Abstract
OBJECTIVES
To determine whether weight loss in older adults may be a marker of impending burden of multimorbidity regardless of initial weight, testing the hypotheses that obesity but not overweight in elderly adults is associated with greater number of diseases than normal weight and that obese older adults who lose weight over time have the greatest burden of multimorbidity.
DESIGN
Longitudinal cohort study (Invecchiare in Chianti Study).
SETTING
Community.
PARTICIPANTS
Individuals aged 60 and older at baseline followed for an average of 4 years (N = 1,025).
MEASUREMENTS
Multimorbidity was measured as number of diagnosed diseases. Baseline body mass index (BMI) was categorized as normal weight (<25.0 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30.0 kg/m2). Loss of weight was defined as decrease over time in BMI of at least 0.15 kg/m2 per year. Age, sex, and education were covariates.
RESULTS
Baseline obesity was cross-sectionally associated with high multimorbidity and greater longitudinal increase of multimorbidity than normal weight (P = .005) and overweight (P < .001). Moreover, obese participants who lost weight over follow-up had a significantly greater increase in multimorbidity than other participants, including obese participants who maintained or gained weight over time (P = .005). In nonobese participants, changes in weight had no effect on changes in multimorbidity over time. Sensitivity analyses confirmed that one specific disease did not drive the association and that competing mortality did not bias the association.
CONCLUSION
Loss of weight in obese older persons is a strong biomarker of impending expansion of multimorbidity. Older obese individuals who lose weight should receive thoughtful medical attention.
Keywords: multimorbidity, BMI, weight loss, obesity, older adults
Aging is associated with rising susceptibility to many diseases, which clinically results in the co-occurrence of multiple chronic conditions, or multimorbidity.1,2 Multimorbidity is the most common chronic condition in the older population and increases the risk of premature death, hospitalization, loss of physical functioning, depression, polypharmacy, and worsening quality of life, translating into a substantial economic burden for health systems.3–9 Identifying biomarkers or “phenotypes” that predict faster and greater accumulation of chronic diseases with aging may help develop strategies to prevent and reduce the burden of multimorbidity.
Previous literature has widely explored the role of body composition and its change over time as predictors of higher risk of specific diseases, but whether body mass index (BMI) and its longitudinal changes may be biomarkers of expansion in overall multimorbidity in elderly adults has not been investigated.
In particular, although obesity and weight gain in middle age are well-established risk factors for future development of major chronic diseases, the recommended BMI to achieve optimum health in older adults is still debated.10 Recent evidence has suggested that being slightly overweight in older age may be protective, with higher mortality risk for those who are normal weight and obese.11
Several studies have also explored the association between changes in weight or BMI and mortality risk in older adults.12–21 Although not all are consistent, most studies reported greater mortality risk associated with loss of weight than with weight gain, especially in obese older adults. Illness-related loss of weight may account for the described association, but previous studies have rarely explored markers of multimorbidity as potential mechanisms for the association between weight loss and greater risk of death.
Two previous studies that examined the cross-sectional associations between BMI and multimorbidity in middle-aged adults found that multimorbidity is strongly associated with higher BMI,22,23 but whether this association is confirmed also at older ages and holds true longitudinally has not been adequately investigated. Therefore, this study was performed to explore how BMI and its changes over time may contribute to multimorbidity in older adults.
It was hypothesized that higher baseline BMI would be associated with higher cross-sectional multimorbidity and faster prospective accumulation of diseases in older adults and that faster decline in BMI, especially in obese individuals, would be associated with greater increase in number of chronic diseases than in the rest of the population.
METHODS
Study Design and Setting
The present analysis used data from the Invecchiare in Chianti (Aging in the Chianti Area, InCHIANTI) study, a longitudinal population-based study of older people living in the Chianti area, Tuscany, Italy. The study was designed to identify factors contributing to mobility decline and disability in older persons. A detailed description of the sampling procedures and data collection methods has been previously published.24 Briefly, participants were randomly selected from the city registries of Greve in Chianti and Bagno a Ripoli, two small, rural towns in the Tuscan countryside, using a multistage sampling method. Baseline data were collected in 1998 to 2000; the 3-year follow-up took place in 2001 to 2003, the 6-year follow-up in 2004 to 2006, and the 9-year follow-up in 2007 to 2009. The Italian National Research Council on Aging ethical committee ratified the study protocol, and participants provided written consent to participate.
Participants
Of the 1,203 participants aged 60 and older at enrollment, 1,025 had completed information on BMI and multimorbidity and were included in the present analysis. Of these, 796 participated in the 3-year follow up visit, 666 in the 6-year follow up visit, and 533 in the 9-year follow up visit. Average follow-up was 4 years.
The design of the study is shown in Figure S1.
Multimorbidity
In recent years, the study of multimorbidity has gained considerable interest in the literature, although no standard operational definition of multimorbidity has emerged. One of the most common approaches is to define the co-occurrence of multiple chronic conditions as count of number of diseases.25 For this purpose, including (at least) the 12 most prevalent chronic diseases with high effect or burden in a given population has been suggested.26 Following these recommendations, a priori a list of 15 candidate chronic conditions were selected that could be reliably adjudicated based on the data available and known to have high prevalence and be associated with high disability and mortality risk in older adults (described below). As in previous studies, multimorbidity was operationalized as number of diagnosed diseases (according to standard clinical criteria) in each participant at each time point.27 Multimorbidity has previously been conventionally defined as the presence of at least two chronic conditions at the same time in the same individual; this study aimed at exploring changes over time in multimorbidity according to changes in BMI, so the count of diagnoses was considered as a continuous variable, without using any specific cut points. The terms multimorbidity and multiple chronic conditions are used interchangeably throughout the article.
Chronic Diseases
The presence of 15 chronic conditions was ascertained at baseline and follow-up visits. Most (hypertension, diabetes mellitus, coronary artery disease, congestive heart failure, stroke, chronic obstructive pulmonary disease, cancer, Parkinson’s disease, hip fracture, lower extremity joint disease) were defined using standard criteria and algorithms similar to those used in the Women’s Health and Aging Study.28 Anemia was defined as a hemoglobin level less than 12 g/dL in women and less than 13 g/dL in men,29 renal failure as a glomerular filtration rate estimated using the Cockroft–Gault equation of less than 30 mL/min, peripheral arterial disease as ankle–brachial index measured using a Doppler stethoscope less than 0.9,30 cognitive impairment as an age- and education-corrected Mini Mental State Examination score less than 24, and depression as a score of 20 or greater on the Center for Epidemiologic Studies Depression Scale.31
Table S1 shows the prevalence of diseases included in the operationalization of multimorbidity at baseline.
Body Mass Index
BMI was calculated as weight measured in kilograms divided by height measured in meters squared (kg/m2). Following the World Health Organization guidelines, based on baseline BMI, participants were classified as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), or obese (≥30.0 kg/m2). Because only four participants had a baseline BMI less than 18.5 kg/m2 (but >18.0 kg/m2), they were merged with the normal-weight group.
Change in BMI
Individual changes in BMI over time were estimated from linear mixed-effects models using all of the longitudinal crude data. For analytical purposes, individual change in BMI was further categorized as decreasing BMI versus no decreasing BMI. Decreasing BMI was defined as a decline greater than 0.15 kg/m2 per year (corresponding to 1 standard deviation below the average of the population); no decreasing BMI included the rest of the sample (stable or increasing BMI).
Covariates
Education was expressed as years of school attendance. Because serum interleukin (IL)-6 levels are known to be significantly associated with obesity and multimorbidity, baseline IL-6, measured in duplicate using high-sensitivity enzyme-linked immunosorbent assays, was also used as a covariate.32
Statistical Analysis
Baseline and longitudinal characteristics of the study population are reported according to categories of baseline BMI (normal weight, overweight, obese) and presented as means and standard deviations, medians and interquartile ranges, or percentages. Linear mixed models were used first to explore the cross-sectional and longitudinal associations between baseline BMI and number of diseases, adjusting for age, sex, education, and baseline IL-6 level. Then, longitudinal trajectories of multimorbidity over time of different baseline BMI categories (normal weight, overweight, obese) were compared, adjusting for baseline age, sex, and education. The independent effects of baseline BMI and changes over time in BMI on multimorbidity rates of change were also explored. Whether a faster decline in BMI over time was associated with a greater increase in multimorbidity over time, independent of baseline age, sex, education, and baseline BMI, was first tested. Then, to better understand the complex relationship between baseline BMI, change in BMI, and multimorbidity, participants were divided into four groups according to baseline BMI (obese vs not obese) and change in BMI over follow-up (decreasing vs stable or increasing BMI): not obese–not decreasing BMI (reference), not obese–decreasing BMI, obese–not decreasing BMI, obese–decreasing BMI. Therefore, longitudinal trajectories of multimorbidity of the four groups were compared, independent of the covariates. Sensitivity analyses were performed to address the possibility that baseline BMI and change in BMI were associated with the incidence of specific chronic conditions, suggesting that specific diseases instead of global multimorbidity might have driven the original results. First, whether specific incident diagnoses during follow-up were different between the four groups considered in the original analysis was investigated by fitting generalized liner models to test differences in incident diagnosis for each of 15 candidate conditions. Then, to exclude whether these differences may have driven the association with overall multimorbidity, the original analysis was rerun, excluding from the original list of candidate diseases conditions that had significant differences in incidence between the groups. Finally, supplemental survival analyses in participants who completed the 6- and 9-year follow-up were performed to exclude the possibility of a survival bias.
All analyses were performed using SAS version 9.3 (SAS institute, Inc., Cary, NC).
RESULTS
Characteristics of the Study Population at Baseline
The baseline population included 1,025 participants aged 60 and older (mean age 73.8 ± 7.2), of whom 572 (55.8%) were female. Mean BMI was 27.5 ± 4.1 kg/m2, and mean number of chronic diseases was 1.7 ± 1.4. After adjusting for age and sex, obese participants had significantly more diseases (1.9) than nonobese participants (1.6) (Table 1). No significant difference in number of diseases was found between overweight and normal-weight participants.
Table 1.
Participant Characteristics According to Baseline Body Mass Index (BMI, kg/m2) Categories at Baseline and Follow-Up Visits
| Characteristic | <25.0 kg/m2 | 25.0–29.9 kg/m2 | ≥30.0 kg/m2 | P Trenda |
|---|---|---|---|---|
| Baseline | ||||
| n | 297 | 472 | 256 | |
| Age, mean ± SD | 75.5 ± 7.7 | 73.1 ± 7.0 | 72.9 ± 6.5 | |
| Female, % | 55.6 | 51.3 | 64.4 | |
| BMI, kg/m2, mean ± SD | 22.8 ± 1.6 | 27.5 ± 1.3 | 32.8 ± 2.7 | |
| Number of diseases, mean ± SD | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.9 ± 0.1 | .02 |
| 3-year follow-up | ||||
| n | 226 | 371 | 185 | |
| Age, mean ± SD | 77.5 ± 7.3 | 75.5 ± 6.5 | 75.2 ± 6.2 | |
| Female, % | 52.6 | 50.9 | 65.8 | |
| BMI, kg/m2, mean ± SD | 22.5 ± 2.1 | 26.4 ± 1.9 | 31.3 ± 3.1 | |
| Number of diseases, mean ± SD | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.7 ± 0.1 | .001 |
| 6-year follow-up | ||||
| n | 185 | 307 | 174 | |
| Age, mean ± SD | 79.0 ± 6.4 | 77.3 ± 5.7 | 77.9 ± 5.8 | |
| Female, % | 56.7 | 50.8 | 65.5 | |
| BMI, kg/m2, mean ± SD | 23.2 ± 2.5 | 27.1 ± 2.5 | 31.6 ± 3.4 | |
| Number of diseases, mean ± SD | 2.4 ± 0.1 | 2.5 ± 0.1 | 3.0 ± 0.1 | <.001 |
| 9-year follow-up | ||||
| n | 143 | 254 | 136 | |
| Age, mean ± SD | 81.3 ± 5.8 | 79.6 ± 5.3 | 79.9 ± 4.9 | |
| Female, % | 58.7 | 51.6 | 61.8 | |
| BMI, kg/m2, mean ± SD | 23.1 ± 2.7 | 27.2 ± 2.7 | 31.3 ± 3.5 | |
| Number of diseases, mean ± SD | 2.9 ± 0.1 | 2.8 ± 0.1 | 3.8 ± 0.1 | <.001 |
SD = standard deviation.
Age- and sex -adjusted P trend.
Baseline BMI and Presence and Prospective Development of Multimorbidity
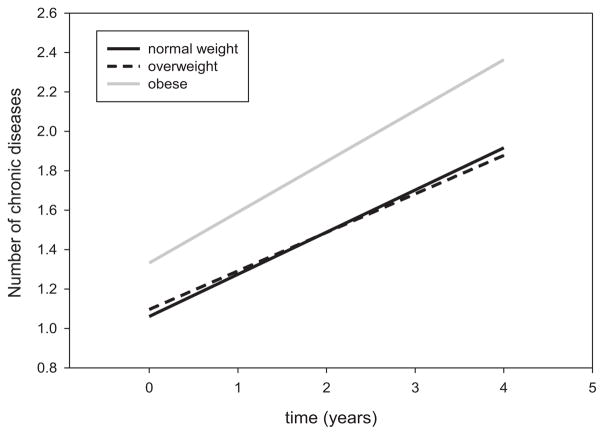
The number of chronic diseases increased on average by 0.2 per year (P < .001) in the whole study population. In exploratory analyses, higher baseline BMI was significantly associated with higher cross-sectional and greater longitudinal increase in number of chronic diseases, independent of baseline age, sex, and education (Model I, Table S2). The association was still statistically significant after adjusting for baseline IL-6 (Model II, Table S2). Using liner mixed models, time-trajectories of multimorbidity over the follow-up according to different baseline BMI categories were estimated and compared (Figure 1). Baseline obesity was significantly associated with greater cross-sectional multimorbidity (P = .005) and greater longitudinal increase in multimorbidity (P < .001) than normal weight and overweight (Table 2). No significant differences in baseline multimorbidity and rates of change in multimorbidity were observed between participants who were normal weight and overweight at baseline (P = .178).
Figure 1.
Mixed models were used to estimate trajectories of multimorbidity over time (average follow-up, 4 years) according to different baseline body mass index (BMI) categories (obesity (n = 256, gray line), overweight (n = 472, dashed dark line), normal weight (n = 256, solid dark line)).
Table 2.
Results from Linear Mixed Model Testing Comparing Cross-Sectional and Longitudinal Associations Between Baseline Body Mass Index (BMI) Category (Normal Weight, Overweight, Obese) and Number of Diseases, Independent of Baseline Age, Sex, and Education
| Baseline BMI Category | Beta Coefficient (Standard Error) | P-Value |
|---|---|---|
| Baseline multimorbidity | ||
| Overweight vs normal weight | 0.016 (0.09) | .87 |
| Obesity vs normal weight | 0.24 (0.11) | .03 |
| Obesity vs overweight | 0.23 (0.10) | .03 |
| Expansion in multimorbidity over time | ||
| Overweight vs normal weight | −0.018 (0.014) | .18 |
| Obesity vs normal weight | 0.044 (0.016) | .005 |
| Obesity vs overweight | 0.062 (0.014) | <.001 |
Decline in BMI and Rate of Change in Multimorbidity
In the overall study population, BMI declined at the rate of 0.05 kg/m2 per year (P < .001). Independent of age, sex, education, and baseline BMI, greater decline in BMI tended to be associated with increase in multimorbidity (P = .06, Table S3).
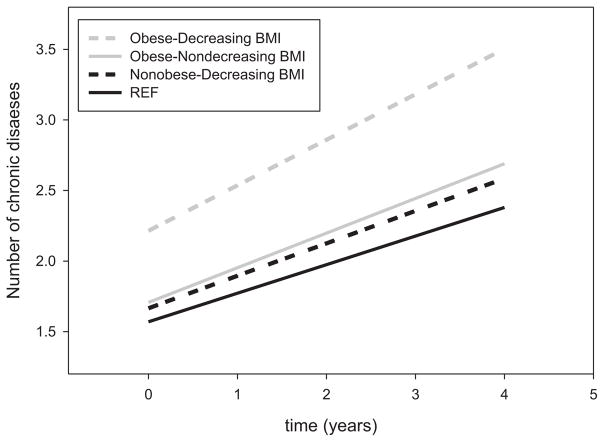
Obese participants who experienced decline in BMI had significantly higher multimorbidity at baseline and greater increase in multimorbidity over time than the other three groups (Figure 2; Table 3). In particular, obese participants with decreasing BMI had a significantly greater increase in multimorbidity than obese participants with stable or increasing BMI (P = .005) and than nonobese participants regardless of their BMI changes. In nonobese participants, loss of weight was not associated with greater increase in multimorbidity than for participants with stable or increasing weight (P = .14).
Figure 2.
Mixed models were used to estimate trajectories of multimorbidity over time (average follow-up, 4 years) according to four groups based on the presence and absence of baseline obesity and decreasing or not decreasing body mass index (BMI) over time (not obese–not decreasing BMI (reference, solid dark line), n = 494; not obese–decreasing BMI (dashed dark line), n = 103; obese–not decreasing BMI (solid gray line), n = 149; obese–decreasing BMI (dashed gray line), n = 50).
Table 3.
Results from Linear Mixed Model Testing Comparing Cross-Sectional and Longitudinal Associations Between Number of Diseases Between Four Groups Based on Baseline Obese or Not Obese and Decreasing or Not Decreasing Body Mass Index (BMI) over Time
| Group | Beta Coefficient (Standard Error) | P-Value |
|---|---|---|
| Baseline multimorbidity | ||
| Not obese–decreasing BMI vs not obese–not decreasing BMI | 0.09 (0.13) | .41 |
| Obese–not decreasing vs not obese–not decreasing BMI | 0.14 (0.11) | .20 |
| Obese–decreasing BMI vs not obese–not decreasing BMI | 0.64 (0.18) | <.001 |
| Obese–not decreasing BMI vs not obese–decreasing BMI | 0.41 (0.15) | .79 |
| Obese–decreasing BMI vs not obese–decreasing BMI | 0.55 (0.21) | .009 |
| Obese–decreasing BMI vs obese–not decreasing BMI | 0.51 (0.19) | .009 |
| Expansion in multimorbidity over time | ||
| Not obese–decreasing BMI vs not obese–not decreasing BMI | 0.027 (0.018) | .14 |
| Obese–not decreasing vs not obese–not decreasing BMI | 0.043 (0.016) | .008 |
| Obese–decreasing BMI vs not obese–not decreasing BMI | 0.120 (0.024) | <.001 |
| Obese–not decreasing BMI vs not obese–decreasing BMI | 0.016 90.022) | .47 |
| Obese–decreasing BMI vs not obese–decreasing BMI | 0.092 (0.028) | .001 |
| Obese–decreasing BMI vs obese–not decreasing BMI | 0.077 (0.027) | .005 |
Sensitivity Analysis
Sensitivity analyses were performed to address the possibility that the incidence of specific conditions was associated with loss of weight in obese adults. Two conditions—chronic kidney disease (CKD) (P = .002) and anemia (P < .001)—developed more frequently in participants who lost weight (whether they were obese and not at baseline) than in the reference group of nonobese participants who did not lose weight (P = .003 and P = .004 for anemia, P = .005 and P = .01 for CKD). To verify whether anemia and CKD fully explained the results of increase of multimorbidity in obese individuals who lost weight, the original analysis were rerun excluding anemia and CKD from the count of diseases. The results were unchanged even when both conditions were excluded, with obese older adults who lost weight over time having not only the most diseases at baseline, but also the greatest increase in multimorbidity over follow-up.
Finally, to address the possibility that greater loss to follow-up because of higher mortality of participants who were obese at baseline and did not experience decline in weight could explain the findings, supplemental survival analyses were performed in participants who completed the 6- and 9-year follow-up visit (Table S4). Under this hypothesis, the substantial deterioration of health leading to early mortality in these subjects would have been missed in the analysis. The results from these supplemental survival analyses showed that participants who were obese at baseline and declined in BMI over follow-up had significantly higher subsequent mortality than the reference group (nonobese–not decreasing BMI), after adjustment for baseline age and sex (hazard ratio = 2.02, P = .002 for mortality after 6 years; hazard ratio = 2.43, P = .003 for mortality after 9 years). In contrast, there was no difference in mortality between those who were obese at baseline and did not decline in BMI over follow-up and the reference group (nonobese–not decreasing BMI). These results demonstrated that, not only was there no survival bias, but also health deterioration in obese older persons who lost weight was probably underestimated because of competing mortality.
DISCUSSION
The present study investigated the complex and dynamic relationship between BMI and its fluctuations over time and longitudinal changes in health status, operationalized as multimorbidity, in older participants of the InCHIANTI Study. Baseline obesity was found to be significantly associated with higher cross-sectional presence and greater longitudinal increase in multimorbidity over the follow-up period than baseline overweight and normal weight, although no significant differences in baseline and longitudinal multimorbidity were observed between baseline normal weight and overweight. In addition, participants who were obese at baseline and lost weight over follow-up had a significantly greater longitudinal increase in multimorbidity than all other participants, even those who were obese at baseline but maintained or gained weight over time. In nonobese older adults, loss of weight was not associated with significantly greater increase in multimorbidity than stable or increasing weight over time.
Although there is a general consensus that midlife obesity is associated with greater risk of future development of many chronic diseases and lower likelihood of successful aging, optimal BMI for older adults is controversial.10,33 Recent studies have shown that being slightly overweight in middle and older age is associated with better health and longevity than normal weight, although these results are still debated.11 In addition, in older adults, loss of weight has been associated with higher mortality risk than weight gait.12–21
To the best of the knowledge of the authors, no previous studies have fully investigated whether weight loss in older adults is a marker of impending expansion in multimorbidity regardless of initial weight.
Regarding the biological mechanisms that may link weight loss to accumulation of chronic conditions, previous studies found that chronic inflammation and high resting metabolic rate are associated with greater prospective burden of multimorbidity in older adults.27,34 Therefore, weight loss may be the result of the co-occurrence of multiple wasting conditions rather than the cause (reverse causation).10 Loss of weight may also occur at a preclinical stage in the disease history and start several years before diagnosis, as previous reports showing that chronic conditions that contribute directly to weight loss may remain undiagnosed for years have suggested.10,18
These findings make several innovative contributions to the current literature. First, weight loss was found to be associated with increase in multimorbidity in obese but not nonobese older adults. Although the mechanism for this association remains unclear, it offers an important clue to identifying individuals with impending decline in health at an early stage and has relevance for research. Weight loss is considered to be one of the general frailty diagnostic criteria, but it might be that it adds important information only when applied to frankly obese individuals. Although further investigations in larger populations and validation of these findings are required for their translation into clinical recommendations, obese older adults who lose weight should receive thoughtful medical attention. Second, this study contributes to the controversy over whether being slightly overweight in old age is associated with higher or lower probability of living a long and healthy life.10 Neither cross-sectional nor longitudinal evidence was found that overweight is associated with a different burden of multimorbidity than normal weight.
A further interesting finding of this study was that participants who were obese at baseline and lost weight during follow-up not only experienced a larger increase in multimorbidity, but also had higher incidence rates of anemia and CKD, two diseases that are strongly associated with frailty, although even when the contribution of these two conditions was factored out from the computation of multimorbidity, the results were substantially unchanged. These findings suggest that rising multimorbidity in this specific group of individuals results from greater global susceptibility to disease, one of the main features of frailty.
The average difference in increase in number of diseases between groups over the average follow-up time may not appear dramatic (Figure 2), but from a clinical perspective, developing an additional chronic disease that is severe enough to surpass a standard diagnostic threshold is not a trivial event, particularly for its prognostic effect. Previous observational studies reported increasing risk of mobility loss and incident functional dependency with increasing number of chronic diseases, and even one newly diagnosed chronic condition has been found to be associated with nearly twice the likelihood of functional dependency onset over 12, 24, and 36 months of follow-up.35–38 Furthermore, in the current study, an additional disease is added on average in a group of individuals (obese participants who subsequently lost weight) that already had a higher level of multimorbidity at baseline than the rest of the population (Figure 2), highlighting that its occurrence is even more meaningful.
This study has several limitations. First, the large loss of participants during follow-up might have affected the findings. Supplemental mortality analyses were performed to address the high rate of loss during follow-up due to death; the results showed no evidence of survival bias but suggested that the risk of rapid increase in multimorbidity in obese older persons who lost weight was probably underestimated because of competing mortality. Second, the absence of participants with BMI less than 18.5 (underweight) at baseline in the sample population might affect the generalizability of the results, which should be confirmed in further studies including larger, more-diverse populations. Third, information about intentional weight loss was not collected in the InCHIANTI Study because the practice of prescribing weight-loss interventions (dietary restriction) in the older Italian population is uncommon. Therefore, in the present analysis, weight loss was assumed to be unintentional. Fourth, only BMI was used as measure of body composition, with information on visceral obesity or sarcopenic obesity not included. BMI is only an approximate measure of adiposity and does not take into account change in body composition that occurs with aging, but because BMI has the advantage of being cheap and easy to use for physicians and general practitioners as a screening tool to closely monitor weight changes in older adults, the findings may be easier to translate into clinical practice. Finally, information on disease severity was not considered as part of the definition of multimorbidity, although strict thresholds were used for defining diseases to minimize the chance of over-diagnosis. Severity classification systems that homogeneously quantify the effect of diseases on health are not available, which is probably why the numeric estimates of multimorbidity tend to be lower than those that other studies have reported.39
In conclusion, loss of weight in obese older adults is strongly associated with greater increase in multimorbidity than in nonobese older adults and even in obese older adults who maintain or gain weight. Loss of weight in nonobese older adults is not associated with a significantly greater increase in multimorbidity than stable or increasing weight. Although further investigations in larger, more-diverse populations in terms of culture and body composition characteristics (including underweight or extremely obese individuals) are required to confirm the generalizability of the findings, loss of weight in obese older adults should be considered a “phenotype” of impending deterioration of health status that requires fast, thoughtful medical attention.
Supplementary Material
Acknowledgments
Conflict of Interest: No author has any conflict of interests.
The InCHIANTI baseline study (1998–2000) was supported as a targeted project (ICS110.1/RF97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (NIA) (Contracts 263 MD 9164 and 263 MD 821336); the InCHIANTI Follow-up 1 study (2001–03) was funded by the NIA (Contracts N.1-AG-1–1 and N.1-AG-1–2111); and the InCHIANTI Follow-up 2 and 3 studies (2004–10) were financed by the NIA (Contract N01-AG-5–0002), supported in part by the Intramural Research Program of the NIA, National Institutes of Health, Baltimore, Maryland.
Dr. Boyd was supported by Paul Beeson Career Development Award NIA K23 AG032910, AFAR, The John A. Hartford Foundation, The Atlantic Philanthropies, The Starr Foundation, and an anonymous donor.
Dr. Fabbri and Dr. Ferrucci had full access to all of the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis.
Author Contributions: All authors contributed to this paper.
Sponsor’s Role: None.
Footnotes
Additional Supporting Information may be found in the online version of this article:
Figure S1. Design of the study.
Table S1. Prevalence of chronic diseases included in the evaluation of multimorbidity at baseline (N = 1,025).
Table S2. Linear mixed models testing the association between baseline body mass index (BMI) and presence and prospective development of multimorbidity, independent of baseline age, sex, education (Model I) and baseline, age, sex, education, and baseline interleukin (IL-6) (Model II).
Table S3. Linear mixed model testing the association between change in body mass index (BMI) overtime and presence and prospective development of multimorbidity, independent of baseline age, sex, education, and baseline BMI.
Table S4. Survival analyses testing differences in mortality after 6 and 9 years of follow-up in the four groups defined for the present study and based on baseline body mass index (BMI) and change in BMI over time.
Please note: Wiley-Blackwell is not responsible for the content, accuracy, errors, or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Smith SM, O’Dowd T. Chronic diseases: What happens when they come in multiples? Br J Gen Pract. 2007;57:268–270. [PMC free article] [PubMed] [Google Scholar]
- 2.Fortin M, Soubhi H, Hudon C, et al. Multimorbidity’s many challenges. BMJ. 2007;334:1016–1017. doi: 10.1136/bmj.39201.463819.2C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition—multimorbidity. JAMA. 2012;307:2493–2494. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogeli C, Shields AE, Lee TA, et al. Multiple chronic conditions: Prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22:391–395. doi: 10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menotti A, Mulder I, Nissinen A, et al. Prevalence of morbidity and multimorbidity in elderly male populations and their impact on 10-year all-cause mortality: The FINE study (Finland, Italy, Netherlands, Elderly) J Clin Epidemiol. 2001;54:680–686. doi: 10.1016/s0895-4356(00)00368-1. [DOI] [PubMed] [Google Scholar]
- 6.Fortin M, Bravo G, Hudon C, et al. Relationship between multimorbidity and health-related quality of life of patients in primary care. Qual Life Res. 2006;15:83–91. doi: 10.1007/s11136-005-8661-z. [DOI] [PubMed] [Google Scholar]
- 7.Townsend A, Hunt K, Wyke S. Managing multiple morbidity in mid-life: A qualitative study of attitudes to drug use. BMJ. 2003;327:837. doi: 10.1136/bmj.327.7419.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayliss EA, Bayliss MS, Ware JE, Jr, et al. Predicting declines in physical function in persons with multiple chronic medical conditions: What we can learn from the medical problem list. Health Qual Life Outcomes. 2004;2:47. doi: 10.1186/1477-7525-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortin M, Bravo G, Hudon C, et al. Psychological distress and multimorbidity in primary care. Ann Fam Med. 2006;4:417–422. doi: 10.1370/afm.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontana L, Hu FB. Optimal body weight for health and longevity: Bridging basic, clinical, and population research. Aging Cell. 2014;13:391–400. doi: 10.1111/acel.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winter JE, Macinnis RJ, Wattanapenpaiboon N, et al. BMI and all-cause mortality in older adults: A meta-analysis. Am J Clin Nutr. 2014;99:875–890. doi: 10.3945/ajcn.113.068122. [DOI] [PubMed] [Google Scholar]
- 12.Newman AB, Yanez D, Harris T, et al. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49:1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 13.Wannamethee SG, Shaper AG, Walker M. Weight change, weight fluctuation, and mortality. Arch Intern Med. 2002;162:2575–2580. doi: 10.1001/archinte.162.22.2575. [DOI] [PubMed] [Google Scholar]
- 14.Somes GW, Kritchevsky SB, Shorr RI, et al. Body mass index, weight change, and death in older adults: The Systolic Hypertension in the Elderly Program. Am J Epidemiol. 2002;156:132–138. doi: 10.1093/aje/kwf019. [DOI] [PubMed] [Google Scholar]
- 15.Corrada MM, Kawas CH, Mozaffar F, et al. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol. 2006;163:938–949. doi: 10.1093/aje/kwj114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen ND, Center JR, Eisman JA, et al. Bone loss, weight loss, and weight fluctuation predict mortality risk in elderly men and women. J Bone Miner Res. 2007;22:1147–1154. doi: 10.1359/jbmr.070412. [DOI] [PubMed] [Google Scholar]
- 17.Harrington M, Gibson S, Cottrell RC. A review and meta-analysis of the effect of weight loss on all-cause mortality risk. Nutr Res Rev. 2009;22:93–108. doi: 10.1017/S0954422409990035. [DOI] [PubMed] [Google Scholar]
- 18.Alley DE, Metter EJ, Griswold ME, et al. Changes in weight at the end of life: Characterizing weight loss by time to death in a cohort study of older men. Am J Epidemiol. 2010;172:558–565. doi: 10.1093/aje/kwq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold AM, Newman AB, Cushman M, et al. Body weight dynamics and their association with physical function and mortality in older adults: The Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2010;65A:63–70. doi: 10.1093/gerona/glp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CG, Boyko EJ, Nielson CM, et al. Mortality risk in older men associated with changes in weight, lean mass, and fat mass. J Am Geriatr Soc. 2011;59:233–240. doi: 10.1111/j.1532-5415.2010.03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy RA, Patel KV, Kritchevsky SB, et al. Weight change, body composition, and risk of mobility disability and mortality in older adults: A population-based cohort study. J Am Geriatr Soc. 2014;62:1476–1483. doi: 10.1111/jgs.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Booth HP, Prevost AT, Gulliford MC. Impact of body mass index on prevalence of multimorbidity in primary care: Cohort study. Fam Pract. 2014;31:38–43. doi: 10.1093/fampra/cmt061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agborsangaya CB, Ngwakongnwi E, Lahtinen M, et al. Multimorbidity prevalence in the general population: The role of obesity in chronic disease clustering. BMC Public Health. 2013;13:1161. doi: 10.1186/1471-2458-13-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 25.Goodman RA, Posner SF, Huang ES, et al. Defining and measuring chronic conditions: Imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013;10:E66. doi: 10.5888/pcd10.120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fortin M, Stewart M, Poitras ME, et al. A systematic review of prevalence studies on multimorbidity: Toward a more uniform methodology. Ann Fam Med. 2012;10:142–151. doi: 10.1370/afm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabbri E, An Y, Zoli M, et al. Aging and the burden of multimorbidity: Associations with inflammatory and anabolic hormonal biomarkers. J Gerontol A Biol Sci Med Sci. 2015;70A:63–70. doi: 10.1093/gerona/glu127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guralnik JM, Fried LP, Simonsick EM, et al. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. [Google Scholar]
- 29.Woodman R, Ferrucci L, Guralnik J. Anemia in older adults. Curr Opin Hematol. 2005;12:123–128. doi: 10.1097/01.moh.0000154030.13020.85. [DOI] [PubMed] [Google Scholar]
- 30.McDermott MM, Fried L, Simonsick E, et al. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: The Women’s Health and Aging Study. Circulation. 2000;101:1007–1012. doi: 10.1161/01.cir.101.9.1007. [DOI] [PubMed] [Google Scholar]
- 31.Milaneschi Y, Bandinelli S, Penninx BW, et al. The relationship between plasma carotenoids and depressive symptoms in older persons. World J Biol Psychiatry. 2012;13:588–598. doi: 10.3109/15622975.2011.597876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh-Manoux A, Sabia S, Bouillon K, et al. Association of body mass index and waist circumference with successful aging. Obesity (Silver Spring) 2014;22:1172–1178. doi: 10.1002/oby.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fabbri E, An Y, Schrack J, et al. Energy metabolism and the burden of multi-morbidity in older adults. Results from the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2014 Nov 18; doi: 10.1093/gerona/glu209. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guralnik JM, LaCroix AZ, Abbott RD, et al. Maintaining mobility in late life. I. Demographic characteristics and chronic conditions. Am J Epidemiol. 1993;137:845–857. doi: 10.1093/oxfordjournals.aje.a116746. [DOI] [PubMed] [Google Scholar]
- 36.Boult C, Kane RL, Louis TA, et al. Chronic conditions that lead to functional limitation in the elderly. J Gerontol. 1994;49:M28–M36. doi: 10.1093/geronj/49.1.m28. [DOI] [PubMed] [Google Scholar]
- 37.Kriegsman DMW, Deeg DJH, Stalman WAB. Comorbidity of somatic chronic diseases and decline in physical functioning: The Longitudinal Aging Study Amsterdam. J Clin Epidemiol. 2004;57:55–65. doi: 10.1016/S0895-4356(03)00258-0. [DOI] [PubMed] [Google Scholar]
- 38.Wolff JL, Boult C, Boyd C, et al. Newly reported chronic conditions and onset of functional dependency. J Am Geriatr Soc. 2005;53:851–855. doi: 10.1111/j.1532-5415.2005.53262.x. [DOI] [PubMed] [Google Scholar]
- 39.Salive ME. Multimorbidity in older adults. Epidemiol Rev. 2013;35:75–83. doi: 10.1093/epirev/mxs009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.