Abstract
Background
Most studies have failed to identify any prognostic value of the current T-stage protocol for pancreatic ductal adenocarcinoma (PDAC) by the American Joint Committee on Cancer and the Union for International Cancer Control unless some grouping was performed.
Methods
To document the parameters included in this T-stage protocol, 223 consecutive pancreatoduodenectomy specimens with PDAC were processed by a uniform grossing protocol.
Results
Peripancreatic soft tissue (PST) involvement, the main pT3 parameter, was found to be inapplicable and irreproducible due to lack of a true capsule in the pancreas and variability in the amount and distribution of adipose tissue. Furthermore, 91 % of the cases showed carcinoma in the adipose tissue, presumably representing the PST, and thus were classified as pT3. An additional 4.5 % were qualified as pT3 due to extension into adjacent sites. The T-stage defined as such was not found to have any correlation with survival (p = 0.4). A revised T-stage protocol was devised that defined pT1 as 2 cm or smaller, pT2 as >2–4 cm, and pT3 as larger than 4 cm. This revised protocol was tested in 757 consecutive PDACs. The median and 3-year survival rates of this size-based protocol were 26, 18, 13 months, and 40 %, 26 %, 20 %, respectively (p < 0.0001). The association between higher T-stage and shorter survival persisted in N0 cases and in multivariate modeling. Analysis of the Surveillance, Epidemiology, and End Results database also confirmed the survival differences (p < 0.0001).
Conclusions
This study showed that resected PDACs are already spread to various surfaces of the pancreas, leaving only about 4 % of PDACs to truly qualify as pT1/T2, and that the current T-stage protocol does not have any prognostic correlation. In contrast, as shown previously in many studies, size is an important prognosticator, and a size-based T-stage protocol is more applicable and has prognostic value in PDAC.
The current T-stage protocol for pancreatic ductal adenocarcinoma (PDAC) by the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) (7th edition) uses tumor size for the early stages of tumor (pT1: ≤2 cm; pT2: >2 cm) and describes pT3 as the spread of the tumor beyond the pancreas, with this further defined as peripancreatic soft tissue (PST) involvement, common bile duct (CBD) involvement, or extension to the duodenum.1
Most major studies investigating the prognostic factors in PDAC have failed to identify the prognostic relevance for this T-stage2–5 unless regrouping was performed. Moreover, the type of grouping used to achieve statistical significance has varied from study to study, with some studies advocating pT1/pT2 vs pT3/pT46–10 and others combining pT1, pT2, and pT3 vs pT411,12 or combining Tis, pT1, and pT2 vs pT3 vs pT4.13 It is not clear whether the failure of the current T-stage system is due to the variability in interpretation of the parameters, the clinical insignificance of the parameters selected, or both.
In this study, the applicability and prognostic relevance of the main T-stage parameters in the current AJCC protocol were analyzed both through a critical analysis of the current guidelines and prospectively in a well-characterized cohort of 223 PDACs subjected to a uniform grossing pathologic documentation protocol.14,15 The findings showed that the current T-stage scheme is inapplicable and insignificant. Therefore, a size-based protocol was devised and tested on 757 consecutive PDACs in our institutional databases and on 10,554 PDACs in the SEER database and found to have significant prognostic correlation.
METHODS AND DESIGN
Assessment of the Current Guidelines on T-Stage Parameters
The definitions provided in the main texts on the topic including the UICC/AJCC staging book (7th edition),1 the cancer synoptic webpage of the College of American Pathologists (CAP),16 documents prepared by American College of Surgeons, the World Health Organization (WHO) classification of tumors of the digestive system,17 and the AFIP fascicle were evaluated.18
Cases for the Analysis of Current T-Stage Parameters
In this study, 223 consecutive pancreatoduodenectomies containing PDAC were prospectively processed according to a uniform grossing protocol (see later) designed to allow proper documentation of the pertinent T-stage parameters.14,15 To have a more uniform cohort, the study included only conventional PDACs without any neoadjuvant therapy that had undergone pancreatoduodenectomy at Emory and Wayne State Universities and were grossed by the authors personally. The complete pathology material of the cases was re-reviewed by three pathologists to confirm pertinent parameters. Unusual pancreatic adenocarcinoma variants such as colloid were excluded as well as nonductal tumors (acinar, neuroendocrine, pancreatoblastoma, solid pseudopapillary tumors) and secondary cancers (e.g., ampullary, CBD cancers) using stringent criteria.19,20
Sampling Protocol
The grossing protocol used for this purpose has been described in detail in other publications and bears negligible cost.14,15 Briefly, it is a standardized approach in which the soft tissues covering the pancreatic head are removed before dissection of the pancreatic head. This approach can be adopted and used with ease by ordinary gross room personnel in a conventional gross room using routine procedures. An important part of this protocol is the orange-peeling of the soft tissues covering the pancreas, which are presumed to be the closest representation of PST. In each case, the CBD is opened in the plane that goes through both the CBD and the main pancreatic duct to establish the interaction of the tumor with these two ducts. Similarly, the tumor invasion of the duodenum or ampulla is documented per standard grossing protocols. The size of the invasive carcinoma is routinely documented in pathology gross rooms.
A Size-Based Staging Protocol
During this study, the current parameters that define pT3 (e.g., PST or CBD involvement; see Supplemental Text) were found to be inapplicable or prognostically irrelevant (see “Results” Section). Therefore, a size-based definition of pT3 was devised, with pT3 defined as a tumor larger than 4 cm.
Because the median size of the tumors in this study was 3.2 cm (see “Results” section) and because a cutoff of 3 cm might provide more significance, it was thought that because 2 cm is already used to define pT1 versus pT2 in the AJCC/UICC tumor-node-metastasis (TNM) staging, a cutoff of 4 cm would be more appropriate for pT3. This also parallels the European Neuroendocrine Tumour Society (ENETS) T-stage scheme for pancreatic neuroendocrine tumors (NETs), which findings have found to be a stronger prognosticator of NETs than the AJCC/UICC T-stage. Because most authors believe that pT4 is to be reserved for unresectable cases1 or cases that require more than a classic pancreatoduodenectomy operation, we kept pT1 to pT3 for the resected cases analyzed in this study.
This size-based staging system was tested with 757 consecutive PDACs, including the well-characterized cohort of 223 cases resected at Emory and Wayne State Universities between 2004 and 2013. Tumor size information was obtained from the pathology reports.
Analysis of SEER Database
For comparison with the clinical cohort, data on patients with pancreatic cancer were obtained from structured queries of the publicly available SEER database. Our analysis included PDACs diagnosed between 2004 and 2010. Cases without complete information on clinicopathologic characteristics relevant to survival were excluded, resulting in a sample of 10,554 cases.
Statistical Analyses
Kaplan–Meier survival curves were estimated and compared between all T-stages using the log-rank test. Survival time for all analyses was set at zero after the first 3 months of follow-up evaluation. This was done to avoid bias because individuals with less than 3 months of follow-up evaluation were excluded from the study.
Multivariate analysis was conducted using Cox proportional hazard models, with adjustment for age, sex, AJCC N-stage, margin status, and lymphovascular/perineural invasions. The proportional hazard assumption was evaluated using log–log survival curves. To evaluate the discriminatory power of the models, the concordance probability estimate (CPE) and Akaike’s Information Criterion (AIC) were calculated.21 All analyses were conducted using SAS software version 9.3 (SAS Institute, Cary, NC) and SPSS version 22 (IBM Corp., Armonk, NY), except for the CPE, which was calculated using the CPE package in R (R Core Team, Vienna, Austria).
RESULTS
Study Population
The study enrolled 223 patients with ordinary PDAC, all processed according to the uniform grossing protocol defined earlier. The mean age of the patients was 66 years (range 33–87 years), and 57 % of the patients were female (n = 126). The median tumor size was 3.2 cm (range 0.7–7 cm). The majority of the patients (78 %) had lymph node metastasis (pN1). The follow-up period ranged from 3.2 to 154 months (median 18 months). At the last follow-up visit, 139 of the patients (62 %) had died of disease, and 84 (38 %) were alive with disease (Table 1).
TABLE 1.
Clinicopathologic features of the cases
| Institutional cohort (n = 223) n (%) | SEER database (n = 9296) n (%) | |
|---|---|---|
| Gender | ||
| Female | 126 (56.5) | 4639 (50) |
| Male | 97 (43.5) | 4657 (50) |
| Age (years) | ||
| Median (range) | 66 (33–87) | 66 (4–85) |
| Mean | 65 | 65 |
| Tumor size (cm) | ||
| Median | 3.2 | 3.3 |
| Mean | 3.3 | 3.7 |
| AJCC T staging | ||
| T1 | 4 (1.8) | 655 (7) |
| T2 | 4 (1.8) | 1697 (18) |
| T3 | 213 (95.5) | 6207 (67) |
| T4 | 2 (0.9) | 737 (8) |
| Proposed T staging | ||
| T1 (≤2 cm) | 29 (13) | 1573 (17) |
| T2 (> 2 to ≤4 cm) | 142 (64) | 5097 (55) |
| T3 (> 4 cm) | 52 (23) | 2626 (28) |
| AJCC N staging | ||
| N0 | 49 (22) | 3756 (40) |
| N1 | 174 (78) | 5540 (60) |
| LNs examined | ||
| Median (range) | 18 (3–45) | 11 (1–89) |
| LNs with metastasis | ||
| Median (range) | 3 (1–20) | 2 (1–47) |
| Outcome | ||
| Dead | 139 (62) | 5636 (61) |
| Alive | 84 (38) | 3660 (39) |
| Survival (months) | ||
| Median | 18 | 16 |
Institutional cohort processed according to a uniform grossing protocol, with authors confirming the diagnosis/stage by re-reviewing the entire pathology material
SEER Surveillance, Epidemiology, and End Results, AJCC American Joint Committee on Cancer, LN lymph node
PST Involvement
In 91 % of the cases, foci of carcinoma were identified in the orange-peeled PST. The highest incidence (58 %) was detected in the PST of the uncinate (superior mesenteric artery) margin (Table 2).
TABLE 2.
Frequency of local spread patterns of ordinary pancreatic ductal adenocarcinomas (PDACs) analyzed in 223 cases by careful grossing
| Spread location | % |
|---|---|
| CBD (any part of CBD) | 97 |
| CBD (extra-pancreatic) | 5 |
| Duodenum | 74 |
| Ampulla | 50 |
| Peripancreatic soft tissuea | 91 |
| Uncinate (SMA) margin | 58 |
| Superior pancreatic | 20 |
| Anterior pancreatic | 29 |
| Anterior pancreaticoduodenal | 19 |
| Posterior pancreatic | 23 |
| Posterior pancreaticoduodenal | 19 |
| Inferior pancreatic | 16 |
| LN metastasis | 79 |
CBD common bile duct, SMA superior mesenteric artery, LN lymph node
Peripancreatic soft tissue: adipose tissue covering the pancreas removed during the orange-peeling approach for LN harvesting; does not represent complete or perpendicular sampling
In 48 % of the cases, the foci of the carcinoma were insidious and away from the main tumor, represented as isolated solitary (naked) ducts. These ducts were either deceptively benign-appearing or mimicking pancreatic intraepithelial neoplasia (PanIN)22 (Supplemental Fig. 2). Notably, some of these foci gave the impression of intravascular spread. The involvement of PST was not found to have any statistical correlation with clinical outcome (p = 0.9).
CBD Involvement
When defined as any part of CBD, 97 % of the PDACs were found to have CBD involvement, rendering this parameter irrelevant.22 If defined as extra-pancreatic part of CBD, then CBD involvement seemed to be present in 5 % of the cases. Regardless, CBD involvement was found to have no prognostic impact (p = 0.9).
Duodenum/Ampulla Involvement
Neither duodenum involvement (74 %) nor ampullary involvement (50 %) (with muscularis involvement included) was found to have any association with clinical outcome (p = 0.89 and 0.14, respectively).
Testing of the Current T-Stage Protocol
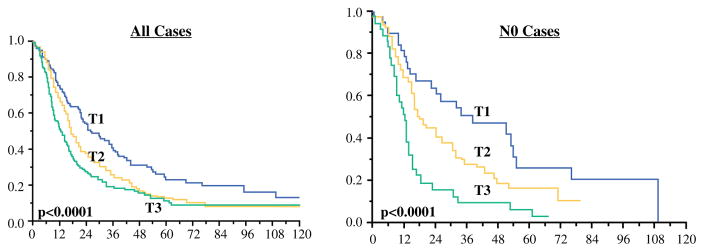
With PST involvement defined as the presence of carcinoma foci in the orange-peeled soft tissues, CBD involvement defined as extra-pancreatic, and duodenum involvement defined as including muscularis, 95.5 % of the PDAC cases in this study had to be classified as pT3, leaving only 1.8 % of the cases to qualify as pT1 and 1.8 % as pT2. This staging system had no prognostic value because the 1-year survival rates for pT1 vs pT2 vs pT3 vs pT4 were respectively 75, 75, 65, and 50 %, and the 3-year survival rates were respectively 25, 0, 28, and 0 % (p = 0.4).
Testing of the Size-Based T-Stage Protocol
When pT1 was defined as 2 cm or smaller, pT2 as>2 to 4 cm, and pT3 as larger than 4 cm and tested in 223 ordinary PDACs, they broke down into respective categories of 13, 64, and 23 %. The median overall survival times in this cohort were respectively 27, 24, and 9 months. The 1-year survival rates were respectively 79, 69, and 47 %, and 3-year survival rates were respectively 37, 28, and 21 % (p = 0.004). However, the difference between pT1 and pT2 did not reach statistical significance due to the small number of T1 cases (p = 0.5).
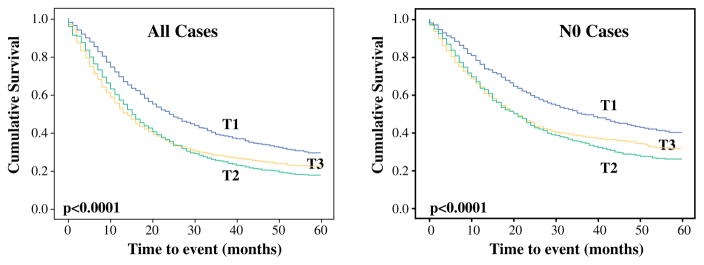
For this reason, the proposed size-based T-staging protocol also was tested in 757 consecutive ordinary PDACs. The distributions of pT1, pT2, and pT3 in this cohort were respectively 20, 56, and 24 %, and the median overall survival periods were respectively 26, 17.5, and 13 months. The 1-year survival rates were respectively 75, 67, and 51 %, and the 3-year survival rates were respectively 40, 26, and 20 % (p <0.0001 for pT1 vs pT2 vs pT3; p = 0.002 for pT1 vs pT2; p < 0.0001 for pT1 vs pT3; and p = 0.01 for pT2 vs pT3).
Lymph node status information was available in 476 cases. When cases without lymph node metastasis (n = 180) were analyzed separately, the prognostic significance persisted. The median overall survival times for pT1 vs pT2 vs pT3 were respectively 38, 18, and 12.5 months. The 1-year survival rates were respectively 82, 72, and 52 %, and the 3-year survival rates were respectively 51.5, 28, and 10 % (p < 0.0001 for pT1 vs pT2 vs pT3; p = 0.04 for pT1 vs pT2; p < 0.0001 for pT1 vs pT3; and p = 0.002 for pT2 vs pT3) (Table 3,Fig. 1). Analysis of the SEER database confirmed the survival differences between these T-stages, although the pT3 group had crossover for pT2. The median overall survival times for pT1 vs pT2 vs pT3 were 24, 15, and 14 months. The 1-year survival rates were respectively 70, 58, and 53 %, and the 3-year survival rates were respectively 39, 25, and 28 % (p < 0.0001 for pT1 vs pT2 vs pT3; p < 0.0001 for pT1 vs pT2; p < 0.0001 for pT1 vs pT3; and p = 0,6 for pT2 vs pT3).
TABLE 3.
Median survivals for proposed (size-based) T-stages (institutional cohort)
| All cases
|
N0 cases
|
|||||||
|---|---|---|---|---|---|---|---|---|
| T1 (≤2 cm) | T2 (>2 to ≤4 cm) | T3 (>4 cm) | p Value | T1 (≤2 cm) | T2 (>2 to ≤4 cm) | T3 (> 4 cm) | p Value | |
| n (%) | 151 (20) | 421 (56) | 185 (24) | < 0.0001 | 46 (25) | 97 (54) | 37 (21) | < 0.0001 |
| Median (months) | 26 | 17.5 | 13 | 38 | 18 | 12.5 | ||
| 1 Year (%) | 75 | 67 | 51 | 82 | 72 | 52 | ||
| 3 Year (%) | 40 | 26 | 20 | 51.5 | 28 | 10 | ||
FIG. 1.
Comparison of survival between proposed (size-based) T-stages in the institutional cohort. Left All cases. Right Cases without lymph node metastasis (pN0)
The results were similar when separate analyses were performed for cases without lymph node metastasis. The median overall survival periods for pT1 vs pT2 vs pT3 were respectively 36, 21, and 21 months. The 1-year survival rates were 77, 64, and 64 %, and the 3-year survival rates were respectively 50, 35, and 38 % (p < 0.0001 for T1 vs T2 vs T3; p < 0.0001 for pT1 vs pT2; p < 0.0001 for pT1 vs pT3; and p = 0,4 for pT2 vs pT3) (Table 4, Fig. 2).
TABLE 4.
Median survivals for proposed (size-based) T-stages (SEER database)
| All cases (n = 9296)
|
N0 cases (n = 3756)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| T1 (≤2 cm) | T2 (> 2 to ≤4 cm) | T3 (> 4 cm) | p Value | T1 (≤2 cm) | T2 (> 2 to ≤4 cm) | T3 (> 4 cm) | p Value | |
| n (%) | 1573 (17) | 5097 (55) | 2626 (28) | <0.0001 | 823 (22) | 1889 (50) | 1044 (28) | < 0.0001 |
| Median (months) | 24 | 15 | 14 | 36 | 21 | 21 | ||
| 1 Year (%) | 70 | 58 | 53 | 77 | 64 | 64 | ||
| 3 Year (%) | 39 | 25 | 28 | 50 | 35 | 38 | ||
SEER Surveillance, Epidemiology, and End Results
FIG. 2.
Comparison of survival between proposed (size-based) T-stages in the Surveillance, Epidemiology, and End Results (SEER) database. Left All cases. Right Cases without lymph node metastasis (pN0)
In the multivariable Cox regression model adjusted for age, sex, N-stage, margin status, and lymphovascular/perineural invasions, the association between a higher proposed T-stage and shorter survival persisted. Using the T1 group as the reference, the adjusted hazard ratios (HRs) and 95 % confidence intervals (CIs) for T2 and T3 were respectively 1.03 (0.60–1.78) and 1.83 (CI 0.99–3.38) for all the cases in our cohort (p = 0.01). In these models, age also was statistically significantly associated with survival. The HR was 1.2 (95 % CI 1.0–1.4; p = 0.02) for a 10-year increase in age.
The multivariate analysis of the SEER database included age, sex, and N-stage. In these analyses, using the T1 group as the reference, the adjusted HR and 95 % CI were 1.39 (1.28–1.50) for T2 and 1.47 (1.35–1.60) for T3 (p < 0.0001).
DISCUSSION
In this study, the applicability and clinical relevance of the current T staging was investigated in a well-characterized and stringently defined cohort of ordinary PDAC patients who had undergone pancreatoduodenectomy and had been processed according to a protocol allowing the documentation of the spread patterns of PDAC.14,15 The findings showed that even after the definitional ambiguities are overcome, the current AJCC/UICC T staging for the pancreas is both impractical and lacks prognostic correlation.
An analysis of the current guidelines indicates that one of the main parameters defining T3 spread beyond the pancreas is primarily defined as involvement of PST.1,16–18 As this study further discovered, PST is not a well-defined structure. Also, because PDACs are highly insidious tumors with subtle spread patterns, proper documentation of the true PST either requires extensive perpendicular sampling (as advocated by Verbeke and Esposito/Schlitter protocols23–25), which is costly and time consuming, or necessitates shaved removal and examination of the soft tissues covering the pancreatic head using a method such as orange-peeling.14,15 In this study, the latter approach showed that these soft tissues covering the pancreas are involved in more than 90 % of the resected PDACs in the head. Together with other parameters discussed later, this leaves only about 4 % of cases to qualify as pT1/pT2, thus negating the usability of the current T staging.
Naturally, in the vast majority of the literature, the reported incidence of T1 and T2 is much higher than in our study2,26–30 because proper sampling of PST was not performed as in our study with the orange-peeling approach. In most pathology laboratories, because random sampling is used to document PST, a significant proportion of cases get understaged as T1/T2 because soft tissue involvement is missed. Therefore, it can be safely assumed that the extent of tumor infiltration into PST is a factor of larger tumor size, so it is not surprising that in studies with random sampling of PST, incidental discovery of carcinoma in these sections correlates with the outcome as a reflection of larger tumors.31 However, our study illustrates that with careful sampling and documentation, the true PST is in fact involved with carcinoma in 91 % of PDACs. Furthermore, the involvement of PST was not found to have any significant association with prognosis. This also accords with reports in the literature that in most major studies, no linear correlation could be documented between the current T-stage protocol and prognosis unless some regrouping was performed to achieve significance.2,6–13
The commonness of PST involvement, especially with carcinomatous foci away from the main tumor, also highlights the fact that PDACs typically are spread far beyond what meets the eye at the time of diagnosis, commonly and extensively involving the soft tissues covering the pancreas. This “horses-out-of-the-barn” phenomenon most likely is also responsible for the rapid recurrence and dissemination of pancreatic cancer seen in virtually all cases. At the same time, however, considering that resected PDACs have an incomparably longer survival than nonresected cases,32 this may argue in favor of performing pancreatoduodenectomy even for borderline resectable cases.33–35
The analysis of the current staging guidelines also highlights the challenges in applicability of the remaining pT3-defining parameters as well. For example, CBD involvement is interpreted variably in different guideline documents, with some advocating the inclusion of any CBD segment,1 and others stating only extra-pancreatic biliary involvement as the sole criterion.18 Both definitions present their own applicability challenges. Defining CBD involvement as extra-pancreatic requires a “magic bullet” spread, whereas involvement of the mid-proximal CBD, which is extra-pancreatic, requires extensive local spread of the tumor, rendering it unresectable and thus not amenable to pT staging or entailing intramural spread through the CBD wall, intramucosally or through the perineural spread, which indicates the diagnosis of primary CBD carcinoma instead. Additionally, no good definition of extra-pancreatic CBD exists because CBD has a variable pancreatic covering depending on the individual and the sampling. Moreover, most sampling protocols do not specifically focus on the extra-pancreatic CBD, except for the CBD margin. If, instead, the entire CBD, including the intra-pancreatic compartment, is regarded as the criterion for pT3, then it is involved in 97 % of the cases,22 essentially rendering it an irrelevant parameter for stratification purposes.
Thus, this study fundamentally shows what has already been noted in the literature regarding the lack of linear prognostic correlation for the AJCC/UICC T staging for pancreatic head cancers of the ordinary PDAC type.2–5 However, it also shows that the main reason for this failure is the applicability and irreproducibility of the pT3 parameters as defined currently.
One potential T-stage model that can replace the current inapplicable one is the model based on size, which already is the main defining parameter for T1 and T2 in the pancreas. This model also has been used very successfully with many solid organ cancers, including breast and lung cancers.1 Numerous studies also have found it to be a strong prognosticator in PDACs. Actually, in many major studies investigating the prognostic factors in PDACs, size stands out as one of the most important parameters.5,36–43 The number that seems to be recurrent in these studies as a stratifier is 3–3.5 cm,37,38,40–43 which essentially is the median size of tumor in resected cancers. Therefore, a cutoff of 4 cm to define T3 appears to be the most logical. This also is the cutoff adopted by ENETS for T staging of pancreatic NETs.44 Defined as such in this study, 13 % of the PDACs fell into the pT1 category and 64 % into the pT2 category, compared with the about 4 % categorization as pT1/T2 together in the current T-stage classification. Notably, the median size of the invasive carcinoma in the current study (3.2 cm) and the percentage of tumors smaller than 2 cm are very comparable with those reported in other large institution-based studies.40,45,46 In Japan, this figure is consistently reported to be about 10 %, presumably due to more careful grossing protocols.47,48 However, in the SEER database2 and ESPAC publications,28 the percentage of small cancers seems to be much higher than in these institutional cohorts, which is presumably attributable to the mis-recordings of non-PDAC cancers such as intraductal papillary mucinous neoplasm-associated carcinomas in the SEER database and other nonpathologically verified databases.
This size-based T-stage, unlike the current T-stage classification, was found to be highly significant prognostically both in our institutional cohort and in the SEER database (Figs. 1 and 2), as well as in a multivariate model. The prognostic significance persists in cases without lymph node metastasis as well. Although the survival difference between T1 and T2 in our well-characterized cohort of 223 cases did not reach statistical significance due to small numbers; the separation of T1 and T2 was highly significant both in our larger cohort of 757 PDACs and in the SEER database. The survival of T3 versus T2 also was greatly different in our institutional cohort, whereas in the SEER database, the pT3 group had the rather expected cross over the pT2 due to the inclusion of benevolent cancer types (e.g., acinar, pancreatoblastoma, solid-pseudopapillary cancers), selectively clustering in the T3 group due to their commonly large size at presentation.
It should be noted that different approaches have been used for pathologic examination of pancreatic specimens.49–56 However, it does not matter which grossing approach is used as long as proper sampling of PST is performed and complete harvesting of lymph nodes is achieved. Our protocol is a very straightforward one that involves separation of PST before cutting of the pancreatic head to avoid underevaluation of this tissue, and this method currently is used in many institutions. This proper sampling of PST accounts for the higher percentage of current AJCC pT3 cases in our institutional cohort (96 %). Similarly, before establishment of this approach, the median number of regional lymph nodes examined in our own institutions was very low (respectively 6 and 7 in our two institutions and also similar to the median number of 7 in the SEER database).14,15,57–59 Currently, with the orange-peeling approach, our median lymph node number of 17 is similar to those reported from most experienced and high-volume institutions.45,46
The application of a better lymph node harvesting approach also may account for the higher lymph node metastasis rate in our institutional cohort (79 %) than the rates recorded in SEER (60 %, Table 1), CONKO (70 %),26,27 and ESPAC (54 %),28,29 as well as for the higher percentage of pN2 cases60 (pN1: 26 %; pN2: 51 %) than in the SEER database (pN1: 33 %; pN2: 27 %). Notably, a careful review of the literature shows that the data from high-volume institutions also have a lymph node metastasis rate of nearly 80 %.40,43,45
Naturally, we recognize that moving to a different staging system has implications and comes with its challenges, such as hampered comparison with earlier data. On the other hand, it is very clear that virtually no institution-based studies have been able to confirm a direct and linear correlation for the current AJCC T staging. Thus, without question, we need an applicable and clinically relevant T-staging system for the pancreas.
In summary, this study demonstrated that PDACs are found to be spread beyond what meets the eye when proper dissection and careful microscopic examination is performed, which also renders the current T staging of PDACs by AJCC/UICC as impractical, with only about 4 % of cases qualifying as pT1 and pT2. The current AJCC T staging also has no prognostic correlation, as amply demonstrated in the literature and in this study. Therefore, a different T-staging protocol was devised that defines pT1 as 2 cm or smaller, pT2 as >2–4 cm, and pT3 as larger than 4 cm. This proposed T-staging protocol was found to have linear prognostic value and should be used to replace the current T-staging protocol.
Supplementary Material
Footnotes
Presented in part at the annual meeting of the United States and Canadian Academy of Pathology, March 2014, at San Diego, CA, USA.
Electronic supplementary material
The online version of this article (doi:10.1245/s10434-016-5093-7) contains supplementary material, which is available to authorized users.
DISCLOSURE
Volkan Adsay has a Grant from National Institutes of Health (#5P50 CA62924). Lauren M. Postlewait has received funding from the Katz Foundation. For the remaining authors none were declared. This study is also supported in parts by funds generously donated by Monastra Foundation.
References
- 1.Edge SB, Byrd DR, Compton CG, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7. New York: Springer; 2010. [Google Scholar]
- 2.Park H, An S, Eo SH, et al. Survival effect of tumor size and extrapancreatic extension in surgically resected pancreatic cancer: proposal for improved T classification. Hum Pathol. 2014;45:2341–6. doi: 10.1016/j.humpath.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 3.Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–8. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–94. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 5.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrone CR, Pieretti-Vanmarcke R, Bloom JP, et al. Pancreatic ductal adenocarcinoma: long-term survival does not equal cure. Surgery. 2012;152:S43–9. doi: 10.1016/j.surg.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magistrelli P, Antinori A, Crucitti A, et al. Prognostic factors after surgical resection for pancreatic carcinoma. J Surg Oncol. 2000;74:36–40. doi: 10.1002/1096-9098(200005)74:1<36::aid-jso9>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.Kent TS, Sachs TE, Sanchez N, Vollmer CM, Jr, Callery MP. Conditional survival in pancreatic cancer: better than expected. HPB Oxford. 2011;13:876–80. doi: 10.1111/j.1477-2574.2011.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richter A, Niedergethmann M, Sturm JW, Lorenz D, Post S, Trede M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg. 2003;27:324–9. doi: 10.1007/s00268-002-6659-z. [DOI] [PubMed] [Google Scholar]
- 10.de Jong MC, Li F, Cameron JL, et al. Reevaluating the impact of tumor size on survival following pancreaticoduodenectomy for pancreatic adenocarcinoma. J Surg Oncol. 2011;103:656–62. doi: 10.1002/jso.21883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber A, Kehl V, Mittermeyer T, et al. Prognostic factors for survival in patients with unresectable pancreatic cancer. Pancreas. 2010;39:1247–53. doi: 10.1097/MPA.0b013e3181e21b1b. [DOI] [PubMed] [Google Scholar]
- 12.Takai S, Satoi S, Toyokawa H, et al. Clinicopathologic evaluation after resection for ductal adenocarcinoma of the pancreas: a retrospective, single-institution experience. Pancreas. 2003;26:243–9. doi: 10.1097/00006676-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Hartwig W, Hackert T, Hinz U, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254:311–9. doi: 10.1097/SLA.0b013e31821fd334. [DOI] [PubMed] [Google Scholar]
- 14.Adsay NV, Basturk O, Altinel D, et al. The number of lymph nodes identified in a simple pancreatoduodenectomy specimen: comparison of conventional vs orange-peeling approach in pathologic assessment. Mod Pathol. 2009;22:107–12. doi: 10.1038/modpathol.2008.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adsay NV, Basturk O, Saka B, et al. Whipple made simple for surgical pathologists: orientation, dissection, and sampling of pancreaticoduodenectomy specimens for a more practical and accurate evaluation of pancreatic, distal common bile duct, and ampullary tumors. Am J Surg Pathol. 2014;38:480–93. doi: 10.1097/PAS.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Washington K, Berlin J, Branton P, Burgart LJ. [Accessed 25 Jan 2016];CAP cancer reporting protocols: protocol for the examination of specimens from patients with carcinoma of the exocrine pancreas. 2013 http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/pancreasexo-13protocol-3201.pdf.
- 17.Bosman FT, Carneiro FHHR, Theise ND. WHO classification of tumors of digestive system. Lyon: WHO Press; 2010. [Google Scholar]
- 18.Hruban R, Pitman MB, Klimstra DS. Tumors of the Pancreas. Washington, DC: American Registry of Pathology; 2007. [Google Scholar]
- 19.Adsay V, Ohike N, Tajiri T, et al. Ampullary region carcinomas: definition and site-specific classification with delineation of four clinicopathologically and prognostically distinct subsets in an analysis of 249 cases. Am J Surg Pathol. 2012;36:1592–608. doi: 10.1097/PAS.0b013e31826399d8. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez RS, Bagci P, Kong SY, et al. Distal common bile duct adenocarcinoma: analysis of 47 cases and comparison with pancreatic and ampullary ductal carcinomas (abstract) Mod Pathol. 2014;25:109A. doi: 10.1038/modpathol.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gönen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92:965–70. [Google Scholar]
- 22.Oliva IV, Bandyopadhyay S, Coban I, et al. Incidence and significance of common bile duct involvement in resected pancreatic ductal adenocarcinomas: should it be represented in the TNM staging? (abstract) Mod Pathol. 2009;22:319A. [Google Scholar]
- 23.Verbeke CS. Resection margins and R1 rates in pancreatic cancer-are we there yet? Histopathology. 2008;52:787–96. doi: 10.1111/j.1365-2559.2007.02935.x. [DOI] [PubMed] [Google Scholar]
- 24.Esposito I, Kleeff J, Bergmann F, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008;15:1651–60. doi: 10.1245/s10434-008-9839-8. [DOI] [PubMed] [Google Scholar]
- 25.Schlitter AM, Esposito I. Definition of microscopic tumor clearance (R0) in pancreatic cancer resections. Cancers Basel. 2010;2:2001–10. doi: 10.3390/cancers2042001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–81. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 27.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–77. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 28.Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–85. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 29.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 30.Sinn M, Striefler JK, Sinn BV, et al. Does long-term survival in patients with pancreatic cancer really exist? Results from the CONKO-001 study. J Surg Oncol. 2013;108:398–402. doi: 10.1002/jso.23409. [DOI] [PubMed] [Google Scholar]
- 31.Hamidian Jahromi A, Zibari GB, Jafarimehr E, et al. Peripancreatic soft tissue involvement: independent outcome predictor in patients with resected pancreatic adenocarcinoma. Int Surg. 2014;99:62–70. doi: 10.9738/INTSURG-D-13-00112.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De La Cruz MS, Young AP, Ruffin MT. Diagnosis and management of pancreatic cancer. Am Fam Physician. 2014;89:626–32. [PubMed] [Google Scholar]
- 33.Yamada S, Fujii T, Sugimoto H, et al. Aggressive surgery for borderline resectable pancreatic cancer: evaluation of National Comprehensive Cancer Network guidelines. Pancreas. 2013;42:1004–10. doi: 10.1097/MPA.0b013e31827b2d7c. [DOI] [PubMed] [Google Scholar]
- 34.Evans DB, Farnell MB, Lillemoe KD, Vollmer C, Jr, Strasberg SM, Schulick RD. Surgical treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1736–44. doi: 10.1245/s10434-009-0416-6. [DOI] [PubMed] [Google Scholar]
- 35.Katz MH, Crane CH, Varadhachary G. Management of borderline resectable pancreatic cancer. Semin Radiat Oncol. 2014;24:105–12. doi: 10.1016/j.semradonc.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Rohan VS, Hsu JT, Liu KH, et al. Long-term results and prognostic factors in resected pancreatic body and tail adenocarcinomas. J Gastrointest Cancer. 2013;44:89–93. doi: 10.1007/s12029-012-9448-4. [DOI] [PubMed] [Google Scholar]
- 37.Ueda M, Endo I, Nakashima M, et al. Prognostic factors after resection of pancreatic cancer. World J Surg. 2009;33:104–10. doi: 10.1007/s00268-008-9807-2. [DOI] [PubMed] [Google Scholar]
- 38.You DD, Lee HG, Heo JS, Choi SH, Choi DW. Prognostic factors and adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. J Gastrointest Surg. 2009;13:1699–706. doi: 10.1007/s11605-009-0969-5. [DOI] [PubMed] [Google Scholar]
- 39.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–72. doi: 10.1016/s0002-9610(05)80406-4. discussion 72–63. [DOI] [PubMed] [Google Scholar]
- 40.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas–616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–79. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 41.Moon HJ, An JY, Heo JS, Choi SH, Joh JW, Kim YI. Predicting survival after surgical resection for pancreatic ductal adenocarcinoma. Pancreas. 2006;32:37–43. doi: 10.1097/01.mpa.0000194609.24606.4b. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto G, Muta M, Tsuruta K, Horiguchi S, Karasawa K, Okamoto A. Tumor size significantly correlates with postoperative liver metastases and COX-2 expression in patients with resectable pancreatic cancer. Pancreatology. 2007;7:167–73. doi: 10.1159/000104241. [DOI] [PubMed] [Google Scholar]
- 43.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210–1191. [DOI] [PubMed] [Google Scholar]
- 44.Kloppel G, Rindi G, Perren A, Komminoth P, Klimstra DS. The ENETS and AJCC/UICC TNM classifications of the neuroendocrine tumors of the gastrointestinal tract and the pancreas: a statement. Virchows Arch. 2010;456:595–7. doi: 10.1007/s00428-010-0924-6. [DOI] [PubMed] [Google Scholar]
- 45.House MG, Gonen M, Jarnagin WR, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11:1549–55. doi: 10.1007/s11605-007-0243-7. [DOI] [PubMed] [Google Scholar]
- 46.Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–8. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 47.Japan Pancreas Society. Classification of pancreatic carcinoma. 3. Tokyo: Kanehara; 2011. English. [Google Scholar]
- 48.General rules for the study of pancreatic cancer. 6. Tokyo: Kanehara; 2013. Japanese, revised. [Google Scholar]
- 49.Staley CA, Cleary KR, Abbruzzese JL, et al. The need for standardized pathologic staging of pancreaticoduodenectomy specimens. Pancreas. 1996;12:373–80. doi: 10.1097/00006676-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Luttges J, Zamboni G, Kloppel G. Recommendation for the examination of pancreaticoduodenectomy specimens removed from patients with carcinoma of the exocrine pancreas: a proposal for a standardized pathological staging of pancreaticoduodenectomy specimens including a checklist. Dig Surg. 1999;16:291–6. doi: 10.1159/000018738. [DOI] [PubMed] [Google Scholar]
- 51.Chatelain D, Flejou JF. Pancreatectomy for adenocarcinoma: prognostic factors, recommendations for pathological reports. Ann Pathol. 2002;22:422–31. [PubMed] [Google Scholar]
- 52.Verbeke CS, Gladhaug IP. Resection margin involvement and tumour origin in pancreatic head cancer. Br J Surg. 2012;99:1036–49. doi: 10.1002/bjs.8734. [DOI] [PubMed] [Google Scholar]
- 53.Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232–7. doi: 10.1002/bjs.5397. [DOI] [PubMed] [Google Scholar]
- 54.Verbeke CS, Menon KV. Redefining resection margin status in pancreatic cancer. HPB Oxford. 2009;11:282–9. doi: 10.1111/j.1477-2574.2009.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.The Royal College of Pathologists of United Kingdom Standards and Datasets for Reporting Cancers. [Accessed 24 Dec 2013];Data set for the histopathological reporting of carcinomas of the pancreas, ampulla of vater, and common bile duct. 2013 http://www.rcpath.org/NR/rdonlyres/954273A2-3F01-4B97-B0F6-C136231DF65F/0/datasethistopathologicalreportingcarcinomasmay10.pdf.
- 56.Maksymov V, Hogan M, Khalifa MA. An anatomical-based mapping analysis of the pancreaticoduodenectomy retroperitoneal margin highlights the urgent need for standardized assessment. HPB Oxford. 2013;15:218–23. doi: 10.1111/j.1477-2574.2012.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15:165–74. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 58.Tomlinson JS, Jain S, Bentrem DJ, et al. Accuracy of staging node-negative pancreas cancer: a potential quality measure. Arch Surg. 2007;142:767–23. doi: 10.1001/archsurg.142.8.767. discussion 773–764. [DOI] [PubMed] [Google Scholar]
- 59.Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol. 2006;13:1189–200. doi: 10.1245/s10434-006-9016-x. [DOI] [PubMed] [Google Scholar]
- 60.Basturk O, Saka B, Balci S, et al. Substaging of lymph node status in resected pancreatic ductal adenocarcinoma has strong prognostic correlations: proposal for a revised N classification for TNM staging. Ann Surg Oncol. 2015;22(Suppl 3):1187–95. doi: 10.1245/s10434-015-4861-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.