Significance
General anesthetics are an important class of drugs that suppress the activity of neurons in the brain to produce reversible unconsciousness. In addition, several general anesthetics, including the intravenous drug, propofol, and the inhaled gas, isoflurane, cause pain/irritation upon administration and a reduction in blood pressure. These side effects arise from activation of the transient receptor potential ankyrin repeat 1 (TRPA1) ion channel but the underlying mechanisms are unknown. In this study we identify a potential binding pocket for propofol and isoflurane, including several critical amino acid residues, located in the channel pore region of TRPA1. These results confirm an important role for the pore region in regulating channel activity. Further, the information may be useful in designing drugs to counter anesthetic-mediated activation of TRPA1.
Keywords: general anesthetic, TRPA1, propofol, isoflurane, A-967079
Abstract
General anesthetics suppress CNS activity by modulating the function of membrane ion channels, in particular, by enhancing activity of GABAA receptors. In contrast, several volatile (isoflurane, desflurane) and i.v. (propofol) general anesthetics excite peripheral sensory nerves to cause pain and irritation upon administration. These noxious anesthetics activate transient receptor potential ankyrin repeat 1 (TRPA1), a major nociceptive ion channel, but the underlying mechanisms and site of action are unknown. Here we exploit the observation that pungent anesthetics activate mammalian but not Drosophila TRPA1. Analysis of chimeric Drosophila and mouse TRPA1 channels reveal a critical role for the fifth transmembrane domain (S5) in sensing anesthetics. Interestingly, we show that anesthetics share with the antagonist A-967079 a potential binding pocket lined by residues in the S5, S6, and the first pore helix; isoflurane competitively disrupts A-967079 antagonism, and introducing these mammalian TRPA1 residues into dTRPA1 recapitulates anesthetic agonism. Furthermore, molecular modeling predicts that isoflurane and propofol bind to this pocket by forming H-bond and halogen-bond interactions with Ser-876, Met-915, and Met-956. Mutagenizing Met-915 or Met-956 selectively abolishes activation by isoflurane and propofol without affecting actions of A-967079 or the agonist, menthol. Thus, our combined experimental and computational results reveal the potential binding mode of noxious general anesthetics at TRPA1. These data may provide a structural basis for designing drugs to counter the noxious and vasorelaxant properties of general anesthetics and may prove useful in understanding effects of anesthetics on related ion channels.
General anesthetics have been used for over 150 y, but the molecular mechanisms for their actions are only just emerging. There is good evidence that these drugs produce anesthesia by acting on ligand-gated ion channels and, in particular, by enhancing the activity of γ-aminobutyric receptors type A (GABAA) receptors. Although mutagenesis and photolabeling studies have identified putative binding sites for anesthetics on GABAA and related receptors, the precise molecular mechanisms remain unclear (1). In addition to producing inhibitory effects in the CNS, many general anesthetics are noxious. The i.v. drugs propofol and etomidate commonly used for the induction of anesthesia elicit “burning” pain upon injection (2, 3). Furthermore, several inhalational anesthetics, including isoflurane and desflurane, produce neurogenic respiratory irritation that limits their use as induction agents. Previous studies have shown that transient receptor potential ankyrin repeat 1 (TRPA1), a nociceptive ion channel and target for mustard oil (allyl isothiocyanate, or AITC) and wasabi (4, 5), is the principal sensory nerve target of noxious anesthetics (6, 7). Furthermore, propofol-evoked vascular “pain” (6), isoflurane-induced mechanical hyperalgesia (7), and desflurane-induced bronchoconstriction (7, 8) are abolished in TRPA1-null animals. Moreover, TRPA1-null mice exhibit faster isoflurane-induced anesthesia associated with less respiratory irritation, compared with wild-type (WT) mice or TRPV1-null mice (9). In addition to nociception, TRPA1 located in the vascular endothelium contributes to the vasorelaxant effects of anesthetics such as propofol (10).
The molecular mechanisms, however, underlying anesthetic sensing by TRPA1 are unknown. Our earlier data suggest that noxious general anesthetics interact directly with TRPA1 (6, 11). First, activation of TRPA1 is retained in cell-free patches, suggesting a membrane-delimited action. Second, the ability of anesthetics to activate TRPA1 (isoflurane and desflurane are active; halothane and sevoflurane are inactive) does not correlate with the ability of anesthetics to partition into the membrane, arguing against signaling via membrane fluidity. Third, long-chain alcohols (that are predicted to mimic volatile anesthetic binding) exhibit a carbon-chain length cutoff at TRPA1 between octanol and decanol, consistent with their binding to a defined molecular pocket on TRPA1.
Previous studies have identified two key domains required for TRPA1 activation. Electrophilic compounds are known to activate TRPA1 through covalent modification of cysteines and a lysine in the N-terminal region (12, 13). In addition, the S5/S6 domain may serve as a target region for nonelectrophilic agonists, including menthol (14), eudesmol (15), and protons (16), or several antagonists (17, 18), including the potent inhibitor A-967079 (14, 19–21). Furthermore, a recent electron cryo-microscopy (cryo-EM) study (22) has revealed the high-resolution structure of TRPA1 bound to an agonist or antagonists. Similar to TRPV1 (23), the structure shows four subunits assembling to form a channel, with each subunit composed of six transmembrane (TM) segments (S1–S6) and large intracellular N- and C-terminal domains. Unexpectedly, C-terminal α-helices are revealed to form a coiled coil and a TRP-domain helix, not previously predicted for TRPA1, which runs parallel to the membrane and links the intracellular and S6 domains. Two pore helices are located between S5 and S6, the second of which defines the extracellular mouth and cation selectivity. Furthermore, two sites of restriction in pore helix 1 and S6 are proposed to form upper and lower gates, respectively. Notably, the study identified a binding pocket for the potent antagonist A-967079, formed by S5, S6, and pore helix 1, which is consistent with an important role for this region in channel gating.
Here we use phylogenetic analysis, combined with molecular modeling and functional studies of TRPA1, to identify critical sites for activation by general anesthetics. We reveal that isoflurane and propofol share a potential binding pocket with A-967079, located in the S5, S6, and the first pore helix. Furthermore, we show that these noxious general anesthetics interact with a distinct set of amino acids required for their agonistic effects.
Materials and Methods
Cell Culture.
HEK293F cells were cultured in DMEM supplemented with 10% FBS, 1 mM sodium pyruvate, 1% 100× MEM nonessential amino acids and 1% penicillin/streptomycin (HyClone) at 37 °C in a water-saturated atmosphere containing 5% CO2. Cell cultures were seeded in a culture flask (25 cm2) (Sarstedt Inc.) and subcultured twice a week. For electrophysiology, cells were plated on poly-d-lysine–coated coverslips, transiently transfected with WT or mutant TRPA1 using lipofectamine, and used for experiments within 1–2 d.
Site-Directed Mutagenesis and Chimeras.
TRPA1 mutants were generated by PCR mutagenesis using a QuikChange Site-Directed Mutagenesis Kit (Agilent) and confirmed by sequencing. Chimeric Drosophila/mouse TRPA1 constructs were a gift from A. Patapoutian, The Scripps Research Institute, La Jolla, CA.
Electrophysiology.
Whole-cell, voltage-clamp recordings were performed by using an EPC8 patch-clamp amplifier (HEKA Electronics) that was controlled by the program Pulse (version 8.65, HEKA Electronics). The bath solution contained 140 mM NaCl, 4 mM KCl, 1 mM MgCl2, 1.2 mM CaCl2, 10 mM Hepes, and 5 mM glucose, pH 7.3. The pipette solution contained 140 mM CsCl, 10 mM Hepes, 10 mM EGTA, and 2 mM Mg-ATP, pH 7.3. Solutions were applied via a valve-controlled gravity-fed perfusion system. A 200-ms ramp from −100 mV to +100 mV or +200 mV was used to measure the voltage-dependent properties of the channels.
Volatile General Anesthetics and Chemicals.
Saturated stock solutions of volatile general anesthetics were prepared in gas-tight bottles by dissolving excess anesthetic agents in bath solutions overnight as previously described (6). From these stock solutions fresh dilutions were made up every 40–60 min. Concentrations of anesthetics in the bath solutions were verified by using a modified head-space gas chromatography method.
Molecular Modeling.
The structure of human TRPA1 revealed by cryo-EM [Protein Data Bank (PDB) ID 3J9P] (22) was used for our docking studies. Ligands were prepared using LigPrep (Schrodinger). Docking was performed using induced-fit protocol (24) (Schrodinger) with the OPLS3 force field (25). The residues used to define the center of the docking box were the six residues (Ser-873, Thr-874, Leu-881, Phe-944, Val-948, Ile-950) required for A-967079 antagonism as well as Phe-909 identified in ref. 22 within the pocket formed by S5, S6, and the first pore helix.
Results
Phylogenetic Analysis Reveals a Critical Role for the S5 Region in Anesthetic Activation.
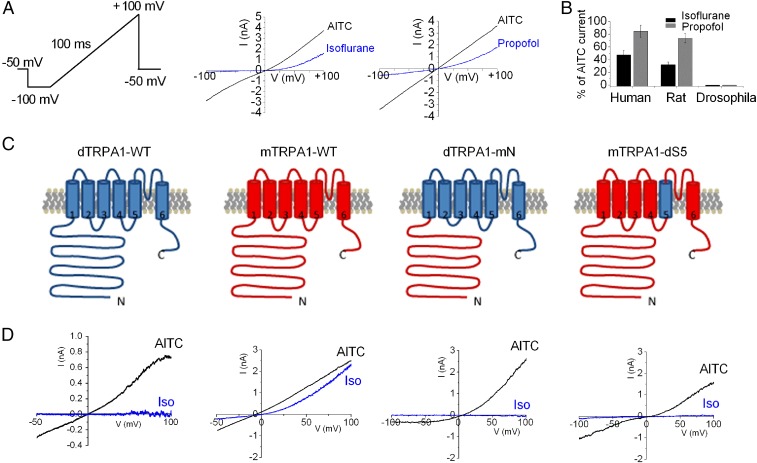
TRPA1 channels from Drosophila to human are sensitive to electrophilic agonists such as AITC (26), and we asked whether the same held true for general anesthetics. Fig. 1A shows that isoflurane (0.9 mM) or propofol (100 µM) activated outwardly rectified currents in voltage-clamped HEK293 cells expressing human or rat TRPA1. Note that the rectification in the presence of isoflurane and propofol arises from the voltage dependence of TRPA1 gating as well as an additional inhibitory effect of anesthetics at negative potentials (6). The mean current (measured at +100 mV) evoked by isoflurane or propofol was ∼30–40% and ∼70–80% of the maximal response obtained with AITC (Fig. 1B). In contrast, the Drosophila ortholog (dTRPA1) was completely insensitive to these anesthetics (Fig. 1B). Thus, these data reveal that the pungent anesthetics isoflurane and propofol are agonists of mammalian but not Drosophila TRPA1. To identify the molecular basis for activation by general anesthetics, we reasoned that chimeras of mammalian and Drosophila TRPA1 channels might allow us to identify critical domains. As the N-terminal domain is essential for activation by electrophiles (12, 13), to test whether anesthetics would share a similar binding site, we first considered a dTRPA1-mN chimera, in which the mouse 720-amino-acid N terminus is exchanged for the Drosophila N terminus (Fig. 1C). Fig. 1D shows that substituting the mouse N terminus fails to confer isoflurane sensitivity to dTRPA1, although the chimera retains sensitivity to AITC. Thus, the N terminus alone does not appear to mediate sensitivity to volatile anesthetics. We next studied the mTRPA1-dS5 construct, in which the mouse protein contains the fifth transmembrane domain of the Drosophila protein. The whole-cell recordings (Fig. 1D) show that isoflurane sensitivity is completely abolished in this chimera. Therefore, these data suggest that the S5 region is critical for mediating activation by volatile anesthetics.
Fig. 1.
Phylogenetic analysis reveals a critical role for the S5 domain in anesthetic activation. (A) Representative currents recorded from rTRPA1-expressing HEK293 cells during a 100-ms voltage ramp from −100 to +100 mV in response to isoflurane (0.9 mM), propofol (1 mM), or AITC (100 µM, black trace). The baseline current in the absence of agonists was subtracted. (B) Pungent general anesthetics activate rat and human TRPA1 but not Drosophila. Mean current measured at +100 mV induced by isoflurane (0.9 mM) and propofol (1 mM) from HEK293 cells expressing rat, human, and Drosophila TRPA1. Data are mean ± SEM from 3 to 10 cells. (C) Schematic of WT dTRPA1, WT mTRPA1, dTRPA1-mN, mTRPA1-dTM5 receptors. (D) Current-voltage relationship for responses to isoflurane (Iso, blue traces) and AITC (black traces) in HEK293 cells transfected with chimeric TRPA1 channels shown in C. Note that baseline currents were subtracted.
A Single-Amino-Acid Residue in S5 of TRPA1 Is Critical for Sensing Isoflurane.
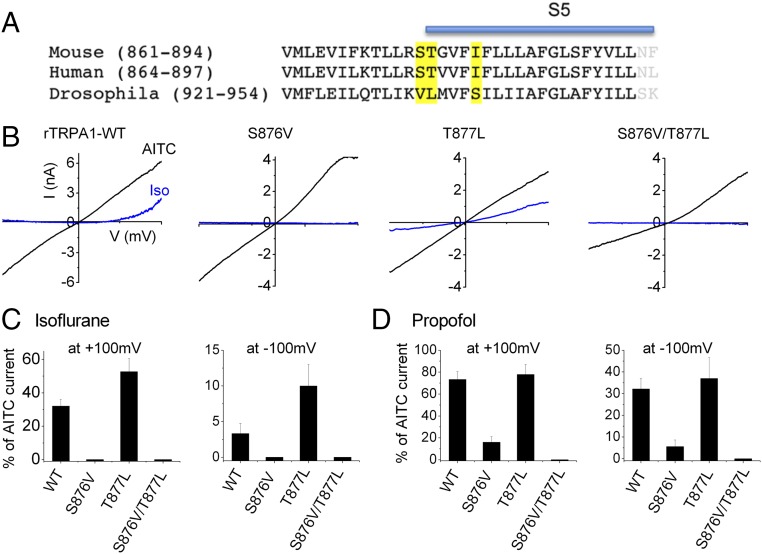
Next, we sought to identify the amino acids responsible for the species-specific differences in TRPA1 anesthetic sensing. An inspection of the protein sequence (Fig. 2A) revealed several divergent amino acids between mammalian and Drosophila TRPA1 in the S5 domain (highlighted in yellow). Notably, Ser-876 and Thr-877 are implicated in menthol sensitivity of rodent TRPA1 (14). Accordingly, we exchanged these amino acid residues with the corresponding Drosophila residues and studied the anesthetic sensitivity of these mutants under voltage clamp. Strikingly, replacing Ser-876 alone completely inhibited activation by isoflurane; however, this mutant channel nonetheless displayed normal sensitivity to AITC (Fig. S1). In contrast, mutating Thr-877 did not block isoflurane activation; rather, currents in this Thr-877Leu mutant channel were larger compared with WT TRPA1, probably reflecting an important role for Thr-877 in the inhibitory effects of general anesthetics. Replacing both of these residues (Ser-876Val/Thr-877Leu) produced the same response as the single Ser-876Val mutation (Fig. 2 B and C). Similarly, we found that mutating Ser-876 but not Thr-877 greatly reduced sensitivity to propofol, and responses to propofol were abolished in double-mutant receptors (Fig. 2D).
Fig. 2.
S876 in TRPA1 S5 domain is critical for sensing isoflurane. (A) Alignment of S5 region used in chimeras from mouse, human, and Drosophila TRPA1. Amino acids in dTRPA1 divergent from the mammalian sequence are marked in yellow. (B) Representative I–V traces (−100 to +100 mV) evoked by isoflurane (0.9 mM, blue traces) and AITC (100 µM, black traces) for WT and mutant (S876V, T877L, and S876V/T877L) rTRPA1. (C and D) Mean currents evoked by isoflurane and propofol (1 mM) measured at +100 mV and −100 mV in HEK293 cells expressing WT and mutant rTRPA1. Data are mean ± SEM from three to six cells.
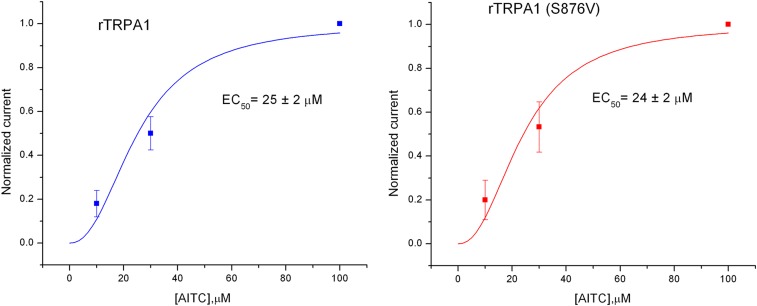
Fig. S1.
AITC sensitivity in rat WT TRPA1 and S876V mutants. Currents were recorded at −100 mV (n = 3–4). The smooth lines are best-fits to a Hill function.
Isoflurane Competitively Disrupts TRPA1 Antagonist A-967079.
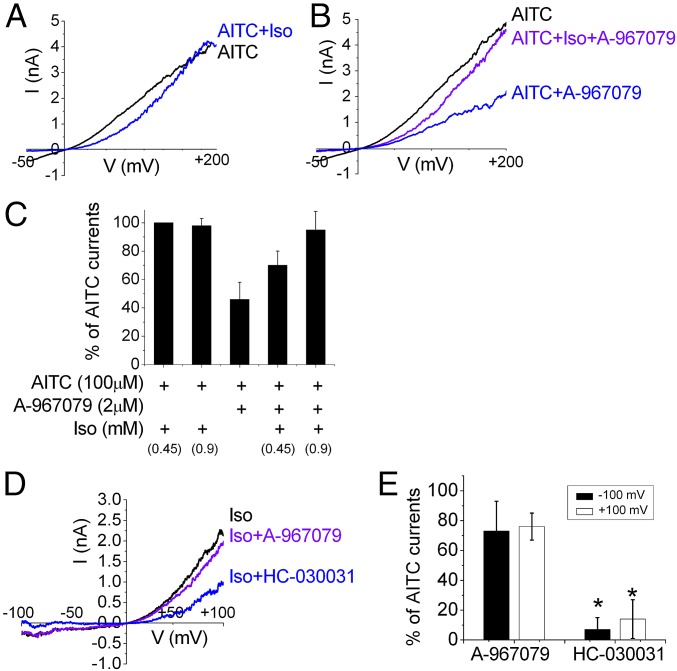
Phylogenetic studies (14, 20, 21) and molecular modeling (19, 22) of TRPA1 have identified a requirement for Ser-876 and Thr-877 (rat sequence) along with five other residues (Leu-884, Phe-912, Phe-947, Val-951, and Ile-953) located in S5, S6, and the first pore helix for sensitivity to the potent antagonist A-967079. These residues are predicted to form a binding pocket for A-967079, and the antagonist may form H-bond interactions with Ser-876 and Thr-877 and π–π interactions with Phe-912. We therefore hypothesized that pungent anesthetics and A-969079 may share an overlapping binding pocket on TRPA1. To test this hypothesis, we asked whether isoflurane could compete with A-967079 and abolish the antagonistic effects of A-967079 in rTRPA1. We first measured the inhibitory effects of A-967079 on AITC-evoked responses. To separate the direct inhibitory effects of A-967079 and isoflurane on TRPA1, we recorded currents at high positive potentials (+200 mV) where the inhibitory effect of isoflurane is removed (Fig. 3A) (6) but A-967079 inhibition is retained (Fig. 3B). Fig. 3B shows that whereas A-967079 (2 µM) inhibited the AITC-evoked current by 50%, the addition of isoflurane relieved this inhibition. Fig. 3C summarizes these data and shows that isoflurane decreases the A-967079 block in a concentration-dependent manner consistent with a competitive mechanism. Similarly, we found that A-967079 (2 µM) only weakly inhibited currents evoked by isoflurane (0.9 mM) by 20% (Fig. 3 D and E). In contrast, HC-030031 (2 µM), a less potent antagonist that binds at a site distinct from A-967079 (19, 22), was significantly more effective at suppressing isoflurane-evoked currents with ∼80% inhibition (Fig. 3E, *P < 0.01). Taken together, these data support isoflurane binding and displacing A-967079 from an overlapping site.
Fig. 3.
Isoflurane inhibits A-967079 antagonism in a concentration-dependent manner. Representative current-voltage traces for rTRPA1 activated by AITC in the presence or absence of isoflurane (0.9 mM) (A) and in the presence of A-967079 (2 µM) (B). (C) Mean current (% of AITC alone) measured at +200 mV induced by AITC plus isoflurane and/or A-967079 (n = 6–9). Isoflurane relieves the inhibitory effect of A-967079. (D and E) Inhibition of isoflurane-evoked currents by A-967079 or HC-030031 (both 2 µM) measured at −100 and +100 mV (n= 4–5; *P < 0.01 compared with response to HC-030031).
Introducing a Putative Binding Pocket for A-967079 in dTRPA1 Recapitulates Isoflurane Agonism.
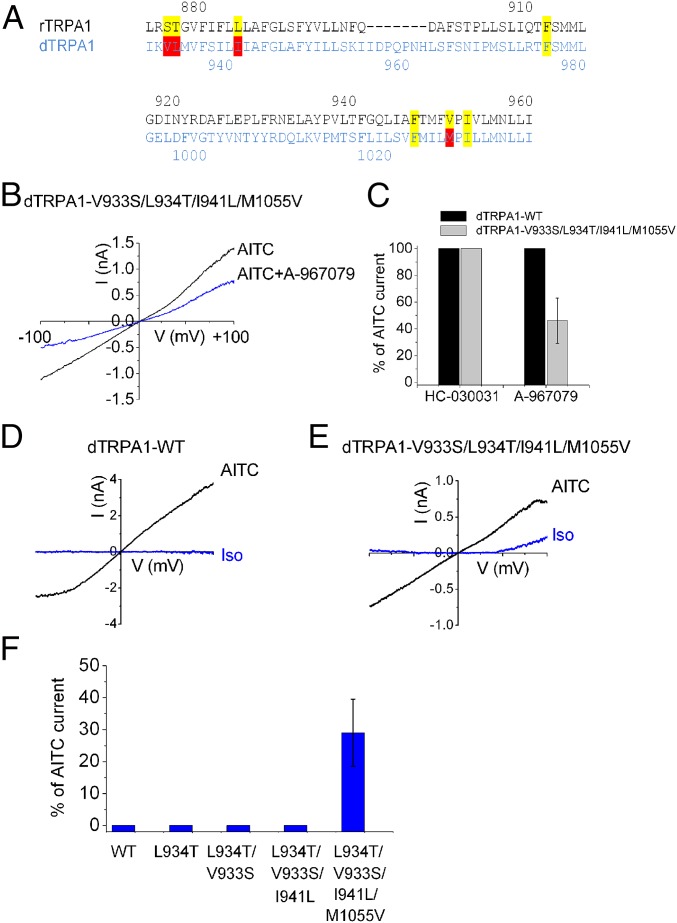
Because our data predict that isoflurane and A-967079 bind to a similar site, we asked whether anesthetic sensitivity in dTRPA1 could be recapitulated by introduction of the critical residues defining the A-967079–binding pocket. In addition to Ser-876 and Thr-877, sequence alignment shows that dTRPA1 lacks the corresponding amino acid residues for Leu-884 and Val-952 (Ile-941 and Met-1015) (Fig. 4A). Accordingly, we found that dTRPA1 channels were insensitive to A-967079 (Fig. 4C). However, introduction of the four missing amino acid residues (Val-933-Ser/Leu-934-Thr/Ile-941-Leu/Met-1055-Val) into dTRPA1 conferred sensitivity with ∼50% inhibition of AITC-evoked currents (Fig. 4C). In contrast, these channels remained insensitive to the unrelated antagonist, HC-030031. These results confirm that these residues do form a binding pocket for A-967079. Next, we tested for sensitivity to isoflurane. Fig. 4 D–F shows that isoflurane agonism was gained after replacement of all four critical residues and that the isoflurane efficacy (29 ± 10.5% of AITC, n = 6, +100 mV) was similar to that observed with rTRPA1 (32 ± 3.4% of AITC, n = 8). Thus, these data suggest that the residues required to form a putative binding pocket for A-969079 are also necessary for the agonistic action of anesthetics.
Fig. 4.
Introduction of the A-967079 binding “pocket” into dTRPA1 confers sensitivity to isoflurane. (A) Alignment of S5, S6, and the first pore helix from rat and Drosophila TRPA1. Amino acids implicated in the sensitivity of rTRPA1 to the potent antagonist A-967079 are labeled yellow, and nonidentical residues in dTRPA1 are marked red. (B and C) AITC (500 µM)-evoked currents in WT and V933S/L934T/I941L/M1055V mutant dTRPA1 in the presence of 10 µM A-961079 (n = 6) or 20 µM HC-030031 (n = 4). (D and E) Representative I–V traces in response to isoflurane (0.9 mM, blue traces) and AITC (500 µM, black traces) obtained from voltage ramps for cells expressing WT and V933S/L934T/I941L/M1055V dTRPA1. (F) Mean current responses of isoflurane (measured at +100 mV, blue columns) in HEK293 cells expressing WT dTRPA1 (n = 8) and mutants (n = 5, 23, 3, and 6 for L934, V933S/L934T, V933S/L934T/I941L, and V933S/L934T/I941L/M1055V, respectively).
Molecular Modeling and Experimental Validations Identify Key Residues Involved in Anesthetic Binding.
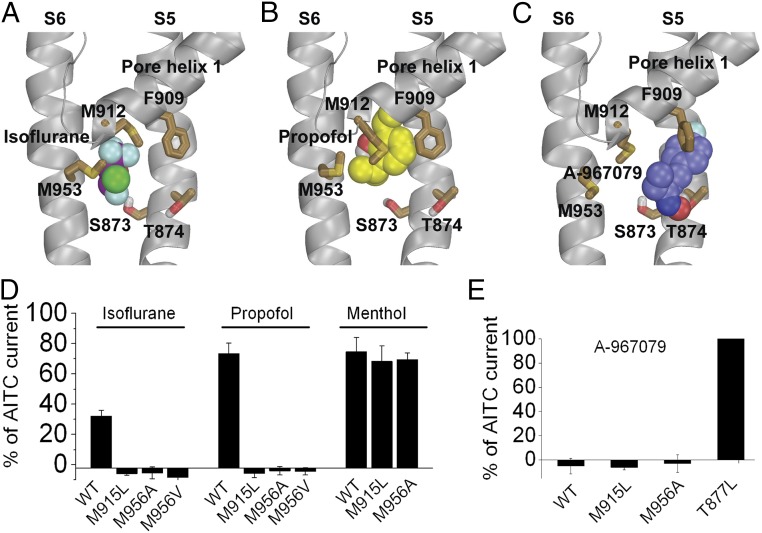
As described above, our mutagenesis data showed that residues forming the binding pocket for A-967079 are also required for the agonist action of anesthetics (Fig. 4F). To identify other amino acid residues involved in direct interactions with general anesthetics, we docked isoflurane and propofol to a binding pocket formed by these residues within S5 and S6 helices and the pore helix 1 using the structure of human TRPA1 revealed by cryo-EM (PDB ID 3J9P) (22). As a control, we first docked A-967079 and found that it prefers a pose (Fig. 5C) similar to that revealed in the cryo-EM structure (22) in which the antagonist forms a π–π interaction with Phe-909 of the first pore helix, whereas its hydroxyl group forms a hydrogen bond with Thr-874 in agreement with our mutagenesis data (Fig. 5E). Our docking results indicate that isoflurane prefers a pose in which its CF2 forms a halogen bond with Ser-873 (distance is ∼3 Å) (Fig. 5A), in agreement with our mutagenesis results (Fig. 2C). In addition to Ser-873, the CF3 group of isoflurane forms a halogen bond with Met-912 (distance is ∼3.3 Å) (27), whereas the Cl group forms a halogen bond with Met-953 (distance between Cl and sulfur of Met-953 is 3.3 Å). Similarly, we found that propofol binds in the same binding pocket as isoflurane with one of its isopropyl groups within 4 Å of residues Ser-873 [consistent with our mutagenesis results (Fig. 2D)] and Met-953 and its phenyl group forming a sulfur-aromatic interaction with Met-912 (Fig. 5B). Thus, the binding modes of isoflurane and propofol overlap with that of A-967079, and this supports our finding that isoflurane disrupts A-967079 antagonism, presumably by competitively inhibiting the binding of A-967079.
Fig. 5.
Molecular modeling and functional analysis identify TRPA1 residues involved in binding isoflurane and propofol. (A–C) Binding modes of isoflurane (A), propofol (B), and A-967078 (C) in human TRPA1 revealed by molecular docking. Note that Met-912 and Met-953 interact with isoflurane and propofol but not A-967078. (D) Mean currents (measured at +100 mV) evoked by isoflurane (0.9 mM), propofol (1 mM), or menthol (1 mM) in HEK293 cells expressing WT (n = 3) or mutant (n = 6–11) rTRPA1s. (E) Inhibition of AITC responses by A-967079 in WT (n = 5) and mutant (n = 4–6) rTRPA1s.
Our molecular modeling predicts critical roles for Met-912 and Met-953 (in human TRPA1) in the binding of noxious general anesthetics, and we therefore tested the function of rTRPA1 channels lacking these corresponding residues (Met-915 and Met-956). Fig. 5D shows that isoflurane and propofol agonism were completely abolished in these mutants (Met-915Leu, Met-956Ala, and Met-956Val). In contrast, these mutant channels retained sensitivity to both menthol and A-967079 (Fig. 5 D and E). Thus, these methionines represent critical and selective sites for noxious general anesthetics.
Discussion
General anesthetics are important drugs that suppress CNS activity, leading to reversible unconsciousness, immobility, and amnesia. In addition, several general anesthetics, including the i.v. agent propofol and the inhalants isoflurane and desflurane, activate the TRPA1 ion channel located in peripheral sensory nerves to cause pain/irritation upon administration. In this study we have identified a putative anesthetic binding pocket in TRPA1 and critical amino acids required for anesthetic sensing. We observed species-specific differences in anesthetic sensitivity between mammalian and Drosophila TRPA1 and exploited this finding using chimeric TRPA1 proteins to identify an important role for S5. Interestingly, S5 along with S6 and pore helix 1 form a binding pocket for the TRPA1 antagonist A-967079 (14, 19–22), and we found that isoflurane disrupted the effects of A-967079. In contrast, isoflurane did not disrupt the inhibitory effects of HC-030031, an unrelated antagonist not predicted to interact with the A-967079–binding pocket (19, 22). Therefore, these findings support the hypothesis that isoflurane binds to this same pocket and displaces A-967079 in a competitive manner. Importantly, in support of this hypothesis we found that reconstituting the required residues for the A-967079–binding pocket in Drosophila TRPA1 confers sensitivity to both A-967079 and anesthetics. Finally, using molecular modeling we predicted three amino acid residues (human TRPA1 Ser-873, Met-912, and Met-953) to interact with both isoflurane and propofol. Significantly, mutagenesis of each of these residues abolished the agonistic effects of isoflurane and propofol (Figs. 2 C and D and 5E). In contrast, mutagenesis of Met-912 and Met-953 did not affect responses to A-967079 or menthol, suggesting that loss of anesthetic sensitivity was not due to an allosteric mechanism but that these residues are direct and selective sites for anesthetic binding. Ser-873 also contributes to the A-967079/anesthetic–binding pocket, and therefore functional deficits in S873V mutants may reflect both a direct binding interaction and an allosteric mechanism.
These data underscore an important role for the pore domain in TRPA1 gating. Indeed, in addition to menthol and A-967079, there is accumulating evidence that this region is critical for the actions of several agonists and antagonists. Activation by the hop oil eudesmol requires Ser-873 (15), the inhibitory effects of monoterpenes require both Ser-873 and Thr-874 (18), and inhibition by 6-methyl-5-[2-(trifluoromethyl)phenyl]-1H-indazole requires Thr-874, Val-876, and Phe-877 and, to a lesser extent, Met-956 (17). However, it remains unclear whether or not these compounds directly bind to these residues. In contrast, cryo-EM analysis of TRPA1 indicates that A-967079 but not HC-030031 binds the pore region. Although not unequivocal, our experimental observation that isoflurane exhibits apparent competition with A-967079, but not with HC-030031, supports a direct interaction of the anesthetic with the pore domain consistent with our modeling. Interestingly, a recent study identified isoflurane binding to the pore region of the bacterial voltage-gated sodium channel NaChBac, which is structurally homologous to TRP channels (28).
If anesthetics and A-967079 share an overlapping binding pocket, then how do they produce opposite functional effects on TRPA1 gating? The structural information gleaned from cryo-EM studies of TRPV1 in the apo and ligand-bound states (29) suggest that S5, S6, and the pore helix are mobile elements in gating and similar rearrangements of these structures are predicted for TRPA1 (22). Activation of TRPV1 is accompanied by tilting of the pore helix and a shift of the lower S6 region away from the central canal leading to a widening of the upper and lower gates, respectively (29). In TRPA1, A-967079 interacts with Phe-909 in pore helix 1 and with Ser-873/Thr-874 in S5 and may therefore stabilize and prevent movement of these structures (22). In contrast, our data reveal isoflurane and propofol to be wedged between Met-912 in pore helix 1 and Met-953 in S6, in close proximity to the upper (Asp-915) and lower gates (Ile-957 and Val-961), and therefore this interaction may induce translation and widening of the upper and/or lower gates to account for partial agonism.
The precise molecular mechanisms by which anesthetics modulate their key target receptors in the CNS remain unclear. The low potency of anesthetics, in particular, volatile agents such as isoflurane, makes identifying binding sites challenging. Although mutagenesis studies have identified numerous amino acid residues in GABAA receptors critical for the effects of general anesthetics, whether or not these constitute binding sites (versus allosteric effects) is uncertain. Conversely, photolabeling studies have identified numerous binding sites for volatile anesthetics on GABAA and nicotinic acetylcholine receptors (nAChRs), not all of which are predicted to affect channel function (1). Furthermore, these studies suggest discrete binding sites for different anesthetics. For example, photolabeling GABAA receptors with azietomidate reveals a binding pocket at the interface between β- and α-subunits (30), whereas photolabeling with propofol yields an intrasubunit site of the β3-subunit (31). In addition, Mihic and colleagues discovered a domain in the α1-subunit of GABAA receptors (including Ser-270) that is important for mediating the effect of certain volatile anesthetics and etomidate, but not propofol (32). Moreover, a single point mutation in the GABAA receptor β3-subunit abolishes sensitivity to the agents, propofol and etomidate, without significantly affecting responses to volatile drugs (33). In contrast, our study shows that isoflurane and propofol share the same binding pocket and interact with serine and methionine residues to produce activation of TRPA1. Interestingly, methionine residues may represent prime targets for anesthetics. For example, azietomidate photolabels α–Met-236 and β–Met-286 in GABAA receptors (30), and TDBzl-etomidate (a positive allosteric regulator of nAChRs) photobels γ–Met-299, which lies in the interface between α- and γ-subunits of Torpedo nAChRs (34).
The noxious and hemodynamic effects of certain anesthetics limit their clinical use. In particular, propofol, the most widely used i.v. anesthetic worldwide, produces pain on injection that cannot be fully prevented with current strategies and hypotension, both of which are largely attributable to activation of TRPA1 (6, 10). Thus, the structural information of propofol binding to TRPA1 identified in this study may aid in the design of compounds that can counter these effects, including drugs that block propofol binding without otherwise affecting TRPA1 gating.
Acknowledgments
This study was supported by NIH Grant R21NR012065 and in part by the Intramural Research Program of the NIH, NIDA.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1618144114/-/DCSupplemental.
References
- 1.Forman SA, Miller KW. Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels. Can J Anaesth. 2011;58(2):191–205. doi: 10.1007/s12630-010-9419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Picard P, Tramèr MR. Prevention of pain on injection with propofol: A quantitative systematic review. Anesth Analg. 2000;90(4):963–969. doi: 10.1097/00000539-200004000-00035. [DOI] [PubMed] [Google Scholar]
- 3.Tan CH, Onsiong MK. Pain on injection of propofol. Anaesthesia. 1998;53(5):468–476. doi: 10.1046/j.1365-2044.1998.00405.x. [DOI] [PubMed] [Google Scholar]
- 4.Jordt S-E, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427(6971):260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 5.Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124(6):1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Matta JA, et al. General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci USA. 2008;105(25):8784–8789. doi: 10.1073/pnas.0711038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eilers H, et al. Pungent general anesthetics activate transient receptor potential-A1 to produce hyperalgesia and neurogenic bronchoconstriction. Anesthesiology. 2010;112(6):1452–1463. doi: 10.1097/ALN.0b013e3181d94e00. [DOI] [PubMed] [Google Scholar]
- 8.Satoh J, Yamakage M. Desflurane induces airway contraction mainly by activating transient receptor potential A1 of sensory C-fibers. J Anesth. 2009;23(4):620–623. doi: 10.1007/s00540-009-0786-8. [DOI] [PubMed] [Google Scholar]
- 9.Li F, et al. Transient receptor potential A1 activation prolongs isoflurane induction latency and impairs respiratory function in mice. Anesthesiology. 2015;122(4):768–775. doi: 10.1097/ALN.0000000000000607. [DOI] [PubMed] [Google Scholar]
- 10.Sinha S, Sinharoy P, Bratz IN, Damron DS. Propofol causes vasodilation in vivo via TRPA1 ion channels: Role of nitric oxide and BKCa channels. PLoS One. 2015;10(4):e0122189. doi: 10.1371/journal.pone.0122189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornett PM, Matta JA, Ahern GP. General anesthetics sensitize the capsaicin receptor transient receptor potential V1. Mol Pharmacol. 2008;74(5):1261–1268. doi: 10.1124/mol.108.049684. [DOI] [PubMed] [Google Scholar]
- 12.Hinman A, Chuang H-H, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103(51):19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macpherson LJ, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445(7127):541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 14.Xiao B, et al. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J Neurosci. 2008;28(39):9640–9651. doi: 10.1523/JNEUROSCI.2772-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohara K, et al. Identification of significant amino acids in multiple transmembrane domains of human transient receptor potential ankyrin 1 (TRPA1) for activation by eudesmol, an oxygenized sesquiterpene in hop essential oil. J Biol Chem. 2015;290(5):3161–3171. doi: 10.1074/jbc.M114.600932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de la Roche J, et al. The molecular basis for species-specific activation of human TRPA1 protein by protons involves poorly conserved residues within transmembrane domains 5 and 6. J Biol Chem. 2013;288(28):20280–20292. doi: 10.1074/jbc.M113.479337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moldenhauer H, Latorre R, Grandl J. The pore-domain of TRPA1 mediates the inhibitory effect of the antagonist 6-methyl-5-(2-(trifluoromethyl)phenyl)-1H-indazole. PLoS One. 2014;9(9):e106776. doi: 10.1371/journal.pone.0106776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takaishi M, Uchida K, Fujita F, Tominaga M. Inhibitory effects of monoterpenes on human TRPA1 and the structural basis of their activity. J Physiol Sci. 2014;64(1):47–57. doi: 10.1007/s12576-013-0289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klement G, et al. Characterization of a ligand binding site in the human transient receptor potential ankyrin 1 pore. Biophys J. 2013;104(4):798–806. doi: 10.1016/j.bpj.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakatsuka K, et al. Identification of molecular determinants for a potent mammalian TRPA1 antagonist by utilizing species differences. J Mol Neurosci. 2013;51(3):754–762. doi: 10.1007/s12031-013-0060-2. [DOI] [PubMed] [Google Scholar]
- 21.Banzawa N, et al. Molecular basis determining inhibition/activation of nociceptive receptor TRPA1 protein: A single amino acid dictates species-specific actions of the most potent mammalian TRPA1 antagonist. J Biol Chem. 2014;289(46):31927–31939. doi: 10.1074/jbc.M114.586891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulsen CE, Armache J-P, Gao Y, Cheng Y, Julius D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015;520(7548):511–517. doi: 10.1038/nature14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504(7478):107–112. doi: 10.1038/nature12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherman W, Day T, Jacobson MP, Friesner RA, Farid R. Novel procedure for modeling ligand/receptor induced fit effects. J Med Chem. 2006;49(2):534–553. doi: 10.1021/jm050540c. [DOI] [PubMed] [Google Scholar]
- 25.Harder E, et al. OPLS3: A force field providing broad coverage of drug-like small molecules and proteins. J Chem Theory Comput. 2016;12(1):281–296. doi: 10.1021/acs.jctc.5b00864. [DOI] [PubMed] [Google Scholar]
- 26.Kang K, et al. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464(7288):597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilcken R, Zimmermann MO, Lange A, Joerger AC, Boeckler FM. Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J Med Chem. 2013;56(4):1363–1388. doi: 10.1021/jm3012068. [DOI] [PubMed] [Google Scholar]
- 28.Kinde MN, et al. Fluorine-19 NMR and computational quantification of isoflurane binding to the voltage-gated sodium channel NaChBac. Proc Natl Acad Sci USA. 2016;113(48):13762–13767. doi: 10.1073/pnas.1609939113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao E, Liao M, Cheng Y, Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature. 2013;504(7478):113–118. doi: 10.1038/nature12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li G-D, et al. Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J Neurosci. 2006;26(45):11599–11605. doi: 10.1523/JNEUROSCI.3467-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yip GMS, et al. A propofol binding site on mammalian GABAA receptors identified by photolabeling. Nat Chem Biol. 2013;9(11):715–720. doi: 10.1038/nchembio.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mihic SJ, et al. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389(6649):385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 33.Jurd R, et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17(2):250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 34.Nirthanan S, Garcia G, III, Chiara DC, Husain SS, Cohen JB. Identification of binding sites in the nicotinic acetylcholine receptor for TDBzl-etomidate, a photoreactive positive allosteric effector. J Biol Chem. 2008;283(32):22051–22062. doi: 10.1074/jbc.M801332200. [DOI] [PMC free article] [PubMed] [Google Scholar]