Significance
Some bilaterally symmetrical animals show genetically programmed differences between their left and right sides, for example the placement of the heart and viscera in humans and other vertebrates. We dissect the regulation of this in embryos of amphioxus, a close relative of vertebrates that develops extraordinary asymmetries as a larva. We demonstrate a system in which asymmetric expression of the signal gene Nodal is controlled by positive and negative feedback loops with its own inhibitors. When this system is disrupted, embryos develop mirror-image symmetry with two “left” sides or two “right” sides. Comparison with other animals shows how this complex regulatory mechanism has evolved by addition of new feedback regulation to a more ancient signaling interaction.
Keywords: amphioxus, Nodal, left–right asymmetry, TALEN, embryonic development
Abstract
Many bilaterally symmetrical animals develop genetically programmed left–right asymmetries. In vertebrates, this process is under the control of Nodal signaling, which is restricted to the left side by Nodal antagonists Cerberus and Lefty. Amphioxus, the earliest diverging chordate lineage, has profound left–right asymmetry as a larva. We show that Cerberus, Nodal, Lefty, and their target transcription factor Pitx are sequentially activated in amphioxus embryos. We then address their function by transcription activator-like effector nucleases (TALEN)-based knockout and heat-shock promoter (HSP)-driven overexpression. Knockout of Cerberus leads to ectopic right-sided expression of Nodal, Lefty, and Pitx, whereas overexpression of Cerberus represses their left-sided expression. Overexpression of Nodal in turn represses Cerberus and activates Lefty and Pitx ectopically on the right side. We also show Lefty represses Nodal, whereas Pitx activates Nodal. These data combine in a model in which Cerberus determines whether the left-sided gene expression cassette is activated or repressed. These regulatory steps are essential for normal left–right asymmetry to develop, as when they are disrupted embryos may instead form two phenotypic left sides or two phenotypic right sides. Our study shows the regulatory cassette controlling left–right asymmetry was in place in the ancestor of amphioxus and vertebrates. This includes the Nodal inhibitors Cerberus and Lefty, both of which operate in feedback loops with Nodal and combine to establish asymmetric Pitx expression. Cerberus and Lefty are missing from most invertebrate lineages, marking this mechanism as an innovation in the lineage leading to modern chordates.
Bilaterians share three primary developmental axes. The anterior–posterior (AP) and dorsal–ventral (DV) axes define bilaterally symmetrical organization. The third axis, orthogonal to these, is known as the medial–lateral or left–right (LR) axis and displays mirror-image symmetry. However, many bilaterian species deviate consistently from true symmetry, raising fundamental questions of how symmetry is broken and how different developmental programs can unfold on the left and right sides of an organism (1). In vertebrates, this includes asymmetric development of the heart and viscera, disruption of which during embryogenesis causes a range of human disorders (2).
Correct LR organization in vertebrates is regulated by a gene cassette in which right-sided Cerberus (Cer) and left-sided Nodal and Lefty regulate left-sided expression of the Pitx family gene Pitx2 and hence morphological LR asymmetry (3). Cer expression on the right of the embryonic node is required to repress Nodal signaling, which happens by Cer protein binding directly to Nodal protein. This restricts the ability of Nodal protein to activate the expression of the Nodal gene, leading to an up-regulation of Nodal on the left of the embryo. Lefty expression is also up-regulated by Nodal and, like Cer, acts as an extracellular inhibitor of Nodal. Coexpression of Nodal and Lefty can act as an activator–inhibitor system in the sense originally described by Turing (4). Nodal on the left of the embryo activates expression of Pitx2, which directs the development of left-sided morphology (3).
Several studies have sought to dissect the evolutionary history of Nodal signaling and its regulation of LR asymmetry. Notably, asymmetric expression of Nodal and Pitx in gastropod mollusc embryos plays a role in the development of LR asymmetry, including the coiling of the shell (5, 6). Asymmetric expression of Nodal and/or Pitx has also been reported in some other lophotrochozoans, including Pitx in a brachiopod and an annelid and Nodal in a brachiopod (7, 8). These data can be interpreted to suggest an ancestral role for Nodal and Pitx in regulating bilaterian LR asymmetry, however the picture is complicated by data from other lineages. First, in ecdysozoan lineages, Nodal appears to have been lost (7, 9), whereas Pitx is generally symmetrically expressed (8) and, where studied, does not function in LR asymmetry (10, 11). Second, some lophotrochozoans do not show asymmetric Pitx expression (8, 12, 13). Third, functional studies on protostome Nodal signaling are currently restricted to molluscs (5), so it is unclear whether the regulatory connection between Nodal and Pitx is conserved even in those species that show asymmetric expression. An additional complication is that Cer and Lefty are also absent from many invertebrate bilaterian lineages (9, 14). The extracellular inhibition of Nodal by the proteins encoded by these two genes is a critical component of Nodal signaling in vertebrates (4, 15) and is required for both the generation and maintenance of asymmetric Nodal and Pitx2.
To examine the evolutionary history of Nodal signaling and its regulation, we turned to amphioxus, the basal chordate lineage and one that forms a typical chordate body plan while also developing pronounced LR asymmetries (16). During amphioxus embryogenesis, the mouth opens on the left side of the head, while the gill slits, which initiate opening at the ventral midline, later extend up the right side. Other endodermal organs are similarly asymmetric, and somites also show pronounced LR asymmetry. Cer, Nodal, Lefty, and Pitx are asymmetrically expressed in amphioxus, and pharmacological manipulation of the Alk4/5/7 Tgfβ receptor disrupts LR development, suggesting a function for Nodal in amphioxus LR asymmetry (17, 18). However, Nodal function has not been directly tested, and it is not known if Pitx regulates LR asymmetric morphology. The functions of Cer and Lefty are also untested, so it is not clear if and how they regulate Nodal signaling.
To test these regulatory interactions in amphioxus, we developed transcription activator-like effector nucleases (TALEN)-based methods of removing gene function and heat-shock promoter (HSP)-driven methods of overexpression and deployed them to address the function of all four genes. We show that right-sided Cer represses Nodal expression and find that when Cer function is impaired Nodal becomes bilateral. Nodal activates its own expression and also that of Lefty. Lefty feeds back to repress Nodal, restricting its expression to the left side. This results in left-sided activation of Pitx. We further show that these regulatory steps are essential for normal LR asymmetry to develop. The knockdown or overexpression of Cer, Nodal, Lefty, or Pitx results in embryos with either two phenotypic left sides or two phenotypic right sides, with bilateral duplication of most normally lateralized structures in each case. These data show the full regulatory cassette, with both inhibitors acting to restrict Nodal expression to the left side, is present in amphioxus and controls LR morphology. We conclude this pathway represents an ancient chordate character, with the full antagonist-based regulation of Nodal already present in the common ancestor of the chordates.
Results
Cer, Nodal, Lefty, and Pitx are known to be asymmetrically expressed in amphioxus embryos (18–21), however the relative timing of their expression has yet to be elucidated. We therefore examined whether these genes are spatiotemporally activated in patterns consistent with a regulatory hierarchy. Staged in situ hybridization demonstrated that right-sided Cer preceded lateral restriction of Nodal, Lefty, and Pitx (SI Appendix, Fig. S1). Furthermore, lateralized expression of Lefty and Pitx preceded that of Nodal (SI Appendix, Fig. S1).
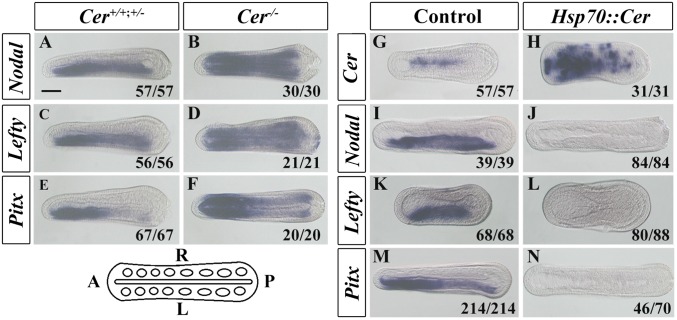
These temporal expression patterns are consistent with Cer regulating the lateralization of the other genes. To test this, we generated a TALEN knockout heterozygous Cer line (SI Appendix, Figs. S2 and S3). Crossing Cer+/− animals yielded Cer+/+, Cer+/−, and Cer−/− genotypes in the expected ratio (SI Appendix, Fig. S4), and we assessed the expression of Nodal, Lefty, and Pitx in these embryos. Although Cer+/− and wild-type embryos maintained wild-type expression, Cer−/− embryos showed bilateral expression of Nodal, Lefty, and Pitx (Fig. 1 A–F). This indicates Cer is necessary to restrict the expression of Nodal, Lefty, and Pitx to the left side of amphioxus embryos. To test whether Cer was also sufficient to regulate these genes, we induced overexpression of Cer using a heat-regulated HSP::Cer construct (SI Appendix, Fig. S5). This generated widespread ectopic Cer expression in 100% (31/31) of embryos (Fig. 1H) and abolished left-sided expression of Nodal, Lefty, and Pitx in 100% (84/84), 95% (80/88), and 66% (46/70) of embryos, respectively (Fig. 1 J, L, and N). Combined, these data show Cer is necessary and sufficient for regulating the lateral expression of Nodal, Lefty, and Pitx.
Fig. 1.
Cer regulates Nodal, Lefty, and Pitx expression in amphioxus. (A–F) Expression of Nodal, Lefty, and Pitx in Cer wild-type/heterozygous (Cer+/+, Cer+/−) or homozygous mutant (Cer−/−) embryos. (G–N) Expression of Cer, Nodal, Lefty, and Pitx in Control (uninjected but heat-shocked) and Cer misexpressed (Hsp70::Cer-injected and heat-shocked) embryos. Numbers at the bottom right of each panel indicate the number of times the phenotype shown was observed out of the total number of manipulated embryos. (Scale bar, 50 μm.) All embryos are in dorsal view with anterior to left as indicated in the sketch at the bottom left.
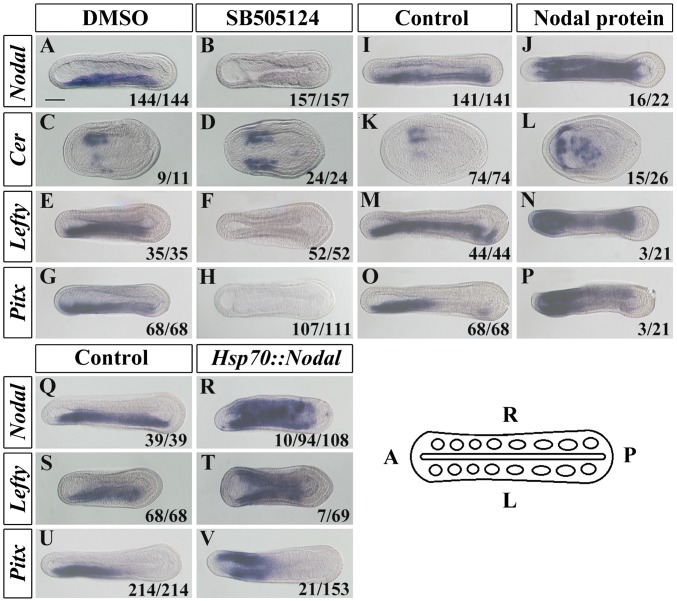
Nodal signaling is transduced by Tgfβ receptors of the Alk4/5/7 family (22). Pharmacological inhibition of Alk4/5/7 in amphioxus has been reported to block left-sided Nodal, Lefty, and Pitx expression (18), and we found the same in our experiments (Fig. 2 B, F, and H). However, the Alk4/5/7 receptor may also mediate signaling by other Tgfβ family ligands, including Activin and Tgfβ (23), raising uncertainty as to the ligand involved in its activation in amphioxus in vivo. Nodal in amphioxus also has an earlier developmental role (24), precluding its inhibition by the TALEN method as embryos would not develop to the stage where LR asymmetry can be addressed. We therefore tested the effect of inducible and ectopic Nodal on amphioxus LR asymmetry. Overexpression of Nodal using an HSP::Nodal construct (Fig. 2 R, T, and V and SI Appendix, Fig. S5), or administration of Nodal protein to developing embryos (Fig. 2 J, N, and P), induced ectopic right-sided expression of Nodal, Lefty, and Pitx. We also found that Nodal manipulation fed back on Cer expression, with Alk4/5/7 inhibition inducing ectopic left-sided Cer (as previously reported in ref. 18) and Nodal overexpression inhibiting Cer expression in the first somites (Fig. 2 D and L). These results confirm previous inference of the necessity of Nodal for lateralised Pitx expression in amphioxus, demonstrate that it is also sufficient, and further show that Nodal also negatively regulates Cer expression. Because we have shown that Cer inhibits Nodal expression, this suggests a negative feedback loop in which Nodal represses the expression of its own inhibitor.
Fig. 2.
Nodal regulates Cer, Nodal, Lefty, and Pitx expression in amphioxus. (A–H) Expression patterns of Nodal, Cer, Lefty and Pitx in control (DMSO) and Nodal pathway inhibitor (SB505124)-treated embryos. Embryos were treated from the midgastrula stage until they were collected for gene expression analysis (the 3-somite stage for Cer and around the 8-somite stage for other genes). (I–P) Expression patterns of Nodal, Cer, Lefty, and Pitx in Control (untreated) and Nodal protein-treated embryos. Embryos were cultured with 8 μg/mL of mouse recombinant Nodal protein for the same times as above. (Q–V) Expression patterns of Nodal, Lefty, and Pitx in control (uninjected but heat-shocked) and Nodal misexpressed (Hsp70::Nodal-injected and heat-shocked) embryos. Numbers at the bottom right of each panel indicate the number of times the phenotype shown was observed, out of the total number of embryos examined. (Scale bar, 50 μm.) All images are dorsal views with anterior to left as indicated in the sketch at the bottom right.
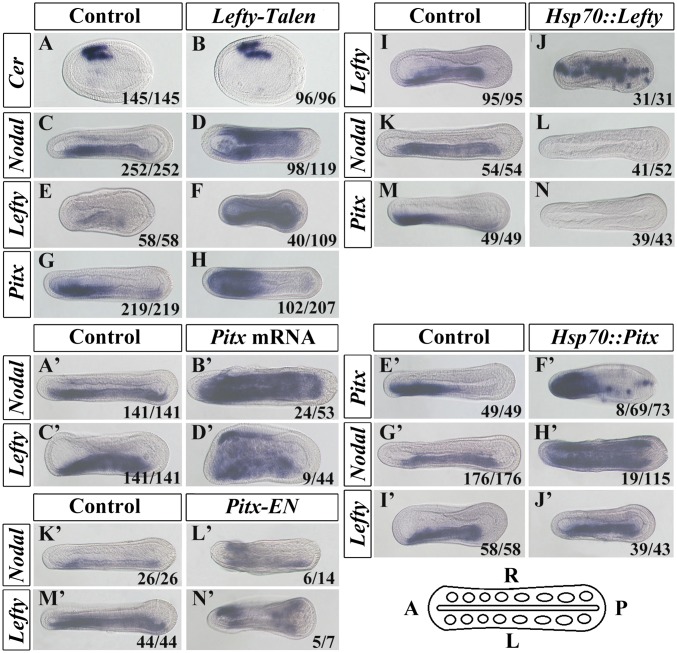
In vertebrate embryos, Lefty also acts as an extracellular inhibitor of Nodal protein, and experiments in an echinoderm suggest this might also be the case in this lineage (25). To test the function of Lefty in amphioxus, we generated embryos in which the Lefty gene was targeted by injection of a TALEN mRNA (SI Appendix, Fig. S6). In 82.4% (98/118) of injected embryos, Nodal expression became bilateral (Fig. 3D). Furthermore, Pitx and Lefty itself also became bilateral, in 49.3% (102/207) and 36.7% (40/109) of embryos, respectively (Fig. 3 H and F). To test the sufficiency of Lefty to inhibit normal left-sided gene expression, we also injected embryos with an inducible HSP::lefty construct (SI Appendix, Fig. S5). In the majority of heat-shocked embryos, left-sided expression of Nodal and Pitx was lost (Fig. 3 L and N). These data demonstrate that, in amphioxus, Lefty can act to inhibit Nodal expression. In the absence of Lefty, Nodal is ectopically expressed on the right-hand side (Fig. 3D). As Nodal up-regulates Lefty and Pitx (Fig. 2 N, P, T, and V), this probably in turn leads to the ectopic expression of Lefty and Pitx seen in Lefty TALEN-injected embryos (Fig. 3 F and H).
Fig. 3.
Lefty inhibits Nodal, Lefty, and Pitx expression, and Pitx overexpression or knockdown induces bilateral expression of Nodal and Lefty. (A–H) Expression patterns of Cer, Nodal, Lefty, and Pitx in control (uninjected) and Lefty TALEN mRNA-injected embryos. (I–N) Expression patterns of Nodal, Lefty, and Pitx in control (uninjected but heat-shocked) and Lefty misexpressed (Hsp70::Lefty-injected and heat-shocked) embryos. (A’–D’) Expression patterns of Nodal and Lefty in control (uninjected) and Pitx mRNA-injected embryos. (E’–J’) Expression patterns of Nodal, Lefty, and Pitx in control (uninjected but heat-shocked) and Pitx misexpressed (Hsp70::Pitx-injected and heat-shocked) embryos. (K’–N’) Expression patterns of Nodal and Lefty in control (uninjected) and Pitx–En mRNA-injected embryos. Numbers at the bottom right of each panel indicate the number of times the phenotype shown was observed, out of the total number of embryos examined. (Scale bar, 50 μm.) All images are dorsal views with anterior to left as indicated in the sketch at the bottom right.
Pitx homeobox genes are conserved targets of Nodal signaling in vertebrates (3), echinoderms (26), and probably also molluscs (5). In vertebrates, asymmetric Pitx2 is required for correct LR morphogenesis, but studies suggest that Pitx2 does not feed back to regulate Nodal expression (27). Although the experiments described above show that Cer, Lefty, and Nodal can regulate Pitx expression in amphioxus, they do not establish whether Pitx might also regulate their expression. To test this, we sought to up- and down-regulate Pitx expression, while monitoring its impact on the expression of Nodal and Lefty. Up-regulation of Pitx by mRNA injection caused significant disruption to early development, although many of the embryos that did survive through to the neurula stage and beyond displayed ectopic right-sided expression of Nodal (45.3%: 24/53) and Lefty (20.5%: 9/44) (Fig. 3 B’ and D’). To abrogate this early developmental impact of Pitx mRNA injection, we injected an inducible HSP::Pitx construct (SI Appendix, Fig. S5). In heat-induced HSP::Pitx-injected embryos, 16.5% (19/115) showed ectopic right-sided Nodal expression (Fig. 3H’). None showed ectopic Lefty expression (Fig. 3J’). To block Pitx function, we designed a fusion construct linking amphioxus Pitx DNA-binding domain to the Engrailed Repressor (EnR) domain (SI Appendix, Fig. S5) and injected the mRNA encoding this into amphioxus embryos. As with Pitx mRNA injection, many such embryos developed with significant abnormalities before the neurula stage, precluding their analysis for LR defects. However, in embryos that reached the neurula stage and displayed approximately normal AP and DV axial organization, ectopic activation of both Lefty and Nodal was observed on the right side in 71.4% (5/7) and 42.9% (6/14) of cases, respectively (Fig. 3 L’ and N’).
In some cases, LR asymmetry phenotypes derived from manipulating gene expression early in development might be explained by disturbed AP/DV organization, which in vertebrates may affect the midline and hence increase the incidence of laterality defects (28). Although this consideration might apply to embryos injected with Pitx mRNA or Pitx–EnR mRNA, which would be translated very early in development, the HSP::Pitx construct was only activated by heat shock after the midgastrula stage, and this also induced ectopic right-sided Nodal expression (Fig. 3H’). These data suggest that, unlike vertebrates, in amphioxus Pitx feeds back to regulate Nodal signaling.
The experiments described above show that Cer represses Nodal, that Nodal represses Cer and activates Lefty and Pitx, that Lefty represses Nodal, and that Pitx activates Nodal. An assumption is that left-sided Pitx will drive the development of left-sided morphology. To test this, we allowed embryos in which Cer, Nodal, Lefty, or Pitx expression had been manipulated to grow to the late embryonic or larval stages, so we could monitor the impact on the development of LR asymmetric characters.
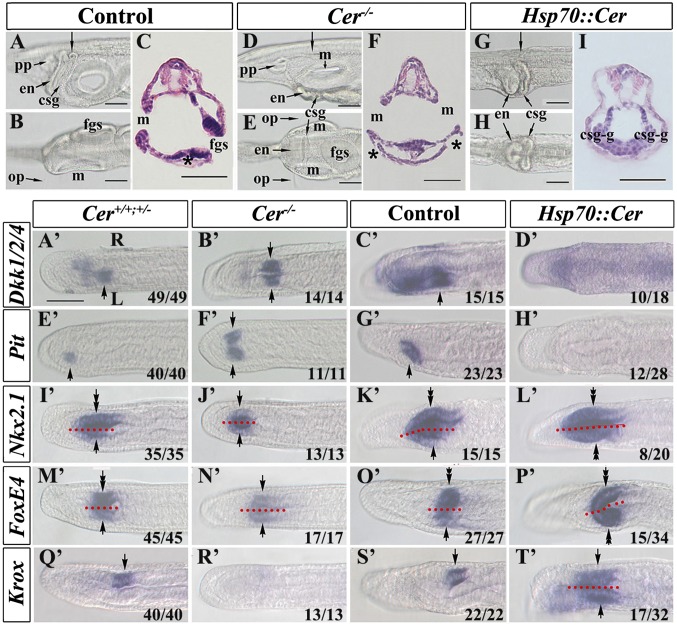
Amphioxus larvae are extremely asymmetric, with the mouth positioned on the left side of the head and the gill slits, which initiate opening at the ventral midline, expanding to spread up the right side. Several internal systems are also distinctly organized across the LR axis of the head and pharynx, including the club-shaped gland, preoral pit, and endostyle (Fig. 4 A–C). A suit of asymmetrically expressed marker genes (18) including Krox, FoxE4, Nkx2.1, Pit, and Dkk1/2/4 mark the LR displacement of these structures (Fig. 4 A’, E’, I’, M’, and Q’). To determine the impact of manipulating Cer expression on LR morphology, we allowed manipulated embryos to grow to the larval stage before analyzing morphology and LR marker gene expression. Cer−/− embryos develop a consistent two-left-side (2-left) phenotype, with a mouth opening on each side, duplication of the left-sided structures such as the preoral pit and proximal club-shaped gland, and loss of the right-sided distal club-shaped gland (Fig. 4 D–F and SI Appendix, Fig. S7). The gill slits, while still initiating and opening at the ventral midline, were also affected as they failed to extend up the right side (Fig. 4 D–F and SI Appendix, Fig. S7). Conversely, HSP::Cer larvae developed the reciprocal phenotype, with two–right-side (2-right) and concomitant duplication of most right-sided structures (Fig. 4 G–I and SI Appendix, Fig. S7). The gill slits were also affected: The first slit appeared to initiate at the ventral midline and like the Cer−/− phenotype failed to extend up either side, however unlike Cer−/− embryos, it also failed to properly open. LR marker gene expression precisely matched this morphology, confirming the duplication and loss of reciprocal structures in the 2-left and 2-right phenotypes (Fig. 4 A’–T’). We also allowed embryos in which Nodal or Lefty had been up- or down-regulated to grow to the larval stage. These manipulations also produced embryos with asymmetry defects, with Nodal overexpression and Lefty knockdown resulting in 2-left phenotypes and Nodal knockdown or Lefty overexpression resulting in 2-right phenotypes (SI Appendix, Fig. S7). As discussed above, injection of Pitx mRNA caused early developmental malformations, however a small number of such embryos did pass through to the larval stage and among them 6.1% exhibited 2-left phenotypes. Likewise, Pitx overexpression via the HSP::Pitx construct, which did not affect early development, also produced 2-left phenotypes in 10.2% of cases. No 2-right phenotypes were observed in either manipulation. Embryos injected with Pitx–EnR were severely malformed by the larval stage, and few pharyngeal structures could be discerned. However, in 7% of cases, paired bilateral mouths were observed, approximating the 2-left phenotype. This is consistent with the ectopic right-sided activation of Nodal in Pitx–EnR-injected embryos (Fig. 3 L’ and N’). These data indicate that the asymmetric expression of the Cer–Nodal–Lefty–Pitx cassette regulates the LR morphology of the larva in a predictable way: Any side on which Nodal–Pitx are activated becomes phenotypically left. If they are not activated, it becomes phenotypically right.
Fig. 4.
Cer mutation or misexpression affects development of amphioxus LR asymmetry and the expression of organ-specific marker genes. (A–C) Asymmetrical morphologies of control (wild-type, Cer+/−, or Hsp70::Cer-uninjected but heat-hocked) larvae. (A) Left lateral view of the pharyngeal region focused on the right side. (C) Transverse section of larva from A (arrow). (D–F) Symmetrical morphologies of Cer mutant larvae. (D) Left lateral view of the pharyngeal region focused on the sagittal plane. (E) Dorsal view of the pharyngeal region focused on the ventral side. (F) Transverse section of larva from D (arrow). (G–I) Symmetrical morphologies of Cer misexpressed larvae. (G) Left lateral view of the pharyngeal region focused on the sagittal plane. (H) Dorsal view of the pharyngeal region focused on the ventral side. (I) Transverse section of larva from G (arrow). (A’–D’) Expression of Dkk1/2/4 in the region destined to develop into the mouth opening (arrows). The expression domain is detected on the left side of Cer+/+ or Cer+/− siblings or control embryos and on both sides of Cer−/− mutant embryos but is lost in Cer misexpressed embryos. (E’–H’) Expression of Pit in the prospective preoral pit (arrows). Pit transcripts are detected on the left side of Cer+/+ or Cer+/− siblings or control embryos and on both sides of Cer−/− mutants but are undetectable in Cer misexpressed embryos. (I’–L’) Expression of Nkx2.1 in the prospective endostyle. Expression of Nkx2.1 in the endostyle can be divided into the left-side part (arrows) and the right-side part (tandem arrows) along the sagittal plane (indicated by red dotted lines). Although its expression in the left side is duplicated in Cer−/− mutants and lost in Cer misexpressed embryos, its expression in the right side is lost in Cer−/− mutants and duplicated in Cer misexpressed embryos. (M’–P’) Expression of FoxE4 in the prospective csg. The csg comprises two parts: the left-sided duct (arrows) and the right-sided glandular region (tandem arrows). FoxE4 expression in the duct is duplicated in Cer−/− mutants and lost in Cer misexpressed embryos, but its expression in the glandular region is duplicated in Cer misexpressed embryos and lost in Cer−/− mutants. (Q’–T’) Expression of Krox in the duct of the prospective csg (arrows). Its expression is lost in Cer−/− mutants but duplicated in Cer misexpressed embryos. Embryos in A’–T’ are in dorsal view with anterior to left. On A’, L marks the left side and R the right side, and this applies to all panels from A’ to T’. Numbers at the bottom right of each panel indicate the number of times the phenotype shown was observed, out of the total number of embryos examined. In some images, red dotted lines separate the left and right of the embryos along the midline. Genotypes as depicted in Fig. 1. Asterisks in C and F denote the external opening of the csg. csg, club-shaped gland; csg-g, right-sided glandular region of csg; en, endostyle; fgs, first gill slit; m, mouth; op, oral papillae; pp, preoral pit. (Scale bar, 50 μm.) The scale bar in A’ also applies to B’–T’.
Discussion
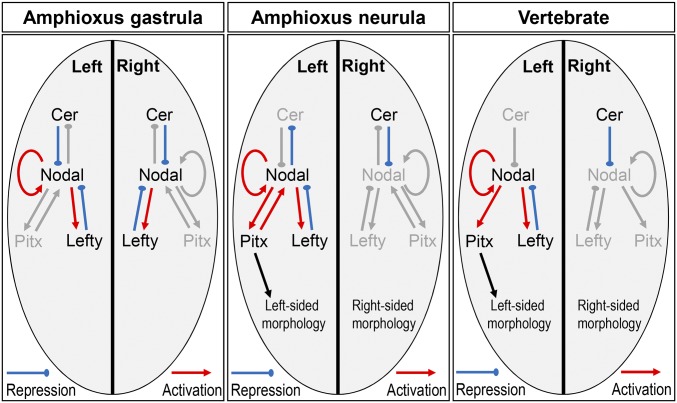
This study shows that the Cer–Nodal–Lefty–Pitx cassette functions in amphioxus to regulate LR asymmetry. Our data allow us to construct a regulatory model for the left and right side of amphioxus embryos (Fig. 5). The model suggests competitive feedback on the left side of the amphioxus embryo, with Nodal repressing the expression of its upstream repressor (Cer), while activating the expression of its downstream repressor (Lefty) and activator (Pitx). In vertebrates, both Cer and Lefty repress Nodal signaling by directly binding to Nodal protein, whereas Nodal up-regulates its own expression and that of Lefty (4, 15). Thus, in both lineages, the establishment of LR identify is dependent on the appropriate regulation of Nodal via positive and negative feedback mediated by extracellular factors. Their focus is establishing asymmetric Pitx as the downstream effector, and disruption of this process in amphioxus or vertebrates leads to embryos with disrupted LR asymmetry. The morphological outcome in amphioxus is predictable based on the gene expression; essentially, if Pitx expression is activated on a side of the embryo, it will develop left-sided morphology, whereas if Pitx is absent, right-sided morphology will develop. Manipulations that yield bilateral Pitx distributions generate unviable embryos with 2–left- or 2–right-sided organization, with duplication of the respective pharyngeal and endodermal structures including the mouth, endostyle, and club-shaped gland. These morphologies show that, once handedness is established in amphioxus, the two sides develop independently and autonomously according to their gene expression and are not noticeably influenced by what is happening on the other side of the embryo.
Fig. 5.
A gene regulatory network model for the control of LR asymmetry in amphioxus. At the gastrula stage, bilateral expression of Cer, Nodal, and Lefty are observed, but Pitx is not expressed, indicating Nodal regulation of Pitx is blocked. During the late gastrula and early neurula stages, Cer expression is maintained on the right side of the embryo. Cer continues to represses Nodal expression, and as a consequence, downstream activation of Nodal and Lefty is lost, and Pitx is not activated. In the absence of Pitx, right-sided morphology develops. On the left of the embryo, Cer expression is lost during the late gastrula to early neurula stage. Nodal is therefore not inhibited and can activate the expression of itself, Pitx, and Lefty. Left-sided Pitx expression results, and hence left-sided morphology develops. An equivalent diagram summarizing the interactions in vertebrates is shown for comparison.
Although the core Cer–Nodal–Lefty–Pitx pathway appears conserved between amphioxus and vertebrates, we also note a difference: In amphioxus, Pitx feeds back on the regulation of Nodal expression. Our model (Fig. 5) suggests that in amphioxus the propagation of “leftness” downstream of asymmetric Nodal may therefore depend on competition between positive feedback from Pitx and negative feedback from Lefty. This regulatory link is not seen in vertebrates or echinoderms (where Nodal regulation of Pitx expression has been demonstrated) (26), suggesting it may be an amphioxus novelty.
There are also some aspects of amphioxus morphology on which our work sheds light. The amphioxus mouth is unusual in that it opens on the left side of the head, rather than the midline as in most animal lineages, including vertebrates. This has led to speculation that the amphioxus mouth is a derived structure, possibly evolving from the equivalent of a left-sided coelomic pore and not homologous to mouths in other chordates (17). Our demonstration of its dependence on the Cer–Nodal–Lefty–Pitx pathway supports this theory, as the mouth is regulated as a left-sided rather than a midline structure.
Amphioxus gill slits are also unusual. The first gill slit openings, known as the primary gill slits, appear first at the ventral midline and then extend only up the right side of the head (29). Through larval development, gill slits are hence right-sided only. However, at metamorphosis, another set of gill slit openings appear, known as the secondary gill slits. These initiate also on the right side of the larva, dorsal to the primary slits. As these secondary slits extend, the primary slits move ventrally and eventually come to lie on the left side of the head, such that the final adult morphology has symmetrical gill slits. This has led to an assumption, dating from the 1800s, that the slits that lie on the right side of the larval head are in fact homologous to the left-sided slits in other deuterostomes (30). In our experiments, the primary slits initiate but fail to extend in 2-left embryos or to open at all in 2-right embryos. That they initiate is consistent with their positioning at the ventral midline, suggesting this does not require LR axial information. Failure to extend in either sort of embryo suggests subsequent morphogenesis requires both left- and right-sided information. Furthermore, that the slits develop further in 2-left embryos than in 2-right embryos is in keeping with the historical view that the primary slits are, primitively, left-sided structures.
We do not know what breaks symmetry in amphioxus. Studies in some vertebrates, in the urochordate Halocynthia, and in echinoderms suggest a role for cilia in symmetry breaking (31–33). Although the amphioxus embryo is heavily ciliated at the stages at which the model suggests symmetry is broken, as yet there is no evidence for a functional role for cilia in amphioxus. We consider the timing of expression and regulatory interactions consistent with the proposal by Blum et al. for a cilia-based mechanism localized to the gastrocoel roof (31). Whatever the symmetry-breaking mechanism, our data suggest it might work in two ways. Symmetric Cer precedes asymmetric right-sided Cer. This in turn precedes asymmetric Nodal expression, but asymmetric Lefty and Pitx expression are detectable before asymmetric Nodal expression yet are regulated by Nodal. Thus, the symmetry-breaking mechanism might act to repress Cer expression on the left, allowing Nodal protein to activate Lefty and Pitx. Nodal autoregulates, explaining the lag in asymmetric Nodal expression comparted to Lefty and Pitx. Under this model, Nodal negative feedback on Cer would act to lock in this state. We do not know precisely how Nodal feeds back on Cer, although inhibition of the Alk4/5/7 receptor shows it works through this pathway.
The second possibility is that, as Nodal feeds back negatively on Cer, the symmetry-breaking mechanism might act to liberate Nodal from Cer repression. Because Cer represses Nodal by direct protein–protein binding (15), this could be via some additional extracellular factor preventing or disrupting this interaction. Under this model, Nodal would then repress Cer and regulate Lefty and Pitx as above. Although compatible with much of our data, this does not easily account for the early onset of asymmetric Cer compared with Lefty and Pitx. It is also not how the system is thought to work in vertebrates (Fig. 5), where there is so far no evidence for Nodal feeding back on Cer. We hence consider it a less parsimonious explanation.
Our study shows that full complexity of the Cer–Nodal–Lefty–Pitx cassette was established by the common ancestor of the chordates and inherited by their vertebrate descendants. Pitx and Nodal are also involved in LR specification in molluscs (5) and may be asymmetrically expressed in some other lophotrochozoans (7, 8), leading to speculation that the role of Nodal signaling in LR development was acquired early in animal evolution. However, the roles of Cer and Lefty may be more recent innovations. Cer is a member of the larger Cer/DAN/Gremlin family of Tgfβ signaling regulators. Clear Cer genes have only been identified in chordates, although one study suggests they may be older and lost by many lineages (9). Lefty is a divergent member of the Tgfβ family. It is so far found only in chordates and ambulacrarians and in the latter does function in LR asymmetry (9, 25). Therefore, it is unlikely that Cer and Lefty are more broadly involved in LR asymmetry across protostomes, and we suggest their incorporations into regulation of LR asymmetry are innovations of chordates and deuterostomes, respectively, with negative feedback providing more nuanced control of Nodal signaling in these lineages.
Materials and Methods
Animal and Embryo Cultivation and Embryo Microinjection.
Amphioxus (Branchiostoma floridae) were originally acquired from Jr-Kai Yu, Institute of Cellular and Organismic Biology, Academia Sinica, Taipei, Taiwan, and the colony was maintained under previously described conditions (34, 35). Gametes were obtained using the thermal-shock method (from 20 °C to 26 °C) (34). Fertilization and subsequent culturing of the embryos were carried out essentially as described previously (36) at 27–28 °C unless otherwise stated. Injection solution was prepared containing 20% (vol/vol) glycerol, 5 mg/mL Texas Red dextran (Life Technology Co.), and 0.25 μg/μL plasmid DNA or 0.15–1.5 μg/μL synthesized mRNA. Embryo microinjection was performed as previously described (36).
Larval Cultivation and Detection of Cer Mutations in Founder and F1 Amphioxus.
Cer TALEN mRNA-injected embryos and larvae were reared at 24 °C and fed with unicellular algae Dicrateria zhanjiangensis, and juveniles and adults were reared in a same way as a previous description (34, 35). Each F0 founder was crossed with a wild-type animal, respectively. About 30–50 embryos from each cross were collected and lysed with Animal Tissue Direct PCR Kit (Foregene Co.) for mutation efficiency and mutation site analysis (34). Progeny of crosses carrying frame-shift mutations were maintained. F1 genotypes were determined in a similar way as described for F0 by cutting a tiny tip of the tail of each animal. The F1 animals carrying identical frame-shift mutations were maintained and intercrossed to generate F2 generation. The F2 embryos were fixed at different developmental stages with 4% (wt/vol) PFA–Mops–EGTA for in situ hybridization analysis or morphological examination. Animal husbandry and mutation detection are described in detail in SI Appendix, SI Materials and Methods.
Plasmid Construct Preparation.
The full length of coding sequences of Cer, Nodal, Lefty, and Pitx genes was amplified from a gastrula cDNA library and then ligated into a pGEM-T-Easy vector (Promega Co.) separately. Further, they were recombined into expression vectors pXT7 or P1. The P1 is a construct initially derived from vector pAcGFP1-1 (Clontech Co.) by integrating a 790-bp regulatory region of B. belcheri Hsp70 gene upstream of the GFP coding sequence (37). A Pitx–EN-dominant negative construct was made by fusing the Pitx DNA-binding domain and the Drosophila EnR domain in the pXT7 vector. TALENs targeting Cer and Lefty genes were designed and assembled as previously described (38). TALEN binding sites and primer sequences used for cloning and TALEN efficiency assay are shown in SI Appendix, Tables S1–S5. The details for the preparation are described in SI Appendix, SI Materials and Methods.
In Vitro Synthesized mRNA and Plasmid DNA Preparation.
All plasmid DNAs were prepared using Plasmid Mini Kit (Omega Co.) and linearized with either BamHI (for pXT7-derived constructs) or SacI (for TALEN constructs), extracted by phenol-chloroform, and dissolved in RNase-free water. In vitro mRNA synthesis was conducted using either T7 (for pXT7-derived constructs) or T3 (for TALEN constructs) mMESSAGE mMACHINE kit (Ambion Co.).
Heat-Shock Experiment.
Embryos injected with Hsp70::Cer, Hsp70::Nodal, Hsp70::Lefty, or Hsp70::Pitx DNA plasmids were raised at 25 °C, and heat-shocked at 34 °C in a water bath for 30 min at the early gastrula stage. Uninjected embryos at the same stage were also heat-shocked and used as controls. After heat shock, the embryos were returned to 25 °C and allowed to develop until they were fixed with 4% (wt/vol) PFA–Mops–EGTA at desired stages for in situ or morphological analysis.
SB505124 and Nodal Protein Treatment and in Situ Hybridization.
Embryos were treated with 50 μM SB505124 (Sigma Co.) or 8 μg/mL recombinant mouse Nodal protein (R&D Co.) in four-well dishes [coated with 1.5% (wt/vol) agarose] from early gastrula, and then were fixed at required stages for in situ hybridization or cultured continuously for morphological observation. The experiments of in situ hybridization were performed essentially according to a previous description (39). All details are described in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Jr-Kai Yu for providing B. floridae animals, and Dr. Dominic Norris for comments on the manuscript. This work was supported by National Natural Science Foundation of China Grants 31471986, 31372188, and 31672246 and Fundamental Research Funds for the Central Universities of China Grant 20720160056.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620519114/-/DCSupplemental.
References
- 1.Palmer AR. Symmetry breaking and the evolution of development. Science. 2004;306(5697):828–833. doi: 10.1126/science.1103707. [DOI] [PubMed] [Google Scholar]
- 2.Bisgrove BW, Morelli SH, Yost HJ. Genetics of human laterality disorders: Insights from vertebrate model systems. Annu Rev Genomics Hum Genet. 2003;4:1–32. doi: 10.1146/annurev.genom.4.070802.110428. [DOI] [PubMed] [Google Scholar]
- 3.Vandenberg LN, Levin M. A unified model for left-right asymmetry? Comparison and synthesis of molecular models of embryonic laterality. Dev Biol. 2013;379(1):1–15. doi: 10.1016/j.ydbio.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller P, et al. Differential diffusivity of Nodal and Lefty underlies a reaction-diffusion patterning system. Science. 2012;336(6082):721–724. doi: 10.1126/science.1221920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grande C, Patel NH. Nodal signalling is involved in left-right asymmetry in snails. Nature. 2009;457(7232):1007–1011. doi: 10.1038/nature07603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuroda R, Endo B, Abe M, Shimizu M. Chiral blastomere arrangement dictates zygotic left-right asymmetry pathway in snails. Nature. 2009;462(7274):790–794. doi: 10.1038/nature08597. [DOI] [PubMed] [Google Scholar]
- 7.Grande C, Martín-Durán JM, Kenny NJ, Truchado-García M, Hejnol A. Evolution, divergence and loss of the Nodal signalling pathway: New data and a synthesis across the Bilateria. Int J Dev Biol. 2014;58(6-8):521–532. doi: 10.1387/ijdb.140133cg. [DOI] [PubMed] [Google Scholar]
- 8.Martin-Duran JM, Vellutini BC, Hejnol A. Embryonic chirality and the evolution of spiralian left-right asymmetries. Philos Trans R Soc Lond B Biol Sci. 2016;371(1710):20150411. doi: 10.1098/rstb.2015.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenny NJ, et al. The Lophotrochozoan TGF-β signalling cassette—Diversification and conservation in a key signalling pathway. Int J Dev Biol. 2014;58(6-8):533–549. doi: 10.1387/ijdb.140080nk. [DOI] [PubMed] [Google Scholar]
- 10.Vorbrüggen G, et al. Embryonic expression and characterization of a Ptx1 homolog in Drosophila. Mech Dev. 1997;68(1-2):139–147. doi: 10.1016/s0925-4773(97)00139-1. [DOI] [PubMed] [Google Scholar]
- 11.Westmoreland JJ, McEwen J, Moore BA, Jin Y, Condie BG. Conserved function of Caenorhabditis elegans UNC-30 and mouse Pitx2 in controlling GABAergic neuron differentiation. J Neurosci. 2001;21(17):6810–6819. doi: 10.1523/JNEUROSCI.21-17-06810.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Currie KW, Pearson BJ. Transcription factors lhx1/5-1 and pitx are required for the maintenance and regeneration of serotonergic neurons in planarians. Development. 2013;140(17):3577–3588. doi: 10.1242/dev.098590. [DOI] [PubMed] [Google Scholar]
- 13.März M, Seebeck F, Bartscherer K. A Pitx transcription factor controls the establishment and maintenance of the serotonergic lineage in planarians. Development. 2013;140(22):4499–4509. doi: 10.1242/dev.100081. [DOI] [PubMed] [Google Scholar]
- 14.Namigai EK, Kenny NJ, Shimeld SM. Right across the tree of life: The evolution of left-right asymmetry in the Bilateria. Genesis. 2014;52(6):458–470. doi: 10.1002/dvg.22748. [DOI] [PubMed] [Google Scholar]
- 15.Piccolo S, et al. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397(6721):707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boorman CJ, Shimeld SM. The evolution of left-right asymmetry in chordates. BioEssays. 2002;24(11):1004–1011. doi: 10.1002/bies.10171. [DOI] [PubMed] [Google Scholar]
- 17.Kaji T, Reimer JD, Morov AR, Kuratani S, Yasui K. Amphioxus mouth after dorso-ventral inversion. Zoological Lett. 2016;2:2. doi: 10.1186/s40851-016-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soukup V, et al. The Nodal signaling pathway controls left-right asymmetric development in amphioxus. Evodevo. 2015;6:5. doi: 10.1186/2041-9139-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onai T, Yu JK, Blitz IL, Cho KW, Holland LZ. Opposing Nodal/Vg1 and BMP signals mediate axial patterning in embryos of the basal chordate amphioxus. Dev Biol. 2010;344(1):377–389. doi: 10.1016/j.ydbio.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasui K, Zhang S, Uemura M, Saiga H. Left-right asymmetric expression of BbPtx, a Ptx-related gene, in a lancelet species and the developmental left-sidedness in deuterostomes. Development. 2000;127(1):187–195. doi: 10.1242/dev.127.1.187. [DOI] [PubMed] [Google Scholar]
- 21.Yu JK, Holland LZ, Holland ND. An amphioxus nodal gene (AmphiNodal) with early symmetrical expression in the organizer and mesoderm and later asymmetrical expression associated with left-right axis formation. Evol Dev. 2002;4(6):418–425. doi: 10.1046/j.1525-142x.2002.02030.x. [DOI] [PubMed] [Google Scholar]
- 22.Reissmann E, et al. The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev. 2001;15(15):2010–2022. doi: 10.1101/gad.201801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136(22):3699–3714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- 24.Yu JK, et al. Axial patterning in cephalochordates and the evolution of the organizer. Nature. 2007;445(7128):613–617. doi: 10.1038/nature05472. [DOI] [PubMed] [Google Scholar]
- 25.Duboc V, Lapraz F, Besnardeau L, Lepage T. Lefty acts as an essential modulator of Nodal activity during sea urchin oral-aboral axis formation. Dev Biol. 2008;320(1):49–59. doi: 10.1016/j.ydbio.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Duboc V, Röttinger E, Lapraz F, Besnardeau L, Lepage T. Left-right asymmetry in the sea urchin embryo is regulated by nodal signaling on the right side. Dev Cell. 2005;9(1):147–158. doi: 10.1016/j.devcel.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Ryan AK, et al. Pitx2 determines left-right asymmetry of internal organs in vertebrates. Nature. 1998;394(6693):545–551. doi: 10.1038/29004. [DOI] [PubMed] [Google Scholar]
- 28.Roessler E, Muenke M. Midline and laterality defects: Left and right meet in the middle. BioEssays. 2001;23(10):888–900. doi: 10.1002/bies.1130. [DOI] [PubMed] [Google Scholar]
- 29.Hatschek B. Studien über entwicklung des amphioxus. Arbeiten aus den Zoologischen Instituten der Universität Wien und der Zoologischen Station in Triest. 1882;4(1):1–88. [Google Scholar]
- 30.Willey A. Amphioxus and the Ancestry of the Vertebrates. Macmillan and Co.; New York: 1894. [Google Scholar]
- 31.Blum M, Feistel K, Thumberger T, Schweickert A. The evolution and conservation of left-right patterning mechanisms. Development. 2014;141(8):1603–1613. doi: 10.1242/dev.100560. [DOI] [PubMed] [Google Scholar]
- 32.Nishide K, Mugitani M, Kumano G, Nishida H. Neurula rotation determines left-right asymmetry in ascidian tadpole larvae. Development. 2012;139(8):1467–1475. doi: 10.1242/dev.076083. [DOI] [PubMed] [Google Scholar]
- 33.Tisler M, et al. Cilia are required for asymmetric nodal induction in the sea urchin embryo. BMC Dev Biol. 2016;16(1):28. doi: 10.1186/s12861-016-0128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li G, Shu Z, Wang Y. Year-round reproduction and induced spawning of Chinese amphioxus, Branchiostoma belcheri, in laboratory. PLoS One. 2013;8(9):e75461. doi: 10.1371/journal.pone.0075461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li G, Yang X, Shu Z, Chen X, Wang Y. Consecutive spawnings of Chinese amphioxus, Branchiostoma belcheri, in captivity. PLoS One. 2012;7(12):e50838. doi: 10.1371/journal.pone.0050838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Li G, Feng J, Yang X, Wang YQ. An efficient microinjection method for unfertilized eggs of Asian amphioxus Branchiostoma belcheri. Dev Genes Evol. 2013;223(4):269–278. doi: 10.1007/s00427-013-0441-0. [DOI] [PubMed] [Google Scholar]
- 37.Li D, et al. Isolation and functional analysis of the promoter of the amphioxus Hsp70a gene. Gene. 2012;510(1):39–46. doi: 10.1016/j.gene.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 38.Li G, et al. Mutagenesis at specific genomic loci of amphioxus Branchiostoma belcheri using TALEN method. J Genet Genomics. 2014;41(4):215–219. doi: 10.1016/j.jgg.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu JK, Holland LZ. Amphioxus whole-mount in situ hybridization. Cold Spring Harb Protoc. 2009;2009(9):pdb.prot5286. doi: 10.1101/pdb.prot5286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.