Abstract
We compared the growth levels and pathogenicities of the Mycobacterium tuberculosis MT103 clinical strain with those of the ΔpurC mutant strain MYC1551, which is auxotrophic for purine in wild-type and gamma interferon receptor (IFN-γR)-deficient mice. The ΔpurC strain MYC1551 grew initially in both wild-type and IFN-γR-deficient mice upon aerosol infection, but it grew much less than strain MT103 did. Despite the comparable bacterial burdens of the mice, IFN-γR-deficient mice succumbed to infection with ΔpurC strain MYC1551 from necrotic pneumonia within 6 weeks of those infected with MT103. In conclusion, the ΔpurC mutant MYC1551 displays reduced growth but retains pathogenicity. Therefore, the use of mutant strains of M. tuberculosis as live vaccines may not be recommended.
Tuberculosis (TB) remains a major global public health issue, with a projected mortality of 90 million cases over the next 3 decades without any further interventions (7). Vaccination with the attenuated strain of Mycobacterium bovis BCG has been effective against severe forms of TB in children, but the levels of protection vary in different geographical locations, and the strain is not effective against the adult form of TB (3). Considerable efforts have been made to identify specific mycobacterial antigens involved in the protective immune response to TB (8, 15). The possibility of using live mutant strains of mycobacteria as vaccines has also been explored (1, 11, 12, 17). An advantage of using attenuated live vaccines instead of subunit vaccines is that the former may provide a greater complement of antigens, since live vaccines produce most antigens that are normally expressed in vivo. Live attenuated strains of Mycobacterium tuberculosis should express antigens which the BCG vaccine lacks and which may be important in the immune response to TB (10). Attenuated auxotrophic mutants of bacterial pathogens have already shown potential as live vaccine candidates (5, 19). Further, severely immunodeficient mice have survived BCG auxotrophic infection, although they succumbed to wild-type (WT) BCG infections (1, 12, 14). It was therefore proposed that attenuated BCG strains could present potentially safe and useful vaccines against TB infection (10) and, specifically, that they could be used in patients infected with human immunodeficiency virus. Strain BCG and M. tuberculosis ΔpurC and ΔleuD mutants that are auxotrophic for purine have been tested with animal models (1, 12, 14), confirming reduced virulence after intravenous infections in mice.
Here, we determined the pathogenicity of MYC1551, the ΔpurC mutant from clinical strain MT103, with a physiological model of aerosol infection, aiming to understand whether the mutant strain has reduced pathogenicity in an immunosuppressed host. In view of the critical role that gamma interferon (IFN-γ) plays in controlling mycobacterial infections (4, 6, 9), we used immunodeficient IFN-γ receptor-deficient (IFN-γR−/−) mice (13).
The M. tuberculosis ΔpurC strain MYC1551 is lethal in IFN-γR−/− mice.
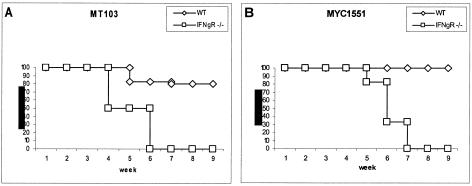
Since the auxotrophic ΔpurC strain MYC1551 is attenuated and does not grow in in vitro-cultured macrophages (14), we wanted to determine whether this mutant has reduced pathogenicity in an immunodeficient host. Therefore, IFN-γR−/− mice were infected with WT MT103 or the ΔpurC mutant MYC1551 by aerosol exposure (100 CFU per lung) and compared to WT mice. The immunodeficient IFN-γR−/− mice infected with MT103 started to lose body weight within 2 weeks (data not shown) and succumbed to infection between weeks 4 and 6 (Fig. 1A), and those infected with the ΔpurC mutant MYC1551 lost weight within 4 weeks and survived slightly longer but succumbed to infection in weeks 5 through 7 (Fig. 1B). By contrast, most of the WT mice (8 of 10) infected with either MT103 or the ΔpurC mutant MYC1551 survived (Fig. 1). As MYC1551 has a reduced capacity for in vitro intracellular growth in resting macrophages (14), we wanted to determine whether MYC1551 has a reduced capacity for growth in the host in vivo.
FIG. 1.
The M. tuberculosis ΔpurC mutant MYC1551 causes rapid lethality in IFN-γR−/− mice. WT mice and IFN-γR−/− mice were exposed to aerogenic infections (100 CFU) with the WT MT103 (A) and ΔpurC mutant MYC1551 (B) strains. Each group contained 10 mice. The values on the y axes indicate percent survival.
Reduced bacterial burdens in M. tuberculosis ΔpurC strain MYC1551-infected mice.
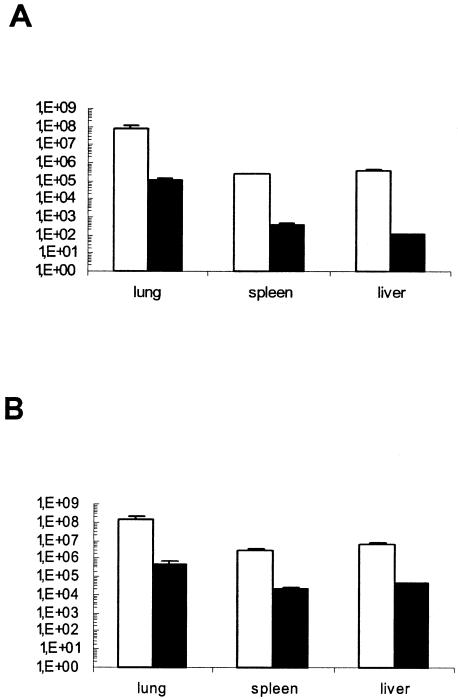
Organs of infected mice were assessed for mycobacterial growth at 4 weeks of infection. Mycobacterial loads of MT103 in the lungs, spleens, and livers of IFN-γR−/− mice were higher (for lungs and livers, P < 0.05; for spleens, P < 0.001) than those in WT mice (Fig. 2). The ΔpurC mutant MYC1551 grew much less in vivo upon aerosol infection, but some growth was preserved, unlike after intravenous infection (17). The bacterial counts in these lungs, spleens, and livers were significantly lower than the bacterial counts in those with MT103 infections (P < 0.01) (Fig. 2).
FIG. 2.
MYC1551 mutant mycobacteria have reduced growth in vivo. The bacterial burdens in the lung, spleen, and liver were determined 4 weeks after aerosol infection of WT (A) and IFN-γR−/− (B) mice infected with the WT MT103 (open bars) and the ΔpurC mutant MYC1551 (filled bars). Bars represent mean CFU values (n = 5), with error bars indicating standard deviations. The data are representative of two independent experiments.
The bacterial loads in lungs and especially in spleens and livers of IFN-γR−/− mice infected with the ΔpurC mutant MYC1551 were significantly higher than those in the organs of WT mice (lungs, P < 0.05; spleens and livers, P < 0.01) (Fig. 2). Indeed, MYC1551 CFU counts in organs were 3 (in lungs) and 10 (in spleens and livers) times higher in IFN-γR−/− mice, which suggests that there is residual growth, not an absence of growth as reported previously (17), which is IFN-γ sensitive. Therefore, the data suggest that the mutant mycobacteria have a reduced capacity for growth in normal and IFN-γR−/− hosts, and we wanted to determine whether the inflammatory changes might be reduced.
The M. tuberculosis ΔpurC mutant MYC1551 causes acute pneumonia in IFN-γR−/− mice.
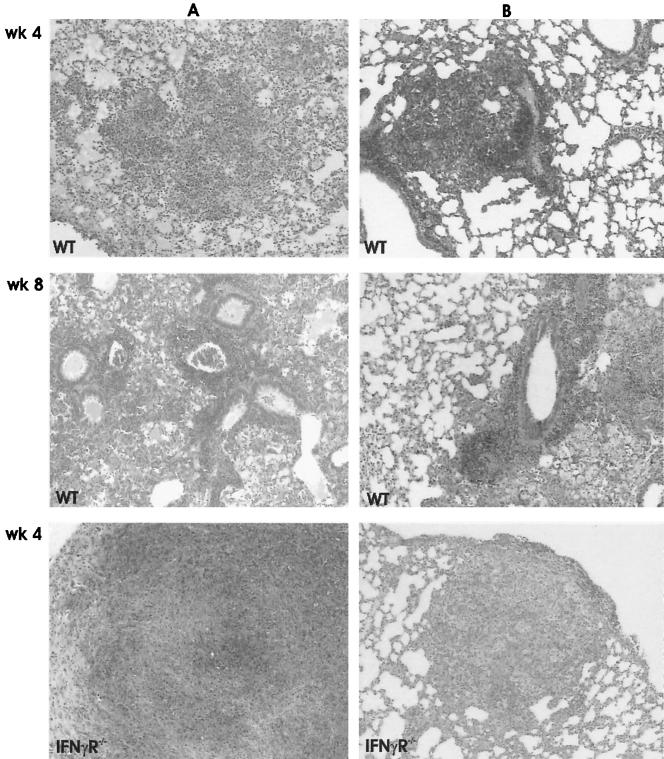
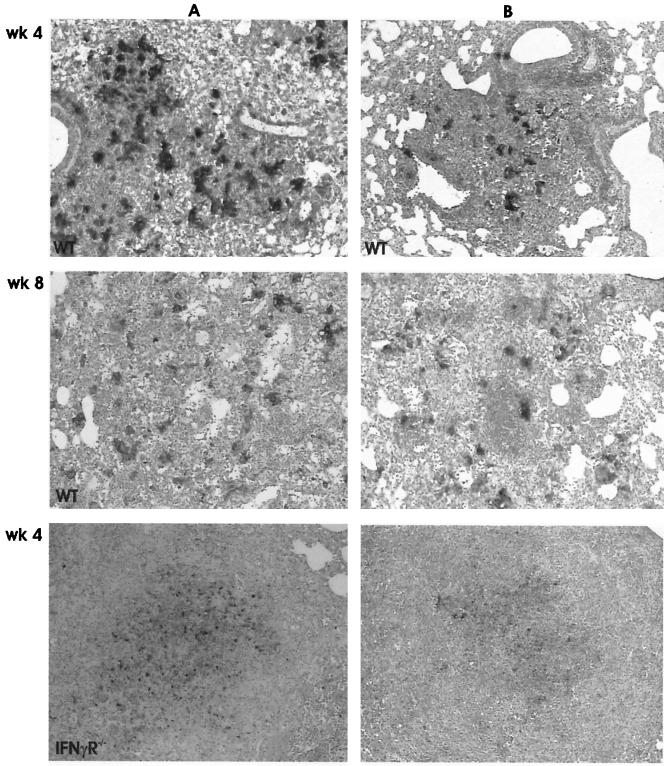
The inflammatory and granuloma responses were investigated by light microscopy at 4 and 8 weeks after aerosol infection. In MT103-infected WT mice, typical lung granulomas consisting of activated macrophages and abundant lymphocytes were found at 4 weeks after infection, a response that increased slightly at 8 weeks (Fig. 3A). MT103 infections in IFN-γR−/− mice caused necrotic pneumonia with abundant extracellular growth of mycobacteria in the absence of proper granuloma formation (Fig. 3A). In addition, there were many small granulomas in the liver (data not shown).
FIG. 3.
Diminished inflammatory and granuloma responses in the lungs of WT mice compared to IFN-γR−/− mice upon infection with the WT MT103 (A) and the mutant MYC1551 (B). Shown are images of confluent inflammation and necrosis in IFN-γR−/− mice infected with the MT103 and MYC1551 strains. These images are representative micrographs of the lungs from WT mice at 4 and 8 weeks and IFN-γR−/− mice at 4 weeks after infection. Sections were stained with hematoxylin and eosin and are representative of five mice each. Magnification, ×80.
The infection of WT mice with the M. tuberculosis ΔpurC mutant MYC1551 induced an increase in peribronchial cellular infiltration, with the formation of small granulomas, between weeks 4 and 8 (Fig. 3B). By contrast, MYC1551 caused extensive pneumonia in IFN-γR−/− mice at 4 weeks, with diffuse mononuclear cellular infiltration in the absence of granuloma and necrosis, which was lethal. Therefore, the MYC1551 mutant strain retained pathogenicity, as observed in the immunodeficient IFN-γR−/− mice, even with lower organ bacterial burdens.
Reduced iNOS expression in the absence of IFN-γR signaling.
Mycobacterial infection causes activation of inducible nitric oxidase (iNOS) in infected macrophages. Activated macrophages can be detected in organs by staining them for their iNOS immunoreactivity. At 4 weeks, WT mice infected with the MT103 strain, or to a lesser extent, the ΔpurC mutant MYC1551, displayed iNOS immunoreactivity in the lungs (Fig. 4A), as well as in the liver and spleen (data not shown). iNOS expression was reduced in IFN-γR−/− mice infected with either strain (Fig. 4B). Nitrotyrosine, which is the end product of the iNOS reaction, was detected in the lungs of WT mice, and the level detected was very similar to that obtained from iNOS staining of these tissues (data not shown). As expected, in IFN-γR−/− mice, nitrotyrosine immunoreactivity was diminished, concomitant with reduced iNOS expression. Therefore, the ΔpurC mutant MYC1551 has an attenuated capacity to induce iNOS expression. In the absence of IFN-γR signaling, reduced activation of macrophages occurred and no proper granulomas were formed.
FIG. 4.
Mice infected with the mutant MYC1551 display reduced activation of pulmonary macrophages compared to mice infected with the WT MT103. Expression levels of iNOS in the lungs of WT and IFN-γR−/− mice at 4 weeks after infection were determined as described previously (8, 18). WT and IFN-γR−/− mice were infected with the MT103 (A) and MYC1551 (B) strains, and representative immunohistochemistry sections from five mice each are shown. Magnification, ×80.
In conclusion, we demonstrated here that the M. tuberculosis ΔpurC mutant MYC1551 displayed reduced growth in WT mice but had 0.5- to 2-log-higher growth levels in the lungs and in the livers and spleens, respectively, of IFN-γR−/− mice. Although the organ CFU counts of the ΔpurC mutant MYC1551 were about 2 logs lower than those of the parent strain in IFN-γR−/− mice, the mutant mycobacteria were still pathogenic in the immunodeficient host. The heightened sensitivity of IFN-γ−/− or IFN-γR−/− mice to mycobacteria is well documented (4, 6, 9). Therefore, the use of such an immunodeficient model to test reduced pathogenicities of mycobacterial mutants is justified. Our data indicate that the ΔpurC MYC1551 auxotrophic mutant displays a growth disadvantage in vivo but causes death in IFN-γR−/− mice. These findings are relevant for the extrapolation of recombinant vaccine strategies for the use of live vaccines in immunodepressed patients.
Acknowledgments
Grants from The Wellcome Trust, NRF, and MRC of South Africa supported this work.
Editor: F. C. Fang
REFERENCES
- 1.Bange, F. C., A. M. Brown, and W. R. Jacobs, Jr. 1996. Leucine auxotrophy restricts growth of Mycobacterium bovis BCG in macrophages. Infect. Immun. 64:1794-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers, M. A., A. Williams, D. Gavier-Widén, A. Whelan, G. Hall, P. D. Marsh, B. R. Bloom, W. R. Jacobs, and R. G. Hewinson. 2000. Identification of a Mycobacterium bovis BCG auxotrophic mutant that protects guinea pigs against M. bovis and hematogenous spread of Mycobacterium tuberculosis without sensitization to tuberculin. Infect. Immun. 68:7094-7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 4.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford, R. M., L. Van De Verg, L. Yuan, T. L. Hadfield, R. L. Warren, E. S. Drazek, H.-S. H. Houng, C. Hammack, K. Sasala, T. Polsinelli, J. Thompson, and D. L. Hoover. 1996. Deletion of purE attenuates Brucella melitensis infection in mice. Infect. Immun. 64:2188-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 7.Dye, C., B. G. Williams, M. A. Espinal, and M. C. Raviglione. 2002. Erasing the world's slow stain: strategies to beat multidrug-resistant tuberculosis. Science 295:2042-2046. [DOI] [PubMed] [Google Scholar]
- 8.Flynn, J. L. 2004. Immunology of tuberculosis and implications in vaccine development. Tuberculosis (Edinburgh) 84:93-101. [DOI] [PubMed] [Google Scholar]
- 9.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guleria, I., R. Teitelbaum, R. A. McAdam, G. Kalpana, W. R. Jacobs, Jr., and B. R. Bloom. 1996. Auxotrophic vaccines for tuberculosis. Nat. Med. 2:334-337. [DOI] [PubMed] [Google Scholar]
- 11.Hingley-Wilson, S. M., V. K. Sambandamurthy, and W. R. Jacobs, Jr. 2003. Survival perspectives from the world's most successful pathogen, Mycobacterium tuberculosis. Nat. Immunol. 4:949-955. [DOI] [PubMed] [Google Scholar]
- 12.Hondalus, M. K., S. Bardarov, R. Russell, J. Chan, W. R. Jacobs, Jr., and B. R. Bloom. 2000. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect. Immun. 68:2888-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 259:1742-1745. [DOI] [PubMed] [Google Scholar]
- 14.Jackson, M., S. W. Phalen, M. Lagranderie, D. Ensergueix, P. Chavarot, G. Marchal, D. N. McMurray, B. Gicquel, and C. Guilhot. 1999. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect. Immun. 67:2867-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orme, I. M., D. N. McMurray, and J. T. Belisle. 2001. Tuberculosis vaccine development: recent progress. Trends Microbiol. 9:115-118. [DOI] [PubMed] [Google Scholar]
- 16.Pavelka, M. S., Jr., B. Chen, C. L. Kelley, F. M. Collins, and W. R. Jacobs, Jr. 2003. Vaccine efficacy of a lysine auxotroph of Mycobacterium tuberculosis. Infect. Immun. 71:4190-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelicic, V., M. Jackson, J. M. Reyrat, W. R. Jacobs, Jr., B. Gicquel, and C. Guilhot. 1997. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 94:10955-10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambandamurthy, V. K., X. Wang, B. Chen, R. G. Russell, S. Derrick, F. M. Collins, S. L. Morris, and W. R. Jacobs, Jr. 2002. A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nat. Med. 8:1171-1174. [DOI] [PubMed] [Google Scholar]
- 19.Simmons, C. P., S. J. Dunstan, M. Tachedjian, J. Krywult, A. L. M. Hodgson, and R. A. Strugnell. 1998. Vaccine potential of attenuated mutants of Corynebacterium pseudotuberculosis in sheep. Infect. Immun. 66:474-479. [DOI] [PMC free article] [PubMed] [Google Scholar]