49d) expression and function. Unexpectedly, EVL/VASP dKO T cells did not exhibit alterations in shear-resistant adhesion to, or in crawling on, primary endothelial cells under physiologic shear forces. Instead, deletion of EVL and VASP impaired T-cell diapedesis. Furthermore, T-cell diapedesis became equivalent between control and EVL/VASP dKO T cells upon α4 integrin blockade. Overall, EVL and VASP selectively mediate activated T-cell trafficking by promoting the diapedesis step of transendothelial migration in a α4 integrin-dependent manner.
Activated T-cell trafficking across the vascular endothelium is essential for ongoing immune surveillance of tissues and for effective immune responses to conditions such as infection and cancer. Conversely, in situations of immune dysregulation, inhibition of self-reactive T-cell trafficking represents a promising target for therapeutic immunomodulation. Disruption of these pathways, such as by antibody blockade of α4 integrins, is a highly effective approach to immunomodulation (1, 2). However, the molecular mechanisms by which chemokine receptor and adhesion molecule signaling induce the T-cell cytoskeletal machinery to promote extravasation are not yet fully elucidated.
Transendothelial migration (TEM), the process by which T cells extravasate from the blood into tissues, is characterized by four distinct steps: rolling along the vascular wall, arrest or adhesion, intravascular crawling, and diapedesis across the endothelial barrier (3). Surface adhesion molecules play well-characterized roles in each step of the process. For example, the initial rolling step of TEM is facilitated by interactions between T-cell and endothelial selectins, whereas the adhesion, intravascular crawling, and diapedesis steps of TEM are mainly regulated by chemokine- and shear force-stimulated modulation of lymphocyte function-associated antigen 1 (LFA-1, αLβ2 integrin, CD11a/CD18) and very late antigen 4 (VLA-4, α4β1 integrin, CD49d/CD29) interactions with intracellular adhesion molecule 1 (ICAM-1) and vascular cellular adhesion molecule 1 (VCAM-1), respectively. Dynamic cytoskeletal changes occur throughout the process of TEM (4, 5); however, the regulation of these cytoskeletal changes is not completely understood.
The lymphocyte actin-myosin cytoskeleton is composed of networks of linear and branched actin filaments, cross-linked by class-II nonmuscle myosin. We have previously shown that inhibition of myosin-IIA, the main class-II myosin protein expressed in lymphocytes, alters T-cell trafficking, motility, and TEM (6–9). Although numerous studies have focused on the upstream regulatory signaling cascades that control actin network remodeling during migration (5, 10), less is understood about how downstream effectors of branched and linear actin polymerization might participate in lymphocyte TEM. Two major families of effector proteins of linear actin polymerization are expressed in lymphocytes. The Formin family, which is thought to nucleate new linear actin filament production, has been implicated in T-cell activation and egress from the thymus (11–13). Less is known about the Ena/VASP (vasodilator-stimulated phosphoprotein) family of cytoskeletal effectors in T cells.
The Ena/VASP family is composed of three members: mammalian-enabled (Mena), which is not typically expressed in hematopoietic cells; VASP; and Ena-VASP–like (EVL) (14, 15). These proteins coordinate monomeric actin recruitment to the barbed end of the actin filament, prevent actin filament capping, and can play a role in actin filament bundling (16–20). Structurally, EVL and VASP share significant homology, containing an N-terminal EVH1 domain, which regulates cellular localization; and a C-terminal EVH2 domain, which facilitates tetramerization, binds filamentous actin (F-actin), and is thought to be responsible for actin polymerization (21–24). Ena/VASP proteins are capable of compensating for deletion of one another, but there is some evidence that they are differentially regulated (15).
EVL, and especially VASP, are implicated in the motility, adhesion, and sensory capacity of many cell types. EVL and VASP localize to filopodia tips (25) as well as to adhesive sites, such as fibroblast focal adhesions (24). This localization pattern is consistent with a role in migration and adhesion. Fibroblasts lacking EVL and VASP produce shorter filopodia, and a slower-moving lamellipodium, which paradoxically leads to enhanced fibroblast motility (26, 27). Platelets from VASP-knockout mice demonstrate enhanced vascular adhesion (28), whereas inside-out signaling through β2 integrins is impaired in VASP-deficient neutrophils (29).
Only a few studies have examined the role of Ena/VASP proteins in T cells, showing that Ena/VASP proteins can contribute to actin remodeling during T-cell receptor (TCR) signaling (24, 30). However, previous studies did not focus on the role of EVL and VASP in T-cell development or T-cell motility. Therefore, we sought to elucidate the mechanisms by which EVL and VASP might influence T-cell adhesion, migration, and trafficking in vivo.
Results
Deletion of EVL and VASP Does Not Significantly Affect T-Cell Development.
To determine if deletion of Ena/VASP proteins altered T-cell trafficking in vivo, we used EVL/VASP double-knockout (dKO) mice (generously provided by Frank Gertler, Massachusetts Institute of Technology, Cambridge, MA) (31, 32). We confirmed EVL and VASP deletion in T cells, and verified that Mena was not up-regulated as a compensatory mechanism in EVL/VASP dKO T cells by Western blot analysis (Fig. S1). We then analyzed if EVL/VASP deficiency caused defects in T-cell development. Flow cytometry analysis of lymphocyte populations in the thymus and secondary lymphoid organs showed no gross defects in T-cell development and normal proportions of mature CD4 and CD8 T cells in the periphery of EVL/VASP dKO mice (Fig. S2).
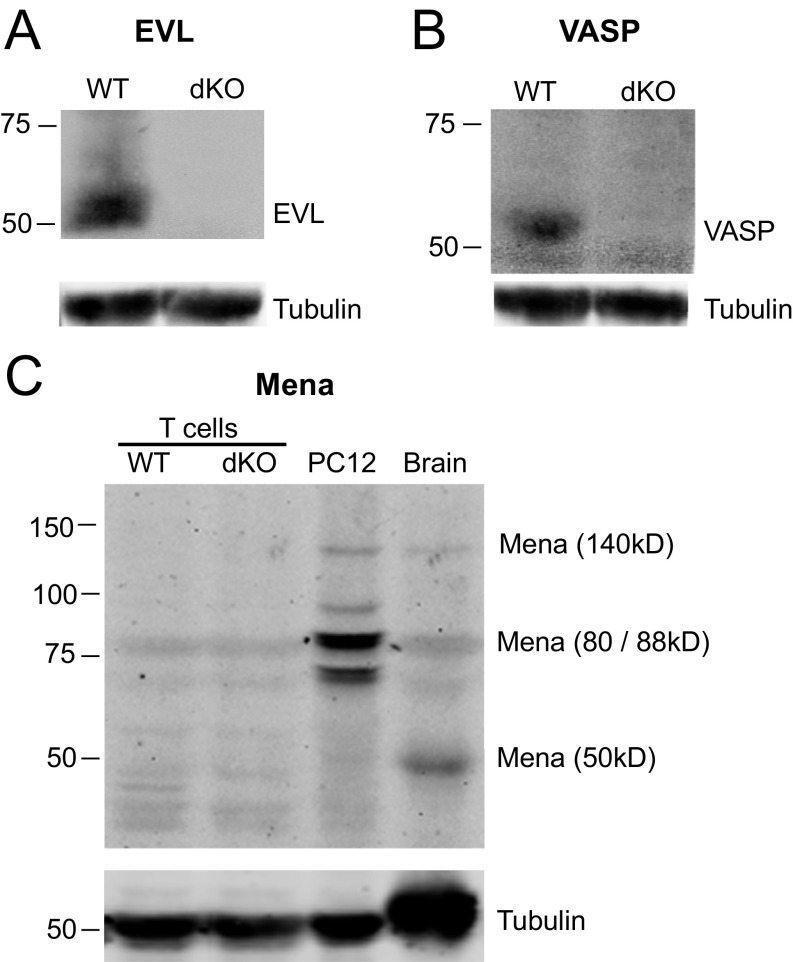
Fig. S1.
EVL/VASP dKO T cells do not express EVL, VASP, or Mena proteins. T-cell lysates were obtained from activated T cells 5–6 d postactivation. (A) EVL protein is expressed in WT T cells, but is not expressed in EVL/VASP dKO T cells. (B) VASP protein is expressed in WT T cells, but is not expressed in EVL/VASP dKO T cells. (C) Compared with WT T cells, Mena protein levels do not increase in EVL/VASP dKO cells to compensate for deletion of EVL and VASP. Brain lysates and PC12 cells were used as positive controls for Mena expression. Data are representative of at least two independent experiments.
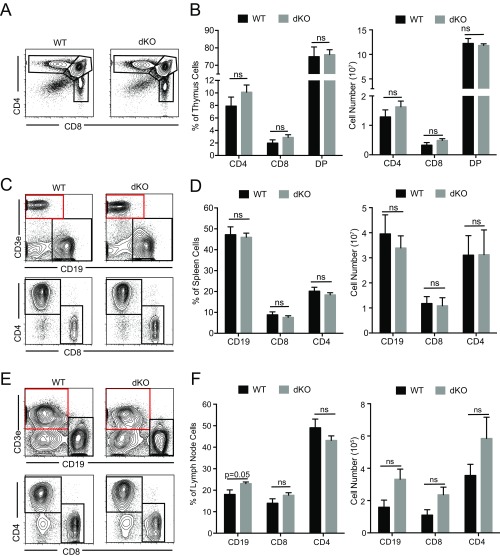
Fig. S2.
T-cell development and lymphocyte populations in peripheral lymphoid organs are not altered in EVL/VASP dKO mice. (A and B) Thymus CD4, CD8, and double-positive T-cell fractions are maintained in EVL/VASP dKO mice both by frequency and by number. (C and D) Spleen CD4, CD8, and B-cell populations are equivalent in EVL/VASP dKO and WT mice, both by frequency and by number. (E and F) Peripheral lymph node CD4, CD8, and B-cell populations are not reduced in EVL/VASP dKO mice, both by frequency and by number. In C and E the red squares highlight the gates used for the Lower panels. Data in A, C, and E are representative of five independent experiments; data in B, D, and F are the mean of five independent experiments. Error bars are SEM; statistics are t tests. ns, not significant.
Deletion of Both EVL and VASP Selectively Impairs Activated CD4 T-Cell Trafficking into Secondary Lymphoid Tissues.
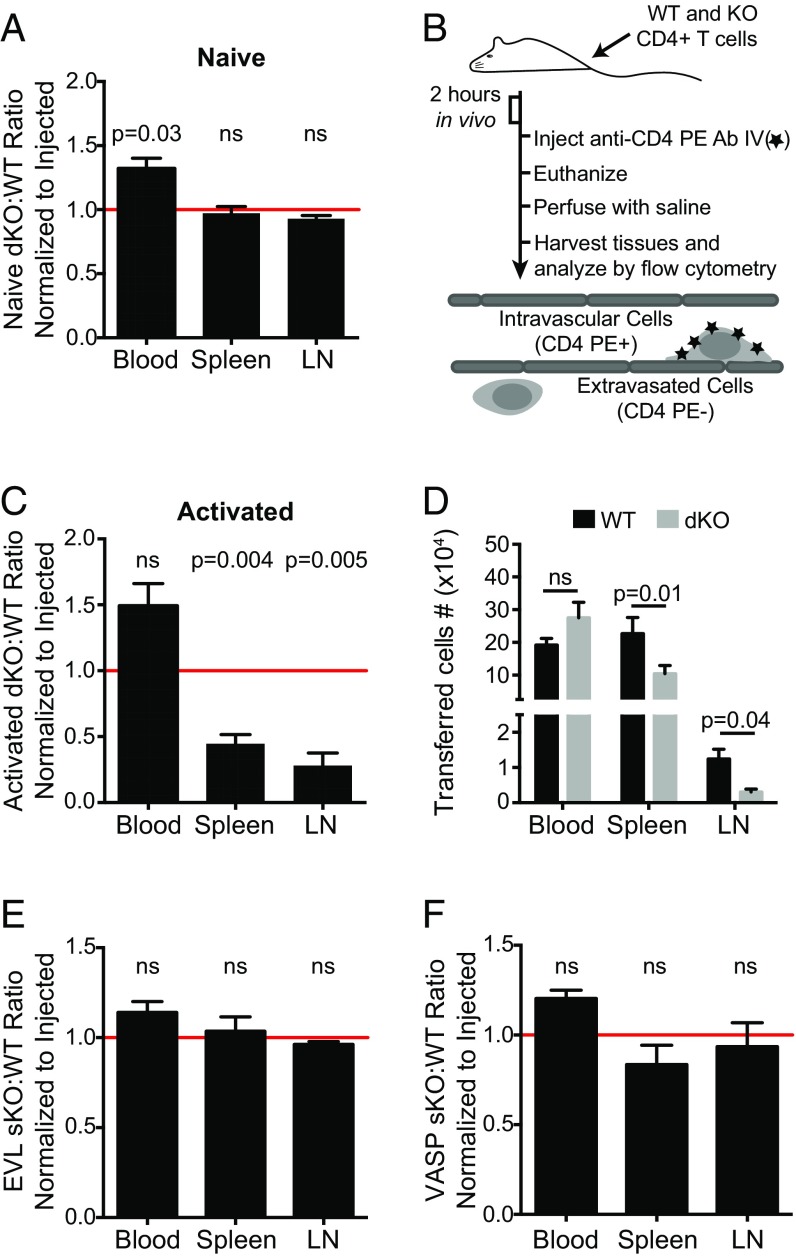
Next, we used coadoptive transfers of WT control and EVL/VASP dKO T cells to study the T-cell–intrinsic effect of EVL and VASP deficiency on trafficking in vivo. Consistent with the normal pattern of development and homeostatic trafficking, dKO and WT naïve CD4 T cells had equivalent homing to the spleen and lymph nodes after intravenous adoptive transfer in to WT recipient mice (Fig. 1A).
Fig. 1.
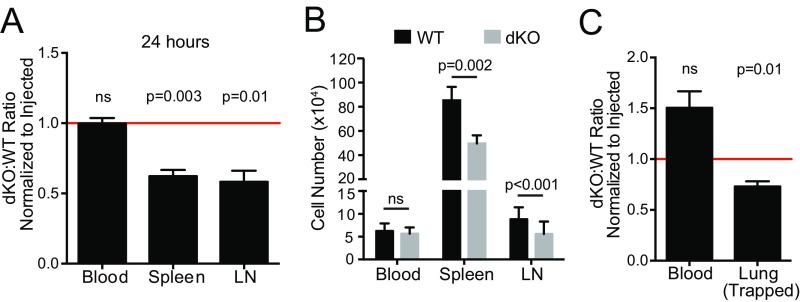
Deletion of both EVL and VASP selectively inhibits activated but not naïve CD4 T-cell trafficking to secondary lymphoid organs. (A) Naïve T-cell trafficking is not affected by EVL and VASP deficiency. Differentially dye-labeled naïve WT and EVL/VASP dKO T cells were coadoptively transferred intravenously at a 1:1 ratio and T-cell trafficking to lymphoid tissues was quantified by flow cytometry 2 h after adoptive transfer. The dKO:WT ratio was normalized to the ratio in the injected sample to account for possible minor variations in the injection mixture (typically < 10%). (B) Experimental set-up for cotransfer of activated WT and KO cells, including intravascular staining method to distinguish intravascular T cells from those that have extravasated into the tissue of interest. (C–F) Dye-labeled CD4 WT and single-KO or dKO activated T cells were coadoptively transferred at a 1:1 ratio and T-cell trafficking to tissues 2 h after adoptive transfer was quantified by flow cytometry as above. A ratio below 1.0 (horizontal red line) indicates impaired homing of KO T cells. (C) Quantification of activated T-cell trafficking. Ratio of extravasated WT and EVL/VASP dKO activated T cells, harvested and analyzed as above. (D) Number of WT and dKO T cells recovered from the indicated tissues from data in C. (E and F) Trafficking of EVL (E) and VASP (F) single-KO activated T cells relative to WT controls. Data are the average from a minimum of three independent experiments. Error bars are SEM. Statistics are one-sample t test compared with a hypothetical value of 1.0 (A, C, E, F) or paired t test (D). LN, lymph node; ns, not significant.
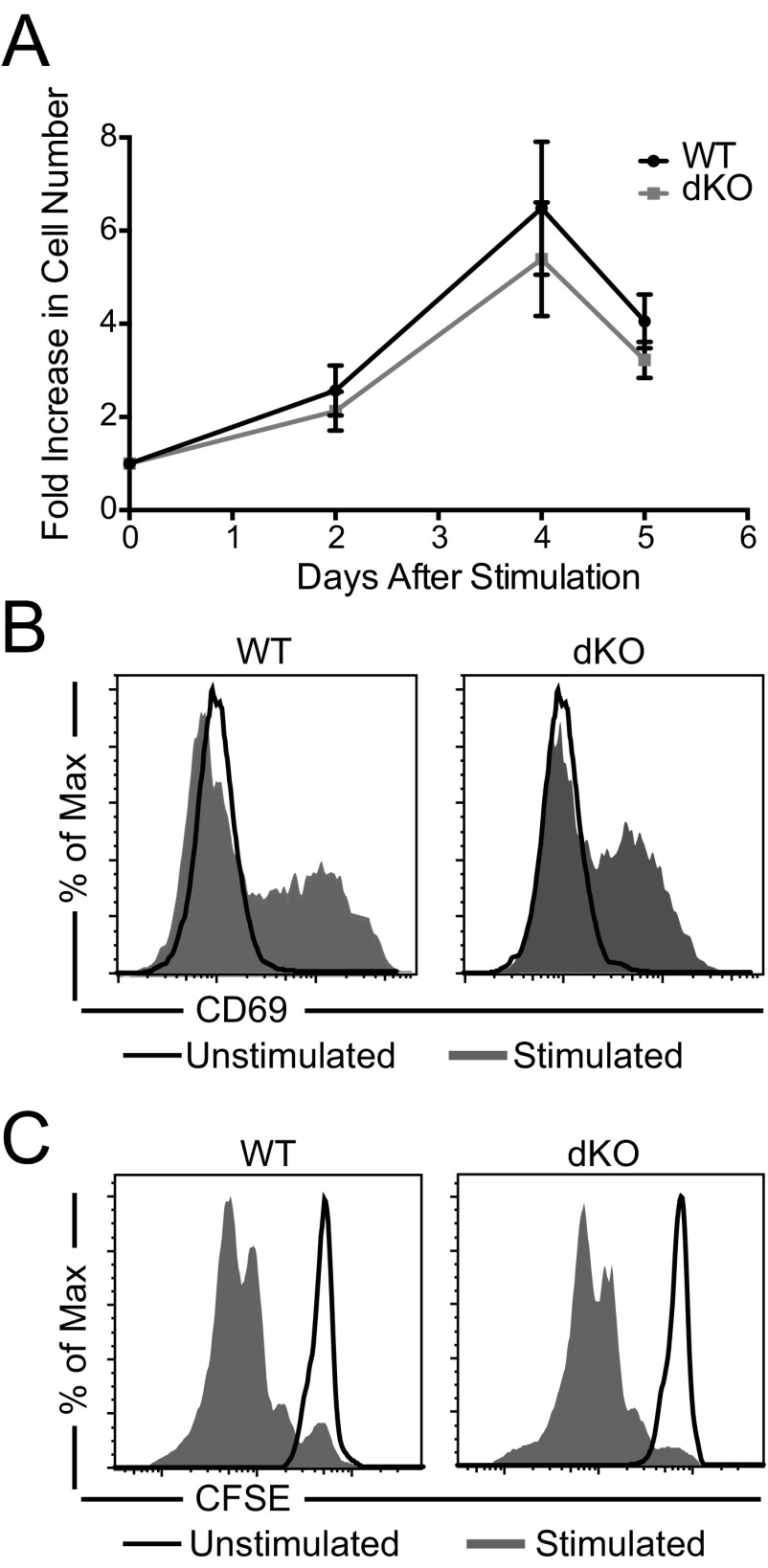
Activated T cells have different requirements for migration and trafficking than their naïve counterparts (33–36). Therefore, we investigated if Ena/VASP family proteins are specifically required for activated T-cell trafficking. We first established that, upon ex vivo polyclonal activation with CD3 and CD28 stimulation, T-cell proliferation kinetics and activation profiles were similar for WT and EVL/VASP dKO T cells (Fig. S3). Activated T cells can become lodged in the vasculature (particularly in the lungs) (37, 38), potentially because of their increased size and adhesion properties. Therefore, we used an established technique (39, 40) to distinguish extravasated T cells from those stuck intravascularly by injecting intravenously a fluorophore-conjugated anti-CD4 antibody immediately before euthanasia of the recipient mice (Fig. 1B). Only extravasated cells (T cells negative for intravascular anti-CD4 staining) were considered to have infiltrated a tissue.
Fig. S3.
Polyclonal CD3-induced T-cell activation is not affected by deletion of EVL and VASP. (A) Time-course of the fold-increase in cell numbers after T-cell activation by anti-CD3 and anti-CD28 of EVL/VASP dKO T cells. (B) Representative CD69 expression on WT and EVL/VASP dKO T cells 48 h after activation. (C) Representative CFSE dilutions of WT and EVL/VASP dKO T cells 48 h after activation. Data in A are the mean of seven independent experiments (error bars are SEM); data in B and C are representative of two independent experiments.
In this setting, in vitro-activated CD4 T cells maintain expression of CCR7 and can recirculate to secondary lymphoid organs. To determine if homeostatic trafficking of activated T cells was affected by Ena/VASP deficiency, we cotransferred control and EVL/VASP dKO T cells into unimmunized recipient mice. Following intravenous adoptive transfer, activated dKO CD4 T cells on average exhibited a 2.2-fold reduction in spleen trafficking and a 3.3-fold reduction in lymph node trafficking 2 h after adoptive transfer compared with WT controls (Fig. 1 C and D). This activated dKO T-cell trafficking defect still persisted 24 h posttransfer (Fig. S4 A and B).
Fig. S4.
Impairment in activated EVL/VASP dKO T-cell trafficking to peripheral lymphoid organs is maintained 24 h posttransfer, and does not result from trapping in the lung. (A) Trafficking of activated WT and EVL/VASP dKO T cells in peripheral lymphoid tissues harvested 24 h after T-cell transfer, expressed as a ratio and normalized to the ratio in the injected sample. A ratio below 1.0 (red line) indicates impaired homing of dKO T cells. (B) Number of WT and dKO T cells recovered from the indicated tissues from data in A. (C) Activated WT and EVL/VASP dKO T cells trapped in the blood vessels of the lung 2 h after transfer (measured by intravascular staining). Data expressed as a ratio and normalized to the ratio in the injected sample. Data are the mean of four independent experiments; error bars are SEM. Statistics in A and C are one-sample t test compared with a hypothetical value of 1.0; statistics in B are paired t tests. ns, not significant.
Furthermore, the intravascular staining used to quantify T cells that had entered tissues versus cells that remained adhered within blood vessels also indicated that the defect in activated EVL/VASP dKO T-cell trafficking was not a result of selective trapping of these cells in the lung microvasculature, the first capillary bed encountered after intravenous adoptive transfer. In fact, significantly more WT than dKO T cells were recovered from inside the lung microvasculature (Fig. S4C). In keeping with these data, there is a trend toward more EVL/VASP dKO T cells than control cells in the blood compartment, suggesting that these cells may be unable to gain full access to tissues from the blood (Fig. 1 C and D).
We next analyzed if deletion of EVL or VASP alone was sufficient to impair activated T-cell trafficking to secondary lymphoid organs. Consistent with the likelihood of redundant or compensatory functions between Ena/VASP proteins, single-knockout of EVL or VASP did not give rise to activated T-cell trafficking defects (Fig. 1 E and F).
EVL and VASP Deletion Impairs Activated CD4 T-Cell Trafficking to Sites of Inflammation.
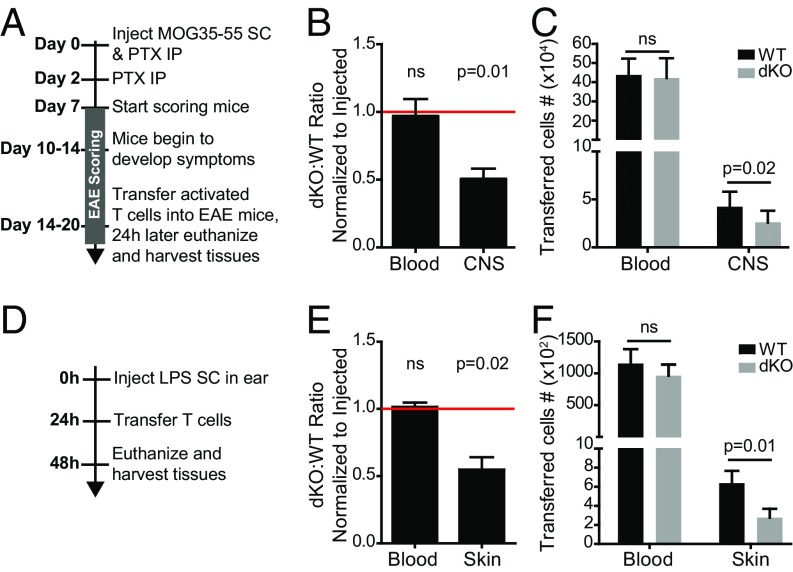
We next sought to determine if activated CD4 T-cell trafficking to sites of inflammation was affected by Ena/VASP protein deficiency. Because the vascular endothelial barrier in the central nervous system (CNS) is particularly restrictive, we first investigated EVL/VASP-deficient activated T-cell trafficking to the CNS in the context of autoimmune inflammation, using a mouse model of multiple sclerosis: experimental autoimmune encephalomyelitis (EAE). Twenty-four hours after adoptive transfer of T cells into mice with ongoing EAE, we quantified the number of transferred T cells that had extravasated into the CNS using the intravascular staining technique described above. Activated dKO T cells exhibited on average a 2.0-fold reduction in trafficking to the CNS relative to control T cells (Fig. 2 A–C). Next, we analyzed T-cell trafficking to the inflamed skin, using lipopolysaccharide (LPS) as the inflammatory stimulus. Recipient mice were treated by subcutaneous LPS injections in the ear and 24 h later activated control and dKO T cells were intravenously transferred into the recipient mice. Quantification of transferred T cells that had extravasated into the inflamed skin of the ear showed a 1.8-fold reduction in dKO T-cell trafficking to this site (Fig. 2 D–F).
Fig. 2.
EVL and VASP deletion inhibits activated CD4 T-cell trafficking to the CNS during EAE and to the inflamed skin. (A) Experimental set-up for cotransfer of WT and EVL/VASP dKO activated T cells into mice with ongoing EAE. Activated, dye-labeled polyclonal CD4 WT and dKO T cells were coadoptively transferred at a 1:1 ratio, and were harvested 24 h posttransfer from the blood and CNS (brain and spinal cord). (B) Activated T-cell trafficking during EAE was quantified by flow cytometry, shown as the ratio of dKO:WT T cells normalized to the ratio in the injected sample. (C) Number of WT and dKO T cells recovered from the indicated tissues from data in B. (D) Experimental set-up to quantify activated T-cell trafficking to the inflamed skin. Twenty-four hours after LPS-induced inflammation in the ears, WT and dKO activated T cells were coadoptively transferred at a 1:1 ratio, and were harvested 24 h posttransfer from the blood and ears of the recipient mice. (E) Ratio of dKO:WT T cells recovered from blood and ears, normalized to the ratio in the injected sample. (F) Number of WT and dKO T cells recovered from the indicated tissues from data in E. Data are the average of four independent experiments. Error bars are SEM. Statistics are one-sample t test compared with a hypothetical value of 1.0 (B, E) or paired t test (C, F). IP, intraperitoneal; ns, not significant; SC, subcutaneous.
Deletion of EVL and VASP Reduces Chemokine-Triggered Actin Polymerization but Does Not Impair Activated T-Cell Chemotaxis in Vitro.
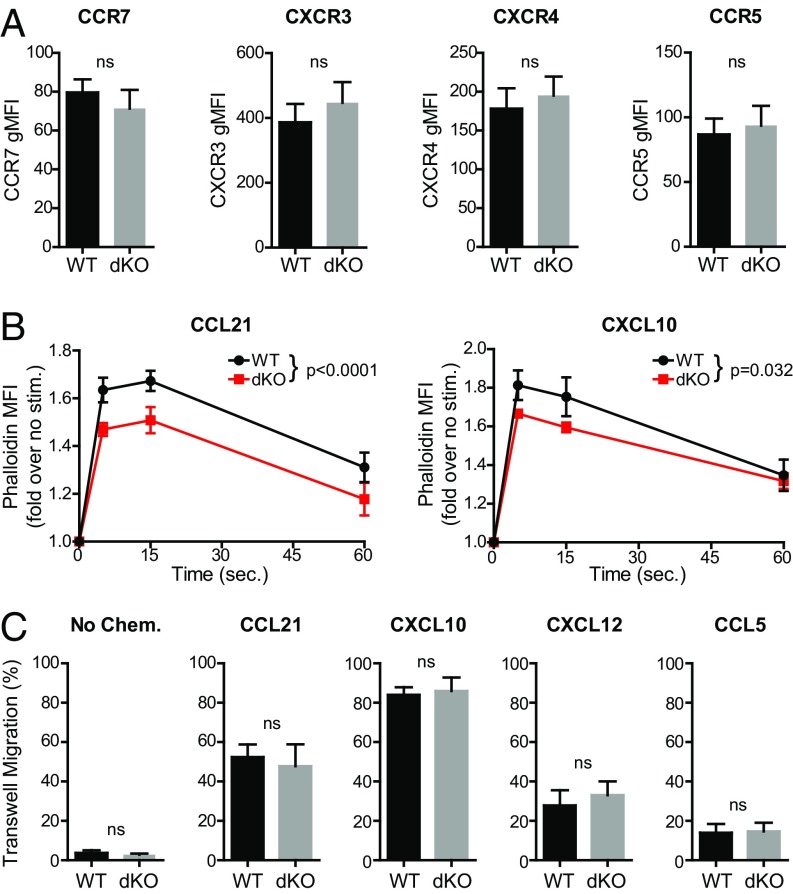
Chemokine signaling is instrumental in mediating leukocyte migration and trafficking (41, 42). Therefore, we analyzed if the trafficking defect of Ena/VASP-deficient activated T cells could be because of an altered ability to respond to chemokine stimulation. First, we measured the expression of chemokine receptors involved in both homeostatic and inflammatory trafficking. Our data showed no difference in the expression of CCR7, CXCR3, CXCR4, and CCR5 between control and EVL/VASP dKO CD4 T cells (Fig. 3A).
Fig. 3.
Deletion of both EVL and VASP in activated CD4 T cells reduces chemokine-triggered actin polymerization but does not impair chemotaxis. (A) Quantification of chemokine receptor expression in WT and EVL/VASP dKO activated T cells; data shown as gMFI. (B) Time-course analysis of actin polymerization in WT and dKO activated T cells in response to 100 ng/mL CCL21 stimulation (WT vs. dKO curve comparison P < 0.0001) or 100 ng/mL CXCL10 stimulation (WT vs. dKO curve comparison P = 0.032), measured by flow cytometry quantification of fluorescent phalloidin staining. (C) Chemotactic migration across 5-μm pore Transwell chambers in the absence of chemokine, or in the presence of CCL21 (100 ng/mL), CXCL10 (100 ng/mL), CXCL12 (1 μg/mL), or CCL5 (100 ng/mL) in the bottom wells as indicated. Data are the average of at least three independent experiments; error bars are SEM; statistics are paired t tests in A and C, or two-way ANOVA in B. ns, not significant.
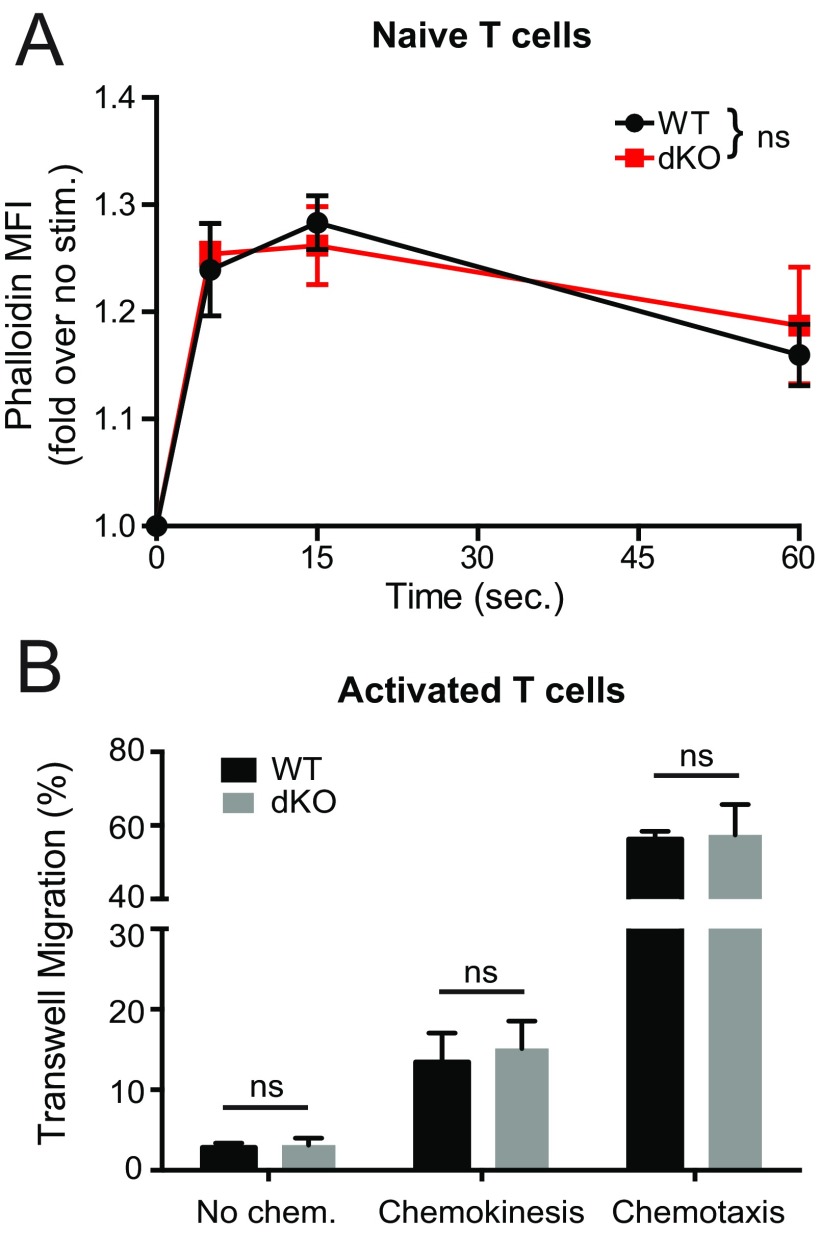
Ena/VASP proteins are cytoskeletal effectors that promote actin filament polymerization (14). During migration, chemokine stimulation can trigger actin network remodeling and promote motility (34). Therefore, we analyzed if Ena/VASP deficiency impaired actin polymerization in response to chemokine stimulation. To this end, we measured the F-actin content in control and EVL/VASP dKO T cells before and after chemokine stimulation in a time-course analysis. Our results showed that dKO-activated T cells had a small but significant defect in actin polymerization promoted by CCL21 stimulation and, to a lesser degree, by CXCL10 (Fig. 3B). However, when we analyzed actin polymerization in response to CCL21 in naïve T cells, we did not see a defect in EVL/VASP dKO cells (Fig. S5A). This finding suggests a different reliance on Ena/VASP proteins for actin polymerization between naïve and activated T cells.
Fig. S5.
Normal chemokine-induced actin polymerization in naïve EVL/VASP dKO T cells, and normal chemokinesis and chemotaxis of EVL/VASP dKO activated T cells. (A) Time-course analysis of actin polymerization in WT and dKO naïve T cells in response to 100 ng/mL CCL21 stimulation (WT vs. dKO curve comparison not significant), measured by flow cytometry quantification of fluorescent phalloidin staining. (B) Activated T-cell migration across 5-μm pore Transwell chambers, either in the absence of chemokine or with CCL21 (1 μg/mL) both in the upper and lower wells (chemokinesis), or with CCL21 (1 μg/mL) only in the lower well (chemotaxis). Data are the mean of three independent experiments; error bars are SEM. Statistics in A are two-way ANOVA, statistics in B are paired t tests. ns, not significant.
Based on this result, we then measured chemokine-stimulated migration using Transwell chambers. There were no significant differences in migration in the absence of chemokine, or in chemotaxis toward CCL21, CXCL10, CXCL12, or CCL5 in the lower chamber between control and EVL/VASP dKO-activated T cells (Fig. 3C). Furthermore, chemokinetic migration in response to CCL21 in both the upper and lower chambers was also unaffected (Fig. S5B). Although we detected slightly reduced chemokine-mediated actin polymerization in EVL/VASP-deficient activated T cells, overall these data showing normal chemotaxis suggest that the strong trafficking defect of EVL/VASP dKO T cells in vivo is not explained by an overall defect in migratory capacity or chemokine sensing.
Activated CD4 EVL/VASP dKO T Cells Are Deficient in α4 Integrin Expression and Function.
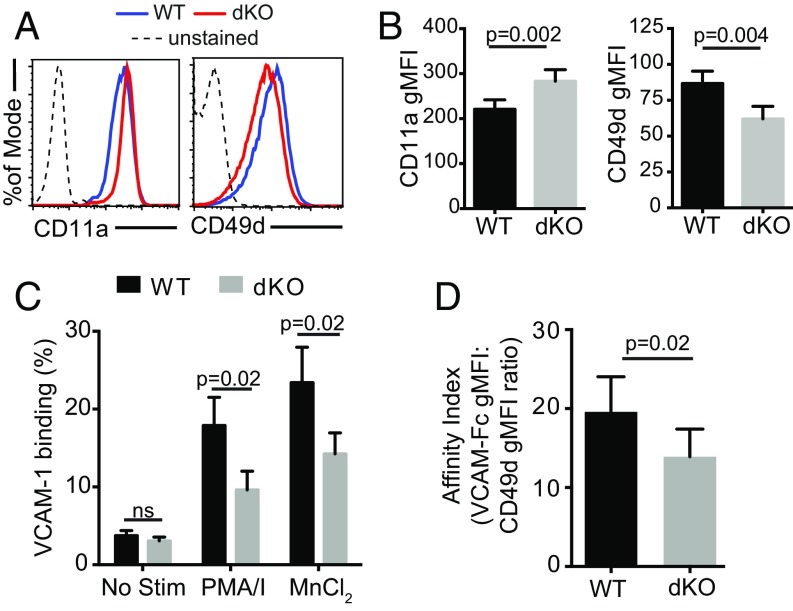
Ena/VASP proteins are reported to affect adhesion and integrin function (28, 43). Therefore, having ruled-out impaired chemotaxis, we next examined the expression and function of key integrins involved in extravasation to determine if the trafficking impairment we observed was because of integrin defects. Flow cytometry analysis revealed that activated EVL/VASP dKO T cells expressed on average 28% less CD49d, but 28% more CD11a (Fig. 4 A and B).
Fig. 4.
Activated EVL/VASP dKO CD4 T cells have a deficit in α4 integrin (CD49d) expression and function. (A) Examples of CD11a and CD49d expression on WT and EVL/VASP dKO activated T cells by flow cytometry. (B) Quantification of CD11a and CD49d surface expression in activated WT and dKO T cells; data shown as gMFI. (C) CD49d function measured as soluble VCAM-1 binding to T cells in response to the indicated stimuli (MnCl2: manganese chloride; PMA/I: PMA and ionomycin). (D) Affinity for VCAM-1 calculated as PMA/ionomycin-elicited VCAM-1 binding normalized to surface expression of CD49d by gMFI. Data in A are representative of 10 independent experiments; data in B are the average of ten experiments; data in C and D are the average of three independent experiments. Error bars are SEM. All P values are paired t tests. ns, not significant.
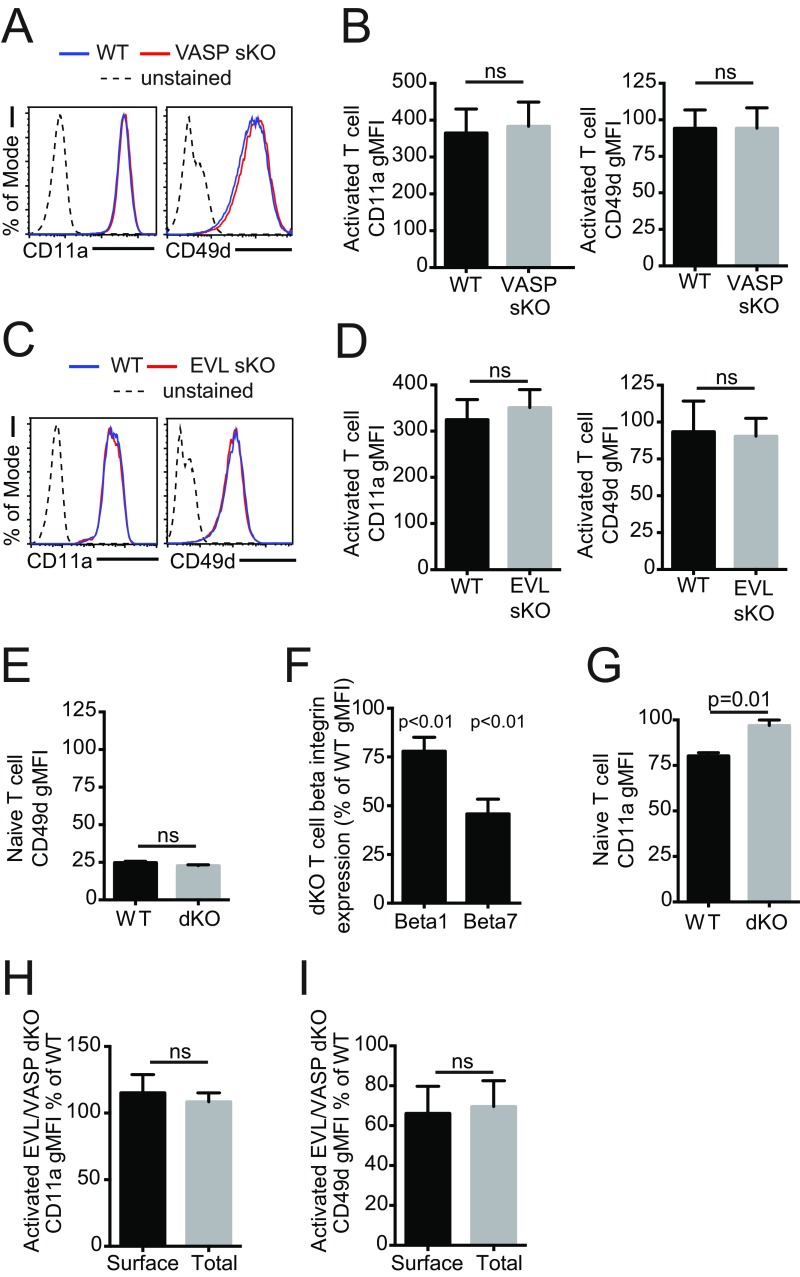
CD49d, the α4 subunit of the integrins α4β1 (VLA-4) and α4β7 (LPAM-1), is primarily expressed on antigen-experienced T cells, which may explain why only activated dKO T cells exhibited a trafficking defect in vivo. Consistent with their normal trafficking phenotype, activated EVL or VASP single-knockout T cells did not exhibit reduced CD49d expression, nor did naïve dKO T cells (which only expressed negligible levels of CD49d) (Fig. S6 A–E). Additionally, expression of the α4 integrin binding partners β1 (CD29) and β7 in activated cells was also reduced in activated dKO T cells compared with WT T cells (Fig. S6F). Furthermore, total expression versus surface expression of CD11a and CD49d were similarly affected in activated dKO T cells, indicating that the defect is not a result of altered integrin trafficking from intracellular stores to the cell surface (Fig. S6 H and I).
Fig. S6.
Integrin expression profiles on activated and naïve EVL and VASP KO T cells. (A) Examples of CD11a and CD49d expression by flow cytometry in activated VASP sKO T cells. (B) Quantification of CD11a and CD49d expression in activated VASP sKO T cells. (C) Examples of CD11a and CD49d expression by flow cytometry in activated EVL sKO T cells. (D) Quantification of CD11a and CD49d expression in activated EVL sKO T cells. (E) Quantification of expression of CD49d in naïve WT and EVL/VASP dKO T cells by flow cytometry. (F) Quantification of CD29 (β1 integrin) and β7 integrin expression in activated WT and EVL/VASP dKO T cells, shown as the percentage of expression in dKO T cells relative to WT T cells by gMFI. (G) Quantification of CD11a expression in naïve WT and EVL/VASP dKO T cells by flow cytometry. (H) Surface expression and total expression (measured by staining after permeabilization) of CD11a in activated T cells, shown as the percentage of expression in dKO relative to WT T cells by gMFI. (I) Surface expression and total expression (measured by staining after permeabilization) of CD49d in activated T cells, shown as the percentage of expression in dKO T cells relative to WT T cells by gMFI. Data in A and C are representative of three independent experiments; data in B and D–I are the mean of at least three independent experiments. Error bars are SEM; statistics are paired t tests except in F, which are one-sample t test compared with a hypothetical value of 100. ns, not significant.
Integrin activity can be regulated by conformation, which influences integrin affinity for ligands (34, 44). The main CD49d ligands are fibronectin and VCAM-1, with the latter expressed on vascular endothelial cells. Therefore, to determine if CD49d function was compromised in EVL/VASP-deficient T cells, we measured WT and dKO T-cell binding to soluble VCAM-1. In the absence of stimulation, there was very low basal VCAM-1 binding capacity and no difference between WT and dKO activated T cells. However, in response to phorbol myristate acetate (PMA)/ionomycin stimulation or treatment with MnCl2 (which exogenously forces integrins into a high-affinity conformation), a lower percent of dKO cells bound VCAM-1 (Fig. 4C). Because dKO T cells expressed less CD49d, we normalized for this difference in expression to evaluate CD49d affinity for VCAM. We calculated a VCAM-1/CD49d affinity index for WT and dKO T cells upon stimulation with PMA/ionomycin by normalizing the geometric mean fluorescence intensity (gMFI) of bound VCAM-1 to the surface expression of CD49d (also by gMFI) (Fig. 4D). This analysis showed significantly decreased CD49d affinity for VCAM-1 on a per receptor basis upon deletion of EVL and VASP. Taken together, these data show that deletion of EVL and VASP decreases CD49d expression on the T-cell surface and impairs inside-out signaling activation of CD49d.
Activated EVL/VASP dKO CD4 T Cells Are Competent in Shear-Resistant Adhesion and Crawling.
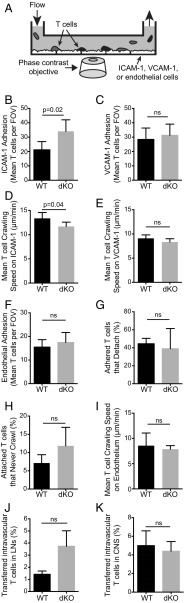
To determine how the perturbation in CD49d activity we observed could influence the impaired trafficking phenotype of EVL/VASP-deficient T cells, we then investigated if decreased expression and function of CD49d in EVL/VASP-deficient T cells caused impaired adhesion or motility on integrin ligands. Using flow chambers that recapitulate the physiologic shear forces of the microvasculature (Fig. 5A), we first quantified shear-resistant adhesion to integrin ligands in the presence of chemokine. Compared with controls, EVL/VASP dKO T-cell adhesion to ICAM-1 was actually moderately improved (Fig. 5B), consistent with increased CD11a expression, and no significant differences were observed in shear-resistant adhesion to VCAM-1 (Fig. 5C). We then analyzed dKO T-cell 2D migration on ICAM-1– or VCAM-1–coated surfaces under flow. Time-lapse spinning-disk confocal microscopy was used to image T cells, and the mean crawling speed of dKO T cells compared with WT was found to be slightly slower on ICAM-1 (13% less) (Fig. 5D) and equivalent on VCAM-1–coated surfaces (Fig. 5E), suggesting that adhesion and motility on integrins is not severely impaired in dKO T cells.
Fig. 5.
EVL/VASP dKO activated CD4 T cells are competent in shear-resistant adhesion and migration. (A) Schematic of the experimental set-up for measuring T-cell adhesion and motility under physiologic shear forces. (B and C) WT and EVL/VASP dKO T-cell shear-resistant adhesion to ICAM-1 (B), or to VCAM-1 (C). (D and E) Mean WT and dKO T-cell crawling speed under flow on ICAM-1 in the presence of CCL21 (D) or on VCAM-1 in the presence of CCL21 (E). (F) Quantification of WT and dKO T-cell shear-resistant adhesion to TNF-α–activated primary microvascular brain endothelial cells in the presence of CCL21. (G) Detachment of initially adhered T cells from activated endothelial monolayers after shear flow increase from 0.2 to 2 dyne/cm2. (H) Hyperadhesiveness of WT and dKO T cells to primary brain endothelial cells, measured as the frequency of adhered T cells that failed to crawl. (I) Mean T-cell crawling speed under shear forces on activated brain endothelial monolayers. (J) Percentage of in vivo adoptively transferred WT and dKO T cells recovered from lymph nodes that remained in the vasculature 2 h posttransfer (identified by intravascular staining). (K) Percentage of transferred T cells recovered from the CNS that remained intravascular, 24 h posttransfer. Data represent the average of a minimum of three independent experiments; error bars are SEM; P values are paired t tests. ns, not significant.
Next, adhesion to primary endothelial cells was quantified in vitro to determine if T-cell adhesion to the vascular wall was likely to be inhibited in vivo. Primary brain microvascular endothelial cells were grown to confluence in flow chambers, activated with TNF-α 24 h before imaging (to up-regulate adhesion molecule expression), and pretreated with CCL21 30 min before imaging (to stimulate T-cell adhesion). Fluorescently dyed T cells were flowed through the chamber and imaged with fluorescent and phase-contrast spinning-disk microscopy to quantify the number of adhered T cells. Surprisingly, deletion of EVL and VASP did not alter shear-resistant T-cell adhesion to primary brain endothelial cells (Fig. 5F), and detachment from the endothelial monolayer under flow conditions was also unchanged (Fig. 5G). Furthermore, no statistically significant change was noted in the percentage of EVL/VASP dKO T cells that adhered to the endothelial monolayer but never crawled (Fig. 5H). Similarly, T-cell crawling speed on the endothelial monolayer under flow conditions, an in vitro model for intravascular crawling in vivo, was unaffected by EVL and VASP deletion (Fig. 5I). Taken together, these data indicate that despite differences in integrin expression and function, EVL/VASP dKO T cells are capable of normal adhesion and migration on endothelial cells in vitro, suggesting that a different mechanism caused the trafficking impairment of EVL/VASP-deficient T cells.
Furthermore, using the intravascular staining method described above to quantify T cells that remained intravascular in our in vivo trafficking experiments, we did not see a significant difference or defect in the frequency of EVL/VASP dKO activated T cells remaining in the vasculature of isolated lymph nodes from recipient mice 2 h posttransfer (Fig. 5J). In fact, there might be a trend toward increased numbers of dKO T cells adhered to the lymph node vasculature. We also did not observe a significant difference in the frequency of EVL/VASP dKO T cells that are found in the CNS microvasculature of mice with EAE, 24 h after adoptive transfer (Fig. 5K). Although this is not a direct readout of adhesion to the microvasculature, it further indicates that the impairment in activated dKO T-cell trafficking is unlikely to be because of a deficiency in the adhesion step of extravasation.
Deletion of EVL and VASP Inhibits the Diapedesis Step of Transendothelial Migration in Vitro.
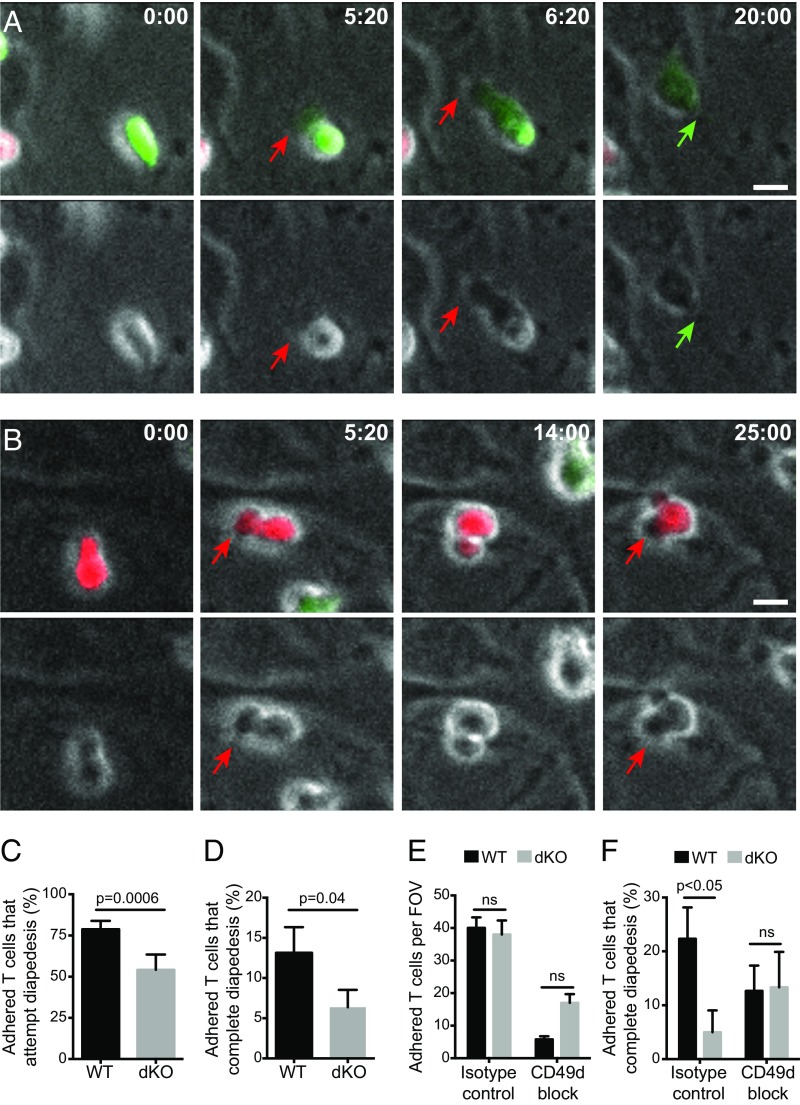
In addition to playing a role in adhesion and intravascular crawling, integrins are crucially important for the diapedesis step of TEM (33, 45). Therefore, we investigated whether the trafficking defect of EVL/VASP-deficient T cells could be caused by defects in migration through the endothelial barrier during extravasation. For these experiments, we imaged T-cell TEM on primary brain endothelial cells in the flow-chamber system described above using phase contrast and spinning disk confocal microscopy to determine the effects of EVL and VASP deletion on T-cell diapedesis in vitro. Using phase-contrast microscopy, with the plane of focus set to the endothelial monolayer, T cells attached on the endothelium (above the plane of focus) appear to have a white halo surrounding them. In contrast, T cells that have undergone diapedesis and the protrusions produced during that process lose this halo (6, 46) (Fig. 6 A and B and Movies S1 and S2). Collected images were analyzed to determine the frequency and timing of diapedesis attempts, defined as T-cell production of fluorescent protrusions lacking a phase halo, and diapedesis completions, defined as events in which the T-cell body follows after the protrusion and the entire T-cell loses its halo. This analysis revealed that deletion of EVL and VASP significantly impaired both T-cell diapedesis attempts and completions (Fig. 6 C and D), indicating that the mechanism of reduced dKO T-cell trafficking in vivo lies at the stage of diapedesis.
Fig. 6.
EVL/VASP-deficient activated CD4 T cells are impaired in the diapedesis step of transendothelial migration in vitro through a CD49d-dependent mechanism. Fluorescently labeled WT and EVL/VASP dKO activated T cells were flowed on primary brain microvascular endothelial monolayers and imaged by time-lapse fluorescent and phase-contrast microscopy. (A and B) Representative examples of diapedesis completion (A) and diapedesis attempts without completion (B), as visualized by phase microscopy. (Upper) Fluorescence overlay on phase channel; (Lower) phase channel alone. WT T cells are in green, dKO T cells in red. Red arrows indicate diapedesis attempts (extension of protrusions), green arrows indicate diapedesis completion. (Scale bars, 5 μm.) Timestamps are minutes:seconds. (C and D) Quantification of the percentage of adhered WT and dKO T cells that attempted (C) or completed (D) diapedesis. (E) Shear-resistant adhesion of WT and dKO T cells to a monolayer of primary endothelial cells with or without CD49d blockade (overall ANOVA P = 0.0002). (F) Frequency of adhered T cells that completed diapedesis with or without CD49d-blockade (overall ANOVA P = 0.005). Images in A and B are representative of six independent experiments; data are the average of six (C and D) or four (E and F) independent experiments. Error bars are SEM. P values are paired t tests (C and D) or one-way ANOVA with post hoc Tukey test (E and F). ns, not significant.
CD49d-Independent Diapedesis Does Not Require EVL and VASP.
Finally, having identified a defect in CD49d function in the EVL/VASP dKO T cells, we examined how CD49d affected the diapedesis of control and EVL/VASP-deficient T cells. To this end, we preincubated T cells with CD49d-blocking or isotype control antibodies before introducing the T cells into flow chambers, and then imaged for 30 min and analyzed the imaging data, as described above. As expected, shear-resistant adhesion of both control and dKO T cells to the endothelial monolayer was dramatically impaired by CD49d blockade. However, there was a trend toward more EVL/VASP dKO than WT T cells adhering to the endothelial monolayer with CD49d blockade, suggesting that they may use alternate adhesion molecules more effectively (Fig. 6E). As opposed to control antibody treatment, which confirmed a significant reduction in TEM of the dKO T cells, CD49d blockade greatly reduced the number of adhered T cells on the endothelial monolayer and resulted in a similar ability of the adhered WT and dKO T cells to complete diapedesis (Fig. 6F). These data support the idea that EVL and VASP promote T-cell diapedesis via a CD49d-using mechanism, and that impaired CD49d function in EVL/VASP-deficient T cells is a mechanism for their in vivo defect in T-cell trafficking.
Discussion
This report on the role of EVL and VASP in T-cell migration and trafficking identifies a key role for the actin effector proteins EVL and VASP in activated, but not naïve T-cell trafficking, and determines that the trafficking defect in EVL/VASP dKO T cells occurs at the diapedesis step of TEM. EVL/VASP deficiency resulted in impaired activated T-cell trafficking to the CNS during autoimmune neuroinflammation, to the inflamed skin and to secondary lymphoid organs. Nonetheless, based on the varying endothelial barrier properties and adhesion molecules used for extravasation into different tissues, it is possible that the Ena/VASP family may have a more or less profound impact on trafficking to different anatomical sites.
The observation that deletion of EVL and VASP impaired activated T-cell trafficking in vivo is consistent with the literature implicating Ena/VASP proteins in cellular motility (27, 47–51). Although we identified reduced actin polymerization in response to chemokine triggering in activated EVL/VASP dKO T cells, surprisingly we found no major effect on activated T-cell chemotaxis or crawling in vitro. The finding that shear-resistant T-cell adhesion to primary brain endothelial cells was not affected by deletion of EVL and VASP was even more surprising, as Ena/VASP proteins are established negative regulators of platelet adhesion to the vascular wall (28).
To determine the mechanism of the trafficking defect of EVL/VASP-deficient T cells, we analyzed adhesion molecule function and observed alterations in expression and activity of T-cell integrins upon deletion of EVL and VASP. Surface and total expression of CD49d was decreased in EVL/VASP dKO T cells, whereas CD11a expression was slightly but significantly increased. Shear-resistant adhesion to purified ICAM-1 was enhanced upon deletion of EVL and VASP, as expected based on CD11a levels. However, although CD49d expression was reduced in EVL/VASP dKO T cells, there was no corresponding decrease in shear-resistant adhesion to VCAM-1. Because surface integrins need to be activated to achieve a high-affinity conformation, differences in expression do not necessarily correspond to alterations in function. However, when we quantified CD49d affinity for VCAM-1, we found that EVL/VASP dKO T cells not only bound less soluble VCAM-1 than WT control cells, but were also less capable of increasing their VCAM-1 binding capacity in response to PMA/ionomycin stimulation. This finding suggests that EVL and VASP positively regulate both expression and activation of CD49d.
These data suggest that differential regulation of CD49d and CD11a upon deletion of EVL/VASP could explain the preservation of endothelial adhesion we observed, with increased CD11a compensating for decreased CD49d. Indeed, upon CD49d blockade, dKO T cells trended toward increased endothelial adhesion relative to WT controls, suggesting that they may have developed enhanced CD49d-independent adhesion mechanisms in response to poor CD49d function. However, because adhesion to and crawling on endothelial cells is unaffected in EVL/VASP dKO T cells, simple alterations in the adhesive functions of these integrins do not provide a sufficient explanation for the trafficking defects we observed in vivo. In contrast, our in vitro TEM data point to a critical nonredundant role for EVL and VASP in diapedesis, suggesting that the requirement for Ena/VASP family proteins in activated T-cell trafficking occurs specifically at the diapedesis step of TEM.
Our finding that EVL and VASP are required for diapedesis but not adhesion, motility, or chemotaxis is currently unique among actin cytoskeletal regulators. For example, our previous work (6) has indicated that the cytoskeletal effector myosin-IIA affects both T-cell motility and diapedesis, and deletion of mDia1, another actin effector protein, both impairs thymocyte development and produces defects in chemotactic migration and in vivo homing of naïve T cells (11). Similarly, deletion of the cytoskeletal regulators Rap1, RIAM, talin, or RAPL impair chemokine-stimulated ICAM-1 adhesion and naïve T-cell trafficking (52–54), whereas CRK proteins regulate T-cell adhesion, chemotaxis, and diapedesis, leading to reduced T-cell trafficking selectively to inflamed tissues (55). In contrast, recent reports show that Kindlin-3 is not required for diapedesis, although it has been shown to play an important role in adhesion and CNS trafficking more generally (56, 57).
We propose that the unique and selective role of EVL and VASP in activated T-cell diapedesis is related to alterations in CD49d activity. Poor trafficking of activated EVL/VASP dKO T cells correlated with decreased CD49d expression and function. Conversely, naïve EVL/VASP dKO T cells trafficked normally and their CD49d expression, although very low, was not significantly different from the low level expressed in control T cells. Furthermore, activated EVL or VASP single-knockout T cells expressed normal CD49d levels, possibly explaining the normal trafficking pattern they exhibit. Taken together, these data identify impaired CD49d expression and function, which are known to regulate activated T-cell homing to peripheral lymph nodes and sites of inflammation (58), as a mechanism mediating the impairment in EVL/VASP-deficient T-cell diapedesis and trafficking.
In line with this notion, control and EVL/VASP dKO cells that adhered to endothelial monolayers despite CD49d blockade were equally likely to undergo diapedesis in vitro. This finding suggests that EVL and VASP are specifically required for CD49d-dependent diapedesis, and opens up new questions about how such a specific requirement might be regulated.
The role of CD49d and VCAM-1 in T-cell extravasation is well established. Various studies have reported that ligation of VCAM-1 stimulates changes in endothelial permeability, triggering dissociation of vascular endothelial (VE) protein tyrosine-phosphatase (VE-PTP) from VE-cadherin, phosphorylation of VE-cadherin at tyrosine 731, and ultimately, down-regulation of VE-cadherin from endothelial cell junctions (59–61). T-cell protrusions that are generated during the “intravascular crawling” stage of extravasation have previously been proposed to trigger chemokine depot-initiated diapedesis (35). It has also been suggested that filopodia generated by T cells can promote the sensing of permissive sites for diapedesis (62). Ena/VASP family proteins are involved in generating membrane protrusions such as filopodia (20, 63, 64) and localize to the microspikes generated upon ligation of the TCR (24). Consistent with this finding, our data showed reduced protrusions initiating diapedesis by EVL/VASP-deficient T cells. Furthermore, activated CD49d clusters at the leading edge of migrating T cells (65), and the microvilli that form at the leukocyte-endothelial cell interface in migrating leukocytes are rich in CD49d but not CD18 integrins (66). Further study may allow determination of whether EVL and VASP coordinate the presentation of CD49d on T-cell protrusions, which are able to trigger the opening of endothelial junctions via signaling through VCAM-1.
Similar to our results using EVL/VASP dKO T cells, conditional knockout of CD49d in mice results in impaired T-cell trafficking to an inflamed CNS (67). Interference with CD49d-mediated T-cell trafficking via monoclonal antibody blockade has made natalizumab a very effective treatment for multiple sclerosis (1, 2) and Crohn’s disease (68). This therapy targets activated or memory T cells without significantly impairing naïve T-cell surveillance. However, use of natalizumab is limited in patients who are infected with the JC Virus, as they are at risk for progressive multifocal leukoencephalopathy, a fatal complication (69, 70). Natalizumab-treated patients can also experience increased susceptibility to urinary and respiratory tract infections, which can trigger relapses (71).
Our work supports the idea that CD49d has a specific, EVL/VASP-dependent function during the diapedesis step of TEM, which is distinct from its roles in adhesion and intravascular crawling. Although EVL/VASP-directed therapies would need to be targeted to lymphocytes to avoid effects on other cells, our work suggests that further studies of EVL and VASP in T cells could identify therapeutically useful ways to more selectively modulate CD49d activity and activated T-cell trafficking.
Materials and Methods
Ethics Statement.
All experiments involving mice were conducted in compliance with the NIH's Guide for the Care and Use of Laboratory Animals (72) and with the approval by the Institutional Animal Care and Use Committee of National Jewish Health (Protocol #AS2811-02-17). All efforts were made to minimize mouse suffering.
Mice.
KO mice lacking EVL and VASP were generated, respectively, by Kwiatkowski et al. (31) and Aszódi et al. (32) (EVL/VASP dKO mice were generously provided by Frank Gertler, Massachusetts Institute of Technology, Cambridge, MA). These mice were on a 129 × C57BL/6 mixed background and bred in-house at National Jewish Health. Single EVL or VASP KO and WT 129 × C57BL/6 mice were derived from the double-KO mice and were maintained in parallel. Recipient mice used for homeostatic trafficking assays were 129S1 × C57BL/6 F1 hybrid mice (Stock #101043, The Jackson Laboratory). For EAE induction and short-term CNS trafficking, as well as skin trafficking experiments, CD45.1 congenically marked C57BL/6 recipient mice (Strain #564) were purchased from Charles River. CD45.1 C57BL/6 mice were also used to provide third-party WT splenocytes for T-cell activations.
T-Cell Isolation.
Naïve CD4+ T cells were isolated and purified by negative selection. Briefly, spleens, as well as mesenteric, brachial, axial, and inguinal lymph nodes, were harvested and single-cell suspensions were made by passing tissues through 100-μm sterile filters. T cells were purified using StemCell EasySep magnetic isolation kits. Naive CD4 T-cell selection kits were used for naïve cells; whereas total CD4 T-cell selection kits were used when T cells were then subsequently activated in vitro.
T-Cell Activation and Culture.
T cells were cultured using R10: RPMI 1640 with the addition of l-glutamine, penicillin, streptomycin, and β-mercaptoethanol (all purchased from Invitrogen) and 10% (vol/vol) FCS (Fisher Scientific). Purified CD4+ T cells were activated in vitro with plate-bound anti-CD3 and soluble anti-CD28 antibodies (BioXcell), in the presence of third-party WT feeder splenocytes. Feeder cells that could be identified by a congenic marker (CD45.1) were harvested from third-party mouse spleens as above, and red blood cells were lysed in 175 mM ammonium chloride. Splenocytes were then irradiated at 1,500 rads, and mixed in a 2:1 ratio with purified T cells. Next, 2 µg/mL soluble anti-CD28 (clone PV-1) was added to the mixture, and cells were plated at 2 × 106/mL in a 24-well plate that had been coated with 2 µg/mL anti-CD3 (clone 2C11) for 1–2 h at 37 °C. On day 2 postactivation, cells were resuspended at 1 × 106/mL in fresh R10 + 10 U/mL recombinant human interleukin 2 (rIL2) (obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH from Maurice Gately, Hoffmann-La Roche Inc., Basel, Switzerland). On day 4 postactivation, cells were resuspended at 2 × 106/mL in fresh R10 + 10 U/mL rIL2. Before use on day 5, dead cells were removed by Histopacque-1119 (Sigma-Aldrich) density gradient, and cells were resuspended at 2 × 106/mL in R10 + 10 units/mL rIL2.
Antibody Clones Used for Flow Cytometry.
Antibody clones used for flow cytometry were: CD4 (GK1.5), CD5 (53-7.3), CD8a (53-6.7), CD11a (M17/4), CD19 (6D5), CD25 (PC61), CD29 (HMB1-1), CD44 (IM7), CD45.1 (A20), CD49d (R1.2), CD62L (Mel14), CD69 (H1.2F3), CD197 (4B12), CCR5 (7A4), CCR7 (4B12), CXCR3 (CXCR3-173), CXCR4 (L276F12). All purchased from Biolegend or eBioscience.
Antibodies Used for Western Blot.
Antibodies used for Western blot were: VASP (9A2, CellSignaling); EVL (ab108406, Abcam); Mena (sc-135988, Santa Cruz); mouse anti-Tubulin (Sigma-Aldrich); secondary antibodies (Licor donkey anti-mouse IR680 and IR800, donkey anti-rabbit IR800).
Dye-Labeling T Cells.
Naïve or activated CD4 EVL/VASP dKO and WT T cells were differentially labeled with either 2 µM carboxy-fluorescein diacetate succinimidyl ester (CFSE; Invitrogen) or 1 µM Violet Proliferation Dye (VPD; eBiosciences), mixed at a 1:1 ratio, and used for in vitro adhesion and migration assays under flow, as well as in vivo trafficking experiments. Between experimental repeats, dyes were swapped to control for potential effects of the dyes.
In Vivo Adoptive Transfer and Intravascular Staining.
For homeostatic trafficking in untreated recipient mice, 5 × 106 dKO and WT dye-labeled cells were transferred intravenously into recipient mice which were then euthanized 2 or 24 h after adoptive transfer and peripheral lymph nodes, spleen, blood, and lungs were harvested. Intravascular staining of cells remaining in blood vessels was performed via intravenous injection of 3 µg PE- or APC-conjugated anti-CD4 antibody (GK1.5) 3 min before euthanasia (39). After euthanizing with CO2, blood was then harvested by cardiocentesis, and the peripheral vasculature and lungs were fully perfused through the heart with saline. Single-cell suspensions were generated from lymph nodes and spleen by passing organs through a 100-µm filter. Blood and splenic red blood cells were lysed in 175 mM ammonium chloride. Lungs were minced, digested in DNase and Collagenase D for 30 min, passed through a 100-µM filter, and spun through a Histopacque-1119 density gradient to isolate leukocytes. One to three recipient mice were typically used per experiment, depending on cell numbers available.
Induction of EAE and Scoring.
EAE was induced using MOG induction kits from Hooke Laboratories according to their protocol. Briefly, WT female CD45.1 C57BL/6 mice of at least 8 wk of age were immunized with 200 µg of MOG35–55 peptide emulsified in complete Freund’s adjuvant injected subcutaneously, followed by intraperitoneal injection of 200 ng pertussis toxin on the day of induction and the following day. Typical EAE onset was within 10–15 d postimmunization. Mice were monitored and scored daily for development of EAE based on the following 0–5 scoring criteria: 0, no disease; 1 limp tail; 2, weakness or partial paralysis of hind limbs; 3, full paralysis of hind limbs; 4, complete hind limb paralysis and partial front limb paralysis; 5, complete paralysis of front and hind limbs or moribund state. Mice with a score ≥ 4 were euthanized immediately. Mice with scores of 2 or greater were used for T-cell trafficking experiments. These procedures were approved and carried out in accordance to the regulations of the Institutional Animal Care and Use Committees of National Jewish Health, and all efforts were made to minimize mouse suffering. Five mice were induced per group.
CNS Trafficking.
For CNS trafficking, 5 × 106 WT and dKO activated T cells were transferred IV into acutely ill EAE mice (score ≥ 2.0, see above), and recipients were euthanized 24 h after adoptive transfer following intravascular staining of cells as above. Intravascular staining and saline perfusion after cardiocentesis was performed as above. Blood, brain, and spinal cord were isolated and single-cell suspensions were generated from brain and spinal cord by passing tissues through a 100-µm filter. A 70%/30% (vol/vol) percoll gradient was used to isolate leukocytes. Total leukocytes in all samples were manually enumerated on a hemocytometer, and adoptively transferred cells were quantified by flow cytometry (CyAN, Beckman Coulter). One to three recipient mice were typically used per experiment, depending on availability of sufficiently ill recipient mice.
Skin Trafficking.
Inflammation was induced in the ears of CD45.1 recipient mice via subcutaneous injection of 20 μg LPS. Dye-labeled control or dKO activated T cells were transferred intravenously 24 h after the induction of inflammation. Then, 24 h after transfer of T cells, mice were injected intravenously with 3 μg of CD4-APC and then euthanized 3 min later. Blood was collected via cardiocentesis and the mouse was gravity-perfused with saline. Ears were removed, peeled apart, cut into small pieces, and placed into 10 mL digestion media [RPMI with 10% (vol/vol) FBS, 0.25 mg/mL DNase, Roche, 0.786 Wunsch U/mL collagenase; Roche]. Ears were digested for 45 min at 37 °C with occasional mixing. After digestion, the remaining ear tissue was further mechanically dissociated and then filtered out on a 100-μM nylon mesh strainer followed by rinsing with RPMI with 10% (vol/vol) FBS. Lymphocytes were then separated from debris using Histopaque-1119, washed and resuspended in FACS buffer for staining. Blood and nondraining lymph nodes and spleen were also harvested and processed as described above. All samples were stained with anti–CD45.1-BUV-395 before analysis by flow cytometry using a LSR Fortessa (Beckton Dickinson). Transferred T cells that fully extravasated were identified as dye-positive (CFSE or VPD), CD4-APC–negative, and CD45.1-BUV395–negative. Two recipient mice were used per experiment.
Actin Polymerization Assay.
Activated T cells were stimulated with either 1 μg/mL or 0.1 μg/mL of chemokine (Peprotech) for 5, 15, or 60 s at 37 °C in 2% (wt/vol) BSA in RPMI. The reaction was stopped using 4% (wt/vol) paraformaldehyde (Electron Microscopy Sciences) in PBS and the cells were fixed for 10 min. T cells were then permeabilized with saponin (Sigma-Aldrich) buffer [0.5% (wt/vol) saponin, 2% (vol/vol) FBS, and 0.05% sodium azide in PBS] for 30 min at room temperature. Cells were stained with a 1:50 dilution of Phalloidin-Alexa Fluor-647 (Life Technologies) in saponin buffer for 30 min and then washed twice. Analysis was done using a Beckman-Coulter Cyan flow cytometer.
Transwell Migration.
Wells of a 24-well plate were prepared containing RPMI with 2% (wt/vol) BSA and 10 mM Hepes, with or without indicated chemokines (CCL21, CXCL10, CXCL12, and CCL5, all from Peprotech). For chemotaxis assays, 1 × 106 control or dKO T cells were added to the top chambers of 5-μm Transwell plates (Corning) and allowed to migrate for 1 h at 37 °C in the presence or absence of chemokines in the lower well. For chemokinesis, chemokine was present in both upper and lower wells. As a standard, 2 × 105 cells (20% of input cells added to Transwells) were placed directly into bottom wells with no Transwell to calculate the percentage of migrated cells. Each condition was set up in duplicate Transwells. Migrated T cells were collected from the bottom wells and 25 μL of cell counting beads (123count eBeads, eBioscience) were added. Each sample was quantified for a fixed period (30 s) using a flow cytometer (CyAn ADP Beckman Coulter). The number of cells counted during this time was normalized to the number of beads counted to adjust for any variations in flow rate during the run.
Soluble VCAM-1 Binding Assay.
T cells were resuspended in RPMI without phenol red, supplemented with 5% (wt/vol) BSA (Sigma-Aldrich). Experimental wells were set up in duplicate, each with 0.5 × 106 cells in 25 µL RPMI + BSA medium in a round-bottom 96-well plate. Twenty-five microliters of R10 medium, MnCl2 treatment medium (R10 with 4 nM MnCl2), or PMA/ionomycin treatment medium (R10 with 50 nm/mL PMA and 1 μg/mL ionomycin) were added to each experimental well. The plate was gently vortexed and then incubated for 5 min at 37 °C. Next, 50 µL VCAM-Fc (1 mg/mL; R&D Systems) was added to each well and incubated for 10 min at 37 °C. Samples were then transferred to ice and 100 µL ice-cold RPMI + BSA medium was added to each well. Samples were washed once in 200 µL ice-cold RPMI + BSA medium, and were then resuspended in 100 µL APC-conjugated anti–human-Fc antibody (clone HP6017) in RPMI + BSA (1 µL per test). Samples were incubated on ice for 1 h and then washed three times. Cells were then filtered through Nytex filters, and samples were analyzed by flow cytometry (CyAN, Beckman Coulter).
Microscopy.
We used a 3i (Intelligent Imaging Innovations) Marianas spinning-disk confocal microscope system equipped with a Zeiss inverted stand and a Yokogawa spinning disk unit. We imaged through a 20× phase-contrast objective. CFSE- and VPD-labeled lymphocytes were excited with the 488 and 445 laser lines, respectively. Endothelial cells stained with A647-conjugated CD31 antibody were excited with the 640 laser line. Appropriate emission wavelengths were acquired for each channel. Time-lapse images were acquired every 20 s for 30 min, at three or four separate stage positions per run. Three XY planes (with 3-μm z-spacing) were acquired to accommodate potential z-drift because of alterations in shear-flow speed. Acquisition was managed using Slidebook software (3i, v6.0).
Adhesion and Crawling on ICAM-1 and VCAM-1.
A 1:1 mixture of differently dyed T cells was resuspended in RPMI without phenol red, supplemented with 2% (wt/vol) BSA (R&D) 10 mM Hepes at 4 × 106/mL. T cells were perfused into a flow chamber (μ-slide VI, IBIDI) that had been coated with ICAM-1 or VCAM-1 (1 mg/mL) in PBS for 1 h at 37 °C. Cells were initially perfused into the chamber at 0.2 dyne/cm2 shear-flow for 5 min, and then the shear-flow was raised to 2 dyne/cm2. Spinning-disk confocal fluorescence images were acquired every 20 s for 30 min.
Endothelial Adhesion, Crawling, and Diapedesis Under Flow.
A 1:1 mixture of differentially dyed WT and dKO T cells was resuspended in RPMI without phenol red, supplemented with 2% (wt/vol) BSA, and 10 mM Hepes at 2 × 106/mL. T cells were perfused into a flow chamber (μ-slide VI, IBIDI) coated with a monolayer of mouse primary brain microvascular endothelial cells (Cell Biologics) that had been activated with TNF-α 24 h before imaging, and treated with CCL21 30 min before imaging. The endothelial cells were also labeled with APC-conjugated anti-CD31 antibody (clone 390) to visualize the endothelial cell membrane and monolayer integrity; this staining does not perturb the T-cell TEM process (73, 74). T cells were initially perfused into the chamber at 0.2 dyne/cm2 shear-flow for 5 min, and then the shear-flow was raised to 2 dyne/cm2. Phase contrast and fluorescence images were acquired every 20 s for 30 min using a spinning-disk confocal microscope.
In CD49d (α4 integrin) blockade experiments, a 1:1 mixture of control and EVL/VASP dKO T cells at 10 × 106 cells/mL was pretreated with a mixture of two CD49d-blocking antibodies (clones 9C10 and R1-2) at 2 µg/mL each, for 30 min on ice before imaging. IgG2b antibody was used as an isotype control. Cells were then perfused into a flow chamber at 10 × 106 cells/mL as above.
Quantification of Adhesion, Crawling, and Diapedesis Under Flow.
Manual scoring and automated quantification were completed on blinded images, using Imaris software (Bitplane). Adhesion (cells per field of view 1 min after the increase in flow) was quantified using the Imaris “spot” algorithm. In some cases the number of cells per field-of-view was normalized between experiments to account for a different number of T-cell input. Mean crawling speed was quantified by following fluorescent cells using the Imaris “track” algorithm. In endothelial TEM experiments, cells that never crawled, cell detachment, and diapedesis attempts and completions were also scored manually. Briefly, T cells that contained partial regions that lost and then regained the white phase contrast ring in a stepwise manner were scored as having attempted diapedesis, whereas T cells that underwent a stepwise darkening and completely lost the white phase contrast ring were scored as having completed diapedesis. Diapedesis data were filtered to exclude all cells that were not present in the field of view for at least 13 min (the mean time required to complete diapedesis).
Statistical Analysis.
Prism software (GraphPad) was used to graph the data and calculate statistical significance. The statistical significance of single comparison data were determined by performing paired or unpaired Student’s t tests, or one-sample t tests versus a hypothetical ratio of 1.0, as appropriate. One-way ANOVA with Tukey posttests was used for multiple comparisons. Two-way ANOVA was used for comparison of data with two independent variables.
Supplementary Material
Acknowledgments
We thank F. Gertler and R. Fassler for the gift of the vasodilator-stimulated phosphoprotein (VASP) and Ena-VASP–like knockout mice; M. Gebert, R. Long, and D. Tracy for help with mouse genotyping and colony maintenance; R. Lindsay for technical help with some experiments; J. Loomis and S. Sobus for expert technical assistance with cell sorting; J. Loomis for microscope maintenance; and P. Henson, R. Kedl, and R. Torres for reagents and comments on the manuscript. This work was funded in part by National Institute of Allergy and Infectious Diseases/NIH Awards R01AI125553 and R56AI105111 (to J.J.); awards from the Dana Foundation (Brain-Immuno Imaging grant), National Multiple Sclerosis Society (Pilot grant), and American Society of Hematology (Bridge grant) (all to J.J.); NIH training Grant T32AI007405 (to M.L.E.); and NIH/National Center for Advancing Translational Sciences Colorado CTSI TL1 Grant 8TL1TR00155 (to M.L.E.); NIH Training Grant T32AI007405 (to S.B.T.). The spinning-disk confocal microscope used was acquired thanks to Shared Instrumentation Grant Award S10RR029218. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other funding agencies.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701886114/-/DCSupplemental.
References
- 1.Miller DH, et al. International Natalizumab Multiple Sclerosis Trial Group A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348(1):15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- 2.Polman CH, et al. AFFIRM Investigators A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 3.Ley K, Laudanna C, Cybulsky MII, Nourshargh S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 4.Stroka KMM, Hayenga HNN, Aranda-Espinoza H. Human neutrophil cytoskeletal dynamics and contractility actively contribute to trans-endothelial migration. PLoS One. 2013;8(4):e61377. doi: 10.1371/journal.pone.0061377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nourshargh S, Hordijk PLL, Sixt M. Breaching multiple barriers: Leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11(5):366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 6.Jacobelli J, Estin Matthews M, Chen S, Krummel MF. Activated T cell trans-endothelial migration relies on myosin-IIA contractility for squeezing the cell nucleus through endothelial cell barriers. PLoS One. 2013;8(9):e75151. doi: 10.1371/journal.pone.0075151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobelli J, et al. Confinement-optimized three-dimensional T cell amoeboid motility is modulated via myosin IIA-regulated adhesions. Nat Immunol. 2010;11(10):953–961. doi: 10.1038/ni.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobelli J, Bennett FC, Pandurangi P, Tooley AJ, Krummel MF. Myosin-IIA and ICAM-1 regulate the interchange between two distinct modes of T cell migration. J Immunol. 2009;182(4):2041–2050. doi: 10.4049/jimmunol.0803267. [DOI] [PubMed] [Google Scholar]
- 9.Jacobelli J, Chmura SA, Buxton DB, Davis MM, Krummel MF. A single class II myosin modulates T cell motility and stopping, but not synapse formation. Nat Immunol. 2004;5(5):531–538. doi: 10.1038/ni1065. [DOI] [PubMed] [Google Scholar]
- 10.Vicente-Manzanares M, Ma X, Adelstein RSS, Horwitz ARR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10(11):778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakata D, et al. Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J Exp Med. 2007;204(9):2031–2038. doi: 10.1084/jem.20062647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenmann KMM, et al. T cell responses in mammalian diaphanous-related formin mDia1 knock-out mice. J Biol Chem. 2007;282(34):25152–25158. doi: 10.1074/jbc.M703243200. [DOI] [PubMed] [Google Scholar]
- 13.Gomez TSS, et al. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 2007;26(2):177–190. doi: 10.1016/j.immuni.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: Regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 15.Vanderzalm P, Garriga G. Losing their minds: Mena/VASP/EVL triple knockout mice. Dev Cell. 2007;13(6):757–758. doi: 10.1016/j.devcel.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Hansen SD, Mullins RD. VASP is a processive actin polymerase that requires monomeric actin for barbed end association. J Cell Biol. 2010;191(3):571–584. doi: 10.1083/jcb.201003014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bear JEE, et al. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109(4):509–521. doi: 10.1016/s0092-8674(02)00731-6. [DOI] [PubMed] [Google Scholar]
- 18.Bear JEE, Gertler FBB. Ena/VASP: Towards resolving a pointed controversy at the barbed end. J Cell Sci. 2009;122(Pt 12):1947–1953. doi: 10.1242/jcs.038125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chesarone MAA, Goode BLL. Actin nucleation and elongation factors: mechanisms and interplay. Curr Opin Cell Biol. 2009;21(1):28–37. doi: 10.1016/j.ceb.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Applewhite DA, et al. Ena/VASP proteins have an anti-capping independent function in filopodia formation. Mol Biol Cell. 2007;18(7):2579–2591. doi: 10.1091/mbc.E06-11-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferron F, Rebowski G, Lee SH, Dominguez R. Structural basis for the recruitment of profilin-actin complexes during filament elongation by Ena/VASP. EMBO J. 2007;26(21):4597–4606. doi: 10.1038/sj.emboj.7601874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailly M. Ena/VASP family: New partners, bigger enigma. Dev Cell. 2004;7(4):462–463. doi: 10.1016/j.devcel.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Benz PMM, et al. Differential VASP phosphorylation controls remodeling of the actin cytoskeleton. J Cell Sci. 2009;122(Pt 21):3954–3965. doi: 10.1242/jcs.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambrechts A, et al. cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. J Biol Chem. 2000;275(46):36143–36151. doi: 10.1074/jbc.M006274200. [DOI] [PubMed] [Google Scholar]
- 25.Tokuo H, Ikebe M. Myosin X transports Mena/VASP to the tip of filopodia. Biochem Biophys Res Commun. 2004;319(1):214–220. doi: 10.1016/j.bbrc.2004.04.167. [DOI] [PubMed] [Google Scholar]
- 26.Bear JE, et al. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell. 2000;101(7):717–728. doi: 10.1016/s0092-8674(00)80884-3. [DOI] [PubMed] [Google Scholar]
- 27.Barzik M, McClain LMM, Gupton SLL, Gertler FBB. Ena/VASP regulates mDia2-initiated filopodial length, dynamics, and function. Mol Biol Cell. 2014;25(17):2604–2619. doi: 10.1091/mbc.E14-02-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massberg S, et al. Enhanced in vivo platelet adhesion in vasodilator-stimulated phosphoprotein (VASP)-deficient mice. Blood. 2004;103(1):136–142. doi: 10.1182/blood-2002-11-3417. [DOI] [PubMed] [Google Scholar]
- 29.Deevi RK, et al. Vasodilator-stimulated phosphoprotein regulates inside-out signaling of beta2 integrins in neutrophils. J Immunol. 2010;184(12):6575–6584. doi: 10.4049/jimmunol.0903910. [DOI] [PubMed] [Google Scholar]
- 30.Krause M, et al. Fyn-binding protein (Fyb)/SLP-76-associated protein (SLAP), Ena/vasodilator-stimulated phosphoprotein (VASP) proteins and the Arp2/3 complex link T cell receptor (TCR) signaling to the actin cytoskeleton. J Cell Biol. 2000;149(1):181–194. doi: 10.1083/jcb.149.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwiatkowski AVV, et al. Ena/VASP is required for neuritogenesis in the developing cortex. Neuron. 2007;56(3):441–455. doi: 10.1016/j.neuron.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Aszódi A, et al. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO J. 1999;18(1):37–48. doi: 10.1093/emboj/18.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shulman Z, et al. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity. 2009;30(3):384–396. doi: 10.1016/j.immuni.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alon R, Shulman Z. Chemokine triggered integrin activation and actin remodeling events guiding lymphocyte migration across vascular barriers. Exp Cell Res. 2011;317(5):632–641. doi: 10.1016/j.yexcr.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Shulman Z, et al. Transendothelial migration of lymphocytes mediated by intraendothelial vesicle stores rather than by extracellular chemokine depots. Nat Immunol. 2011;13(1):67–76. doi: 10.1038/ni.2173. [DOI] [PubMed] [Google Scholar]
- 36.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291(5512):2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 37.Galkina E, et al. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J Clin Invest. 2005;115(12):3473–3483. doi: 10.1172/JCI24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson KGG, et al. Cutting edge: Intravascular staining redefines lung CD8 T cell responses. J Immunol. 2012;189(6):2702–2706. doi: 10.4049/jimmunol.1201682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson KGG, et al. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 2014;9(1):209–222. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pereira JP, An J, Xu Y, Huang Y, Cyster JG. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat Immunol. 2009;10(4):403–411. doi: 10.1038/ni.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thelen M, Stein JV. How chemokines invite leukocytes to dance. Nat Immunol. 2008;9(9):953–959. doi: 10.1038/ni.f.207. [DOI] [PubMed] [Google Scholar]
- 42.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 43.Schmit MA, et al. Vasodilator phosphostimulated protein (VASP) protects endothelial barrier function during hypoxia. Inflammation. 2012;35(2):566–573. doi: 10.1007/s10753-011-9347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Wang H. Integrin signalling and function in immune cells. Immunology. 2012;135(4):268–275. doi: 10.1111/j.1365-2567.2011.03549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol. 2015;15(11):692–704. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- 46.Cinamon G, Alon R. A real time in vitro assay for studying leukocyte transendothelial migration under physiological flow conditions. J Immunol Methods. 2003;273(1-2):53–62. doi: 10.1016/s0022-1759(02)00418-0. [DOI] [PubMed] [Google Scholar]
- 47.Laurent V, et al. Role of proteins of the Ena/VASP family in actin-based motility of Listeria monocytogenes. J Cell Biol. 1999;144(6):1245–1258. doi: 10.1083/jcb.144.6.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michael M, Vehlow A, Navarro C, Krause M. c-Abl, Lamellipodin, and Ena/VASP proteins cooperate in dorsal ruffling of fibroblasts and axonal morphogenesis. Curr Biol. 2010;20(9):783–791. doi: 10.1016/j.cub.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lafuente EMM, et al. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell. 2004;7(4):585–595. doi: 10.1016/j.devcel.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 50.Neel NFF, et al. VASP is a CXCR2-interacting protein that regulates CXCR2-mediated polarization and chemotaxis. J Cell Sci. 2009;122(Pt 11):1882–1894. doi: 10.1242/jcs.039057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Evans IR, Wood W. Drosophila blood cell chemotaxis. Curr Opin Cell Biol. 2014;30:1–8. doi: 10.1016/j.ceb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katagiri K, et al. Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat Immunol. 2004;5(10):1045–1051. doi: 10.1038/ni1111. [DOI] [PubMed] [Google Scholar]
- 53.Klapproth S, et al. Loss of the Rap-1 effector RIAM results in leukocyte adhesion deficiency due to impaired β2 integrin function in mice. Blood. 2015;126(25):2704–2712. doi: 10.1182/blood-2015-05-647453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su W, et al. Rap1 and its effector RIAM are required for lymphocyte trafficking. Blood. 2015;126(25):2695–2703. doi: 10.1182/blood-2015-05-644104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang Y, et al. CRK proteins selectively regulate T cell migration into inflamed tissues. J Clin Invest. 2015;125(3):1019–1032. doi: 10.1172/JCI77278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen SJ, et al. The integrin coactivator Kindlin-3 is not required for lymphocyte diapedesis. Blood. 2013;122(15):2609–2617. doi: 10.1182/blood-2013-04-495036. [DOI] [PubMed] [Google Scholar]
- 57.Moretti FA, et al. Kindlin-3 regulates integrin activation and adhesion reinforcement of effector T cells. Proc Natl Acad Sci USA. 2013;110(42):17005–17010. doi: 10.1073/pnas.1316032110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Issekutz TB. Inhibition of in vivo lymphocyte migration to inflammation and homing to lymphoid tissues by the TA-2 monoclonal antibody. A likely role for VLA-4 in vivo. J Immunol. 1991;147(12):4178–4184. [PubMed] [Google Scholar]
- 59.Schulte D, et al. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J. 2011;30(20):4157–4170. doi: 10.1038/emboj.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nottebaum AFF, et al. VE-PTP maintains the endothelial barrier via plakoglobin and becomes dissociated from VE-cadherin by leukocytes and by VEGF. J Exp Med. 2008;205(12):2929–2945. doi: 10.1084/jem.20080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wessel F, et al. Leukocyte extravasation and vascular permeability are each controlled in vivo by different tyrosine residues of VE-cadherin. Nat Immunol. 2014;15(3):223–230. doi: 10.1038/ni.2824. [DOI] [PubMed] [Google Scholar]
- 62.Song KH, et al. T cells sense biophysical cues using lamellipodia and filopodia to optimize intraluminal path finding. Integr Biol. 2014;6(4):450–459. doi: 10.1039/c4ib00021h. [DOI] [PubMed] [Google Scholar]
- 63.Schirenbeck A, et al. The bundling activity of vasodilator-stimulated phosphoprotein is required for filopodium formation. Proc Natl Acad Sci USA. 2006;103(20):7694–7699. doi: 10.1073/pnas.0511243103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lebrand C, et al. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron. 2004;42(1):37–49. doi: 10.1016/s0896-6273(04)00108-4. [DOI] [PubMed] [Google Scholar]
- 65.Hyun Y-MM, Chung H-LL, McGrath JLL, Waugh REE, Kim M. Activated integrin VLA-4 localizes to the lamellipodia and mediates T cell migration on VCAM-1. J Immunol. 2009;183(1):359–369. doi: 10.4049/jimmunol.0803388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abitorabi MA, Pachynski RK, Ferrando RE, Tidswell M, Erle DJ. Presentation of integrins on leukocyte microvilli: A role for the extracellular domain in determining membrane localization. J Cell Biol. 1997;139(2):563–571. doi: 10.1083/jcb.139.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rothhammer V, et al. α4-Integrins control viral meningoencephalitis through differential recruitment of T helper cell subsets. Acta Neuropathol Commun. 2014;2:27. doi: 10.1186/2051-5960-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sandborn WJ, et al. International Efficacy of Natalizumab as Active Crohn’s Therapy (ENACT-1) Trial Group Evaluation of Natalizumab as Continuous Therapy (ENACT-2) Trial Group Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353(18):1912–1925. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- 69.Bloomgren G, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366(20):1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 70.Major EO, Frohman E, Douek D. JC viremia in natalizumab-treated patients with multiple sclerosis. N Engl J Med. 2013;368(23):2240–2241. doi: 10.1056/NEJMc1214233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pellegrino P, et al. Efficacy of vaccination against influenza in patients with multiple sclerosis: The role of concomitant therapies. Vaccine. 2014;32(37):4730–4735. doi: 10.1016/j.vaccine.2014.06.068. [DOI] [PubMed] [Google Scholar]
- 72. National Research Council (2011) Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC), 8th Ed.
- 73.Woodfin A, et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. 2011;12(8):761–769. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Christofidou-Solomidou M, Nakada MT, Williams J, Muller WA, DeLisser HM. Neutrophil platelet endothelial cell adhesion molecule-1 participates in neutrophil recruitment at inflammatory sites and is down-regulated after leukocyte extravasation. J Immunol. 1997;158(10):4872–4878. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.