Abstract
Mutations in the gene encoding pyrin are associated with autoinflammatory disorder Familial Mediterranean Fever (FMF). A FMF-knock-in mouse strain that expresses chimeric pyrin protein with a V726A mutation (MefvV726A/V726A) was generated to model human FMF. This mouse strain shows an autoinflammatory disorder that is prevented by genetic deletion of IL-1 (IL-1) receptor or apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC). ASC-mediated cell death leads to the release of IL-1α and IL-1β, both of which signal through IL-1 receptor. Furthermore, caspase-1 and caspase-8 can interact with ASC to mediate secretion of IL-1 cytokines. The specific IL-1 cytokine instigating development of FMF and the enzymatic caspase involved in its secretion currently are unknown. In this study, we show that the autoinflammation observed in MefvV726A/V726A mice is mediated specifically by IL-1β and not IL-1α. Furthermore, the disorder is dependent on the caspase-1–ASC axis, whereas caspase-8 is dispensable. Concurrently, aberrant IL-1β release by MefvV726A/V726A monocytes in response to stimulation with lipopolysaccharide also is dependent on the caspase-1–ASC axis. In conclusion, our studies have uncovered a specific role for caspase-1–mediated IL-1β release in the manifestation of FMF.
Familial Mediterranean Fever (FMF) is an autosomal-recessive, autoinflammatory disorder characterized by episodic fever and neutrophil-mediated inflammation of serosal tissues. The disorder is highly prevalent in Jewish, Armenian, Arab, and Turkish families geographically located in the Mediterranean basin.1 Sequence alterations in the gene encoding pyrin (MEFV; alias marenostrin), are associated with FMF.2, 3 MEFV encodes a protein of 781 amino acids with an N-terminal pyrin, a central boxed-box and coiled coil domain, and a C-terminal PRY/SPRY (B30.2) domain.4 Nearly one third of mutations found in patients with FMF are located in exon 10 of the MEFV gene (http://fmf.igh.cnrs.fr/ISSAID/infevers/schema.php?n=1, last accessed October 10, 2016). In human MEFV, exon 10 encodes for the B30.2 domain. This domain, however, is absent in the murine ortholog.5 To study the effect of these mutations, a collection of FMF-knock in (FMF-KI) mouse strains are generated that express a chimeric pyrin protein harboring the human B30.2 domain with FMF-associated mutations.5 One such FMF-KI strain is represented as MefvV726A/V726A and expresses murine pyrin spliced to the B30.2 domain with a valine to alanine substitution at amino acid position 726. The MefvV726A/V726A strain develops an autoinflammatory disorder characterized by runted growth and neutrophilia, which can be prevented by genetic deletion of apoptosis-associated speck-like protein (ASC) or IL-1–receptor (IL-1R) signaling.5 A mouse strain with V726A as one allele and wild type as another, represented as MefvV726A/+, does not show the signs of autoinflammation observed in FMF-KI strains, highlighting the recessive nature of the disease.5 FMF-KI mouse strains harboring other FMF-associated pyrin mutations within the B30.2 domain such as M680I and M694V develop a less severe form of autoinflammatory disease with similar characteristics.5
IL-1α and IL-1β are members of the IL-1 family of cytokines that signal through the same receptor, IL-1R6; however, the two cytokines have different modes of release and downstream inflammatory consequences.7, 8, 9 IL-1α is released from necrotic and pyroptotic cells as an alarmin and does not require processing by caspases for its biological activity.7, 10, 11, 12 IL-1β, however, requires proteolytic cleavage by an inflammasome complex for its maturation, followed by release from the cell via pyroptosis.13, 14 Both caspase-1 and caspase-8 associate with ASC, and can have either complementary or redundant function in the release of IL-1 cytokines.15, 16, 17, 18, 19, 20 Although both IL-1α and IL-1β are released consequent to lytic cell death and perpetuate inflammatory signal through IL-1R, they mediate distinct neutrophil-driven autoinflammatory diseases. We previously showed that although IL-1α promotes disease in a mouse model of neutrophilic dermatosis,10 IL-1β drives neutrophil-mediated disease in a mouse model of osteomyelitis.16, 21, 22
Previous studies have shown contradictory roles for wild-type and mutant pyrin (harboring mutations associated with FMF) in IL-1β processing and inflammation.1, 4, 23, 24, 25, 26, 27 Pyrin recently was identified as an inflammasome-forming sensor that responds to Rho inactivation induced by bacterial infections.28, 29 Loss of pyrin also affects caspase-8 processing and consequent apoptosis.23 These findings suggest that pyrin may mediate the release of IL-1 cytokines through distinct mechanisms. Therefore, identification of the specific IL-1 cytokine involved in FMF pathogenesis and the upstream regulatory process governing its release would clarify the immunopathogenesis of FMF.
In this study, we show that IL-β is the specific IL-1 cytokine that induces the autoinflammatory disorder in a mouse model of FMF. Furthermore, the caspase-1–ASC axis is required to drive the systemic inflammation in vivo and for aberrant IL-1β release in monocytes. Caspase-8, however, is dispensable for disease progression in this model of autoinflammatory disorder. Overall, we have shown a pathogenic role of IL-1β and described caspase-1 as a critical upstream inflammatory caspase regulating IL-1β release in a mouse model of FMF.
Materials and Methods
Mice
MefvV726A/+,5 MefvV726A/V726A,5 Il1b−/−,30 Il1a−/−,31 Casp1−/−Casp11−/−,12 Ripk3−/−Casp8−/−,32 and Asc−/−33 mice have been described previously. MefvV726A/+ mice were bred with Il1b−/−, Il1a−/−, Casp1−/−Casp11−/−, or Ripk3−/−Casp8−/− mice to generate MefvV726A/V726A mice lacking each of these components. Mice were maintained in a specific pathogen-free facility and animal studies were approved by the St. Jude Children's Research Hospital Committee on the Use and Care of Animals.
Cellular Processing and Analysis
Mice were monitored weekly for weight gain starting from 3 to 4 weeks of age and euthanized at 8 to 12 weeks of age for systemic analysis. Peritoneal cells were harvested by peritoneal lavage in RPMI media (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA), nonessential amino acids (Gibco), and penicillin-streptomycin (Sigma, St. Louis, MO). Blood was collected through cardiac puncture, and 50 μL was added to anticoagulant EDTA for cellular analysis. The spleen was homogenized and passed through a 40-μm filter to obtain a single-cell suspension. Both blood and spleen were subjected to red blood cell lysis and washed in complete media. Left lobes of lung were homogenized, filtered, and subjected to a 33% Percoll (GE, Boston, MA) gradient centrifugation to remove debris and obtain a single-cell suspension. Cells obtained from the peritoneal cavity, blood, spleen, and lung were fixed with 2% paraformaldehyde (Affymetrix, Santa Clara, CA) and stained for cellular analysis.
Flow Cytometry
The following antibodies were used for cell-surface staining: CD11b (M1/70), CD19 (6D5), CD3 (17A2), and Gr-1 (RB6-8C5) (eBiosciences, San Diego, CA). Cells were stained, run on a LSRII flow cytometer (BD Biosciences, Franklin Lakes, NJ), and analyzed with FlowJo software version 10.2 (FlowJo LLC, Ashland, OR). After removal of doublets, B cells were gated as CD19+ and T cells were gated as CD3+; CD11b+ and polymorphonuclear leukocytes (CD11b+Gr1+) were gated on cells negative for both CD3 and CD19.
Histology and Microscopy Analysis
Liver, kidney, and colon tissues were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. A board-certified pathologist (P.V.) analyzed the hematoxylin and eosin sections for the presence of inflammation and signs of tissue damage in a blinded manner.
Cell Culture and Stimulation
Monocytes were obtained by culturing cells from bone marrow in Dulbecco's modified Eagle's media (Gibco) supplemented with 30% media conditioned by L929 fibroblasts, 10% fetal bovine serum, nonessential amino acids, and penicillin-streptomycin for 3 days. Nonadherent monocytes were collected, counted, and plated at a density of 1 × 106 cells per milliliter for stimulation. Cells were stimulated with lipopolysaccharide (LPS; InvivoGen, San Diego, CA) (200 ng/mL) for 48 hours. At the end of stimulation, 120 μL of supernatant was removed for enzyme-linked immunosorbent assay. Cells and supernatants then were lysed with RIPA buffer containing protease inhibitors and phosphatase inhibitors (Calbiochem, Billerica, MA), and boiled in SDS sample buffer for Western blot analysis. Caspase-1 activation was assessed by Western blot using the anti–caspase-1 p20 antibody (1:3000) from Adipogen (San Diego, CA) (AG-20B-0042-C100) as previously described.17
Enzyme-Linked Immunosorbent Assay
Cytokines in the serum and cell-culture supernatants were measured by enzyme-linked immunosorbent assay, according to the manufacturer's instructions. The IL-1β and multiplex enzyme-linked immunosorbent assay kits were obtained from eBiosciences and Millipore (San Diego, CA), respectively.
Statistical Analysis
All statistical analyses were performed using Prism software version 6.0 (GraphPad Software, San Diego, CA). The t-test or one-way analysis of variance was used with the Fisher least significance difference or Dunn's post-test as indicated. Two-way analysis of variance was used to analyze the kinetics of body weight gain and spleen composition. In most analyses, MefvV726A/+ mice were used as a control to show the degree of disease or protection. P < 0.05 was considered significant.
Results
Runting and Systemic Inflammation in MefvV726A/V726A Mice Are Mediated Specifically by IL-1β and Not IL-1α
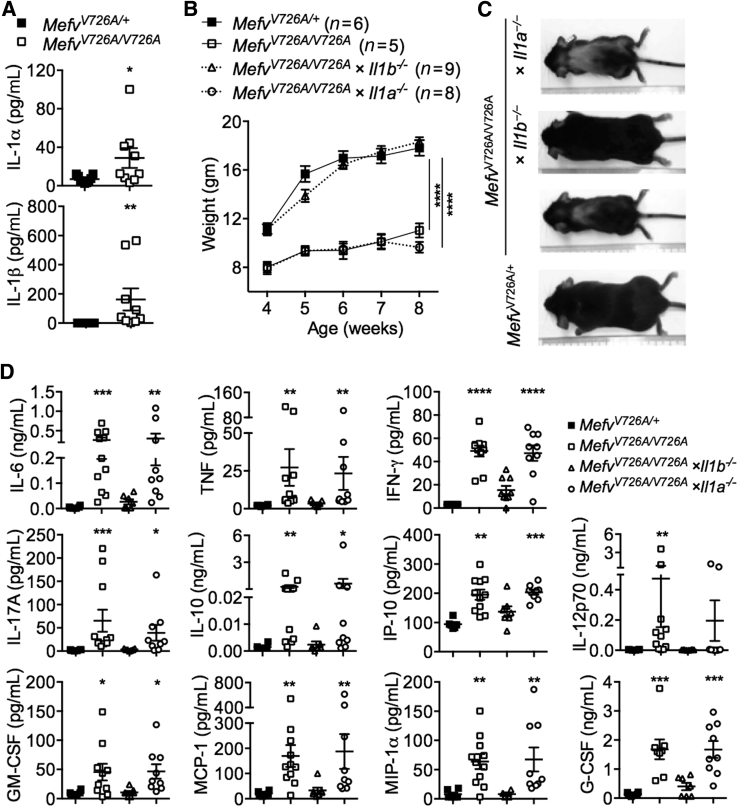
We found significantly increased levels of IL-1α and IL-1β in the serum of MefvV726A/V726A mice in comparison with control MefvV726A/+ mice (Figure 1A), confirming data from a previous study.5 To dissect the role of individual IL-1 cytokines in this model of FMF, MefvV726A/V726A mice lacking either IL-1α or IL-1β were generated and tracked for clinical signs of disease, including body weight and an increase in levels of inflammatory mediators.
Figure 1.
Runting and systemic inflammation in MefvV726A/V726A mice is mediated specifically by IL-1β, but not IL-1α. A: Levels of IL-1α and IL-1β in serum samples. B and C: Body weights of the indicated number of female mice (B) and representative whole-body image (C) of mice at 8 weeks of age. D: Cytokine levels in serum samples. A and D: The x axis in column graphs shows mice strains indicated in separate legend keys. Data are expressed as means ± SEM (A, B, and D). n = 7 to 10 for each genotype (A, B, and D). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001 versus MefvV726A/+ by [t-test (A), two-way analysis of variance (B), and the Kruskal–Wallis test followed by the Dunn post-test (D)]. IFN, interferon; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; MCP-1, monocyte chemoattractant protein-1; MIP-1α, macrophage inflammatory protein-1α; TNF, tumor necrosis factor.
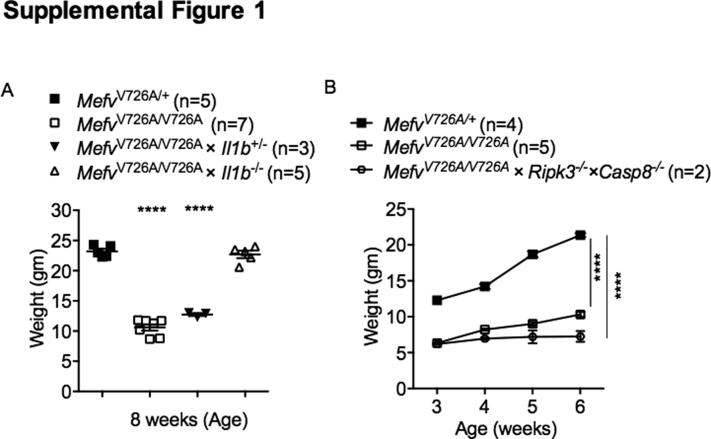
Although MefvV726A/V726A mice gained significantly less weight than MefvV726A/+ mice, genetic deletion of IL-1β completely protected MefvV726A/V726A mice from growth retardation and wasting (Figure 1, B and C). Heterozygosity of Il1b in MefvV726A/V726A mice did not prevent runting, showing that a complete loss of IL-1β is required for protection (Supplemental Figure S1A). Genetic loss of IL-1α, on the other hand, did not affect body weight gain in MefvV726A/V726A mice (Figure 1, B and C), suggesting that IL-1α is dispensable for disease progression. A previous study showed that inflammatory mediators that drive clinical manifestations of wasting are increased in the serum of MefvV726A/V726A mice.5 As expected, the levels of cytokines and chemokines including IL-6, tumor necrosis factor, interferon-γ, IL-17A, IL-10, IP-10, IL-12p70, granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, and granulocyte colony-stimulating factor were increased significantly in serum of MefvV726A/V726A mice compared with the levels in MefvV726A/+ mice (Figure 1D). Genetic ablation of IL-1β, and not IL-1α, in MefvV726A/V726A mice reduced the level of all cytokines to that observed in MefvV726A/+ mice (Figure 1D). Collectively, these data show that IL-1β but not IL-1α is the cytokine that drives hyperinflammation and wasting in a mouse model of FMF.
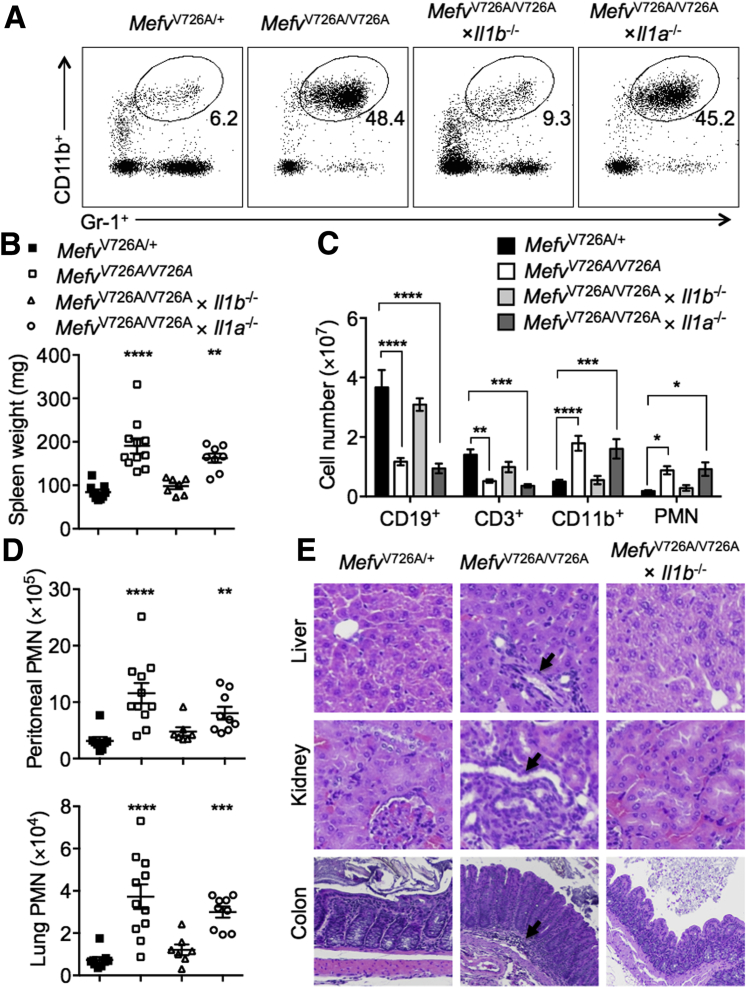
IL-1β Is a Critical Driver of Neutrophilia and Multiorgan Damage Observed in MefvV726A/V726A Mice
Systemic neutrophilia and inflammation of the serosal tissues is one of the hallmarks of FMF.1, 5 Inflammation in MefvV726A/V726A mice further leads to splenomegaly and a change in cellular composition, such that the number of B and T lymphocytes is reduced drastically, whereas the total number of CD11b+ cells and neutrophils (polymorphonuclear leukocytes) is increased.5 Genetic deletion of IL-1β protected MefvV726A/V726A mice from an increased proportion of neutrophils in blood, an increase in splenic weight, and alterations in the splenic cellular composition (Figure 2, A–C). Furthermore, increased neutrophil infiltration observed in the peritoneal cavity and lungs of MefvV726A/V726A mice also was prevented after genetic loss of IL-1β (Figure 2D). All of these features of neutrophil infiltration in MefvV726A/V726A mice were unaffected by genetic deletion of IL-1α (Figure 2, A–D). The loss of IL-1β further protected MefvV726A/V726A mice against histologic perturbations observed in the liver, kidney, and colon that include moderate and multifocal cellular infiltration (Figure 2E). MefvV726A/V726A mice also showed signs of organ damage such as bile duct hyperplasia in the liver, glomerulopathy, necrosis, and proteinosis in the kidney and mucosal hyperplasia in the colon. These clinical features of organ damage were prevented by genetic ablation of IL-1β (Figure 2E). Altogether, these data show that IL-1β is a critical driver of inflammation and consequent clinical manifestations in a mouse model of FMF.
Figure 2.
IL-1β is the critical driver of neutrophilia and organ dysfunction observed in MefvV726A/V726A mice. A: Representative dot plot showing the proportion of polymorphonuclear leukocytes (PMNs) in the blood of indicated mice. The cells were gated on single cells; the percentage of cells identified as PMN is noted on each plot. B–D: Spleen weight (B), spleen cellular composition (C), and PMN infiltration (D) in the peritoneal cavity and lung. E: Representative hematoxylin and eosin–stained sections of liver, kidney, and colon tissues. Black arrows indicate granulocytic infiltration in the tissues. B and D: The x axis in the column graphs shows mice strains indicated in the legend key. Data are expressed as means ± SEM (B–D). n = 7 to 10 for each genotype (B–D). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001 versus MefvV726A/+ by using the Kruskal–Wallis test followed by the Dunn post-test (B and D) and two-way analysis of variance (C). Original magnification, ×20 (E).
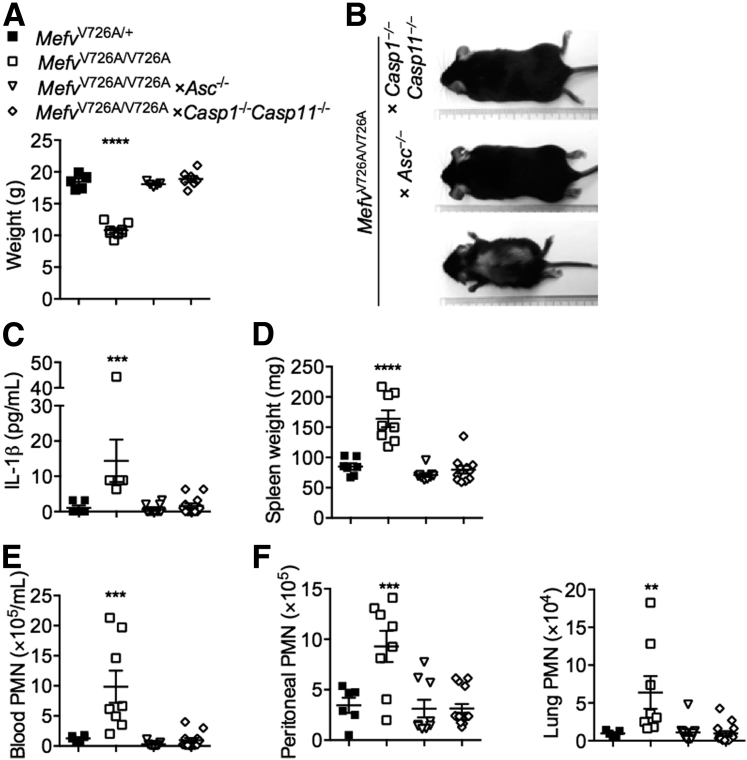
Autoinflammatory Disease in MefvV726A/V726A Mice Is Dependent on the Caspase-1–ASC Axis
The autoinflammatory disorder observed in MefvV726A/V726A mice is rescued by the loss of inflammasome adaptor ASC, implicating a role for inflammasome in the release of IL-1β in this disorder.5 After certain infectious and sterile insults, both caspase-1 and caspase-8 associate with ASC to promote IL-1β processing.15, 17, 18, 20 In addition, caspase-8 can process IL-1β independently of caspase-1.15, 16, 18, 19, 20, 21, 34 To determine whether this autoinflammatory disorder was owing to caspase-1 or caspase-8, we generated MefvV726A/V726A mice lacking either caspase-1 or caspase-8. Genetic deletion of caspase-8 is embryonically lethal, but this lethality is rescued by further deletion of receptor-interacting protein kinase 3 (Ripk3−/−).32, 35 We therefore generated MefvV726A/V726A × Ripk3−/− × Casp8−/− mice to assess the role of caspase-8 in this disorder. We tracked MefvV726A/V726A mice lacking caspase-1 or caspase-8 and RIPK3 for body weight gain, an indicator of wasting and systemic inflammation in this mouse model. Although MefvV726A/V726A mice lacking caspase-1 gained body weight to the same extent as MefvV726A/+ mice (Figure 3, A and B), loss of caspase-8 did not protect against growth retardation and runting (Supplemental Figure S1B). Furthermore, the increase in the IL-1β level observed in the serum of MefvV726A/V726A mice was prevented by genetic deletion of ASC or caspase-1 (Figure 3C). Other signs of systemic inflammation such as splenomegaly (Figure 3D), systemic neutrophilia, and multiorgan neutrophil infiltration (Figure 3, E and F) observed in MefvV726A/V726A mice also were prevented by genetic loss of ASC or caspase-1. These data show a critical role for the ASC–caspase-1 axis in driving the IL-1β–mediated autoinflammatory disorder in MefvV726A/V726A mice. The Casp1−/− mouse strain additionally lacked caspase-11, but a functional role for caspase-11 in this disease model can be ruled out because caspase-11 is known to engage the NLRP3 inflammasome to process IL-1β,12 and loss of NLRP3 in MefvV726A/V726A mice did not protect against autoinflammation.5
Figure 3.
Autoinflammatory disease in MefvV726A/V726A mice is dependent on the caspase-1–ASC axis. A and B: Body weights of female mice (A) and representative whole-body image (B) of mice at 8 weeks of age. C–F: Level of IL-1β in serum samples (C), spleen weight (D), total number of circulating polymorphonuclear leukocytes (PMNs) per milliliter of blood (E), and PMN enumeration (F) in the peritoneal cavity and lung of indicated mice. The x axis in the column graphs shows mice strains indicated in the legend key (A and C–F). Data are expressed as means ± SEM (A and C–F). n = 5 to 11 for each genotype (A and C–F). ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001 versus MefvV726A/+ (A and C–F, one-way analysis of variance followed by the Fisher least significance difference test).
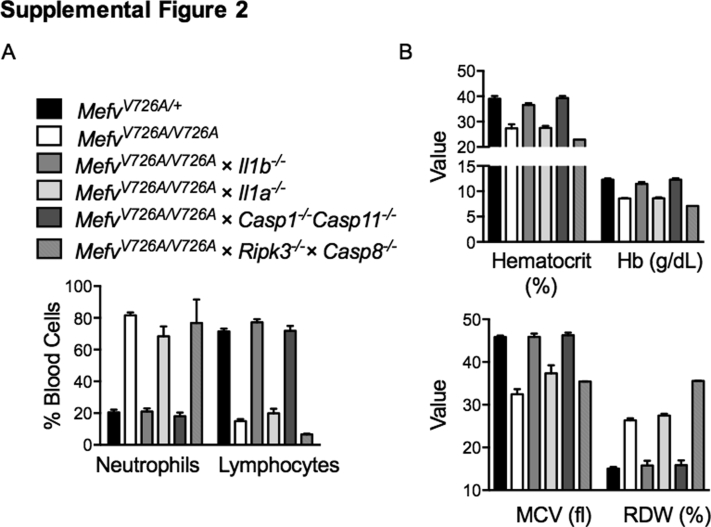
Anemia has been observed in patients with FMF36 and is a common feature of multiple autoinflammatory disorders.37 Inflammatory cytokines including IL-1β have been ascribed an inhibitory role in erythropoiesis leading to anemia.38, 39 We analyzed the parameters of anemia such as hematocrit, hemoglobin level, mean corpuscular volume, and red cell distribution width in the blood of FMF-KI mice lacking IL-1α, IL-1β, caspase-1, or caspase-8 (Ripk3−/−). Anemia was observed in MefvV726A/V726A mice as shown by lower hematocrit and hemoglobin levels (Supplemental Figure S2B). Similar to the inflammatory features of neutrophilia and lymphopenia, anemia observed in MefvV726A/V726A mice also was dependent on the Casp1–IL-1β axis (Supplemental Figure S2, A and B). Lower mean corpuscular volume and higher red cell distribution width is indicative of iron deficiency and is consistent with the role of IL-1 in iron homeostasis and anemia.40, 41
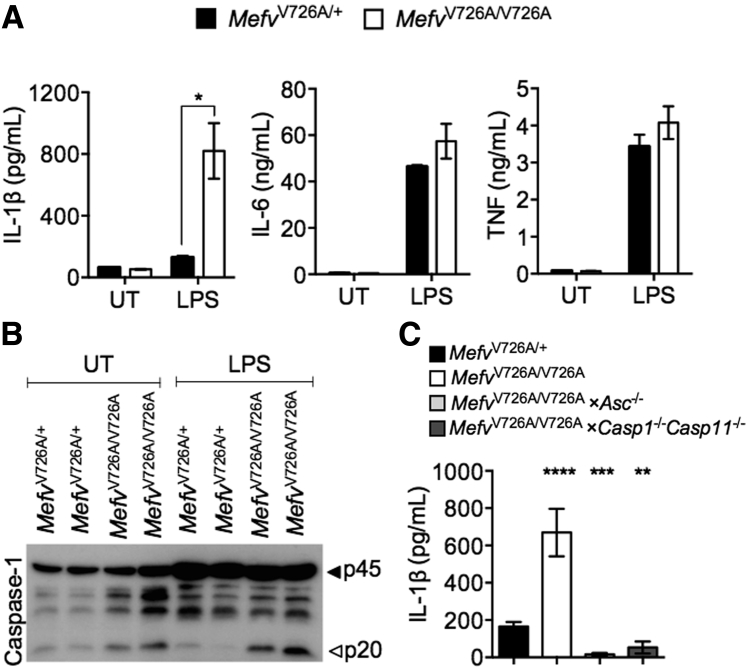
Chronic LPS Exposure Leads to Overt Inflammasome Activation and IL-1β Release in MefvV726A/V726A Monocytes
Immune cells from MefvV726A/V726A mice and peripheral blood mononuclear cells isolated from patients with FMF have shown aberrant IL-1β release in response to LPS stimulation.5, 42 In line with these observations, we observed an increase in IL-1β release and caspase-1 cleavage in MefvV726A/V726A monocytes after 48 hours of LPS stimulation (Figure 4, A and B). Release of IL-1β from MefvV726A/V726A monocytes was abolished in the absence of ASC or caspase-1 (Figure 4C). FMF-associated pyrin mutations also have been ascribed a role in promoting NF-κB signaling.27 However, production of NF-κB signaling-dependent cytokines IL-6 and tumor necrosis factor was similar between MefvV726A/V726A and MefvV726A/+ monocytes (Figure 4A). Therefore, aberrant caspase-1 activation drives the production of IL-1β, which leads to overt inflammation and its deleterious consequences in this mouse model of FMF.
Figure 4.
Chronic lipopolysaccharide (LPS) exposure leads to overt inflammasome activation and IL-1β release in MefvV726A/V726A monocytes. Monocytes stimulated with LPS (200 ng/mL) for 48 hours were analyzed for cytokine release (A and C) and caspase-1 processing (B). Data are expressed as means ± SEM. n ≥3 independent experiments. ∗P < 0.05, , ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001 versus with MefvV726A/+ [two-way (A) or one-way (C) analysis of variance followed by the Fisher least significance difference test]. TNF, tumor necrosis factor; UT, untreated.
Discussion
FMF is an autoinflammatory disorder marked by increased production of inflammatory mediators and neutrophilia in both humans42 and mice.5 Both IL-1α and IL-1β are capable of instigating sterile inflammation and we previously have shown that these cytokines drive distinct neutrophil-mediated autoinflammatory disorders.10, 34 Although a reason for this specificity currently is unknown, a possible reason could be the nature of cell death perturbed in each autoinflammatory disorder.9 Although necrotic cell death releases bioactive IL-1α, IL-1β requires caspase-1– and/or caspase-8–mediated maturation and release. Thus, depending on the type of cell death that is instigated, either IL-1α or IL-1β could initiate and promote sterile inflammation, driving the disease pathogenesis. In this study, we showed that in a mouse model of FMF, aberrant caspase-1 activation mediated the maturation and release of IL-1β. Overt IL-1β production led to systemic and multiorgan hyperinflammation observed in the FMF-KI mice. Genetic deletion of IL-1β or caspase-1 in the FMF-KI mice provided protection against multiple parameters of the disorder including runting/wasting, neutrophilia, and anemia, suggesting that the clinical basis of these features is aberrant IL-1β production. Furthermore, IL-1α–mediated IL-1R signaling and caspase-8–mediated IL-1β processing was dispensable for disease pathogenesis.
It has been shown previously that the autoinflammatory disease in FMF-KI mice is instigated by cells of the hematopoietic compartment and is independent of the adaptive immune system.5 This suggests that the disease is perpetuated through IL-1β production by innate immune cells. FMF-KI cells of either granulocytic or monocytic lineage release higher amounts of IL-1β in response to LPS stimulation in vitro (data not shown), suggesting that both of these populations might contribute to this IL-1β–mediated disorder.
Although both anti–IL-1R and anti–IL-1β antibodies are used to treat human patients with FMF,43, 44, 45, 46, 47 our studies provide a mechanistic rationale for specific blockade of IL-1β as the therapy of choice in patients with FMF. Compared with anti–IL-1R, anti–IL-1β therapy has the benefits of a longer biological half-life and an absence of side effects associated with IL-1α blockade.48 Our studies further indicate that inhibition of caspase-1 activity would provide a therapeutic benefit to patients with FMF.
Recent studies have identified that protein kinase N1–mediated phosphorylation of pyrin (at serine 242) allows its association with 14-3-3 proteins, and this interaction inhibits pyrin inflammasome activation.49, 50 FMF-associated mutations in the B30.2 domain inhibit the interaction between pyrin and protein kinase N1, and reduce the inhibitory control on pyrin activation.49 Our work, along with the earlier-mentioned findings, define the upstream molecular mechanisms involved in IL-1β release during FMF pathogenesis. How mutations in the C-terminal B30.2 domain affect phosphorylation at an amino acid position within the N-terminus domain is currently unknown. Mutations in the B30.2 domain reduce the threshold for pyrin activation, release pyrin from inhibitory control, and promote inflammasome activation even in the absence of pyrin-specific stimuli. This promotes aberrant IL-1β production, which drives the pathogenesis in this autoinflammatory disorder. Recent studies also have highlighted the role of pyrin inflammasome in another autoinflammatory disorder called hyperimmunoglobulonemia D syndrome.49, 51 Future studies aimed at elucidating the molecular underpinnings of pyrin activation and inflammasome regulation by the disease-associated mutations would help identify molecular targets for therapy in patients with FMF and other pyrin-associated disorders.
Acknowledgments
We thank Dr. Dan Kastner (NIH) for the MefvV726A/+ and MefvV726A/V726A mice; Dr. Ankit Malik, Amanda Burton, and Daniel Horn for technical assistance; and Drs. Si Ming Man, Prajwal Gurung, and Subbarao Malireddi for review of the manuscript. We apologize to our colleagues whose work could not be cited because of space limitations.
D.S. and T.-D.K. conceptualized the project; D.S. and B.R.S. executed the experiments; P.V. performed the histologic assessment; D.S. conducted the data analysis; D.S. and T.-D.K. wrote the manuscript; and T.-D.K. acquired funding and provided resources and overall supervision.
Footnotes
Supported by NIH grants AR056296, CA163507, AI101935, and AI124346 (T.-D.K.), and the American Lebanese Syrian Associated Charities.
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2016.10.015.
Supplemental Data
Supplemental Figure S1.
IL-1β haploinsufficiency or caspase-8 deficiency does not protect MefvV726A/V726A mice from systemic wasting. Body weights of indicated mice at 8 weeks of age. ∗∗∗∗P < 0.0001 versus MefvV726A/+ by using one-way (A) or two-way (B) analysis of variance followed by the Fisher least significance difference test.
Supplemental Figure S2.
Anemia observed in MefvV726A/V726A mice is dependent on the caspase-1–IL-1β axis. A: Percentage of neutrophils and lymphocytes in the blood of indicated mice. B: Features of anemia including hemoglobin levels (Hb), hematocrit, mean corpuscular volume (MCV), and red cell width distribution (RDW) in the blood of indicated mice. n = 2 to 6 for each genotype.
References
- 1.Chae J.J., Aksentijevich I., Kastner D.L. Advances in the understanding of familial Mediterranean fever and possibilities for targeted therapy. Br J Haematol. 2009;146:467–478. doi: 10.1111/j.1365-2141.2009.07733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The International FMF Consortium Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell. 1997;90:797–807. doi: 10.1016/s0092-8674(00)80539-5. [DOI] [PubMed] [Google Scholar]
- 3.Bernot A., Clepet C., Dasilva C., Devaud C., Petit J.L., Caloustian C., Cruaud C., Samson D., Pulcini F., Weissenbach J., Heilig R., Notanicola C., Domingo C., Rozenbaum M., Benchetrit E., Topaloglu R., Dewalle M., Dross C., Hadjari P., Dupont M., Demaille J., Touitou I., Smaoui N., Nedelec B., Méry J.P., Chaabouni H., Delpech M., Grateau G. A candidate gene for familial Mediterranean fever. Nat Genet. 1997;17:25–31. doi: 10.1038/ng0997-25. [DOI] [PubMed] [Google Scholar]
- 4.Papin S., Cuenin S., Agostini L., Martinon F., Werner S., Beer H.D., Grutter C., Grutter M., Tschopp J. The SPRY domain of Pyrin, mutated in familial Mediterranean fever patients, interacts with inflammasome components and inhibits proIL-1beta processing. Cell Death Differ. 2007;14:1457–1466. doi: 10.1038/sj.cdd.4402142. [DOI] [PubMed] [Google Scholar]
- 5.Chae J.J., Cho Y.H., Lee G.S., Cheng J., Liu P.P., Feigenbaum L., Katz S.I., Kastner D.L. Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1beta activation and severe autoinflammation in mice. Immunity. 2011;34:755–768. doi: 10.1016/j.immuni.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garlanda C., Dinarello C.A., Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C.J., Kono H., Golenbock D., Reed G., Akira S., Rock K.L. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 8.Rider P., Carmi Y., Guttman O., Braiman A., Cohen I., Voronov E., White M.R., Dinarello C.A., Apte R.N. IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187:4835–4843. doi: 10.4049/jimmunol.1102048. [DOI] [PubMed] [Google Scholar]
- 9.England H., Summersgill H.R., Edye M.E., Rothwell N.J., Brough D. Release of interleukin-1alpha or interleukin-1beta depends on mechanism of cell death. J Biol Chem. 2014;289:15942–15950. doi: 10.1074/jbc.M114.557561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lukens J.R., Vogel P., Johnson G.R., Kelliher M.A., Iwakura Y., Lamkanfi M., Kanneganti T.D. RIP1-driven autoinflammation targets IL-1alpha independently of inflammasomes and RIP3. Nature. 2013;498:224–227. doi: 10.1038/nature12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P., Allen H., Banerjee S., Franklin S., Herzog L., Johnston C., McDowell J., Paskind M., Rodman L., Salfeld J., Towne E., Tracey D., Wardwell S., Wei F.Y., Wong W., Kamen R., Seshadri T. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401–411. doi: 10.1016/0092-8674(95)90490-5. [DOI] [PubMed] [Google Scholar]
- 12.Kayagaki N., Warming S., Lamkanfi M., Vande Walle L., Louie S., Dong J., Newton K., Qu Y., Liu J., Heldens S., Zhang J., Lee W.P., Roose-Girma M., Dixit V.M. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 13.Kanneganti T.D., Lamkanfi M., Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 15.Maelfait J., Vercammen E., Janssens S., Schotte P., Haegman M., Magez S., Beyaert R. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukens J.R., Gurung P., Vogel P., Johnson G.R., Carter R.A., McGoldrick D.J., Bandi S.R., Calabrese C.R., Vande Walle L., Lamkanfi M., Kanneganti T.D. Dietary modulation of the microbiome affects autoinflammatory disease. Nature. 2014;516:246–249. doi: 10.1038/nature13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurung P., Anand P.K., Malireddi R.K., Vande Walle L., Van Opdenbosch N., Dillon C.P., Weinlich R., Green D.R., Lamkanfi M., Kanneganti T.D. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192:1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Man S.M., Tourlomousis P., Hopkins L., Monie T.P., Fitzgerald K.A., Bryant C.E. Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1beta production. J Immunol. 2013;191:5239–5246. doi: 10.4049/jimmunol.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vince J.E., Wong W.W., Gentle I., Lawlor K.E., Allam R., O'Reilly L., Mason K., Gross O., Ma S., Guarda G., Anderton H., Castillo R., Hacker G., Silke J., Tschopp J. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Gringhuis S.I., Kaptein T.M., Wevers B.A., Theelen B., van der Vlist M., Boekhout T., Geijtenbeek T.B. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 21.Gurung P., Burton A., Kanneganti T.D. NLRP3 inflammasome plays a redundant role with caspase 8 to promote IL-1beta-mediated osteomyelitis. Proc Natl Acad Sci U S A. 2016;113:4452–4457. doi: 10.1073/pnas.1601636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cassel S.L., Janczy J.R., Bing X., Wilson S.P., Olivier A.K., Otero J.E., Iwakura Y., Shayakhmetov D.M., Bassuk A.G., Abu-Amer Y., Brogden K.A., Burns T.L., Sutterwala F.S., Ferguson P.J. Inflammasome-independent IL-1beta mediates autoinflammatory disease in Pstpip2-deficient mice. Proc Natl Acad Sci U S A. 2014;111:1072–1077. doi: 10.1073/pnas.1318685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chae J.J., Komarow H.D., Cheng J., Wood G., Raben N., Liu P.P., Kastner D.L. Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol Cell. 2003;11:591–604. doi: 10.1016/s1097-2765(03)00056-x. [DOI] [PubMed] [Google Scholar]
- 24.Yu J.W., Wu J., Zhang Z., Datta P., Ibrahimi I., Taniguchi S., Sagara J., Fernandes-Alnemri T., Alnemri E.S. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ. 2006;13:236–249. doi: 10.1038/sj.cdd.4401734. [DOI] [PubMed] [Google Scholar]
- 25.Seshadri S., Duncan M.D., Hart J.M., Gavrilin M.A., Wewers M.D. Pyrin levels in human monocytes and monocyte-derived macrophages regulate IL-1beta processing and release. J Immunol. 2007;179:1274–1281. doi: 10.4049/jimmunol.179.2.1274. [DOI] [PubMed] [Google Scholar]
- 26.Yu J.W., Fernandes-Alnemri T., Datta P., Wu J., Juliana C., Solorzano L., McCormick M., Zhang Z., Alnemri E.S. Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol Cell. 2007;28:214–227. doi: 10.1016/j.molcel.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chae J.J., Wood G., Richard K., Jaffe H., Colburn N.T., Masters S.L., Gumucio D.L., Shoham N.G., Kastner D.L. The familial Mediterranean fever protein, pyrin, is cleaved by caspase-1 and activates NF-kappaB through its N-terminal fragment. Blood. 2008;112:1794–1803. doi: 10.1182/blood-2008-01-134932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H., Yang J., Gao W., Li L., Li P., Zhang L., Gong Y.N., Peng X., Xi J.J., Chen S., Wang F., Shao F. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513:237–241. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 29.Gavrilin M.A., Mitra S., Seshadri S., Nateri J., Berhe F., Hall M.W., Wewers M.D. Pyrin critical to macrophage IL-1beta response to Francisella challenge. J Immunol. 2009;182:7982–7989. doi: 10.4049/jimmunol.0803073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shornick L.P., De Togni P., Mariathasan S., Goellner J., Strauss-Schoenberger J., Karr R.W., Ferguson T.A., Chaplin D.D. Mice deficient in IL-1beta manifest impaired contact hypersensitivity to trinitrochlorobenzone. J Exp Med. 1996;183:1427–1436. doi: 10.1084/jem.183.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuki T., Nakae S., Sudo K., Horai R., Iwakura Y. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. Int Immunol. 2006;18:399–407. doi: 10.1093/intimm/dxh379. [DOI] [PubMed] [Google Scholar]
- 32.Oberst A., Dillon C.P., Weinlich R., McCormick L.L., Fitzgerald P., Pop C., Hakem R., Salvesen G.S., Green D.R. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanneganti T.D., Ozoren N., Body-Malapel M., Amer A., Park J.H., Franchi L., Whitfield J., Barchet W., Colonna M., Vandenabeele P., Bertin J., Coyle A., Grant E.P., Akira S., Nunez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 34.Lukens J.R., Gross J.M., Calabrese C., Iwakura Y., Lamkanfi M., Vogel P., Kanneganti T.D. Critical role for inflammasome-independent IL-1beta production in osteomyelitis. Proc Natl Acad Sci U S A. 2014;111:1066–1071. doi: 10.1073/pnas.1318688111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaiser W.J., Upton J.W., Long A.B., Livingston-Rosanoff D., Daley-Bauer L.P., Hakem R., Caspary T., Mocarski E.S. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Celkan T., Celik M., Kasapcopur O., Ozkan A., Apak H., Ocak S., Arisoy N., Yildiz I. The anemia of familial Mediterranean fever disease. Pediatr Hematol Oncol. 2005;22:657–665. doi: 10.1080/08880010500278681. [DOI] [PubMed] [Google Scholar]
- 37.de Jesus A.A., Goldbach-Mansky R. Monogenic autoinflammatory diseases: concept and clinical manifestations. Clin Immunol. 2013;147:155–174. doi: 10.1016/j.clim.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voulgari P.V., Kolios G., Papadopoulos G.K., Katsaraki A., Seferiadis K., Drosos A.A. Role of cytokines in the pathogenesis of anemia of chronic disease in rheumatoid arthritis. Clin Immunol. 1999;92:153–160. doi: 10.1006/clim.1999.4736. [DOI] [PubMed] [Google Scholar]
- 39.Johnson C.S., Pourbohloul S.C., Furmanski P. Negative regulators of in vivo erythropoiesis: interaction of IL-1 alpha and TNF-alpha and the lack of a strict requirement for T or NK cells for their activity. Exp Hematol. 1991;19:101–105. [PubMed] [Google Scholar]
- 40.van Deuren M., Kroot J.J., Swinkels D.W. Time-course analysis of serum hepcidin, iron and cytokines in a C282Y homozygous patient with Schnitzler's syndrome treated with IL-1 receptor antagonist. Haematologica. 2009;94:1297–1300. doi: 10.3324/haematol.2009.005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee P., Peng H., Gelbart T., Wang L., Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci U S A. 2005;102:1906–1910. doi: 10.1073/pnas.0409808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibrahim J.N., Jounblat R., Delwail A., Abou-Ghoch J., Salem N., Chouery E., Megarbane A., Medlej-Hashim M., Lecron J.C. Ex vivo PBMC cytokine profile in familial Mediterranean fever patients: involvement of IL-1beta, IL-1alpha and Th17-associated cytokines and decrease of Th1 and Th2 cytokines. Cytokine. 2014;69:248–254. doi: 10.1016/j.cyto.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 43.Chae J.J., Wood G., Masters S.L., Richard K., Park G., Smith B.J., Kastner D.L. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production. Proc Natl Acad Sci U S A. 2006;103:9982–9987. doi: 10.1073/pnas.0602081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Hilst J., Moutschen M., Messiaen P.E., Lauwerys B.R., Vanderschueren S. Efficacy of anti-IL-1 treatment in familial Mediterranean fever: a systematic review of the literature. Biologics. 2016;10:75–80. doi: 10.2147/BTT.S102954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alpa M., Roccatello D. Canakinumab as rescue therapy in familial Mediterranean fever refractory to conventional treatment. Drug Des Devel Ther. 2015;9:1983–1987. doi: 10.2147/DDDT.S69117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belkhir R., Moulonguet-Doleris L., Hachulla E., Prinseau J., Baglin A., Hanslik T. Treatment of familial Mediterranean fever with anakinra. Ann Intern Med. 2007;146:825–826. doi: 10.7326/0003-4819-146-11-200706050-00023. [DOI] [PubMed] [Google Scholar]
- 47.Cetin P., Sari I., Sozeri B., Cam O., Birlik M., Akkoc N., Onen F., Akar S. Efficacy of interleukin-1 targeting treatments in patients with familial Mediterranean fever. Inflammation. 2015;38:27–31. doi: 10.1007/s10753-014-0004-1. [DOI] [PubMed] [Google Scholar]
- 48.Dinarello C.A. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 2010;29:317–329. doi: 10.1007/s10555-010-9229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park Y.H., Wood G., Kastner D.L., Chae J.J. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol. 2016;17:914–921. doi: 10.1038/ni.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masters S.L., Lagou V., Jeru I., Baker P.J., Van Eyck L., Parry D.A., Lawless D., De Nardo D., Garcia-Perez J.E., Dagley L.F., Holley C.L., Dooley J., Moghaddas F., Pasciuto E., Jeandel P.Y., Sciot R., Lyras D., Webb A.I., Nicholson S.E., De Somer L., van Nieuwenhove E., Ruuth-Praz J., Copin B., Cochet E., Medlej-Hashim M., Megarbane A., Schroder K., Savic S., Goris A., Amselem S., Wouters C., Liston A. Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Sci Transl Med. 2016;8:332ra45. doi: 10.1126/scitranslmed.aaf1471. [DOI] [PubMed] [Google Scholar]
- 51.Akula M.K., Shi M., Jiang Z., Foster C.E., Miao D., Li A.S., Zhang X., Gavin R.M., Forde S.D., Germain G., Carpenter S., Rosadini C.V., Gritsman K., Chae J.J., Hampton R., Silverman N., Gravallese E.M., Kagan J.C., Fitzgerald K.A., Kastner D.L., Golenbock D.T., Bergo M.O., Wang D. Control of the innate immune response by the mevalonate pathway. Nat Immunol. 2016;17:922–929. doi: 10.1038/ni.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]