Abstract
The extent to which vascular calcification is reversible and the possible mechanisms are unclear. To address this, calcified aortas from uremic mice were transplanted orthotopically into normal mice, and the calcium content, histology, and minerals of the allografts were compared with the nontransplanted donor aorta. Calcium content decreased immediately after transplantation but remained constant thereafter, with 68% ± 12% remaining after 34 weeks. X-ray diffraction showed the presence of apatite in both donor aortas and allografts. Osteoclasts were absent in the allografts and there was no expression of the macrophage marker CD11b, the osteoclast marker tartrate-resistant acid phosphatase, or carbonic anhydrase II. The initial loss of calcium was less in heavily calcified aortas and was associated with an increase in the Ca/P ratio from 1.49 to 1.63, consistent with a loss of nonapatitic calcium. The results indicate that vascular calcification persists after reversal of uremia, because of a lack of active resorption of apatite. This failure to resorb established calcifications may contribute to the severity of vascular calcification and suggests that therapy should be aimed at prevention.
Calcification of the medial layer of arteries is common in patients with chronic kidney disease, diabetes, or advanced age,1, 2, 3, 4, 5 and is thought to be detrimental because of arterial stiffening.6 Although therapies exist to slow or prevent calcification in chronic kidney disease, the extent to which this process is reversible remains unclear. Although reversibility of uremic vascular calcification has been reported in humans,7 this has not been observed in any large interventional studies aimed at correcting disordered mineral metabolism in patients with chronic kidney disease or end-stage renal disease. Studies in patients before and after renal transplantation have shown regression8 or no change9 in coronary artery calcification. However, this calcification is largely atherosclerotic10 and can progress after transplantation as a result of other metabolic factors.11 Studies in uremic animals have been limited to an atherosclerotic model in mice in which sevelamer appeared to reverse some calcification.12 Reversibility of medial calcification has been shown in nonuremic animal models13, 14, 15 and in patients with generalized arterial calcification of infancy.16 However, we did not observe significant reversal of calcification after aortic transplantation in an animal model of this disorder.17
Vascular calcifications are composed primarily of apatitic mineral with varying amounts of whitlockite,18, 19 both of which are highly insoluble under physiological conditions20 and thus are not expected to disappear spontaneously. Apatite appears not to form spontaneously from calcium and phosphate ions, whereas initially brushite (CaHPO4 2H2O) and/or other intermediates (including amorphous calcium phosphate) that are more soluble may be present in variable amounts in calcified vessels.21, 22 Thus, some spontaneous reversal of vascular calcification in the early, pre-apatitic stage is possible. However, apatite is quite soluble at an acidic pH, and resorption of bone, where apatite is abundant, requires acid-producing osteoclasts. Thus, it is likely that a similar process involving acidic conditions would be required to remove established vascular calcifications. Osteoclasts are occasionally observed in calcified arteries but they are rare and osteoclast-mediated resorption has not been shown.23 Alternatively, resorption potentially could be mediated by acid produced from smooth muscle cells, which is consistent with the up-regulation of carbonic anhydrase II in warfarin-mediated vascular calcification15 and the vascular calcification observed in mice lacking this enzyme.24
To determine the extent to which the medial arterial calcification observed in uremia is reversible, calcified aortas from uremic mice fed a high-phosphate diet were transplanted into normal mice. Calcium content, mineral analysis, and histology were examined in allografts over time and compared with segments of the donor aortas that were not transplanted.
Materials and Methods
Uremic Vascular Calcification
Uremia was induced in C57BL6 mice by feeding adenine as previously described.25 Powdered adenine was mixed with standard rodent chow at 0.45% (wt/wt). Vascular calcification was induced by adding sodium phosphate (neutral mixture of Na2HPO4 and NaH2PO4) to the diet at a final content of 2% and by s.c. injections of calcitriol (1 μg/kg) 3 times per week.
Transplantation
Transplantation was performed as previously described.17 Segments (4 to 5 mm) of infrarenal aortas were dissected carefully from the uremic mice and the remaining aorta was saved for histology and measurement of calcium content. A corresponding segment of aorta was removed from a healthy littermate and replaced with the calcified aorta via end-to-end anastomoses with 11-0 monofilament sutures. The allografts and anastomotic sites were pretreated with heparin (200 U/mL) to prevent thrombosis. Sex was not matched except that male aortas were not transplanted into female mice to avoid rejection based on Y-chromosome–specific epitopes. Animals were sacrificed and aortas were removed at the end of the study. Tissue within 0.5 mm of each side of the suture lines was excluded from analyses. All animal procedures were approved by the Institutional Animal Care and Use Committee of Emory University.
Staining Procedures
Freshly isolated aortas were stained with a freshly prepared solution of one volume of ethanol saturated with alizarin red S and 24 volumes of 0.5% KOH for 12 hours. Staining with hematoxylin and eosin and von Kossa were performed on 5-μm–thick sections of formalin-fixed aortas mounted in OCT medium according to standard procedures. Staining for tartrate-resistant acid phosphatase was performed with a leukocyte acid phosphatase kit (387A-1KT; Sigma-Aldrich, St. Louis, MO). Immunohistochemistry was performed using polyclonal rabbit antibody against carbonic anhydrase II and a rabbit monoclonal antibody against CD11b (both from Abcam, Cambridge, MA).
Measurement of Aortic Calcium and Phosphate
Calcium content was measured as previously described,17 with some changes. After staining with alizarin red S, the aortas were destained with 0.05% KOH, rinsed in physiological saline without calcium, and extracted in 50 μL of 1 mol/L HCl overnight. Calcium content in the extracts then was measured by the cresophthalein method. Preliminary testing showed that the prior alizarin red staining and destaining did not remove calcium or interfere with the assay. Phosphate was measured colorimetrically by the molybdate method.26
X-Ray Diffraction
Measurements were performed with a D8 DISCOVER diffractometer (Bruker-AXS, Inc., Billerica, MA), equipped with a copper X-ray tube (wavelength, 1.54056 Å). Multiple paraffin sections from individual aortas/allografts were detached with a fine scalpel blade from glass slides and pooled and homogenized by grinding between two glass slides.
Statistical Analysis
Differences in calcium content were analyzed by t-test, with P < 0.05 considered significant.
Results
Previous studies have shown that mice were more sensitive than rats to adenine with a dietary content of 0.45% sufficient to cause renal failure25 compared with 0.75% in rats. However, mice are more resistant to vascular calcification, necessitating the use of a higher dietary phosphate level (2% versus 0.73% to 1.06%) and larger doses of calcitriol (1000 ng/kg versus 40 to 100 ng/kg) than in rats. Adenine was started at age 117 ± 7 days and continued for 127 ± 8 days. Plasma urea, calcium, and phosphorus values were 21.6 ± 1.3 mmol/L, 1.65 ± 0.12 mmol/L, and 4.25 ± 0.38 mmol/L, respectively, in uremic mice, and 2.45 ± 0.17 mmol/L, 1.83 ± 0.07 mmol/L, and 2.52 ± 0.09 mmol/L in control mice. The calcium content in the aortas of uremic mice was 1155 ± 270 nmol/mg, but varied considerably from 22 to 4262 nmol/mg. Calcium content in normal aorta, measured in recipient aortas, was 5.4 ± 0.4 nmol/mg. This value was subtracted from that in the calcified aortas to determine the amount of excess calcium.
Segments of calcified abdominal aorta approximately 4 to 5 mm in length were removed from uremic mice and placed orthotopically into the abdominal aorta of nonuremic littermates. A total of 36 successful transplantations were performed in recipients aged 243 ± 11 days, of which 13 were female into female, 8 were female into male, and 15 were male into male. Male to female transplantation was not performed because of concern regarding possible rejection owing to genes present on the Y chromosome. There were three perioperative deaths. The allografts were harvested at 2 to 3, 5 to 6, 11 to 15, or 33 to 34 weeks after transplantation and were compared with untransplanted aorta from the respective donors. All but two allografts had an excess calcium content that was the same or lower than in the donor aorta. Two allografts (11 and 13 weeks after transplant) showed very large proportional increases in calcium content compared with the donor aorta (292% and 573% of donor content) despite small absolute increases in calcium content (22 and 143 nmol/mg). This was owing to the minimal calcification of the donor aortas (12 and 30 nmol/mg); to avoid substantial skewing of the data these two allografts were omitted from the analyses. We previously have shown that there is no change in calcium content when normal aortas are transplanted into normal mice.17
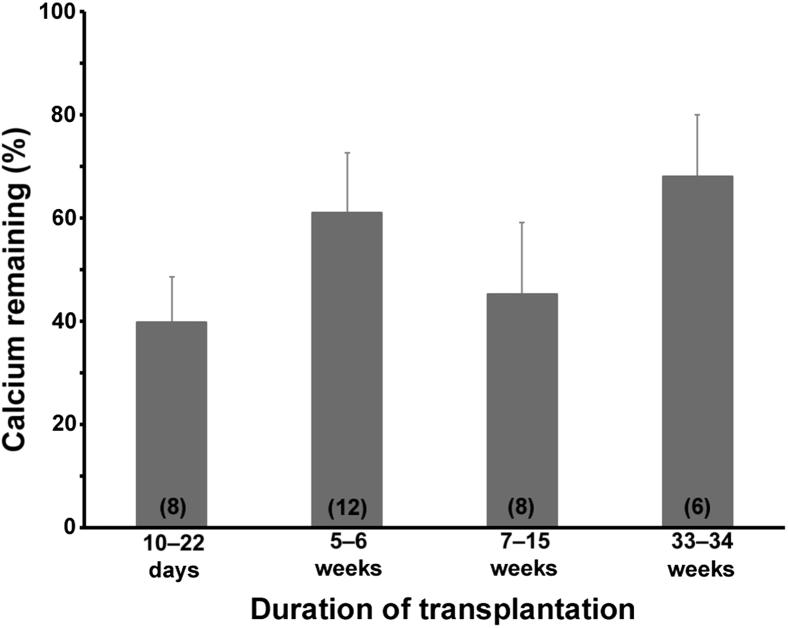
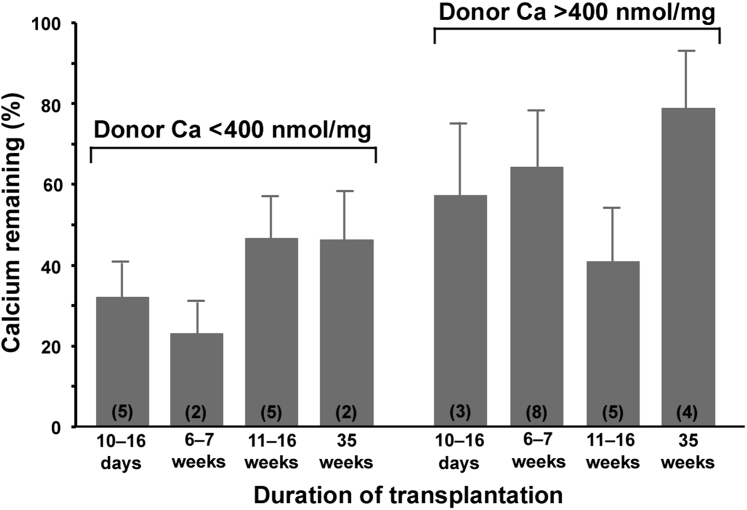
For the remaining 34 allografts, the excess calcium was 52% ± 5% of that in the paired donor aortas (range, 4% to 109%; P < 0.0001) and, as shown in Figure 1, the decrease occurred entirely within the first 2 weeks and did not progress with longer transplant durations. Calcium contents for each transplantation are shown in Supplemental Figure S1. There was no decrease in the calcium content when calcified aortas were placed in tissue culture medium (Dulbecco's modified Eagle's medium) in a CO2 incubator for 24 hours (data not shown), suggesting that there is not an immediate dissolution of calcium after harvesting of the uremic aortas. Although the calcium content of the allografts appeared to increase over time, this was not statistically significant and likely represents variability caused by heterogeneity in calcification. There was no significant correlation between the percentage of remaining calcium and the duration of uremia (r = 0.22), age at transplantation (r = 0.26), the transplant duration (r = 0.26), or the donor aortic calcium content (r = 0.05). The percentage of remaining calcium was greater in allografts from heavily calcified (>400 nmol/mg; n = 20) versus less calcified (<400 nmol/mg; n = 14) donor aortas (60.4 ± 7.5 versus 38.2 ± 5.8; P = 0.037), but the temporal pattern was unchanged (Figure 2). Calcium contents >400 nmol/mg result in dense, concentric calcification.
Figure 1.
Excess calcium content of aortic allografts expressed as a percentage of the calcium content of the donor aorta. Error bars denote standard errors. Numbers within parentheses indicate the number of allografts. Each group was significantly less than 100% (by paired t-test), but there was no difference between the groups (P = 0.30 by analysis of variance).
Figure 2.
Excess calcium content of aortic allografts from mildly and heavily calcified donor aortas expressed as a percentage of the calcium content of the donor aorta. Error bars denote standard errors. Numbers within parentheses indicate the number of allografts. There were no differences between groups by analysis of variance.
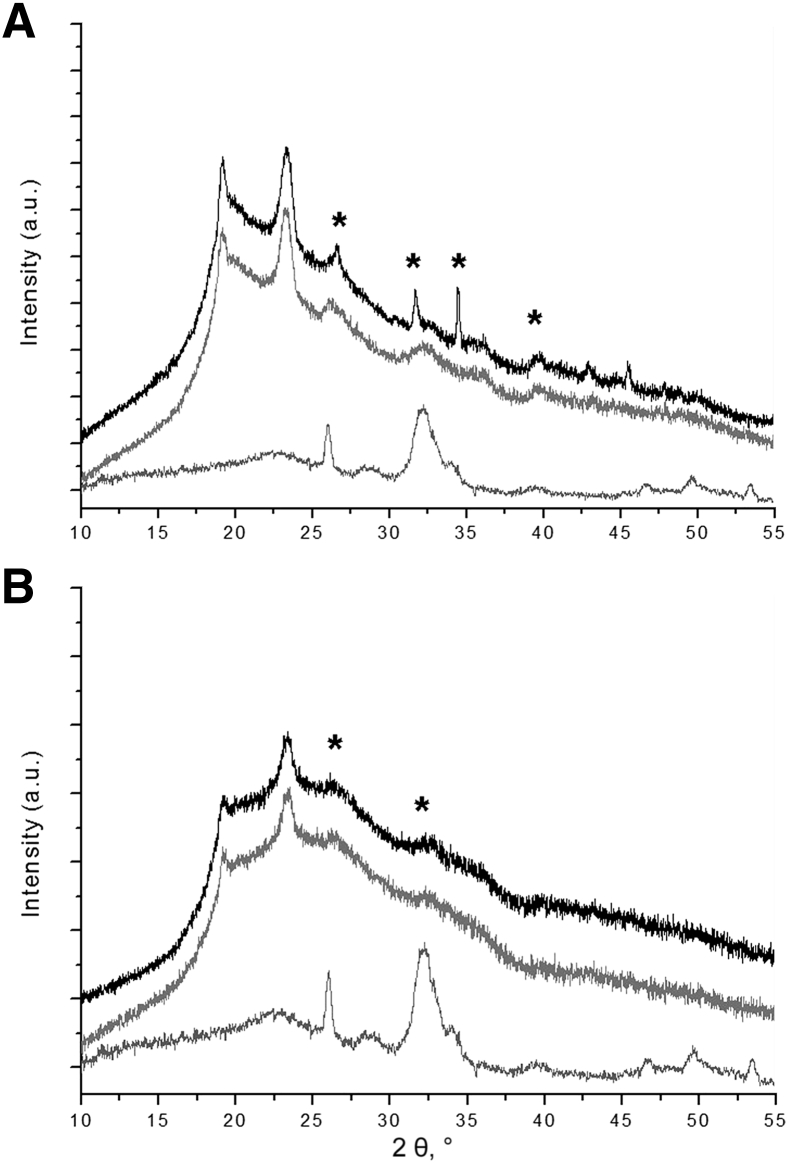
The ratio of calcium to phosphate also was compared in allografts and nontransplanted donor aortas. The ratio in allografts was 1.63 ± 0.04, close to that of pure hydroxyapatite (1.67). The ratio in the donor aortas was significantly lower (1.49 ± 0.05; P < 0.05), consistent with a greater proportion of nonapatitic precursors or amorphous compounds. X-ray diffraction showed the presence of apatite in calcified uremic aortas and allografts (Figure 3). This apatitic phase had a poor crystalline structure typical of the biological apatite observed in bone, as shown by the broader diffraction peaks. However, peak broadening was enhanced, and their intensities were much lower in allografts than that observed in uremic aortas, suggesting a reduction in the apatitic mineral phase in the transplanted aortas.
Figure 3.
X-ray diffraction of calcified aortas. A: Uremic calcified aortas (top two spectra). B: Allografts at 2 weeks (top spectra) and at 8 months (middle spectra). Bottom spectra are X-ray diffraction patterns of mouse bone apatite. Asterisks indicate hydroxyapatite peaks. The two unlabeled peaks to the left of the asterisks derive from the OCT embedding medium. a.u., arbitrary unit.
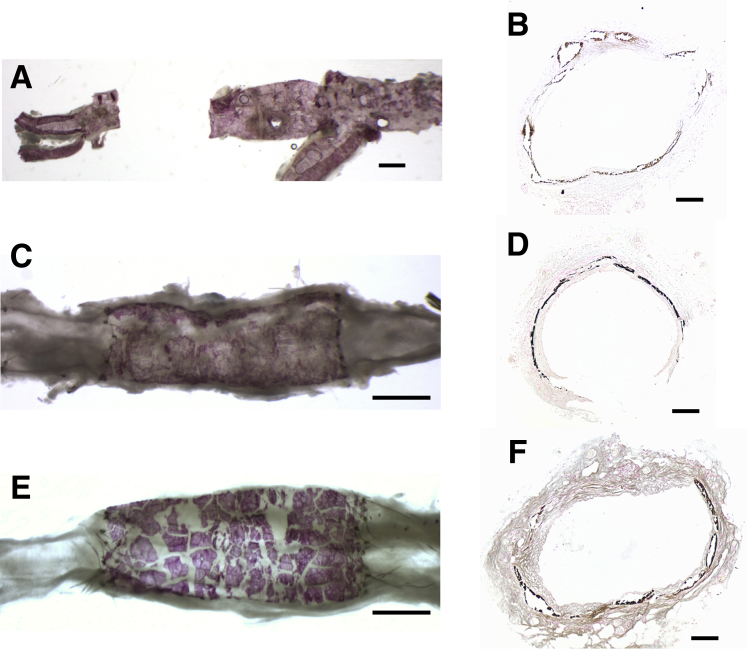
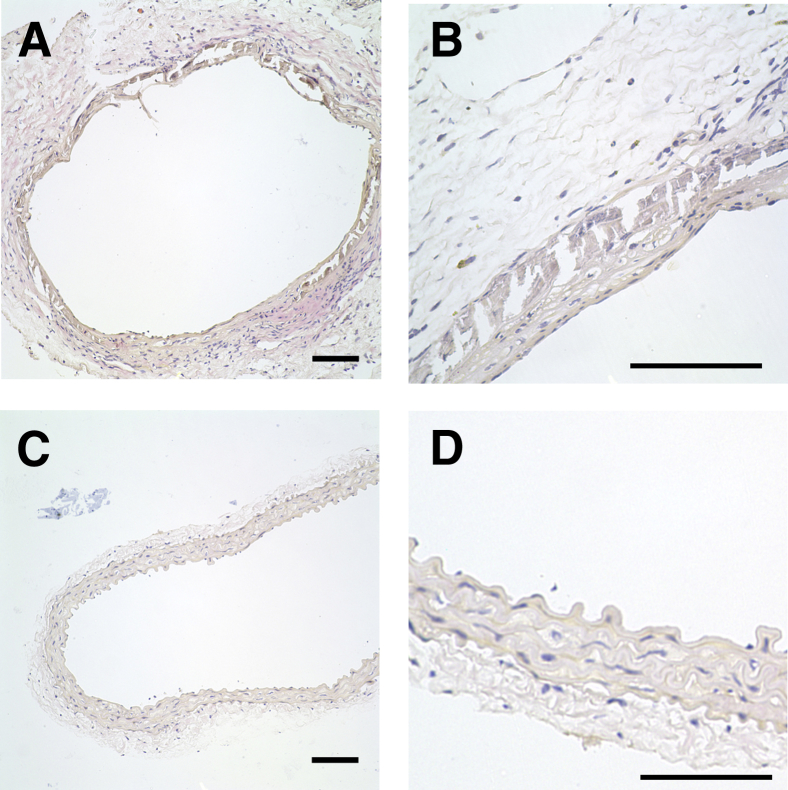
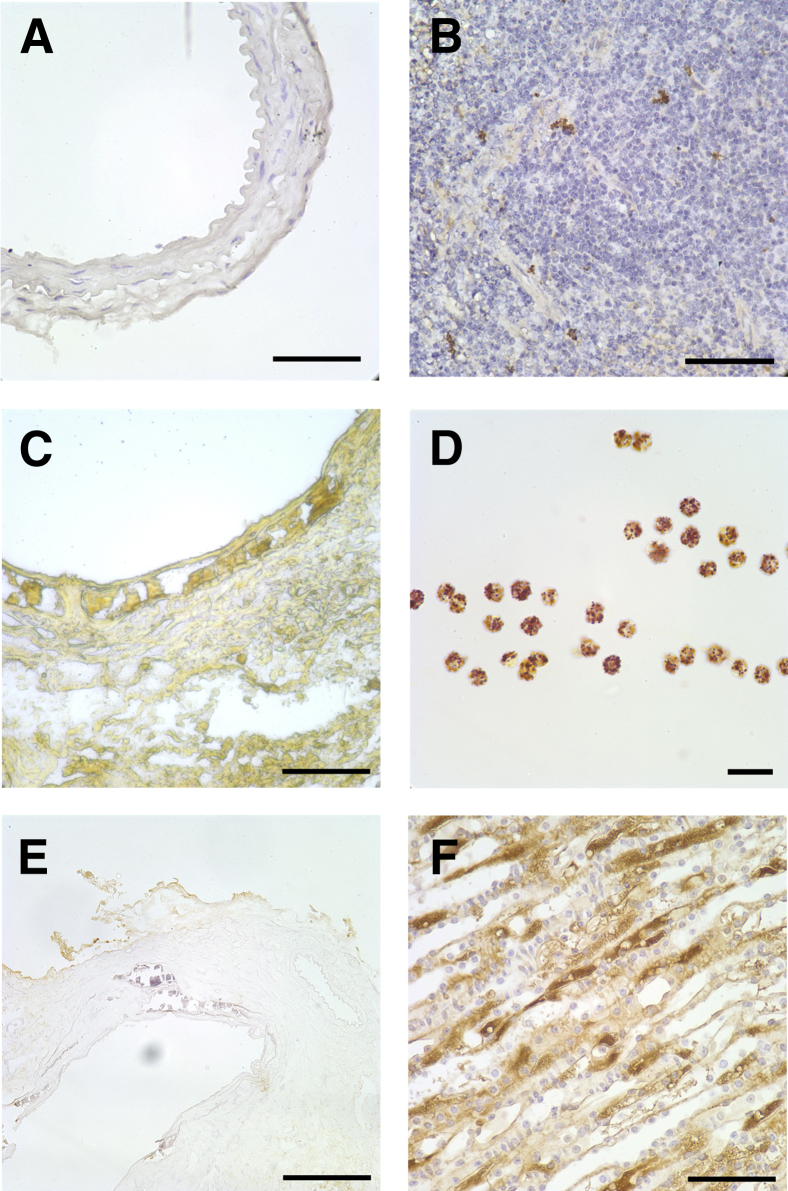
Figure 4 shows the macroscopic and microscopic appearance of the aortic calcifications as shown by alizarin red staining of the luminal surface or by von Kossa staining of formalin-fixed sections. The calcification was exclusively medial, as observed with the adenine model in rats. There was occasional fracturing of the calcified internal elastic lamina and sloughing of the intima during the preparation for histology. Consistent with humans and in other animal models, the calcification was heterogeneous, but there was no obvious difference in the pattern between allografts and donor aortas, or with the duration of transplantation. Staining of allografts with hematoxylin and eosin did not show any inflammatory cell infiltrates or multinucleated cells (Figure 5). The absence of inflammatory cells and osteoclasts was confirmed by staining for CD11b, a marker of murine macrophages (Figure 6, A and B), and staining for tartrate-resistant acid phosphatase (Figure 6, C and D). There was also no cellular staining for carbonic anhydrase II (Figure 6, E and F). In each case, at least five separate allografts (four sections per allograft) were examined.
Figure 4.
Calcification of donor aortas and allografts. A, C, and E: Alizarin red stain. B, D, and F: von Kossa stain. A: Donor aorta. C: Allograft from aorta shown in A, 10 weeks after transplantation. E: Heavily calcified allograft 34 weeks after transplantation. B: Donor aorta. D: Allograft from aorta shown in B, 6 weeks after transplantation. F: Allograft 35 weeks after transplantation. Some fracturing of the calcifications occurred during preparation of the vessels. Scale bars: 1 mm (A, C, and E); 100 μm (B, D, and F).
Figure 5.
Histology of calcified aortic allografts (hematoxylin and eosin staining). A: Seventy-five days after transplant (low magnification). B: Thirty-nine days after transplant (high magnification). C: Noncalcified segment from same allograft as in B. D: Enlarged view of C. Scale bars = 100 μm.
Figure 6.
Histochemistry of aortic allografts. A: Immunohistochemistry for CD11b in a transplanted aorta. B: Positive staining for CD11b in mouse spleen. C: Histochemistry for tartrate-resistant acid phosphatase in a heavily calcified allograft. D: Positive staining for acid phosphatase in human neutrophils. E: Immunohistochemistry for carbonic anhydrase II in a calcified allograft. F: Positive staining for CA II in mouse kidney. Scale bars: 100 μm (A, B, E, F); 50 μm (C); 20 μm (D).
Discussion
Aortic transplantation provides a unique opportunity to examine the reversibility of vascular calcification because a normal internal milieu is restored immediately. Immunologic changes related to transplantation are minimized by using littermates, and this was confirmed by the absence of cellular infiltrates and macrophages. This model has been used previously to distinguish between local and systemic factors in vascular calcification, also without any signs of cell-mediated rejection.17, 25 Calcification of the aortas renders the transplantation more challenging; however, despite that, the surgical survival rate was greater than 90%.
The major finding in this study was the stable persistence of vascular calcifications after transplantation for up to 34 weeks. Presumably, the calcification process was halted by transplantation into normal mice, but it is possible that the calcifications could serve as nucleation sites for further calcification under normal conditions. Injury associated with transplantation does not contribute to the persistence of the calcifications because we previously have shown no calcification in aortic allografts from normal mice.17, 25
No evidence was found for active resorption of the calcifications. Apatite is highly insoluble under physiological conditions and requires an acid environment for dissolution that, in bone, is created by osteoclasts. Presumably, this could also be accomplished through phagocytosis by macrophages, with subsequent dissolution in acidic lysosomes, but this would be limited to small isolated crystals or small crystal aggregates. However, there was no histologic evidence of cellular infiltrates, multinucleated cells, macrophages, or osteoclasts. Although osteoclasts occasionally are observed in calcified human arteries23 and heart valves,27 their presence is very rare, and evidence that they actively resorb calcifications is lacking.
Another potential mechanism for resorption of calcification is acid generation by adjacent smooth muscle cells, which would require carbonic anhydrase to generate carbonic acid. Up-regulation of carbonic anhydrase II has been shown in smooth muscle cells of calcified aortas from rats treated with warfarin,15 and its absence in mice results in vascular calcification.24 However, there was no detectable expression of carbonic anhydrase II in calcified uremic aortas, either before or after transplantation. Whether the discrepancy with the warfarin-treated rats is because of the different model of vascular calcification is unclear.
Despite the absence of active resorption, approximately half the calcium content disappeared from the calcified allografts within the first 2 weeks. Presumably, this occurred through spontaneous dissolution of more soluble precursors of apatite such as brushite (CaHPO4·2H2O) and/or amorphous calcium phosphate,21, 22 which is supported by the calcium/phosphorus ratios. In allografts, this ratio was 1.63, which is close to that for stochiometric apatite (1.67). However, the ratio was lower in donor aortas, consistent with the presence of brushite (Ca/P = 1.0) and/or amorphous calcium phosphate. The fact that the proportional loss of calcium tended to be less in more heavily calcified allografts supports this interpretation because more heavily calcified aortas should have a proportionally higher apatite content. However, the X-ray diffraction data suggest some loss of apatite in allografts as well. If true, the mechanism is unclear and must be limited to a specific fraction of apatite because further removal of calcifications over time was not observed.
Previous animal studies on the reversibility of medial arterial calcification have been limited to models in which the underlying cause could be removed.13, 14, 15 In a model of acute calcitriol toxicity in rats, there was progressive resolution of the calcification during the 9 weeks after calcitriol was stopped.14 However, 28% of the calcium content still remained after 9 weeks, and longer time points were not examined. It is unlikely that significant quantities of apatite would form during just 8 days of calcitriol treatment. In a model of warfarin-induced vascular calcification over 6 weeks, calcification was not reduced and actually continued to increase after warfarin was stopped.13 Reversal was observed when high doses of vitamin K were added, with 60% of the calcium remaining after 6 weeks, similar to the proportion of residual calcium in transplanted uremic aortas. Similarly, 53% of warfarin-induced vascular calcification in another study remained 18 weeks after stopping the warfarin.15
Reversal of vascular calcification has been shown most clearly in generalized arterial calcification of infancy. This disorder is due to a deficiency of ectonucleotide pyrophosphatase pyrophosphorylase, an enzyme that produces pyrophosphate, a key inhibitor of ectopic calcification.28, 29 Complete radiologic resolution of calcifications has been reported in children even without specific therapy.16, 30, 31, 32, 33, 34 However, we observed no regression of aortic calcification when aortas from a mouse model of this disease were transplanted into normal mice.17 The mechanism of reversal has not been explored and whether this is related to continued growth of the arteries during development or whether calcification remains histologically is unclear.
Although reversal of uremia has not been examined, evidence for some reversal of atherosclerotic calcification has been shown in uremic mice after treatment with sevelamer.12 However, it is not known whether this was because of reversal of the atherosclerosis or the calcification. There was no reversal of aortic calcification when uremic rats were switched from a high-phosphate diet to a low-phosphate diet for 28 days, although there may have been some reversal in rats treated with a calcimimetic.35 In contrast, reversal of aortic valve calcification in uremic rats was shown after removal of adenine and supplemental phosphate from the diet.36 The mechanism of the calcium resorption was not investigated.
Whether uremic vascular calcification is reversible in humans remains unclear. A 39% decrease in coronary artery calcification, similar to that observed in mouse aortic allografts, was found 6 months after renal transplantation in 31 patients.8 However, no change was noted after renal transplantation in another study.9 Although renal failure was eliminated, this experimental approach was complicated by the metabolic derangements in transplant patients that can cause progression of atherosclerosis and the accompanying calcification.11 Treatments to correct altered mineral metabolism in patients with renal failure can slow progression of vascular calcification, but reversal has not been shown. Treatment with etidronate appeared to reduce coronary artery calcification in some, but not all, patients with end-stage renal disease.37
In summary, there was no evidence of active resorption of uremic vascular calcification when calcified aortas were transplanted into normal animals. Although there was rapid disappearance of a portion of the excess calcium in uremic aortas, the majority of the excess calcium, likely representing poorly crystalline apatite, remains over time, with no evidence of removal. The absence of active resorption may contribute to the severity of vascular calcification and supports a preventative approach to vascular calcification.
Footnotes
Supported by NIH grant K08 DK079176 (K.A.L.).
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2016.10.006.
Supplemental Data
Individual data for donor and transplanted uremic aortas. The times indicate the transplantation duration.
References
- 1.Abou-Hassan N., D'Orsi E.T., D'Orsi C.J., O'Neill W.C. The risk for medial arterial calcification in CKD. Clin J Am Soc Nephrol. 2012;7:275–279. doi: 10.2215/CJN.06490711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehto S., Niskanen L., Suhonen M., Ronnemaa T., Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 3.Iribarren C., Go A.S., Tolstykh I., Sidney S., Johnston S.C., Spring D.B. Breast vascular calcification and risk of coronary heart disease, stroke, and heart failure. J Womens Health. 2004;13:381–389. doi: 10.1089/154099904323087060. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhury U.K., Airan B., Mishra P.K., Kothari S.S., Subramaniam G.K., Ray R., Singh R., Venugopal P. Histopathology and morphometry of radial artery conduits: basic study and clinical application. Ann Thorac Surg. 2004;78:1614–1622. doi: 10.1016/j.athoracsur.2004.03.105. [DOI] [PubMed] [Google Scholar]
- 5.Everhart J.E., Pettitt D.J., Knowler W.C., Rose F.A., Bennett P.H. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988;31:16–23. doi: 10.1007/BF00279127. [DOI] [PubMed] [Google Scholar]
- 6.Blacher J., Guerin A.P., Pannier B., Marchais S.J., London G.M. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 7.Verberckmoes R., Bouillon R., Krempien B. Disappearance of vascular calcifications during treatment of renal osteodystrophy. Ann Intern Med. 1975;82:529–533. doi: 10.7326/0003-4819-82-4-529. [DOI] [PubMed] [Google Scholar]
- 8.Abedi S.A., Tarzamni M.K., Nakhjavani M.R., Bohlooli A. Effect of renal transplantation on coronary artery calcification in hemodialysis patients. Transplant Proc. 2009;41:2829–2831. doi: 10.1016/j.transproceed.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Moe S.M., O'Neill K.D., Resterova M., Fineberg N., Persohn S., Meyer C.A. Natural history of vascular calcification in dialysis and transplant patients. Nephrol Dial Transplant. 2004;19:2387–2393. doi: 10.1093/ndt/gfh303. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura S., Ishibashi-Ueda H., Niizuma S., Yoshihara F., Horio T., Kawano Y. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. 2009;4:1892–1990. doi: 10.2215/CJN.04320709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marechal C., Coche E., Goffin E., Dragean A., Schlieper G., Nguyen P., Floege J., Kanaan N., Devuyst O., Jadoul M. Progression of coronary artery calcification and thoracic aorta calcification in kidney transplant recipients. Am J Kidney Dis. 2012;59:258–269. doi: 10.1053/j.ajkd.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Mathew S., Lund R.J., Strebeck F., Tustison K.S., Geurs T., Hruska K.A. Reversal of the adynamic bone disorder and decreased vascular calcification in chronic kidney disease by sevelamer carbonate therapy. J Am Soc Nephrol. 2007;18:122–130. doi: 10.1681/ASN.2006050490. [DOI] [PubMed] [Google Scholar]
- 13.Schurgers L.J., Spronk H.M.H., Soute B.A.M., Schiffers P.M., DeMey J.G.R., Vermeer C. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood. 2007;109:2823–2831. doi: 10.1182/blood-2006-07-035345. [DOI] [PubMed] [Google Scholar]
- 14.Bas A., Lopez I., Perez J., Rodriguez M., Aguilera-Tejero E. Reversibility of calcitriol-induced medial artery calcification in rats with intact renal function. J Bone Miner Res. 2006;21:484–490. doi: 10.1359/JBMR.051211. [DOI] [PubMed] [Google Scholar]
- 15.Essalihi R., Dao H.H., Gilbert L.A., Bouvet C., Semerjian Y., McKee M.D., Moreau P. Regression of medial elastocalcinosis in rat aorta. A new vascular function for carbonic anhydrase. Circulation. 2005;112:1628–1635. doi: 10.1161/CIRCULATIONAHA.104.528984. [DOI] [PubMed] [Google Scholar]
- 16.Otero J.E., Gottesman G.S., McAlister W.H., Mumm S., Madson K.L., Kiffer-Moreira T., Sheen C., Millan J.L., Ericson K.L., Whyte M.P. Severe skeletal toxicity from protracted etidronate therapy for generalized arterial calcification of infancy. J Bone Miner Res. 2013;28:419–430. doi: 10.1002/jbmr.1752. [DOI] [PubMed] [Google Scholar]
- 17.Lomashvili K.A., Narisawa S., Millan J.L., O'Neill W.C. Vascular calcification is dependent on plasma levels of pyrophosphate. Kidney Int. 2014;85:1351–1356. doi: 10.1038/ki.2013.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeGeros R.Z., Contiguglia S.R., Alfrey A.C. Pathological calcifications associated with uremia. Calcif Tissue Res. 1973;13:173–185. doi: 10.1007/BF02015408. [DOI] [PubMed] [Google Scholar]
- 19.Verberckmoes S.C., Persy V., Behets G.J., Neven E., Hufkens A., Zebger-Gong H., Muller D., Haffner D., Querfeld U., Bohic S., De Broe M.E., D'Haese P.C. Uremia-related vascular calcification. More than apatite deposition. Kidney Int. 2007;71:298–303. doi: 10.1038/sj.ki.5002028. [DOI] [PubMed] [Google Scholar]
- 20.O'Neill W.C. Vascular calcification: not so crystal clear. Kidney Int. 2007;71:282–283. doi: 10.1038/sj.ki.5002119. [DOI] [PubMed] [Google Scholar]
- 21.Neuman W.F., Neuman M.W. University of Chicago Press; Chicago, IL: 1958. The Chemical Dynamics of Bone Mineral. [Google Scholar]
- 22.Johnsson M.S., Nancollas G.H. The role of brushite and octacalcium phosphate in apatite formation. Crit Rev Oral Biol Med. 1992;3:61–82. doi: 10.1177/10454411920030010601. [DOI] [PubMed] [Google Scholar]
- 23.Han K.H., Hennigar R.A., O'Neill W.C. The association of bone and osteoclasts with vascular calcification. Vasc Med. 2015;20:527–533. doi: 10.1177/1358863X15597076. [DOI] [PubMed] [Google Scholar]
- 24.Spicer S.S., Lewis S.E., Tashian R.E., Schulte B.A. Mice carrying a CAR-2 null allele lack carbonic anhydrase 11 immunohistochemically and show vascular calcification. Am J Pathol. 1989;134:947–954. [PMC free article] [PubMed] [Google Scholar]
- 25.Lomashvili K.A., Wang X., O'Neill W.C. Role of local versus systemic vitamin D receptors in vascular calcification. Arterioscler Thromb Vasc Biol. 2014;34:146–151. doi: 10.1161/ATVBAHA.113.302525. [DOI] [PubMed] [Google Scholar]
- 26.Cogan E.B., Birrell G.B., Griffith O.H. A robotics-based automated assay for inorganic and organic phosphates. Anal Biochem. 1999;271:29–35. doi: 10.1006/abio.1999.4100. [DOI] [PubMed] [Google Scholar]
- 27.Mohler E.R., Gannon F., Reynolds C., Zimmerman R., Keane M., Kaplan F.S. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 28.Rutsch F., Vaingankar S., Johnson K., Goldfine I., Maddux B., Schauerte P., Kalhoff H., Sano K., Boisvert W.A., Superti-Furga A., Terkeltaub R.A. PC-1 nucleotide triphosphate pyrophosphohydrolase deficiency in idiopathic infantile arterial calcification. Am J Pathol. 2001;158:543–554. doi: 10.1016/S0002-9440(10)63996-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lomashvili K.A., Cobbs S., Hennigar R.A., Hardcastle K.I., O'Neill W.C. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol. 2004;15:1392–1401. doi: 10.1097/01.asn.0000128955.83129.9c. [DOI] [PubMed] [Google Scholar]
- 30.Sholler G.F., Yu J.S., Bale P.M., Hawker R.E., Celermajer J.M., Kozlowski K. Generalized arterial calcification of infancy: three case reports, including spontaneous regression with long-term survival. J Pediatr. 1984;105:257–260. doi: 10.1016/s0022-3476(84)80123-7. [DOI] [PubMed] [Google Scholar]
- 31.Marrott P.K., Newcombe K.D., Becroft D.M., Friedlander D.H. Idiopathic infantile arterial calcification with survival to adult life. Pediatr Cardiol. 1984;5:119–122. doi: 10.1007/BF02424963. [DOI] [PubMed] [Google Scholar]
- 32.Ciana G., Trappan A., Bembi B., Benettoni A., Maso G., Zennaro F., Ruf N., Schnabel D., Rutsch F. Generalized arterial calcification of infancy: two siblings with prolonged survival. Eur J Pediatr. 2006;165:258–263. doi: 10.1007/s00431-005-0035-6. [DOI] [PubMed] [Google Scholar]
- 33.Miyai K., Ariyasu D., Numakura C., Yoneda K., Nakazato H., Hasegawa Y. Hypophosphatemic rickets developed after treatment with etidronate disodium in patient with generalized arterial calcification in infancy. Bone Rep. 2015;3:57–60. doi: 10.1016/j.bonr.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreira C.R., Ziegler S.G., Gupta A., Groden C., Hsu K.S., Gahl W.A. Treatment of hypophosphatemic rickets in generalized arterial calcification of infancy (GACI) without worsening of vascular calcification. Am J Med Genet A. 2016;170A:1308–1311. doi: 10.1002/ajmg.a.37574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez I., Mendoza F.J., Guerrero F., Almaden Y., Henley C., Aguilera-Tejero E., Rodriguez M. The calcimimetic AMG 641 accelerates regression of extraosseous calcification in uremic rats. Am J Physiol Renal Physiol. 2009;296:F1376–F1385. doi: 10.1152/ajprenal.90737.2008. [DOI] [PubMed] [Google Scholar]
- 36.Shuvy M., Abedat S., Beeri R., Danenberg H.D., Planer D., Ben-Dov I.Z., Meir K., Sosna J., Lotan C. Uraemic hyperparathyroidism causes a reversible inflammatory process of aortic valve calcification in rats. Cardiovasc Res. 2008;79:492–499. doi: 10.1093/cvr/cvn088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nitta K., Akiba T., Suzuki K., Uchida K., Watanabe R.Y., Majima K., Aoki T., Nihei H. Effects of cyclic intermittent etidronate therapy on coronary artery calcification in patients receiving long-term hemodialysis. Am J Kidney Dis. 2004;44:680–688. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual data for donor and transplanted uremic aortas. The times indicate the transplantation duration.