Abstract
Purpose
The complement system is involved in the pathogenesis of age-related macular degeneration (AMD). Because activated microglia are also associated with AMD, we studied the relationship between complement anaphylatoxin receptors and microglial recruitment.
Methods
We assessed the effect of anaphylatoxin C3a receptor (C3aR) and C5a receptor (C5aR) knockout (KO) on light damage–induced migration of microglia/macrophages into the mouse outer retina via immunofluorescence and real-time quantitative PCR.
Results
We found that the mRNA levels of C3, C5, C3aR, C5aR, and two activators of the complement alternative pathway, Cfb and Cfd, were all upregulated after light exposure. Retinal Iba1-positive microglia/macrophages express receptors for C3a and C5a. Light damage increased the number of retinal Iba1-positive cells and the mRNA levels of Iba1. Compared with the wild-type (WT) mice, these increases were attenuated in the C5aR KO mice but not in the C3aR KO mice.
Conclusions
C5aR but not C3aR promoted the recruitment of microglia/macrophages. These divergent properties of complement anaphylatoxins in the light damage model provide a rationale for testing the differential effects of these receptors in additional retinal and neurodegeneration models.
Introduction
Age-related macular degeneration (AMD), the leading cause of irreversible blindness in the United States [1], is thought to be a disease of chronic inflammation. Evidence from human retinas and animal models implicates activation of the complement system [2-5]. In humans, complement factor 3 (C3) and activated C3 were discovered in drusen, extracellular deposits representing the earliest clinical finding in AMD [6]. Additionally, polymorphisms within genes encoding complement proteins increase the risk of AMD [7-12]. Last, patients with AMD have elevated serum levels of primary and activated complement products [13].
Mouse models have been useful for studying the contributions of complement to the end-stage pathologies of AMD, which include degeneration of the photoreceptors and the RPE (dry AMD), and choroidal neovascularization (CNV; wet AMD). Mice lacking complement factor D, an initiator of the alternative pathway, are protected from photoreceptor degeneration following light damage [14]. Similarly, mice lacking either complement anaphylatoxin receptor C3aR or C5aR were found to have decreased CNV following laser-induced injury [15]. Interestingly, divergent functions of C3aR and C5aR in the mouse retina have been suggested. Mice with knockout (KO) of C3aR but not C5aR have progressive retinal cell loss and decreased function by electroretinography, before and following light damage, compared to wild-type (WT) mice [16].
Identifying mechanisms through which complement anaphylatoxins modulate inflammation in the retina may provide a rationale for targeted drug design. Chemotactic recruitment of monocytes is a well-established function of anaphylatoxins, which has been demonstrated in the mouse choroid [15] but not in the retina. The relationship between complement and microglia may be important as microglia serve as resident immunological sensors of the retina that can rapidly transform to reactive phagocytes following insults [17]. In humans and animals, phagocytic activity of microglia is thought to promote degeneration of retinal photoreceptors [18-20]. Here, we seek to elucidate the relationship between complement anaphylatoxins and microglia in the light damage model using C3aR and C5aR KO mice.
Methods
Animals
Male C3aR (stock number: 005712) and C5aR (stock number: 006845) knockout mice on a BALB/cJ background (about 2 months old), as well as wild-type (WT) BALB/cJ mice (stock number: 000651), were purchased from Jackson Laboratory (Bar Harbor, ME). Procedures complied with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. The eyes of the C3aR KO (2 months of age), C5aR KO (5 months of age), and age- and sex-matched WT mice were enucleated after the mice were euthanized at day 7 following light damage for morphologic analysis. Euthanasia was carried out by first giving an IP injection of (in mg/kg bodyweight): 80 ketamine (Par Pharmaceutical, Spring Valley, NY), 10 xylazine (LIoyd Inc., Shenandoah, IA), and 2 acepromazine (Boehringer Ingelheim Vetmedica, Inc. St. Joseph, MO), followed by cervical dislocation. Eyes were enucleated for real-time quantitative PCR (qPCR) and immunohistochemistry 2 days following light damage, a time point at which we previously found the number of retinal microglia/macrophages peaked [21].
Light damage paradigm
Without any previous dark adaptation, the mice were exposed to 10 k lux of cool white light-emitting diode (LED) light in a well-ventilated room continuously for 4 h from 12:00 PM to 4:00 PM. LED arrays (4NFLS-x2160–24V-x, Superbrightled, St. Louis, Missouri) were placed outside the cage, above and on both sides, at a distance of 25 cm from the center of the cage. The maximum irradiance was in the blue band (about 380 to 485 nm), at 43 Watt/m2. After the exposure to light, the mice were placed in the normal light-dark cycle before they were euthanized.
Real-time qPCR
RNA was isolated from neurosensory retina (NSR) samples (RNeasy Kit; Qiagen, Valencia, CA) according to the manufacturer’s protocol. cDNA was synthesized with reverse transcription reagents (TaqMan; Applied Biosystems, Darmstadt, Germany) according to the manufacturer’s protocol [21]. cDNA was synthesized with reverse transcription reagents (TaqMan; Applied Biosystems, Darmstadt, Germany) according to the manufacturer’s protocol. Real-time qPCR was used to analyze the expression of genes in the NSR samples obtained from the eye of each mouse that was not used for immunostaining. Samples were obtained from the WT, C3aR KO, and C5aR KO mice without light damage (n = 4 for each genotype) and then were compared to the samples from those genotypes 2 days after light damage (n = 4 for each genotype). The probes used were ionized calcium binding adaptor 1 (Iba1, Mm00479862_g1), complement component 3 (C3, Mm01232779_m1), complement component 5 (C5, Mm00439275_m1), complement component 3a receptor 1 (C3aR, Mm02620006_s1), complement component 5a receptor 1(C5aR, Mm00500292_s1), complement factor b (Cfb, Mm00433909_m1), and complement factor d (Cfd, Mm01143935_g1). Eukaryotic 18S rRNA (Hs99999901_s1) was used as an endogenous control. Real-time qPCR (TaqMan; ABI, Foster City, CA) was performed on a sequence detection system (Prism Model 7500; ABI) using the ΔΔCT method, which provides normalized expression values. All reactions were performed in technical triplicates (three qPCR replicates per qPCR probe for each of the four mice). One eye was used to obtain the qPCR samples from each mouse.
Immunofluorescence on cryosections and image analysis
As previously described [21], the globes were immersion fixed in 4% paraformaldehyde (PFA), and the eyecups were generated by removing the anterior segment. The eyecups were infiltrated in 30% sucrose overnight and embedded in Tissue-Tek optimum cutting temperature (OCT; Sakura Finetek, Torrance, CA). Immunofluorescence was performed on 10-μm-thick sections from the superior retina about 300 μm away from the optic nerve head. The primary antibodies against a microglia/macrophage marker, ionized calcium binding adaptor 1 (Iba1; Wako Chemicals USA, Inc., Richmond, VA) and translocator protein (TSPO; EPR5384, Abcam, Cambridge, MA), C5aR1 (ab117579, Abcam), and fluorescein isothiocyanate (FITC)–conjugated C3aR antibody (MA5–17475, Thermo Scientific, Waltham, MA) were used at 1:200 dilution. Primary antibody was detected using fluorophore-labeled secondary antibodies (711–165–152, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Control sections were treated identically but without primary antibody. The sections were analyzed with fluorescence microscopy with identical exposure parameters (Model TE300 microscope; Nikon, Tokyo, Japan, with ImagePro software; Media Cybernetics, Silver Springs, MD).
Statistical analysis
Statistical analyses for the complement profile by real-time qPCR were performed in GraphPad Prism 6.0 (San Diego, CA) using the unpaired Student t test. Iba1-positive cell quantification and qPCR for Iba1 were performed with one-way ANOVA with a Tukey post hoc test comparing the mean of each group with the mean of every other group. Comparison of the outer nuclear layer (ONL) nuclei was performed with one-way ANOVA with post hoc pairwise comparisons using Bonferroni adjustment. A p value of less than 0.05 was considered statistically significant.
Results
Upregulation of complement genes after light damage
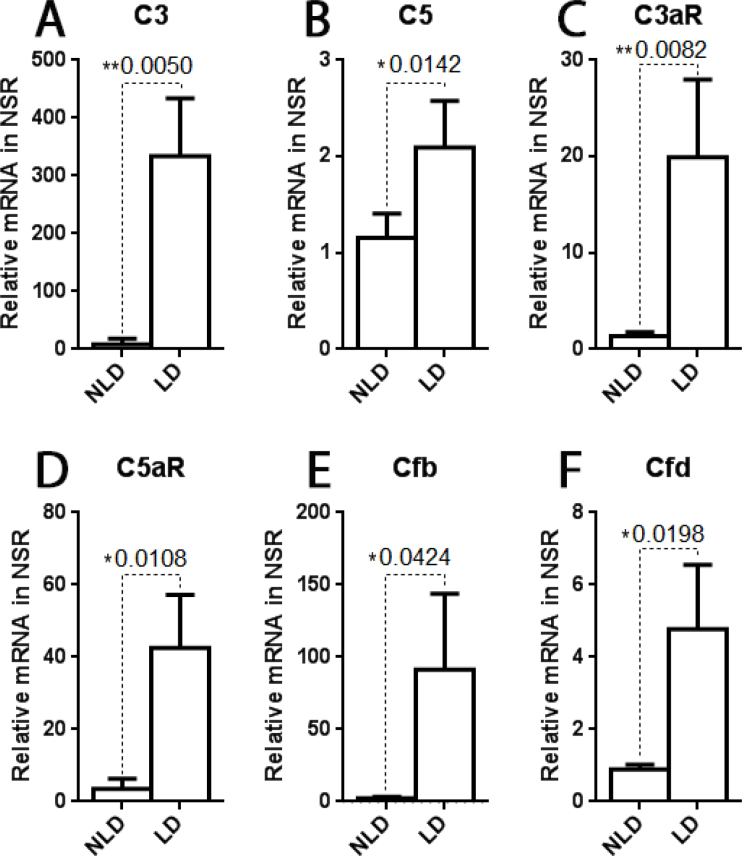
Several genes in the complement pathway were upregulated in the neural retina by light damage. At day 2 following light damage, a time point at which we previously found high levels of microglia/macrophage retinal infiltration [21], the mRNA levels of C3, C5, C3aR, and C5aR were all significantly increased in the light-damaged retinas compared with the non-light-damaged retinas (Figure 1A–D). Additionally, two activators of the alternative complement pathway, which could produce anaphylatoxin, Cfb and Cfd, were significantly upregulated after light damage (Figure 1E,F).
Figure 1.
Relative mRNA levels measured with qPCR. There were significantly higher levels of C3, C5, C3aR, C5aR, Cfb, and Cfd mRNA in the retina at 2 days after light damage compared with the non-light-damaged control retinas. n = 4, *p<0.05, **p<0.01.
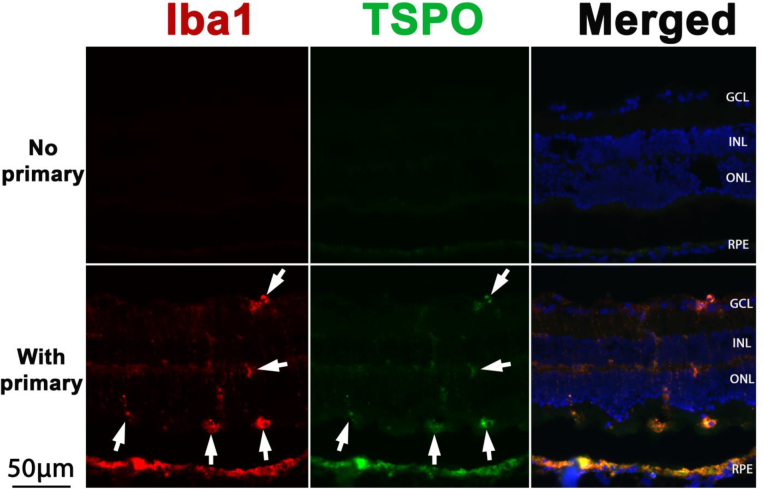
Iba1-positive microglia/macrophages also are TSPO-positive
To investigate the staining specificity of the Iba1 antibody, we performed double immunolabeling with Iba1 and TSPO antibodies. Figure 2 shows that the signals from Iba1 (red in Figure 2) and TSPO (another marker of activated murine microglia/macrophages [22], green in Figure 2) were colocalized (merged images in Figure 2). This result provides confirmation that Iba1-positive cells (white arrows in Figure 2) were activated microglia/macrophages.
Figure 2.
Double immunolabeling for Iba1 and TSPO. Fluorescence photomicrographs show Iba1 (red) and TSPO (green) in the retinas at day 2 after light damage. Retinal sections from wild-type (WT) mice labeled with secondary antibody served as a negative control (upper row). The merged images show colocalization of Iba1 and TSPO (white arrows). GCL = ganglion cell layer, INL = inner nuclear layer, ONL = outer nuclear layer, RPE = retinal pigment epithelium. Scale bar equals 50 μm.
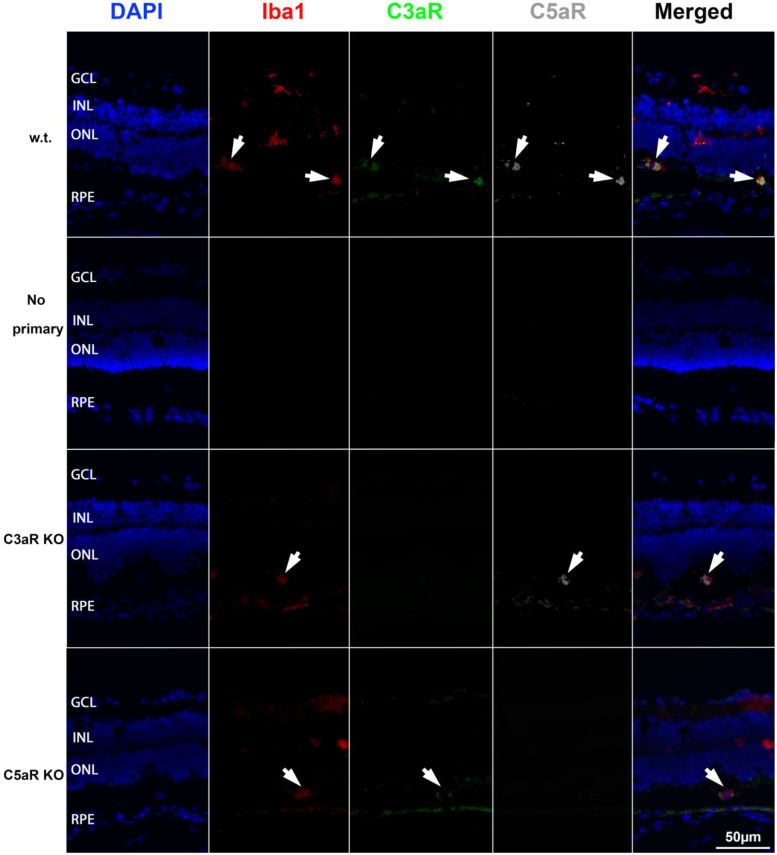
Iba1-positive microglia/macrophages express C3aR and C5aR
Triple immunolabeling for Iba1 (marker of microglia/macrophages), C3aR, and C5aR (white arrows in Figure 3) showed that the Iba1-positive microglia/macrophages in the retina coexpress C3aR and C5aR (the first row in Figure 3), mainly in the outer retina. In the no primary antibody controls, there was no labeling (the second row in Figure 3). Sections of the C3aR KO and C5aR KO retinas served as negative controls for C3aR and C5aR labeling, respectively. In the merged images, colocalization of Iba1, C3aR, and C5aR demonstrate that activated Iba1-positive microglia/macrophages express receptors for C3a and C5a.
Figure 3.
Triple immunolabeling for Iba1, C3aR, and C5aR. Fluorescence photomicrographs show Iba1 (red), C3aR (green), and C5aR (dark gray) in the retinas at day 2 after light damage. Retinal sections from the C3aR knockout (KO) mice and the C5aR KO mice were used as controls for antibody specificity. Staining of the wild-type (WT) retinal sections with (first row) and without (second row) primary antibody are shown. The merged images show colocalization of Iba1, C3aR, and C5aR (white arrows). GCL = ganglion cell layer, INL = inner nuclear layer, ONL = outer nuclear layer, RPE = retinal pigment epithelium. Scale bar equals 50 μm.
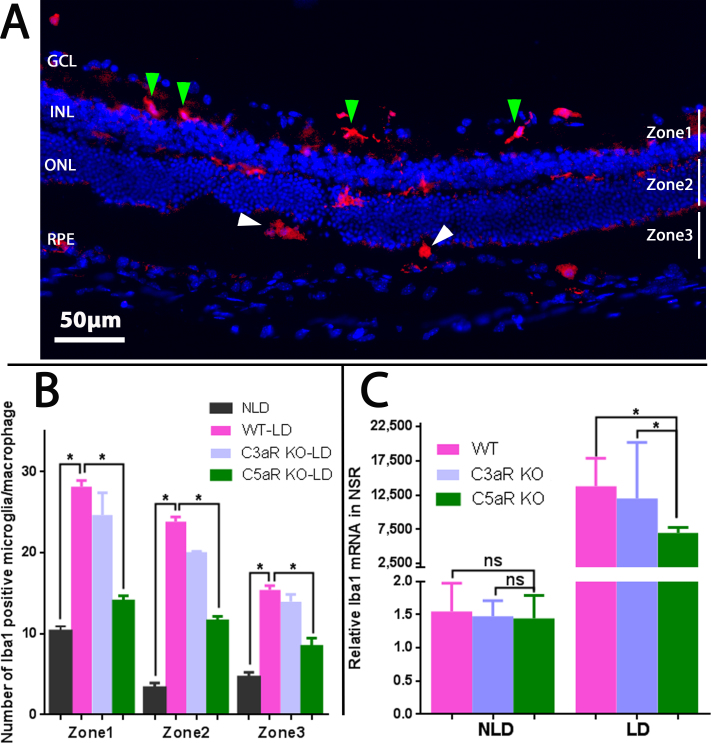
Number, distribution, and morphology of Iba1-positive microglia/macrophages
The mouse retinas were divided into three zones to study microglia/macrophages. Zone 1 spans from the ganglion cell layer to the outer plexiform layer, zone 2 comprises the ONL, while zone 3 contains the photoreceptor inner/outer segments (IS/OS) and the RPE layer (Figure 4A). In the non-light-damaged retinas, microglia/macrophages were primarily localized to the inner retina, and only a few were observed in the ONL, IS/OS, or RPE [23,24].
Figure 4.
Fluorescence photomicrographs and quantification of Iba1-labeled retinal microglia/macrophages following light damage. A: Longitudinal branched (green arrowheads) and amoeboid-shaped (white arrowheads) microglia/macrophages are shown in different zones of the wild-type (WT) retina. B: Counting of Iba1-positive microglia/macrophages revealed increased numbers in the light-damaged retinas compared with the non-light-damaged retinas. There were significantly fewer Iba1-positive microglia/macrophages in all zones of the C5aR knockout (KO) retinas compared to the WT and C3aR KO retinas. n = 4, *p<0.05. GCL = ganglion cell layer, INL = inner nuclear layer, ONL = outer nuclear layer, RPE = retinal pigment epithelium. C: Relative mRNA levels of Iba1 measured with real-time quantitative PCR (qPCR). No statistically significant differences were observed in the mRNA levels of Iba1 among the non-light-damaged retinas of the WT, C3aR KO, and C5aR KO mice. There were statistically significantly lower mRNA levels of Iba1 in the C5aR KO retinas compared with the WT and C3aR KO retinas after light damage. n = 4, *p<0.05.
The number, distribution, and morphology of microglia/macrophages changed following light damage. The microglia in zone 1 displayed a branched morphology (green arrowheads in Figure 4A) but were amoeboid in zone 3 (white arrowheads in Figure 4A). The total number of microglia/macrophages in the entire retina was increased by 3.5-fold at day 2 after light damage in the WT mice (Figure 4B) with an increased percentage of microglia/macrophages located in zones 2 and 3 in the WT light-damaged retinas compared with the non-light-damaged retinas. These results suggest that microglia migrated from the inner retina toward the outer retina, in agreement with previous reports [23-25]. However, in the C5aR KO light-damaged retinas, but not in the C3aR KO light-damaged retinas, the number of microglia/macrophages was significantly reduced in all three zones when compared to WT light-damaged retinas (Figure 4B).
The mRNA levels of Iba1 in retinas at day 2 after light damage
Before the light damage, there were no differences in the mRNA levels of the microglia/macrophage marker Iba1 among the WT, C3aR KO, and C5aR KO mice (the non-light-damaged group in Figure 4C). Light damage induced a statistically significant increase in the mRNA level of Iba1 in all genotypes. However, the mRNA levels of Iba1 were significantly lower in the C5aR KO light-damaged group compared with the WT light-damaged group (p = 0.016) and to the C3aR KO light-damaged group (p = 0.042; light-damaged group in Figure 4C). There was no difference between the WT light-damaged group and the C3aR KO light-damaged group (p = 0.65).
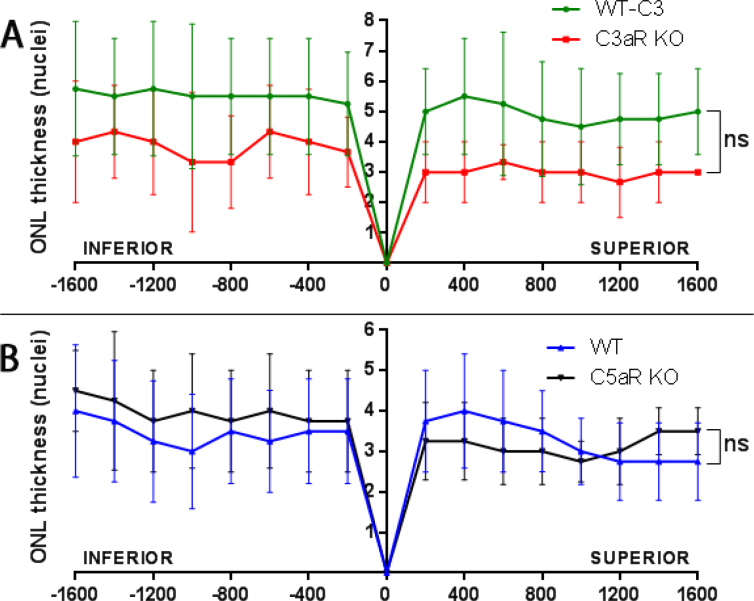
Quantification of the ONL nuclei at day 7 after light damage
Because there were fewer activated microglia/macrophages in the C5aR KO retina after light damage, we tested whether this would protect against light damage. At day 7 following light damage, morphologic analysis was performed, and the numbers of ONL nuclei were quantified in sagittal sections through the optic nerve head. We observed thinning of the ONL and loss of photoreceptor IS/OS in the WT, C3aR KO, and C5aR KO retinas. Counting of the ONL nuclei revealed no statistically significant difference between the WT and C3aR KO retinas (Figure 5A) or between the WT and C5aR KO retinas (Figure 5B).
Figure 5.
Quantification of ONL nuclei at day 7 following light damage. Plot of the number of nuclei per column in the outer nuclear layer (ONL; means ± standard deviation, SD). There was no statistically significant difference between the wild-type (WT; n = 4, green line in A) and C3aR knockout (KO) retinas (n =3, red line in A). Similarly, no statistically significant difference was observed between the WT (n = 4, blue line in A) and C5aR KO retinas (n = 4, black line in B).
Discussion
In this study, we compared the microglia/macrophage response in the retina following light damage in WT mice versus mice lacking one of two complement anaphylatoxin receptors, C3aR or C5aR. Consistent with previous studies, we found that following retinal insult, highly branched microglia, normally located predominantly in the inner retina, migrate to all layers of the retina, and transform into an amoeboid morphology in the outer retina [23-26]. Before the light exposure, there was no difference in the resident Iba1-positive microglia/macrophages in the retinas among the WT, C3aR KO, and C5aR KO mice. In contrast, the microglial/macrophage response was impaired in the light-damaged mice that lacked C5aR but not C3aR, suggesting that C5aR is important for the recruitment of microglia/macrophages 2 days following light insult, a time of high-level microglial infiltration in this light damage model. It has been shown that although C3a and C5a are chemoattractants, C5a causes robust and prolonged activation of various signaling pathways in many cell types, while the response with C3a is generally transient and weak [27]. The present data are consistent with this concept.
As previously reported, the complement system was activated by light exposure [21,28]. The qPCR data also showed upregulation of the mRNA levels of genes involved in the complement cascade, including C3, C5, C3aR, C5aR, Cfb, and Cfd. Cfb activates the alternative pathway by generating C3 convertase after cleavage (of a proenzyme) by Cfd. After C3 is cleaved by C3 convertase, C5 convertase forms and generates the anaphylatoxin C5a. Although we were unable to measure C5a directly with enzyme-linked immunosorbent assay (ELISA) due to low levels of C5 in the neural retina, the mRNA levels of Cfb, Cfd, C3, and C5 in the retina were significantly increased following light damage suggesting that more C5a would be generated. As immunolabeling showed C5aR on Iba1 microglia/macrophages, and C5aR KO light-damaged mice have fewer retinal microglia/macrophages, we posit that direct action of C5a is necessary for the recruitment of these cells. Confirming our observations of C5aR-dependent microglia/macrophage expansion, the increase in the mRNA levels of Iba1 in the retina was attenuated in the light-damaged mice that lacked C5aR but not C3aR. Diminished microglia/macrophage recruitment in the C5aR KO mice did not result in a change in the extent of the ONL loss induced by light damage. This may reflect the moderate reduction in microglial recruitment or indicate that microglial recruitment is a consequence but not a cause of the loss of photoreceptors in this model.
A recently published study showed that C3aR/C5aR double KO mice developed less severe uveitis than the control mice [29], supporting the roles of these receptors in retinal inflammation. Previous work by Yu et al. demonstrated that mice lacking C3aR but not C5aR had progressive retinal cell loss, retinal dysfunction, and increased dysfunction following light damage [16]. Those findings suggest that C3aR but not C5aR is necessary for retinal protection. Accordingly, we also discovered divergent functions of these anaphylatoxin receptors by demonstrating that C5aR but not C3aR is required for migration and activation of microglia/macrophages following light damage. Taken together, these studies suggest that at least in the retinal light damage model, C5aR is proinflammatory whereas C3aR can be protective. However, this distinction in immune function between C3aR and C5aR function may not be generalizable outside the retina as both anaphylatoxins were found to contribute to CNV and macrophage recruitment in choroid tissue [15].
Iba1 is a common marker to microglia and macrophages, and therefore, our observations cannot distinguish between these cell types. In a study of laser-induced injury in mice, basal levels of microglia were found in a 2:1 ratio with macrophages, but both populations expanded to almost equal levels at 2 days after laser injury [30]. Small lesion studies performed in other areas of the mouse central nervous system suggest that acute microglial expansion is derived mostly from native cells, with only a minority infiltrating from the circulation [31]. Likewise, in a study of microglia following optic nerve injury in mice, most proliferating cells were determined to be locally derived, with 33% of microglia proliferating [32]. However, our disease model may have a different profile for blood–retina barrier breakdown than either of these previous studies and therefore may not have comparable numbers of infiltrating immune cells.
A recently published paper showed the ability of complement factor H (CFH) to inhibit subretinal mononuclear phagocyte clearance, and the AMD-associated CFH (H402) variant had an increased capacity to inhibit the clearance [33]. Thus, this CFH variant may cause subretinal microglia/macrophage accumulation by increasing the amount of C5a to attract these cells and by inhibiting their clearance. In summary, our findings in conjunction with previous work highlight differing functions of anaphylatoxin receptors in the retina. These differences may inform new therapeutic directions. Specific inhibition of C5aR could be useful in blocking harmful microglia-mediated inflammation while sparing retinal-protective effects of C3a. Because microglia are known to express and deposit C3 into retinal tissues following light damage [34], it is possible that broad inhibition of microglia may be less desirable as evidenced by retinal degeneration and dysfunction of mice lacking C3 and C3aR [16]. We propose that the specific blockade of C5aR could maintain protective basal action of microglia while preventing deleterious over-activation. Further study of C3aR and C5aR in additional mouse models of retinal degeneration will be needed to evaluate the usefulness of these complements as potential targets for treatment of retinal disease.
Acknowledgments
This work was supported by R01EY015240, R01EY023709, NIH CTSA KL2TR000139, Research to Prevent Blindness, the FM Kirby Foundation, the Paul and Evanina Bell Mackall Foundation Trust, a gift in memory of Dr. Lee F. Mauger.
References
- 1.Klein R, Chou C-F, Klein BEK, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol Chic Ill. 1960;2011:75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- 2.Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001;73:887–96. doi: 10.1006/exer.2001.1094. [DOI] [PubMed] [Google Scholar]
- 3.Zipfel PF, Lauer N, Skerka C. The role of complement in AMD. Adv Exp Med Biol. 2010;703:9–24. doi: 10.1007/978-1-4419-5635-4_2. [DOI] [PubMed] [Google Scholar]
- 4.Grassmann F, Heid IM, Weber BHF. Genetic risk models in age-related macular degeneration. Adv Exp Med Biol. 2014;801:291–300. doi: 10.1007/978-1-4614-3209-8_37. [DOI] [PubMed] [Google Scholar]
- 5.Kawa MP, Machalinska A, Roginska D, Machalinski B. Complement system in pathogenesis of AMD: dual player in degeneration and protection of retinal tissue. J Immunol Res. 2014;2014:483960. doi: 10.1155/2014/483960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134:411–31. doi: 10.1016/s0002-9394(02)01624-0. [DOI] [PubMed] [Google Scholar]
- 7.Edwards AO, Ritter R, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 8.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 9.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, Smith RT, AMD Genetics Clinical Study Group Hageman GS, Dean M, Allikmets R. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38:458–62. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yates JRW, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, Clayton DG, Hayward C, Morgan J, Wright AF, Armbrecht AM, Dhillon B, Deary IJ, Redmond E, Bird AC, Moore AT, Genetic Factors in AMD Study Group Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:553–61. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- 12.Seddon JM, Yu Y, Miller EC, Reynolds R, Tan PL, Gowrisankar S, Goldstein JI, Triebwasser M, Anderson HE, Zerbib J, Kavanagh D, Souied E, Katsanis N, Daly MJ, Atkinson JP, Raychaudhuri S. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet. 2013;45:1366–70. doi: 10.1038/ng.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machalińska A, Dziedziejko V, Mozolewska-Piotrowska K, Karczewicz D, Wiszniewska B, Machaliński B. Elevated plasma levels of C3a complement compound in the exudative form of age-related macular degeneration. Ophthalmic Res. 2009;42:54–9. doi: 10.1159/000219686. [DOI] [PubMed] [Google Scholar]
- 14.Rohrer B, Guo Y, Kunchithapautham K, Gilkeson GS. Eliminating complement factor D reduces photoreceptor susceptibility to light-induced damage. Invest Ophthalmol Vis Sci. 2007;48:5282–9. doi: 10.1167/iovs.07-0282. [DOI] [PubMed] [Google Scholar]
- 15.Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ, Ambati J. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci USA. 2006;103:2328–33. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu M, Zou W, Peachey NS, McIntyre TM, Liu J. A novel role of complement in retinal degeneration. Invest Ophthalmol Vis Sci. 2012;53:7684–92. doi: 10.1167/iovs.12-10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T. Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res. 2015;45:30–57. doi: 10.1016/j.preteyeres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res. 2003;76:463–71. doi: 10.1016/s0014-4835(02)00332-9. [DOI] [PubMed] [Google Scholar]
- 19.Cruz-Guilloty F, Saeed AM, Echegaray JJ, Duffort S, Ballmick A, Tan Y, Betancourt M, Viteri E, Ramkhellawan GC, Ewald E, Feuer W, Huang D, Wen R, Hong L, Wang H, Laird JM, Sene A, Apte RS, Salomon RG, Hollyfield JG, Perez VL. Infiltration of proinflammatory m1 macrophages into the outer retina precedes damage in a mouse model of age-related macular degeneration. Int J Inflamm. 2013;xxx:503725. doi: 10.1155/2013/503725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimm C, Remé CE. Light damage as a model of retinal degeneration. Methods Mol Biol. 2013;935:87–97. doi: 10.1007/978-1-62703-080-9_6. [DOI] [PubMed] [Google Scholar]
- 21.Song D, Song Y, Hadziahmetovic M, Zhong Y, Dunaief JL. Systemic administration of the iron chelator deferiprone protects against light-induced photoreceptor degeneration in the mouse retina. Free Radic Biol Med. 2012;53:64–71. doi: 10.1016/j.freeradbiomed.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholz R, Caramoy A, Bhuckory MB, Rashid K, Chen M, Xu H, Grimm C, Langmann T. Targeting translocator protein (18 kDa) (TSPO) dampens pro-inflammatory microglia reactivity in the retina and protects from degeneration. J Neuroinflammation. 2015;12:201. doi: 10.1186/s12974-015-0422-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collier RJ, Wang Y, Smith SS, Martin E, Ornberg R, Rhoades K, Romano C. Complement deposition and microglial activation in the outer retina in light-induced retinopathy: inhibition by a 5–HT1A agonist. Invest Ophthalmol Vis Sci. 2011;52:8108–16. doi: 10.1167/iovs.10-6418. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C, Shen J-K, Lam TT, Zeng HY, Chiang SK, Yang F, Tso MO. Activation of microglia and chemokines in light-induced retinal degeneration. Mol Vis. 2005;11:887–95. [PubMed] [Google Scholar]
- 25.Kohno T, Inomata H, Taniguchi Y. Identification of microglia cell of the rat retina by light and electron microscopy. Jpn J Ophthalmol. 1982;26:53–68. [PubMed] [Google Scholar]
- 26.Lee JE, Liang KJ, Fariss RN, Wong WT. Ex vivo dynamic imaging of retinal microglia using time-lapse confocal microscopy. Invest Ophthalmol Vis Sci. 2008;49:4169–76. doi: 10.1167/iovs.08-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schraufstatter IU, DiScipio RG, Zhao M, Khaldoyanidi SK. C3a and C5a Are Chemotactic Factors for Human Mesenchymal Stem Cells, Which Cause Prolonged ERK1/2 Phosphorylation. J Immunol. 2009;182:3827–36. doi: 10.4049/jimmunol.0803055. [DOI] [PubMed] [Google Scholar]
- 28.Scholz R, Sobotka M, Caramoy A, Stempfl T, Moehle C, Langmann T. Minocycline counter-regulates pro-inflammatory microglia responses in the retina and protects from degeneration. J Neuroinflammation. 2015;12:209. doi: 10.1186/s12974-015-0431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Bell BA, Yu M, Chan CC, Peachey NS, Fung J, Zhang X, Caspi RR, Lin F. Complement anaphylatoxin receptors C3aR and C5aR are required in the pathogenesis of experimental autoimmune uveitis. J Leukoc Biol. 2015;(September) doi: 10.1189/jlb.3A0415-157R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eter N, Engel DR, Meyer L, Helb HM, Roth F, Maurer J, Holz FG, Kurts C. In vivo visualization of dendritic cells, macrophages, and microglial cells responding to laser-induced damage in the fundus of the eye. Invest Ophthalmol Vis Sci. 2008;49:3649–58. doi: 10.1167/iovs.07-1322. [DOI] [PubMed] [Google Scholar]
- 31.Ladeby R, Wirenfeldt M, Garcia-Ovejero D, Fenger C, Dissing-Olesen L, Dalmau I, Finsen B. Microglial cell population dynamics in the injured adult central nervous system. Brain Res Brain Res Rev. 2005;48:196–206. doi: 10.1016/j.brainresrev.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Wohl SG, Schmeer CW, Witte OW, Isenmann S. Proliferative response of microglia and macrophages in the adult mouse eye after optic nerve lesion. Invest Ophthalmol Vis Sci. 2010;51:2686–96. doi: 10.1167/iovs.09-4537. [DOI] [PubMed] [Google Scholar]
- 33.Calippe B, Augustin S, Beguier F, Charles-Messance H, Poupel L, Conart JB, Hu SJ, Lavalette S, Fauvet A, Rayes J, Levy O, Raoul W, Fitting C, Denèfle T, Pickering MC, Harris C, Jorieux S. Sullivan PM9, Sahel JA, Karoyan P, Sapieha P, Guillonneau X, Gautier EL, Sennlaub F. Complement Factor H Inhibits CD47-Mediated Resolution of Inflammation. Immunity. 2017;46:261–72. doi: 10.1016/j.immuni.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Rutar M, Natoli R, Kozulin P, Valter K, Gatenby P, Provis JM. Analysis of complement expression in light-induced retinal degeneration: synthesis and deposition of C3 by microglia/macrophages is associated with focal photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2011;52:5347–58. doi: 10.1167/iovs.10-7119. [DOI] [PubMed] [Google Scholar]