Abstract
BACKGROUND
Colorectal cancer, as the second leading cause of cancer-related deaths among men and women in the United States, represents an important area for public health intervention. Although colorectal cancer screening can prevent cancer and detect disease early when treatment is most effective, few organized public health screening programs have been implemented and evaluated. From 2005 to 2009, the Centers for Disease Control and Prevention funded 5 sites to participate in the Colorectal Cancer Screening Demonstration Program (CRCSDP), which was designed to reach medically underserved populations.
METHODS
The authors conducted a longitudinal, multiple case study to analyze program implementation processes. Qualitative methods included interviews with 100 stakeholders, 125 observations, and review of 19 documents. Data were analyzed within and across cases.
RESULTS
Several themes related to CRCSDP implementation emerged from the cross-case analysis: the complexity of colorectal cancer screening, the need for teamwork and collaboration, integration of the program into existing systems, the ability of programs to use wisdom at the local level, and the influence of social norms. Although these themes were explored independently from 1 another, interaction across themes was evident.
CONCLUSIONS
Colorectal cancer screening is clinically complex, and its screening methods are not well accepted by the general public; both of these circumstances have implications for program implementation. Using patient navigation, engaging in transdisciplinary teamwork, assimilating new programs into existing clinical settings, and deferring to local-level wisdom together helped to address complexity and enhance program implementation. In addition, public health efforts must confront negative social norms around colorectal cancer screening.
Keywords: colorectal cancer screening, program evaluation, qualitative evaluation, interdisciplinary communication, early detection of cancer, cancer screening tests, collaboration, taboo, program implementation
INTRODUCTION
Screening for colorectal cancer, a disease that claimed over 53,000 American lives in 2007,1 is effective in reducing both incidence and mortality.2 Screening detects disease early, when treatment is most effective, and screening by colonoscopy may also prevent colorectal cancer entirely by removing premalignant polyps.3 As the second leading cause of cancer-related deaths among men and women in the United States,4 colorectal cancer is an important area for public health intervention.5 A more complete description of the colorectal cancer burden is found elsewhere in this supplement to Cancer.6
In 2005, the Centers for Disease Control and Prevention (CDC) initiated the Colorectal Cancer Screening Demonstration Program (CRCSDP) to promote colorectal cancer screening. Funded from 2005 to 2009, its purpose was to explore the feasibility of organized colorectal cancer screening programs for medically underserved populations in the United States.7 Across the country, 5 sites received funding: Suffolk County, New York; Baltimore City, Maryland; Nebraska; St. Louis, Missouri; and Greater Seattle, Washington.
As a condition of funding, sites were required to provide colorectal cancer screening for average risk, low-income, uninsured or under-insured adults using any test recommended by the US Preventive Services Task Force (USPSTF).8 In addition, recipients were mandated to implement other program components to support quality screening: public education and outreach, patient support, tracking and follow-up, quality assurance, and data monitoring. Sites were encouraged to develop program models best fitting the needs of medically underserved individuals within their localities.
To determine overall program feasibility, the CDC conducted an evaluation of the CRCSDP, including a longitudinal multiple case study to describe program implementation, an assessment of patient characteristics and clinical outcomes, and an examination of program costs. In the evaluation, we assessed 2 time periods—program start-up and program implementation. Program start-up involved the first 9 to 12 months of the project, when sites were hiring staff, developing policies and procedures, and putting provider contracts in place. The program implementation phase was the remaining project period, during which colorectal cancer screening services were provided. Results from program start-up, including a description of the 5 program models, have been published elsewhere.7,9–11
In this report, we introduce the multiple case study evaluation of the CRCSDP’s implementation phase. Two other articles in this supplement also draw from the case study.12,13 Our purpose is to elucidate themes from the implementation of the CRCSDP to inform state, tribal, territorial, and other organizations planning or implementing colorectal cancer screening programs. Our insights into the management of colorectal cancer screening programs are intended to advance public health efforts and contribute to more efficient and effective programs in the future. We focus on 4 areas in this report. First, we discuss the methods of the case study. Next, we present themes that emerged during program implementation based on qualitative data analysis. Then, we relate these findings to the literature about colorectal cancer screening and compare what we observed during CRCSDP’s start-up. Finally, we identify limitations of the study and offer considerations and recommendations for the implementation of similar programs.
METHODS
We conducted a rigorous multiple case study to assess program implementation, because qualitative methods provide nuances of meaning, depth of understanding, and an ascertainment of context in analyzing complex situations and processes.14,15 Case study methods used for the start-up phase have been described previously.9,10 Here, we present the case study methods that were used to evaluate the implementation phase of the CRCSDP over the 4-year program period. Each of the 5 CRCSDP sites represented a single case, and the CRCSDP served as the overall evaluand.14,16 The longitudinal nature of the case study and the inclusion of all 5 cases permitted us to document and interpret what happened over time and to compare implementation processes across sites. By using a team approach,17,18 we collected and analyzed information through various qualitative methods, including individual and group interviews, document review, and participant observation.
Sample
Each of the 5 CDC-funded CRCSDP sites represented a unique exemplar in this multiple case study. Each case was bound by the required program components (eg, client recruitment, screening and diagnostic services, quality assurance, patient support).14,16 Within each site, evaluators selected data sources from the broadest possible range of individuals involved in program implementation, narrowing and focusing to fewer individuals in subsequent data collections as the programs moved to closure. We interviewed 50 individuals at early implementation (from September to December 2007) and 32 individuals while the CRCSDP was nearing the end of its funding period (from June to August 2009). We began with a comprehensive selection of interviewees involved with the CRCSDP and ended with a more purposeful19,20 sample, selecting individuals who were chosen for their depth of knowledge of the program. Participants in the case study included site staff, stakeholders, and CDC program consultants serving as liaisons between the CDC and site staff. Program directors at each site assisted the evaluation team in developing a list of potential interviewees (ie, staff and stakeholders).
Data Collection
All interviews were audio-recorded and transcribed verbatim by a professional transcription service. Given resource constraints, interviews in 2007 were conducted by telephone. In 2009, interviews were conducted in-person at each CRCSDP site. Interviewers supplemented the recordings with standard, structured field notes.15,21
Interview questions about program implementation centered on program successes, challenges, lessons learned, partnerships, staff skills, relationships with providers, and overall program management. Final interviews explored the program life cycle, program close out, and sustainability planning efforts.
The CDC case study team conducted participant observation17,22 and recorded in-field notes, both face-to-face and during monthly telephone conferences between the CDC program consultant and key site staff, including the program director, the program manager (sometimes the same individual), and data specialists who managed each site’s clinical data. We recorded field notes during the 2009 visit to each of the 5 sites and at 2 reverse-site visits held in 2007 and 2008, in which site personnel met together in Atlanta with CDC staff and national experts on colorectal cancer screening.
Our documents included the annual grantee funding applications with supporting materials and the subsequent reports generated by the 5 sites. Data collected during implementation involved reviewing 19 documents, more than 125 observations, 82 interviews with 100 individuals (singly or in pairs), and a group interview at each of the sites.
Analysis
Data were analyzed inductively20 and abductively23,24 after each wave of interviews. Atlas.ti25 (version 5.6.1; Atlas.ti Scientific Software Development GmbH, Berlin, Germany), a qualitative analysis software program, was used to manage the material generated by the interviews.26 Although we used some of the concepts from the start-up analysis for the implementation data,12 data were distinct enough for the development of a new codebook. The first set of implementation data (2007) was coded by 1 team member, and a second member provided support when questions arose. Two members of the team shared the data coding for the second round of interviews (2009). Working together, the coders achieved an acceptable inter-rater reliability of 86%.27–32 Once coding was complete, the team identified common ideas among the responses of staff first within and then across the sites and classified codes and corresponding quotations into themes.16
Trustworthiness
Qualitative methods of data analysis are evaluated for both the research process and the product. Terms like trustworthiness, understanding, authenticity, and credibility are used interchangeably to describe what Mishler33 calls validation, a process(es) in which claims are made for and we evaluate the trustworthiness of reported observations, generalizations, and interpretations.33 The following strategies, commonly in qualitative research,17,20,34,35 were used to establish the trustworthiness of the case study analysis: triangulation of data, negative case analysis, member checking, and maintenance of a detailed audit trail. We triangulated data collection methods through interviews, participant observation and document analysis, data sources across personnel at the sites, and data collectors and analyzers among the research team to ensure multiple positions for our research. Negative case analysis involves intentionally looking for cases that contradict or challenge the researchers’ interpretations of the data, which results in a more nuanced and robust analysis. Member checking, soliciting participants’ views of the accuracy of the research findings and interpretations, also bolstered the credibility of our interpretations. Finally, the team maintained a detailed audit trail, documenting the evaluation methods and processes, to make our procedures explicit.
Reflexivity, or locating the researchers in the work, is another technique for enhancing the trustworthiness of the qualitative report.15,17 Recognizing the manner in which researchers’ perspectives, experiences, and values influence how data are interpreted is underscored by Charmaz.36 Reflexivity is used to document and track these differences.15,17,36–38 Thus, we note that the case study team was composed of 3 CDC evaluators (Amy DeGroff, Jennifer Boehm, and Elizabeth Rohan) and 2 external evaluators contracted from the University of Georgia (Judith Preissle and Rebecca Glover-Kudon). The evaluators have backgrounds in public health, education, anthropology, sociology, public policy, and oncology social work, providing a multidisciplinary team. Although it inevitably represented the CDC as the grantor to the interviewees, the team worked to minimize this power differential. With each round of interviews, evaluators repeated the confidentiality agreement and stressed the respondents’ opportunities to speak candidly about their experiences with the CRCSDP for the purposes of program improvement.
RESULTS
Several themes related to CRCSDP implementation emerged from the cross-case analysis: the complexity of colorectal cancer screening compared with screening for other cancers, teamwork and collaboration, integration of the program into existing systems, the ability of programs to use wisdom at the local level, and the influence of social norms. Figure 1 depicts a heuristic of these themes and serves as an organizational structure for presenting results. The figure’s fixed form belies the fluidity of concepts within themes and between and among ideas. For example, a concern voiced repeatedly by participants across the sites was CDC’s policy to exclude from eligibility those patients who exhibited potential symptoms of colorectal cancer (eg, rectal bleeding). This reflected the CDC’s emphasis on a public health screening program for prevention and early detection in the asymptomatic population rather than a diagnostic or treatment program for those with symptoms. Nevertheless, the requirement posed challenges for site personnel at various levels of implementation, and the concern is mentioned repeatedly throughout the results for its multiple and intersecting implications. An in-depth analysis of themes on program recruitment, crucial to program implementation, is detailed elsewhere in this supplement to Cancer12 and, thus, is not included in our report.
Figure 1.
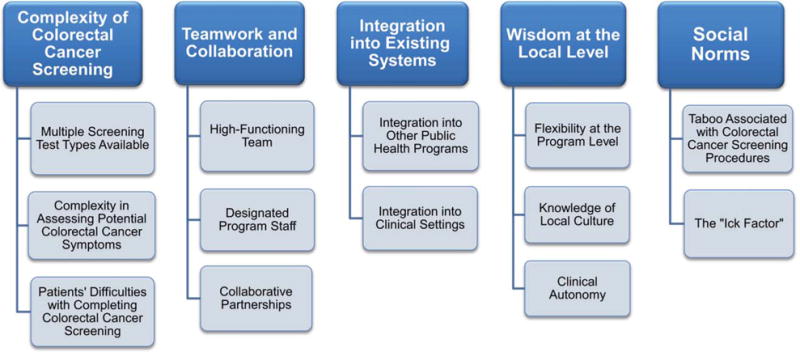
Implementation themes that emerged from cross-case analysis are illustrated. Those themes include the complexity of colorectal cancer screening, team work and collaboration, integration into existing systems, wisdom at the local level, and the influence of social norms.
Complexity of Colorectal Cancer Screening
Respondents across sites repeatedly commented on the multifaceted nature of colorectal cancer screening—the sheer complexity of it—and how that translated into complexity in implementing their screening programs. Although many factors contribute to the complexity of colorectal cancer screening, those aspects identified most frequently in CRCSDP’s implementation were the multiple types of tests available to screen for colorectal cancer, the assessment of whether a prospective candidate for screening already was exhibiting potential colorectal cancer symptoms, and, for patients, the overall difficulty of completing colorectal cancer screening.
Multiple screening test types available
Although medical screenings for most other cancers depend on a single type of test, colorectal cancer screening can be accomplished through a single test or a combination of tests, including colonoscopy, fecal occult blood test (FOBT), fecal immunochemical test (FIT), sigmoidoscopy, computed tomography (CT) colonography, and fecal deoxyribonucleic acid (DNA) testing. CRCSDP sites were required to use any screening method recommended by the USPSTF. At the time the program started, these included annual FOBT, sigmoidoscopy, or double-contrast barium enema every 5 years or colonoscopy every 10 years.8 Three sites initially selected FOBT (followed by colonoscopy for those with FOBT-positive results), and 2 sites chose colonoscopy. Complicating matters, a patient’s risk for colorectal cancer has implications for the type of screening test used. At all CRCSDP sites, patients were assessed for risk (eg, family history of colorectal cancer, personal history of polyps) at enrollment; and, for FOBT programs, those patients deemed high risk were referred directly to screening colonoscopy.
The variety of screening tests added to the complexity of implementing the CRCSDP in 2 ways. First, as noted above, each program chose among 4 different test types and dealt with the sequelae of their choice(s). For some sites, their test choice made CRCSDP implementation more laborious. For example, some sites observed that, counterintuitively, their choice of FOBT—a simpler test—was administratively more complicated. A program manager from the health department explained that, although patients in her state’s CDC-funded National Breast and Cervical Cancer Early Detection Program (NBCCEDP) could be enrolled in a variety of ways, the complexity and burden of determining the appropriate test type for the colorectal cancer program necessitated a single point of entry—the health department—because they believed it was too burdensome to be handled in a primary care practice. She said, “It’s because (we’ve) got to apply the (eligibility) algorithms that actually are kind of complicated. How does that look for a physician’s office?”
Site staff also observed that the general public was confused about the variety of test types available and reported that patients did not always understand why they were being offered a particular test. A staff member from a site that was using FOBT described how community uncertainty about the various screening tests challenged their program. She said:
“So, when you say colon cancer screening, 1 of 3 things happens. 1) They interpret it as a colonoscopy, so they don’t call in (to enroll), because they are scared; 2) they interpret it as colonoscopy, and they are okay with (having 1, but, when) they call in, they get an FOBT kit, and then they are angry; or 3) they call in saying they don’t know what (colorectal cancer screening) is, and FOBT gets explained to them. So … some people were complaining about false advertisement. They were assuming that a colon cancer screening was a colonoscopy when there are (actually) several techniques that can be done.”
Complexity in assessing potential colorectal cancer symptoms
Assessing whether a patient exhibited colorectal cancer symptoms also contributed to the complexity of implementation, because symptoms of colorectal cancer (eg, rectal bleeding, abdominal pain, a change in bowel habits, and weight loss) can be difficult to distinguish from symptoms of other conditions. This site staff member explained, “Vague, smaller symptoms (are usually) symptoms of hemorrhoids, because technically, a little blood on the tissue (toilet paper) generally is going to be a hemorrhoid.”
One of the CDC’s program policies, as stated above, deemed individuals who were assessed as symptomatic for colorectal cancer ineligible for the CRCSDP. Consequently, sites developed complex algorithms for determining clinical eligibility for patients, posing implementation challenges for 4 of the 5 sites. The policy had repercussions for how sites approached—and often struggled with—assessing clinical symptoms, providing general public education about the program, recruiting patients for the program, and addressing the concerns of clinicians who were troubled by this policy.
The Suffolk County site in New York worked around the complexity of symptom assessment and, thus, did not struggle with the ramifications of the policy. Rather than relying on multiple providers at the community health centers—in which clients were recruited to assess patients for clinical eligibility—this site had a physician on its team who conducted the assessment for clinical eligibility on all patients who were referred to the program. The physician’s clinical expertise facilitated the assessment process, including identifying medical resources for clients who were deemed ineligible for the CDC-funded program. A site staff member said the following:
“We needed someone with medical skills to handle the precolonoscopy (telephone) assessment in terms of identifying any potential medical ineligibility and making sure (to) refer (patients who are not eligible) to the appropriate source. … This individual, in our case, is a physician. I think … a nurse practitioner or a PA (Physician Assistant) could be trained to do it.”
Patients’ difficulties with completing colorectal cancer screening
Finally, patients’ difficulties in completing any of the screening tests made the process complex. This was especially true for colonoscopy, which can involve a patient’s having a precolonoscopy medical examination, taking time off work the day before the colonoscopy to prepare (ie, “prep” or clean out) the bowel, taking time off work for the colonoscopy itself, and arranging for an escort to and from the procedure. As a staff member at a site exclusively using colonoscopy said, “We’ve learned to appreciate, to use the term loosely, just how complicated it is … to get 1 person through the program.” Understanding these difficulties, the CDC required sites to provide support services for patients to facilitate the screening process.
On the basis of community needs, each site defined its own approach to providing patient support. The comprehensiveness of these services ranged from using health department staff, to providing little more than telephone reminders with patients at 1 site, and to providing a dedicated, full-time patient navigator at another site.39 More intensive support services, that is, patient navigation, were used more often for patients undergoing colonoscopy than for those completing FOBT. To assist patients with their colonoscopies, navigators provided education about colorectal cancer screening and the bowel preparation process, ensured that patients had access to laxative supplies, conducted reminder and follow-up calls, assisted with logistical barriers, and served as liaison between the patient and clinical staff. A staff member who was discussing the difficulty patients had with colonoscopy procedures claimed that, “Patient support services make or break the program.”
It is noteworthy that patient support services helped the CRCSDP reach its intended population—the medically underserved. Navigators, by garnering the trust of patients, provided a conduit to the medical system for populations who otherwise did not have access to primary care. A site staff member explained, “In the population we’re serving, in an urban setting … there’s not a natural trust (in) the hospital or the medical system.”
This issue of complexity is central to understanding the CRCSDP’s implementation. Interviewees noted that they had significantly and continually underestimated how much time, staff, and overall effort were needed to implement the program. Although underestimating effort may be typical of any new program, the seasoned health care providers across the CRCSDP sites agreed on the unique complexity of colorectal cancer screening.
Teamwork and Collaboration
Teamwork is essential in clinical oncology care,40–43 and collaboration was evident at each of the 5 CRCSDP sites. Three aspects of working together to enhance program implementation emerged from the data: a high-functioning team, designated program staff, and collaborative partnerships.
High-functioning team
Having a high-functioning team was essential for implementing the CRCSDP. For our purposes, “team” refers to site staff, that is, the group of staff within the grantee agency leading the CRCSDP. Staff across sites described a high-functioning team as exhibiting “good communication skills, teamwork, team building, encouraging and supporting 1 another, validating successes, and (discussing) problems … in a way that preserves the integrity of the people who are involved.” Data suggest that high-functioning teams with diverse expertise were key to implementing the CRCSDP, given its complexity. Effective implementation demanded coordination among staff with varied, but complementary, experience and expertise: for example, clinical aspects of colorectal cancer screening, health education, data management, and contract management. Site staff recognized the necessity for an interdisciplinary approach: “Each team member brought (his or her) own skills and abilities to the broader program.”
Although team structures varied across sites—from hierarchical to horizontal—role definition, a well defined division of labor, and clear communication were indispensable to team functioning. Sites also benefited from teams of consistent, tenured staff. Four of the 5 sites had almost no turnover during the 4-year program period, enhancing team cohesion and supporting the development of institutional program knowledge.
The notion of teamwork—which became more pronounced over time—was so critical to implementation that, when asked to identify program champions, site staff were reluctant to name just 1 individual. Instead, they named the whole team, indicating a shared responsibility for and commitment to the program’s success: “Not 1 person is responsible for all of this … It takes a team of people to understand it, to work together, and be committed to it.”
Designated program staff
Participants emphasized the necessity of having 1 or more designated staff, as part of their teams, fully funded by the program to implement the CRCSDP. They noted the substantial effort required to implement a brand new public health program. However, for multiple reasons—hiring restrictions, efforts to integrate staff across several public health programs, and preserving program funding for screening services—several sites initially relied on existing staff to administer the CRCSDP. Some of these staff members were assigned part time to the CRCSDP, and others were supported entirely with non-CDC resources but were assigned to the CRCSDP in addition to their other responsibilities.
Consequently, some staff felt pulled in several directions when working across multiple public health programs. Site staff emphasized that, at times, the CRCSDP was all-consuming, rendering them unable to meet their other responsibilities. Conversely, staff reported feeling compromised in their efforts to implement the CRCSDP while working for other programs. One site staff member described the day-to-day angst of the staffing arrangement: “I would probably say half my day is spent on … (CRCSDP).… It’s turned out to be a lot (of work), and I still have 2 other full-time jobs I have to deal with.” Some sites adjusted staffing plans by hiring full-time individuals for the CRCSDP. For other sites, resource constraints limited the capacity to make staffing changes, and they struggled with this dilemma over the 4 years.
Designated staff also were integral to program implementation at local provider sites where screening services were delivered. Provider sites were typically overburdened community health clinics, university hospitals, or specialty clinics facing significant resource limitations and patient populations with extensive needs. Without a designated staff member within a provider site to champion the program and facilitate its integration there, implementation could easily falter. Over time, the Greater Seattle, Baltimore City, and St. Louis sites benefited from recruiting and financially supporting some level of staffing within the provider sites. One provider site staff member said, “If you’re going to have facilities doing the screening, there needs to be a person of some sort, I don’t care (what the role is), but a contact person (onsite).” Funding staff in these clinics fostered clinic-level program integration and ensured oversight and accountability.
Collaborative partnerships
Collaboration with external partners advanced CRCSDP implementation by extending program resources and increasing legitimacy. Some relationships with partners were formalized through memoranda of understanding, whereas others remained informal. Overall, staff were adamant that, “you can’t do this (program) in a vacuum.”
To extend resources beyond what the program itself could provide, sites needed to work with other agencies. For example, collaborative partnerships were essential in meeting the CDC’s mandate to secure resources for treatment of patients diagnosed with cancer through the CRCSDP. In Nebraska, cancer centers across the state committed to providing cancer treatment resources through written memoranda of understanding. Partners supported other program components as well—the Baltimore City site involved the American Cancer Society’s call center to field questions about the program and assist with client recruitment. Leveraging such partnerships extended program resources and enhanced program implementation. State Comprehensive Cancer Control (CCC) Programs helped foster these partnerships. A site staff member remarked that, “Having a really good relationship with the CCC Program has been absolutely critical in (our state).… (The CCC Program director) has so many contacts in the cancer world in the state, and she’s really pulled a lot of things together for us.
Partnerships also advanced the program’s stature in the community, providing both tacit and active endorsement and furthering the program’s credibility among a wider audience. For example, medical advisory boards often included well respected leaders in the field of colorectal cancer who actively promoted and implemented the CRCSDP. In 1 site, a leading gastrointestinal surgeon made presentations to physicians across the state about the value and effectiveness of FOBT. Several sites also were able to enlist colorectal cancer survivors as partners to endorse their programs, providing personal testimonials to promote colorectal cancer screening.
Across sites and circumstances, site staff discussed how teamwork and collaborative partnerships were essential to CRCSDP implementation. Cooperation among members of a high-functioning team, having funded staff in key roles, and collaborating with partners to extend the reach or resources of the program were crucial.
Integration into existing systems
The CRCSDP, as a new program, was integrated into existing social organizations and structures, or systems, while maintaining a distinct program identity.13 Throughout the course of the program, sites achieved various degrees of integration into local, existing systems.
Integration into existing public health programs
To establish the CRCSDP, 4 of the 5 sites built on their NBCCEDP, capitalizing on existing program infrastructure, such as experienced staff, provider and partner networks, and data management systems. Such integration benefited the CRCSDP sites by facilitating implementation; however, instances occurred in which integration proved problematic, suggesting the necessity for a more careful consideration of the degree to which integration with existing public health programs was both feasible and desirable.
One example of sites’ use of existing infrastructure to support implementation was the established network of NBCCEDP providers and partners, which offered a ready pool of familiar (or well known) primary care providers. Respondents claimed that building on these existing relationships helped the CRCSDP gain legitimacy in the medical community and fostered provider interest in working with this new program. One site staff member said, “Our breast and cervical health program … has a good reputation in the medical community here, so the partnership (with the NBCCEDP) has been really helpful.” A staff member from another site explained that the willingness of their providers to work with the CRCSDP hinged on the trusting relationships they had built over the course of the NBCCEDP: “I think a lot has to do with (the fact that) they trust the program. We’ve been around for a long time, and they know they can trust us with breast and cervical, and so I think maybe that has helped.”
Nevertheless, integration with existing programs did not always facilitate implementation. This was especially evident with the CRCSDP policy that excluded symptomatic patients, because the NBCCEDP allows symptomatic women to be served. Across sites, staff repeatedly commented on the difficulty of integrating programs, including promoting the CRCSDP and the NBCCEDP together, given the discrepancy in eligibility criteria between the 2 programs, as the following quote indicates:
“It’s kind of hard for providers to wrap their heads around (the idea) that, with this program, we can’t enroll symptomatic clients. But, in the breast and cervical, if you have a symptomatic client, that’s when you really rev up and work and get a resolution to that abnormality.”
In some instances, integration across screening programs led to confusion among clients and providers. Nebraska’s Every Woman Matters program integrated the CRCSDP with 2 other CDC-funded programs: the NBCCEDP and Well Integrated Screening and Evaluation for Women Across the Nation (WISEWOMAN), which focuses on cardiovascular health. Although site staff promoted the 3 programs together, they needed to ensure that patients and providers understood that the programs were, indeed, distinct. One Nebraska site staff member noted, “(Patients) have confusion, sometimes, if they are clients of the Every Woman Matters program. They think they’re automatically enrolled in the colorectal program.” Providers are often similarly confused.
The program in Suffolk County, New York was a stand-alone program that did not integrate with other programs within their system. Still, this program achieved a great deal of success and provides a counter-example to the models of integration described above.
Integration into clinical settings
Site staff members’ understanding of provider sites’ systems and processes, such as patient flow processes, data collection systems and related forms, and treatment plans, was fundamental to integrating the CRCSDP into those clinical settings. Some of this expertise was gained from each program’s medical advisory board, which was convened by the sites during start-up to provide clinical guidance. In addition, some site staff had first-hand clinical knowledge and experience, which augmented integration of this public health program into clinical settings. This site staff member described how he used his knowledge of clinical settings to empathize with provider site staff:
“Well, I think (it’s important) definitely to work within the existing clinical systems as much as you can. By that I mean when I go out to the different clinics or when I’m training new clinicians on this program, I think I use my experience (of having) work(ed) in a clinic to relate to them that I understand the mindset.”
Supporting integration into clinical settings (eg, provider sites) exemplifies the flexibility CRCSDP sites built into their programs, a pattern emphasized in the section below. Integration of the CRCSDP into existing systems, although fundamental to furthering program implementation, took time, demanded careful consideration, and required support.
Wisdom at the local level: In vivo theme
Wisdom at the local level was a theme that emerged from the data in vivo, that is, derived directly from respondents’ own words. One site staff member described the CRCSDP as “a marvelous program, if we could just loosen up some of the (CDC-imposed) criteria and (be) allow(ed to use) some wisdom at the local level (we’d be even better off).” Site staff appreciated having flexibility at the program level and being able to use their knowledge of the local environment to enhance program implementation. Most staff wished for the clinical autonomy to incorporate local wisdom.
Flexibility at the program level
Because the CRCSDP was a demonstration program, the CDC permitted flexibility across sites for many aspects of implementation, including the program models used, screening test type(s) selected, and overall program management. Staff across all sites commented that it was useful to have “enough flexibility to let go of things that weren’t necessary” and to change things that were not working. Site staff also noted the importance of “tailor(ing) what they’re doing to their own situation, their own resources, their own strengths.” This flexibility, intentionally built into the CDC’s design of the CRCSDP, was mirrored at sites with decentralized programs (Baltimore City, Greater Seattle, and St. Louis) that contracted with multiple provider sites to offer screenings. Staff from these sites realized that their program designs and implementation activities needed to be “flexible so that (they could) provide for the context of each provider.” This flexibility at the provider site level corresponded to the flexibility at the program level of the CRCSDP.
Conversely, many site staff aspired for greater flexibility at the program level on clinical eligibility, because they disagreed with the CDC’s policy of excluding symptomatic patients. One site staff member explained that the population their program intended to serve was particularly vulnerable to experiencing gastrointestinal symptoms because of their life circumstances, including challenges of communicating across diverse cultural expressions of pain:
“(Excluding people with symptoms) is … not optimal. The reality is our patient population is stressed, low income, uninsured, multiethnic—and half of them have abdominal symptoms. (These symptoms are) so common that (the policy) excludes (a lot of people who need to be screened).”
Knowledge of local culture
Site staff’s knowledge of local cultures was evident both in program models and in implementation activities. For example, Nebraska’s program offered limited support services to patients to help them through the screening process. In explaining that the state is comprised of many small towns with a deep-rooted culture of neighbors helping each other, site staff repeatedly noted, “Nebraskans will help Nebraskans.” In their view, the health department had less need to provide logistical support to patients in that program. In addition, Nebraska patients were asked to contribute a small fee for their colonoscopies so that the CDC resources could be used to screen more people, which paradoxically reflects both the neighbors-helping-neighbors culture and the self-sufficiency culture in that state. A site staff member from Nebraska explained that their patients took pride in paying the fee.
Site staff in Greater Seattle also described how they considered local cultures in designing their program, as this comment illustrates:
“From my perspective working out of the local County Public Health Department, the more local you can make programs like this work (and) the more you can build up the fabric of your community long term, the better off you’re going to be, because you are building up from within the communities and not trying to impose it on top. I think especially in our state, (which) tends to (favor) local control anyway, the idea that you would have a state health department tell somebody here in Seattle what to do and how to do it just doesn’t seem to make sense to me.”
During the course of CRCSDP implementation, the Greater Seattle program introduced a shared decision-making model that they believed was more consistent with their community culture and, consequently, would be more effective in recruiting clients. This model was intended to encourage a discussion between patient and provider about whether the patient preferred FOBT or colonoscopy, as opposed to having providers impose a particular test. The shared decision-making model resonated with the local control, grass-roots culture of Greater Seattle, as described previously.
Clinical autonomy
More clinical autonomy was desired for 2 clinical decisions—establishing the colonoscopy rescreening interval and allowing individuals who had symptoms into the program. Site staff were occasionally confronted with endoscopists who prescribed a shorter interval for rescreening than recommended by the USPSTF guidelines8 followed by the CDC. In these situations, site staff and provider site coordinators, many of whom were nurses, described feeling uncomfortable with questioning a gastroenterologist’s decision while trying to enforce CDC program guidelines. At times, adhering to program policies meant challenging a physician’s clinical autonomy, a difficulty of CRCSDP implementation.
In addition, site staff wished for more clinical autonomy to allow symptomatic patients into the program. Site staff expressed frustration with being responsible for implementing a policy they did not like, as noted above; concomitantly, many providers struggled with the stifled autonomy. They wished to include patients with “certain (potential colorectal cancer) symptoms” given their clinical and ethical sensibilities. One site staff member described the difficulty clinicians—both primary care providers and gastroenterologists—had with excluding symptomatic patients:
“(There was) tremendous pushback from providers about not being able to see symptomatic people. … It was a hurdle for us to get over as far as getting providers to buy into doing this program. … It just runs contrary to a provider’s nature when you exclude people who are symptomatic.”
Overall, these data suggest that allowing sites to use their local wisdom can enhance implementation by supporting responsive, strengths-based management. Many professionals at each CRCSDP site had years of experience working with patients in their communities. They not only wanted their knowledge to be recognized and respected, but they also wanted to use what they knew to be good practice. They wanted to be empowered to use this knowledge, that is, “wisdom at the local level,” to implement the CRCSDP.
Social Norms
Although it was not a factor that was considered much at the outset, by the end of the CRCSDP, site staff reflected on the effect of social norms on the implementation and performance of their programs. The predominant social norms repeatedly confronted by site staff were the taboos associated with colorectal cancer screening procedures and the perception of many individuals that the screening tests are embarrassing and unpleasant. These norms mean that colorectal cancer screening is not as widely accepted—or discussed among friends—as are other cancer screening tests. One site staff member conceded, “There isn’t the level of community support (for colorectal cancer) like (there is) for breast cancer.”
The taboo associated with colorectal cancer screening procedures
The taboo site staff described ranged from their awkwardness with discussing stool to patients’ aversion to colonoscopy because of homophobia or a history of sexual abuse. The taboo was reflected in reluctance among some site staff and primary care providers to speak frankly with patients about colorectal cancer screening. Repeatedly, respondents described awkwardness in directly discussing bowel movements with patients. One site staff remarked: “I was really embarrassed. I was calling the patient up, and I didn’t really quite know how to say your stool. Do you talk about stool? Do you talk about bowel movements? Do you call it poop?” Another site staff member described similar discomfort from staff at a provider site, which prompted them to provide training to their staff, “The medical assistants for a while were, like, ew (gross), I don’t want to talk about that with the patient.”
Some respondents described a stronger reaction, explaining the taboo around having something inserted into the anus, particularly for men. One site staff member focused on homophobia: “I think they find (colonoscopy) frightening … and something that they don’t consider wholesome or correct.” Another directly and poignantly addressed the aversion to colonoscopy because of an individual’s history of sexual abuse:
“I think it’s a huge sexual taboo, and it’s not even talked about. No one (from our program) has once raised the idea that putting something up another’s rectum implies sodomy abuse. And so, to talk with someone who has suffered sexual abuse and explain what colonoscopy is … and that they’re getting anesthesia (and going to be asleep), they’re absolutely terrified (about) what’s going to happen and that they’re not in control. I have had 2 transgender patients, and, you know, their stories are very complex. I met them down there (in the colonoscopy suite) and literally held their hand(s) while they went to sleep.”
As site staff came to recognize the impact of the taboo associated with colorectal cancer screening, they confronted it more directly. They emphasized that the CRCSDP forced the issue of talking about body parts associated with colorectal cancer screening, which, in turn, decreased the stigma associated with the taboo. One site staff claimed, “(Colorectal cancer screening messages are) all over the place now … from me, from the providers, and so everybody’s a lot more comfortable about the whole issue.”
The “ick factor” associated with colorectal cancer screening
Related to the taboo about colorectal cancer screening is the public’s perception that such screening procedures are embarrassing and unpleasant, prompting the “ick factor.” Site staff suggested that many individuals avoid colorectal cancer screening because they are reluctant to deal with their stool directly in an FOBT or indirectly by using laxatives to clean out their bowels in preparation for colonoscopy. One respondent described what she observed as the general public’s perception of FOBT, saying, “People don’t want to do (FOBT). They’re messy. They think, oh, I have to smear my stool … and they don’t (want to do it). I think it’s 1 of those gross factors that they don’t want to deal with.”
Overall, site staff noted that social norms around colorectal cancer screening discouraged screening, affecting not only how sites recruited clients to the program12 but also how staff interacted with patients once enrolled. Site staff did concede, however, that both widespread acceptance of breast cancer screening and the existence of the CRCSDP have helped to increase the social acceptability of colorectal cancer screening. This site staff member compared previous shifts in social norms about breast cancer screening to currently shifting social norms about colorectal cancer screening:
“Let’s look at the general public. People just aren’t getting screening for colon cancer, whether they have insurance or they don’t. … I certainly lived in an era when women started getting mammograms, and it took us a while to decide that was something we wanted to do. It was painful. It had stigma. You didn’t talk about that part of your body.”
Challenging the social norms that undermine the social acceptability of colorectal cancer screening was imperative to successful CRCSDP implementation, a concept that developed throughout the course of the program.
DISCUSSION
Several interacting themes emerged from the cross-case analysis with implications for implementing the CRCSDP. Notably, the technical features of colorectal cancer screening contribute to its complexity. However, social norms, including taboos around human eliminatory functions and the “ick factor” in the screening procedures, interact with the clinical complexity to further complicate program implementation. Moreover, because both clinicians and the general public are susceptible to these social norms, addressing the provider-patient interaction may be necessary to enhance not only patient care but also implementation of colorectal cancer screening programs. Similarly, local wisdom may be applied in making decisions about program policies and integrating colorectal cancer screening into existing clinical systems and public health programs. Teamwork and collaboration interact with each of the other themes, because those are the mechanisms through which the work is accomplished. In documenting program implementation processes, our results elaborate on issues raised in extant literature on colorectal cancer screening2,3,44–59 and offer valuable insights into other clinical and public health professionals who aim to advance colorectal cancer screening more broadly.
The themes from our analysis have implications for practice. These data suggest that the overall complexity of colorectal cancer screening is a function of many factors that are not readily simplified, and implementation of the CRCSDP reflected this complexity in both planning7,10 and delivery. Site staff, as noted above, had underestimated the complexity involved in colorectal cancer screening and, thus, had significantly and continually underestimated how much effort was needed to implement the CRCSDP. To help manage the complexity of implementing a colorectal cancer screening program, public health practitioners, clinicians, and other stakeholders must be empowered to use their “wisdom at the local level” throughout program implementation. Specifically, programs could use their wisdom and knowledge of the local cultures to provide education to the public about the variety of test types available for colorectal cancer screening and to promote shared decision making between providers and patients to accommodate patients’ test preferences. In addition, these data indicate that incorporating patient navigation services (or similar patient support services) into the CRCSDP helped patients traverse the complicated terrain of colorectal cancer screening, supporting similar findings from previous research.39,47,51,54,60,61
These data also suggest that a transdisciplinary approach to the implementation of a complex public health program like the CRCSDP is preferable to multidisciplinary or interdisciplinary approaches. Multidisciplinary models of practice involve bringing together practitioners from different disciplines to work on a particular project or program, yet each practitioner views the issue within his or her own disciplinary framework.62–64 Interdisciplinary practice goes a step further, integrating different perspectives; however, practitioners remain grounded in their own disciplines.62–64 Building on both multidisciplinarity and interdisciplinarity, transdisciplinary teamwork transcends disciplinary boundaries to accommodate complexity and create new conceptual frameworks from which to understand and resolve issues on the task or process at hand.62–66 The goal of transdisciplinarity is to gain an understanding of the world in its complexity and not just a part of it.62 The high-functioning teamwork described by CRCSDP site staff was transdisciplinary. CRCSDP team members—and their collaborating partners—came to the program with different disciplinary backgrounds, but they transcended those boundaries to design, implement, and address difficulties in a complex public health program. By practicing transdisciplinary public health, practitioners, researchers, and evaluators can identify and address the complexities inherent in implementing a complex program like the CRCSDP.
Furthermore, these data suggest that successful implementation of screening for 1 type of cancer (eg, breast cancer) may not assure success in colorectal cancer screening. While capitalizing on existing program infrastructure often enhances the implementation of a new public health program, especially during the start-up phase,9 it cannot be assumed that wholesale integration is feasible or even preferable. While integration has been emphasized in public health, including for chronic diseases,67 careful consideration must be given to the similarities and differences between and among programs. Assimilation—not necessarily integration—may be a more meaningful goal. Integration means combining different programs; assimilation means preserving distinct programs as they become part of a larger whole. In this way programs can maximize efficiencies and capitalize on their experience while maintaining separate policies and procedures, where needed, and adequate staffing for carrying out a new program. This conception of assimilation can help inform other public health or other health care programs intending to integrate cancer screening or other chronic disease programs.
Finally, the influence of social norms on the uptake of colorectal cancer screening cannot be underestimated. Our data suggest that social norms about feces68 and the processes involved in colorectal cancer screening,44,45,49,50 particularly colonoscopy, tend to be negative and, thus, undermine more widespread colorectal cancer screening. Previous research asserts that a screening test has to be acceptable both to the target population and to health care professionals.69,70 If social norms associated with colorectal cancer screening procedures are not addressed, then they remain a strong, invisible force against improved screening prevalence. Consequently, public health efforts, including policy initiatives and national campaigns, such as Screen for Life, must contribute to shifting social norms and normalizing colorectal cancer screening. Advancing social norms about colorectal cancer screening requires transdisciplinary teamwork grounded in an ecologic model71 that recognizes the influence of multiple sectors, including families, primary care providers, faith-based organizations, workplaces, health care institutions, private and public insurers, media, and policy makers.
Like in any qualitative inquiry, our findings are not statistically generalizable to other settings. Although the results are specific to these 5 unique cases, our analysis provides sufficiently detailed documentation to allow readers to decide on the naturalistic generalizabilty16 (ie, transferability) of the findings to their own settings.20 In addition, our results do not include patients’ views of the CRCSDP or attitudes from the communities that were served by the programs, nor do we have input from site staff who left their positions. To mitigate these limitations, we used rigorous methods, including studying each of the 5 sites intensively, performing member checks, and generating continual documentation across time. Furthermore, we considered competing explanations for the patterns we developed, and our reports have been scrutinized by experts in public health who were not directly involved in the study. Finally, 3 of the 5 evaluators were from the CDC, which may have influenced the responses from participants either to present a more positive view of the program or to speak less candidly about problems that arose. However, such “insider” effects were moderated by the inclusion of 2 external evaluators. In addition, because respondents appeared to be as willing to speak candidly about problems and challenges as they were to speak about successes, we believe that any “insider” effects were inconsequential. These case study results provide depth to our understanding of the myriad issues CRCSDP staff addressed during implementation that augments the quantitative evaluations provided elsewhere in this supplement to Cancer.6,11,72
The CRCSDP, as a demonstration project, offered a unique opportunity to evaluate the implementation of a novel public health program. Our results provide valuable insights for the implementation of similar efforts in the future. Although some of the implementation issues faced by the CRCSDP were unique to colorectal cancer screening, many of the themes we identified may be applied to other types of screening programs and perhaps to other chronic disease programs.
Acknowledgments
FUNDING SUPPORT
The Colorectal Cancer Screening Demonstration Program evaluated in this supplement was funded by the Centers for Disease Control and Prevention Funding Opportunity Number RFA AA030.
Footnotes
The articles in this supplement were commissioned based on participation in evaluating the Centers for Disease Control and Prevention-funded Colorectal Cancer Screening Demonstration Program.
The opinions or views expressed in this supplement are those of the authors and do not necessarily reflect the opinions or recommendations of the journal editors, the American Cancer Society, John Wiley & Sons, Inc., or the Centers for Disease Control and Prevention.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.US Cancer Statistics Working Group. Incidence and Mortality Web-based Report. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2010. United States Cancer Statistics: 1999–2007. Available at www.cdc.gov/uscs. [Accessed January 30, 2013. [Google Scholar]
- 2.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 3.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Vital signs: colorectal cancer screening among adults aged 50–75 years. MMRW Morbid Mortal Wkly Rep. 2010;59:1–5. [PubMed] [Google Scholar]
- 5.Frieden TR. A framework for public health action: the health impact pyramid. Am J Public Health. 2010;100:590–595. doi: 10.2105/AJPH.2009.185652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seeff LC, Royalty J, Helsel WE, et al. Clinical outcomes from the CDC’s Colorectal Cancer Screening Demonstration Program. Cancer. 2013;119(suppl 15):2820–2833. doi: 10.1002/cncr.28163. [DOI] [PubMed] [Google Scholar]
- 7.Seeff LC, DeGroff A, Tangka F, et al. Development of a federally funded demonstration colorectal cancer screening program [serial online] Prev Chronic Dis. 2008;5:A64. [PMC free article] [PubMed] [Google Scholar]
- 8.Preventive Services US, Force Task. Screening for Colorectal Cancer: Clinical Summary of US Preventive Services Task Force Recommendation. 2008 Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/colocancer/colosum.htm. Accessed February 24, 2011.
- 9.DeGroff A, Boehm J, Goode Green S, Holden D, Seeff LC. Facilitators and challenges to start-up of the colorectal cancer screening demonstration program [serial online] Prev Chronic Dis. 2008;5:A39. [PMC free article] [PubMed] [Google Scholar]
- 10.DeGroff A, Holden D, Goode Green S, Boehm J, Seeff LC, Tangka F. Start-up of the colorectal cancer screening demonstration program [serial online] Prev Chronic Dis. 2008;5:A38. [PMC free article] [PubMed] [Google Scholar]
- 11.Tangka FK, Subramanian S, Bapat B, et al. Cost of starting colorectal cancer screening programs: results from 5 federally funded demonstration programs [serial online] Prev Chronic Dis. 2008;5:A47. [PMC free article] [PubMed] [Google Scholar]
- 12.Boehm JE, Rohan EA, Preissle J, DeGroff A, Glover-Kudon R. Recruiting patients into the CDC’s Colorectal Cancer Screening Demonstration Program: strategies and challenges across 5 sites. Cancer. 2013;119(suppl 15):2914–2925. doi: 10.1002/cncr.28161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glover-Kudon R, DeGroff A, Rohan EA, Preissle J, Boehm J. Developmental milestones across the programmatic life cycle: implementing the CDC’s Colorectal Cancer Screening Demonstration Program. Cancer. 2013;119(suppl 15):2926–2939. doi: 10.1002/cncr.28166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin RK. Case Study Research: Designs and Methods. 4th. Thousand Oaks, CA: SAGE; 2009. [Google Scholar]
- 15.Riessman C, editor. Qualitative Studies in Social Work Research. Thousand Oaks, CA: SAGE; 1994. [Google Scholar]
- 16.Stake RE. Multiple Case Study Analysis. New York: The Guilford Press; 2006. [Google Scholar]
- 17.Padgett DK, editor. The Qualitative Research Experience. Belmont, CA: Wadsworth/Thomson Learning; 2004. [Google Scholar]
- 18.Guest G, McQueen KM, editors. Handbook for Team-Based Qualitative Research. Lanham, MD: Altamira Press; 2008. [Google Scholar]
- 19.Patton MQ. Qualitative Research and Evaluation Methods. 3rd. Thousand Oaks, CA: SAGE; 2002. [Google Scholar]
- 20.LeCompte ME, Preissle J. Ethnography and Qualitative Design in Educational Research. 2nd. San Diego, CA: Academic Press; 1993. [Google Scholar]
- 21.DeWalt KM, DeWalt BR. Participant Observation: A Guide for Fieldworkers. Walnut Creek, CA: Altamira Press; 2002. [Google Scholar]
- 22.Emerson RM, Fretz RI, Shaw LL. Participant observation and field-notes. In: Atkinson P, Coffey A, Delamont S, Lofland J, Sofland L, editors. Handbook of Ethnography. London: SAGE; 2001. pp. 352–368. [Google Scholar]
- 23.Shank G. Shaping qualitative research in educational psychology. Contemp Educ Psychology. 1994;19:340–359. [Google Scholar]
- 24.Shank G. Abductive strategies in educational research. Am J Semiotics. 1987;5:275–290. [Google Scholar]
- 25.ATLAS.ti [computer program] Berlin, Germany: Atlas.ti Scientific Software Development GmbH; 2010. [Google Scholar]
- 26.Lewins A, Silver C. Using Software in Qualitative Research: A Step-by-Step Guide. Los Angeles, CA: SAGE; 2007. [Google Scholar]
- 27.Richards L. Handling Qualitative Data: A Practical Guide. Thousand Oaks, CA: SAGE; 2005. [Google Scholar]
- 28.Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. 2nd. Thousand Oaks, CA: SAGE; 1994. [Google Scholar]
- 29.Hruschka DJ, Schwartz DC, St John DC, Picone-Decaro E, Jenkins RA, Carey JW. Reliability in coding open-ended data: lessons learned from HIV behavioral research. Field Methods. 2010;16:307–330. [Google Scholar]
- 30.Boyatzis RE. Reliability is consistency of judgment. In: Boyatzis RE, editor. Transforming Qualitative Information: Thematic Analysis and Code Development. Thousand Oaks, CA: SAGE; 1998. pp. 144–159. [Google Scholar]
- 31.MacQueen KM, McLellan E, Kay K, Milstein B. Codebook development for team-based qualitative analysis. Cultural Anthropol Methods. 1998;10:31–36. [Google Scholar]
- 32.Harry B, Sturges KM, Klingner JK. Mapping the process: an exemplar of process and challenge in grounded theory analysis. Educ Res. 2005;34:3–13. [Google Scholar]
- 33.Mishler E. Validation in inquiry-guided research: the role of exemplars in narrative studies. Harvard Educ Rev. 1990;60:415–442. [Google Scholar]
- 34.Lincoln YS, Guba EG. Naturalistic Inquiry. Beverly Hills, CA: SAGE; 1985. [Google Scholar]
- 35.Denzin NK, Lincoln YS, editors. The Sage Handbook of Qualitative Research. 3rd. Thousand Oaks, CA: SAGE; 2005. [Google Scholar]
- 36.Charmaz K. Constructing Grounded Theory: A Practical Guide through Qualitative Analysis. London, United Kingdom: SAGE; 2006. [Google Scholar]
- 37.Dowling M. Approaches to reflexivity in qualitative research. Nurs Res. 2006;13:7–21. doi: 10.7748/nr2006.04.13.3.7.c5975. [DOI] [PubMed] [Google Scholar]
- 38.Mauthner NS, Doucet A. Reflexive accounts and accounts of reflexivity in qualitative data analysis. Sociology. 2003;37:413–431. [Google Scholar]
- 39.Cavanagh MF, Lane DS, Messina CR, Anderson JC. Clinical case management and navigation for colonoscopy screening in an academic medical center. Cancer. 2013;119(suppl 15):2894–2904. doi: 10.1002/cncr.28156. [DOI] [PubMed] [Google Scholar]
- 40.Rohan E. Laboring at the Edge: Effects of Repeated Exposure to Death and Dying on Oncology Doctors, Nurses, and Social Workers. Saarbrucken, Germany: VDM, Verlag Dr. Muller; 2009. [Google Scholar]
- 41.Rohan E, Bausch J. Climbing Everest: oncology work as an expedition in caring. J Psychosoc Oncol. 2009;27:84–118. doi: 10.1080/07347330802616043. [DOI] [PubMed] [Google Scholar]
- 42.Stearns N, Lauria M, Hermann J, Fogelberg P, editors. Professional Issues in Oncology Social Work. Atlanta, GA: American Cancer Society; 2003. [Google Scholar]
- 43.Penson RT, Dignan FL, Canellos GP, Picard CL, Lynch TJ., Jr Burnout: caring for the caregivers. Oncologist. 2000;5:425–434. [PubMed] [Google Scholar]
- 44.Bass SB, Gordon TF, Ruzek SB, et al. Perceptions of colorectal cancer screening in urban African American clinic patients: differences by gender and screening status. J Cancer Educ. 2011;26:121–128. doi: 10.1007/s13187-010-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beeker C, Kraft JM, Southwell BG, Jorgensen CM. Colorectal cancer screening in older men and women: qualitative research findings and implications for intervention. J Commun Health. 2000;25:263–278. doi: 10.1023/a:1005104406934. [DOI] [PubMed] [Google Scholar]
- 46.Briss P, Rimer B, Reilley B, et al. Promoting informed decisions about cancer screening in communities and healthcare systems. Am J Prev Med. 2004;26:67–80. doi: 10.1016/j.amepre.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Chen LA, Santos S, Jandorf L, et al. A program to enhance completion of screening colonoscopy among urban minorities. Clin Gastroenterol Hepatol. 2008;6:443–450. doi: 10.1016/j.cgh.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Chen X, White MC, Peipins LA, Seeff LC. Increase in screening for colorectal cancer in older Americans: results from a national survey. J Am Geriatr Soc. 2008;56:1511–1516. doi: 10.1111/j.1532-5415.2008.01796.x. [DOI] [PubMed] [Google Scholar]
- 49.DeBourcy AC, Lichtenberger S, Felton S, Butterfield KT, Ahnen DJ, Denberg TD. Community-based preferences for stool cards versus colonoscopy in colorectal cancer screening. J Gen Intern Med. 2007;23:169–174. doi: 10.1007/s11606-007-0480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geiger TM, Miedema BW, Geana MV, Thaler K, Rangneka NJ, Cameron GT. Improving rates for screening colonoscopy: analysis of the health information national trends (HINTS I) data. Surg Endosc. 2008;22:527–533. doi: 10.1007/s00464-007-9673-2. [DOI] [PubMed] [Google Scholar]
- 51.Lasser KE, Murillo J, Lisboa S, Casimir AN, Fletcher RH, Ayanian JZ. Colorectal cancer screening among ethnically diverse, low-income patients. Arch Intern Med. 2011;171:906–912. doi: 10.1001/archinternmed.2011.201. [DOI] [PubMed] [Google Scholar]
- 52.Green AR, Peters-Lewis A, Percac-Lima S, et al. Barriers to screening colonoscopy for low-income Latino and white patients in an urban community health center. J Gen Intern Med. 2008;23:834–840. doi: 10.1007/s11606-008-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miles A, Cockburn J, Smith RA, Wardle J. A perspective from countries using organized screening programs. Cancer. 2004;101:1201–1213. doi: 10.1002/cncr.20505. [DOI] [PubMed] [Google Scholar]
- 54.Nash D, Azeez S, Vlahov D, Schori M. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006;83:231–243. doi: 10.1007/s11524-006-9029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rimer BK, Briss PA, Zeller PK, Chan ECY, Woolf SH. Informed decision making: What is its role in cancer screening? Cancer. 2004;101:1214–1228. doi: 10.1002/cncr.20512. [DOI] [PubMed] [Google Scholar]
- 56.Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17:1623–1630. doi: 10.1158/1055-9965.EPI-07-2838. [DOI] [PubMed] [Google Scholar]
- 57.Ward SH, Parameswaran L, Bass SB, Paranjape A, Gordon TF, Ruzek SB. Resident physicians’ perceptions of barriers and facilitators to colorectal cancer screening for African Americans. J Natl Med Assoc. 2010;102:303–311. doi: 10.1016/s0027-9684(15)30602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zapka JG, Lemon SC, Puleo E, Estabrook B, Luckmann R, Erban S. Patient education for colon cancer screening: a randomized trial of a video mailed before a physical examination. Ann Intern Med. 2004;141:683–692. doi: 10.7326/0003-4819-141-9-200411020-00009. [DOI] [PubMed] [Google Scholar]
- 59.Seeff LC, Nadel MR, Klabunde CN, et al. Patterns and predictors of colorectal cancer test use in the adult US population. Cancer. 2004;100:2093–2103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 60.Percac-Lima S, Grant RW, Green A, et al. A patient-tailored navigator program for colorectal cancer screening in a community health center: a randomized controlled trial. J Gen Intern Med. 2008;23:237–238. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hendren S, Griggs JJ, Epstein RM, et al. Study protocol: a randomized controlled trial of patient navigation-activation to reduce cancer health disparities [serial online] BMC Cancer. 2010;10:551. doi: 10.1186/1471-2407-10-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gelhert S, Murray A, Sohmer D, McClintock M, Conzen S, Olopade O. The importance of transdisciplinary collaborations for understanding and resolving health disparities. Soc Work Public Health. 2010;25:408–422. doi: 10.1080/19371910903241124. [DOI] [PubMed] [Google Scholar]
- 63.Kessel F, Rosenfield PL. Toward transdisciplinary research—historical and contemporary perspectives. Am J Prev Med. 2008;35:S225–S234. doi: 10.1016/j.amepre.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 64.Satterfield JM, Spring B, Brownson RC, et al. Toward a transdisciplinary model of evidence-based practice. Milbank Q. 2009;87:368–390. doi: 10.1111/j.1468-0009.2009.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosenfield PL. The potential of transdisciplinary research for sustaining and extending linkages between the health and social sciences. Soc Sci Med. 1992;35:1343–1357. doi: 10.1016/0277-9536(92)90038-r. [DOI] [PubMed] [Google Scholar]
- 66.Holmes JH, Lehman A, Hade E, et al. Challenges for multilevel health disparities research in a transdisciplinary environment. Am J Prev Med. 2008;35(2 suppl):S182–S192. doi: 10.1016/j.amepre.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Slonim AB, Callaghan C, Daily L, et al. Recommendations for integration of chronic disease programs: are your programs linked [serial online]? Prev Chronic Dis. 2007;4:A34. [PMC free article] [PubMed] [Google Scholar]
- 68.Praeger D. Poop Culture. Los Angeles, CA: Feral House; 2007. [Google Scholar]
- 69.Meissner HI, Smith RA, Rimer BK, et al. Promoting cancer screening: learning from experience. Cancer. 2004;101:1107–1117. doi: 10.1002/cncr.20507. [DOI] [PubMed] [Google Scholar]
- 70.Meissner HI, Vernon SW, Rimer BK, et al. The future of research that promotes cancer screening. Cancer. 2004;101:1251–1259. doi: 10.1002/cncr.20510. [DOI] [PubMed] [Google Scholar]
- 71.Zapka JG, Lemon SC. Interventions for patients, providers, and health care organizations. Cancer. 2004;101:1165–1187. doi: 10.1002/cncr.20504. [DOI] [PubMed] [Google Scholar]
- 72.Subramanian S, Tangka FKL, Hoover S, et al. Costs of planning and implementing the CDC’s Colorectal Cancer Screening Demonstration Program. Cancer. 2013;119(suppl 15):2855–2862. doi: 10.1002/cncr.28158. [DOI] [PMC free article] [PubMed] [Google Scholar]


