Significance
We have recently reported that glutamine synthetase (GS) is negatively regulated by glutamine through a feedback loop involving the E3 ubiquitin ligase CRL4CRBN. However, the molecular events that take place at each step of the pathway are not well understood. Here, we show that valosin-containing protein (VCP)/p97, is required for GS degradation. It acts downstream of CRL4CRBN. p97 extracts ubiquitylated GS subunits from the decamer so that they can be degraded by the proteasome. Interestingly, p97 is also required for immunomodulatory drug-induced degradation of all four known CRL4CRBN neosubstrates, including Ikaros family zinc finger proteins 1 (IKZF1) and 3 (IKZF3), casein kinase 1α (CK1α), and the translation termination factor GSPT1, which accounts for antitumor activity of these drugs. Our findings could have important implications for patient responsiveness to cancer therapy with immunomodulatory drugs.
Keywords: VCP/p97, glutamine synthetase, CRBN, substrates, degradation
Abstract
Glutamine synthetase (GS) plays an essential role in metabolism by catalyzing the synthesis of glutamine from glutamate and ammonia. Our recent study showed that CRBN, a direct protein target for the teratogenic and antitumor activities of immunomodulatory drugs such as thalidomide, lenalidomide, and pomalidomide, recognizes an acetyl degron of GS, resulting in ubiquitylation and degradation of GS in response to glutamine. Here, we report that valosin-containing protein (VCP)/p97 promotes the degradation of ubiquitylated GS, resulting in its accumulation in cells with compromised p97 function. Notably, p97 is also required for the degradation of all four known CRBN neo-substrates [Ikaros family zinc finger proteins 1 (IKZF1) and 3 (IKZF3), casein kinase 1α (CK1α), and the translation termination factor GSPT1] whose ubiquitylation is induced by immunomodulatory drugs. Together, these data point to an unexpectedly intimate relationship between the E3 ubiquitin ligase CRL4CRBN and p97 pathways.
Glutamine plays important roles in many cellular processes, including oxidative metabolism and ATP generation, biosynthesis of proteins, lipids, and nucleic acids, and cell growth and proliferation through the regulation of the mTOR signaling pathway, translation, and autophagy (1, 2). In mammals, it is the most abundant amino acid in plasma with a concentration of 0.5–0.9 mM (3), accounting for ∼20% of its free amino acid pool. Glutamine synthetase (GS) is the only enzyme that is capable of de novo synthesis of glutamine and also functions to detoxify glutamate and ammonia, depending on tissue localization. Skeletal muscles and lungs are major sites of glutamine synthesis, whereas cells of the gut and the immune system, such as lymphocytes and macrophages, consume large amounts of glutamine in plasma (4). GS protects neurons against excitotoxicity by converting glutamate into glutamine in brain, detoxifies ammonia in liver, and maintains physiologic pH in kidney (5). In an attempt to investigate the role of GS in development, He et al. generated GS-knockout mice and reported that GS is essential in early embryogenesis, because deletion of the murine GLUL gene causes lethality at the blastocyst stage (embryonic day 3.5) (6). Interestingly, mouse ES cells maintain pluripotency and proliferate when grown in the absence of exogenous glutamine (7). However, inhibition of GS with the small molecule methionine sulfoximine (MSO) is sufficient to block the proliferation of ES cells in glutamine-free medium (7). In humans, congenital systemic glutamine deficiency caused by homozygous GS mutations results in multiorgan failure and neonatal death (8).
Recent studies highlight the importance of glutamine metabolism in metabolic reprogramming, because many tumor cells display “glutamine addiction” (9). Activation of oncogenes such as MYC, KRAS, and HIF1α and/or loss of tumor suppressor genes including p53 can directly mediate the reprogramming of glutamine metabolism by selectively activating their downstream signaling or metabolic pathways (1, 4, 10, 11). As a result, some tumor cells require large amounts of exogenous glutamine to generate building blocks and energy for their growth and survival. In contrast, various tumor cell lines with high expression levels of GS enzyme can synthesize glutamine de novo and can grow and proliferate in the absence of exogenous glutamine (12–14).
Befitting its critical role in nitrogen metabolism, GS activity is tightly regulated. Pioneering studies by Stadtman’s group (15) and others demonstrated that bacterial GS is subject to complex feedback regulation by glutamine and downstream metabolites by reversible adenylylation and deadenylylation of a specific tyrosine residue, resulting in the inactivation of GS (16–18). In contrast to the well-defined regulation of bacterial GS, the molecular mechanism underlying the regulation of GS activity in mammalian cells is poorly understood. Before the discovery of ubiquitin-dependent proteolysis, it was proposed that glutamine inactivates GS through an uncharacterized degradation mechanism (19–22). Interestingly, the C-terminal region of bacterial GS, which contains the tyrosine that is adenylylated, is missing in mammalian GS. In contrast, eukaryotic GS has a highly conserved N-terminal extension that does not exist in prokaryotic GS (23). We recently reported that endogenous GS protein levels in multiple cell types and different mouse tissues are negatively regulated by glutamine via the E3 ubiquitin ligase CRL4CRBN (24). CRBN, a direct protein target for thalidomide teratogenicity and antitumor activity of immunomodulatory drugs, including lenalidomide and pomalidomide and a novel CRBN modulator CC-885 (25–31), recognizes an acetylated motif (called an “acetyl degron”) of GS, leading to ubiquitylation and subsequent degradation of GS in response to glutamine (24). However, the molecular events that take place at each step of the pathway are not well understood. For example, one of the fundamental questions is how the ubiquitin–proteasome system (UPS) manages to degrade individual subunits of a homodecameric enzyme complex.
Valosin-containing protein (VCP)/p97, a homohexameric AAA ATPase, promotes a number of cellular processes, including ubiquitin-dependent protein degradation, endoplasmic reticulum-associated degradation (ERAD), and autophagy (32). p97 working in concert with different adaptors mediates the extraction of ubiquitylated proteins from organelles, chromatin, and protein complexes and delivers them for proteasome- and autophagy-mediated protein degradation. One of the major functions of p97 is thought to be the disassembly of protein complexes, presumably by converting chemical energy generated from ATP hydrolysis into mechanical force used for conformational changes of target proteins (33). Mutations in p97 cause inclusion body myopathy associated with Paget’s disease of bone and frontotemporal dementia (IBMPFD) (34, 35) and a small fraction of familial amyotrophic lateral sclerosis (ALS) cases (36). Transgenic and knockin mouse models have been generated to investigate how these mutations contribute to the pathogenesis of IBMPFD and ALS (37–40). Because of its pivotal role in maintaining the cellular protein homeostasis important for tumor cell growth and survival, p97 is of particular interest as an anticancer drug target. Recently we developed the reversible and ATP-competitive p97 inhibitors DBeQ and ML240 (41, 42). Subsequent optimization of ML240 resulted in the identification of CB-5083 (43), which is currently being tested in phase I clinical trials. CB-5083 exhibits potent antitumor activity in both multiple myeloma and solid tumor xenograft models (44). However, the precise mechanisms by which p97 regulates substrates under physiological conditions remain poorly understood, and only a limited number of substrates have been studied in great detail.
Results
Glutamine Induces GS Disassembly in a Ubiquitylation- and p97-Dependent Manner.
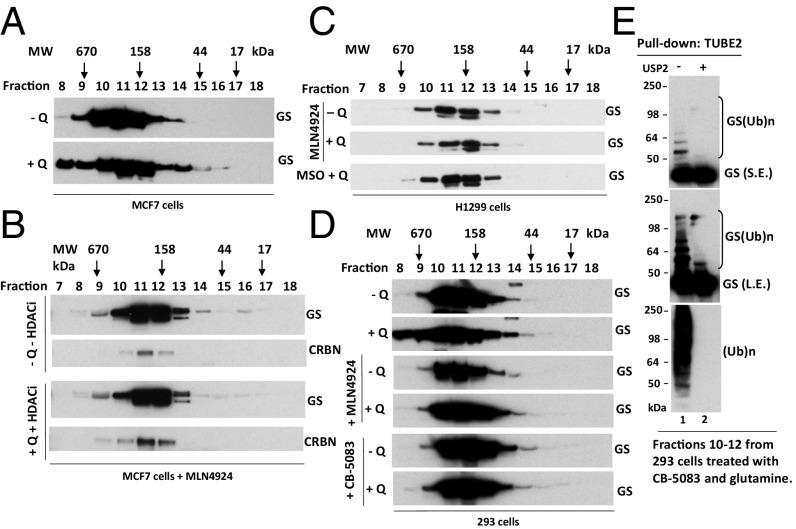
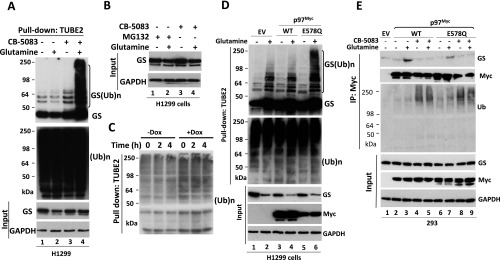
The active sites of the proteasome are enclosed in a chamber, access to which is governed by portals with a diameter of ∼13 Å (45, 46). Because this diameter is narrower than the diameter of most globular proteins, substrates must be disengaged from binding partners and unfolded before being threaded into the proteasome’s proteolytic chamber. Because GS is a homodecamer composed of two pentameric rings stacked upon each other to form a structure 90 × 110 Å (23), it is not known how the UPS mediates its glutamine-induced degradation. It is possible that a high concentration of glutamine can destabilize the GS oligomer and facilitate the degradation of individual monomers. To test this idea, we performed a series of gel filtration experiments with cell lysates prepared from glutamine-starved cells and glutamine-treated cells in the presence or absence of different chemical inhibitors. Fractions from each sample were collected and analyzed by immunoblotting. As shown in Fig. 1A, endogenous GS eluted primarily as a single peak corresponding to a homodecameric protein complex (∼440 kDa) in glutamine-starved cells. Upon glutamine treatment, GS fractionated as a broader peak with “tails” of both higher and lower molecular weight (MW), both of which were suppressed by the addition of the NEDD8-activating enzyme inhibitor MLN4924 (Fig. 1 B–D) (47). Moreover, glutamine-induced spreading of the GS peak was also blocked by the GS inhibitor MSO, which inhibited glutamine-induced GS ubiquitylation and degradation (Fig. S1) (48). Interestingly, the p97 inhibitor CB-5083 also blocked glutamine-induced spreading of the GS peak (Fig. 1D) but did not block GS ubiquitylation (Fig. 1E). We show later that this effect of CB-5083 may be caused by the competitive displacement of GS by other substrates that accumulate upon inhibition of p97. Taken together, our data suggest that glutamine-triggered GS ubiquitylation may promote the recruitment of p97 to initiate the disassembly of the decamer, inducing a shift of a fraction of the GS pool to higher and lower MW, respectively.
Fig. 1.
Glutamine alters the apparent assembly state of GS in an ubiquitylation- and p97-dependent manner. (A and B) MCF7 cells were starved of glutamine for 24 h and then were pretreated with (A) or without (B) 2 µM pan cullin–RING ubiquitin ligase inhibitor MLN4924 for 30 min, followed by the addition of 4 mM glutamine plus the histone deacetylase (HDAC) inhibitors suberoylanilide hydroxamic acid (SAHA) (2 µM) and NAM (10 µM) (+Q +HDACi) or not (−Q −HDACi) for 2 h. Cell lysates were fractionated on a Superdex 200 gel filtration column. Individual fractions were concentrated by trichloroacetic acid (TCA) precipitation and analyzed by SDS/PAGE and immunoblotting with the indicated antibodies. (C) As in A and B, except that H1299 cells were starved of glutamine for 24 h and then were pretreated with MLN4924 (2 μM) or the GS inhibitor MSO (2 mM) for 30 min, followed by the addition (or not) of 4 mM glutamine for 2 h. (D) As in A and B, except that HEK293 cells were starved of glutamine for 24 h and then were pretreated with MLN4924 (2 μM) or the p97 inhibitor CB-5083 (10 μM) for 30 min, followed by the addition (or not) of 4 mM glutamine for 2 h. (E) Fractions 10–12, prepared from HEK293 cells treated with CB-5083 and glutamine (used in D, bottom panel), were combined and subjected to pulldown with TUBE2 resin, followed by treatment with or without USP2. The bound fractions were analyzed by SDS/PAGE and immunoblotting with antibodies against GS and ubiquitin. L.E., long exposure; S.E., short exposure; (Ub)n, polyubiquitin.
Fig. S1.
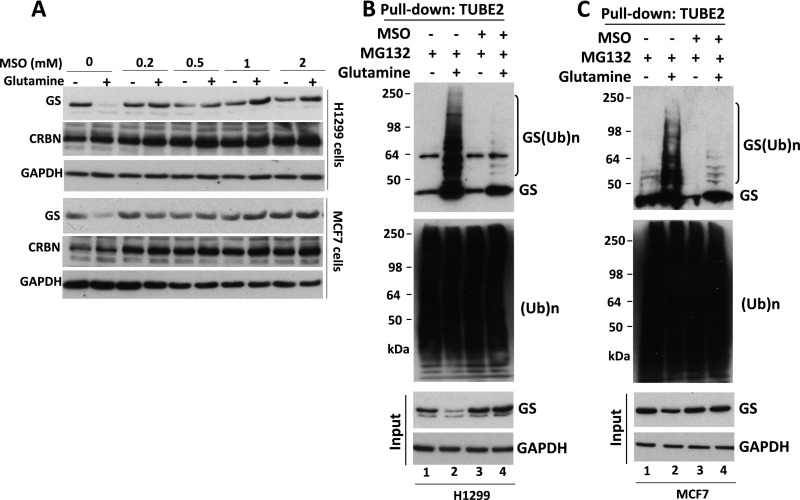
(Related to Fig. 1) The GS inhibitor MSO suppresses glutamine-induced GS ubiquitylation and degradation. (A) H1299 and MCF7 cells were starved of glutamine for 24 h. Cells were pretreated with the GS inhibitor MSO for 30 min, followed by the addition (or not) of 4 mM glutamine for 8 h. Cell extracts were analyzed by SDS/PAGE and immunoblotting with antibodies against GS, CRBN, and GAPDH. (B and C) H1299 (B) and MCF7 (C) cells were starved of glutamine for 24 h and then were pretreated with MG132 (10 μM) in the presence or absence of MSO (1 mM) for 30 min, followed by the addition (or not) of 4 mM glutamine for 3 h. Cell lysates were fractionated on a TUBE2 resin. The bound fractions and lysate samples (inputs) were analyzed by SDS/PAGE and immunoblotting with antibodies against GS, ubiquitin, and GAPDH. (Ub)n, polyubiquitin.
p97 Interacts with Endogenous GS and Promotes Degradation of GS in a Glutamine-Dependent Manner.
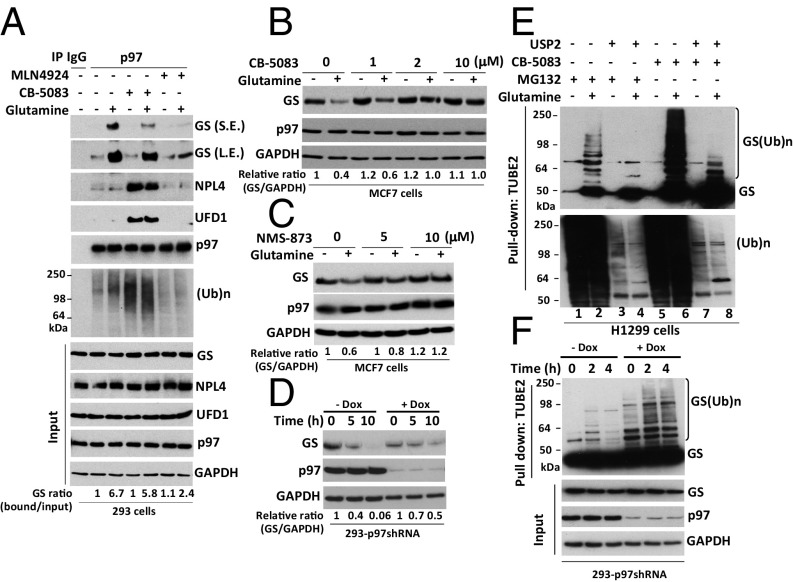
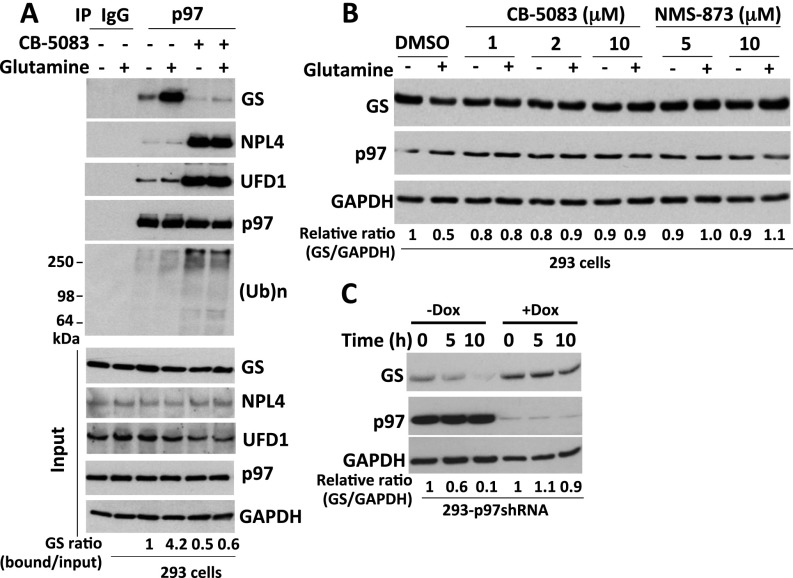
Based on the above observations, we hypothesized that p97 may be recruited to ubiquitylated GS to regulate its degradation. To investigate this hypothesis, we first performed immunoprecipitation (IP) experiments using an antibody specific to p97 and found that glutamine greatly enhanced the binding of endogenous, apparently unubiquitylated GS to endogenous p97 (Fig. 2A). This binding nevertheless was likely dependent on ubiquitylation, because it was greatly reduced by pretreatment of cells with the pan–Cullin-RING ligase (CRL) inhibitor MLN4924 (Fig. 2A). The role of ubiquitylation in GS binding to p97 is considered in more detail in the next section. GS also was recovered, albeit in reduced amounts, in p97 immunoprecipitates from cells treated with the p97 inhibitor CB-5083 following the activation of GS degradation (Fig. 2A). We suggest that this reduction was caused by competition from other p97 substrates that accumulated upon treatment with CB-5083; this notion is consistent with the observation that much greater amounts of UFD1•NPL4 and high-MW ubiquitin conjugates coimmunoprecipitated with p97 from cells treated with CB-5083. In support of this idea, the recovery of GS in p97 immunoprecipitates was essentially eliminated if a bolus of p97 substrates was accumulated by CB-5083 treatment before the activation of GS degradation with glutamine (Fig. S2A; note that the design of this experiment is similar to that of Fig. 1D).
Fig. 2.
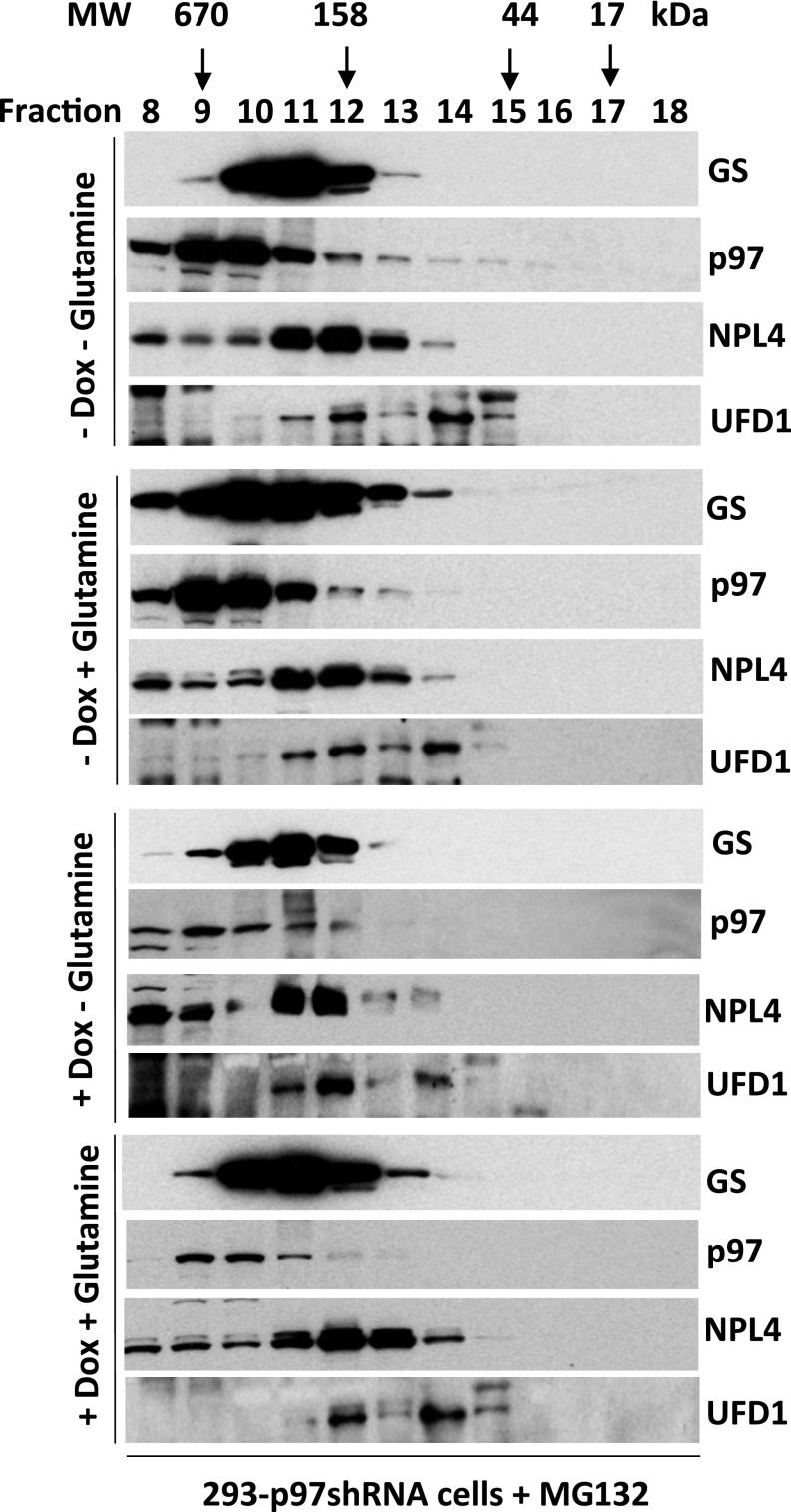
p97 interacts with endogenous GS and promotes glutamine-induced degradation of GS. (A) HEK293 cells were starved of glutamine for 24 h, were pretreated (or not) with MLN4924 (2 μM) for 30 min, and then were treated (or not) with 4 mM glutamine for 2 h. For CB-5083 treatment, after the addition of 4 mM glutamine for 90 min, cells were treated with CB-5083 (10 μM) for 30 min. Protein extracts were immunoprecipitated with mouse IgG control or p97 antibodies, followed by Western blot analysis with the indicated antibodies. The ratio of GS bound to p97 normalized to input GS is shown. L.E., long exposure (the long exposure blot was quantified); S.E., short exposure; (Ub)n, polyubiquitin. (B and C) MCF7 cells were starved of glutamine for 24 h and were pretreated with the p97 inhibitors CB-5083 (B) or NMS-873 (C) for 30 min, followed by addition (or not) of 4 mM glutamine for 4 h. Cell extracts were analyzed by SDS/PAGE and immunoblotting with antibodies against GS, p97, and GAPDH. The relative ratios of GS:GAPDH, normalized to lane 1, are shown. (D) HEK293 cells stably expressing doxycycline (Dox)-inducible shRNA targeting p97 were either mock-treated or induced with doxycycline (1 µg/mL) for 48 h and then were starved of glutamine for 24 h, followed by the addition of 4 mM glutamine for the indicated times. Cell lysates were analyzed by SDS/PAGE and immunoblotting with antibodies against GS, p97, and GAPDH. The relative ratios of GS:GAPDH, normalized to lane 1, are shown. (E) H1299 cells were starved of glutamine for 24 h and then were pretreated with MG132 (10 μM) or CB-5083 (10 μM) for 30 min, followed by the addition (or not) of 4 mM glutamine for 3 h. Cell lysates were fractionated on a TUBE2 resin, followed by treatment with or without USP2. The bound fractions and lysate samples were analyzed by SDS/PAGE and immunoblotting with antibodies against GS and ubiquitin. Input is shown in Fig. S3B. (F) HEK293 cells stably expressing doxycycline-inducible shRNA targeting p97 were mock-treated or were induced with doxycycline (1 µg/mL) for 48 h and then were starved of glutamine for 24 h, followed by the addition of 4 mM glutamine for the indicated times. Cell lysates were fractionated on a TUBE2 resin, and both lysate samples and the bound fractions were analyzed by SDS/PAGE and immunoblotting with antibodies against GS, ubiquitin (shown in Fig. S3C), p97, and GAPDH.
Fig. S2.
(Related to Fig. 2) p97 interacts with endogenous GS and is required for glutamine-induced GS degradation. (A) HEK293 cells were starved of glutamine for 24 h and were pretreated (or not) with CB-5083 (10 μM) for 30 min, followed by the addition (or not) of 4 mM glutamine for 2 h. Protein extracts were immunoprecipitated with mouse IgG control or p97 antibodies, followed by Western blot analysis with the indicated antibodies. The ratios of GS bound to p97 normalized to input GS are shown. (Ub)n, polyubiquitin. (B) HEK293 cells were starved of glutamine for 24 h and were pretreated with the p97 inhibitor NMS-873 or CB-5083 for 30 min, followed by the addition (or not) of 4 mM glutamine for 4 h. Cell extracts were analyzed by SDS/PAGE and immunoblotting with antibodies against GS, p97, and GAPDH. The relative ratio of GS:GAPDH, normalized to lane 1, is shown. (C) HEK293 cells stably expressing doxycycline (Dox)-inducible shRNA targeting p97 were mock-treated or were induced with doxycycline (1 µg/mL) for 48 h and then were starved of glutamine for 24 h, followed by the addition of 4 mM glutamine and the HDAC inhibitors (2 μM SAHA+10 mM NAM) for the indicated times. Cell extracts were analyzed by SDS/PAGE and immunoblotting with antibodies against GS, p97, and GAPDH. The GS:GAPDH ratio for each sample was calculated, normalized to untreated cells, and is indicated below the bottom immunoblot in each panel.
The enhanced binding of GS to p97 in cells treated with glutamine suggests that p97 might modulate glutamine-induced degradation of GS. Consistent with this idea, inhibition of p97 by CB-5083 or NMS-873 blocked glutamine-induced GS degradation (Fig. 2 B and C and Fig. S2B). Glutamine-induced GS degradation also was inhibited upon depletion of p97 by shRNA knockdown (Fig. 2D and Fig. S2C).
Because glutamine promotes GS degradation by the UPS, we next sought to determine the effect of p97 inactivation on GS ubiquitylation. We treated glutamine-starved cells with the proteasome inhibitor MG132 or the p97 inhibitor CB-5083 in the presence or absence of 4 mM glutamine and then enriched ubiquitin conjugates on a tandem ubiquitin-binding entity (TUBE2) resin. Immunoblotting of the bound fraction and input (cell lysate) with antibodies against GS or ubiquitin revealed that inhibition of either p97 or the proteasome in glutamine-treated cells resulted in a significant increase in ubiquitin-conjugated GS forms, which were deconjugated with the deubiquitylating enzyme ubiquitin C-terminal hydrolase 2 (USP2) (Fig. 2E and Fig. S3A). A similar result was obtained upon shRNA knockdown of p97 (Fig. 2F). Consistent with the results obtained with p97 ATPase inhibitors, overexpression of the ATPase-deficient p97E578Q mutant (49) caused accumulation of polyubiquitylated GS upon glutamine activation, even though the mutant protein was able to bind GS, albeit with slightly reduced efficiency (Fig. S3 D and E).
Fig. S3.
(Related to Fig. 2) p97 controls GS ubiquitylation status in response to glutamine. (A) H1299 cells were starved of glutamine for 24 h and then were pretreated with CB-5083 (10 μM) for 30 min, followed by the addition (or not) of 4 mM glutamine for 3 h. Cell lysates were fractionated on a TUBE2 resin. The bound fractions and lysate samples were analyzed by SDS/PAGE and immunoblotting with antibodies against GS, ubiquitin, and GAPDH. (Ub)n, polyubiquitin. (B) Western blot analysis showing input protein levels for Fig. 3B. (C) The bound fractions shown in Fig. 2F were analyzed by SDS/PAGE and immunoblotting with anti-ubiquitin antibody. (D) The ATP hydrolysis-deficient p97 E578Q mutant promotes the accumulation of ubiquitylated GS in response to glutamine. H1299 cells were transfected with empty plasmid (EV) or with plasmids expressing WT p97Myc or the p97Myc-E578Q mutant. Twenty-four to thirty-six hours after transfection, cells were starved of glutamine for 24 h, followed by the addition (or not) of 4 mM glutamine for 2 h. Total ubiquitinated proteins were affinity-purified using TUBE2-agarose. Bound fractions and cell lysates (input) were analyzed by SDS/PAGE and immunoblotting with the indicated antibodies. (E) The ATP hydrolysis-deficient p97 E578Q mutant binds endogenous GS. HEK293 cells were transfected with empty plasmid or with plasmids expressing WT p97Myc or the p97Myc-E578Q mutant. Twenty-four hours after transfection, cells were starved of glutamine for 24 h and were pretreated (or not) with CB-5083 (10 μM) for 30 min followed by the addition (or not) of 4 mM glutamine for 2 h. Cell extracts were immunoprecipitated with anti-Myc antibody, and the precipitated and input fractions were evaluated by SDS/PAGE and immunoblotting with the indicated antibodies.
It is worth noting that substantial amounts of apparently unubiquitylated GS coprecipitated with p97 (Fig. 2A and Fig. S2A). However, large amounts of unmodified GS were also pulled down along with ubiquitylated GS on TUBE2 resin (Fig. 2 E and F and Fig. S3 A and D). The association of apparently unmodified GS with TUBE2 resin was specific, in that it was strongly stimulated by glutamine (best seen in Fig. 2E). We suggest that p97 is recruited to GS decamers that contain a small number of ubiquitylated GS subunits and a much larger number of unmodified subunits, to extract the ubiquitylated GS subunits from the unmodified subunits.
Accumulation of Ubiquitylated GS upon p97 Inhibition Is Dependent on CRL4CRBN Activity.
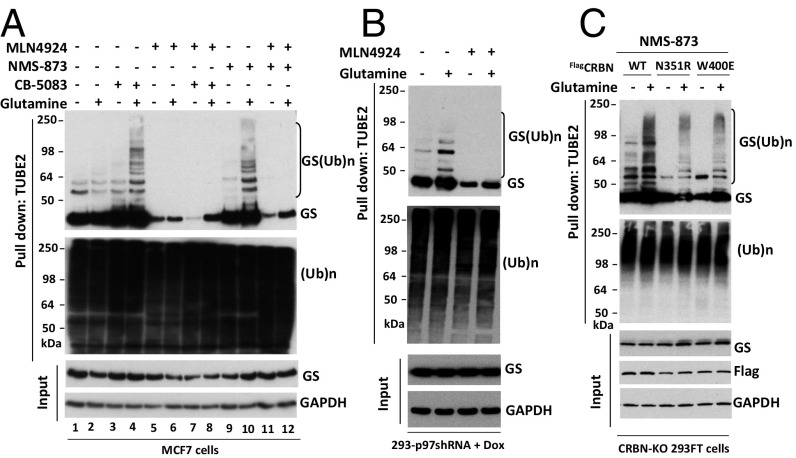
We recently reported that CRL4CRBN directly mediates the glutamine-induced ubiquitylation of GS (24). Next, we tested whether CRL4CRBN and p97 function on the same pathway to regulate glutamine-induced GS degradation. We reasoned that, if p97 functions downstream of CRL4CRBN, the exceptionally strong accumulation of ubiquitylated GS induced by p97 inhibitors should be blunted in cells in which CRL4CRBN is inhibited. To address this issue, MCF7 cells were cotreated with the p97 inhibitors CB-5083 or NMS-873 with or without the pan-CRL inhibitor MLN4924 and then were activated with 4 mM glutamine for 3 h. TUBE2 pulldown experiments showed that the accumulation of polyubiquitylated GS caused by CB-5083 or NMS-873 was completely abolished in MLN4924-treated cells (Fig. 3A). In keeping with the point made in the preceding paragraph, loss of ubiquitylated GS caused a parallel reduction in the recovery of apparently unmodified GS on TUBE2 resin. A similar epistatic effect of MLN4924 was observed in cells depleted of p97 by shRNA knockdown (Fig. 3B). The effect of MLN4924 was caused specifically by the inhibition of CRL4CRBN, because accumulation of ubiquitylated GS in response to NMS873 was blunted in CRBN-knockout 293FT cells expressing substrate binding-defective N351R or W400E mutants of CRBN (Fig. 3C) (24).
Fig. 3.
Inhibition of p97 promotes the accumulation of ubiquitylated GS in a CRL4CRBN-dependent manner. (A) MCF7 cells were starved of glutamine for 24 h and then were pretreated (or not) with CB-5083 (10 μM), NMS-873 (10 μM), or MLN4924 (2 μM) for 30 min as indicated in the figure, followed by the addition (or not) of 4 mM glutamine for 3 h. Cell lysates were fractionated on a TUBE2 resin, and both lysate samples and the bound fractions were analyzed by SDS/PAGE and immunoblotting with antibodies against GS, ubiquitin, and GAPDH. (Ub)n, polyubiquitin. (B) HEK293 cells stably expressing doxycycline (Dox)-inducible shRNA targeting p97 were induced with doxycycline (1 µg/mL) for 48 h and then were starved of glutamine for 24 h. Cells were pretreated (or not) with MLN4924 (2 μM) for 30 min, followed by the addition (or not) of 4 mM glutamine for 2 h. Cell lysates were fractionated on a TUBE2 resin, and both lysate samples and the bound fractions were analyzed by SDS/PAGE and immunoblotting with antibodies against GS, ubiquitin, and GAPDH. (C) CRBN-KO 293FT cells stably expressing WT FlagCRBN or the indicated mutants were starved of glutamine for 24 h. Cells were pretreated with NMS-873 (10 μM) for 30 min, followed by the addition (or not) of 4 mM glutamine for 2 h. Cell lysates were fractionated on a TUBE2 resin, and both lysate samples and the bound fractions were analyzed by SDS/PAGE and immunoblotting with antibodies against GS, ubiquitin, Flag, and GAPDH.
The p97 Adaptor Complex UFD1•NPL4 Interacts with Ubiquitylated GS upon Glutamine Activation.
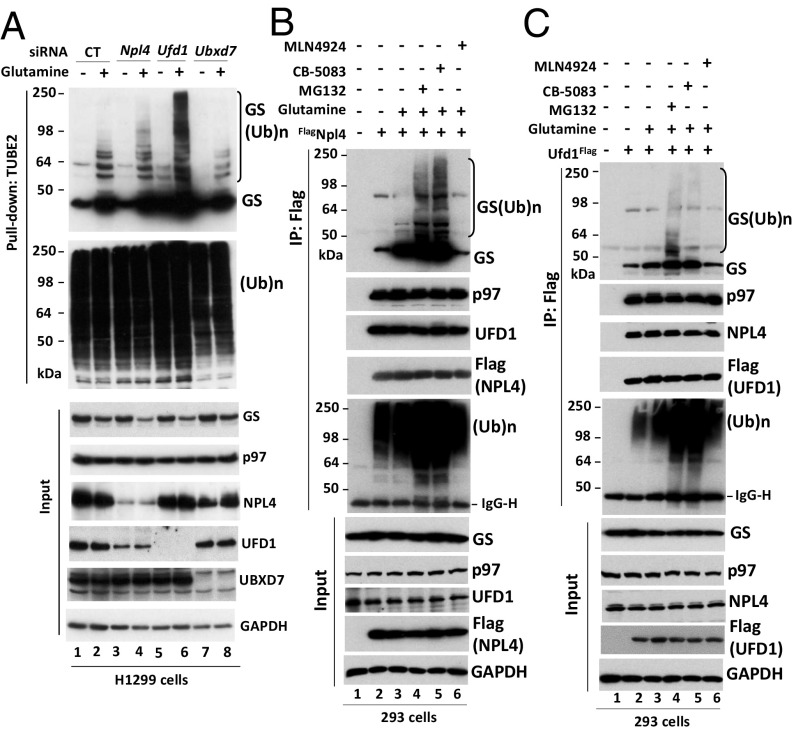
One of the central roles of p97 is to bind and extract ubiquitylated proteins from stable protein complexes, membranes, or chromatin (32). This process typically involves an adaptor protein, which may link ubiquitylated substrates to p97. To test whether a p97 adaptor participates in glutamine-induced GS ubiquitylation and degradation, we used siRNAs to knock down UBX domain-containing protein 7 (UBXD7), a direct binding partner of cullins 2 (CUL2) and 4 (CUL4) (50, 51), and the heterodimer ubiquitin fusion degradation protein (UFD1)•nuclear protein localization protein 4 (NPL4), which is well known to target ubiquitylated substrates for p97-dependent proteasomal degradation (52, 53). Depletion of UFD1, but not UBXD7, caused ubiquitylated GS to accumulate upon glutamine treatment (Fig. 4A), suggesting that UFD1•NPL4 participates in glutamine-induced GS degradation. Consistent with observations in the TUBE2 pulldowns, glutamine stimulated the interaction between UFD1•NPL4 and both ubiquitylated and apparently unmodified GS (Fig. 4 B and C). Inhibition of proteasome by MG132 or p97 by CB-5083 significantly enhanced this interaction, which was abolished by blocking GS ubiquitylation with MLN-4924 (Fig. 4 B and C). Despite these interactions, depletion of either UFD1 or NPL4 did not block glutamine-induced degradation of GS (Fig. 4A). Either UFD1•NPL4 plays a facilitating role that is not essential for GS degradation, or other adaptors can compensate for UFD1•NPL4 when it is absent.
Fig. 4.
The p97 adaptor complex UFD1•NPL4 interacts with ubiquitylated GS upon the addition of glutamine. (A) H1299 cells were transfected with control (CT) siRNA or with siRNAs that target NPL4, UFD1, or UBXD7. Forty-eight hours after transfection, cells were starved of glutamine for 24 h, followed by the addition (or not) of 4 mM glutamine for 2 h. Total ubiquitinated proteins were affinity-purified from cell lysates using TUBE2-agarose. Bound fractions and cell lysates (input) were analyzed by SDS/PAGE and immunoblotting with the indicated antibodies. (Ub)n, polyubiquitin. (B and C) HEK293 cells were transiently transfected with empty vector or with plasmids encoding FlagNPL4 (B) or UFD1Flag (C). After 24-h transfection, cells were starved of glutamine for 24 h and were pretreated (or not) with MG132 (20 μM), CB-5083 (10 μM), or MLN4924 (2 μM) for 30 min, followed by the addition (or not) of 4 mM glutamine for 2 h. Cell lysates were immunoprecipitated with anti-Flag resin. Precipitated and input fractions were analyzed by SDS/PAGE and immunoblotting with the indicated antibodies.
p97 Mediates the Disassembly of GS upon Inhibition of the Proteasome.
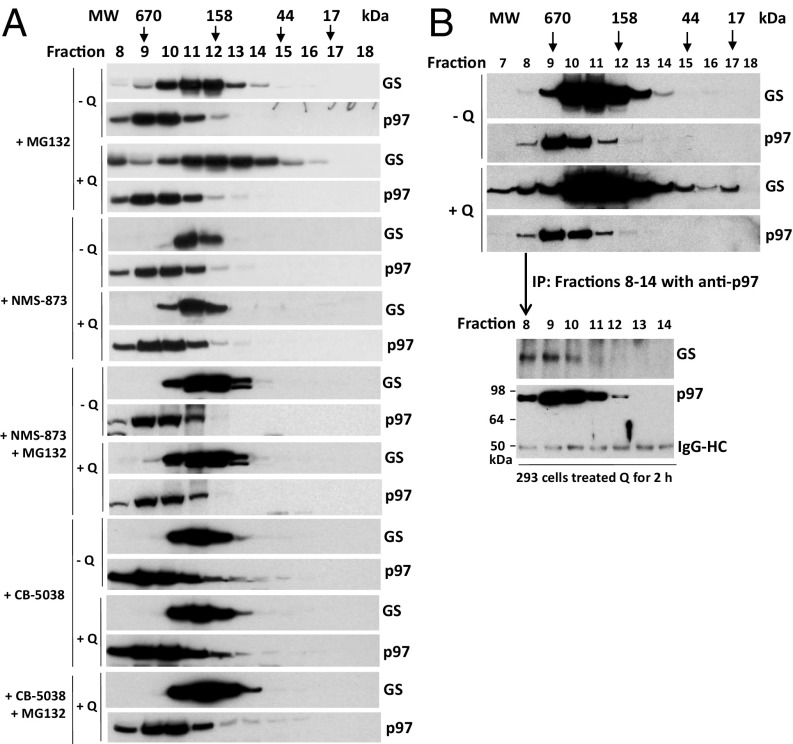
Our observation that p97 binds ubiquitylated GS and promotes its proteasome-dependent degradation suggests that p97 might mediate the disassembly of ubiquitylated GS subunits from a homodecamer, thereby enabling their degradation by the proteasome. This notion is consistent with the observations reported in Fig. 1 that the appearance of a low-MW pool of GS upon gel filtration of cell lysate was dependent upon glutamine, NEDD8 conjugation, and p97 activity. We next sought to explore more fully whether p97 mediates glutamine-induced disassembly of GS by treating MCF7 cells with glutamine in the presence of proteasome and/or p97 inhibitors. Gel filtration experiments indicated that inhibition of the proteasome accentuated the glutamine-induced spreading of the GS peak into higher- and lower-MW complexes and that this spreading was completely blocked by inhibition of p97 with CB-5083, NMS873, or shRNA knockdown (Fig. 5A and Fig. S4). The spreading of the GS peak to lower MW in cells treated with glutamine plus MG132 was likely caused by the disassembly of the decamer into smaller oligomers; we suggest that the spreading to higher MW was caused, at least in part, by the binding of GS to p97, because both proteins coeluted in high-MW fractions, GS bound p97 and UFD1•NPL4 (Figs. 2A and 4 B and C), and the high-MW complex was absent when p97 function was inhibited with small molecules or shRNA knockdown. As a more robust test of this proposal, we treated glutamine-starved cells with or without glutamine and fractionated the cell lysates by gel filtration (Fig. 5B). Fractions from across the peak of the glutamine-treated sample were subjected to IP with anti-p97 followed by immunoblotting for GS. A clear association of GS with p97 was observed in fractions that correspond to the high-MW tail of the GS peak.
Fig. 5.
p97 mediates the disassembly of GS upon inhibition of the proteasome. (A) MCF7 cells were grown to 80–90% confluence in 15-cm plates and were starved of glutamine for 24 h. Cells were pretreated (or not) with MG132 (10 μM), NMS-873 (10 µM), or CB-5083 (10 μM) for 30 min and then were treated with 4 mM glutamine (+Q) or not (−Q) for 6 h. Cell lysates were fractionated on a Superdex 200 gel filtration column. Individual fractions were concentrated by TCA precipitation and analyzed by SDS/PAGE and immunoblotting with the indicated antibodies. (B) HEK293 cells were starved of glutamine for 24 h and then were treated with 4 mM glutamine (+Q) or not (−Q) for 2 h. Cell lysates were fractionated on a Superdex 200 gel filtration column. (Upper) Individual fractions were concentrated by TCA precipitation and analyzed by SDS/PAGE and immunoblotting with antibodies against GS and p97. (Lower) Fractions 8–14, prepared from HEK293 cells treated with 4 mM glutamine, were immunoprecipitated with p97 antibody, followed by Western blot analysis with the indicated antibodies. A band at ∼50 kDa represents IgG heavy chains (IgG-HC).
Fig. S4.
(Related to Fig. 5) Depletion of p97 by shRNA blocks glutamine-induced GS disassembly. HEK293 cells stably expressing doxycycline (Dox)-inducible shRNA targeting p97 were mock-treated or were induced with doxycycline (1 µg/mL) for 48 h, starved of glutamine for 24 h, and then pretreated with MG132 (10 μM) for 30 min, followed by the addition (or not) of 4 mM glutamine for 6 h. Cell lysates were fractionated on a Superdex 200 gel filtration column. Individual fractions were concentrated by TCA precipitation and analyzed by SDS/PAGE and immunoblotting with the indicated antibodies.
Immunomodulatory Drug-Induced Degradation of CRBN Neosubstrates Requires p97.
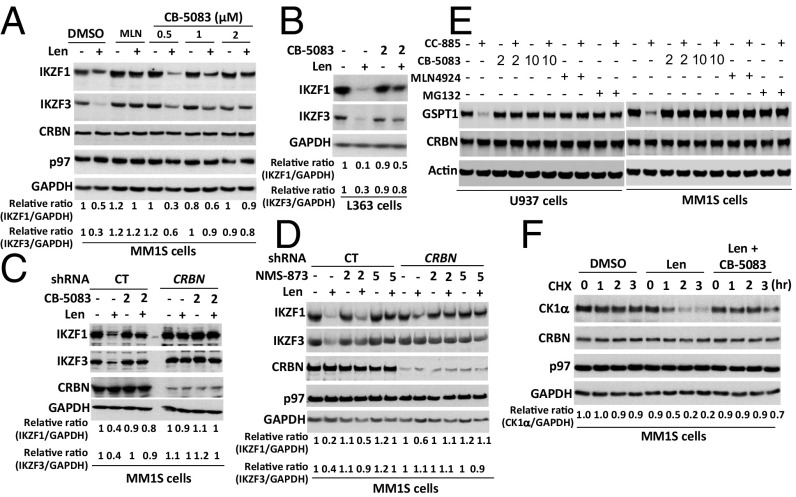
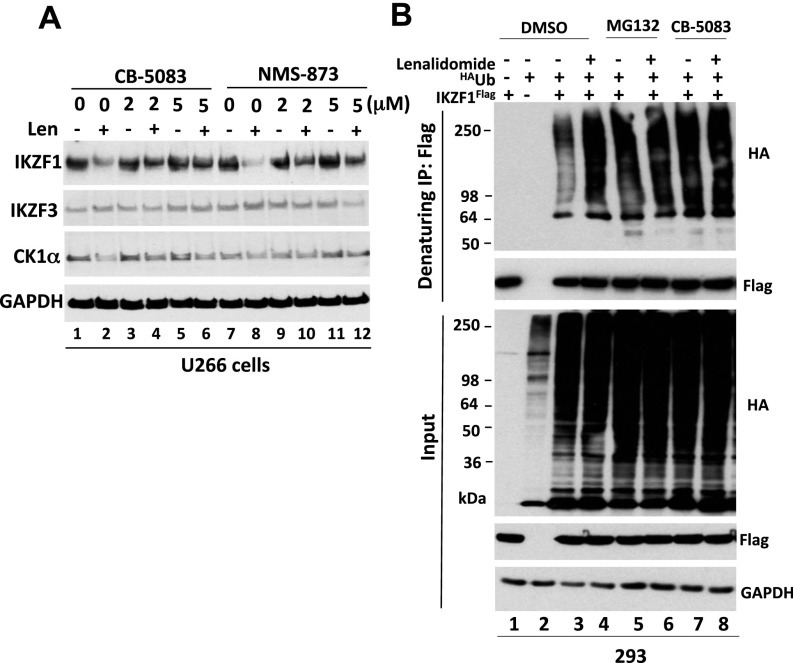
To investigate a potential role for p97 in regulating the degradation of other CRL4CRBN substrates, we extended our study to immunomodulatory drug-induced degradation of CRL4CRBN neosubstrates (28, 29, 54). CB-5083 blocked the lenalidomide-induced degradation of the lymphoid transcription factors Ikaros family zinc finger proteins 1 (KZF1) and 3 (IKZF3) in MM.1S and L363 multiple myeloma cells (Fig. 6 A–C). A similar blockade of the lenalidomide effect was observed in MM.1S (Fig. 6D) and U266 (Fig. S5A) cells treated with NMS873. Control experiments verified that the effect of lenalidomide on IKZF1 and IKZF3 was CRBN-dependent (Fig. 6 C and D). Inhibition of p97 with CB-5083 or proteasome with MG132 caused the accumulation of ubiquitylated IKZF1 regardless of whether lenalidomide was added (Fig. S5B), suggesting that p97 was required for both its constitutive and lenalidomide-induced degradation. To ask if p97 inhibition also affects the degradation of other CRBN neosubstrates induced by the CRBN modulator CC-885, we examined the effect of CB5083 on CC-885–induced degradation of the translation termination factor GSPT1 (30). Similar to MLN4924 and MG132, pretreatment with CB5083 completely blocked the CC-885–induced degradation of GSPT1 in U937 AML cells and MM1S myeloma cells (Fig. 6E). Finally, to confirm that CB5083 was indeed blocking neosubstrate proteolysis, we performed a cycloheximide chase experiment to monitor lenalidomide-induced degradation of casein kinase 1α (CK1α) (31). As shown in Fig. 6F, lenalidomide induced rapid loss of CK1α, but this effect was completely blocked upon inhibition of p97 with CB5083.
Fig. 6.
p97 is required for immunomodulatory drug-induced degradation of CRBN neosubstrates. (A) MM1S cells were pretreated with the indicated doses of CB-5083 or 1 µM MLN4924 (MLN) for 30 min, followed by the addition of lenalidomide (Len) (10 μM) for 4 h. Cell lysates were fractionated by SDS/PAGE and immunoblotted for the indicated endogenous proteins. The relative ratios of IKZF1:GAPDH or IKZF3, normalized to lane 1, are shown here and in B–D. (B) L363 cells were pretreated with CB-5083 (2 μM) for 30 min, followed by the addition (or not) of lenalidomide (10 μM) for 4 h. Cell lysates were fractionated by SDS/PAGE and immunoblotted for the indicated endogenous proteins. (C) MM1S cells stably expressing control (CT) or CRBN shRNAs were pretreated with CB-5083 (2 μM) for 30 min, followed by the addition (or not) of lenalidomide (10 μM) for 5 h. Cell lysates were analyzed by immunoblotting with the indicated antibodies. (D) As in C, except that cells were pretreated (or not) with NMS-873 (2 or 5 μM) for 30 min. (E) U937 and MM1S cells were pretreated (or not) with CB-5083 (2 or 10 μM), MLN4924 (1 μM), or MG132 (10 μM) for 1 h, followed by treatment with 10 nM CC-885 for an additional 2 h. Whole-cell extracts were subjected to immunoblot analysis. (F) Cells were pretreated with CB-5083 (2 μM) and/or lenalidomide (10 μM) for 30 min followed by the addition of cyclohexamide (CHX) (100 μg/mL). At the indicated times after the addition of cyclohexamide, samples were harvested for immunoblot analysis. The relative ratios of CK1α:GAPDH, normalized to lane 1, are shown.
Fig. S5.
(Related to Fig. 6) (A) Lenalidomide-induced degradation of CRBN neosubstrates requires p97. U266 cells were pretreated with the indicated doses of CB-5083 or NMS-873 for 30 min, followed by the addition (or not) of lenalidomide (Len) (10 μM) for 4 h. Cell lysates were fractionated by SDS/PAGE and immunoblotted for the indicated endogenous proteins. (B) Depletion of p97 or proteasome activity promotes the accumulation of ubiquitylated IKZF1, suggesting that p97 functions downstream of IKZF1 ubiquitylation. HEK293 cells were transfected with plasmids encoding HAUb and IKZF1Flag. After 24 h of transfection, the cells were pretreated with 20 µM MG132 or 10 µM CB-5083 for 30 min followed by the addition (or not) of 10 µM lenalidomide for 3 h. Denatured lysate proteins were immunoprecipitated with anti-Flag antibody. The input lysates and bound fractions were evaluated by SDS/PAGE and immunoblotting with antibodies against the HA and Flag tags.
Discussion
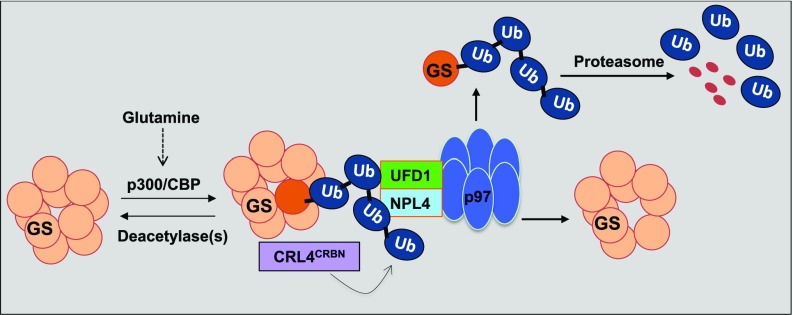
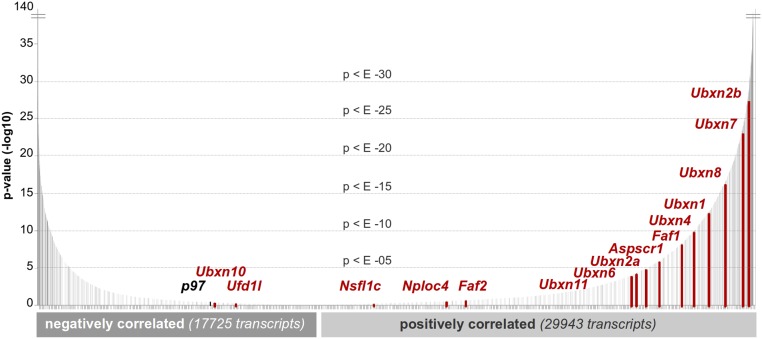
In this study, we uncover a role for p97 in regulating GS. As illustrated in Fig. 7, glutamine induces CRL4CRBN-dependent ubiquitylation of GS subunits. Subsequently, p97•UFD1•NPL4 binds to ubiquitylated GS and promotes the disassembly of the homodecamer, thereby enabling degradation of the ubiquitylated subunits by the proteasome. Currently, we do not know how acetylation, ubiquitylation, and extraction of individual subunits are coordinated. Pulldown experiments with a ubiquitin-binding resin reveal that large amounts of unmodified GS are retrieved in a manner that depends on factors that promote GS ubiquitylation. This finding suggests that one or a small number of subunits are ubiquitylated per homodecamer. Once these subunits are extracted and degraded by the proteasome, the fate of the remaining unmodified subunits is unclear, but we anticipate that they reassemble to form a smaller number of homodecamers. A number of questions about the mechanism and regulation of GS degradation remain to be addressed, including the means by which glutamine levels are monitored, the mechanism by which p97 extracts ubiquitylated subunits from GS decamers, and the role of adaptor proteins in this process. Interestingly, bioinformatic analysis of cell lines from the Cancer Cell Line Encyclopedia (CCLE) (55) revealed significant covariance between the expression of mRNAs encoding CRBN and multiple p97 adaptors (Fig. S6), suggesting diverse functional connections between these pathways.
Fig. 7.
Proposed model for the role of p97 in mediating regulated degradation of CRL4CRBN substrates, including GS and lenalidomide-induced neosubstrates. For the sake of simplicity, one subunit of GS homodecamer is ubiquitylated by CRL4CRBN, segregated by the p97–UFD1–NPL4 complex, and subsequently degraded by the proteasome upon the addition of glutamine. Ub, ubiquitin.
Fig. S6.
(Related to Fig. 3) CRBN expression covaries significantly with p97 and its adaptors. Transcript expression values (expressed as TPM) were obtained from RNA-seq performed on cancer cell lines in the 935 CCLE. These data were generated by the CTD2 network (https://ctd2.nci.nih.gov/dataPortal/) established by the National Cancer Institute’s Office of Cancer Genomics. For each detected transcript, significance of correlation with Crbn across 935 samples was determined. Data are plotted in terms of increasing significance for transcripts positively and negatively correlated with CRBN. p97 and its adaptors, as a whole, are significantly correlated with CRBN mRNA expression as determined by Kolmogorov–Smirnov testing.
In the brain, GS is highly expressed in astrocytes and plays a critical role in regulating the glutamine–glutamate cycle between astrocytes and neurons (56). In astrocytes, GS combines glutamate and ammonia to form glutamine, which then is transferred to neurons and converted to glutamate by mitochondrial glutaminase (57). Glutamate is sequestered in synaptic vesicles and then is released as a neurotransmitter into the synaptic cleft, after which it is taken up by astrocytes to complete the cycle. The excessive accumulation of glutamate can induce excitotoxicity or neuronal cell death (58). Notably, it was reported that specific depletion of GS in murine astrocytes in the brain leads to death at postnatal day 3 (59). In addition, deregulated GS activity and/or increased glutamate levels in the cerebrospinal fluid have been implicated in neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and ALS (60–63). Our findings that p97 directly controls GS degradation have potential implications for human diseases, in particular ALS, which is characterized by the progressive degeneration of motor neurons. Riluzole, an inhibitor of glutamate release, is the only effective drug for ALS, and it prolongs survival by 3–6 mo (64). Administration of the GS inhibitor MSO to SOD1G93A transgenic mice, which develop a phenotype similar to ALS in humans, decreases both glutamine and glutamate levels in the brain and significantly extends the lifespan of these mice (65, 66). Therefore, it will be of particular interest in future studies to investigate whether possible defects in the degradation of GS and other p97 substrates caused by p97 mutations identified in ALS patients may contribute to pathogenesis or serve as early diagnostic markers of the disease.
Recent work has shown that immunomodulatory drugs trigger CRBN-dependent ubiquitylation of IKZF1, IKZF3, CK1α, and GSPT1, which is followed by their degradation (28–31, 54). Remarkably, all these immunomodulatory drug-induced degradations are dependent on p97. Thus, all five CRBN substrates/neosubstrates examined in this work exhibit striking dependence on p97. We know of no other ubiquitylation pathway, other than ERAD, in which the substrates uniformly exhibit dependence on p97 for their degradation. This unique feature of CRL4CRBN may be a coincidental consequence of the oligomerization status of its known substrates or may point to an obligate functional relationship between these proteins. Further studies are required to illuminate the role of p97 in regulating immunomodulatory drug-induced degradation of these CRBN neosubstrates. GSPT1, in a binary complex with eukaryotic peptide chain release factor subunit 1 (eRF1), functions as a polypeptide chain release factor (67), whereas IKZF1/3 proteins are known to recruit the Mi-2/nucleosome remodeling and deacetylase (NuRD) complex to specific genomic targets (68, 69). By analogy to other degradation pathways in which p97 plays a critical role (70, 71), it is possible that p97 mediates the extraction of ubiquitylated GSPT1 from eRF1 and ubiquitylated IKZF1/3 proteins from chromatin before their degradation by the proteasome.
Our observations point to an unexpected role for p97 in the mechanism of action of immunomodulatory drugs, and this role could have important implications for patient responsiveness to immunomodulatory drug therapy. CRBN expression levels do not correlate with responsiveness to immunomodulatory drugs across MM cell lines (72). Among six cell lines that are resistant to immunomodulatory drugs, only two, RPMI-8226 and KMS11, express low levels of CRBN protein. In striking contrast, the other four intrinsically resistant cell lines (KMS34, LP-1, KMS12BM, and JJN-3) have higher basal levels of CRBN than the immunomodulatory drug-sensitive MM cell lines (72), suggesting that other factors, possibly including p97 and its adaptors, play a critical role in regulating the CRBN-dependent anti-myeloma activity of immunomodulatory drugs.
SI Experimental Procedures
Materials and Cell Lines.
Lenalidomide (Chem-Pacific), suberoylanilide hydroxamic acid (SAHA/Vorinostat; Sigma), MLN4924 (Pevonedistat; Active Biochem), MG132 (Millipore), NMS-873 (Calbiotech), and CB-5083 (a kind gift from Seth Cohen, University of California, San Diego, La Jolla, CA) were dissolved in DMSO at room temperature and were stored at −80 °C until use. MSO and nicotinamide (NAM) from Sigma were dissolved in distilled water and kept at −80 °C and 4 °C, respectively.
NCI-H1299 cells (ATCC no. CRL-5803), MCF7 cells (ATCC no. HTB-22), MDA-MB-231 cells (ATCC no. HTB-26), and HEK293 cells (CRL-1573) were purchased from ATCC. CRBN-knockout 293FT cells were kindly provided by William Kaelin, Dana Farber Cancer Institute, Boston. Cells were grown in DMEM supplemented with 10% (vol/vol) heat-inactivated FBS (Atlanta Biologicals), 2 mM glutamine, and penicillin-streptomycin. Human multiple myeloma cell lines, including MM.1S and U266 purchased from ATCC and L363 cells (kindly provided by Francesco Parlati, Calithera Biosciences, South San Francisco, CA) were maintained in Roswell Park Memorial Institute (RPMI)-1640 medium containing 10% FBS supplemented with 2 mM glutamine and penicillin-streptomycin. All cell cultures were checked periodically for mycoplasma contamination. Cell lines also were authenticated by short tandem repeat DNA profiling analysis by Laragen, Inc.
Antibodies.
Anti-glutamine synthetase (C-20; sc-6640-R), anti-GAPDH conjugated to HRP (FL-335 HRP; sc-25778 HRP), anti-IKZF1 (H-100; sc-13039), and anti-VCP/p97 (H-120; sc-20799) antibodies used for Western blots were from Santa Cruz Biotechnology. Anti-NPL4 (13489) and Anti-UFD1 antibodies (13789) were from Cell Signaling Technology. Anti-ubiquitin (P4D1-A11; 05–944) and anti-UBXD7 (AB10037) antibodies were from EMD Millipore. Anti-CRBN (HPA045910) and anti-Myc HRP (A5598) antibodies were from Sigma. Anti-IKZF3 antibody (NBP2-24495) was from Novus Biologicals. Anti-Flag HRP-conjugated antibody (600-403-383) was from Rockland Immunochemicals. For secondary antibodies, HRP goat anti-rabbit IgG (PI-1000) and HRP horse anti-mouse IgG (PI-2000) were from Vector Laboratories. For IP experiments, anti-VCP antibody (ab11433) was from Abcam, and EZview Red anti-Flag M2 (F2426) and EZview Red anti–c-Myc affinity gels (E6654) were from Sigma.
RNAi-Mediated Knockdown.
For siRNA transfection, control (nontargeting; sc-78528), NPL4 (sc-61227), UFD1 (sc-41689), and UBXD7 (sc-78377) siRNAs were purchased from Santa Cruz Biotechnology. Cells were grown to 50% confluence in 10-cm plates and then were transfected with 50 nM (final concentration) of siRNAs using Opti-MEM medium and Lipofectamine RNAiMAX transfection reagent according to the manufacturer’s protocol (Thermo Fisher Scientific). To achieve maximally effective siRNA knockdown, cells were transfected again 24 h later. Beginning 24 h after the second siRNA transfection, cells were starved of glutamine for 24 h, followed by the addition of 4 mM glutamine. Subsequently, cells were harvested and analyzed. For shRNA-mediated knockdown of p97, the HEK-293-p97sh cell line (DTC-139) expressing doxycycline-inducible p97-specific shRNA from the pTRIPZ-p97sh lentiviral construct was used as previously described (73).
Plasmids.
Lentiviral vectors directing the expression of WT FlagCRBN (RDB2915: pCDH-Flag-CRBN) and its mutants (RDB3186: pCDH-Flag-CRBN-W400E; RDB3187: pCDH-Flag-CRBN-N351R), constructed in pCDH-T2AcGFP-MSCV (System Biosciences), were recently reported (24). pcDNA3.1-3xFlagNPL4 plasmid (RDB3314) and pcDNA3.1-UFD11xFlag plasmid (RDB3315) were a kind gift from David Chan (California Institute of Technology, Pasadena, CA) (74). pcDNA3.1-p97Myc-His WT (RDB2001) and pcDNA3.1-p97Myc-His E578Q mutant (RDB2002) plasmids were a gift from Phyllis Hanson, Washington University in St. Louis, St. Louis (75). The full-length cDNA of IKZF1-V2 was amplified from the lentiviral vector plenti-UBCgate-IKZF1-V2-3xHA-pGK-PUR (a kind gift from William Kaelin) (29) and then was subcloned into pCMV6-Flag-Myc tags (pCMV6-IKZF1Flag-Myc; RDB3318). All cDNAs cloned into mammalian expression vectors were confirmed by DNA sequencing (Laragen).
Immunoblot Analysis and IP.
The protocols were performed as described previously (24, 76).
For Fig. 6E, U937 (ATCC) and MM1S (ATCC) cells were maintained in RPMI 1640 tissue-culture medium (Invitrogen) supplemented with 10% FBS, 1× sodium pyruvate, 1× nonessential amino acids, 100 U/mL penicillin, and 100 µg/mL streptomycin. After incubation with DMSO, CB5083, MLN4924, or MG132 for 1 h, cells were treated with CC-885 for an additional 2 h. Cells then were washed twice with ice-cold 1× PBS and were lysed in buffer A [50 mM Tris⋅HCl, 150 mM NaCl, 1% Triton X-100, a Complete Protease Inhibitor tablet (Roche), and a Phosphatase Inhibitor Cocktail tablet (Roche)]. Whole-cell extracts were harvested and subjected to immunoblot analysis with the following antibodies: rabbit anti-GSPT1 polyclonal antibody (ab49878; Abcam), rabbit anti-CRBN monoclonal antibody (CRBN65; Celgene), mouse anti-Actin monoclonal antibody (A5316; Sigma-Aldrich), goat anti-mouse IRDye-800 antibody (926–32210; LI-COR), and goat anti-rabbit IRDye-800 antibody (926–32211; LI-COR).
Cycloheximide Chase Experiments.
MM.1S cells were seeded overnight in complete medium in 24-well plates (1 × 105 cells per well) and then were pretreated with CB-5083 (2 μM) and/or lenalidomide (10 μM) for 30 min, followed by the addition of 100 µg/mL cycloheximide. At the indicated times after the addition of cycloheximide, samples were harvested for immunoblot analysis.
TUBE2 Pulldown.
Cells grown to 80% confluence in 15-cm plates and starved of glutamine for 24 h were pretreated with the proteasome inhibitor MG132 (10 μM), the NEDD8-activating enzyme inhibitor MLN4924 (1–2 μM), or the p97 inhibitors CB-5038 (10 μM) or NMS-873 (10 μM) for 0.5 h, followed by the addition (or not) of 4 mM glutamine for 2–4 h. The cells were lysed in IP lysis buffer [25 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100] containing a protease inhibitor mixture, MG132 (10 μM), and 10 mM N-ethylmaleimide (NEM; Sigma). Whole-cell protein extracts were incubated with 20 μL of TUBE2 agarose beads (Boston Biochem) for 2 h with rotation at 4 °C. Beads were washed three times with IP lysis buffer, and bound proteins were eluted in 60 μL 1.5× SDS sample buffer, boiled for 5 min, and subjected to Western blot analysis.
For USP2 treatment, after washing five times with IP lysis buffer and two times with ubiquitylation buffer [50 mM Tris⋅HCl (pH 8.0), 10 mM MgCl2, 0.2 mM CaCl2, and 1 mM DTT] plus the protease inhibitor mixture MG132 (20 μM), the beads were incubated at 30 °C for 2 h in 30 μL of ubiquitylation buffer containing 1.5 μL USP2 (E-504; Boston Biochem). USP2-treated samples were mixed with 30 μL 2× SDS sample buffer, boiled for 5 min, and subjected to Western blot analysis.
In Vivo Ubiquitylation Assays.
The in vivo ubiquitylation assays were performed under denaturing IP conditions (24, 76). HEK293 cells were grown in 10-cm plates to 60% confluence and then were transiently transfected with 4 µg pCMV6-IKZF1Flag-Myc and 0.5 µg pcDNA3- HAUbiquitin (HAUb) and/or empty vector plasmid (pcDNA3.1) using FuGENE-HD transfection reagent (Promega). After 24 h of transfection, the cells were pretreated with MG132 (20 µM) or CB-5083 (10 µM) for 30 min followed by the addition of 10 µM lenalidomide for 3 h. Cells were harvested using trypsin, lysed in 0.3 mL denaturing IP lysis buffer [1% SDS, 50 mM Tris, 10 mM DTT (pH 7.5)], and boiled for 5 min. Subsequently, denatured proteins were diluted 10× in native IP lysis buffer [25 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100] containing a protease inhibitor mixture, MG132 (10 μM), and 10 mM N-ethylmaleimide (NEM) (Sigma) and then were immunoprecipitated with anti-Flag resin. IP washing steps were performed using IP lysis buffer supplemented with 0.3 M NaCl. The input lysates and bound fractions were evaluated by SDS/PAGE and immunoblotting with antibodies against the HA and Flag tags.
Gel Filtration.
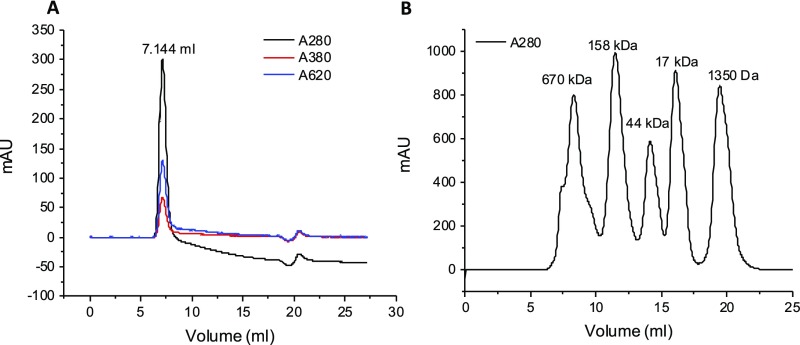
Cells grown in 15-cm plates (∼50–60% confluence) were starved of glutamine by culturing in glutamine-free DMEM supplemented with 10% FBS and penicillin/streptomycin for 24 h and then were pretreated with different inhibitors, including SAHA+NAM, MLN4924, MSO, MG132, NMS-873, and CB-5083, for 30 min, followed by the addition of 4 mM glutamine for 2 h (short treatment) or 6 h (long treatment). Cells were harvested and lysed in 1 mL of cell lysis buffer [25 mM Tris (pH 7.5), 250–300 mM NaCl, 1% Triton X-100] containing a protease inhibitor mixture, 1 mM DTT, and 10 mM NEM. Cell lysates were sonicated briefly and cleared by centrifugation at 18,000 × g for 10 min at 4 °C. After filtering through a 0.22-μm Ultrafree-MC centrifugal filter device (Millipore), soluble protein extracts (500 μL) were applied to a Superdex 200 10/300 GL (GE Healthcare Life Sciences) gel filtration column connected to an ÄKTA FPLC system (GE Healthcare Life Sciences). The void volume was determined using blue dextran (Sigma; D5751) as shown in Fig. S7A. MW estimation is based on the protein standard profile generated in house, as shown in Fig. S7B. Individual fractions of 1 mL in protein storage buffer [30 mM Tris (pH 7.5), 150 mM NaCl, 1 mM DTT] were collected and concentrated by 10% TCA precipitation, followed by washing with 1 mL ice-cold acetone. The pellets were air-dried and resuspended in 100 μL 1.5× SDS sample buffer, boiled for 5 min, and subjected to Western blotting analysis with the indicated antibodies.
Fig. S7.
(A) Void volume determination of Superdex 200 10/300 GL. Blue dextran (400 μL, 1 mg/mL) was injected into a Superdex 200 10/300 GL column and was monitored at wavelengths of 280, 380, and 620 nm (A280, absorbance at 280 nm; A380, absorbance at 380 nm; A620, absorbance at 620 nm). (B) Chromatogram for protein standard of Superdex 200 10/300 GL. Gel filtration standard (500 μL) (Bio-Rad; 151–1901) was injected into a Superdex 200 10/300 GL column and was monitored at a wavelength of 280 nm. mAU, milli absorbance unit.
Assessment of CRBN and p97 Complex Covariance.
RNA-sequencing (RNA-seq) transcripts per million (TPM) values were obtained for CCLE cell lines. These data were generated by the Cancer Target Discovery and Development (CTD2) network (https://ocg.cancer.gov/programs/ctd2/data-portal) established by the National Cancer Institute’s Office of Cancer Genomics (https://ocg.cancer.gov/ctd2-data-project/translational-genomics-research-institute-quantified-cancer-cell-line-encyclopedia). Original data and scripts used to retrieve data are available upon request. The significance of Crbn covariance (P value) with all detected transcripts was calculated from Pearson’s correlation coefficients. To determine if the group of p97 and its adaptors covary significantly with Crbn, Kolmogorov–Smirnov testing (α = 0.01) was used. The distribution of all transcripts’ covariance with CRBN was determined to be significantly different from the distribution of CRBN covariance with p97 and its adaptors, indicating that p97 and its adaptors covary significantly with Crbn expression.
Acknowledgments
We thank Dr. William Kaelin (Dana Farber Cancer Institute) for the CRBN-KO 293FT cell line and plasmids; Dr. Francesco Parlati (Calithera Biosciences) for L363 cells; Dr. David Chan (California Institute of Technology) for plasmids; Dr. Yuyong Ma and Dr. Seth Cohen (University of California, San Diego) for CB-5083; and all the members of the R.J.D. laboratory for helpful discussions, particularly E. Blythe, R. Verma, and W. den Besten. T.V.N. is supported by a Fellow Award from the Leukemia & Lymphoma Society. R.J.D. is an Investigator of the Howard Hughes Medical Institute (HHMI), and this work was supported in part by the HHMI and in part by NIH Grant GM-065997.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700949114/-/DCSupplemental.
References
- 1.Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16(10):619–634. doi: 10.1038/nrc.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicklin P, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136(3):521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergström J, Fürst P, Norée LO, Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol. 1974;36(6):693–697. doi: 10.1152/jappl.1974.36.6.693. [DOI] [PubMed] [Google Scholar]
- 4.Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: Cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123(9):3678–3684. doi: 10.1172/JCI69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor L, Curthoys NP. Glutamine metabolism: Role in acid-base balance*. Biochem Mol Biol Educ. 2004;32(5):291–304. doi: 10.1002/bmb.2004.494032050388. [DOI] [PubMed] [Google Scholar]
- 6.He Y, Hakvoort TB, Vermeulen JL, Lamers WH, Van Roon MA. Glutamine synthetase is essential in early mouse embryogenesis. Dev Dyn. 2007;236(7):1865–1875. doi: 10.1002/dvdy.21185. [DOI] [PubMed] [Google Scholar]
- 7.Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518(7539):413–416. doi: 10.1038/nature13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Häberle J, et al. Congenital glutamine deficiency with glutamine synthetase mutations. N Engl J Med. 2005;353(18):1926–1933. doi: 10.1056/NEJMoa050456. [DOI] [PubMed] [Google Scholar]
- 9.Wise DR, Thompson CB. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem Sci. 2010;35(8):427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23(1):27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330(6009):1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 12.Kung HN, Marks JR, Chi JT. Glutamine synthetase is a genetic determinant of cell type-specific glutamine independence in breast epithelia. PLoS Genet. 2011;7(8):e1002229. doi: 10.1371/journal.pgen.1002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tardito S, et al. Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nat Cell Biol. 2015;17(12):1556–1568. doi: 10.1038/ncb3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bott AJ, et al. Oncogenic Myc induces expression of glutamine synthetase through promoter demethylation. Cell Metab. 2015;22(6):1068–1077. doi: 10.1016/j.cmet.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stadtman ER. The story of glutamine synthetase regulation. J Biol Chem. 2001;276(48):44357–44364. doi: 10.1074/jbc.R100055200. [DOI] [PubMed] [Google Scholar]
- 16.Schutt H, Holzer H. Biological function of the ammonia-induced inactivation of glutamine synthetase in Escherichia coli. Eur J Biochem. 1972;26(1):68–72. doi: 10.1111/j.1432-1033.1972.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 17.Meyer JM, Stadtman ER. Glutamine synthetase of pseudomonads: Some biochemical and physicochemical properties. J Bacteriol. 1981;146(2):705–712. doi: 10.1128/jb.146.2.705-712.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung HK, Rhee SG. Separation of glutamine synthetase species with different states of adenylylation by chromatography on monoclonal anti-AMP antibody affinity columns. Proc Natl Acad Sci USA. 1984;81(15):4677–4681. doi: 10.1073/pnas.81.15.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul J, Fottrell PF. Mechanism of D-glutamyltransferase repression in mammalian cells. Biochim Biophys Acta. 1963;67:334–336. doi: 10.1016/0006-3002(63)91836-5. [DOI] [PubMed] [Google Scholar]
- 20.Demars R. The inhibition by glutamine of glutamyl transferase formation in cultures of human cells. Biochim Biophys Acta. 1958;27(2):435–436. doi: 10.1016/0006-3002(58)90367-6. [DOI] [PubMed] [Google Scholar]
- 21.Arad G, Freikopf A, Kulka RG. Glutamine-stimulated modification and degradation of glutamine synthetase in hepatoma tissue culture cells. Cell. 1976;8(1):95–101. doi: 10.1016/0092-8674(76)90190-2. [DOI] [PubMed] [Google Scholar]
- 22.Crook RB, Tomkins GM. Effect of glutamine on the degradation of glutamine synthetase in hepatoma tissue-culture cells. Biochem J. 1978;176(1):47–52. doi: 10.1042/bj1760047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krajewski WW, et al. Crystal structures of mammalian glutamine synthetases illustrate substrate-induced conformational changes and provide opportunities for drug and herbicide design. J Mol Biol. 2008;375(1):217–228. doi: 10.1016/j.jmb.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen TV, et al. Glutamine triggers acetylation-dependent degradation of glutamine synthetase via the thalidomide receptor cereblon. Mol Cell. 2016;61(6):809–820. doi: 10.1016/j.molcel.2016.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito T, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327(5971):1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 26.Zhu YX, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118(18):4771–4779. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Girona A, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26(11):2326–2335. doi: 10.1038/leu.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krönke J, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343(6168):301–305. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu G, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343(6168):305–309. doi: 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matyskiela ME, et al. A novel cereblon modulator recruits GSPT1 to the CRL4(CRBN) ubiquitin ligase. Nature. 2016;535(7611):252–257. doi: 10.1038/nature18611. [DOI] [PubMed] [Google Scholar]
- 31.Krönke J, et al. Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS. Nature. 2015;523(7559):183–188. doi: 10.1038/nature14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer H, Bug M, Bremer S. Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat Cell Biol. 2012;14(2):117–123. doi: 10.1038/ncb2407. [DOI] [PubMed] [Google Scholar]
- 33.Yamanaka K, Sasagawa Y, Ogura T. Recent advances in p97/VCP/Cdc48 cellular functions. Biochim Biophys Acta. 2012;1823(1):130–137. doi: 10.1016/j.bbamcr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Watts GD, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36(4):377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 35.Weihl CC, Pestronk A, Kimonis VE. Valosin-containing protein disease: Inclusion body myopathy with Paget’s disease of the bone and fronto-temporal dementia. Neuromuscul Disord. 2009;19(5):308–315. doi: 10.1016/j.nmd.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson JO, et al. ITALSGEN Consortium Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron. 2010;68(5):857–864. doi: 10.1016/j.neuron.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Custer SK, Neumann M, Lu H, Wright AC, Taylor JP. Transgenic mice expressing mutant forms VCP/p97 recapitulate the full spectrum of IBMPFD including degeneration in muscle, brain and bone. Hum Mol Genet. 2010;19(9):1741–1755. doi: 10.1093/hmg/ddq050. [DOI] [PubMed] [Google Scholar]
- 38.Badadani M, et al. VCP associated inclusion body myopathy and paget disease of bone knock-in mouse model exhibits tissue pathology typical of human disease. PLoS One. 2010;5(10):e13183. doi: 10.1371/journal.pone.0013183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weihl CC, Miller SE, Hanson PI, Pestronk A. Transgenic expression of inclusion body myopathy associated mutant p97/VCP causes weakness and ubiquitinated protein inclusions in mice. Hum Mol Genet. 2007;16(8):919–928. doi: 10.1093/hmg/ddm037. [DOI] [PubMed] [Google Scholar]
- 40.Yin HZ, et al. Slow development of ALS-like spinal cord pathology in mutant valosin-containing protein gene knock-in mice. Cell Death Dis. 2012;3:e374. doi: 10.1038/cddis.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chou TF, et al. Reversible inhibitor of p97, DBeQ, impairs both ubiquitin-dependent and autophagic protein clearance pathways. Proc Natl Acad Sci USA. 2011;108(12):4834–4839. doi: 10.1073/pnas.1015312108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chou TF, Li K, Frankowski KJ, Schoenen FJ, Deshaies RJ. Structure-activity relationship study reveals ML240 and ML241 as potent and selective inhibitors of p97 ATPase. ChemMedChem. 2013;8(2):297–312. doi: 10.1002/cmdc.201200520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou HJ, et al. Discovery of a first-in-class, potent, selective, and orally bioavailable inhibitor of the p97 AAA ATPase (CB-5083) J Med Chem. 2015;58(24):9480–9497. doi: 10.1021/acs.jmedchem.5b01346. [DOI] [PubMed] [Google Scholar]
- 44.Anderson DJ, et al. Targeting the AAA ATPase p97 as an approach to treat cancer through disruption of protein homeostasis. Cancer Cell. 2015;28(5):653–665. doi: 10.1016/j.ccell.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubin DM, Finley D. Proteolysis. The proteasome: A protein-degrading organelle? Curr Biol. 1995;5(8):854–858. doi: 10.1016/s0960-9822(95)00172-2. [DOI] [PubMed] [Google Scholar]
- 46.Löwe J, et al. Crystal structure of the 20S proteasome from the archaeon T. acidophilum at 3.4 A resolution. Science. 1995;268(5210):533–539. doi: 10.1126/science.7725097. [DOI] [PubMed] [Google Scholar]
- 47.Soucy TA, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458(7239):732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 48.Huang YF, Wang Y, Watford M. Glutamine directly downregulates glutamine synthetase protein levels in mouse C2C12 skeletal muscle myotubes. J Nutr. 2007;137(6):1357–1362. doi: 10.1093/jn/137.6.1357. [DOI] [PubMed] [Google Scholar]
- 49.Ye Y, Meyer HH, Rapoport TA. Function of the p97-Ufd1-Npl4 complex in retrotranslocation from the ER to the cytosol: Dual recognition of nonubiquitinated polypeptide segments and polyubiquitin chains. J Cell Biol. 2003;162(1):71–84. doi: 10.1083/jcb.200302169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.den Besten W, Verma R, Kleiger G, Oania RS, Deshaies RJ. NEDD8 links cullin-RING ubiquitin ligase function to the p97 pathway. Nat Struct Mol Biol. 2012;19(5):511–516, S511. doi: 10.1038/nsmb.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexandru G, et al. UBXD7 binds multiple ubiquitin ligases and implicates p97 in HIF1alpha turnover. Cell. 2008;134(5):804–816. doi: 10.1016/j.cell.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer HH, Wang Y, Warren G. Direct binding of ubiquitin conjugates by the mammalian p97 adaptor complexes, p47 and Ufd1-Npl4. EMBO J. 2002;21(21):5645–5652. doi: 10.1093/emboj/cdf579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bays NW, Wilhovsky SK, Goradia A, Hodgkiss-Harlow K, Hampton RY. HRD4/NPL4 is required for the proteasomal processing of ubiquitinated ER proteins. Mol Biol Cell. 2001;12(12):4114–4128. doi: 10.1091/mbc.12.12.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gandhi AK, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.) Br J Haematol. 2014;164(6):811–821. doi: 10.1111/bjh.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barretina J, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483(7391):603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schousboe A, Scafidi S, Bak LK, Waagepetersen HS, McKenna MC. Glutamate metabolism in the brain focusing on astrocytes. Adv Neurobiol. 2014;11:13–30. doi: 10.1007/978-3-319-08894-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Behar KL, Rothman DL. In vivo nuclear magnetic resonance studies of glutamate-gamma-aminobutyric acid-glutamine cycling in rodent and human cortex: The central role of glutamine. J Nutr. 2001;131(9 Suppl):2498S–2504S; discussion 2523S-2494S. doi: 10.1093/jn/131.9.2498S. [DOI] [PubMed] [Google Scholar]
- 58.Foran E, Trotti D. Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009;11(7):1587–1602. doi: 10.1089/ars.2009.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He Y, et al. Glutamine synthetase deficiency in murine astrocytes results in neonatal death. Glia. 2010;58(6):741–754. doi: 10.1002/glia.20960. [DOI] [PubMed] [Google Scholar]
- 60.Gunnersen D, Haley B. Detection of glutamine synthetase in the cerebrospinal fluid of Alzheimer diseased patients: A potential diagnostic biochemical marker. Proc Natl Acad Sci USA. 1992;89(24):11949–11953. doi: 10.1073/pnas.89.24.11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tumani H, Shen G, Peter JB, Brück W. Glutamine synthetase in cerebrospinal fluid, serum, and brain: A diagnostic marker for Alzheimer disease? Arch Neurol. 1999;56(10):1241–1246. doi: 10.1001/archneur.56.10.1241. [DOI] [PubMed] [Google Scholar]
- 62.Bos IW, et al. Increased glutamine synthetase but normal EAAT2 expression in platelets of ALS patients. Neurochem Int. 2006;48(4):306–311. doi: 10.1016/j.neuint.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 63.Rothstein JD, et al. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol. 1990;28(1):18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- 64.Hardiman O, van den Berg LH, Kiernan MC. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7(11):639–649. doi: 10.1038/nrneurol.2011.153. [DOI] [PubMed] [Google Scholar]
- 65.Bame M, Pentiak PA, Needleman R, Brusilow WS. Effect of sex on lifespan, disease progression, and the response to methionine sulfoximine in the SOD1 G93A mouse model for ALS. Gend Med. 2012;9(6):524–535. doi: 10.1016/j.genm.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 66.Ghoddoussi F, et al. Methionine sulfoximine, an inhibitor of glutamine synthetase, lowers brain glutamine and glutamate in a mouse model of ALS. J Neurol Sci. 2010;290(1-2):41–47. doi: 10.1016/j.jns.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 67.Hoshino S, et al. Molecular cloning of a novel member of the eukaryotic polypeptide chain-releasing factors (eRF). Its identification as eRF3 interacting with eRF1. J Biol Chem. 1998;273(35):22254–22259. doi: 10.1074/jbc.273.35.22254. [DOI] [PubMed] [Google Scholar]
- 68.Sridharan R, Smale ST. Predominant interaction of both Ikaros and Helios with the NuRD complex in immature thymocytes. J Biol Chem. 2007;282(41):30227–30238. doi: 10.1074/jbc.M702541200. [DOI] [PubMed] [Google Scholar]
- 69.Harker N, et al. The CD8alpha gene locus is regulated by the Ikaros family of proteins. Mol Cell. 2002;10(6):1403–1415. doi: 10.1016/s1097-2765(02)00711-6. [DOI] [PubMed] [Google Scholar]
- 70.Verma R, Oania R, Fang R, Smith GT, Deshaies RJ. Cdc48/p97 mediates UV-dependent turnover of RNA Pol II. Mol Cell. 2011;41(1):82–92. doi: 10.1016/j.molcel.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raman M, Havens CG, Walter JC, Harper JW. A genome-wide screen identifies p97 as an essential regulator of DNA damage-dependent CDT1 destruction. Mol Cell. 2011;44(1):72–84. doi: 10.1016/j.molcel.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gandhi AK, et al. Measuring cereblon as a biomarker of response or resistance to lenalidomide and pomalidomide requires use of standardized reagents and understanding of gene complexity. Br J Haematol. 2014;164(2):233–244. doi: 10.1111/bjh.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Radhakrishnan SK, den Besten W, Deshaies RJ. p97-dependent retrotranslocation and proteolytic processing govern formation of active Nrf1 upon proteasome inhibition. eLife. 2014;3:e01856. doi: 10.7554/eLife.01856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chan NC, et al. Degradation of the deubiquitinating enzyme USP33 is mediated by p97 and the ubiquitin ligase HERC2. J Biol Chem. 2014;289(28):19789–19798. doi: 10.1074/jbc.M114.569392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dalal S, Rosser MF, Cyr DM, Hanson PI. Distinct roles for the AAA ATPases NSF and p97 in the secretory pathway. Mol Biol Cell. 2004;15(2):637–648. doi: 10.1091/mbc.E03-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Nguyen T, et al. SUMO-specific protease 1 is critical for early lymphoid development through regulation of STAT5 activation. Mol Cell. 2012;45(2):210–221. doi: 10.1016/j.molcel.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]