Abstract
We investigated the role of interleukin-17 (IL-17)/IL-17 receptor (IL-17R)-mediated signaling in the protective immunity against Toxoplasma gondii. IL-17R−/− mice developed a normal adaptive immunity against the parasite. However, increased mortality in the knockout animals can be attributed to a defect in the migration of polymorphonuclear leukocytes to infected sites during early infection.
While the induction of a cell-mediated response is essential for protection against Toxoplasma gondii, the initial innate immune response led by neutrophils has also been reported to be critical for successful resolution of the infection (1, 6, 13). Depletion studies have shown that their loss leads to exacerbation of infection (3, 13). The factors involved in the development of this neutrophil response against infection have not been well studied. Recently, interleukin 17 (IL-17) has been shown to be one of the major cytokines involved in the development and recruitment of neutrophils (18). In the present study, the role of IL-17 receptor (IL-17R)-mediated signaling in the generation of protective immunity against an intracellular parasite was evaluated for the first time.
IL-17R−/− mice are more susceptible to T. gondii than are parental wild types.
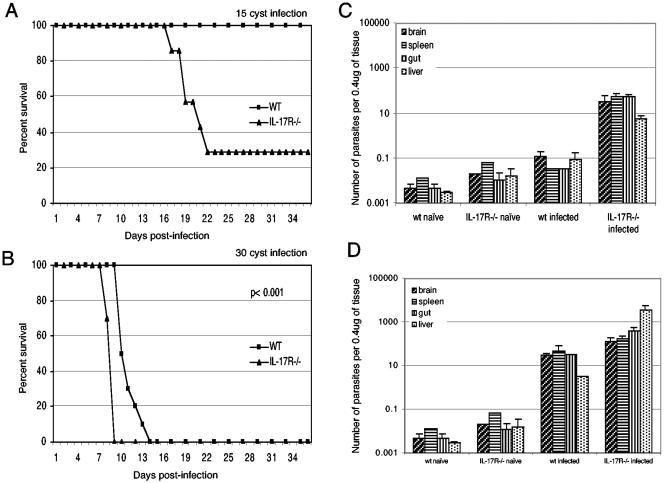
To determine if a lack of IL-17 signaling can alter the susceptibility to T. gondii infection, 5- to 6-week-old female IL-17R−/− mice (Amgen Inc., Thousand Oaks, Calif.) and parental C57BL/6 mice (Jackson Laboratories, Bar Harbor, Maine) were challenged perorally with 15 cysts of the 76K strain of T. gondii. As seen in Fig. 1A, nearly 80% of the knockout mice succumbed to infection by day 22 postinfection (p.i.). Conversely, none of the wild-type mice died or exhibited any signs of clinical sickness, and they survived until the termination of the experiment. When the inoculum was increased to 30 cysts, parental control animals survived longer (P < 0.001) but ultimately succumbed to the infection (Fig. 1B).
FIG. 1.
IL-17R−/− mice are susceptible to oral T. gondii infection and display a high parasite burden. Survival of IL-17R−/− mice infected with different doses of cysts (15 [A] or 30 [B] cysts/mouse) is shown. Data are represented as the cumulative percentage of two experiments (n = 10). The statistical analysis was performed using the Kaplan-Meier test (16). Shown is the number of parasites per microgram of tissue DNA in the organs of IL-17R−/− mice infected with 15 (C) or 30 (D) cysts of T. gondii (n = 3). Statistical analysis was performed using an unpaired Student's t test as already described (12).
To determine if the susceptibility of IL-17R−/− mice was due to increased parasite burden, tissues from both knockout and parental mice were analyzed by quantitative PCR as already described (4). The relative abundance of the B1 gene, a genetic marker for T. gondii, was determined at day 14 p.i. in the animals receiving the low dose (15 cysts) and at day 7 p.i. in those infected with a higher dose (30 cysts). As shown in Fig. 1C, a low-dose infection resulted in an approximately 2 log increase of parasite burden in the tissues (spleen, liver, gut, and brain) of knockout animals compared to wild-type mice (P < 0.05). The levels in the parental controls were close to uninfected levels, indicating that they were able to resolve the infection. Similarly, when the mice were infected with a higher parasite dose, tissues of the knockout mice had a significantly higher parasite burden than the parental strain, with the liver displaying the largest difference (P < 0.05) (Fig. 1D).
IL-17R−/− mice display less severe tissue damage in response to T. gondii infection.
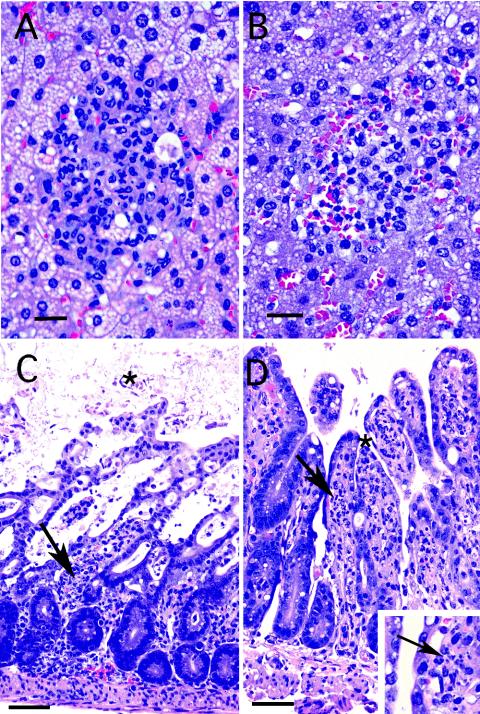
Previous studies have reported that C57BL/6 mice infected orally with a high dose of T. gondii cysts develop a severe inflammatory response (8, 15). To determine if lack of IL-17R can alter the inflammatory response, tissues from mice at day 7 p.i were subjected to histopathological analysis as previously described (4). As expected, the liver from parental C57BL/6 mice showed extensive fatty change in hepatocytes (Fig. 2A), while IL-17R-knockout mice had slightly less severe changes (Fig. 2B). As shown in Fig. 2C, the small intestine of the wild-type animals exhibited extensive necrosis and hemorrhage as previously described (10), but the IL-17R−/− animals showed much less mucosal damage (Fig. 2D). T. gondii tachyzoites were evident in the lamina propria of the IL-17R−/− mice (Fig. 2, insert), while the multiple fields from the wild-type mice did not show any presence of parasites.
FIG. 2.
Photomicrographs of the liver and ileum from IL-17R−/− and wild-type mice infected with 30 cysts of T. gondii at day 7 p.i. (A) Wild-type (WT) liver: extensive fatty change is seen in hepatocytes with small foci of mixed PMN and lymphocytic infiltration. Bar, 10 μM. (B) IL17R−/− liver: less severe fatty change is seen in hepatocytes with lymphocytic inflammation similar to that seen in the parental animals, including some PMNs. Bar, 10 μM. (C) WT small intestine: extensive necrosis and hemorrhage, with loss of the superficial mucosa and blood and cellular debris in bowel lumen (star). Small foci of mixed inflammatory cells including PMNs are seen in the lamina propria (arrow). Bar, 75 μM. (D) IL 17R−/− small intestine: the superficial mucosa is preserved (star), and a mixed inflammatory infiltrate including PMNs is evident within the lamina propria (arrow). Bar, 75 μM. (Inset) Close-up with arrow pointing to tachyzoites in lamina propria of a knockout animal.
PMN influx into tissues is inhibited in IL-17R−/− mice.
Our present studies demonstrated that although IL-17R−/− mice exhibit increased mortality from toxoplasma infection, they develop a normal antigen-specific T-cell immunity and NK-cell response against the parasite (data not shown). As neutrophils are important in host defense to T. gondii (1, 13) and IL-17 has been implicated in polymorphonuclear leukocytes (PMN) granulopoeisis and recruitment (9, 11, 18), we were interested in evaluating the trafficking of PMNs to the tissues of animals infected with 30 cysts of the 76K strain of T. gondii. Hematoxylin and eosin-stained sections of liver and intestine were examined, and relative PMN indices were determined by examination under an Olympus Van Ox microscope. Multiple sections of each tissue were counted in a blinded manner (7). At days 2 and 4 p.i., a decreased PMN index was observed in both tissues in the IL-17R−/− mice (Table 1). This difference was more pronounced in the intestine, but the number of PMNs began to increase in both tissues by day 6 p.i.
TABLE 1.
Relative PMN indices in the tissues of T. gondii-infected mice
| Tissue | PMN indexa at day p.i.:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0
|
2
|
4
|
6
|
|||||
| Mouse 1 | Mouse 2 | Mouse 1 | Mouse 2 | Mouse 1 | Mouse 2 | Mouse 1 | Mouse 2 | |
| Wild-type liver | + | + | ++ | +++ | +++ | +++ | +++ | ++ |
| IL-17R−/− liver | + | + | + | + | ++ | + | ++ | ++ |
| Wild-type gut | + | ++ | ++++ | ++++ | +++ | +++ | +++ | +++ |
| IL-17R−/− gut | + | + | + | + | + | + | ++ | ++ |
+, no, or rare, single PMN within inflammatory nodules; ++, few, or less than one-third, of the cells in the inflammatory nodules are PMNs; +++, moderate, or one- to two-thirds of the cells in the nodule are PMNs; ++++, two-thirds or more of the cells are PMNs.
Neutrophil migration into the peritoneal cavity is significantly decreased in IL-17R−/− mice after T. gondii infection.
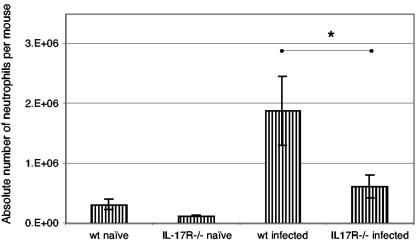
In order to assess the ability of IL-17R−/− mice to recruit neutrophils, wild-type and knockout animals were injected intraperitoneally with 2 × 106 RH strain tachyzoites. Four hours later, peritoneal exudate cells were collected (2 × 105/sample) and cytospun (700 rpm for 5 min) onto glass microscope slides (Fisher Scientific, Pittsburgh, Pa.) pretreated with Vectabond (Vector Lab, Burlingame, Calif.) by using cytofunnels (Thermo Shandon, Pittsburgh, Pa.). To determine the number of neutrophils, differential counts (300 cells/slide) were performed using a Diff-Quick stain (IMEB, San Marco, Calif.) (5). Previous studies (2) have shown that intraperitoneal infection of mice with tachyzoites of T. gondii results in a rapid recruitment of PMNs into the peritoneal cavity. As shown in Fig. 3, the number of neutrophils in the peritoneum of the wild-type mice as a result of T. gondii infection was significantly higher than that of the IL-17R−/− mice 4 h p.i. (P < 0.05).
FIG. 3.
Defective PMN influx in IL-17R−/− mice early after T. gondii infection. The number of neutrophils was determined by differential counting. *, P < 0.05. The results are representative of two experiments.
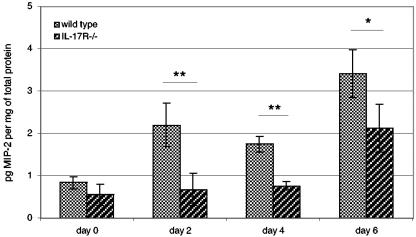
To determine if a defect in neutrophil recruitment is accompanied by decreased macrophage inflammatory protein 2 (MIP-2) levels, serum from the infected animals was evaluated for the chemokine at days 0, 2, 4, and 6 p.i. by using an enzyme-linked immunosorbent assay kit (R&D Systems) according to the manufacturer's protocol (14, 17). As shown in Fig. 4, there was no difference between control and knockout mice in the initial chemokine level (day 0). However, at days 2 and 4 p.i., levels of MIP-2 in the control animals increased significantly (P < 0.02) over those in the knockout mice, which remained at concentrations similar to those of uninfected animals (Fig. 4). By day 6, the level of MIP-2 in the knockout mice began to increase but was still significantly less than in the parental control animals (P < 0.05).
FIG. 4.
Defective chemokine production in IL-17R−/− mice infected with T. gondii. IL-17R−/− mice (three mice/group) were infected with 15 cysts of T. gondii. Data are presented as means ± standard deviations of individual mice. Significant differences between treatment pairs are indicated by lines. **, P < 0.02; *, P ≤ 0.05. The assay was performed twice with similar results.
In summary, as stated above, PMNs appear to play an important role during early T. gondii infection (5). The mechanism of rapid induction of neutrophil response during T. gondii infection has not been described. The present study demonstrates for the first time that early neutrophil induction during T. gondii infection is dependent on IL-17-mediated signaling. A diminished response in IL-17R−/− mice was associated with failure to produce the chemokine MIP-2 early in infection. Based on our findings, we hypothesize that T. gondii infection induces a strong and early neutrophil response. The neutrophils clear the parasites during initial stages of infection so that adaptive immunity, which is induced later, is not overburdened. In the presence of suboptimal levels of neutrophils, parasite load is not efficiently reduced and adaptive immunity is unable to handle this elevated burden, which ultimately leads to increased mortality.
Acknowledgments
We are thankful to Joseph Chaiban for his help in phenotypic studies.
This work was supported by National Institutes of Health grant A133325 awarded to I.A.K.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Alexander, J., T. M. Scharton-Kersten, G. Yap, C. W. Roberts, F. Y. Liew, and A. Sher. 1997. Mechanisms of innate resistance to Toxoplasma gondii infection. Philos. Trans. R. Soc. London B 352:1355-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliss, S. K., B. A. Butcher, and E. Y. Denkers. 2000. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J. Immunol. 165:4515-4521. [DOI] [PubMed] [Google Scholar]
- 3.Bliss, S. K., L. C. Gavrilescu, A. Alcaraz, and E. Y. Denkers. 2001. Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect. Immun. 69:4898-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casciotti, L., K. H. Ely, M. E. Williams, and I. A. Khan. 2002. CD8+-T-cell immunity against Toxoplasma gondii can be induced but not maintained in mice lacking conventional CD4+ T cells. Infect. Immun. 70:434-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Rio, L., S. Bennouna, J. Salinas, and E. Y. Denkers. 2001. CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J. Immunol. 167:6503-6509. [DOI] [PubMed] [Google Scholar]
- 6.Gazzinelli, R., S. Heiny, T. A. Wynn, S. Wolf, and A. Sher. 1993. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell deficient hosts. Proc. Natl. Acad. Sci. USA 90:6115-6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan, I. A., P. M. Murphy, L. Casciotti, J. D. Schwartzman, J. Collins, J.-L. Gao, and G. R. Yeaman. 2001. Mice lacking the chemokine receptor CCR1 show increased susceptibility to Toxoplasma gondii infection. J. Immunol. 166:1930-1937. [DOI] [PubMed] [Google Scholar]
- 8.Khan, I. A., J. D. Schwartzman, T. Matsuura, and L. H. Kasper. 1997. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc. Natl. Acad. Sci. USA 94:13955-13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laan, M., Z.-H. Cui, H. Hoshino, J. Lotvall, M. Sjostrand, D. C. Gruenert, B.-E. Skoogh, and A. Linden. 1999. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 162:2347-2352. [PubMed] [Google Scholar]
- 10.Liesenfeld, O., J. Kosek, J. Remington, and Y. Suzuki. 1996. Association of CD4+ T cell-dependent, interferon-gamma-mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J. Exp. Med. 184:597-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyamoto, M., O. Prause, M. Sjostrand, M. Laan, J. Lotvall, and A. Linden. 2003. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J. Immunol. 170:4665-4672. [DOI] [PubMed] [Google Scholar]
- 12.Neter J., W. Wasserman, and M. H. Kutner 1985. Applied linear statistical models, 2nd ed. R.D. Irwin, Homewood, Ill.
- 13.Sayles, P. C., and L. L. Johnson. July1996. Exacerbation of toxoplasmosis in neutrophil-depleted mice. Nat. Immun. 15:249-258. [PubMed] [Google Scholar]
- 14.Standiford, T., S. Kunkel, M. Greenberger, L. Laichalk, and R. Strieter. 1996. Expression and regulation of chemokines in bacterial pneumonia. J. Leukoc. Biol. 59:24-28. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki, Y., A. Sher, G. Yap, D. Park, L. E. Neyer, O. Liesenfeld, M. Fort, H. Kang, and E. Gufwoli. 2000. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J. Immunol. 164:5375-5382. [DOI] [PubMed] [Google Scholar]
- 16.Tanino, Y., H. Makita, K. Miyamoto, T. Betsuyaku, Y. Ohtsuka, J. Nishihira, and M. Nishimura. 2002. Role of macrophages migration inhibitory factor in bleomycin-induced lung injury and fibrosis in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 283:L156-L162. [DOI] [PubMed] [Google Scholar]
- 17.Xue, M.-L., A. Thakur, and M. Willcox. 2002. Macrophage inflammatory protein-2 and vascular endothelial growth factor regulate corneal neovascularization induced by infection with Pseudomonas aeruginosa in mice. Immunol. Cell Biol. 80:323-327. [DOI] [PubMed] [Google Scholar]
- 18.Ye, P., F. H. Rodriguez, S. Kanaly, K. L. Stocking, J. Schurr, P. Schwarzenberger, P. Oliver, W. Huang, P. Zhang, J. Zhang, J. E. Shellito, G. J. Bagby, S. Nelson, K. Charrier, J. J. Peschon, and J. K. Kolls. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194:519-528. [DOI] [PMC free article] [PubMed] [Google Scholar]