Abstract
Actinobacillus actinomycetemcomitans produces a leukotoxin that selectively kills human leukocytes. Recently, we reported that macrophages are highly sensitive to leukotoxin and that their lysis involves activation of caspase 1. In this study, we show that leukotoxin also induces the production and release of proinflammatory cytokines from human macrophages. The macrophages were challenged with leukotoxin or lipopolysaccharide (LPS) from A. actinomycetemcomitans or LPS from Escherichia coli, and the production and secretion of interleukin-1β (IL-1β), IL-6, and tumor necrosis factor alpha (TNF-α) were determined at the mRNA and protein levels by reverse transcription-PCR and enzyme-linked immunosorbent assay, respectively. Leukotoxin (1 to 30 ng/ml) induced abundant production and secretion of IL-1β, while the effects on IL-6 and TNF-α production were limited. Leukotoxin (1 ng/ml) caused a 10-times-higher release of IL-1β than did LPS (100 ng/ml). The secreted IL-1β was mainly the bioactive 17-kDa protein. At higher concentrations (>30 ng/ml), leukotoxin caused secretion of mainly inactive cytokine, the 31-kDa pro-IL-1β. The presence of specific antibodies to IL-1β or of a caspase 1 inhibitor blocked the secretion and production of the cytokine. Supernatants of leukotoxin-challenged macrophages stimulated bone resorption when tested in a mouse calvarial model. The activity could be blocked by an IL-1 receptor antagonist or specific antibodies to IL-1β. We concluded that A. actinomycetemcomitans leukotoxin can trigger abundant production and secretion of bioactive IL-1β by human macrophages, which is mediated by activation of caspase 1.
Actinobacillus actinomycetemcomitans is a gram-negative coccoid bacillus associated with aggressive periodontitis, especially the localized form that affects young individuals (18, 38). This form of periodontitis is characterized by a rapid loss of alveolar bone, often in close proximity to a periodontal pocket infected with A. actinomycetemcomitans (34, 46, 47). In such pockets, the bacterium may comprise >90% of the microflora (7).
A major virulence pattern of A. actinomycetemcomitans is the production of leukotoxin, an exotoxin of the RTX family (44). The leukotoxin-producing abilities of different strains have been correlated with disease onset (6, 15-17). The toxin selectively kills human leukocytes, and it also induces apoptosis in T lymphocytes and polymorphonuclear leukocytes (24, 25, 33). Therefore, leukotoxin is assumed to contribute to the severity of the periodontal disease by disrupting the local defense mechanisms (14, 19).
Cell lysis requires the interaction of leukotoxin with the transmembrane cell receptor LFA-1 (27). Recently, it was shown that human monocytes/macrophages are lysed at a 10-fold lower concentration than polymorphonuclear leukocytes and lymphocytes by a mechanism that involves activation of a cysteine proteinase, caspase 1 (23). Since caspase 1 is responsible for the activation and secretion of interleukin-1β (IL-1β) (10), this finding implies the possibility that the interaction of leukotoxin with monocytes may activate cytokine production (11, 32).
In periodontitis, alveolar bone loss is caused by the enhanced local formation and activation of osteoclasts (40). Osteoclast differentiation has been suggested to be initiated by proinflammatory cytokines, including IL-1, tumor necrosis factor alpha (TNF-α), and IL-6 (29). Elevated expression of IL-1 in periodontal tissue, as well as increased concentrations of the cytokine in gingival crevicular fluid, correlates with disease progression (3, 12, 30, 35, 36). Furthermore, IL-1 antagonists inhibit inflammatory-cell recruitment and osteoclast formation, and they prevent the loss of periodontal tissues in primate models of experimental periodontitis (1, 8, 9, 13).
Previous studies examining the role of leukotoxin in host-parasite interactions have mainly focused on leukocyte lysis (2, 26, 41). Studies with another RTX toxin, α-hemolysin from Escherichia coli, showed that sublytic concentrations of the toxin can induce renal epithelial cell activation, assessed by Ca2+ oscillations (26, 42). It was suggested that the activation may lead to the gene expression responsible for inflammatory reactions. A recent observation (23) of leukotoxin-stimulated activation of caspase 1 in monocytes implies that this RTX toxin may also be involved in the induction of inflammatory conditions. The present study aimed to examine the production and secretion of proinflammatory osteoclast-activating cytokines by human macrophages exposed to sublytic and lytic concentrations of A. actinomycetemcomitans leukotoxin.
MATERIALS AND METHODS
Leukotoxin and LPS preparations.
Leukotoxin was purified from A. actinomycetemcomitans strain HK 1519 belonging to the highly leukotoxic JP2-like clone (5). The procedure included extraction of leukotoxin from the bacteria with a 300 mM NaCl solution and purification of the toxin from the extract by liquid chromatography, as reported previously (20). The purity of the leukotoxin preparation was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and staining with Coomassie brilliant blue R250 and silver nitrate (31). With a sample load of 1 μg of protein, only a single band was seen at the 116-kDa position. The amount of lipopolysaccharide (LPS) in the leukotoxin preparation was <0.001 μg/mg of total protein, as determined by a Limulus amebocyte lysate-based assay (Coamatic Chromo-LAL; Chromogenix, Mölndal, Sweden).
An LPS-enriched, leukotoxin-free fraction was also obtained by liquid chromatography from the same bacterial extract. The chromatographic separation was run in an AKTA system equipped with a Superose 12 column (Pharmacia, Uppsala, Sweden) using 300 mM NaCl as the elution buffer. The LPS fraction contained <1 μg of protein/mg of LPS, as determined by the Micro BCA protein assay (Pierce, Cheshire, United Kingdom).
In some experiments, LPS from Escherichia coli (O26:B6) was used. This substance was purchased from Difco Laboratories (Detroit, Mich.). Before use, the lyophilized LPS was dissolved in cell culture medium at the concentrations indicated below.
Preparation of macrophages.
Mononuclear leukocytes (MNL) were isolated from an enriched leukocyte fraction (buffy coat) obtained from 450 ml of venous blood. The blood was taken from donors visiting the University Hospital blood bank in Umeå. Informed consent was obtained from all subjects. MNL were isolated by isopycnic centrifugation in Lymphoprep (Nycomed AB, Lidingö, Sweden), as described previously (43). The fraction containing MNL was collected, and the cells were washed three times with phosphate-buffered saline (PBS) (250 × g; 10 min) to remove the platelets. The cell pellet was then resuspended in RPMI 1640 culture medium containing 10% fetal bovine serum (Sigma-Aldrich, St. Louis, Mo.) to yield 5 × 106 cells/ml. This suspension was distributed into 35-mm-diameter petri dishes (8 ml/dish; NUNC A/S, Roskilde, Denmark) and incubated at 37°C in 5% CO2 for 2 h to allow the monocytes to adhere. The nonadherent lymphocytes were removed by two rinses with 1 ml of PBS. The adherent cells were detached from the dish surface by treating them with 0.1% trypsin in PBS for 1 min, washed once with PBS, and resuspended in culture medium (106 cells/ml). This procedure yields ∼95% monocytes (43). The suspension (106 cells/well) was distributed onto 2-cm2 culture dishes (NUNC) and incubated for 20 h at 37°C in 5% CO2 to equilibrate the cells.
Prior to experimentation, the culture medium was replaced by 0.25 ml of fresh medium/well. The adherent mononuclear leukocytes obtained by this procedure are referred to as macrophages below.
Macrophage viability and cytolysis assays.
To determine the effect of leukotoxin on macrophage viability, cells were incubated at 37°C for 3 h in the presence of leukotoxin (concentration range, 0.3 to 100 ng/ml). In preliminary experiments, the 3-h incubation period was found to be suitable for the detection of leukotoxin-induced IL-1β secretion. The number of viable monocytes in the culture was assayed at the end of the incubation by analyzing the uptake of the dye neutral red (4). For this purpose, the culture medium was replaced by fresh medium containing 40 μg of neutral red/ml, and the incubation was continued at 37°C for 2 h. Cells detached during the incubation were harvested by centrifuging (250 × g; 5 min). Detached and adherent cells were rinsed with fixation solution and resuspended in extract solution (1% acetic acid in 50% ethanol). The total amount of soluble dye in the extracts was measured in a spectrophotometer. Cell viability was expressed as the percentage of neutral red uptake found in the controls, i.e., cells not exposed to leukotoxin (21).
Leukotoxin-induced cytolysis was determined as the release of the cytosol enzyme lactate dehydrogenase (LDH) into the culture medium as described earlier (21). Macrophages were incubated for 3 h at 37°C in the presence of up to 100 ng of leukotoxin per ml of culture medium. The mixture was subsequently placed on ice for 10 min, and the cells were pelleted by centrifuging them at 1,000 × g and 4°C for 5 min. The activity of the enzyme released from damaged cells into the supernatant was measured (45) and expressed as the percentage of the total LDH activity (100%) obtained by lysing the cells with 0.1% Triton X-100.
Effect on expression of cytokine mRNA.
The macrophages were incubated in culture medium for 3 h at 37°C in the presence of 1 ng of leukotoxin/ml or 100 ng of LPS/ml from either A. actinomycetemcomitans or E. coli. The leukotoxin concentration chosen was found to be sublytic according to the results of the viability and cytolysis assays (see Results for detailed data). Macrophages in plain culture medium served as controls. At the end of the incubation period, the medium was removed, the cells were lysed in Trizol LS (Life Technologies, Täby, Sweden), and total RNA was extracted following the manufacturer's instructions. cDNA was synthesized from total RNA using a commercially available kit (first-strand cDNA synthesis kit for reverse transcription [RT]-PCR; Roche Diagnostics Scand AB, Bromma, Sweden).
PCR was run for mRNA analyses of the human cytokines IL-1β, IL-6, and TNF-α and of the constitutive enzyme GAPDH (glyceraldehyde-3-phosphate dehydrogenase) using a PCR core kit (Roche Diagnostics Scand AB). The templates were amplified in a Mastercycler gradient (Eppendorf, Hamburg, Germany) employing the following protocol: 94°C for 2 min, 18 to 33 cycles at 94°C for 15 s and annealing at 51 to 60°C for 20 s (IL-1β, 56°C; IL-6, 51°C; TNF-α, 60°C; GAPDH, 57°C) and at 72°C for 25 s, and finally 72°C for 2 min. The amplified products were separated by electrophoresis in a 1.5% agarose gel and stained with ethidium bromide.
The sequences of the PCR primers were as follows: for IL-1β, forward (5′-GAC CTT CCA GGA GAA TGA CC-3′) and reverse (5′-GGC TTA TCA TCT TTC AAC ACG-3′) (PCR product, 332 bp); for IL-6, forward (5′-CCA GGA GCC CAG CTA TGA AC-3′) and reverse (5′-TCA GCC ATC TTT GGA AGG TTC-3′) (PCR product, 306 bp); for TNF-α, forward (5′-TCA GAT CAT CTT CTC GAA CC-3′) and reverse (5′-CAG ATA GAT GGG CTC ATA CC-3′) (PCR product, 358 bp); for GAPDH, forward (5′-CAA CTA CAT GGT TTA CAT GTT C-3′) and reverse (5′-GCC AGT GGA CTC CAC GAC-3′) (PCR product, 163 bp). The design of all PCR primers (Life Technologies) was based on the mRNA sequence information available in the National Center for Biotechnology Information gene bank. The identities of the PCR products were confirmed using a DYEnamic ET terminator cycle-sequencing kit (Amersham Biosciences, Little Chalfont, United Kingdom). The base sequence was analyzed on an ABI 377 XL DNA sequencer (PE Applied Biosystems, Foster City, Calif.).
Effect on cytokine production and secretion.
The macrophages were incubated with the bacterial stimuli as described above. The amounts of IL-1β, IL-6, and TNF-α secreted into the culture medium or retained intracellularly in the macrophages were determined by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (OptEIA; BD Pharmingen, San Diego, Calif.). The amount of pro-IL-1β (biologically inactive precursor) was quantified by an ELISA kit (Quantikine; R&D Systems Inc., Minneapolis, Minn.). To release the intracellular cytokines, the culture medium was removed and 0.25 ml of 0.1% Triton X-100 solution was added to each well. Following a 1-h incubation at room temperature, the macrophage lysate was collected. All samples were stored at −20°C pending analysis.
IL-1β in supernatants was also assayed by an immunoblot technique (22) that allows discrimination of the biologically inactive pro-IL-1β (31 kDa) and the active protein (17 kDa). Briefly, the proteins in the supernatant were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a Polyscreen membrane (NEN Life Science Products, Boston, Mass.), and incubated with polyclonal rabbit anti-human IL-1β antibodies (Sigma-Aldrich) recognizing both pro-IL-1β and active IL-1β. As a secondary antibody, horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Dako, Glostrup, Denmark) was used. The immunoreactive protein bands were visualized on photographic film (Eastman Kodak, Rochester, N.Y.) using the enhanced-chemiluminescence technique (Pierce, Rockford, Ill.).
To determine the involvement of the cysteine proteinase caspase 1 in leukotoxin-induced IL-1β production, experiments were performed in the presence of the caspase 1 inhibitor acetyl-Tyr-Val-Ala-Asp-chloromethylketone (Ac-YVAD-CMK; Calbiochem, La Jolla, Calif.) at a final concentration of 100 μM. To inhibit autocrine activation by secreted IL-1β, antibodies to IL-1β (polyclonal rabbit anti-human; Genzyme Corp., Cambridge, Mass.) were added (10 μg/ml of medium). These antibodies did not interfere with the binding of the monoclonal antibody used in the ELISA. The inhibitor or the antibodies were added to the leukocyte cultures 15 min prior to leukotoxin exposure.
Bone resorption assay.
The bone-resorbing activities of macrophage supernatants were analyzed by measuring the release of minerals from cultured neonatal mouse calvariae (28). The mineral mobilization was determined by the percentage release of 45Ca from prelabeled bones. Briefly, 1- to 2-day-old CsA mice were injected with 1.5 μCi of 45Ca (DuPont, Brussels, Belgium). Five days later, the mice were sacrificed, and six bone fragments were dissected from the parietal part of the calvaria. Following preincubation for 18 to 24 h in alpha minimal essential medium (α-MEM) culture medium (Sigma-Aldrich) containing 0.1% albumin and 1 μM indomethacin, the bone specimens were thoroughly washed with plain α-MEM and placed in culture dishes containing 2 ml of α-MEM. Cell-free supernatants of macrophages (4 × 106/ml) cultured for 3 h in the presence or absence of 1 ng of leukotoxin/ml were added to yield a final concentration of 1%. Plain culture media without macrophage supernatant and with or without recombinant human IL-1β (100 pg/ml; Calbiochem) were used as controls. After a 96-h incubation at 37°C, the bone fragments were demineralized in HCl and the radioactivity in these samples and the media were analyzed using a scintillation counter. The increases in 45Ca release from the bone into the media caused by the different macrophage supernatants were expressed in relation to the release found in the controls, i.e., bone in plain culture medium (100%).
The involvement of IL-1 in bone-resorbing activity induced by the macrophage supernatants was monitored with monoclonal antibodies against human IL-1α or IL-1β (Genzyme Corp.) or by using the human IL-1 receptor antagonist protein (IRAP; Calbiochem). The antibodies were added to the macrophage supernatants 12 h (at 4°C) before they were added to the calvarial bioassay, while IRAP was added to the calvarial assay 3 h (at 37°C) before addition of the supernatants. The final concentrations were 300 ng/ml for IL-1α and IL-1β antibodies and 100 ng/ml for IRAP.
Statistical analysis.
The significance of differences was assessed with one-way analysis of variance (see Fig. 2), the Dunnett two-sided test (see Fig. 4), and the Dunnett T3 test (see Fig. 5). Mean values and standard deviations are shown in the figures. P values of <0.05 were considered indicative of statistically significant differences.
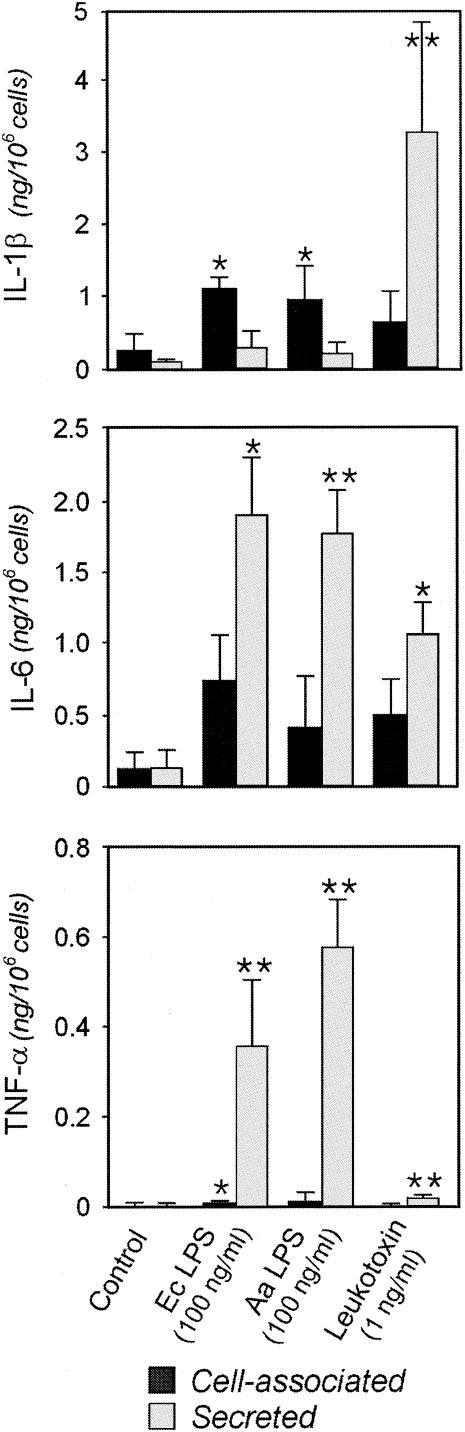
FIG. 2.
Cytokine production (cell-associated or secreted IL-1β, IL-6, and TNF-α) by macrophages exposed to 1 ng of leukotoxin/ml or to 100 ng of lipopolysaccharide/ml from A. actinomycetemcomitans (Aa LPS) or from E. coli (Ec LPS) for 3 h. The cell-associated or secreted amounts of these proteins (active form of IL-1β) were quantified by ELISA. The mean and standard deviation of five experiments performed with macrophages obtained from different donors are shown. P values of <0.05 (*) and <0.01 (**) (one-way analysis of variance) in comparison to the corresponding controls are indicated.
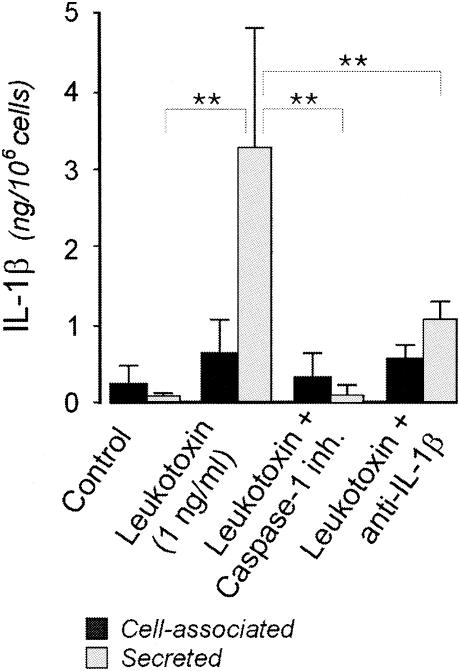
FIG. 4.
IL-1β production (cell associated or secreted) by macrophages exposed to 1 ng of leukotoxin/ml for 3 h in the presence of the caspase 1 inhibitor (inh.) Ac-YVAD-CMK or monoclonal antibodies to IL-1β. The cell-associated and secreted amounts of IL-1β (active form) were quantified by ELISA. The mean and standard deviation of five experiments performed with macrophages obtained from different donors are shown. P values of <0.05 (*) and <0.01 (**) (Dunnett two-sided test) are indicated.
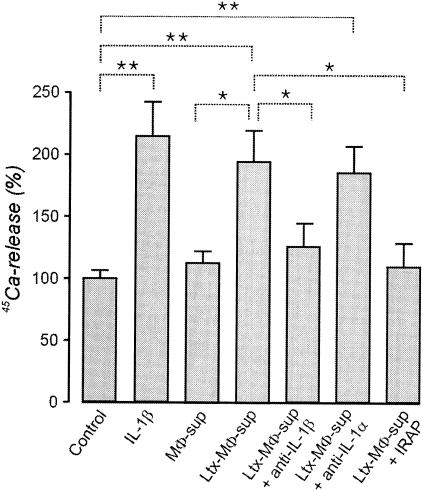
FIG. 5.
Induction of bone resorption by supernatants from macrophages (MΦ-sup) incubated for 3 h with 1 ng of leukotoxin (Ltx)/ml. Monoclonal antibodies against IL-1α, IL-1β, or IRAP were added to some supernatants. The resorption is determined by the mineral mobilization from bone fragments of mouse calvariae prelabeled with 45Ca and incubated for 96 h in culture medium supplemented with 1% macrophage supernatant. The bone resorption is expressed as the percent release of 45Ca. The spontaneous release of 45Ca by the control bones cultured in plain medium without macrophage supernatant was considered 100%. The mean and standard deviation of six experiments are shown. P values of <0.05 (*) and <0.01 (**) (Dunnett T3 test) are indicated.
Nucleotide sequence accession numbers.
The GenBank accession numbers and the positions of the 5′ and 3′ ends of the nucleotides for the predicted PCR products are as follows: IL-1β, NM000576 (335 to 667); IL-6, NM000600 (624 to 1197); TNF-α, NM000594 (399 to 757); GAPDH, BC023632 (153 to 316).
RESULTS
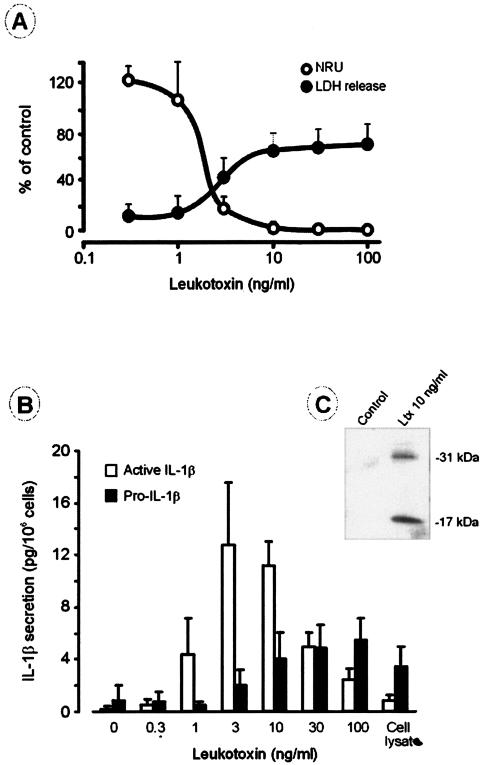
Challenging the cultures of macrophages with A. actinomycetemcomitans leukotoxin for 3 h led to cytolysis when the leukotoxin concentration was ≥3 ng/ml, while only a minor release of LDH was observed at toxin concentrations of ≤1 ng/ml (Fig. 1A). Toxin concentrations of ≤1 ng/ml present in the culture for 3 h left the macrophages viable, with their capacity for neutral red uptake similar to that of the control cultures (Fig. 1A). In all mixtures, extracellular release of both pro-IL-1β and active IL-1β was detected (Fig. 1B). A statistically significant increase in the secretion of active IL-1β was found after a 3-h incubation with ≥1 ng of leukotoxin/ml. The secretion of active IL-1β peaked at 3 ng of leukotoxin/ml, while the release of pro-IL-1β peaked at a toxin concentration of 100 ng/ml. At toxin concentrations ≥30 ng/ml, the secretion of the active IL-1β decreased (Fig. 1B). Immunoblotting of culture supernatants from macrophages exposed to 10 ng of leukotoxin/ml for 60 min showed the presence of the active IL-1β protein (17 kDa), as well as of the inactive precursor (31 kDa) (Fig. 1C).
FIG. 1.
(A) Human macrophage lysis at various concentrations of A. actinomycetemcomitans leukotoxin. The cytotoxic effect was measured as (i) the release of LDH from cells exposed to the toxin for 3 h, with the release expressed as the percentage of the LDH activity in cultures lysed by Triton X-100, and (ii) the neutral red uptake (NRU) by cells exposed to leukotoxin for 20 h, with the uptake expressed as a percentage of the value found in the negative cell cultures (without leukotoxin). Shown are the mean and standard deviation (SD) of four experiments with macrophages obtained from different donors. (B) Effect of A. actinomycetemcomitans leukotoxin on the extracellular release of IL-1β (active form) and pro-IL-1β by human macrophages. The cells were exposed to different concentrations of leukotoxin for 3 h, and the amounts of the two proteins released were quantified by ELISA. Shown are the mean and SD of four experiments with macrophages obtained from different donors. (C) Effect of A. actinomycetemcomitans leukotoxin (Ltx) on the extracellular release of IL-1β (active form; 17 kDa) and pro-IL-1β (31 kDa) by human macrophages. The cells were exposed to leukotoxin for 1 h, and the release of the two proteins was examined by immunoblotting. A representative result of three experiments with macrophages obtained from different donors is shown.
The leukotoxin also induced the synthesis and release of IL-6 and TNF-α from the challenged macrophages (Fig. 2). Compared to the other bacterial stimuli, such as LPS from E. coli and A. actinomycetemcomitans, the leukotoxin exhibited a reduced capacity to induce production and secretion of these cytokines (Fig. 2). While almost all TNF-α was found in the supernatant (secreted into the culture medium), ∼30% or less of the produced IL-6 was recovered in the cell pellets (cell associated). Heat treatment (70°C for 30 min) of the leukotoxin preparation completely abolished cytokine production (data not shown). In the case of IL-1β (the active form), two different patterns were observed. LPS stimulation resulted in the production of mainly (∼80%) cell-associated cytokine, whereas leukotoxin induced substantial production of IL-1β that was mainly secreted (Fig. 2). It should be emphasized that the amount of IL-1β secreted by leukotoxin-challenged (1 ng/ml) macrophages was 25-times greater than that caused by the LPS preparations at 100-times-higher concentration (100 ng/ml).
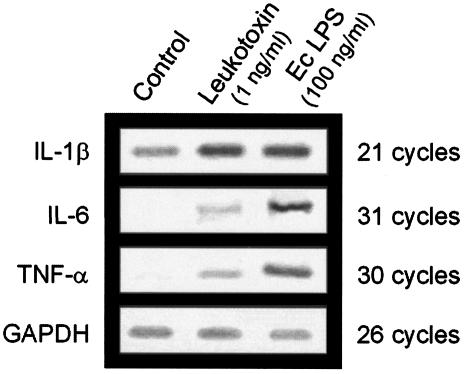
Leukotoxin (1 ng/ml) increased the levels of mRNA for IL-6 and TNF-α, as shown by a semiquantitative RT-PCR assay (Fig. 3). A similar effect was observed with the LPS of E. coli (100 ng/ml) (Fig. 3). Expression of IL-1β mRNA in the control cultures of adherent macrophages was detected after only 21 cycles by RT-PCR. This expression was slightly increased in the presence of leukotoxin or LPS compared to GAPDH mRNA (Fig. 3).
FIG. 3.
Levels of mRNA for the cytokines IL-1β, IL-6, and TNF-α and for the constitutive enzyme GAPDH in macrophages exposed to 1 ng of leukotoxin/ml from A. actinomycetemcomitans or to 100 ng of E. coli LPS (Ec LPS)/ml for 3 h. The corresponding mRNA levels in unchallenged (Control) cells are also included. The experiment was performed in triplicate with macrophages obtained from different donors. Representative results are shown.
Blocking of the caspase 1 or IL-1β activity with a specific inhibitor or specific antibodies, respectively, inhibited the leukotoxin-induced secretion of active IL-1β (Fig. 4), as well as the secretion of IL-6 and TNF-α (data not shown).
The culture supernatants from leukotoxin-challenged macrophages stimulated 45Ca release in the bone resorption assay. This release was similar to that obtained with 100 pg of recombinant human IL-1β/ml (Fig. 5). The bone-resorbing activity caused by the leukotoxin-challenged supernatant was inhibited when IRAP or antibodies to IL-1β were present. In contrast, addition of antibodies to IL-1α had no effect on 45Ca release (Fig. 5). Leukotoxin alone (≤1 μg/ml) also did not stimulate 45Ca release of calvarial bone incubated in plain medium (data not shown).
DISCUSSION
The present findings, together with the results from previous studies (11, 23, 32), indicate that the interaction of A. actinomycetemcomitans leukotoxin with human monocytes may lead to two different events depending on the toxin concentration. At low (<30 ng/ml) concentrations, the toxin induces rapid production and secretion of the proinflammatory cytokines IL-1β, TNF-α, and IL-6 before the cells eventually die. At higher concentrations, the toxin causes rapid cell death with limited secretion of bioactive cytokines. Compared to the classic inflammatory stimulus LPS, the leukotoxin appears to be a more potent inducer of cytokine release, since it caused substantially greater secretion of bioactive IL-1β at a 100-times-lower concentration.
The presence of specific antibodies to IL-1β partially inhibited the leukotoxin-stimulated production and secretion of the cytokine, while complete inhibition was obtained with an inhibitor for caspase 1 (Ac-YVAD-CMK). Caspase 1, a cytosolic cysteine protease previously named IL-1β-converting enzyme, is responsible for the activation and secretion of IL-1β (10, 11, 32). It seems that the release of IL-6 and TNF-α was a secondary event induced by the secreted bioactive IL-1β. The precursor of IL-1β is synthesized as a 31-kDa biologically inactive protein that is stored intracellularly. During activation, caspase 1 cleaves the precursor to a 17-kDa bioactive protein, which is then secreted (10). Leukotoxin appears to trigger not only the mechanism for production but also that for secretion of active IL-1β, the latter involving activation of caspase 1 in monocytes (23). The mechanism for the abundant production of the active cytokine seems to involve two separate steps. The first step is the activation of caspase 1, which is leukotoxin dependent. The active enzyme cleaves intracellular pro-IL-1β, which is then secreted in its active form. The second step is the synthesis of new IL-1β, which is stimulated by the secreted cytokine in an autocrine manner (10). Secretion of active IL-1β was dose dependent and was significantly enhanced at sublytic and low-lytic concentrations of leukotoxin (1 to 30 ng/ml). At higher concentrations, the proportion of pro-IL-β increased, which indicates insufficient conversion of the precursor to the active cytokine. Possibly, cell lysis at high toxin concentrations proceeds too rapidly to allow the mechanisms for activation and secretion to operate fully, and perhaps the rapid cell death involves other mechanisms independent of caspase 1 activation.
The mRNA levels for IL-1β were only marginally increased after exposure of the adherent macrophages to leukotoxin or LPS, while the protein levels were greatly enhanced. This is in line with previous observations showing that transcription of the IL-1β gene in cultures of adherent macrophages is regulated independently of translation (37). Adherence of peripheral blood monocytes to a surface activates transcription of the IL-1β gene without necessarily resulting in production of the protein (10). The IL-6 and TNF-α mRNA levels in the different cultures of macrophages were more in line with the production of the corresponding proteins, with a slight increase caused by the leukotoxin and a substantial enhancement caused by LPS.
The culture supernatant of macrophages that was exposed to sublytic concentrations of leukotoxin (1 ng/ml) stimulated bone resorption in an assay with cultured mouse calvariae. The activity was nearly completely blocked by IL-1β antibodies or an IL-1 receptor antagonist, while IL-1α antibodies had no significant effect. Thus, IL-1β is the most important factor for bone resorption released by leukotoxin-stimulated macrophages.
In general, the roles of bacterial toxins in inflammation have been suggested to depend on their cytolytic activities. A new role of RTX toxins in inflammation has recently been proposed, namely, that sublytic concentrations of the toxins can induce cellular events that promote inflammation (39, 42). Upon chemotactic migration to the site of inflammation, the ability of leukotoxin to activate caspase 1 and induce secretion of bioactive IL-1β in human macrophages may comprise a novel pathogenic mechanism involved in aggressive periodontitis.
Acknowledgments
This study was supported in part by the Research Fund of the Swedish Research Council, Västerbotten County; the Swedish Dental Association; and the Swedish Patent Revenue Fund.
Editor: J. D. Clements
REFERENCES
- 1.Assuma, R., T. Oates, D. Cochran, S. Amar, and D. T. Graves. 1998. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J. Immunol. 160:403-409. [PubMed] [Google Scholar]
- 2.Baehni, P., C. C. Tsai, W. P. McArthur, B. F. Hammond, and N. S. Taichman. 1979. Interaction of inflammatory cells and oral microorganisms. VIII. Detection of leukotoxic activity of a plaque-derived gram-negative microorganism. Infect. Immun. 24:233-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boch, J. A., N. Wara-Aswapati, and P. E. Auron. 2001. Interleukin 1 signal transduction—current concepts and relevance to periodontitis. J. Dent. Res. 80:400-407. [DOI] [PubMed] [Google Scholar]
- 4.Borenfreund, E., and J. A. Puerner. 1985. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 24:119-124. [DOI] [PubMed] [Google Scholar]
- 5.Brogan, J. M., E. T. Lally, K. Poulsen, M. Kilian, and D. R. Demuth. 1994. Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect. Immun. 62:501-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bueno, L. C., M. P. Mayer, and J. M. DiRienzo. 1998. Relationship between conversion of localized juvenile periodontitis-susceptible children from health to disease and Actinobacillus actinomycetemcomitans leukotoxin promoter structure. J. Periodontol. 69:998-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christersson, L. A., and J. J. Zambon. 1993. Suppression of subgingival Actinobacillus actinomycetemcomitans in localized juvenile periodontitis by systemic tetracycline. J. Clin. Periodontol. 20:395-401. [DOI] [PubMed] [Google Scholar]
- 8.Delima, A. J., S. Karatzas, S. Amar, and D. T. Graves. 2002. Inflammation and tissue loss caused by periodontal pathogens is reduced by interleukin-1 antagonists. J. Infect. Dis. 186:511-516. [DOI] [PubMed] [Google Scholar]
- 9.Delima, A. J., T. Oates, R. Assuma, Z. Schwartz, D. Cochran, S. Amar, and D. T. Graves. 2001. Soluble antagonists to interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibits loss of tissue attachment in experimental periodontitis. J. Clin. Periodontol. 28:233-240. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello, C. A. 1996. Biologic basis for interleukin-1 in disease. Blood 87:2095-2147. [PubMed] [Google Scholar]
- 11.Edgeworth, J. D., J. Spencer, A. Phalipon, G. E. Griffin, and P. J. Sansonetti. 2002. Cytotoxicity and interleukin-1β processing following Shigella flexneri infection of human monocyte-derived dendritic cells. Eur. J. Immunol. 32:1464-1471. [DOI] [PubMed] [Google Scholar]
- 12.Gravallese, E. M., and S. R. Goldring. 2000. Cellular mechanisms and the role of cytokines in bone erosions in rheumatoid arthritis. Arthritis Rheum. 43:2143-2151. [DOI] [PubMed] [Google Scholar]
- 13.Graves, D. T., and D. Cochran. 2003. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J. Periodontol. 74:391-401. [DOI] [PubMed] [Google Scholar]
- 14.Guthmiller, J. M., E. T. Lally, and J. Korostoff. 2001. Beyond the specific plaque hypothesis: are highly leukotoxic strains of Actinobacillus actinomycetemcomitans a paradigm for periodontal pathogenesis? Crit. Rev. Oral Biol. Med. 12:116-124. [DOI] [PubMed] [Google Scholar]
- 15.Haraszthy, V. I., G. Hariharan, E. M. Tinoco, J. R. Cortelli, E. T. Lally, E. Davis, and J. J. Zambon. 2000. Evidence for the role of highly leukotoxic Actinobacillus actinomycetemcomitans in the pathogenesis of localized juvenile and other forms of early-onset periodontitis. J. Periodontol. 71:912-922. [DOI] [PubMed] [Google Scholar]
- 16.Haubek, D., O. K. Ennibi, K. Poulsen, S. Poulsen, N. Benzarti, and M. Kilian. 2001. Early-onset periodontitis in Morocco is associated with the highly leukotoxic clone of Actinobacillus actinomycetemcomitans. J. Dent. Res. 80:1580-1583. [DOI] [PubMed] [Google Scholar]
- 17.Haubek, D., and J. Westergaard. 2004. Detection of a highly toxic clone of Actinobacillus actinomycetemcomitans (JP2) in a Moroccan immigrant family with multiple cases of localized aggressive periodontitis. Int. J. Paediatr. Dent. 14:41-48. [DOI] [PubMed] [Google Scholar]
- 18.Henderson, B., S. P. Nair, J. M. Ward, and M. Wilson. 2003. Molecular pathogenicity of the oral opportunistic pathogen Actinobacillus actinomycetemcomitans. Annu. Rev. Microbiol. 57:29-55. [DOI] [PubMed] [Google Scholar]
- 19.Johansson, A., R. Claesson, L. Hänström, G. Sandström, and S. Kalfas. 2000. Polymorphonuclear leukocyte degranulation induced by leukotoxin from Actinobacillus actinomycetemcomitans. J. Periodontal Res. 35:85-92. [DOI] [PubMed] [Google Scholar]
- 20.Johansson, A., L. Hänström, and S. Kalfas. 2000. Inhibition of Actinobacillus actinomycetemcomitans leukotoxicity by bacteria from the subgingival flora. Oral Microbiol. Immunol. 15:218-225. [DOI] [PubMed] [Google Scholar]
- 21.Johansson, A., and S. Kalfas. 1998. Characterization of the proteinase-dependent cytotoxicity of Porphyromonas gingivalis. Eur. J. Oral Sci. 106:863-871. [DOI] [PubMed] [Google Scholar]
- 22.Johansson, A., G. Sandström, R. Claesson, L. Hänström, and S. Kalfas. 2000. Anaerobic neutrophil-dependent killing of Actinobacillus actinomycetemcomitans in relation to the bacterial leukotoxicity. Eur. J. Oral Sci. 108:136-146. [DOI] [PubMed] [Google Scholar]
- 23.Kelk, P., A. Johansson, R. Claesson, L. Hänström, and S. Kalfas. 2003. Caspase 1 involvement in human monocyte lysis induced by Actinobacillus actinomycetemcomitans leukotoxin. Infect. Immun. 71:4448-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korostoff, J., J. F. Wang, I. Kieba, M. Miller, B. J. Shenker, and E. T. Lally. 1998. Actinobacillus actinomycetemcomitans leukotoxin induces apoptosis in HL-60 cells. Infect. Immun. 66:4474-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lally, E. T., E. E. Golub, I. R. Kieba, N. S. Taichman, S. Decker, P. Berthold, C. W. Gibson, D. R. Demuth, and J. Rosenbloom. 1991. Structure and function of the B and D genes of the Actinobacillus actinomycetemcomitans leukotoxin complex. Microb. Pathog. 11:111-121. [DOI] [PubMed] [Google Scholar]
- 26.Lally, E. T., R. B. Hill, I. R. Kieba, and J. Korostoff. 1999. The interaction between RTX toxins and target cells. Trends Microbiol. 7:356-361. [DOI] [PubMed] [Google Scholar]
- 27.Lally, E. T., I. R. Kieba, A. Sato, C. L. Green, J. Rosenbloom, J. Korostoff, J. F. Wang, B. J. Shenker, S. Ortlepp, M. K. Robinson, and P. C. Billings. 1997. RTX toxins recognize a β2 integrin on the surface of human target cells. J. Biol. Chem. 272:30463-30469. [DOI] [PubMed] [Google Scholar]
- 28.Lerner, U. H. 1987. Modifications of the mouse calvarial technique improve the responsiveness to stimulators of bone resorption. J. Bone Miner. Res. 2:375-383. [DOI] [PubMed] [Google Scholar]
- 29.Lerner, U. H. 2004. New molecules in the tumor necrosis factor ligand and receptor superfamilies with importance for physiological and pathological bone resorption. Crit. Rev. Oral Biol. Med. 15:64-81. [DOI] [PubMed] [Google Scholar]
- 30.McGee, J. M., M. A. Tucci, T. P. Edmundson, C. L. Serio, and R. B. Johnson. 1998. The relationship between concentrations of proinflammatory cytokines within gingiva and the adjacent sulcular depth. J. Periodontol. 69:865-871. [DOI] [PubMed] [Google Scholar]
- 31.Merril, C. R. 1990. Gel-staining techniques. Methods Enzymol. 182:477-488. [DOI] [PubMed] [Google Scholar]
- 32.Monack, D. M., C. S. Detweiler, and S. Falkow. 2001. Salmonella pathogenicity island 2-dependent macrophage death is mediated in part by the host cysteine protease caspase-1. Cell Microbiol. 3:825-837. [DOI] [PubMed] [Google Scholar]
- 33.Nalbant, A., C. Chen, Y. Wang, and H. H. Zadeh. 2003. Induction of T-cell apoptosis by Actinobacillus actinomycetemcomitans mutants with deletion of ltxA and cdtABC genes: possible activity of GroEL-like molecule. Oral Microbiol. Immunol. 18:339-349. [DOI] [PubMed] [Google Scholar]
- 34.Novak, M. J., and K. F. Novak. 1996. Early-onset periodontitis. Curr. Opin. Periodontol. 3:45-58. [PubMed] [Google Scholar]
- 35.Rasmussen, L., L. Hanstrom, and U. H. Lerner. 2000. Characterization of bone resorbing activity in gingival crevicular fluid from patients with periodontitis. J. Clin. Periodontol. 27:41-52. [DOI] [PubMed] [Google Scholar]
- 36.Roberts, F. A., R. D. Hockett, Jr., R. P. Bucy, and S. M. Michalek. 1997. Quantitative assessment of inflammatory cytokine gene expression in chronic adult periodontitis. Oral Microbiol. Immunol. 12:336-344. [DOI] [PubMed] [Google Scholar]
- 37.Schindler, R., B. D. Clark, and C. A. Dinarello. 1990. Dissociation between interleukin-1 beta mRNA and protein synthesis in human peripheral blood mononuclear cells. J. Biol. Chem. 265:10232-10237. [PubMed] [Google Scholar]
- 38.Slots, J., and M. Ting. 1999. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontol. 2000 20:82-121. [DOI] [PubMed] [Google Scholar]
- 39.Soderblom, T., C. Oxhamre, E. Torstensson, and A. Richter-Dahlfors. 2003. Bacterial protein toxins and inflammation. Scand. J. Infect. Dis. 35:628-631. [DOI] [PubMed] [Google Scholar]
- 40.Taubman, M. A., and T. Kawai. 2001. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit. Rev. Oral Biol. Med. 12:125-135. [DOI] [PubMed] [Google Scholar]
- 41.Tsai, C. C., W. P. McArthur, P. C. Baehni, B. F. Hammond, and N. S. Taichman. 1979. Extraction and partial characterization of a leukotoxin from a plaque-derived gram-negative microorganism. Infect. Immun. 25:427-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uhlen, P., A. Laestadius, T. Jahnukainen, T. Soderblom, F. Backhed, G. Celsi, H. Brismar, S. Normark, A. Aperia, and A. Richter-Dahlfors. 2000. Alpha-haemolysin of uropathogenic E. coli induces Ca2+ oscillations in renal epithelial cells. Nature 405:694-697. [DOI] [PubMed] [Google Scholar]
- 43.Walsh, L. J., F. Stritzel, K. Yamazaki, P. S. Bird, E. Gemmell, and G. J. Seymour. 1989. Interleukin-1 and interleukin-1 inhibitor production by human adherent cells stimulated with periodontopathic bacteria. Arch. Oral Biol. 34:679-683. [DOI] [PubMed] [Google Scholar]
- 44.Welch, R. A. 2001. RTX toxin structure and function: a story of numerous anomalies and few analogies in toxin biology. Curr. Top. Microbiol. Immunol. 257:85-111. [DOI] [PubMed] [Google Scholar]
- 45.Wroblewski, F., and J. S. Ladue. 1955. Lactic dehydrogenase activity in blood. Proc. Soc. Exp. Biol. Med. 90:210-213. [DOI] [PubMed] [Google Scholar]
- 46.Zambon, J. J. 1985. Actinobacillus actinomycetemcomitans in human periodontal disease. J. Clin. Periodontol. 12:1-20. [DOI] [PubMed] [Google Scholar]
- 47.Zambon, J. J. 1996. Periodontal diseases: microbial factors. Ann. Periodontol. 1:879-925. [DOI] [PubMed] [Google Scholar]