Significance
Lysosomes are found in all neuronal domains, including the soma, axon, and dendrites. How neurons are transported in these domains, however, remains poorly understood. In the present study, we show that a protein ensemble comprising BORC, Arl8, SKIP, and kinesin-1 specifically drives lysosome transport into the axon and not the dendrites. We also show that this mechanism of axonal lysosome transport is essential for maintenance of growth-cone dynamics and turnover of autophagosomes in the distal axon. These findings imply that transport of lysosomes into the axon and the dendrites occurs by different mechanisms, and demonstrate that BORC-regulated lysosome transport is critical for axonal functions.
Keywords: lysosomes, polarized organelle transport, kinesin-1, BORC, autophagosomes
Abstract
The ability of lysosomes to move within the cytoplasm is important for many cellular functions. This ability is particularly critical in neurons, which comprise vast, highly differentiated domains such as the axon and dendrites. The mechanisms that control lysosome movement in these domains, however, remain poorly understood. Here we show that an ensemble of BORC, Arl8, SKIP, and kinesin-1, previously shown to mediate centrifugal transport of lysosomes in nonneuronal cells, specifically drives lysosome transport into the axon, and not the dendrites, in cultured rat hippocampal neurons. This transport is essential for maintenance of axonal growth-cone dynamics and autophagosome turnover. Our findings illustrate how a general mechanism for lysosome dispersal in nonneuronal cells is adapted to drive polarized transport in neurons, and emphasize the importance of this mechanism for critical axonal processes.
Since their discovery by de Duve in the mid-20th century (1), lysosomes have become recognized as the main site for the degradation of a wide variety of biomacromolecules in the endomembrane system of animal cells (2). Indeed, lysosomes degrade extracellular and plasma membrane substrates delivered by way of endocytosis, as well as cytoplasmic substrates engulfed in the process of autophagy. In recent years, lysosomes and their precursor late endosomes (hereafter referred to indistinctly as “lysosomes”) have been found to participate in many other cellular processes, including metabolic signaling, immunity, plasma membrane repair, exocytosis, cell adhesion and migration, and tumor invasion and metastasis (2). The performance of all these functions requires that lysosomes constantly survey the cytoplasmic space in search of targets (3). Bidirectional movement of lysosomes occurs along microtubules (4) and is driven by the plus end-directed kinesin (5) and minus end-directed dynein microtubule motors (6). Various ensembles of small GTPases, their effectors, and other adaptor and regulatory molecules mediate coupling of lysosomes to the microtubule motors, enabling their controlled movement in response to diverse cellular needs (7, 8).
We recently described a multiprotein complex named BORC (for BLOC-One–Related Complex) that regulates coupling of lysosomes to kinesin-1, promoting their radial movement toward the peripheral cytoplasm in nonpolarized cells (9). BORC comprises eight subunits named BLOS1, BLOS2, snapin, KXD1, myrlysin (LOH12CR1), lyspersin (C17orf59), diaskedin (C10orf32), and MEF2BNB (LOC729991). The eight subunits have also been designated BORCS1–BORCS8, respectively, by the Human Gene Nomenclature Committee. This complex associates with the cytosolic aspect of the lysosomal membrane in part through a myristoyl group at the N terminus of myrlysin (9). BORC does not interact with kinesin-1 directly, but does so through the small GTPase Arl8b and its effector SKIP (8, 9). Interference with any link in this chain of interactors impairs anterograde movement of lysosomes and leads to the collapse of the lysosome population to the pericentrosomal region of nonpolarized cells (8–10). As a consequence, cellular processes that depend on lysosome movement, such as endocytic degradation, autophagy, cell adhesion and migration, and antigen presentation, are impaired (9, 11–15).
The mechanisms of organelle distribution in polarized cells such as neurons are far more complex. Unlike nonpolarized cells, neurons have a soma, dendrites, and axon with different organelle compositions. Whereas some organelles are distributed throughout the neuron (e.g., mitochondria), others are largely segregated to the somatodendritic domain (e.g., the Golgi complex) or the axonal domain (e.g., synaptic vesicles) (16). Specific patterns of organelle distribution in neurons are partly the result of the organelles’ ability to move along different microtubule tracks through coupling to different microtubule motors (17, 18). Neuronal microtubules are acentrosomal and exhibit distinct properties in the somatodendritic and axonal domains. Dendritic microtubules are shorter and have mixed orientations, whereas axonal microtubules consist of long bundles with uniform plus end-out orientation (19, 20). In addition, dendritic and axonal microtubules display different posttranslational modifications of tubulin (21) and microtubule-associated proteins (22). These distinct properties determine the specificity and direction of organelle movement by different microtubule motors in different regions of the neuron. Of the ∼45 kinesins encoded in mammalian genomes, many mediate axonal anterograde transport, whereas a few additionally drive transport in the dendrites (23–25). In contrast, there is only one cytoplasmic dynein in mammals that mediates retrograde transport in the axon, as well as transport in the dendrites (26, 27). Dendritic transport mediated by kinesins and dynein is potentially bidirectional because of the mixed polarity of dendritic microtubules.
Lysosomes have been previously reported to occur in all regions of the neuron and to play essential roles in general and neuron-specific processes (28–33). Moreover, lysosome dysfunction underlies many neurodevelopmental and neurodegenerative disorders. Among these are hereditary lysosomal-storage diseases caused by mutations in lysosomal proteins, which often affect the central nervous system (34). In addition, defective lysosome function contributes to the pathogenesis of more common neurological disorders such as Alzheimer’s disease and Parkinson’s disease (35). Although it is clear that lysosomes are essential for proper neuronal function, the segregation of the neuronal cytoplasm into structurally and functionally different domains poses additional challenges to the understanding of lysosome distribution and dynamics. Outstanding questions are: How do lysosomes traffic in different neuronal domains? Is the BORC/kinesin-1–dependent lysosome transport characterized in nonpolarized cells also operational in neurons, and, if so, in what neuronal domains? What neuronal functions are dependent on this transport mechanism?
In this study, we have addressed these questions by using rat hippocampal neurons in primary culture as a model system. We find that lysosomes exhibit a largely nonpolarized distribution, and move bidirectionally in all regions of the neuron. Remarkably, the BORC-Arl8-SKIP-kinesin-1 ensemble controls axonal but not dendritic transport of lysosomes. Indeed, interference with these proteins decreases lysosome transport specifically into the axon. Conversely, its enhancement drives lysosome accumulation at axon terminals. We also show that this mechanism is essential for maintenance of axonal growth-cone dynamics and autophagosome clearance. These findings illustrate how a general mechanism for centrifugal transport of lysosomes in nonpolarized cells is adapted to drive polarized transport of lysosomes in neurons.
Results
Nonpolarized Distribution of Lysosomes in Hippocampal Neurons.
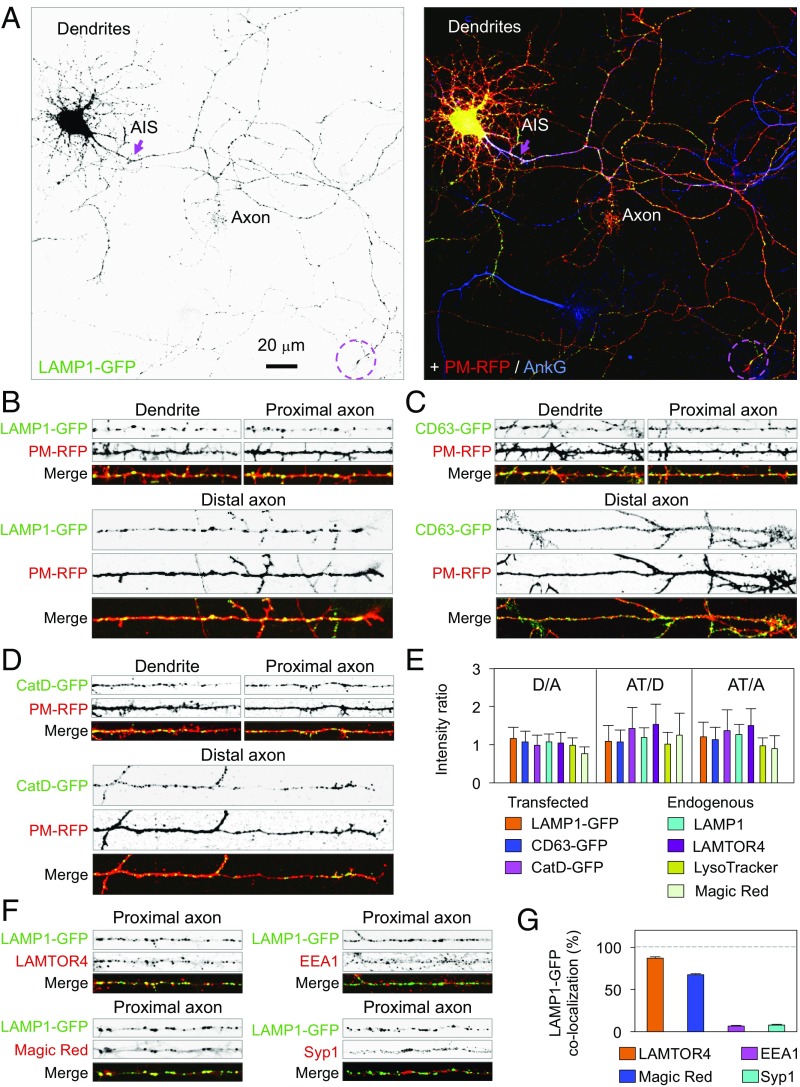
As a first step to analyze the mechanisms that regulate lysosome distribution in neurons, we examined the localization of various endogenous and transgenic lysosomal markers in day in vitro (DIV) 7 or 8 rat hippocampal neurons in primary culture. Immunofluorescence microscopy for the endogenous lysosome-associated membrane protein 1 (LAMP1) (36) or the late endosomal/lysosomal adaptor and MAPK and MTOR activator 4 (LAMTOR4) (37) showed that these proteins were most concentrated in the soma, but were also present in discrete puncta along both the dendrites and the axon (Fig. S1). Staining with the acidic-organelle dye LysoTracker or the fluorogenic cathepsin B substrate Magic Red, both functional markers of lysosomes, showed a similar distribution of fluorescent puncta to the soma, dendrites, and axon (Fig. S1). Expression of various GFP-tagged lysosomal proteins (i.e., LAMP1-GFP, CD63-GFP, or cathepsin D-GFP) by transfection at DIV 4 recapitulated the nonpolarized distribution of the endogenous lysosomal proteins at DIV 7/8 (Fig. 1A–D). In the axon, lysosomes were evenly distributed among the proximal, middle, and distal regions (Fig. 1A–D). Quantification of the mean fluorescence intensity of endogenous and transgenic lysosomal markers in dendrites vs. axon (D/A), axon tips vs. dendrites (AT/D), and axon tips vs. axon (AT/A) from many neurons yielded ratios in the range of 0.8–1.5 (Fig. 1E), confirming that lysosomes have a largely nonpolarized distribution, and are not particularly concentrated in axon tips. The axonal LAMP1-GFP colocalized with endogenous LAMTOR4 and Magic Red product, but not the endogenous early endosomal antigen 1 (EEA1) and synaptic vesicle protein synaptophysin-1 (Syp1; Fig. 1 F and G), confirming that LAMP1-GFP is confined to lysosomes and does not spill over into other axonal organelles. LAMP1-GFP and LAMTOR4 also exhibited a nonpolarized distribution in more mature DIV 17 neurons (Fig. 2 A–C). Taken together, these observations demonstrated the presence of conventional lysosomes in all neuronal domains, and validated LAMP1-GFP as a faithful reporter of this distribution.
Fig. S1.
Nonpolarized distribution of lysosomes in DIV 7/8 hippocampal neurons (Fig. 1). (A) DIV 7 neurons were fixed with 4% paraformaldehyde, 4% sucrose in PBS solution for 20 min at room temperature, followed by 99.8% methanol cooled to −20 °C for 3 min, and stained for endogenous LAMTOR4 (red channel), LAMP1 (green channel), and AnkG (blue channel). (B and C) Live DIV 8 neurons expressing GFP (green channel) and incubated for 30 min with LysoTracker (B) or Magic Red (C) probes (red channels). Arrows point to the AIS. (Bottom) Straightened 50-µm segments of dendrites and proximal axons.
Fig. 1.
Nonpolarized distribution of lysosomes in DIV 7 hippocampal neurons. (A) Fixed DIV 7 neuron expressing LAMP1-GFP (grayscale, green in merge) and PM-RFP (red in merge), and stained for endogenous AnkG (blue in merge). Arrow points to the AIS, and the dashed circle shows an axon tip. (B–D) Fifty-micrometer segments of dendrites and proximal axons and 100-μm segments of distal axons from fixed DIV 7 neurons expressing PM-RFP together with LAMP1-GFP (B), CD63-GFP (C), or cathepsin D (CatD)-GFP (D). Fig. S1 shows immunostaining of endogenous LAMP1 and LAMTOR4 and staining with LysoTracker and Magic Red. (E) D/A, AT/D, and AT/A ratios are represented as the mean ± SD from 25 neurons such as those in B–D and Fig. S1. (F) Fifty-micrometer segments of proximal axons from DIV 7 neurons expressing LAMP1-GFP and stained live with Magic Red or, after fixation, for endogenous LAMTOR4, EEA1, or synaptophysin-1 (Syp1). (G) Quantification of colocalization of 150 LAMP1-GFP–positive particles from 15 neurons per condition analyzed with the different markers shown in F, and expressed as mean ± SD.
Fig. 2.
Nonpolarized distribution of lysosomes in DIV 17 hippocampal neurons. (A) Fixed DIV 17 neuron expressing LAMP1-GFP (green channel) and PM-RFP (red channel) and stained for AnkG (blue channel). (B) Fixed DIV 17 neuron stained for endogenous LAMTOR4 (green channel), the postsynaptic marker PSD-95 (red channel), and AnkG (blue channel). Arrows point to the AIS. (Bottom) Straightened 50-µm segments of dendrites and proximal axons. (C) D/A ratios are represented as the mean ± SD from 20 neurons per condition.
Lysosomes Move Bidirectionally in both Axons and Dendrites.
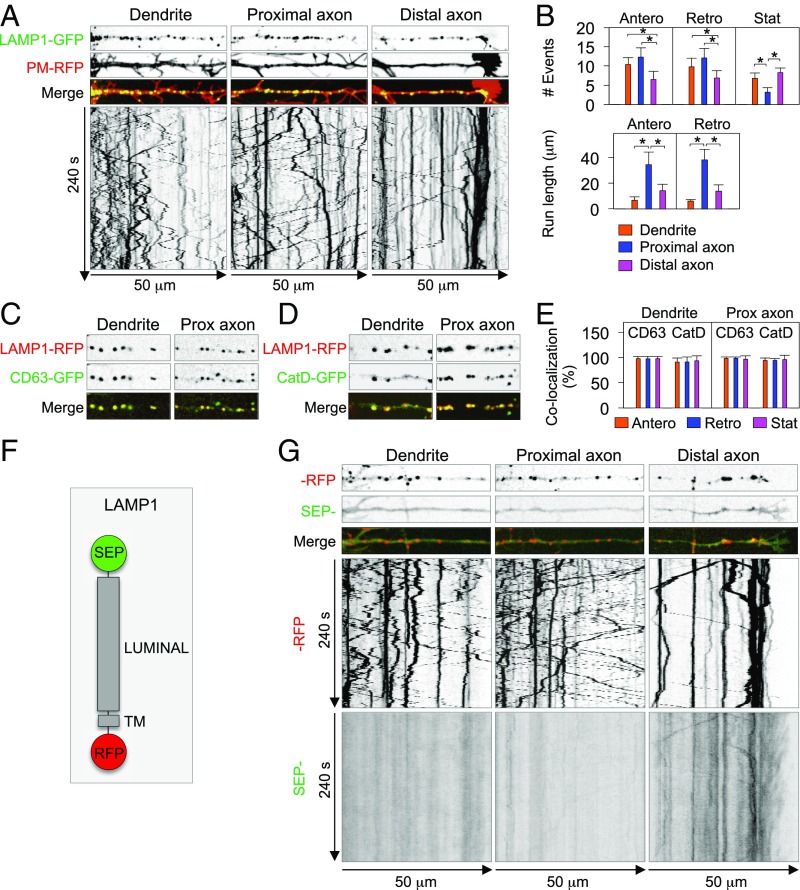
To examine lysosome dynamics in hippocampal neurons, we performed live-cell imaging of LAMP1-GFP in cells coexpressing plasma membrane-targeted RFP (PM-RFP) and stained with a CF640R-conjugated antibody to the axon initial segment (AIS) protein neurofascin (i.e., NF-640R) (38). This imaging revealed bidirectional movement of lysosomes containing LAMP1-GFP in dendrites and axon (Fig. 3A and Movie S1). Motile lysosomes accounted for 74.8% of the total in dendrites (51.5% anterograde, 48.5% retrograde) and 88.4% in the axon (50.4% anterograde, 49.6% retrograde), whereas the rest were stationary (Fig. 3 A and B). The average run length of moving lysosomes was shorter in dendrites than in axons (Fig. 3 A and B), consistent with the different microtubule organization in the two domains. The number and run length of lysosomes moving anterogradely and retrogradely in the proximal axon (Fig. 3 A and B and Movie S1) were not significantly different from those parameters in the midaxon. In the distal axon, including axon tips, however, we saw an increase in the number of stationary lysosomes, particularly at the entrance to the growth cones (Fig. 3 A and B). Additional experiments showed comovement of LAMP1-RFP with CD63-GFP and Cathepsin D-GFP in dendrites and axon (Fig. 3 C–E and Movies S2 and S3), corroborating the identity of the moving vesicles as conventional lysosomes. These results confirmed and expanded previous observations in cortical neurons (30), demonstrating that functional lysosomes have a largely nonpolarized, dynamic distribution in different neuronal types.
Fig. 3.
Bidirectional movement of lysosomes in dendrites and axon. (A) Single frames from Movie S1 (top three strips) and kymographs (bottom strip) of 50-μm segments of a dendrite, proximal axon, and distal axon from a DIV 8 neuron expressing LAMP1-GFP and PM-RFP. The kymographs show LAMP1-GFP moving in anterograde (lines with negative slopes) and retrograde (lines with positive slopes) directions, and in stationary foci (vertical lines), during 240 s of recording. (B) Quantification of the number and run length of LAMP1-GFP particles in dendrites, proximal axon, and distal axon expressed as mean ± SD from eight neurons. Statistical significance for all groups was calculated by one-way ANOVA followed by Dunnett’s test (*P < 0.01). (C and D) Single frames from Movies S2 and S3 showing colocalization of moving and stationary particles containing LAMP1-RFP together with CD63-GFP (C) or CatD-GFP (D) in 50-μm segments of a dendrite and proximal axon. (E) Quantification of the colocalization of LAMP1-RFP with CD63-GFP and CatD-GFP in anterograde, retrograde, and stationary particles in dendrites and proximal axon. A total of 148 and 141 LAMP1-RFP–positive particles from five neurons per condition were analyzed for colocalization with CD63-GFP and CatD-GFP, respectively. Results are expressed as the mean ± SD. (F) Schematic representation of SEP-LAMP1-RFP. (G) Single frames from Movie S4 (top three strips) and kymographs (bottom strip) of 50-μm segments of a dendrite, proximal axon, and distal axon from a DIV 7 neuron expressing SEP-LAMP1-RFP. Note the absence of SEP fluorescence in lysosomes moving in dendrites and proximal axon, and its presence near the axon tip. Also note the more diffuse appearance of SEP fluorescence in the distal axon.
Reduced Acidity of Lysosomes at Axon Tips.
Mature lysosomes in nonneuronal cells have an acidic luminal pH (4.5–5.0). To verify that lysosomes in dendrites and axon are acidic, we fused superecliptic pHluorin (SEP) to the luminal domain of LAMP1-RFP (Fig. 3F) and expressed the chimeric construct (SEP-LAMP1-RFP) in neurons. SEP fluoresces green at neutral pH but is nonfluorescent at pH < 6 (39). In the dendrites and the proximal axon, anterogradely and retrogradely moving lysosomes were positive for RFP but negative for SEP, indicating that they were acidic (Fig. 3 G and Movie S4). In the distal axon, including axon tips, however, we observed some puncta exhibiting both SEP and RFP fluorescence (Fig. 3G). The latter likely represent lysosomes that are less acidic, such as those described in the periphery of nonneuronal cells (40). We also observed more diffuse SEP fluorescence in the distal axon, including the growth cone (Fig. 3G and Movie S4). This fluorescence could result from fusion of lysosomes with the plasma membrane, as previously reported in other cells (29, 41–43).
Interference with the BORC–Arl8b–SKIP–kinesin-1 Ensemble Specifically Impairs Lysosome Transport into the Axon.
A BORC–Arl8b–SKIP–kinesin-1 ensemble was previously shown to drive centrifugal transport of lysosomes in nonpolarized cells (8, 10, 12, 44–46) (Fig. 4A). Given the polarized organization of neurons, we asked whether this mechanism was involved in moving lysosomes into the dendrites and/or the axon. To address this question, we performed shRNA-mediated knockdown (KD) or dominant-negative (DN) interference of the different components of this machinery, and examined the effect of these manipulations on the distribution of LAMP1-RFP in neurons. KD was confirmed by immunostaining, confocal microscopy, and image analysis of the endogenous targets for each shRNA (Fig. S2). We first tested the involvement of kinesin-1, a heterotetramer composed of two kinesin heavy chains (KIF5) and two kinesin light chains (KLC) (47). Neurons express three KIF5 paralogs encoded by different genes: the neuron-specific KIF5A and KIF5C and the ubiquitous KIF5B (48). Treatment with shRNAs targeting all three KIF5 proteins caused a drastic depletion of lysosomes from the axon but not the dendrites (Fig. 4B vs. Fig. 4C). Likewise, overexpression of a KLC1-DN construct encoding the cargo-recognition tetratricopeptide-repeat (TPR) but not the KIF5-interacting heptad-repeat domain (49) specifically reduced the presence of lysosomes in the axon (Fig. 4D). Similar observations were made upon treatment with shRNAs to SKIP, Arl8b, or the myrlysin or lyspersin subunits of BORC (Fig. 4 E–H). The depletion of LAMP-1-RFP–labeled lysosomes from the axon in myrlysin- or lyspersin-KD neurons could be rescued by expression of shRNA-resistant, epitope-tagged forms of the corresponding proteins (Fig. S3). Quantification by image analysis of many neurons showed that interference with the BORC–Arl8b–SKIP–kinesin-1 complex increased the dendrite/axon polarity index of lysosomes from ∼1 in control neurons to 4–8 in the treated neurons (Fig. 4I and Fig. S3). Depletion of lysosomes was uniform throughout the axon, as shown by the largely unchanged ratio of axon tips to the rest of the axon (Fig. 4I and Fig. S3). These observations thus demonstrated a requirement for all of the members of the BORC–Arl8b–SKIP–kinesin-1 ensemble for polarized movement of lysosomes into the axon, but not the dendrites.
Fig. 4.
Reduced localization of lysosomes to the axon caused by interference with components of the BORC/kinesin-1 machinery. (A) Schematic representation of the BORC–Arl8–SKIP–kinesin-1 ensemble (Left) and of the domains involved in interactions (Right). (B–H) DIV 7 neurons coexpressing LAMP1-RFP together with GFP plus control shRNA (B), KIF5A+B+C shRNA (C), KLC1-DN (D), SKIP shRNA (E), Arl8b shRNA (F), myrlysin shRNA (G), or lyspersin shRNA (H). All shRNAs used in this study were shown to be effective at depleting the target proteins (Fig. S2). Arrows point to the AIS, and arrowheads indicate the axon. (I) D/A, AT/D, and AT/A ratios expressed as mean ± SD from 25 neurons analyzed as in B–H. Statistical significance for all groups, including KIF5A+B+C shRNA, KLC1-DN, SKIP shRNA, Arl8b shRNA, myrlysin shRNA, and lyspersin shRNA, was calculated by one-way ANOVA followed by Dunnett’s test (*P < 0.01 vs. control). The effects of myrlysin- and lyspersin-shRNAs could be rescued by expression of the corresponding shRNA-resistant constructs (Fig. S3).
Fig. S2.
KD of KIF5, SKIP, Arl8b, myrlysin, or lyspersin (Fig. 3). (A–E) Neurons transfected at DIV 5 with plasmids encoding GFP together with shRNAs to KIF5A+KIF5B+KIF5C (A), SKIP (B), Arl8b (C), myrlysin (D), or lyspersin (E; green channel) were stained on DIV 7 for the corresponding target proteins (red channel). Arrows point to the AIS. Enlarged images from the dashed boxes (rightmost column) show the absence of the target proteins (red channel) in the axons from neurons expressing the corresponding shRNA (reported by GFP expression; green channel) compared with their presence in the axons of adjacent neurons not expressing the shRNAs. (F) Quantification of fluorescence intensity of the proteins in axon tips of GFP-positive neurons and their neighboring GFP-negative neurons. Values are expressed as the mean ± SD from 15 neurons per condition. Statistical significance between group pairs was calculated by Student’s t test (*P < 0.01 vs. control).
Fig. S3.
Rescue of myrlysin and lyspersin in KD neurons (Fig. 3). (A) DIV 7 neurons expressing LAMP1-RFP (red channel) and myrlysin-shRNA/GFP (green channel) in the absence (Top) or presence (Bottom) of shRNA-resistant myrlysin tagged with the myc epitope (blue channel, Inset). (B) Neurons expressing LAMP1-RFP (red channel) and lyspersin-shRNA/GFP (green channel) in the absence (Top) or presence (Bottom) of shRNA-resistant lyspersin tagged with the myc epitope (blue channel, Inset). Arrows point to the AIS and arrowheads to the axon. Quantification of LAMP1-RFP intensity ratios is shown (Right) as mean ± SD from 20 neurons per condition. Statistical significance for all groups was calculated by one-way ANOVA followed by Dunnett’s test (*P < 0.01, myrlysin-shRNA or lyspersin-shRNA vs. control and rescue).
Kinesin-1 Requires SKIP for Its Ability to Promote Axonal Transport of Lysosomes.
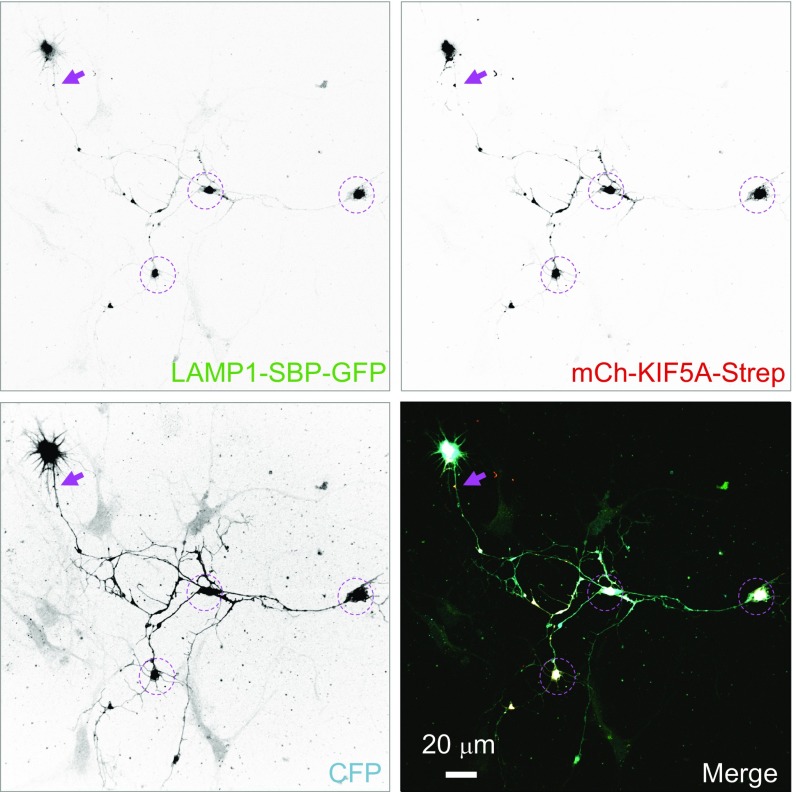
To analyze in more detail the activity of the different components of the BORC/kinesin-1 machinery in moving lysosomes to the axon, we examined the effect of overexpressing different combinations of motor and adaptor proteins on the distribution of LAMP1-RFP. We observed that overexpression of GFP-KIF5A had no effect on lysosome distribution (Fig. 5A). Coexpression of GFP-KIF5A with HA-KLC1 caused only a modest increase in the amount of lysosomes at axon tips relative to the rest of the axon (Fig. 5A). Additional expression of myc-SKIP, however, resulted in strong accumulation of lysosomes at axon tips (Fig. 5B), as reflected by an AT/A ratio of ∼6 (Fig. 5A). For comparison, we showed that forced coupling of lysosomes to the KIF5A motor domain by coexpression of chimeric constructs comprising the motor domain of KIF5A fused to streptavidin and mCherry (mCh-KIF5A-Strep), and LAMP1 fused to the streptavidin-binding peptide and GFP (LAMP1-SBP-GFP), caused massive accumulation of lysosomes at axon tips (Fig. 5A and Fig. S4). In these experiments, D/A ratios did not change significantly (Fig. 5A), but this was because lysosome accumulation occurred at axon tips and not along the axon. Importantly, GFP-KIF5A, HA-KLC1, and myc-SKIP colocalized on lysosomes in the axon (Fig. 5 C–E), consistent with their effects being the consequence of a direct association.
Fig. 5.
Kinesin-1 requires SKIP to drive axonal lysosome transport. (A) Quantification of the effects of overexpressing different combinations of GFP-KIF5A, KLC1-HA, and myc-SKIP. D/A, AT/D, and AT/A ratios are expressed as the mean ± SD from 25 neurons. Statistical significance for all groups was calculated by one-way ANOVA followed by Dunnett’s test (*P < 0.01 vs. control). GFP was used as a control for uniform distribution, and a combination of LAMP1-SBP-GFP with mCh-KIF5A-streptavidin (Fig. S4) as a control for extreme redistribution to axon tips. (B) DIV 7 neuron expressing LAMP1-RFP together with GFP-KIF5A, KLC1-HA, and myc-SKIP. Arrows point to the AIS, and the dashed circle shows an axon tip. (C) Fifty-micrometer segments of a dendrite and the proximal axon and 100-μm-length segment of the distal axon of the neuron shown in B. (D and E) Fluorescence line intensity plots show the accumulation of all proteins at the axon tip (D), and their colocalization in vesicles along the distal axon (E). (F) DIV 7 neuron expressing LAMP1-RFP plus KLC1-HA, myc-SKIP, and a Rigor-KIF5A mutant able to interact with but not walk along microtubules. (G and H) Single images of the initial part of the axon (Top) and kymographs (Bottom) from live DIV8 neurons expressing LAMP1-RFP plus GFP-Rigor-KIF5A (G), or these proteins in combination with KLC1-HA and myc-SKIP (H). Note that expression of Rigor-KIF5A alone does not affect the movement of lysosomes into the axon, whereas Rigor-KIF5A plus KLC1 and SKIP immobilize lysosomes in the initial part of the axon.
Fig. S4.
Forced coupling of lysosomes to kinesin-1 drives lysosomes to axon tips (Figs. 4 and 5). DIV 7 neurons transfected on DIV 5 with plasmids encoding chimeric constructs comprising the motor domain of KIF5A fused to streptavidin and mCherry (mCh-KIF5A-Strep; red channel) and LAMP1 fused to the streptavidin-binding peptide and GFP (LAMP1-SBP-GFP; green channel). Arrows point to the AIS. Note the dramatic redistribution of lysosomes to axon tips (dashed circles).
As another means of demonstrating the importance of SKIP for the effect of kinesin-1 on lysosomes, we examined the effect of overexpressing SKIP together with HA-KLC1 and a GFP-KIF5A G235A “rigor” mutant (Rigor-KIF5A), which can bind to its preferred microtubules but not “walk” along them (50). As previously reported (38, 51), under these conditions, GFP-Rigor-KIF5A decorated microtubule tracks spanning the initial part of the axon, corresponding to the preaxonal exclusion zone (38) and AIS (Fig. 5F). Importantly, whereas expression of GFP-Rigor-KIF5A alone had little or no effect on lysosome motility in the axon (Fig. 4G), coexpression with HA-KLC1 and myc-SKIP arrested lysosomes in the initial part of the axon (Fig. 5 F and H). The failure of GFP-Rigor-KIF5A alone to exert a DN effect on axonal lysosome transport could be the result of incomplete replacement of this protein for endogenous KIF5 isoforms in complex with KLC and SKIP over the time course of the experiments. The coexpression with HA-KLC1 and myc-SKIP, on the contrary, endows the excess GFP-Rigor-KIF5A with the ability to bind, and thus immobilize, lysosomes independently of endogenous KIF5–KLC–SKIP complexes. All these experiments were consistent with SKIP being critical for the ability of kinesin-1 to associate with lysosomes and to drive them down the axon.
Arl8b and SKIP Are Limiting Factors in the Coupling of Lysosomes to Kinesin-1 in Neurons.
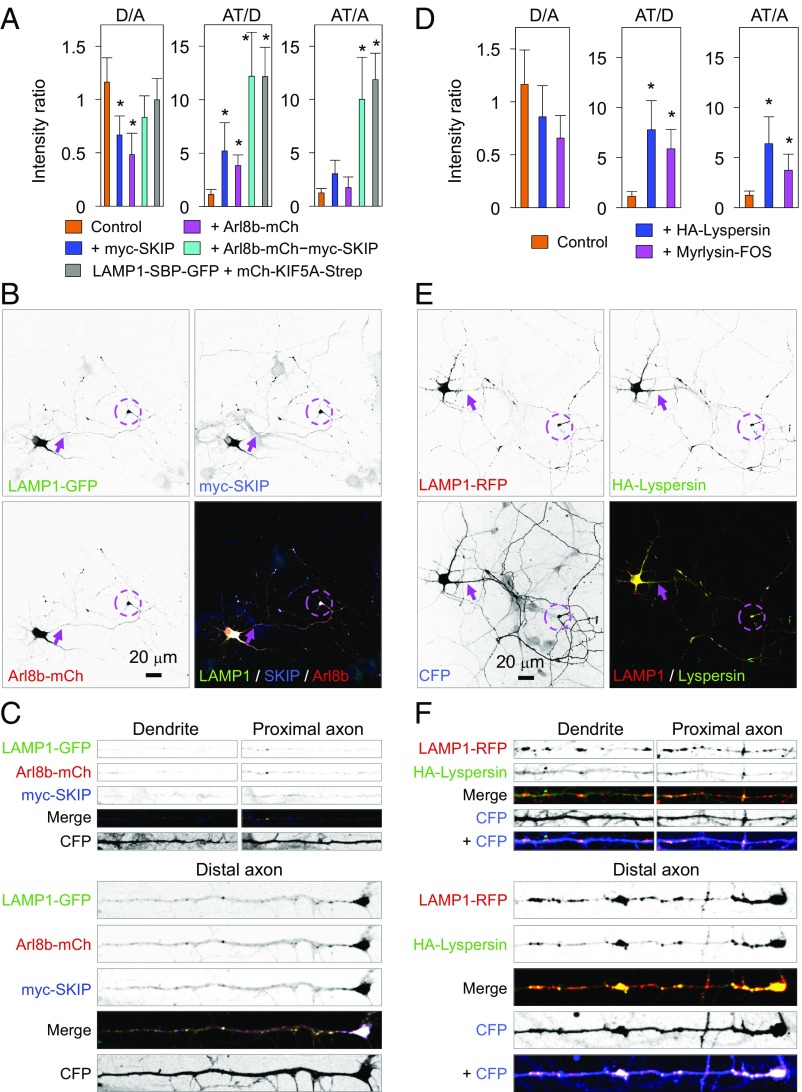
Arl8b and BORC function upstream of SKIP to couple lysosomes to kinesin-1 (8, 9) (Fig. 4A). Single overexpression of Arl8b or SKIP showed association of these proteins with axonal lysosomes (Movie S5), but caused only a modest concentration of lysosomes in axon tips (Fig. 6A). When overexpressed together, however, Arl8b and SKIP promoted robust accumulation of lysosomes in axon tips (Fig. 6 A–C), comparable to that resulting from coexpression of mCh-KIF5A-Strep with LAMP1-SBP-GFP (Fig. 6A and Fig. S4). The strong accumulation of lysosomes at axon tips in neurons overexpressing a combination of Arl8b and SKIP depleted lysosomes from dendrites and axon shafts, explaining why the D/A ratio in these neurons was higher than in those overexpressing Arl8b or SKIP alone, in which accumulation occurred all along the axon (Fig. 6A). These results indicated that Arl8b and its effector SKIP are limiting factors for the coupling of lysosomes to kinesin-1 in neurons.
Fig. 6.
Arl8b-SKIP or BORC subunit overexpression promotes accumulation of lysosomes in axon tips. (A) Quantification of the effects of overexpressing myc-SKIP or Arl8b-mCherry alone, or both proteins together, on the distribution of LAMP1-GFP. (B and C) DIV 7 neuron expressing LAMP1-GFP and SKIP-Arl8b (B) and enlarged regions of a dendrite, proximal axon, and distal axon of this neuron (C). (D) Quantification of the effects of overexpressing HA-lyspersin or myrlysin-FOS on the distribution of LAMP1-GFP. (E and F) DIV 7 neuron expressing LAMP1-RFP, HA-lyspersin, and CFP. In A and D, LAMP1 intensity ratios are the mean ± SD from 25 neurons. Statistical significance for all groups was calculated by one-way ANOVA followed by Dunnett’s test (*P < 0.01 vs. control). In B and E, arrows point to the AIS and dashed circles show axon tips. Comovement of Arl8b-GFP or GFP-SKIP with LAMP-1-RFP is shown in Movie S5. Comovement of myrlysin-GFP with LAMP-1-RFP is shown in Movie S6.
Myrlysin-GFP was also found on lysosomes moving in both directions, as well as in stationary foci (Movie S6). Interestingly, overexpression of myrlysin-FOS or HA-lyspersin caused accumulation of lysosomes in axon tips, albeit to a lesser extent relative to Arl8b plus SKIP (Fig. 6 D–F). These observations indicated that myrlysin and lyspersin have an intrinsic ability to activate downstream effectors of lysosome movement, although full activity may require the other subunits of BORC.
Reduced Growth Cone Dynamics and Aberrant Autophagosomes in Axons from BORC-KD Neurons.
What is the physiological importance of BORC-dependent lysosome transport into axons? As expected, expression of myrlysin shRNA reduced transport of LAMP1-RFP into the axon (Fig. 7 A and B and Movie S7). Interestingly, in the same neurons, we noticed a decrease in the size of axonal growth cones upon depletion of myrlysin (Fig. 7 C and D and Movie S7). Similar decreases in growth cone size were observed in lyspersin- and Arl8b-shRNA–treated neurons (Fig. 7D). Moreover, the shape of the growth cones did not change over time in myrlysin-KD cells, whereas it was highly dynamic, particularly in the peripheral region, in control cells (Fig. 7C and Movie S7). The decreased size and dynamics of the growth cones in myrlysin-KD cells indicates a requirement of lysosomes for growth cone structure and function.
Fig. 7.
Aberrant growth cones and autophagosomes in the axon of myrlysin-KD cells. (A) Single images (top three strips) and kymographs (bottom strip) from Movie S7 of a 100-μm segment of the distal axon from a DIV 8 neuron expressing LAMP1-RFP together with control-shRNA/GFP (Left) or myrlysin-shRNA/GFP (Right). (B) Quantification of the total number of lysosomes moving in the distal axon from neurons transfected as in A and expressed as the mean ± SD from seven neurons per condition. (C) Images of an axonal growth cone at different times from the neurons expressing control or myrlysin-shRNA in A and Movie S7. (D) Quantification of the growth cone area expressed as normalized mean ± SD from 50 growth cones per condition relative to control shRNA. Ctrl, control; Lys, lyspersin; Myr, myrlysin. (E) Single images (Top) and kymographs (Bottom) from movies of a 100-μm-length segment of the distal axon from DIV 8 neurons expressing mCherry-LC3 plus control-, myrlysin-, myrlysin+lyspersin-, or Arl8b-shRNA. (F) Quantification of the number of LC3 particles moving retrogradely in the distal axon and intensity levels of LC3 in the distal axon in neurons transfected as in E. LC3 transport is expressed as the mean ± SD from seven neurons per condition. Intensity levels of LC3 are expressed as normalized mean ± SD from 15 neurons per condition. (G and H) Single images (top two strips) and kymographs (bottom strip) from movies of 100-μm-length segment of the distal axon from DIV 8 neurons expressing mCherry-LC3 plus LAMP1-GFP (G) or LAMP1-BFP2 in the presence of control-shRNA (H, Left) or myrlysin-shRNA (H, Right). Total LC3 levels and LC3/LAMP1 colocalization events are expressed as the mean ± SD from seven neurons per condition. Statistical significance for groups (D, F, and H) was calculated by one-way ANOVA followed by Dunnett’s test. Significance between group pairs (B and G) was calculated by Student’s t test (*P < 0.01 vs. control).
We also observed that control neurons exhibited small vesicles labeled with the autophagy marker LC3, some that were stationary and others that moved mainly in the retrograde direction, as previously reported (30, 31, 52, 53) (Fig. 7 E–H). In contrast, neurons depleted of myrlysin, lyspersin, myrlysin plus lyspersin, or Arl8b exhibited an accumulation of LC3 in large, immobile structures in the distal axon (Fig. 7 E and F). Analysis of mCherry-LC3 and LAMP1-GFP colocalization revealed that most stationary and retrograde mCherry-LC3 vesicles also contained LAMP1-GFP (Fig. 7G) or LAMP1-BFP2 (Fig. 7H) in control neurons. In contrast, the aberrant mCherry-LC3–labeled structures in myrlysin-KD neurons were largely devoid of LAMP1-BFP2 (Fig. 7H). From these experiments we concluded that BORC-dependent anterograde transport of lysosomes is important for mobilization and disposal of autophagosomes from the distal axon. This conclusion is consistent with the notion that fusion of lysosomes with autophagosomes contributes to the dynein-dependent retrograde transport of the resulting autolysosomes, in addition to the degradation of their contents (31, 53).
Discussion
Previous studies demonstrated that BORC, Arl8b, and SKIP function together to couple lysosomes to kinesin-1, promoting their centrifugal transport in nonpolarized cells (8–10, 12, 44–46, 54). Here we show that this ensemble also plays a role in lysosome transport in neurons, but only in the axon and not the dendrites. We also show that BORC/kinesin-1–dependent anterograde transport of lysosomes is required for axonal growth cone structure and dynamics, as well as autophagosome mobilization and disposal.
BORC–Arl8–SKIP–Kinesin-1 Specifically Drives Lysosome Transport into the Axon.
In agreement with previous studies (28–33, 55), fluorescence microscopy of fixed neurons showed the presence of lysosomes in the soma, dendrites, and axon (Figs. 1 and 2 and Fig. S1). Furthermore, live-cell imaging revealed that lysosomes move bidirectionally in all neuronal domains (Fig. 3). These organelles were identified as conventional lysosomes because they contained the lysosomal integral membrane proteins LAMP1 and CD63, the luminal acid hydrolase cathepsin D, and the LAMTOR4 subunit of the ragulator complex that mediates mTORC1 signaling at the lysosome surface. They also contained catalytically active cathepsin B, as revealed by using the Magic Red substrate, and most had an acidic pH, as evidenced by staining with LysoTracker and absence of SEP fluorescence (Figs. 1, 2, and 3 and Fig. S1). We cannot rule out, however, that some of these axonal organelles are late endosomes, as these represent a stage in the maturation to lysosomes, and thus share many properties. In line with previous studies (30, 55, 56), we did observe some heterogeneity among axonal lysosomes. For example, in the distal axon, lysosomes were less mobile, and some were less acidic (Fig. 3). This heterogeneity resembles that recently reported for lysosomes in nonneuronal cells (40).
Lysosomes in the proximal and distal parts of the axon depend on the BORC–Arl8–SKIP–kinesin-1 complex for anterograde transport. Indeed, silencing or inactivation of components of this complex depleted lysosomes from the axon (Fig. 4), whereas their overexpression drove lysosomes to axon tips (Figs. 5 and 6). This latter effect was particularly dramatic for coexpression of Arl8 and SKIP (Fig. 6), suggesting that this GTPase-effector pair is a limiting factor in the chain of interactors. Moreover, we observed that Rigor-KIF5A, in conjunction with KLC and SKIP, arrested lysosome movement at the beginning of the axon (Fig. 5 G and H), consistent with the special role of kinesin-1 in the transport of lysosomes into the axon.
Studies by other groups that used Caenorhabditis elegans as a model system showed that Arl8 (57) and the myrlysin subunit of BORC (known as SAM-4 in this organism) (58) regulate the transport of synaptic vesicle precursors in the axon, although, in this case, through coupling to the kinesin-3 UNC-104. These findings suggest a broader role for BORC and Arl8 in axonal transport of different organelles.
BORC-Dependent Lysosome Transport Promotes Axonal Growth-Cone Dynamics.
Axonal growth cones are highly motile structures involved in axon outgrowth and guidance (59, 60). They comprise a central region enriched in microtubules and transport vesicles and a peripheral region with a high concentration of actin filaments that organize lamellipodia and filopodia. The dynamics of growth cones are dependent on cooperation between the microtubule and actin cytoskeletons, as well as on the delivery of transport vesicles to the leading edge. We observed that lysosomes normally reached the central region of the growth cone (Figs. 1 and 3). Importantly, impairment of lysosome transport by myrlysin, lyspersin, or Arl8b KD caused a dramatic reduction in the size and dynamics of growth cones (Fig. 7 A–D). These effects suggest that lysosome transport is required to maintain the structure and function of growth cones. Lysosomes could deliver adhesion or signaling molecules to the growth cone, as they do to focal adhesions in nonpolarized cells (61–63). This explanation is consistent with the requirement of lysosome exocytosis for the extension and arborization of neurites in sympathetic neurons (29). Alternatively, lysosomes could function to degrade adhesion or signaling molecules in the growth cone. In this regard, ubiquitination of the neuronal L1/NgCAM adhesion molecule was shown to target it for degradation in lysosomes, influencing neurite outgrowth and cell adhesion (64). In addition, Robo receptors for the repulsive axon guidance protein Slit were found to be down-regulated by degradation in late endosomes and lysosomes (65, 66).
Requirement of BORC-Dependent Lysosome Transport for Autophagosome Mobilization and Disposal.
An additional neuronal function that requires BORC-dependent lysosome transport is the turnover of axonal autophagosomes, as evidenced by the accumulation of large, immobile LC3-positive structures in the distal axon of neurons depleted of myrlysin and/or lyspersin or Arl8b (Fig. 7 E and F). Autophagosomes form constitutively in the distal axon and subsequently undergo dynein-mediated retrograde transport (30, 31, 52, 53). As they move toward the proximal axon, they become more acidic and acquire bidirectional movement, probably as a result of fusion with anterogradely moving lysosomes (53). Our observations are consistent with the requirement of anterograde lysosome movement for fusion with autophagosomes and degradation of their contents, including LC3. Moreover, fusion with lysosomes appears to confer on autophagosomes the ability to move retrogradely. This phenomenon was previously attributed to the delivery of a snapin-dynein complex from late endosomes to autophagosomes during fusion (31). The fact that snapin is a subunit of BORC (9) raises the additional possibility that snapin is required for anterograde lysosome transport as part of the BORC–Arl8–SKIP–kinesin-1 ensemble. Fusion with lysosomes then likely enables acquisition of other adaptor or signaling molecules for recruitment of dynein to the resulting autolysosomes.
How Are Lysosomes Transported in Dendrites?
Unlike axonal transport, dendritic transport of lysosomes does not appear to depend on kinesin-1 (Figs. 4–6). In fact, many of the ∼45 mammalian kinesins exclusively mediate axonal transport, and only a handful, including the kinesin-3 proteins KIF1A, KIF1Bβ, KIF1C, and KIF16B and the kinesin-4 proteins KIF21A and KIF21B, mediate organelle transport to axons and dendrites (24, 25, 67). Of these, KIF1A and KIF1Bβ have been reported to move late endosomes and lysosomes in nonneuronal cells (54, 68, 69). It would thus be of interest to test if KIF1A and KIF1Bβ mediate lysosome transport in dendrites. Alternatively, lysosome transport in dendrites could be mediated by dynein, as shown in previous studies (20, 33, 70). Further studies will be needed to assess the relative contributions of different microtubule motors to lysosome transport in dendrites.
Concluding Remarks
Our findings illustrate how a mechanism that mediates radial dispersal of lysosomes in nonpolarized cells is adapted to promote polarized transport of lysosomes into the axon of hippocampal neurons. The specific role of kinesin-1 in this process is likely derived from its preference for microtubules enriched in acetylated and GTP-bound tubulin (51, 54, 71), which are particularly abundant in the axon (51, 72). Alterations in the expression of BORC and kinesin-1 subunits have been linked to psychiatric and neurological disorders (73–76). In light of our findings, it would be of interest to investigate if aberrant transport of lysosomes in the axon contributes to the pathogenesis of these disorders.
Materials and Methods
Recombinant DNA constructs (Table S1), antibodies and other probes (Table S2), culture and transfection of rat hippocampal neurons, fluorescence microscopy, live-cell imaging, image analysis, and statistical analysis are described in SI Materials and Methods. All animal experiments were performed in accordance with National Institutes of Health and US government regulations. Research was conducted under Animal Study Proposals 13–011 and 16–011 approved by the National Institute of Child Health and Human Development Animal Care and Use Committee.
Table S1.
Plasmids
| Insert | Species | Tag | Plasmid | Source |
| Arl8b | Human | C-GFP | pcDNA3.1/CT-GFP | Gift from J. H. Brumell |
| Arl8b | Human | C-mCherry | pmCherry-N1 | This study |
| Cathepsin D | Mouse | C-GFP | pEGFP(A206K)N1 | This study |
| CD63 | Human | C-GFP | pEGFP-N1 | This study |
| KIF5A | Mouse | N-GFP | pEGFP-C1 | Ref. 38 |
| KIF5A motor-streptavidin | Mouse | N-mCherry | pmCherry-C1 | KIF5A1-559 fragment (comprising the motor and part of the coiled-coil domain). A 6×(glycine-serine) linker was introduced between KIF5A and streptavidin |
| KIF5A-Rigor | Mouse | N-GFP | pEGFP-C1 | G235A; ref. 38 |
| KLC1 | Rat | N-HA | pCDNA.3 | Gift from K. Verhey |
| KLC1-DN (KLC1 TPR) | Rat | N-HA | pCDNA.3 | Gift from K. Verhey |
| LAMP1 | Rat | C-RFP | Modified Clontech plasmid | Gift from W. Mothes (Addgene plasmid no. 1817) |
| LAMP1 | Rat | C-GFP | pEGFP-N1 | This study |
| LAMP1 | Rat | N-SEP; C-RFP | Modified Clontech plasmid | SEP subcloned into LAMP1-RFP plasmid |
| LAMP1 | Rat | C-Tag BFP2 | mTag BFP2 | Gift from J. Lippincott-Schwartz |
| LAMP1-SBP | Rat | C-GFP | pEGFP-N1 | A streptavidin-binding peptide (SBP) was subcloned between LAMP1 and GFP |
| LC3B | Human | N-mCherry | pcDNA3.1 | Gift from D. Rubinsztein (Addgene plasmid no. 40827) |
| Lyspersin | Human | N-3xHA | pCI-neo | Gift from J. Pu |
| Lyspersin | Human | N-3xmyc | pCI-neo | Gift from J. Pu |
| Myrlysin | Human | C-FOS | pcDNA3.1 | Ref. 9 |
| Myrlysin | Human | C-3xmyc | pCI-neo | Gift from R. Jia |
| Myrlysin | Human | C-GFP | pEGFP-N1 | Ref. 9 |
| CFP | — | — | pECFP-N1 | Gift from J. Lippincott-Schwartz |
| GFP | — | — | pEGFP-N1 | Clontech |
| PM (FYN-FKBP1A) | Human | C-RFP | pmRFP-N1 | Gift from J. Lippincott-Schwartz |
| SKIP | Human | N-myc | pCMV-myc | Gift from S. Méresse |
| SKIP | Human | N-GFP | pEGFP-C1 | Gift from R. Jia |
| Control shRNA | Rat | C-GFP | SureSilencing shRNA | Qiagen, product no. 336311 |
| Arl8b shRNA | Rat | C-GFP | SureSilencing shRNA | Qiagen, cat. no. KR70305G |
| KIF5A shRNA | Rat | C-GFP | SureSilencing shRNA | Qiagen, cat. no. KR51376G |
| KIF5B shRNA | Rat | C-GFP | SureSilencing shRNA | Qiagen, cat. no. KR43314G |
| KIF5C shRNA | Rat | C-GFP | SureSilencing shRNA | Qiagen, cat. no. KR50494G |
| Lyspersin shRNA | Rat | C-GFP | SureSilencing shRNA | Qiagen, cat. no. KR70126G |
| Myrlysin shRNA | Rat | C-GFP | SureSilencing shRNA | Qiagen, cat. no. KR51251G |
| SKIP shRNA | Rat | C-GFP | SureSilencing shRNA | Qiagen, cat. no. KR55991G |
Table S2.
Antibodies and probes
| Antibody/probe | Source | Cat. No. | Fixation |
| Rabbit anti-LAMTOR4 | Cell Signaling | 12284S | PFA |
| Mouse anti-GFP | Thermo Fisher Scientific | A-11120 | PFA |
| Mouse anti-LAMP1 | NIH | −20 °C methanol | |
| Goat anti-ankyrin G | Santa Cruz Biotechnology | sc-31778 | PFA |
| Mouse anti-neurofascin | NeuroMab UC Davis/NIH | 75–027 | PFA |
| Mouse anti-myc epitope | Santa Cruz Biotechnology | sc-40 | PFA |
| Rabbit anti-HA epitope | Thermo Fisher Scientific | OPA1-10980 | PFA |
| Rabbit anti-SKIP | Thermo Fisher Scientific | PA5-20850 | PFA |
| Rabbit anti-KIF5A+B+C | Abcam | ab62104 | PFA |
| Rabbit anti-Arl8b | Proteintech | 13049–1-AP | PFA |
| Mouse anti-myrlysin | OriGene | TA5070040 | PFA |
| Rabbit anti-lyspersin | Thermo Fisher Scientific | PA5-24496 | PFA |
| Mouse anti-PSD 95 | NeuroMab UC Davis/NIH | 75–028 | PFA |
| CF640R | Biotium | 92245 | — |
| Magic Red | Bio-Rad | ICT937 | — |
| LysoTracker (Red DND-99) | Thermo Fisher Scientific | L7528 | — |
PFA, paraformaldehyde.
SI Materials and Methods
Neuron Culture and Transfection.
Primary cultures of rat hippocampal neurons were prepared at embryonic day 18, and cultured as previously described (38, 77). In most experiments, neurons were transfected with different plasmid constructs using Lipofectamine 2000 at DIV 4/5 and then analyzed at DIV 7/8.
Fluorescence Microscopy and Image Analysis.
Immunofluorescence microscopy of fixed neurons expressing different recombinant constructs was performed as previously described (38, 77). To highlight the entire neuron, including dendrites and axons, neurons were transfected with vectors encoding GFP, CFP, or the plasma membrane marker FYN-FKBP1A-RFP (i.e., PM-RFP). The axon was identified by staining for the AIS marker AnkG. Fluorescence images were obtained using a confocal microscope (LSM780; Zeiss) equipped with 40×/1.3 N.A. and 63×/1.4 N.A. objectives and an appropriate filter combination. All image analysis, including computation of D/A, AT/D, and AT/A intensity ratios, growth cone area, and fluorescence line intensity plots, was performed by using ImageJ version 1.44o as previously described (38).
Live-Cell Imaging.
Neurons transfected at DIV 4/5 were imaged at DIV 7/8 by using a spinning-disk confocal microscope (Marianas; Intelligent Imaging) equipped with a 63×/1.4 N.A. objective. Digital images were acquired with an EM-CCD camera (Evolve; Photometrics). To identify the axon, neurons were stained with a CF640R-conjugated antibody to the AIS protein neurofascin (38). For single-color videos, a laser channel used to visualize a specific fluorescently tagged protein was exposed for 100–200 ms and recorded every 1 s for 240 s. For dual-color videos, different laser channels used to visualize fluorescently tagged proteins were sequentially exposed for 100–200 ms and recorded every 1 s for 240 s. Movement of particles in dendrites, proximal axon, and distal axon was quantified by generating kymographs by using ImageJ version 1.44o as previously described (38, 77).
Statistical Analysis.
All bar graphs in the figures represent the mean ± SD from multiple determinations. The total number of cells analyzed in each experiment is indicated in the figure legends. The statistical significance of differences between two conditions was calculated by Student’s t test. Statistical significance for groups was calculated by one-way ANOVA followed by Dunnett’s test.
Supplementary Material
Acknowledgments
We thank R. Bagshaw, J. H. Brumell, R. Jia, J. Lippincott-Schwartz, S. Méresse, W. Mothes, J. Pu, D. Rubinsztein, and K. Verhey for gifts of reagents and X. Zhu for expert technical assistance. This work was funded by the Intramural Program of National Institute of Child Health and Human Development, NIH Grant ZIA HD001607.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1616363114/-/DCSupplemental.
References
- 1.Appelmans F, Wattiaux R, De Duve C. Tissue fractionation studies. 5. The association of acid phosphatase with a special class of cytoplasmic granules in rat liver. Biochem J. 1955;59:438–445. doi: 10.1042/bj0590438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballabio A. The awesome lysosome. EMBO Mol Med. 2016;8:73–76. doi: 10.15252/emmm.201505966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pu J, Guardia CM, Keren-Kaplan T, Bonifacino JS. Mechanisms and functions of lysosome positioning. J Cell Sci. 2016;129:4329–4339. doi: 10.1242/jcs.196287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matteoni R, Kreis TE. Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol. 1987;105:1253–1265. doi: 10.1083/jcb.105.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollenbeck PJ, Swanson JA. Radial extension of macrophage tubular lysosomes supported by kinesin. Nature. 1990;346:864–866. doi: 10.1038/346864a0. [DOI] [PubMed] [Google Scholar]
- 6.Harada A, et al. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J Cell Biol. 1998;141:51–59. doi: 10.1083/jcb.141.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordens I, et al. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol. 2001;11:1680–1685. doi: 10.1016/s0960-9822(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 8.Rosa-Ferreira C, Munro S. Arl8 and SKIP act together to link lysosomes to kinesin-1. Dev Cell. 2011;21:1171–1178. doi: 10.1016/j.devcel.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pu J, et al. BORC, a multisubunit complex that regulates lysosome positioning. Dev Cell. 2015;33:176–188. doi: 10.1016/j.devcel.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka Y, et al. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell. 1998;93:1147–1158. doi: 10.1016/s0092-8674(00)81459-2. [DOI] [PubMed] [Google Scholar]
- 11.Nakae I, et al. The arf-like GTPase Arl8 mediates delivery of endocytosed macromolecules to lysosomes in Caenorhabditis elegans. Mol Biol Cell. 2010;21:2434–2442. doi: 10.1091/mbc.E09-12-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg S, et al. Lysosomal trafficking, antigen presentation, and microbial killing are controlled by the Arf-like GTPase Arl8b. Immunity. 2011;35:182–193. doi: 10.1016/j.immuni.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki A, et al. Arl8/ARL-8 functions in apoptotic cell removal by mediating phagolysosome formation in Caenorhabditis elegans. Mol Biol Cell. 2013;24:1584–1592. doi: 10.1091/mbc.E12-08-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korolchuk VI, et al. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol. 2011;13:453–460. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michelet X, et al. MHC class II presentation is controlled by the lysosomal small GTPase, Arl8b. J Immunol. 2015;194:2079–2088. doi: 10.4049/jimmunol.1401072. [DOI] [PubMed] [Google Scholar]
- 16.Britt DJ, Farías GG, Guardia CM, Bonifacino JS. Mechanisms of Polarized Organelle Distribution in Neurons. Front Cell Neurosci. 2016;10:88. doi: 10.3389/fncel.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 18.Franker MA, Hoogenraad CC. Microtubule-based transport - basic mechanisms, traffic rules and role in neurological pathogenesis. J Cell Sci. 2013;126:2319–2329. doi: 10.1242/jcs.115030. [DOI] [PubMed] [Google Scholar]
- 19.Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: Uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci USA. 1988;85:8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yau KW, et al. Dendrites in vitro and in vivo contain microtubules of opposite polarity and axon formation correlates with uniform plus-end-out microtubule orientation. J Neurosci. 2016;36:1071–1085. doi: 10.1523/JNEUROSCI.2430-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Y, Brady ST. Post-translational modifications of tubulin: Pathways to functional diversity of microtubules. Trends Cell Biol. 2015;25:125–136. doi: 10.1016/j.tcb.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atherton J, Houdusse A, Moores C. MAPping out distribution routes for kinesin couriers. Biol Cell. 2013;105:465–487. doi: 10.1111/boc.201300012. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins B, Decker H, Bentley M, Luisi J, Banker G. A novel split kinesin assay identifies motor proteins that interact with distinct vesicle populations. J Cell Biol. 2012;198:749–761. doi: 10.1083/jcb.201205070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farkhondeh A, Niwa S, Takei Y, Hirokawa N. Characterizing KIF16B in neurons reveals a novel intramolecular “stalk inhibition” mechanism that regulates its capacity to potentiate the selective somatodendritic localization of early endosomes. J Neurosci. 2015;35:5067–5086. doi: 10.1523/JNEUROSCI.4240-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipka J, Kapitein LC, Jaworski J, Hoogenraad CC. Microtubule-binding protein doublecortin-like kinase 1 (DCLK1) guides kinesin-3-mediated cargo transport to dendrites. EMBO J. 2016;35:302–318. doi: 10.15252/embj.201592929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnapp BJ, Reese TS. Dynein is the motor for retrograde axonal transport of organelles. Proc Natl Acad Sci USA. 1989;86:1548–1552. doi: 10.1073/pnas.86.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapitein LC, et al. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr Biol. 2010;20:290–299. doi: 10.1016/j.cub.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 28.Overly CC, Hollenbeck PJ. Dynamic organization of endocytic pathways in axons of cultured sympathetic neurons. J Neurosci. 1996;16:6056–6064. doi: 10.1523/JNEUROSCI.16-19-06056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arantes RM, Andrews NW. A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons. J Neurosci. 2006;26:4630–4637. doi: 10.1523/JNEUROSCI.0009-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S, Sato Y, Nixon RA. Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer’s-like axonal dystrophy. J Neurosci. 2011;31:7817–7830. doi: 10.1523/JNEUROSCI.6412-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng XT, Zhou B, Lin MY, Cai Q, Sheng ZH. Axonal autophagosomes recruit dynein for retrograde transport through fusion with late endosomes. J Cell Biol. 2015;209:377–386. doi: 10.1083/jcb.201412046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuruta F, Dolmetsch RE. PIKfyve mediates the motility of late endosomes and lysosomes in neuronal dendrites. Neurosci Lett. 2015;605:18–23. doi: 10.1016/j.neulet.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Schwenk BM, et al. The FTLD risk factor TMEM106B and MAP6 control dendritic trafficking of lysosomes. EMBO J. 2014;33:450–467. doi: 10.1002/embj.201385857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballabio A, Gieselmann V. Lysosomal disorders: From storage to cellular damage. Biochim Biophys Acta. 2009;1793:684–696. doi: 10.1016/j.bbamcr.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Nixon RA, Yang DS, Lee JH. Neurodegenerative lysosomal disorders: A continuum from development to late age. Autophagy. 2008;4:590–599. doi: 10.4161/auto.6259. [DOI] [PubMed] [Google Scholar]
- 36.Chen JW, Murphy TL, Willingham MC, Pastan I, August JT. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985;101:85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell. 2012;150:1196–1208. doi: 10.1016/j.cell.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farías GG, Guardia CM, Britt DJ, Guo X, Bonifacino JS. Sorting of dendritic and axonal vesicles at the pre-axonal exclusion zone. Cell Reports. 2015;13:1221–1232. doi: 10.1016/j.celrep.2015.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sankaranarayanan S, De Angelis D, Rothman JE, Ryan TA. The use of pHluorins for optical measurements of presynaptic activity. Biophys J. 2000;79:2199–2208. doi: 10.1016/S0006-3495(00)76468-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson DE, Ostrowski P, Jaumouillé V, Grinstein S. The position of lysosomes within the cell determines their luminal pH. J Cell Biol. 2016;212:677–692. doi: 10.1083/jcb.201507112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaiswal JK, Andrews NW, Simon SM. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J Cell Biol. 2002;159:625–635. doi: 10.1083/jcb.200208154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodríguez A, Webster P, Ortego J, Andrews NW. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J Cell Biol. 1997;137:93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padamsey Z, et al. Activity-dependent exocytosis of lysosomes regulates the structural plasticity of dendritic spines. Neuron. 2017;93:132–146. doi: 10.1016/j.neuron.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hofmann I, Munro S. An N-terminally acetylated Arf-like GTPase is localised to lysosomes and affects their motility. J Cell Sci. 2006;119:1494–1503. doi: 10.1242/jcs.02958. [DOI] [PubMed] [Google Scholar]
- 45.Bagshaw RD, Callahan JW, Mahuran DJ. The Arf-family protein, Arl8b, is involved in the spatial distribution of lysosomes. Biochem Biophys Res Commun. 2006;344:1186–1191. doi: 10.1016/j.bbrc.2006.03.221. [DOI] [PubMed] [Google Scholar]
- 46.Mrakovic A, Kay JG, Furuya W, Brumell JH, Botelho RJ. Rab7 and Arl8 GTPases are necessary for lysosome tubulation in macrophages. Traffic. 2012;13:1667–1679. doi: 10.1111/tra.12003. [DOI] [PubMed] [Google Scholar]
- 47.DeBoer SR, et al. Conventional kinesin holoenzymes are composed of heavy and light chain homodimers. Biochemistry. 2008;47:4535–4543. doi: 10.1021/bi702445j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanai Y, et al. KIF5C, a novel neuronal kinesin enriched in motor neurons. J Neurosci. 2000;20:6374–6384. doi: 10.1523/JNEUROSCI.20-17-06374.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verhey KJ, et al. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J Cell Biol. 2001;152:959–970. doi: 10.1083/jcb.152.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakata T, Hirokawa N. Point mutation of adenosine triphosphate-binding motif generated rigor kinesin that selectively blocks anterograde lysosome membrane transport. J Cell Biol. 1995;131:1039–1053. doi: 10.1083/jcb.131.4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakata T, Niwa S, Okada Y, Perez F, Hirokawa N. Preferential binding of a kinesin-1 motor to GTP-tubulin-rich microtubules underlies polarized vesicle transport. J Cell Biol. 2011;194:245–255. doi: 10.1083/jcb.201104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boland B, et al. Autophagy induction and autophagosome clearance in neurons: Relationship to autophagic pathology in Alzheimer’s disease. J Neurosci. 2008;28:6926–6937. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maday S, Wallace KE, Holzbaur EL. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol. 2012;196:407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guardia CM, Farías GG, Jia R, Pu J, Bonifacino JS. BORC functions upstream of kinesins 1 and 3 to coordinate regional movement of lysosomes along different microtubule tracks. Cell Reports. 2016;17:1950–1961. doi: 10.1016/j.celrep.2016.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Overly CC, Lee KD, Berthiaume E, Hollenbeck PJ. Quantitative measurement of intraorganelle pH in the endosomal-lysosomal pathway in neurons by using ratiometric imaging with pyranine. Proc Natl Acad Sci USA. 1995;92:3156–3160. doi: 10.1073/pnas.92.8.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gowrishankar S, et al. Massive accumulation of luminal protease-deficient axonal lysosomes at Alzheimer’s disease amyloid plaques. Proc Natl Acad Sci USA. 2015;112:E3699–E3708. doi: 10.1073/pnas.1510329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu YE, Huo L, Maeder CI, Feng W, Shen K. The balance between capture and dissociation of presynaptic proteins controls the spatial distribution of synapses. Neuron. 2013;78:994–1011. doi: 10.1016/j.neuron.2013.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng Q, et al. The vesicle protein SAM-4 regulates the processivity of synaptic vesicle transport. PLoS Genet. 2014;10:e1004644. doi: 10.1371/journal.pgen.1004644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tojima T, Kamiguchi H. Exocytic and endocytic membrane trafficking in axon development. Dev Growth Differ. 2015;57:291–304. doi: 10.1111/dgd.12218. [DOI] [PubMed] [Google Scholar]
- 60.Kahn OI, Baas PW. Microtubules and growth cones: Motors drive the turn. Trends Neurosci. 2016;39:433–440. doi: 10.1016/j.tins.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steffan JJ, Williams BC, Welbourne T, Cardelli JA. HGF-induced invasion by prostate tumor cells requires anterograde lysosome trafficking and activity of Na+-H+ exchangers. J Cell Sci. 2010;123:1151–1159. doi: 10.1242/jcs.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steffan JJ, et al. Supporting a role for the GTPase Rab7 in prostate cancer progression. PLoS One. 2014;9:e87882. doi: 10.1371/journal.pone.0087882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schiefermeier N, et al. The late endosomal p14-MP1 (LAMTOR2/3) complex regulates focal adhesion dynamics during cell migration. J Cell Biol. 2014;205:525–540. doi: 10.1083/jcb.201310043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schäfer MK, Schmitz B, Diestel S. L1CAM ubiquitination facilitates its lysosomal degradation. FEBS Lett. 2010;584:4475–4480. doi: 10.1016/j.febslet.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 65.Keleman K, et al. Comm sorts robo to control axon guidance at the Drosophila midline. Cell. 2002;110:415–427. doi: 10.1016/s0092-8674(02)00901-7. [DOI] [PubMed] [Google Scholar]
- 66.Li L, et al. Robo3.1A suppresses slit-mediated repulsion by triggering degradation of Robo2. J Neurosci Res. 2014;92:835–846. doi: 10.1002/jnr.23364. [DOI] [PubMed] [Google Scholar]
- 67.Huang CF, Banker G. The translocation selectivity of the kinesins that mediate neuronal organelle transport. Traffic. 2012;13:549–564. doi: 10.1111/j.1600-0854.2011.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsushita M, Tanaka S, Nakamura N, Inoue H, Kanazawa H. A novel kinesin-like protein, KIF1Bbeta3 is involved in the movement of lysosomes to the cell periphery in non-neuronal cells. Traffic. 2004;5:140–151. doi: 10.1111/j.1600-0854.2003.00165.x. [DOI] [PubMed] [Google Scholar]
- 69.Bentley M, Decker H, Luisi J, Banker G. A novel assay reveals preferential binding between Rabs, kinesins, and specific endosomal subpopulations. J Cell Biol. 2015;208:273–281. doi: 10.1083/jcb.201408056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wiggins LM, Kuta A, Stevens JC, Fisher EM, von Bartheld CS. A novel phenotype for the dynein heavy chain mutation Loa: Altered dendritic morphology, organelle density, and reduced numbers of trigeminal motoneurons. J Comp Neurol. 2012;520:2757–2773. doi: 10.1002/cne.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reed NA, et al. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16:2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 72.Cambray-Deakin MA, Burgoyne RD. Posttranslational modifications of alpha-tubulin: Acetylated and detyrosinated forms in axons of rat cerebellum. J Cell Biol. 1987;104:1569–1574. doi: 10.1083/jcb.104.6.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li M, et al. A human-specific AS3MT isoform and BORCS7 are molecular risk factors in the 10q24.32 schizophrenia-associated locus. Nat Med. 2016;22:649–656. doi: 10.1038/nm.4096. [DOI] [PubMed] [Google Scholar]
- 74.Melo US, et al. Overexpression of KLC2 due to a homozygous deletion in the non-coding region causes SPOAN syndrome. Hum Mol Genet. 2015;24:6877–6885. doi: 10.1093/hmg/ddv388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reid E, et al. A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10) Am J Hum Genet. 2002;71:1189–1194. doi: 10.1086/344210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duarte RR, et al. Genome-wide significant schizophrenia risk variation on chromosome 10q24 is associated with altered cis-regulation of BORCS7, AS3MT, and NT5C2 in the human brain. Am J Med Genet B Neuropsychiatr Genet. 2016;171:806–814. doi: 10.1002/ajmg.b.32445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farías GG, Britt DJ, Bonifacino JS. Imaging the polarized sorting of proteins from the Golgi complex in live neurons. Methods MolBiol. 2016;1496:13–30. doi: 10.1007/978-1-4939-6463-5_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.