Abstract
Intranasal inoculation of mice with Bordetella bronchiseptica produces a transient pneumonia that is cleared over several weeks in a process known to require both neutrophils and lymphocytes. In this study, we evaluated the roles of the chemokines MIG (CXCL9), IP-10 (CXCL10), and I-TAC (CXCL11) and their common receptor, CXCR3. Following bacterial inoculation, message expression of interleukin-1 (IL-1), IL-6, and the neutrophil-attracting chemokines KC, LIX, and MIP-2 was rapidly induced, with maximal expression found at 6 h. In contrast, message expression of gamma interferon, MIG, IP-10, and I-TAC peaked at 2 days. Expression of all of these chemokines and cytokines returned to near baseline by 5 days, despite the persistence of high levels of live bacteria at this time. Induced MIG, IP-10, and I-TAC protein expression was localized in areas of inflammation at 2 to 3 days and was temporally associated with increased levels of CXCR3+ lymphocytes in bronchoalveolar lavage fluid. There was no increase in mortality in mice lacking CXCR3. However, the clearance of bacteria from the lung and trachea was delayed, and the recruitment of lymphocytes and NK cells was slightly decreased, for CXCR3−/− mice relative to CXCR3+/+ mice. We conclude that the CXCR3 receptor-ligand system contributes to pulmonary host defense in B. bronchiseptica infection by recruiting lymphocytes and NK cells into the lung.
Whooping cough (pertussis) has historically been a major cause of morbidity and mortality in humans (16, 20). Although vaccines against its causative agent, Bordetella pertussis, have considerably reduced the incidence of this disease in developed countries, its recent resurgence in some vaccinated populations has led to greater interest in the mechanisms of Bordetella pathogenesis and its interactions with the immune system (20). Unfortunately, animal models of B. pertussis may not provide an accurate representation of the human disease, because humans are the only natural hosts for this organism (15, 16). An alternative approach is to study closely related Bordetella subspecies, such as Bordetella bronchiseptica. Like B. pertussis, B. bronchiseptica is a gram-negative aerobic coccobacillus that infects the respiratory tract (16). Unlike B. pertussis, B. bronchiseptica naturally infects numerous animal species, including mice, rats, rabbits, pigs, and (rarely) humans. It is the causative agent of kennel cough, a major source of morbidity in dogs (12).
Intranasal inoculation of mice with 105 to 106 B. bronchiseptica bacteria produces infection of both the upper and lower respiratory tracts (15). In the lungs, bacterial counts peak at 5 to 7 days and then decrease, and bacteria are eliminated after about 4 weeks (15). Bacteria are cleared from the trachea after 6 weeks but persist indefinitely in the nose. Both innate and adaptive immune mechanisms are necessary for clearance of this organism. Neutropenic, SCID, and RAG-1−/− animals succumb to infection (15). NK cells contribute to host defense against the organism but are not essential, as evidenced by the fact that beige mice are still able to clear the infection (15). High levels of bacteria persist for long periods throughout the respiratory tracts of B-cell-deficient mice, and treatment of these mice with a specific antiserum against B. bronchiseptica causes rapid clearance of the organism, indicating that antibodies play an important role in the resolution of this disease (21). The role of T cells in B. bronchiseptica infection has not been specifically investigated. However, Mills et al. reported that T cells obtained from mice infected with B. pertussis had a Th1 phenotype (26). They further demonstrated that T cells are essential for clearance of B. pertussis in mice, since nude mice (lacking T cells) were unable to eliminate the organism unless T cells were provided by adoptive transfer.
Chemokines are a family of small cytokines that play major roles in directing the selective movement of specific immune cells (7, 30, 58). Chemokines are classified into the C, CC, CXC, and CX3C subsets depending on the number and spacing of conserved cysteines in their sequences. The CXC subgroup can be further divided depending on the presence or absence of a conserved ELR (glutamic acid-leucine-arginine) motif. The ELR+ CXC chemokines have potent chemoattractant activity for neutrophils. In mice, this group includes LIX (43), a close homologue of both human ENA-78 (CXCL5) and GCP-2 (CXCL6) (37), and two chemokines, KC and MIP-2, related to the three human GRO (CXCL1 to CXCL3) proteins (31, 46). Among the ELR− CXC chemokines, MIG (CXCL9), IP-10 (CXCL10), and I-TAC (CXCL11) form a distinct and evolutionarily conserved subgroup (9, 55). MIG, IP-10, and I-TAC are induced by gamma interferon (IFN-γ) in multiple cell types (50, 51, 55) and are chemotactic for lymphocytes bearing CXCR3, their common receptor (32, 33, 56).
Although previous studies have shown that lymphocytes are required for the clearance of B. bronchiseptica infection from the lungs in mice (15), the mechanisms involved in recruiting lymphocytes to the lungs have not been investigated. In this study, we found that the chemokines I-TAC, MIG, and IP-10 were induced during infection and that their expression correlated with increased numbers of lymphocytes expressing CXCR3. To investigate the functional role of CXCR3, we compared the course of infection and cellular responses in wild-type (WT) and CXCR3−/− mice. Inactivation of CXCR3 produced a delay in bacterial clearance and decreases in lymphocyte and NK cell recruitment. However, CXCR3−/− mice were able to clear the infection from the lung, suggesting that other mechanisms are able to compensate for the loss of CXCR3 in this infection.
MATERIALS AND METHODS
Mice.
Female BALB/c mice, housed under specific-pathogen-free conditions, were studied at the age of 2 to 5.5 months. In each experiment, mice were the same age ± 4 days. Mice homozygous for a deletion in the CXCR3 gene that renders it functionally inactive (CXCR3−/−) were generated as described elsewhere (14) and then backcrossed to the BALB/c background for 10 generations. WT (CXCR3+/+) BALB/c mice were purchased from Charles River Laboratories (Cambridge, Mass.). All experimental protocols were approved by the Animal Research Committee of the University of California, Los Angeles.
Bacteria.
B. bronchiseptica strains were grown and prepared as previously described (6, 24). The B. bronchiseptica phase-locked Bvg+ strain RB53 was used to coat enzyme-linked immunosorbent assay (ELISA) plates. The wild-type strain RB50 was used for experimental infections. Mice were lightly anesthetized with isoflurane and inoculated intranasally with 1.5 × 105 RB50 bacteria in 50 μl of phosphate-buffered saline (PBS). The number of viable bacteria in the inoculum was confirmed by plating dilutions on agar plates.
Harvesting of tissues and cells.
Mice were euthanized by exsanguination, under halothane anesthesia, by incision of the inferior vena cava. For determination of bacterial load, the right lung lobes and/or the trachea was homogenized in PBS and plated onto brain heart infusion agar (Becton Dickinson) with streptomycin. The left lung was homogenized in 1.5 ml of guanidine thiocyanate lysis buffer (3), and RNA was extracted by the acid phenol method (3). For immunohistochemistry, the lungs were flushed with PBS through the right ventricle of the heart prior to tracheal cannulation. The lungs were inflated with Carnoy's solution, fixed at 4°C, embedded in paraffin, and sectioned at a thickness of 7 μm.
Cells for flow cytometry were obtained by bronchoalveolar lavage (BAL) or by collagenase digestion of the lung. For BAL, the trachea was cannulated and the lungs were lavaged with PBS plus 0.2 mM EGTA. Red blood cells were then lysed in NH4Cl buffer (19), washed, and resuspended in flow buffer containing 1× PBS, 2% fetal calf serum, and 0.1% sodium azide. For collagenase digestion, lungs were flushed with PBS via the right ventricle of the heart and were then excised and digested with collagenase as described elsewhere (19), except that RPMI 1640 containing 25 mM HEPES (Mediatech, Herndon, Va.) was used. After lysis of red blood cells, undigested tissue was removed by filtration through 100-μm-pore-size cell strainers (Becton Dickinson). For both the BAL and collagenase protocols, cells were counted by use of a hemocytometer following resuspension in flow buffer.
RPA.
The RNase protection assay (RPA) was performed as described previously (28). Riboprobe templates were constructed in pGEM-4Z (Promega, Madison, Wis.) with EcoRI and HindIII sites at the 5′ and 3′ ends, respectively, of cDNA inserts. The sizes and specific cDNA regions used in probes were as follows: for I-TAC, 348 nucleotides (nt) (nt 221 to 568 of GenBank accession no. AF179872); for IP-10, 143 nt (nt 46 to 188 of M86829); for MIG, 300 nt (nt 102 to 401 of M34815); for LIX, 354 nt (nt 135 to 488 of U27267); for MIP-2, 270 nt (nt 66 to 336 of X53798); for interleukin-1β (IL-1β), 239 nt (nt 453 to 691of M15131); for KC, 169 nt (nt 119 to 288 of J04596); and for IL-6, 149 nt (nt 304 to 452 of X54542). A probe for ribosomal protein S2 (102 nt), used as a loading control, has been described previously (38, 43). Plasmids for the MIP-2, KC, MIG, and I-TAC probes were a gift from Iain Campbell (The Scripps Research Institute, La Jolla, Calif.).
RT-PCR.
Levels of IFN-γ mRNA were determined by using semiquantitative reverse transcription-PCR (RT-PCR). Total RNA (2.5 μg/sample) was reverse transcribed by using Superscript II RNase H reverse transcriptase (Invitrogen, Carlsbad, Calif.). The PCR mixture contained 1× PCR buffer (Invitrogen), 1.5 mM MgCl2 (Invitrogen), 100 μM deoxynucleoside triphosphates (25 μM each deoxynucleoside triphosphate) (Stratagene), 0.4 μM (each) primer 5′-TCTGGAGGAACTGGC-3′ and primer 5′-CTGTTGCTGAAGAAGGTA-3′, 1 μl of the reverse transcriptase reaction mixture, and 0.625 U of HotStarTaq (QIAGEN, Valencia, Calif.) in a total volume of 25 μl. The primers amplify a 160-bp segment corresponding to nt 227 to 386 of murine IFN-γ (GenBank accession no. K00083). PCR conditions were 94°C for 1 min (1 cycle), followed by 40 cycles of 94°C for 15 s, 50°C for 30 s, and 70°C for 90 s. Control reactions were performed by using primers 5′-AAGCTGAAGACAAGGAGTGGA-3′ and 5′-TGGGCAGGGAGAACAGG-3′, which amplify a 102-bp fragment corresponding to nt 161 to 262 of the murine S2 cDNA (GenBank accession no. AF283559). The reaction conditions were the same as for IFN-γ, except that the annealing temperature was 55°C, and 38 cycles were performed. For both IFN-γ and S2 reactions, the cycle numbers were determined empirically in order to keep the amplifications in the exponential range. PCR products were separated on 1.5% agarose gels and stained with 0.2 μg of ethidium bromide/ml.
Measurement of specific serum immunoglobulin G (IgG) antibodies against B. bronchiseptica.
Serum levels of IgG antibodies with specificity for B. bronchiseptica were measured by ELISA. Ninety-six-well polystyrene assay plates (Becton Dickinson) coated with whole bacteria were used to trap specific antibodies, which were then detected by using a donkey anti-mouse secondary antibody conjugated to horseradish peroxidase (dilution, 1:2,000; Santa Cruz Biotechnology, Santa Cruz, Calif.). The procedure was performed as described previously (5, 18, 24) with the following modifications: (i) to maximize detection of antibodies to B. bronchiseptica virulence proteins, the phase-locked Bvg+ strain RB53 was used to coat the assay plates; (ii) a standard curve was generated for each ELISA plate by using a reference serum prepared by pooling sera from five WT BALB/c mice with high levels of specific anti-B. bronchiseptica antibodies.
Immunohistochemistry.
Sections were deparaffinized in xylene, rehydrated through a graded alcohol series, and stained with hematoxylin and eosin (H&E) or with goat polyclonal antibodies against I-TAC, MIG, and IP-10 (R&D Systems, Minneapolis, Minn.) at 1.5 μg/ml. Nonimmune goat IgG (Jackson ImmunoResearch Laboratories, West Grove, Pa.) was used as a control. After sections were rinsed in PBS containing 0.1% saponin, endogenous peroxidase activity was quenched by using 3% H2O2, and endogenous biotin was blocked by using a kit from Zymed Laboratories (South San Francisco, Calif.). Sections were incubated in 10% nonimmune rabbit serum (Zymed) for 20 min and then were incubated overnight at 4°C with the primary goat antibodies (in PBS plus 0.1% saponin). Next, sections were incubated with a biotinylated rabbit anti-goat antibody (Zymed) at 25°C for 10 min. The stock secondary antibody was diluted in PBS plus 0.1% saponin as follows: 1:4 for anti-IP-10, 1:12 for anti-MIG, and 1:8 for anti-I-TAC antibody. Following a rinse in PBS plus 0.1% saponin, sections were incubated with avidin-horseradish peroxidase (Zymed). After a rinse in PBS, each slide was developed for 2 min by using a 3,3′-diaminobenzidine (DAB) staining kit (Zymed) and was counterstained with Gill's no. 2 hematoxylin (Fisher Scientific, Fair Lawn, N.J.).
Flow cytometry of BAL cells.
BAL cells were resuspended in flow buffer, preincubated with Fc Block (BD PharMingen, San Diego, Calif.), incubated with a rabbit anti-mouse CXCR3 polyclonal antibody (Zymed) or with normal rabbit Ig (Peprotech, Rocky Hill, N.J.), and then incubated with phycoerythrin (PE)-conjugated donkey anti-rabbit Ig (Jackson ImmunoResearch Laboratories). Finally, the cells were incubated with 7-amino-actinomycin D (7AAD) (Calbiochem, San Diego, Calif.) for dead-cell discrimination by using the method of Schmid et al. (41) and were analyzed on a FACSCalibur flow cytometer (Becton Dickinson). The live (low 7AAD fluorescence) lymphocyte population was selected by using forward and side scatter properties and was then examined in FL2 (PE, one color).
Flow cytometry of collagenase digests.
Flow cytometry staining and analysis were performed as described elsewhere (19) by using antibodies from BD PharMingen. A PE-conjugated anti-mouse CD45 antibody was used for gating on leukocytes, and fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse antibodies against CD3, CD4, or B220 were used for subset analysis. NK cells were stained by using an FITC-conjugated DX5 antibody. Appropriate isotype control antibodies were included. Dead cells were excluded by using 7AAD, as described above.
Statistics.
A standard unpaired t test was used to evaluate differences between two groups if their variances were similar. For unequal variances, the unequal-variances form of the t test was used. Experimental results involving multiple parameters were evaluated by analysis of variance (ANOVA). A log transformation of the data was performed if needed to increase the normality of the data and the similarity of variances. If differences were significant by ANOVA at a P value of <0.05, differences between the single control group and each experimental group were assessed by use of a Dunnett test.
RESULTS
Chemokine expression in the lung during infection with B. bronchiseptica.
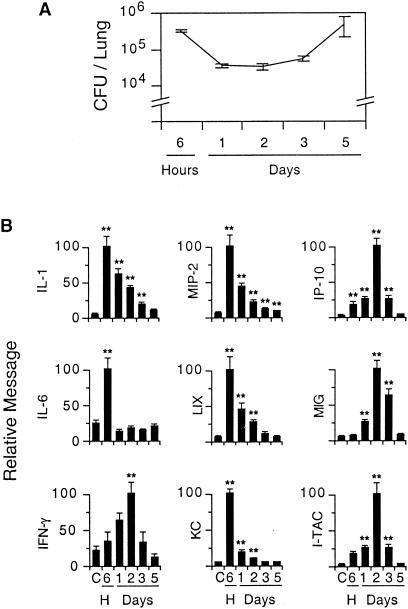
To investigate the expression of selected chemokines and cytokines during the early phases of infection with B. bronchiseptica, 3-month-old BALB/c mice were inoculated intranasally with RB50 (1.5 × 105 CFU in 50 μl of PBS) and then sacrificed at intervals up to 5 days (n = 5 to 6 mice/group). Control animals were inoculated with PBS alone and sacrificed at 3 days. Bacterial loads were determined by colony counts from the right lung. RNA was extracted from the left lung and analyzed by RPA or semiquantitative PCR as described in Materials and Methods. In agreement with previous work (15), lung B. bronchiseptica counts at 24 to 72 h decreased from their initial level and then increased at 5 days (Fig. 1A). Despite the persistence of bacteria in the lung, the expression of the cytokines and chemokines we measured peaked earlier and returned to near baseline by 5 days (Fig. 1B). IL-1 message peaked at 6 h and then decreased slowly, remaining elevated at 5 days relative to control levels. IL-6 was also induced at 6 h but rapidly declined to baseline levels (Fig. 1B). The time courses of the neutrophil-attracting chemokines, LIX, KC, and MIP-2, were similar to that of IL-1, with peak message expression at 6 h, followed by a decline nearly to background by 5 days (Fig. 1B).
FIG. 1.
Transient cytokine and chemokine expression in the lung during the first 5 days after infection with B. bronchiseptica. (A) Bacterial loads in the lung during the 5-day time course. (B) Expression of cytokines and chemokines. The expression of IL-1, IL-6, KC, LIX, MIP-2, I-TAC, MIG, and IP-10 was determined by RPA. IFN-γ expression was determined by RT-PCR. RNA expression is normalized to the murine ribosomal S2 gene. Data are means for different groups; error bars, standard errors of the means. Double asterisks (**) indicate a difference between the indicated group and the PBS control group that is significant at a P value of <0.01 (Dunnett test for multiple comparisons).
In contrast to IL-1, IL-6, and the neutrophil-attracting chemokines, the expression of IFN-γ and the IFN-γ-inducible chemokines I-TAC, MIG, and IP-10 peaked at 48 h after inoculation (Fig. 1B). Levels of IFN-γ and all three of these chemokines decreased between 3 and 5 days, despite a ∼10-fold increase in the number of bacterial CFU during that interval (Fig. 1A). In additional experiments, neither I-TAC nor MIG nor IP-10 was detected by RPA at 7 days (when lung bacterial levels were at their peak) or at later times, up to 35 days (data not shown). These data indicate that I-TAC, MIG, and IP-10 messages are induced in the lung during the early stages of infection with B. bronchiseptica, so these chemokines could potentially play a role in orchestrating leukocyte movement involved in the subsequent clearance of this organism from the lung.
Expression of I-TAC, MIG, and IP-10 proteins in the lung during infection.
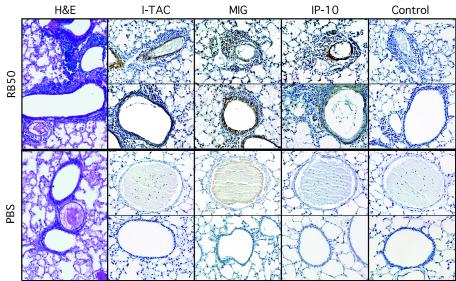
To determine whether I-TAC, MIG, and IP-10 proteins are expressed during infection, and to evaluate their localization, the lungs of WT BALB/c mice (n = 3 to 4/group) were removed 2 days after infection, fixed in Carnoy's solution, and embedded in paraffin. Intranasal inoculation produced patchy areas of intense inflammation, particularly around airways, with other lung areas remaining clear (Fig. 2). Immunohistochemistry using antibodies specific for the three chemokines was performed as described in Materials and Methods. Expression of I-TAC, MIG, and IP-10 proteins was induced in inflamed areas, compared to uninflamed areas and the PBS control (Fig. 2). At 48 h, the three chemokines showed somewhat different patterns of expression: Antibodies against I-TAC and IP-10 gave strong staining of the smooth-muscle layer of vessels in areas with inflammation and stained airways dimly, if at all. Antibodies against MIG, however, stained vessels dimly, if at all, but showed strong staining of airway epithelial cells. Expression of all three chemokines was detected 3 days after infection, as well, but was generally less intense than the staining at 2 days (data not shown). These data indicate that the I-TAC, MIG, and IP-10 proteins, as well as their messages, are expressed in the lung during infection with B. bronchiseptica. No differences in I-TAC, MIG, or IP-10 message or protein expression were detected between infected CXCR3−/− mice and WT mice (data not shown).
FIG. 2.
Immunohistochemical study of I-TAC, MIG, and IP-10 protein expression in the lung during infection with B. bronchiseptica. Leftmost panels show representative H&E staining. Remaining panels show immunohistochemical staining (brown) with antibodies to the CXCR3 ligands or control antibodies, as indicated. Sections shown in the top half of the figure are from lungs collected 48 h after inoculation with B. bronchiseptica (RB50). Sections in the bottom half are from control animals inoculated with PBS. Magnification, ×100. Photomicrographs were taken under the light microscope.
Leukocyte recruitment to the lung during infection with B. bronchiseptica.
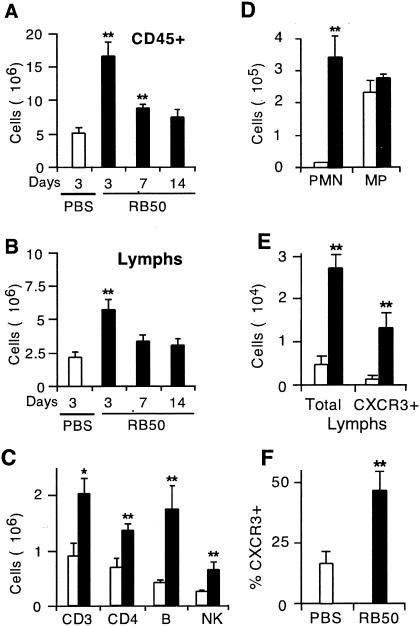
The expression of chemokines in response to infection is expected to recruit leukocytes into the lung. To assess changes in lung leukocyte populations following infection, we prepared single-cell suspensions by collagenase digestion of lungs harvested from WT mice 3, 7, and 14 days after B. bronchiseptica inoculation, or 3 days following PBS (control) inoculation, and analyzed them by flow cytometry, as described in Materials and Methods (n = 3 mice/group). Three days after inoculation, the total number of CD45+ cells and the number of CD45+ lymphocytes were both threefold greater in infected animals than in controls (P ≤ 0.01) (Fig. 3A and B). Similar results were obtained in two replicate experiments (data not shown). Total leukocyte numbers remained elevated above those for the PBS control at 7 days (P ≤ 0.01) but not at 14 days. Lymphocyte numbers were not significantly elevated above those for the PBS control at either 7 or 14 days (Fig. 3B).
FIG. 3.
Leukocyte numbers in the lung during infection with B. bronchiseptica. Cell suspensions from mice inoculated with B. bronchiseptica (filled columns) or with PBS (open columns) were prepared from collagenase digests of the lung (A-C) or from BAL fluid (D-F) and analyzed by counting, differential staining, and flow cytometry. For flow cytometry, a minimum of 30,000 cells was acquired per data point. (A) Total numbers of all leukocytes (CD45+ cells) in collagenase digests 3 to 14 days after infection. (B) Total numbers of lymphocytes in collagenase digests 3 to 14 days after infection. (C) Numbers of CD3, CD4, B, and NK cells in collagenase digests 3 days after infection. (D) Absolute numbers of neutrophils (polymorphonuclear lymphocytes [PMN]) and macrophages (MP) in BAL fluid 3 days after infection. (E) Absolute numbers of total lymphocytes and CXCR3+ lymphocytes in BAL fluid 3 days after infection. (F) Percentage of lymphocytes in BAL fluid that were CXCR3 positive 3 days after infection. For leukocyte and lymphocyte numbers (A and B), statistical significances of differences from the PBS control group were determined by using a Dunnett test. For all other comparisons (C-F), a t test was used to test the significance of differences between the RB50-inoculated group and the PBS control group. *, P < 0.05; **, P ≤ 0.01. Error bars, standard errors of the means.
Lymphocyte subsets were studied by using FITC-conjugated CD3, CD4, B220, and pan-NK antibodies to identify the T-cell, CD4+ T-cell, B-cell, and NK cell subsets. Three days after inoculation, the number of cells in each of these lymphocyte subsets increased 2.0- to 4.4-fold in infected animals compared to the PBS control groups (Fig. 3C). Similar results were seen in a replicate experiment. In both infected and control lungs, the majority of T cells were CD4+. While T cells were more numerous than B cells in the lungs of control animals, the numbers of T and B cells were similar in infected animals, i.e., the increase in the number of B cells (4.4-fold) was greater than the increase in the number of T cells (2.0-fold). The NK cell subset made up less than 15% of all lymphocytes in both infected and control animals (Fig. 3C). Thus, infection with B. bronchiseptica results in recruitment of CD45+ leukocytes, lymphocytes, and each lymphocyte subset to the lung 3 days after infection. The lymphocyte influx at 3 days follows the expression of I-TAC, MIG, and IP-10 (Fig. 1B and 2B) and is therefore consistent with a model in which these chemokines participate in lymphocyte recruitment into the lung.
Neutrophils and CXCR3+ lymphocytes are recruited to the airspaces during B. bronchiseptica infection.
To further evaluate the cell types recruited to the lung during B. bronchiseptica infection, we performed BAL 3 days after intranasal inoculation of WT mice either with RB50 or with PBS alone (n = 3 mice/group). The number of neutrophils in BAL fluid increased 137-fold in infected animals (P < 0.05), but the number of macrophages did not change (Fig. 3D). A replicate experiment showed similar results. In other experiments (data not shown), the increase in the number of neutrophils in BAL fluid occurred as early as 6 h. The recruitment of neutrophils may be due in large part to the expression of KC, LIX, and MIP-2, as described above, since their appearance correlates with the induction of these chemokines.
Expression of CXCR3 by lymphocytes was evaluated by flow cytometry as described in Materials and Methods. The total number of lymphocytes in BAL fluid increased 6.2-fold in infected animals compared to PBS controls (P < 0.01), and the number of CXCR3+ lymphocytes increased nearly 14-fold (P < 0.01) (Fig. 3E). The proportion of BAL lymphocytes that were CXCR3+ increased from 16.3% in controls to 46.3% after infection, a 2.8-fold increase (P < 0.01) (Fig. 3F). This experiment was replicated once, with similar results for all parameters. Thus, a large proportion of the increase in the number of lymphocytes in BAL fluid is due to recruitment of CXCR3+ cells. This observation suggests that this receptor and its ligands could play important roles in the host response to this infection.
CXCR3 is not required for clearance of B. bronchiseptica from the lung.
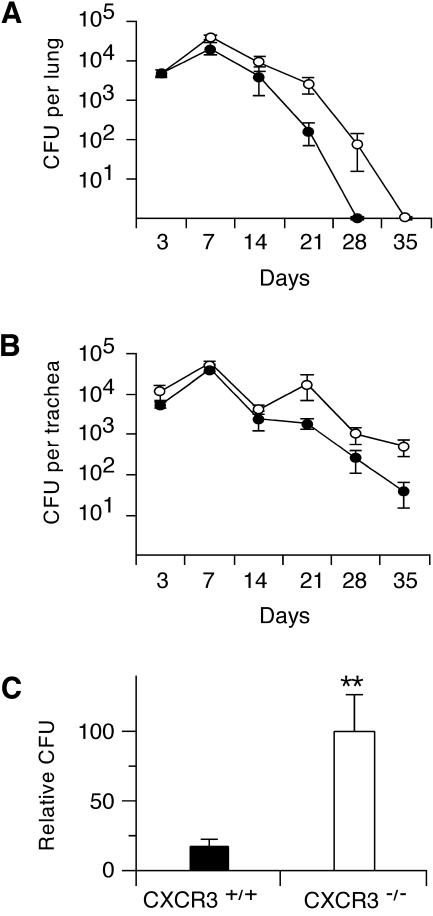
To investigate the functional role of CXCR3 in the immune response to B. bronchiseptica, we compared the clearance of bacteria from the lungs and tracheae of WT (CXCR3+/+) and knockout (CXCR3−/−) mice. WT and knockout mice were inoculated with 1.5 × 105 RB50 bacteria in 50 μl of PBS. Bacterial loads in the lung and trachea were determined at 3, 7, 14, 21, 28, and 35 days after inoculation (n = 5 to 7/group). Serum obtained after euthanasia was frozen in aliquots for later evaluation of antibody responses. Both WT and knockout mice were able to clear the infection from the lungs by 35 days, indicating that CXCR3 is not essential for clearance of the organism (Fig. 4A). Numbers of CFU in the lungs of the two types of mice were identical at 3 days. At all subsequent time points until 35 days, when both groups had cleared the organism, CFU counts in lungs were higher in the knockout animals. Although the differences were not significant at individual time points, the difference between the genotypes was significant by ANOVA (P < 0.01). As observed for WT mice in previous studies (data not shown), clearance of B. bronchiseptica from the trachea was slower than that from the lung in both genotypes (Fig. 4B). Neither the WT nor the knockout mice had cleared the bacteria from the trachea at 35 days. Consistent with the lung results, CFU counts in the trachea were somewhat higher for knockout mice than for WT mice throughout the experiment (P < 0.01 by ANOVA).
FIG. 4.
Clearance of bacteria from the lung and trachea in knockout (CXCR3−/−) and WT (CXCR3+/+) mice. Bacterial loads in lungs (A) and tracheae (B) were higher in knockout mice (open circles) than in WT mice (filled circles) (P < 0.01 by ANOVA). (C) Bacterial loads in the lung were higher at 21 days in knockout mice than in WT mice. The data in panel C were the result of analysis of the 21-day data in panel A (n = 6 per group) together with data from a second, independent experiment (n = 8 per group). Because the absolute numbers of CFU in both groups in the second experiment were substantially greater than those in the first experiment, the absolute CFU data could not be combined directly. Instead, the CFU data in each experiment were first normalized to the mean CFU for all mice (both groups) in that experiment. The normalized data for the two experiments were then combined (total n = 14 per group) and analyzed by using a t test. **, P < 0.01 by t test. Error bars, standard errors of the means.
In a second experiment, numbers of CFU in lungs of WT and knockout mice were measured 21 days after inoculation with 1.5 × 105 bacteria (n = 8 per group). Lung CFU counts were higher in the knockout group than in the WT group at 21 days in both experiments (Fig. 4A; also data not shown), though the differences at 21 days did not quite achieve statistical significance when each experiment was analyzed separately. When the 21-day data from the two experiments were analyzed together (total n = 14 per group), however, the mean bacterial load for the knockout mice was significantly greater (6.0-fold; P ≤ 0.01) than that for the WT mice (Fig. 4C). Taken together, these experiments show that the absence of CXCR3 leads to a modest delay in clearance of bacteria from both the lung and the trachea.
Levels of specific IgG against B. bronchiseptica in the sera of WT and knockout mice were determined by ELISA. Specific antibody was first detected on day 14, and its level increased rapidly thereafter (data not shown). The time courses of antibody production did not differ (by ANOVA) between WT and knockout mice.
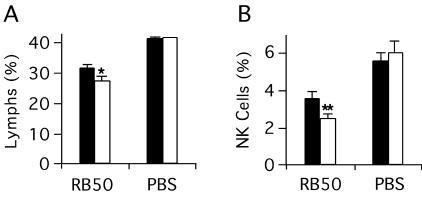
The absence of CXCR3 alters the proportions of lymphocytes and NK cells in the lung.
To determine the effect of CXCR3 inactivation on leukocyte recruitment after infection, we evaluated leukocyte populations by flow cytometry in collagenase lung digests 3 days after infection of WT and knockout mice (n = 3/group). This experiment was performed three times, with similar results. Control experiments, using a PBS inoculum without bacteria, were performed twice. For infected animals, the lymphocyte fraction (as a proportion of all CD45+ leukocytes) was 12% smaller in knockout mice than in WT mice (P < 0.05 by ANOVA) (Fig. 5A). Similarly, the NK cell fraction was about 30% smaller in infected knockout animals than in infected WT animals (P < 0.01) (Fig. 5B). No differences in lymphocyte or NK cell proportions were seen between the two genotypes in the PBS controls. There were no differences between infected WT and knockout animals in the proportions or absolute numbers of the B-cell, T-cell, or granulocyte subsets (data not shown). These data are consistent with the participation of CXCR3 and its ligands in the recruitment of lymphocytes and NK cells to the lung during B. bronchiseptica infection.
FIG. 5.
Differences in leukocyte subset composition in the lungs of knockout and wild-type mice following infection. Single-cell digests of lungs from CXCR3−/− mice (open columns) or CXCR3+/+ mice (filled columns) 3 days after inoculation with B. bronchiseptica RB50 or PBS were examined by flow cytometry. A minimum of 30,000 cells (total cells, live plus dead) was acquired per point; for analysis of the NK cell subset, 100,000 total cells were acquired. Identical gates were used in analysis for all experiments. (A) Percentage of lymphocytes among all CD45+ cells. (B) Percentage of NK cells among all CD45+ cells. Each bar represents the mean of all experimental replicates for a particular group (see the text). Error bars, standard errors of the means. *, P < 0.05 by ANOVA; **, P < 0.01 by ANOVA.
DISCUSSION
In recent years, chemokines have been shown to play roles in host defense against bacteria in numerous models of lung infection in mice, including Klebsiella pneumoniae (2), Streptococcus pneumoniae (10), Pseudomonas aeruginosa (48), Haemophilus influenzae (53), and Legionella pneumophila (45). Chemokine biology in the Bordetella genus remains a relatively unexplored field, and to our knowledge, there are no papers in the literature describing chemokine expression or roles during infection with B. bronchiseptica. Furthermore, most studies on bacterial infection models in the lung have demonstrated key roles for chemokines or chemokine receptors associated with granulocyte or macrophage recruitment; considerably fewer studies have shown roles for chemokines that specialize in the movement of lymphocytes, such as the CXCR3 ligands (42). It is in this context that we examined I-TAC, MIG, and IP-10 and their shared receptor, CXCR3, during infection of the lung with B. bronchiseptica.
In this study, we show that I-TAC, MIG, and IP-10 are induced in the lung during infection with B. bronchiseptica. The I-TAC, MIG, and IP-10 messages all show relatively late peaks of expression, at 48 h (Fig. 1). In contrast, IL-1, IL-6, and the neutrophil chemokines KC, LIX, and MIP-2 are maximally induced by 6 h. The induction of KC, LIX, and MIP-2 is associated with an influx of neutrophils into the lung as early as 6 h that persists up to 3 days (Fig. 3D and data not shown). Considering that neutrophils can express I-TAC, MIG, and IP-10 (11, 51), the delayed expression of these chemokines might be, in part, a function of the time necessary for neutrophil recruitment and activation, as well as a function of the late induction of these chemokines in intrinsic lung cells. Additionally, the late induction of I-TAC, MIG, and IP-10 could be due in part to dependence on the earlier induction of upstream regulatory molecules, particularly IFN-γ, but also possibly IL-1 or other proinflammatory cytokines, as seen in other models (1, 55, 58). For example, Neumann et al. demonstrated that, in a staphylococcal enterotoxin B model of lung inflammation, induction of IP-10 and MIG is largely dependent on IFN-γ (27). In murine cytomegalovirus infection, Salazar-Mather et al. found that the induction of MIG in the mouse liver is dependent on secretion of IFN-γ by NK cells recruited to the liver by MIP-1α (39).
To determine if chemokine expression was associated with the recruitment of immune cells in B. bronchiseptica infection, lung leukocytes were evaluated by flow cytometry following collagenase digestion (Fig. 3A to C). We found that numbers of total leukocytes and lymphocytes are increased at 3 days but return to near baseline by 7 days. The transient influx of leukocytes correlates with the observation that expression of the chemokines and cytokines examined in this study is also transient, with peak induction at 6 to 48 h, followed by a decline to background levels by 5 days (Fig. 1). The intimate association between chemokine expression and inflammatory cell recruitment is demonstrated in the immunohistochemical studies (Fig. 2). Intranasal inoculation results in a patchy distribution of infection and inflammation in the lung, and the expression of I-TAC, MIG, and IP-10 is found in association with the presence of an inflammatory infiltrate.
The decline in chemokine and leukocyte levels to background by 5 to 7 days (Fig. 1B and 3) occurs at a time when the number of viable bacteria in the lung has not yet begun to decrease (Fig. 1A) (15). Similar declines have been seen in a few other mouse infection models. IP-10 and MIG expression peaked 3 days after systemic infection of mice with Rickettsia conorii and then dropped sharply, in both the liver and the lungs, before the bacterial load peaked at 5 days (50). Similarly, levels of IP-10 and other chemokines dropped nearly to background several days before peak viral levels following lung infection with respiratory syncytial virus (RSV) (13). In contrast, chemokine levels generally correlated with bacterial loads in various other mouse lung infection models, including Legionella, Streptococcus, and Chlamydia infection models (17, 45, 52). The differences among these models suggest that organism-specific virulence factors may influence the kinetics and magnitude of some chemokine responses. For example, RSV produces specific proteins (glycoproteins G and SH) associated with suppression of chemokines, including IP-10 (47). Similarly, it is possible that the reduction we observed in I-TAC, MIG, and IP-10 levels between 3 and 5 days, while the number of viable B. bronchiseptica bacteria continued to increase, could be due in part to B. bronchiseptica virulence factors. In particular, B. bronchiseptica produces an adenylate cyclase toxin (15) and also has a type III secretion system, the products (or effectors) of which can inactivate NF-κB in host cells (57). The effects of these factors on host chemokine production could be evaluated in future studies using specific B. bronchiseptica mutant strains.
Although the expression of I-TAC, MIG, and IP-10 in our study was temporally correlated with general leukocyte levels in the lung, these chemokines would be expected to more specifically recruit CXCR3+ lymphocytes to this location. It proved not to be feasible to examine this question using collagenase digests of the lung, because the CXCR3 receptor is apparently proteolyzed during this procedure. However, the number of CXCR3+ lymphocytes present in BAL specimens increased 14-fold (Fig. 3E), and the proportion of lymphocytes expressing CXCR3 increased from 16 to nearly 50% after infection (Fig. 3F). Our data (Fig. 1 to 3) are consistent with the hypothesis that I-TAC, MIG, or IP-10 recruits CXCR3+ cells into the lung in this model, as might be expected, based on the known chemotactic activities of these chemokines for cells bearing the CXCR3 receptor (22, 25, 54). It is also possible that activation of T cells already present in the lung results in upregulation of CXCR3 expression, as has been shown in vitro (34). Given the magnitude of the observed increase in total lymphocyte numbers, the latter mechanism is likely to account for only a minority of the increase in the number of CXCR3+ lymphocytes.
To determine the significance of any lymphocyte recruitment by I-TAC, MIG, or IP-10 via CXCR3 in the host response to B. bronchiseptica, we examined clearance and antibody generation in CXCR3−/− mice. Bacterial loads in these knockout mice were higher than those in WT mice at all time points after 3 days (Fig. 4). However, these differences were modest, and knockout mice were able to clear the infection from the lungs. In addition, levels of specific IgG against B. bronchiseptica did not differ between knockout and WT mice (data not shown). This finding contrasts with recent observations showing that specific IgG levels were significantly decreased in MIG−/− mice infected with the Francisella tularensis live vaccine strain (32). F. tularensis, however, differs considerably from B. bronchiseptica in that the former is a facultatively intracellular organism that has many pathways of transmission, infects mononuclear phagocytes as primary targets, and invades many organs of the body (8, 44).
Although mice lacking CXCR3 were able to clear B. bronchiseptica from the lung, it remained possible that significant changes at the cellular level were occurring as a result of its loss. This has been seen in other models. For example, in another murine model of lung infection (RSV), deletion of a chemokine gene, MIP-1α, resulted in no changes in pathogen load or outcome yet was associated with a marked decrease in the degree of inflammation and leukocyte recruitment to the lung (13). To test for this possibility in our model, we examined inflammation and leukocyte subset composition in the lungs of CXCR3−/− mice. No differences in lung inflammation were readily apparent in H&E-stained lung sections (data not shown), but subtle changes were present in some immune subsets examined by flow cytometry (Fig. 5). Specifically, lymphocytes and NK cells represented a smaller fraction of all leukocytes (CD45+ cells) in the knockout animals. A limitation of the collagenase digestion method used to obtain cells for these flow cytometry studies is that the absolute number of cells obtained per mouse tends to differ considerably between individual mice. For this reason, it was not possible to make statistically meaningful comparisons of absolute lymphocyte numbers or absolute NK cell numbers between the two groups of mice. However, the decrease in the relative parameters does suggest that a decrease in total lymphocyte numbers or in NK numbers could have occurred. Since these cells are known to express CXCR3 (33, 36), this is consistent with the hypothesis that the loss of CXCR3 results in less chemotaxis of these cells into the lung. Decreased numbers of NK cells alone would not be expected to seriously impair host immunity against B. bronchiseptica, since beige mice (lacking NK cells) have little difficulty clearing the organism (15).
Although this study shows that CXCR3 is not required for pulmonary clearance of B. bronchiseptica or antibody production, our data indicate that CXCR3 does contribute to host defense against this organism in WT animals. CXCR3 and its ligands I-TAC, MIG, and IP-10 are upregulated in the lung during infection, and absence of CXCR3 results in a small but statistically significant delay in clearance. The lack of an absolute requirement for CXCR3 is perhaps not surprising, considering that redundancy of immune mechanisms may be essential for robust host defense against the rapid evolution of virulence mechanisms by microorganisms. Redundancy is a well-established and common phenomenon in chemokine biology (4). Lymphocytes can express many other chemokine receptors besides CXCR3, including CCR4, CCR5, CCR7, CCR9, CCR10, CXCR4, and CXCR5 (23, 29, 35, 40, 49), and these could potentially compensate for the loss of CXCR3. CCR5, in particular, is frequently coexpressed with CXCR3 on activated T cells (33).
In conclusion, I-TAC, MIG, and IP-10 are strongly induced in the lung during infection with B. bronchiseptica, and expression of these chemokines is associated with a large increase in the number of CXCR3+ cells in the lung. Inactivation of CXCR3 causes a delay in pulmonary clearance of the organism but does not increase mortality or impair the generation of IgG antibody. We suspect that a double knockout of CXCR3 and another chemokine receptor with a potentially overlapping function, such as CCR5, would exhibit a more dramatic phenotype.
Acknowledgments
We thank Iain Campbell for the gift of plasmids, Marie Burdick and Robert Strieter for advice on the collagenase digestion procedure, Seema Mattoo for assistance with the ELISA system used in this study, and Steve Smale for the use of his flow cytometer. We thank Peggy Cotter for helpful discussions.
This work was supported by National Institutes of Health (NIH) grant RO1 HL57008 (to J.B.S.), NIH grant RO1 AI38417 (to J.F.M.), and NIH National Service Award (NRSA) Institutional Training Grants T32 HD07549 (to D.P.W.) and F32 AI10643 (to A.K.F.-W.).
Editor: V. J. DiRita
REFERENCES
- 1.Aliberti, J. C. S., J. T. Souto, A. Marino, J. Lannes-Vieira, M. M. Teixeira, J. Farber, R. T. Gazzinelli, and J. S. Silva. 2001. Modulation of chemokine production and inflammatory responses in interferon-gamma- and tumor necrosis factor-R1-deficient mice during Trypanosoma cruzi infection. Am. J. Pathol. 158:1433-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, S. C., B. Mehrad, J. C. Deng, G. Vassileva, D. J. Manfra, D. N. Cook, M. T. Wiekowski, A. Zlotnik, T. J. Standiford, and S. A. Lira. 2001. Impaired pulmonary host defense in mice lacking expression of the CXC chemokine lungkine. J. Immunol. 166:3362-3368. [DOI] [PubMed] [Google Scholar]
- 3.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 4.Christopherson, K., and R. Hromas. 2001. Chemokine regulation of normal and pathologic immune responses. Stem Cells 19:388-396. [DOI] [PubMed] [Google Scholar]
- 5.Cotter, P. A., and J. F. Miller. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 62:3381-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotter, P. A., and J. F. Miller. 1997. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol. Microbiol. 24:671-685. [DOI] [PubMed] [Google Scholar]
- 7.Cyster, J. G. 1999. Chemokines and cell migration in secondary lymphoid organs. Science 286:2098-2102. [DOI] [PubMed] [Google Scholar]
- 8.Elkins, K. L., S. C. Cowley, and C. M. Bosio. 2003. Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect. 5:135-142. [DOI] [PubMed] [Google Scholar]
- 9.Farber, J. M. 1997. MIG and IP-10: CXC chemokines that target lymphocytes. J. Leukoc. Biol. 61:246-257. [PubMed] [Google Scholar]
- 10.Fillion, I., N. Ouellet, M. Simard, Y. Bergeron, S. Sato, and M. G. Bergeron. 2001. Role of chemokines and formyl peptides in pneumococcal pneumonia-induced monocyte/macrophage recruitment. J. Immunol. 166:7353-7361. [DOI] [PubMed] [Google Scholar]
- 11.Gasperini, S., M. Marchi, F. Calzetti, C. Laudanna, L. Vicentini, H. Olsen, M. Murphy, F. Liao, J. Farber, and M. A. Cassatella. 1999. Gene expression and production of the monokine induced by IFN-γ (MIG), IFN-inducible T cell alpha chemoattractant (I-TAC), and IFN-γ-inducible protein-10 (IP-10) chemokines by human neutrophils. J. Immunol. 162:4928-4937. [PubMed] [Google Scholar]
- 12.Goodnow, R. A. 1980. Biology of Bordetella bronchiseptica. Microbiol. Rev. 44:722-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haeberle, H. A., W. A. Kuziel, H. J. Dieterich, A. Casola, Z. Gatalica, and R. P. Garofalo. 2001. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1α in lung pathology. J. Virol. 75:878-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock, W. W., B. Lu, W. Gao, V. Csizmadia, K. Faia, J. A. King, S. T. Smiley, M. Ling, N. P. Gerard, and C. Gerard. 2000. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J. Exp. Med. 192:1515-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvill, E. T., P. A. Cotter, M. H. Yuk, and J. F. Miller. 1999. Probing the function of Bordetella bronchiseptica adenylate cyclase toxin by manipulating host immunity. Infect. Immun. 67:1493-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hewlett, E. L. 1995. Bordetella species, p. 2078-2084. In E. L. Mandell, R. G. Douglas, Jr., and J. E. Bennett (ed.), Principles and practice of infectious diseases, 3rd ed. Churchill Livingstone, New York, N.Y.
- 17.Huang, J., M. D. Wang, S. Lenz, D. Y. Gao, and B. Kaltenboeck. 1999. IL-12 administered during Chlamydia psittaci lung infection in mice confers immediate and long-term protection and reduces macrophage inflammatory protein-2 level and neutrophil infiltration in lung tissue. J. Immunol. 162:2217-2226. [PubMed] [Google Scholar]
- 18.Hudson, L., and F. C. Hay. 1989. Practical immunology, 3rd ed. Blackwell Scientific Publications, Oxford, United Kingdom.
- 19.Huffnagle, G. B., R. M. Strieter, T. J. Standiford, R. A. McDonald, M. D. Burdick, S. L. Kunkel, and G. B. Toews. 1995. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J. Immunol. 155:4790-4797. [PubMed] [Google Scholar]
- 20.Kerr, J. R., and R. C. Matthews. 2000. Bordetella pertussis infection: pathogenesis, diagnosis, management, and the role of protective immunity. Eur. J. Clin. Microbiol. Infect. Dis. 19:77-88. [DOI] [PubMed] [Google Scholar]
- 21.Kirimanjeswara, G. S., P. B. Mann, and E. T. Harvill. 2003. Role of antibodies in immunity to Bordetella infections. Infect. Immun. 71:1719-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu, B., A. Humbles, D. Bota, C. Gerard, B. Moser, D. Soler, A. D. Luster, and N. P. Gerard. 1999. Structure and function of the murine chemokine receptor CXCR3. Eur. J. Immunol. 29:3804-3812. [DOI] [PubMed] [Google Scholar]
- 23.Lynch, E. A., C. A. W. Heijens, N. E. Horst, D. M. Center, and W. W. Cruikshank. 2003. IL-16/CD4 preferentially induces Th1 cell migration: requirement of CCR5. J. Immunol. 171:4965-4968. [DOI] [PubMed] [Google Scholar]
- 24.Mattoo, S., J. F. Miller, and P. A. Cotter. 2000. Role of Bordetella bronchiseptica fimbriae in tracheal colonization and development of a humoral immune response. Infect. Immun. 68:2024-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer, M., P. J. Hensbergen, E. M. H. van der Raaij-Helmer, G. Brandacher, R. Margreiter, C. Heufler, F. Koch, S. Narumi, E. R. Werner, R. Colvin, A. D. Luster, C. P. Tensen, and G. Werner-Felmayer. 2001. Cross reactivity of three T cell attracting murine chemokines stimulating the CXC chemokine receptor CXCR3 and their induction in cultured cells and during allograft rejection. Eur. J. Immunol. 31:2521-2527. [DOI] [PubMed] [Google Scholar]
- 26.Mills, K. H., A. Barnard, J. Watkins, and K. Redhead. 1993. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect. Immun. 61:399-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann, B., K. Emmanuilidis, M. Stadler, and B. Holzmann. 1998. Distinct functions of interferon-gamma for chemokine expression in models of acute lung inflammation. Immunology 95:512-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen, T. T., and J. B. Smith. 2002. Ribonuclease protection assay streamlined for high throughput with RNA in formamide and single-step RNase inactivation. BioTechniques 32:502, 504, 506. [DOI] [PubMed] [Google Scholar]
- 29.Ohl, L., G. Henning, S. Krautwald, M. Lipp, S. Hardtke, G. Bernhardt, O. Pabst, and R. Forster. 2003. Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J. Exp. Med. 197:1199-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson, T. S., and K. Ley. 2002. Chemokines and chemokine receptors in leukocyte trafficking. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283:R7-R28. [DOI] [PubMed] [Google Scholar]
- 31.Oquendo, P., J. Alberta, D. Z. Wen, J. L. Graycar, R. Derynck, and C. D. Stiles. 1989. The platelet-derived growth factor-inducible KC gene encodes a secretory protein related to platelet alpha-granule proteins. J. Biol. Chem. 264:4133-4137. [PubMed] [Google Scholar]
- 32.Park, M. K., D. Amichay, P. Love, E. Wick, F. Liao, A. Grinberg, R. L. Rabin, H. W. H. Zhang, S. Gebeyehu, T. M. Wright, A. Iwasaki, Y. M. Weng, J. A. DeMartino, K. L. Elkins, and J. M. Farber. 2002. The CXC chemokine murine monokine induced by IFN-γ (CXC chemokine ligand 9) is made by APCs, targets lymphocytes including activated B cells, and supports antibody responses to a bacterial pathogen in vivo. J. Immunol. 169:1433-1443. [DOI] [PubMed] [Google Scholar]
- 33.Qin, S. X., J. B. Rottman, P. Myers, N. Kassam, M. Weinblatt, M. Loetscher, A. E. Koch, B. Moser, and C. R. Mackay. 1998. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J. Clin. Investig. 101:746-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabin, R. L., M. A. Alston, J. C. Sircus, B. Knollmann-Ritschel, C. Moratz, D. Ngo, and J. M. Farber. 2003. CXCR3 is induced early on the pathway of CD4+ T cell differentiation and bridges central and peripheral functions. J. Immunol. 171:2812-2824. [DOI] [PubMed] [Google Scholar]
- 35.Reiss, Y., A. E. Proudfoot, C. A. Power, J. J. Campbell, and E. C. Butcher. 2001. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J. Exp. Med. 194:1541-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson, M. J. 2002. Role of chemokines in the biology of natural killer cells. J. Leukoc. Biol. 71:173-183. [PubMed] [Google Scholar]
- 37.Rovai, L. E., H. R. Herschman, and J. B. Smith. 1997. Cloning and characterization of the human granulocyte chemotactic protein-2 gene. J. Immunol. 158:5257-5266. [PubMed] [Google Scholar]
- 38.Rovai, L. E., H. R. Herschman, and J. B. Smith. 1998. The murine neutrophil-chemoattractant chemokines LIX, KC, and MIP-2 have distinct induction kinetics, tissue distributions, and tissue-specific sensitivities to glucocorticoid regulation in endotoxemia. J. Leukoc. Biol. 64:494-502. [DOI] [PubMed] [Google Scholar]
- 39.Salazar-Mather, T. P., T. A. Hamilton, and C. A. Biron. 2000. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J. Clin. Investig. 105:985-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schabath, R., G. Muller, A. Schubel, E. Kremmer, M. Lipp, and R. Forster. 1999. The murine chemokine receptor CXCR4 is tightly regulated during T cell development and activation. J. Leukoc. Biol. 66:996-1004. [DOI] [PubMed] [Google Scholar]
- 41.Schmid, I., W. J. Krall, C. H. Uittenbogaart, J. Braun, and J. V. Giorgi. 1992. Dead cell discrimination with 7-amino-actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry 13:204-208. [DOI] [PubMed] [Google Scholar]
- 42.Seiler, P., P. Aichele, S. Bandermann, A. E. Hauser, B. Lu, N. P. Gerard, C. Gerard, S. Ehlers, H. J. Mollenkopf, and S. H. E. Kaufmann. 2003. Early granuloma formation after aerosol Mycobacterium tuberculosis infection is regulated by neutrophils via CXCR3-signaling chemokines. Eur. J. Immunol. 33:2676-2686. [DOI] [PubMed] [Google Scholar]
- 43.Smith, J. B., and H. R. Herschman. 1995. Glucocorticoid-attenuated response genes encode intercellular mediators, including a new C-X-C chemokine. J. Biol. Chem. 270:16756-16765. [DOI] [PubMed] [Google Scholar]
- 44.Tarnvik, A., and L. Berglund. 2003. Tularaemia. Eur. Respir. J. 21:361-373. [DOI] [PubMed] [Google Scholar]
- 45.Tateda, K., T. A. Moore, M. W. Newstead, W. C. Tsai, X. Y. Zeng, J. C. Deng, G. Chen, R. Reddy, K. Yamaguchi, and T. J. Standiford. 2001. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect. Immun. 69:2017-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tekamp-Olson, P., C. Gallegos, D. Bauer, J. McClain, B. Sherry, M. Fabre, S. van Deventer, and A. Cerami. 1990. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its homologues. J. Exp. Med. 172:911-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tripp, R. A., L. Jones, and L. J. Anderson. 2000. Respiratory syncytial virus G and/or SH glycoproteins modify CC and CXC chemokine mRNA expression in the BALB/c mouse. J. Virol. 74:6227-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai, W. C., R. M. Strieter, B. Mehrad, M. W. Newstead, X. Zeng, and T. J. Standiford. 2000. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect. Immun. 68:4289-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uehara, S., K. M. Song, J. M. Farber, and P. E. Love. 2002. Characterization of CCR9 expression and CCL25/thymus-expressed chemokine responsiveness during T cell development: CD3high CD69+ thymocytes and γδ TCR+ thymocytes preferentially respond to CCL25. J. Immunol. 168:134-142. [DOI] [PubMed] [Google Scholar]
- 50.Valbuena, G., W. Bradford, and D. H. Walker. 2003. Expression analysis of the T-cell-targeting chemokines CXCL9 and CXCL10 in mice and humans with endothelial infections caused by rickettsiae of the spotted fever group. Am. J. Pathol. 163:1357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Zandbergen, G., N. Hermann, H. Laufs, W. Solbach, and T. Laskay. 2002. Leishmania promastigotes release a granulocyte chemotactic factor and induce interleukin-8 release but inhibit gamma interferon-inducible protein 10 production by neutrophil granulocytes. Infect. Immun. 70:4177-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, E. J., M. Simard, N. Ouellet, Y. Bergeron, D. Beauchamp, and M. G. Bergeron. 2002. Pathogenesis of pneumococcal pneumonia in cyclophosphamide-induced leukopenia in mice. Infect. Immun. 70:4226-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang, X., C. Moser, J. P. Louboutin, E. S. Lysenko, D. J. Weiner, J. N. Weiser, and J. M. Wilson. 2002. Toll-like receptor 4 mediates innate immune responses to Haemophilus influenzae infection in mouse lung. J. Immunol. 168:810-815. [DOI] [PubMed] [Google Scholar]
- 54.Weng, Y. M., S. J. Siciliano, K. E. Waldburger, A. Sirotinameisher, M. J. Staruch, B. L. Daugherty, S. L. Gould, M. S. Springer, and J. A. Demartino. 1998. Binding and functional properties of recombinant and endogenous CXCR3 chemokine receptors. J. Biol. Chem. 273:18288-18291. [DOI] [PubMed] [Google Scholar]
- 55.Widney, D. P., Y. R. Xia, A. J. Lusis, and J. B. Smith. 2000. The murine chemokine CXCL11 (IFN-inducible T cell alpha chemoattractant) is an IFN-γ- and lipopolysaccharide-inducible glucocorticoid-attenuated response gene expressed in lung and other tissues during endotoxemia. J. Immunol. 164:6322-6331. [DOI] [PubMed] [Google Scholar]
- 56.Xie, J. H., N. Nomura, M. Lu, S. L. Chen, G. E. Koch, Y. M. Weng, R. Rosa, J. Di Salvo, J. Mudgett, L. B. Peterson, L. S. Wicker, and J. A. DeMartino. 2003. Antibody-mediated blockade of the CXCR3 chemokine receptor results in diminished recruitment of T helper 1 cells into sites of inflammation. J. Leukoc. Biol. 73:771-780. [DOI] [PubMed] [Google Scholar]
- 57.Yuk, M. H., E. T. Harvill, and J. F. Miller. 1998. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol. Microbiol. 28:945-959. [DOI] [PubMed] [Google Scholar]
- 58.Zlotnik, A., J. Morales, and J. A. Hedrick. 1999. Recent advances in chemokines and chemokine receptors. Crit. Rev. Immunol. 19:1-47. [PubMed] [Google Scholar]