Abstract
Alternatively activated macrophages (AAMφ) are primarily associated with the chronic stages of parasitic infections and the development of a polarized Th2 response. We have shown that Fasciola hepatica infection of BALB/c mice induces a polarized Th2 response during both the latent and chronic stage of disease. The activation status of macrophages was analyzed in this model of helminth infection by evaluating the expression of genetic markers of alternative activation, namely, Fizz1, Ym1, and Arg1. AAMφ were recruited to the peritoneum of mice within 24 h of F. hepatica infection and after intraperitoneal injection of parasite excretory-secretory (ES) products. Administration of a recombinant antioxidant thioredoxin peroxidase (TPx), which is contained within the ES products, also induced the recruitment of AAMφ to the peritoneum. In vitro studies showed that this recombinant TPx directly converts RAW 264.7 macrophages to an alternatively activated phenotype characterized by the production of high levels of interleukin-10 (IL-10), prostaglandin E2, corresponding with low levels of IL-12. Our data suggest that the Th2 responses induced by the helminth F. hepatica are mediated through the secretion of molecules, one of which is TPx, that induce the recruitment and alternative activation of macrophages.
Macrophages are pivotal to the interaction between the innate and acquired immune systems and are thus critical mediators of many chronic inflammatory diseases. The activity of macrophages is controlled by specific signals that stimulate their development into discrete phenotypes, which differ in terms of receptor expression, effector function, and cytokine secretions. The best-characterized phenotype is the classically activated macrophage, which develops in response to proinflammatory cytokines such as gamma interferon (IFN-γ) (12), microbial infection or bacterial products such as lipopolysaccharide (LPS) or CpG (3, 21). In contrast, macrophages activated by an interleukin-4 (IL-4)/IL-13-dependent signal pathway are classified as alternatively activated macrophages (AAMφ) (14, 20, 55).
Microarray analysis and suppression subtractive hybridization has led to the identification of genes expressed by AAMφ in response to IL-4 and IL-13 (49). These Th2 cytokines induce macrophages to preferentially express arginase 1 (Arg1) to metabolize l-arginine. Indeed, Arg1 is the most highly upregulated gene in mouse peritoneal macrophages, exhibiting a >88-fold increase in response to IL-4 (60). In addition, reports indicate that Fizz1 (found in inflammatory zone), the gene product of which is also associated with experimentally induced asthma (24), and Ym1, an eosinophil chemotactic factor, were found to be abundantly expressed in peritoneal macrophages after implantation of the Th2-inducing parasite Brugia malayi (32).
Although the phenotype of AAMφ may be clearly established, their function and range of activities in vivo has not been fully characterized. It has been proposed that AAMφ have the ability to induce the differentiation of naive T cells to Th2 phenotype directly (50, 56) or by the inhibition of type 1 responses (29, 30) by releasing soluble mediators such as IL-10 and transforming growth factor β (TGF-β). Another consistently reported property of AAMφ is the suppression of lymphocyte proliferation, which is believed to be also mediated by the anti-inflammatory cytokines, IL-10 and TGF-β (8, 19, 30, 56). However, physical separation of T cells from AAMφ prevented this antiproliferative activity, implicating a cell-to-cell receptor-mediated mechanism of suppression (31).
We have previously reported that a murine model of Fasciola helminth infection induces a polarized Th2 immune response (46) coincident with a suppression of Th1 cytokines. Furthermore, infection resulted in the bystander suppression of Th1 responses to a concurrent bacterial infection or immunization with a Th1 inducing vaccine (10). Although we identified a role for IL-4 in the suppression of Th1 responses, with the effect almost completely inhibited in IL-4 knockout mice, it was not questioned whether this was related to the induction of AAMφ by the Th2 cytokine milieu.
Infective Fasciola hepatica larvae penetrate the intestinal wall of the host after ingestion then migrate to the liver via the peritoneum. Here we show that macrophages with an alternatively activated phenotype are recruited to the peritoneal cavity within 24 h after F. hepatica infection. The recruitment of AAMφ to the peritoneum can also be induced by the injection of molecules that are actively secreted by the parasite, one of which has been identified as an antioxidant thioredoxin peroxidase (TPx). Furthermore, we show that TPx may also be directly involved in the activation of macrophages. Treatment of RAW 264.7 macrophages with a recombinant TPx resulted in the induction of Arg1 and the secretion of high levels of IL-10 and prostaglandin E2 (PGE2). These results not only identify a novel role for TPx in the regulation of immune responses during helminth infection but also highlight the suitability of Fasciola infection as a system from which functional insights into macrophage biology could be obtained.
MATERIALS AND METHODS
Animal treatment.
Six- to eight-week-old female BALB/c mice were purchased from Harlan Olac, Ltd. (Oxon, United Kingdom) and maintained according to the guidelines of the Irish Department of Health. Mice were orally infected with 10 metacercariae of F. hepatica (Compton Paddock Laboratories, Berkshire, United Kingdom), which produced infection in 100% of animals. After the experimental period (days 1, 7, 14, and 21 postinfection), mice were analyzed by necropsy, and peritoneal exudate cells (PECs) were harvested by thorough washing of the peritoneal cavity with 10 ml of sterile phosphate-buffered saline (PBS). For analysis of the effect of TPx activity, 4 μg of either peak I (PI; the fraction of parasite excretory-secretory (ES) products which contains TPx) or recombinant TPx was injected intraperitoneally three times per week for 3 weeks, and PECs were harvested as described above 3 days after the final injection. (The protocol of injection and antigen concentration used were designed based on in vitro estimations of antigen secretion/day for adult flukes and the time required to induce sufficient Th2 responses.) As a negative control for TPx activation, a sham fraction, purified from Escherichia coli in the same manner as TPx, and recombinant F. hepatica cathepsin L's were used in all experiments.
Purification of macrophages from PECs.
After determination of viability by trypan blue exclusion, PECs were adjusted to 5 × 106 cells/ml in supplemented minimal essential medium (MEM) and cultured in six-well plates (Costar, Cambridge, Mass.). After 2 to 3 h of incubation at 37°C, nonadherent cells were removed by washing with warm MEM. The remaining adherent cells were highly enriched for macrophages (>95%) as assessed by fluorescence-activated cell sorting staining with the macrophage marker F4/80 (BD Pharmingen, San Diego, Calif.). These cells were removed with cold PBS and a scraper and readjusted to 106 cells/ml for experimentation.
Activation of in vitro macrophages.
Alternatively activated macrophages were generated by treating RAW 264.7 macrophages (ECACC, Salisbury, United Kingdom) with IL-4 (BD Pharmingen) at a concentration of 10 ng/ml in supplemented MEM for 20 h as described previously (43). In other experiments, RAW macrophages were induced to express inducible nitric oxide synthase (iNOS) and secrete NO by incubating them in the presence of 5 μg of LPS (E. coli 111:B4; Sigma, St. Louis, Mo.)/ml.
RNA extraction and RT-PCR.
RNA was recovered from cultured cells by direct addition of Tri-Reagent (Sigma) to the wells. For PECs, 1 ml of cells at a concentration of 106 cells/ml was centrifuged for 6 min at 1,500 rpm, and the resultant pellet was resuspended in Tri-Reagent. Total cellular RNA was subsequently extracted from both preparations according to the manufacturers' specifications. For reverse transcription-PCR (RT-PCR), first-strand cDNA was produced with oligo(dT) primers from 2 μg of total RNA by using avian myeloblastosis virus reverse transcriptase (Promega, Madison, Wis.) at 42°C for 60 min. A 5-μl aliquot of the resultant cDNA was amplified by using primers specific for β-actin (Stratagene, La Jolla, Calif.), Arg1 (43), Fizz1 (43), Ym1 (43), and iNOS (52) under the following conditions: 30 s denaturation at 95°C, 5 s annealing of primers at 55°C, and 12 s elongation at 72°C for 40 cycles. To analyze the range of cytokines expressed by the cells, PCR amplifications were performed with primers specific for IL-12p40 (57), IL-18 (57), IL-10 (59), and TGF-β (5′, 5′-GTTGGGAACGCGTTGCATTT-3′; 3′, 5′-GCGCATAAACTGATCCATGT-3′) over 30 cycles of 30 s denaturation at 95°C, annealing for 30 s at 58°C, and elongation at 72°C for 30 s. All PCR products were electrophoresed on 1% agarose gel and visualized by ethidium bromide staining. Quantification of PCR products was carried out by densitometric analysis of photographic positives of agarose gels by using Un-Scan-It Software (Silk Scientific, Orem, Utah). The quantification method used in these assays is relative because absolute values of mRNA levels could not be determined, as internal controls for specific mRNAs were not used. Values for mRNA are expressed in relative absorbance units and are standardized per unit of β-actin per sample.
Cytokine assays.
Spleens were homogenized and total spleen cells cultured (2 × 106 cells/ml) with ES products or PI. Control stimuli included medium alone or anti-CD3 (2 μg/ml) and phorbol myristate acetate (PMA; 25 ng/ml). Supernatants were removed after 72 h, and the concentrations of interleukin-4 (IL-4), IL-5, and IFN-γ measured by immunoassay using pairs of commercially available monoclonal antibodies (BD Pharmingen) as described previously (46). For in vitro cultures, supernatants from RAW macrophages were harvested 20 h after activation with antigen, and the levels of IL-10 and IL-12 were determined by commercially available sandwich ELISA kits (BD Pharmingen).
Fhe-TPx.
Recombinant TPx was expressed in E. coli BL21 by using plasmid vector pPRO-EXHta with an N-terminal His6 tag (Life Technologies, Rockville, Md.) and purified by using Ni-nitrilotriacetic acid (NTA)-resin (Qiagen, West Sussex, United Kingdom) according to the manufacturer's instructions. The protein was purified further by the method of Frangioni and Neel (18), and the quantity of contaminating LPS present determined to be <0.07 ng by a commercial assay (E-Toxate; Sigma).
SDS-PAGE and Western blot analysis.
Protein samples (25 μg) were mixed 3:1 with sample buffer (125 mM Tris-HCl, 20% glycerol [vol/vol], 4% sodium dodecyl sulfate [SDS], 40 mM dithiothreitol, and 0.01% bromophenol blue), boiled for 5 min, and separated on a sodium dodecyl sulfate-12.5% polyacrylamide gel electrophoresis (PAGE) gel. The proteins were transferred to a nitrocellulose membrane (Schleicher & Schuell, Keene, N.H.), which was then blocked with 5% nonfat dry milk in PBS Tween (0.05% [vol/vol]). The membrane was then incubated with a 1:1,000 dilution of mouse antiserum, raised against recombinant TPx. After a 1-h incubation with this primary antibody, membranes were exposed to alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (Sigma) for 1 h. Finally, the protein bands were visualized by the addition of 3,3′-diaminobenzidine peroxidase substrate.
The anti-TPx antisera was prepared by subcutaneously injecting BALB/c mice three times, 3 weeks apart, with 10 μg of antigen combined with 30 μg of CpG ODN 1826 in alum hydroxide. Immunoglobulin G titers in sera were then determined, and the monospecificity was confirmed by probing lysates of induced and noninduced recombinant bacterial cells.
NO measurement.
Production of NO by macrophages was assessed by measuring the increase in nitrite concentration with the Greiss reagent (Promega, Madison, Wis.). Briefly, 50 μl of culture supernatant was mixed with an equal volume of 1% sulfanilamide-0.1% naphthylethylenediamine dihydrochloride-2.5% H3PO4. Absorbance was measured at 570 nm, and values were quantified against a standard curve of sodium nitrite.
Determination of arginase activity.
After lysis of the cells in Triton X-100, and with l-arginine as a substrate, the arginase activity was determined as previously described (38). One unit of enzyme activity was defined as the amount of enzyme that catalyzes the formation of 1 μmol of urea per min.
PGE2 measurement.
PGE2 concentrations in supernatants from macrophages were measured by using an enzymatic immunoassay kit according to the manufacturer's instructions (Assay Designs, Inc., Ann Arbor, Mich.).
Statistical analysis.
The statistical significance of difference was determined by the two-tailed student t test. P values of <0.05 were considered significant.
RESULTS
Alternatively activated macrophages are induced by parasite infection.
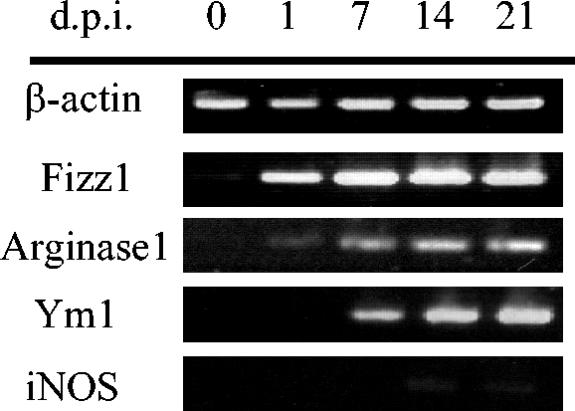
Infection with F. hepatica is associated with a polarized Th2 response that is characterized by the production of high levels of IL-4 and IL-5 from spleen and hepatic and mesenteric lymph nodes in the absence of any detectable IFN-γ (37, 46). To examine the role of innate immune mechanisms in the development of this Th2 response, the status of activation of macrophages during infection was evaluated. The expression of genes coding for Fizz1, Arg1, and Ym1 in adherent PECs was examined at a range of time points after oral infection of BALB/c mice with F. hepatica larvae (Fig. 1). Expression of both Fizz1 and Arg1 was induced in the peritoneal macrophages of infected mice as early as 24 h after infection, although the expression of Arg1 was considerably weaker than that observed for Fizz1. By day 7, the expression of Ym1 was also induced and, together with Fizz1 and Arg1, was continually detected for the duration of the study. In contrast, expression of iNOS, the gene product of which is associated with secretion of IFN-γ (9) and is a marker of classical activation of macrophages, was detected during the latter stages of infection but at very low levels. By day 14 after infection, the majority of infective parasites have reached the liver with extensive tissue migration of the flukes, resulting in severe hepatic fibrosis. In response to such liver injury, inflammatory cells such as macrophages are driven to produce oxygen-derived free radicals, nitric oxide, and inflammatory cytokines in response to the damaging activity of the parasite (6), as indicated by the detection of iNOS.
FIG. 1.
Alternative activation of macrophages in BALB/c mice after an F. hepatica infection. Expression levels of β-actin, Fizz1, Arg1, Ym1, and iNOS were analyzed by RT-PCR in adherent peritoneal exudate cells isolated from mice prior to infection (day 0) and at 1, 7, 14, and 21 days postinfection (d.p.i.). The data shown are from a single mouse and are representative of the findings from four mice examined at each time point (the expression of each gene was similar in each mouse).
Parasite-secreted molecules induce a Th2 type immune response.
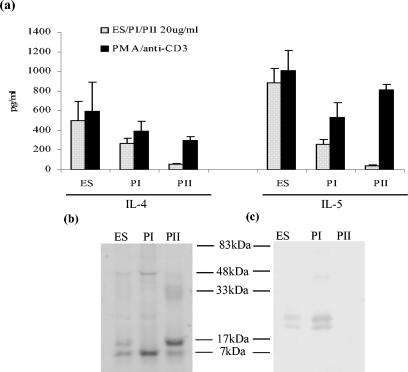
Molecules secreted from various helminth parasites, including F. hepatica, have previously been shown to exert immunomodulatory effects (25, 45, 46). To determine more specifically which molecules secreted by F. hepatica may be involved in inducing AAMφ, parasite ES products prepared by culturing adult worms in vitro, was fractionated by size exclusion chromatography, and two major protein peaks, termed PI (>200 kDa) and PII (60 to 20 kDa), were recovered. When injected into the peritoneum of BALB/c mice every other day over a 3-week period, ES product and PI, but not PII, induced secretion of both IL-4 and IL-5 (Fig. 2a) from spleen cells in the absence of any IFN-γ. This was not due to a failure of IFN-γ production from T cells since stimulation with PMA-anti-CD3 resulted in the secretion of high levels of IFN-γ (64 ng/ml from PBS-treated animals and an average of 67 ng/ml from antigen-treated mice). This result indicated that the F. hepatica PI fraction contained molecules, capable of affecting the polarization of immunity observed during infection.
FIG. 2.
The PI fraction of F. hepatica ES products contains TPx and induces Th2 responses when intraperitoneally injected into BALB/c mice. (a) Antigen-specific production of IL-4 and IL-5 from spleen cells of BALB/c mice injected with ES products or fractions of ES products, termed PI and PII. Spleen cell cultures were stimulated with 20 μg of the relevant injected antigen—ES product, PI, or PII—per ml and PMA-anti-CD3 as a positive control. The data are presented as mean ± the standard error (SE) for four mice. (b) ES product, PI, and PII separated on a SDS-12.5% gel stained with Coomassie blue. (c) ES product, PI, and PII fractions separated by SDS-PAGE and analyzed by Western blots probed with antisera prepared against recombinant TPx.
The PI fraction was of additional interest since we had previously shown that vaccination of cattle with a similar high-molecular-weight fraction induced significant levels of protection against challenge with F. hepatica (13). When adult worm cDNA libraries were screened with serum obtained from these protected cattle they reacted with a clone expressing a novel helminth thiol-specific antioxidant, which we subsequently identified as a member of the TPx family (34, 35). Antisera prepared against the purified recombinant TPx reacted with a protein doublet in F. hepatica ES product and also in PI (Fig. 2b and c). However, Coomassie blue staining of SDS-PAGE gels showed that it was not a major component in these preparations (Fig. 2b).
TPx induced a population of alternatively activated macrophages in vivo.
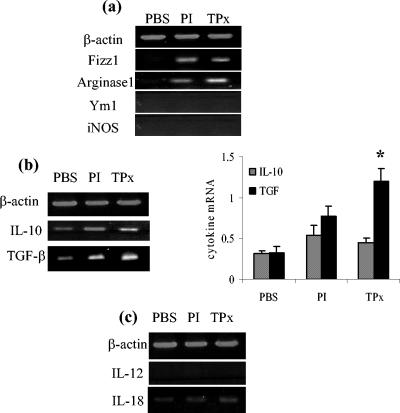
Given the presence of TPx in the Th2-inducing fraction of F. hepatica ES product, we sought to determine whether this enzyme could mimic the induction of AAMφ seen during infection. To replicate the effects of parasite secretions during an infection, nine intraperitoneal injections of PI or recombinant TPx were given over a period of 3 weeks. Three days after the final injection the BALB/c mice were sacrificed, and the peritoneal macrophages were examined by RT-PCR. Both PI and TPx induced Fizz1 and Arg1 gene expression (Fig. 3a), but no expression of Ym1 was detected. Expression of iNOS was not observed, indicating the absence of classically activated macrophages and the recruitment of AAMφ.
FIG. 3.
Injection of PI and purified recombinant TPx induces the recruitment of alternatively activated macrophages into the peritoneum of BALB/c mice. (a) Macrophages recovered from the peritoneum of mice after an intraperitoneal delivery of PI or recombinant TPx exhibit increased expression of gene markers of alternative activation, including Fizz1 and Arg1, although Ym1 is not expressed as assessed by RT-PCR. The cells do not express iNOS, a marker of classically activated macrophages. (b and c) PI and TPx also induce the expression of Th2-associated cytokines IL-10 and TGF-β (b) but not the Th1 cytokines IL-12p40 and IL-18 (c). Agarose gels are representative of results obtained from four animals, and mean data obtained from densitometric analysis of all experiments are shown for IL-10 and TGF-β. Values for mRNA are expressed in relative absorbance units and are standardized per unit of β-actin per sample. ✽, P < 0.05 (for TPx compared to treatment with PBS).
During the chronic phase of many helminth infections when Th2 responses predominate, the cytokine markers of AAMφ, IL-10 and TGF-β, are secreted at relatively high levels (63). TGF-β has been reported to inhibit NO production while increasing arginase activity (4). Such observations are important in the context of the present study since TGF-β apparently favors the production of Th2 responses by inhibiting Th1 induced macrophage activation. After injection of both PI and TPx, a modest increase in gene expression of IL-10 was observed in peritoneal macrophages (Fig. 3b). Although these changes failed to reach statistical significance when analyzed, the changes were reproducible between experiments. Significant levels of TGF-β expression were also induced (Fig. 3b) and correlated with a failure to express gene transcripts for either IL-12 or IL-18 (Fig. 3c).
The establishment of AAMφ by PI and TPx is not due solely to the propensity of this strain of mouse to develop Th2 responses, since peritoneal macrophages from littermates infected with Salmonella enterica serovar Typhimurium (106 CFU/mouse) exhibited a classically activated phenotype with IL-12, IL-18, and iNOS gene expression in the absence of Arg1 (data not shown).
TPx converts cultured macrophages to an alternatively activated phenotype.
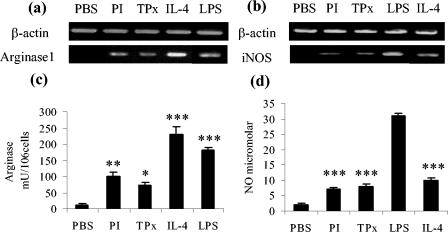
To investigate whether TPx itself is not only capable of recruiting AAMφ in vivo but also of actively driving macrophages toward an alternatively activated phenotype, we treated cultured RAW 264.7 macrophages with either 10 μg of PI or TPx and 20 h later examined both the supernatants and the cells for markers of alternative activation.
It has been widely established that the alternative metabolic pathways of l-arginine metabolism are consistent with the differential activation of macrophages. Production of arginase is induced in alternatively activated macrophages and results in the downregulation of iNOS. Indeed, the balance between NO and arginase reflects the balance between Th1 and Th2 type activities of macrophages (38, 39, 53). After treatment with either PI or TPx, an increase in arginase activity was observed in the lysates of RAW macrophage cells and correlated with the induction of Arg1 gene expression (Fig. 4a and c). In contrast, whereas the levels of NO induced were marginally higher than those for PBS (P < 0.05), neither synthesis of NO or the expression of iNOS was significantly induced compared to RAW macrophages treated with LPS, a classical activator of macrophages (Fig. 4b and d). To complement the in vivo studies, gene expression for Fizz1 and Ym1 was also assessed by RT-PCR. However, no increase in either Ym1 or Fizz1 expression was observed (data not shown). Consistent with this finding, Nair et al. (43) report the expression of Arg1 but not Fizz1 or Ym1 in J774 cell lines treated with IL-4. These authors suggest that the lower plasticity of such a highly differentiated cell line prevented the cells from adapting in response to this cytokine. Furthermore, reports that RAW 264.7 cells failed to upregulate either Ym1 or Ym2 in response to IL-4 has been attributed to the absence of essential signaling components in some clones of this cell line (60).
FIG. 4.
TPx induces cultured RAW macrophages to an alternative activated phenotype. After treatment of RAW macrophages with purified recombinant TPx, the expression of Arg1 (a) and iNOS (b) was measured by RT-PCR (the data are representative of four independent experiments). (c and d) Level of arginase activity and NO, respectively, measured in the culture supernatants of the same cell preparations. The data are presented as the mean ± the SE. ✽, P < 0.05; ✽✽, P < 0.01; ✽✽✽, P < 0.001 (with respect to control treatments, either PBS or LPS).
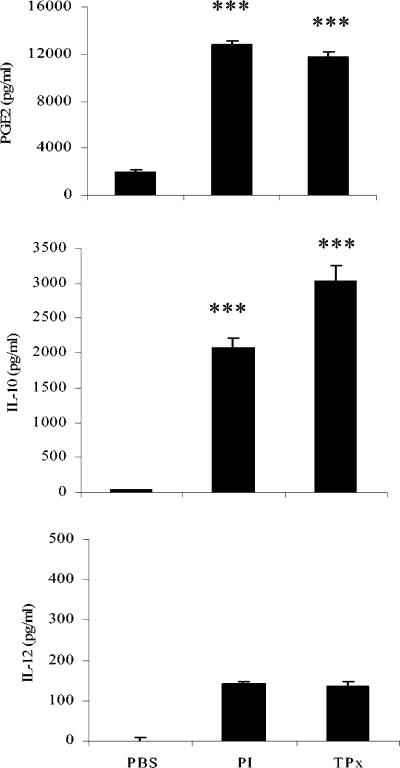
To further establish that PI- and TPx-treated macrophages adopt an alternatively activated phenotype, we examined the production of PGE2, a compound known to be increased during alternative activation of macrophages and to have a significant role in the suppression of IL-12 and IFN-γ production (28, 58). We also measured the production of IL-10 and IL-12, since the secretion of IL-10 by macrophages is archetypal of the alternative pathway of activation, whereas IL-12 is responsible for the polarization of Th1 adaptive responses and therefore the classical pathway of activation. Both PI and TPx induce the secretion of PGE2 and IL-10 but not IL-12 from RAW macrophages (Fig. 5).
FIG. 5.
PI and TPx induce the secretion of anti-inflammatory agents from RAW macrophages. The level of PGE2, IL-10, and IL-12 in the culture supernatants of PI- and TPx-activated macrophages was measured by enzyme-linked immunosorbent assay and compared to cells incubated with PBS alone. The data represents the mean of four experiments ± the SE. ✽✽✽, P < 0.001.
DISCUSSION
During helminth infections, macrophages that undergo changes to an alternatively activated phenotype have been implicated in the regulation of the cytokine environment, leading to preferential induction of Th2 responses seen during the late chronic stage of disease (21, 33). We show here that AAMφ are detected as early as 24 h after an oral infection of F. hepatica larvae. This recruitment of AAMφ can be simulated by intraperitoneally injecting mice with F. hepatica-secreted molecules. Furthermore, we report that TPx secreted by the parasite is at least one molecule responsible for this alternative activation of macrophages.
F. hepatica parasites establish infection by penetrating the intestinal wall and migrating via the peritoneum to the liver. A defining feature of the pathogenesis of this infection is the elevation of Th2 cytokines, a consequence of which is high levels of proline in serum and fibrosis of the liver (6). The extent of liver fibrosis resulting from infection with a related trematode, the blood fluke, Schistosoma mansoni, is also directly influenced by the availability of proline (5, 15, 16). Since l-ornithine, a product of arginase activity, is an essential metabolite for the production of proline, it has been suggested that arginase activity is critically linked to the pathogenesis of parasite infection (22). Murine macrophages, a dominant cell type of schistosoma egg-induced granuloma (54), represent the main cellular source of arginase, production of which appears to be dependent on a type-2-driven immune response (1). Indeed, injection of schistosoma eggs into mice results in the induction of Arg1 by alternatively activated macrophages stimulated with IL-4 and IL-13 (22, 51).
That Th2 cytokines, IL-4 and IL-13, bias macrophages toward an alternatively activated phenotype is well established (21, 36, 43). However, recent studies indicate that AAMφ can occur independently of IL-4/IL-13 signaling. For example, macrophages from T. congolense-infected mice lacking IL-4 and/or IL-13 signaling produced levels of arginase activity similar to infected wild-type BALB/c mice (44). The expression of Ym1, a marker of alternative activation, was also induced in these macrophages in the absence of IL-4/IL-13 signaling (44). Furthermore, in B. malayi-infected C57/BL6 mice (17) and in a model system of vaccination with attenuated schistosomula of S. mansoni (11) the induction of Ym1 expression in AAMφ was also found to be IL-4 independent. In the present study, AAMφ were first detected in the peritoneum at 24 h after infection, more than 7 days before the production of detectable levels of IL-4. These data, combined with the reported observation of juvenile parasites in the peritoneal cavity less than 24 h after oral infection (6), suggests that F. hepatica or molecules secreted by the parasite can induce AAMφ prior to the development of significant levels of IL-4. In support of this, our in vitro studies demonstrated that ES products, including TPx, induced the expression of Arg1 and the production of arginase in RAW macrophages in the absence of Th2 cytokines. This effect was not due to the presence of contaminating LPS, since treatment of the RAW cell line with a sham fraction purified from E. coli in the same manner as TPx had no effect on Arg1 expression or arginase production (data not shown). However, since these events were demonstrated in isolation they do not yet describe a direct link between TPx secretion and IL-4-independent activation of macrophages. In vivo studies with IL-4-, IL-13-, and STAT6-deficient strains of mice are currently under way to answer this question.
Several helminth models have been used for the study of the alternative activation of macrophages, including the injection of schistosome eggs (22), infection with Nippostrongylus brasiliensis (21), Taenia crassiceps (50), and B. malayi (30, 31), but only one previous report describes a molecule, Bm-MIF, that may be involved in the in vivo recruitment of AAMφ (17). Injection of recombinant Bm-MIF induced the upregulation of Ym1 expression by peritoneal macrophages, although the expression of other markers of alternative activation such as Fizz1 or Arg1 was not examined. In the present study, we show that the parasite-secreted molecule, TPx, could not only induce the recruitment and activation of AAMφ in vivo but also modify macrophages in vitro to an alternatively activated phenotype. TPx induced the expression of both Fizz1 and Arg1 in vivo but, in contrast to the study of Falcone et al. (17) with Bm-MIF, no expression of Ym1 was observed. However, expression of Ym1 was detected in AAMφ isolated from mice infected with F. hepatica, indicating that the injection of a purified protein does not completely mimic natural infection and that other molecules secreted by the parasite are influencing macrophage development. In addition, or alternatively, heterogenic populations of AAMφ expressing different gene repertoires in response to different parasite signals may exist. Heterogeneity among macrophages is suggested by a study of macrophage activation during T. congolense infection by Noel et al. (44), who observed an increase in both Fizz1 and Ym1 gene expression in AAMφ elicited from chronically infected C57BL/6 mice but only an increase in the expression of Ym1 in macrophages isolated from infected BALB/c mice. Whether F. hepatica TPx activates one subset of AAMφ whereas other factors induce a different subset is an intriguing possibility that warrants further investigation.
The association of AAMφ with chronic parasite infections has highlighted their role in the induction of Th2 differentiation, but it is unclear how this is mediated. Since reduced levels of IFN-γ and IL-12 and a biased Th2-type response, even to concurrent bacterial and viral antigenic challenges, is observed in helminth infections (2, 10, 27), it has been suggested that suppression of Th1 cytokine secretion by T cells may be a mechanism by which AAMφ influence their function. For example, peritoneal macrophages isolated from mice after B. malayi infection (30) or injection of oligosaccharides from S. mansoni (56) skewed cytokine production of T cells toward a Th2 phenotype characterized by the production of IL-4 (30) or IL-13 (56) while failing to stimulate the production of IFN-γ. The differentiation of IFN-γ-producing Th1 cells was restored by monoclonal antibodies to TGF-β (30) or IL-10 (56), implicating these cytokines in the inhibition of Th1 cytokine production. In support of this hypothesis we show that TPx-activated macrophages produced a number of components including PGE2, IL-10, and TGF-β, which may also be involved in mediating the suppression of IFN-γ production. These results thus provide preliminary evidence of a role for TPx in the immune modulation of host immune responses, a function not previously described for this class of antioxidant. The prediction from this scenario is that secretion of TPx from other pathogens would lead to the induction of AAMφ, which then mediate differentiation of T cells to a Th2 phenotype. It is therefore of interest that a homolog of F. hepatica TPx is produced by eggs of S. mansoni, with its secretion stimulating a significant proliferation of CD4+ T cells in infected mice coincident with egg production and Th1-to-Th2 switching (61).
TPx belongs to a family of thiol-specific antioxidant proteins, which exert a protective role via their peroxidase activity (23, 62). Accumulating evidence suggests that members of this family of proteins regulate various aspects of cellular function by controlling the intracellular redox status of the cell. Typical of this is the regulation of NF-κβ activity, which is central to the gene activation of cytokines (5, 26). As a result of these observations, a paradigm has been proposed on the functional heterogeneity of macrophages based on cytokine propensities, with studies showing that intracellular redox status effects cytokine secretions of macrophages and therefore influences the Th1/Th2 balance (42). For example, depletion of glutathione, a thiol antioxidant, in murine peritoneal macrophages decreased the secretion of IL-12, leading to a switch of T-cell activity from a Th1 profile to a Th2 response (41, 48). In addition, thioredoxin transgenic mice, which overexpress human thioredoxin, demonstrate a long-term T-cell polarization toward Th1 during aging (40). This induction of Th1 seems to develop in response to the enhanced secretion of IL-12 from antigen-presenting cells (47) and is dependent on the enzymatic activity of thioredoxin (7). We demonstrate that, in contrast to this, extracellular delivery of the parasite antioxidant TPx to macrophages results in decreased IL-12 secretion associated with enhanced IL-10 production (Fig. 5). It is possible that in the extracellular environment TPx is acting not as an antioxidant but rather functioning through classical receptor binding. Further studies into the ligation events that may occur at the cell surface and the consequential signal pathways triggered are required. The biochemical connection between extracellular TPx and the intracellular thiol-redox status of macrophages will also need to be determined and may give an insight into the resultant coupling to intracellular redox cycling systems, thus resolving the discrepancy observed in IL-12 regulation.
The present study presents F. hepatica infection of mice as a tractable model for the study of the alternative activation of macrophages, since these cells appear soon after infection and at the initiation of a polarized Th2 response. Unlike in other models, the development of this Th2 response occurs in the absence of a predeveloped Th1 response. Our finding that TPx is at least one helminth molecule involved in directing macrophage activation will be important for elucidating a mechanism of activation, distinguishing heterogeneity between AAMφ in helminth infections as opposed to other disease states, and in deciphering their role in Th2-induction and immune-mediated pathogenesis in helminth infections.
Acknowledgments
Sheila Donnelly and Sandra O'Neill were funded by the Wellcome Trust and Enterprise Ireland, respectively. Mary Sekiya was supported by ildana Biotech of Ireland.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Abdallahi, O. M., H. Bensalem, R. Augier, M. Diagana, M. De Reggi, and B. Gharib. 2001. Arginase expression in peritoneal macrophages and increase circulating polyamine levels in mice infected with Schistosoma mansoni. Cell Mol. Life Sci. 58:1350-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Actor, J. K., M. Shirai, M. C. Kullberg, R. M. Buller, A. Sher, and J. A. Berzofsky. 1993. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th2 cytokine responses as well as delayed virus clearance. Proc. Natl. Acad. Sci. USA 90:948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aderem, A., and R. J. Ultevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 4.Alleva, D. G., C. J. Burger, and K. D. Elgert. 1994. Tumour-induced regulation of suppressor macrophage nitric oxide and TNF-alpha production: role of tumour-derived IL-10, TGF-β, and prostaglandin E2. J. Immunol. 153:1674-1686. [PubMed] [Google Scholar]
- 5.Baeuerle, P. A., and T. Henkel. 1994. Function and activation of NF-κB in the immune system. Annu. Rev. Immunol. 12:141-279. [DOI] [PubMed] [Google Scholar]
- 6.Behm, C. A., and N. C. Sangster. 1999. Pathology, pathophysiology, and clinical aspects, p. 185-201. In J. P. Dalton (ed.), Fasciolosis. CAB International, Wallingford, United Kingdom.
- 7.Bertini, R., O. M. Howard, H. F. Dong, J. J. Oppenheim, C. Bizzarri, R. Sergi, G. Caselli, S. Pagliei, B. Romines, J. A. Wilshire, M. Mengozzi, H. Nakamura, J. Yodoi, K. Pekkari, R. Gurunath, A. Holmgren, L. A. Herzenberg, L. A. Herzenberg, and P. Ghezzi. 1999. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J. Exp. Med. 189:1783-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bird, J. J., D. R. Brown, A. C. Mullen, N. H. Moskowitz, M. A. Mahowald, J. R. Sider, T. F. Gajewski, C. R. Wang, and S. L. Reiner. 1998. Helper T-cell differentiation is controlled by the cell cycle. Immunity 9:229-237. [DOI] [PubMed] [Google Scholar]
- 9.Bogdan, C. 1997. Of microbes, macrophages, and nitric oxide. Behring. Inst. Mitt. 99:58-64. [PubMed] [Google Scholar]
- 10.Brady, M. T., S. M. O'Neill, J. P. Dalton, and K. H. G. Mills. 1999. Fasciola hepatica suppresses a protective Th1 response against Bordetella pertussis. Infect. Immun. 67:5372-5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crabtree, J. E., and R. A. Wilson. 1986. The role of pulmonary cellular reactions in the resistance of vaccinated mice to Schistosoma mansoni. Parasite Immunol. 8:265-285. [DOI] [PubMed] [Google Scholar]
- 12.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune-cell function in mice with disrupted interferon-γ genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 13.Dalton, J. P., S. McGonigle, T. P. Rolph, and S. J. Andrews. 1996. Induction of protective immunity in cattle against infection with Fasciola hepatica by vaccination with cathepsin L proteinase and with hemoglobin. Infect. Immun. 64:5066-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doyle, A. G., G. Herbein, L. J. Montaner, A. J. Minty, D. Caput, P. Ferrara, and S. Gordon. 1994. Interleukin-13 alters the activation state of murine macrophages in vitro: comparison with interleukin 4 and interferon-gamma. Eur. J. Immunol. 24:1441-1445. [DOI] [PubMed] [Google Scholar]
- 15.Dunn, M. A., M. Roijkind, P. K. Hait, and K. S. Warren. 1978. Conversion of arginine to proline in murine schistosomiasis. Gastroenterology 75:1010-1015. [PubMed] [Google Scholar]
- 16.Dunn, M. A., S. Seifter, and P. K. Hait. 1981. Proline trapping in granulomas, the site of collagen biosynthesis in murine schistosomiasis. Hepatology 1:28-32. [DOI] [PubMed] [Google Scholar]
- 17.Falcone, F. H., P. Loke, X. Zang, A. S. MacDonald, R. M. Maizels, J. E. Allen. 2001. A Brugia malayi homolog of macrophage migration inhibitory factor reveals an important link between macrophages and eosinophil recruitment during nematode infection. J. Immunol. 167:5348-5354. [DOI] [PubMed] [Google Scholar]
- 18.Frangioni, J. V., and B. G. Neel. 1993. Solubilization and purification of enzymatically active glutathione-S-transferase (pGEX) fusion proteins. Anal. Biochem. 210:179-187. [DOI] [PubMed] [Google Scholar]
- 19.Gett, A. V., and P. D. Hodgkin. 1998. Cell division regulates the T-cell cytokine repertoire, revealing a mechanism underlying class regulation. Proc. Natl. Acad. Sci. USA 95:9488-9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goerdt, S., and C. E. Orfanos. 1999. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity 10:137-142. [DOI] [PubMed] [Google Scholar]
- 21.Gordon, S. 2003. Alternative activation of macrophages. Nat. Rev. Immunol. 3:23-35. [DOI] [PubMed] [Google Scholar]
- 22.Hesse, M., M. Modolel, A. C. La Flamme, M. Schito, J. M. Fuentes, A. W. Cheever, E. J. Pearce, and T. A. Wynn. 2001. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of l-arginine metabolism. J. Immunol. 167:6533-6544. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann, B., H. J. Hecht, and L. Flohe. 2002. Peroxiredoxins. Biol. Chem. 383:347-364. [DOI] [PubMed] [Google Scholar]
- 24.Holcomb, I. N., R. C. Kabakoff, B. Chan, T. W. Baker, A. Gurney, W. Henzel, C. Nelson, H. B. Lowman, B. D. Wright, N. J. Skelton, G. D. Frantz, D. B. Tumas, F. V. Peale, D. L. Shelton, and C. C. Hebert. 2000. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 19:4046-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holland, M. J., Y. M. Harcus, P. L. Riches, and R. M. Maizels. 2000. Proteins secreted by the parasite nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur. J. Immunol. 30:1977-1987. [DOI] [PubMed] [Google Scholar]
- 26.Jin, D., H. Chae, S. Rhee, and K. Jeang. 1997. Regulatory role for a novel human thioredoxin peroxidase in NF-κB activation J. Biol. Chem. 272:30952-30961. [DOI] [PubMed] [Google Scholar]
- 27.Kullberg, M. C., E. J. Pearce, S. E. Hieny, A. Sher, and J. A. Berzofsky. 1992. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J. Immunol. 148:3264-3270. [PubMed] [Google Scholar]
- 28.Kuroda, E., T. Sugiurs, K. Zeki, Y. Yoshida, and U. Yamashita. 2000. Sensitivity difference to the suppressive effect of prostaglandin E2 amongst mouse strains: a possible mechanism to polarize Th2 response in BALB/c mice. J. Immunol. 164:2386-2395. [DOI] [PubMed] [Google Scholar]
- 29.Lee, S. C., Z. H. Jaffar, K. Wan, S. T. Hogate, and K. Roberts. 1999. Regulation of pulmonary T-cell responses to inhaled antigen: role of Th1- and Th2-mediated inflammation. J. Immunol. 162:6867-6879. [PubMed] [Google Scholar]
- 30.Loke, P., A. S. MacDonald, and J. E. Allen. 2000. Antigen-presenting cells recruited by Brugia malayi induce Th2 differentiation of naive CD4+ T cells. Eur. J. Immunol. 30:1127-1135. [DOI] [PubMed] [Google Scholar]
- 31.Loke, P., A. S. MacDonald, A. Robb, R. M. Maizels, and J.E Allen. 2000. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell-to-cell contact. Eur. J. Immunol. 30:2669-2678. [DOI] [PubMed] [Google Scholar]
- 32.Loke, P., M. G. Nair, J. Parkinson, D. Guiliano, M. Blaxter, and J. E. Allen. 2002. IL-4-dependent alternatively activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 3:7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maizels, R. M., and M. Yazdanbakhsh. 2003. Immuneregulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3:733-744. [DOI] [PubMed] [Google Scholar]
- 34.McGonigle, S., J.P Dalton, and E. R. James. 1998. Peroxiredoxins: a new antioxidant family. Parasitol. Today 14:139-145. [DOI] [PubMed] [Google Scholar]
- 35.McGonigle, S., P. Curley, and J. P. Dalton. 1997. Cloning of peroxiredoxin, a novel antioxidant enzyme, from the helminth parasite Fasciola hepatica. Parasitology 115:101-104. [DOI] [PubMed] [Google Scholar]
- 36.Mills, C. D., K. Kincaid, J. M. Alt, M. J. Heilman, and A. M. Hill. 2000. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 164:6166-6173.10843666 [Google Scholar]
- 37.Mulcahy, G., P. Joyce, and J. P. Dalton. 1999. Immunology of Fasciola hepatica infection, p. 341-376. In J. P. Dalton (ed.), Fasciolosis. CAB International, Wallingford, United Kingdom.
- 38.Munder, M., K. Eichmann, and M. Modolell. 1998. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J. Immunol. 160:5347-5354. [PubMed] [Google Scholar]
- 39.Munder, M., K. Eichmann, J. M. Moran, F. Centeno, G. Soler, and M. Modolell. 1999. Th1/Th2-Regulated expression of arginase isoforms in murine macrophages and dendritic cells. J. Immunol. 163:3771-3777. [PubMed] [Google Scholar]
- 40.Murata, Y., M. Amao, J. Yoneda, and J. Hamuro. 2002. Intracellular thiol redox status of macrophages directs the Th1 skewing in thioredoxin transgenic mice during aging. Mol. Immunol. 38:747-757. [DOI] [PubMed] [Google Scholar]
- 41.Murata, Y., T. Ohteki, S. Koyasu, and J. Hamuro. 2002. IFN-gamma and pro-inflammatory cytokine production by antigen-presenting cells is dictated by intracellular thiol redox status regulated by oxygen tension. Eur. J. Immunol. 32:2866-2873. [DOI] [PubMed] [Google Scholar]
- 42.Murata, Y., T. Shimamura, and J. Hamuro. 2002. The polarization of T(h)1/T(h)2 balance is dependent on the intracellular thiol redox status of macrophages due to the distinctive cytokine production. Int. Immunol. 14:201-212. [DOI] [PubMed] [Google Scholar]
- 43.Nair, M. G., D. W. Cochrane, and J. E. Allen. 2003. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol. Lett. 85:173-180. [DOI] [PubMed] [Google Scholar]
- 44.Noel, W., G. Hassanzadeh, G. Raes, B. Namangala, I. Daems, L. Brys, F. Brombacher, P. D. Baetselier, and A. Beschin. 2002. Infection stage-dependent modulation of macrophage activation in Trypanosoma congolense-resistant and -susceptible mice. Infect. Immun. 70:6180-6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okano, M., A. R. Satoskar, K. Nishizaki, M. Abe, and D. A. Harn. 1999. Induction of Th2 responses and IgE largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J. Immunol. 163:6712-6717. [PubMed] [Google Scholar]
- 46.O'Neill, S. M., K. H. Mills, and J. P. Dalton. 2000. Fasciola hepatica infection downregulates Th1 responses in mice. Parasite Immunol. 22:147-155. [DOI] [PubMed] [Google Scholar]
- 47.Pekkari, K., J. Avila-Carino, A. Bengtsson, R. Gurunath, A. Scheynius, and A. Holmgren. 2001. Truncated thioredoxin (Trx80) induces production of interleukin-12 and enhances CD14 expression in human monocytes. Blood 97:3184-3190. [DOI] [PubMed] [Google Scholar]
- 48.Peterson, J. D., L. A. Herzenberg, K. Vasquez, and C. Waltenbaugh. 1998. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc. Natl. Acad. Sci. USA 95:3071-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raes, G., P. De Baetselier, W. Noel, A. Beschin, F. Brombacher, and G. G. Hassanzadeh. 2002. Differential expression of FIZZ1 and Ym1 in alternatively activated versus classically activated macrophages. J. Leukoc. Biol. 71:597-602. [PubMed] [Google Scholar]
- 50.Rodriguez-Sosa, M., A. R. Satoskar, R. Calderon, L. Gomez-Garcia, R. Saavedra, R. Bojalil, and L. I. Terrazas. 2002. Chronic helminth infection induces alternatively activated macrophages expressing high levels of CCR5 with low interleukin-12 production and Th2 biasing ability. Infect. Immun. 70:3656-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandler, N. G., M. M. Mentink-Kane, A. W. Cheever, and T. A. Wynn. 2003. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J. Immunol. 171:3655-3667. [DOI] [PubMed] [Google Scholar]
- 52.Sasaki, M., Y. Ohara-Nemoto, S. Tajika, M. Kobayashi, C. Yamaura, and S. Kimura. 2001. Antigenic characterisation of a novel Streptococcus aerginosus antigen that induces nitric oxide synthesis by murine peritoneal exudate cells. J. Med. Microbiol. 50:952-958. [DOI] [PubMed] [Google Scholar]
- 53.Sonoki, T., A. Nagasaki, T. Gotoh, M. Takiguchi, M. Takeya, H. Matsuzaki, and M. Mori. 1997. Coinduction of nitric-oxide synthase and arginase I in cultured rat peritoneal macrophages and rat tissues in vivo by lipopolysaccharide. J. Biol. Chem. 272:3689-3693. [DOI] [PubMed] [Google Scholar]
- 54.Stadecker, M. J. 1999. The regulatory role of the antigen presenting cell in the development of hepatic immunopathology during infection with Schistosoma mansoni. Pathobiology 67:269-272. [DOI] [PubMed] [Google Scholar]
- 55.Stein, M., S. Keshav, N. Harris, and S. Gordon. 1992. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176:287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Terrazas, L. I., K. L. Walsh, D. Piskorska, E. McGuire, and D. A. Harn. 2001. The schistosome oligosaccharide lacto-N-neotetraose expands Gr1+ cells that secrete anti-inflammatory cytokines and inhibit proliferation of naive CD4+ cells: a potential mechanism for immune polarization of helminth infections. J. Immunol. 167:5294-5303. [DOI] [PubMed] [Google Scholar]
- 57.Todt, J., J. Sonstein, T. Polak, G. D. Seitzman, B. Hu, and J. L. Curtis. 2000. Repeated intratracheal challenge with particulate antigen modulates murine lung cytokines. J. Immunol. 164:4037-4047. [DOI] [PubMed] [Google Scholar]
- 58.van der Pouw Kraan, T. C., L. C. Boeije, R. J. Smeenk, J. Wijdenes, and L. A. Aarden. 1995. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J. Exp. Med. 181:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verity, C. K., D. P. McManus, and P. J. Brindley. 2002. Cellular responses to Schistosoma japonicum cathepsin D aspartic protease. Parasite Immunol. 24:363-367. [DOI] [PubMed] [Google Scholar]
- 60.Welch, J., L. Escoubet-Lozach, D. B. Sykes, K. Liddiard, D. R. Greaves, and C. K. Glass. 2002. Th2 cytokines and allergic challenge induce Ym1 expression in macrophages by a STAT6-dependent mechanism. J. Biol. Chem. 277:42821-42829. [DOI] [PubMed] [Google Scholar]
- 61.Williams, D. L., H. Asahi, D. J. Botkin, and M. J. Stadecker. 2001. Schistosome infection stimulates host CD4+ T helper cell and B-cell responses against novel egg antigen, thioredoxin peroxidase. Infect. Immun. 69:1134-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wood, Z. A., E. Schroder, J. Robin Harris, and L. B. Poole. 2003. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 28:32-40. [DOI] [PubMed] [Google Scholar]
- 63.Yazdanbakhsh, M., A. van der Biggelaar, and R. M. Maizels. 2001. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduces allergic disease. Trends Immunol. 22:372-377. [DOI] [PubMed] [Google Scholar]