Ischemic stroke—that is, cerebral infarction causing stroke symptoms—is pathophysiologically and phenotypically heterogeneous. To address this heterogeneity, investigators have often chosen to study the genetics of ischemic stroke according to its less-heterogeneous phenotypes. Many systems have been developed and deployed for classifying ischemic stroke into subphenotypes. In pursuit of gene discovery, Malik et al. (1) in PNAS use the popular Trial of Org 10172 Acute Stroke Treatment (TOAST) classification system, which divides ischemic stroke into five main subphenotypes: large artery stroke (LAS), small artery stroke, cardioembolic stroke, stroke of undetermined etiology, and stroke of other defined etiology (rare causes, like arterial dissection). LAS remains a substantial public health problem. Population-based epidemiologic studies have shown that TOAST-defined LAS accounts for about 15% of all ischemic stroke cases (2). Patients with LAS tend to have poorer poststroke outcomes at 3 and 6 mo (3). In young and middle-aged stroke patients, LAS has the highest rates of recurrent stroke and death of any other stroke subtype (4).
A patient with ischemic stroke is classified as having LAS if the cerebral infarct occurs in a perfusion zone distal to blood flow-restricting atherosclerotic stenosis and there is no obvious alternative explanation for the infarct like untreated atrial fibrillation. In medical practice, patients are diagnosed with LAS based on findings of infarction on computed tomography or magnetic resonance (MR) imaging and findings of high-grade (>50% luminal reduction) stenosis on carotid ultrasonography, computed tomography angiography, MR angiography, or catheter-based angiography. Sources of potential cardioembolism are typically excluded based on findings of echocardiography and prolonged electrocardiography. Because of variability in the practice of evaluating patients with stroke and of interpreting test results, the reliability of diagnosing TOAST LAS is only modest. More elaborate web-based algorithmic approaches, like the Causative Classification of Stroke system that was used in the Stroke Genetics Network, reduce, but do not eliminate, the problem of limited reliability (5).
Beyond issues of reliability, there are also concerns about the validity of diagnosing LAS. Presently, atherosclerosis localized to large arteries in the cervical or intracranial circulation is considered causal if it results in high-grade stenosis. In patients who have had stroke or transient ischemic attack in the territory of a carotid artery, the risk of subsequent ipsilateral ischemic stroke is directly proportional to the degree of luminal reduction. Further supporting a causal relationship is the observation that in randomized clinical trials the degree of stroke prevention from carotid endarterectomy is directly related to the degree of carotid stenosis (6). However, degree of stenosis tells only part of the story regarding the relationship between atherosclerosis and stroke. Recent studies using MR-based plaque imaging show that carotid plaque not causing high-grade stenosis (so-called nonstenotic plaque) can also be associated with ischemic stroke. For example, in a study of patients with recent ischemic stroke, carotid intraplaque hemorrhage was a risk factor for recurrent ischemic stroke in individuals with nonstenotic plaque (7). Other plaque characteristics that can be assessed in vivo in stroke patients and that also suggest association with recurrent stroke include fibrotic cap and lipid-rich core. It is becoming increasingly evident that patients diagnosed with LAS and patients diagnosed without LAS are subject to misclassification beyond the intrinsic error rates associated with clinical diagnosis. For some patients with ischemic stroke and high-grade stenosis, the plaque is not causal; and for some patients with ischemic stroke and nonstenotic plaque, the plaque is causal.
Impressively, despite concerns regarding the reliability and validity of diagnosing LAS, in PNAS Malik et al. (1) are able to identify a novel genetic risk factor. To accomplish this, they partnered widely and used a transethnic approach. This approach to risk locus discovery is gaining popularity. The first genomic approach to discovering stroke risk factors was performed in an Icelandic population, taking advantage of a nationwide DNA repository and unparalleled genealogic records (8). Subsequently, the first wave of genome-wide association studies was largely performed in populations exclusively of European ancestry (9). The focus on European populations emerged more out of convenience than conviction that this would be the optimal approach to risk locus discovery. Transethnic meta-analysis of genomic data offers the promise of increased statistical power and improved resolution of fine mapping of loci by taking advantage of differences in local linkage disequilibrium (10). In PNAS, Malik et al. (1) succeed in identifying a new risk locus for LAS using a transethnic exome-wide meta-analysis. Results in European ancestry populations were systematically combined using a random effects model with results in a South Asian population. Even greater population diversity, particularly including individuals of African ancestry, might lead to additional discoveries. Simulation studies suggest that inclusion of African ancestry samples in genome-wide meta-analyses would lead to marked improvement in fine mapping of loci over European-only ancestry meta-analyses (11).
Malik et al. (1) find that a variant in the 3′ untranslated region of the histone-deacetylase 9 (HDAC9) (rs2023938) increased the risk of LAS by 28% [odds ratio (OR) 1.28; 95% CI, 1.16–1.40]. This finding confirms prior work, as the locus of exome-wide significance in the current study is in tight linkage disequilibrium with other loci previously significantly associated with LAS. Others have found genetic association of variants in the HDAC9 locus with LAS in populations of European (12) and Chinese (13) decent. In addition to stroke, HDAC9 variants have also been associated with coronary (14) and peripheral artery (15) disease. HDAC9 variants appear to promote atherosclerosis via increased expression (16). Inhibitors of HDAC9 may represent a new class of drugs targeting prevention of symptomatic atherosclerotic diseases, including ischemic stroke and coronary and peripheral artery disease.
The association of coding variants in alpha-1 antitrypsin (AAT) and disease has been recognized for over half a century. AAT primarily inhibits neutrophil elastase, which is released during active inflammation and leads to breakdown of elastase as part of host defense. Left unchecked, neutrophil elastase has deleterious effects. Patients with the genetic disorder known as AAT deficiency have destruction of lung elastase, leading to emphysema. Now Malik et al. (1) show that a common coding variant [M1 (A213V)] in the gene (serpin family A member 1, SERPINA1) encoding AAT, which is not responsible for AAT deficiency, increases the risk of LAS by 22% (OR, 1.22; 95% CI, 1.13–1.31). The association of the M1 variant and LAS must have come as something of a surprise to investigators, as previous studies showed M1 not to affect circulating levels of AAT or in vitro enzymatic activity in lipid-free buffers. Undeterred, investigators conducted a series of experiments showing that the risk allele appears to alter the function of AAT. Specifically,
In PNAS, Malik et al. succeed in identifying a new risk locus for LAS using a transethnic exome-wide meta-analysis.
investigators found that M1 variation affects binding of AAT to neutrophil elastase in lipid-rich plasma but not expression in atherosclerotic plaque.
Cigarette smoke produces hydrogen peroxide, which can oxidize methionine residues 351 and methionine 358 of AAT, reducing its inhibitory effect on neutrophil elastase, providing a mechanism explaining how cigarette smoking may cause emphysema (17). Knowing that cigarette smoking is a risk factor for stroke, that it can alter AAT, and that a variant in AAT is a risk factor for ischemic stroke, it would be of interest to formally test for gene–environment interactions in future studies.
Finding a relationship between AAT and LAS raises the possibility that therapies designed for AAT deficiency might have an antiatherosclerosis effect in patients with the M1 risk variant and, possibly, even in patients without the risk variant. However, no practical and highly efficacious therapy has been developed for AAT deficiency that would be appropriate to test as a stroke prevention therapy. Intravenous enzyme augmentation is Food and Drug Administration-approved for treating AAT deficiency, but the treatment does not appear to have dramatic effects on hard clinical endpoints (18). Gene therapy using adeno-associated viral vectors has been tried but has not achieved protective levels of AAT (19).
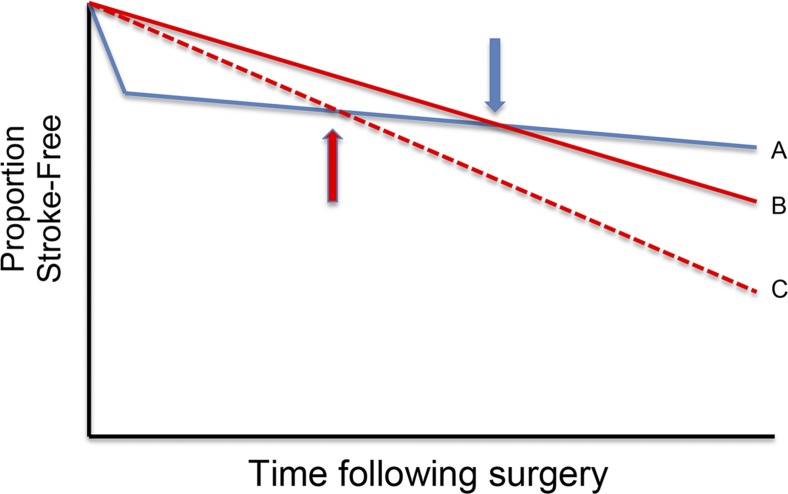
Advances in LAS genetics might help to develop a test for selection of the most appropriate patients for carotid revascularization. Carotid endarterectomy has been shown to reduce the risk of stroke in patients with asymptomatic high-grade carotid atherosclerotic stenosis (20, 21). However, the US Preventative Services Task Force recommends against screening for its presence (22). This recommendation is because of the actuarial reality that the absolute risk of stroke in properly medically managed patients with asymptomatic carotid stenosis can be 1% or even lower (23). A practical way of stratifying risk among patients with asymptomatic carotid stenosis might substantially improve the public health impact of endarterectomy (Fig. 1). It is possible that testing for variants for HDAC9, SERPIN1A, and as yet undiscovered others might be able to serve this function of risk stratification. This would go a long way toward fulfilling the promise of individualizing medical care to optimize stroke prevention.
Fig. 1.
Survival plot of patients with high-grade asymptomatic carotid stenosis undergoing endarterectomy (curve A) and not undergoing endarterectomy (curves B and C). The Asymptomatic Cartoid Atherosclerosis Study found that patients with high-grade asymptomatic carotid stenosis had an up-front risk of stroke and death related to the procedure that was made up for by lower subsequent risk of ipsilateral stroke such that, around 10 months (blue arrow), patients began to have a net benefit relative to the medical management group (gap between A and B). If a subset of patients could be identified to be at high risk of stroke by the use of genetic or other biomarkers (curve C), the crossover point following endarterectomy could be shifted leftward (red arrow) and a greater net benefit could accumulate faster.
Footnotes
The author declares no conflict of interest.
See companion article on page 3613.
References
- 1.Malik R, et al. Common coding variant in SERPINA1 increases the risk for large artery stroke. Proc Natl Acad Sci USA. 2017;114:3613–3618. doi: 10.1073/pnas.1616301114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: Incidence, recurrence, and long-term survival in ischemic stroke subtypes: A population-based study. Stroke. 2001;32:2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 3.Murat Sumer M, Erturk O. Ischemic stroke subtypes: Risk factors, functional outcome and recurrence. Neurol Sci. 2002;22:449–454. doi: 10.1007/s100720200004. [DOI] [PubMed] [Google Scholar]
- 4.Redfors P, et al. Stroke subtype predicts outcome in young and middle-aged stroke sufferers. Acta Neurol Scand. 2012;126:329–335. doi: 10.1111/j.1600-0404.2012.01653.x. [DOI] [PubMed] [Google Scholar]
- 5.McArdle PF, et al. NINDS SiGN Study Agreement between TOAST and CCS ischemic stroke classification: The NINDS SiGN study. Neurology. 2014;83:1653–1660. doi: 10.1212/WNL.0000000000000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnett HJ, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–1425. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 7.Gupta A, et al. Magnetic resonance angiography detection of abnormal carotid artery plaque in patients with cryptogenic stroke. J Am Heart Assoc. 2015;4:e002012. doi: 10.1161/JAHA.115.002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gretarsdottir S, et al. Localization of a susceptibility gene for common forms of stroke to 5q12. Am J Hum Genet. 2002;70:593–603. doi: 10.1086/339252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellenguez C, et al. International Stroke Genetics Consortium (ISGC); Wellcome Trust Case Control Consortium 2 (WTCCC2) Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischemic stroke. Nat Genet. 2012;44:328–333. doi: 10.1038/ng.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris AP. Transethnic meta-analysis of genomewide association studies. Genet Epidemiol. 2011;35:809–822. doi: 10.1002/gepi.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asimit JL, Hatzikotoulas K, McCarthy M, Morris AP, Zeggini E. Trans-ethnic study design approaches for fine-mapping. Eur J Hum Genet. 2016;24:1330–1336. doi: 10.1038/ejhg.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traylor M, et al. Australian Stroke Genetics Collaborative, Wellcome Trust Case Control Consortium 2 (WTCCC2); International Stroke Genetics Consortium Genetic risk factors for ischaemic stroke and its subtypes (the METASTROKE collaboration): A meta-analysis of genome-wide association studies. Lancet Neurol. 2012;11:951–962. doi: 10.1016/S1474-4422(12)70234-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Y, et al. HDAC9 gene is associated with stroke risk in a Chinese population. Exp Biol Med (Maywood) 2013;238:842–847. doi: 10.1177/1535370213494650. [DOI] [PubMed] [Google Scholar]
- 14.Dichgans M, et al. METASTROKE Consortium; CARDIoGRAM Consortium; C4D Consortium; International Stroke Genetics Consortium Shared genetic susceptibility to ischemic stroke and coronary artery disease: A genome-wide analysis of common variants. Stroke. 2014;45:24–36. doi: 10.1161/STROKEAHA.113.002707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsukura M, et al. Genome-wide association study of peripheral arterial disease in a Japanese population. PLoS One. 2015;10:e0139262. doi: 10.1371/journal.pone.0139262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markus HS, et al. Evidence HDAC9 genetic variant associated with ischemic stroke increases risk via promoting carotid atherosclerosis. Stroke. 2013;44:1220–1225. doi: 10.1161/STROKEAHA.111.000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taggart C, et al. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J Biol Chem. 2000;275:27258–27265. doi: 10.1074/jbc.M004850200. [DOI] [PubMed] [Google Scholar]
- 18.Tonelli AR, Brantly ML. Augmentation therapy in alpha-1 antitrypsin deficiency: Advances and controversies. Ther Adv Respir Dis. 2010;4:289–312. doi: 10.1177/1753465810373911. [DOI] [PubMed] [Google Scholar]
- 19.Wozniak J, Wandtke T, Kopinski P, Chorostowska-Wynimko J. Challenges and prospects for alpha-1 antitrypsin deficiency gene therapy. Hum Gene Ther. 2015;26:709–718. doi: 10.1089/hum.2015.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anonymous Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 21.Halliday A, et al. Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): A multicentre randomised trial. Lancet. 2010;376:1074–1084. doi: 10.1016/S0140-6736(10)61197-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonas DE, et al. Screening for asymptomatic carotid artery stenosis: A systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;161:336–346. doi: 10.7326/M14-0530. [DOI] [PubMed] [Google Scholar]
- 23.Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: A prospective, population-based study. Stroke. 2010;41:e11–e17. doi: 10.1161/STROKEAHA.109.561837. [DOI] [PubMed] [Google Scholar]