Abstract
PURPOSE
Community-acquired pneumonia (CAP), acute cough, bronchitis, and lower respiratory tract infections (LRTI) are often caused by infections with viruses or Streptococcus pneumoniae. The prevalence of atypical pathogens Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, and Bordetella pertussis among patients with these illnesses in the ambulatory setting has not been previously summarized. We set out to derive prevalence information from the existing literature.
METHODS
We performed a systematic review of MEDLINE for prospective, consecutive-series studies reporting the prevalence of M pneumoniae, C pneumoniae, L pneumophila and/or B pertussis in outpatients with cough, acute bronchitis, LRTI, or CAP. Articles were independently reviewed by 2 authors for inclusion and abstraction of data; discrepancies were resolved by consensus discussion. A meta-analysis was performed on each pathogen to calculate the pooled prevalence estimates using a random effects model of raw proportions.
RESULTS
Fifty studies met our inclusion criteria. While calculated heterogeneity was high, most studies reported prevalence for each pathogen within a fairly narrow range. In patients with CAP, the overall prevalences of M pneumoniae and C pneumoniae were 10.1% (95% CI, 7.1%–13.1%) and 3.5% (95% CI, 2.2%–4.9%), respectively. Consistent with previous reports, M pneumoniae prevalence peaked in roughly 6-year intervals. Overall prevalence of L pneumophila was 2.7% (95% CI, 2.0%–3.4%), but the organism was rare in children, with only 1 case in 1,765. In patients with prolonged cough in primary care, the prevalence of B pertussis was 12.4% (95% CI, 4.9%–19.8%), although it was higher in studies that included only children (17.6%; 95% CI, 3.4%–31.8%).
CONCLUSIONS
Atypical bacterial pathogens are relatively common causes of lower respiratory diseases, including cough, bronchitis, and CAP. Where surveillance data were available, we found higher prevalences in studies where all patients are tested for these pathogens. It is likely that these conditions are underreported, underdiagnosed, and undertreated in current clinical practice.
Keywords: community acquired pneumonia, cough, respiratory tract infection, Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, Bordetella pertussis
INTRODUCTION
Cough is the 4th most common reason for an office visit to an ambulatory physician, accounting for 2.8% of all visits.1 In primary care, when cough is the patient’s primary complaint, it is most often caused by a virus, but approximately 5% of patients have community-acquired pneumonia (CAP).2 Although viruses and Streptococcus pneumoniae are the most common causes of CAP, some episodes are caused by an atypical bacterial infection such as Mycoplasma pneumoniae, Chlamydophila pneumoniae (also known as Chlamydia pneumoniae), and Legionella pneumophila. Some episodes of non-pneumonia lower respiratory tract infection (LRTI) are caused by the above pathogens as well as by Bordetella pertussis, and the incidence of the latter is increasing in the United States.3
Mycoplasma pneumoniae infection is thought to vary cyclically,4,5 and has been the cause of outbreaks of LRTI.6 Not to be confused with Chlamydia psittaci (which also causes respiratory infections but is contracted from birds), Chlamydophila pneumoniae is more common in children, but has been associated with subsequent serious adult disease as well. A meta-analysis reported an association with lung cancer in patients with previous C pneumoniae infections,7 while others have posited an association with development of asthma.8,9 Legionellosis, better known as Legionnaires’ disease, is caused by L pneumophila and is most commonly diagnosed as a cause of CAP in patients over 50 years of age, and more often in men than women. The organism is found naturally in the environment, and the infection is associated with inhalation of aerosolized water from sources such as hot tubs and cooling towers.10 Recently, increased risk of infection with L pneumophila has also been linked to wet, humid weather.11 Bordetella pertussis is highly communicable and is a source of significant morbidity in children and prolonged symptoms in all patients. Although B pertussis is the only atypical pathogen to have a widely available vaccine, the incidence of B pertussis in the United States is increasing, with more cases in 2012 than any year previously since 1955.3
The prevalence of atypical pathogens, particularly in the outpatient primary care setting, has not been previously summarized. B pertussis and L pneumophila are reported by national surveillance systems in many countries, but they are laboratory-based systems that are subject to significant underreporting.12 The prevalence of C pneumoniae and M pneumoniae vary widely in previous studies of patients with CAP.
Because these atypical pathogens do not respond to beta-lactams, may carry a different prognosis, and can cause serious complications in some patients, it is important to understand their prevalence. Therefore, we performed a meta-analysis to describe the prevalence of atypical pathogens among 2 groups: patients with cough, acute bronchitis, or LRTI in the ambulatory setting and patients diagnosed with CAP. We also compared these “real world” prevalences with the prevalences reported by surveillance systems, where available.
METHODS
Literature Review
We searched MEDLINE for prospective studies that reported the results of testing for M pneumoniae, C pneumoniae, L pneumophila, or B pertussis in outpatients with cough, acute bronchitis, or LRTI, as well as among inpatients and outpatients diagnosed with CAP. In order to reflect contemporary prevalences and microbiology, searches were limited to articles where the majority of data was collected after January 1, 2000. We included articles with abstracts written in English and German (the primary languages of the investigators). Supplemental Appendix A (http://www.annfammed.org/content/14/6/552/suppl/DC1) includes detailed search terms used for each strategy. We also reviewed the reference lists for review articles identified by our search, and of any included studies.
We excluded studies of only or predominantly immunocompromised patients, studies of hospital-acquired infections, studies of special or unusual populations (eg, military recruits), studies of acute exacerbations of chronic obstructive pulmonary disease or asthma, and studies of the etiology of bronchiolitis. Further, we excluded studies set in low- or medium-income countries based on Organisation for Economic Cooperation and Development (OECD) criteria; (Supplemental Appendix B, http://www.annfammed.org/content/14/6/552/suppl/DC1) since we felt that they would not reflect the current practice and epidemiology of the United States. We also excluded case-control studies, case reports, case series and retrospective studies, outbreak investigations, and studies that did not use culture, polymerase chain reaction (PCR), serology, or urine antigen testing (for L pneumophila) to identify pathogens.
Data Abstraction
Two investigators reviewed each abstract to identify articles that should be reviewed in full. Any article selected for full review was examined by both investigators. For each included article, study characteristics and data regarding prevalence were abstracted by both authors. For prevalence data, definite and probable cases were included and possible cases were excluded. Any discrepancies were resolved by consensus discussion.
Surveillance Systems
We used surveillance data reported by high-income members of the OECD.13 The most recent complete data available, from 2012, were abstracted by 2 investigators, with any discrepancies resolved by consensus discussion. For each report, we documented the type of surveillance used, number of cases reported, and total population.
Study Quality
A meta-analysis usually uses a standardized tool to assess the risk of bias.14–16 Unfortunately, there are currently no published tools for assessing bias in studies of disease prevalence. To ensure that the studies included in our meta-analysis were of consistent high quality, we only included studies that met the following criteria: they enrolled consecutive patients, did not gather data from a specialized or unusual population, gathered data prospectively, and used diagnostic tests likely to classify patients accurately as having the pathogen in question.
Analysis
We identified 2 groups for the analysis: patients presenting with acute cough illness or lower respiratory tract symptoms and patients diagnosed with CAP. Where studies reported etiology separately for patients with CAP and those with non-pneumonia LRTI, we report these groups separately as well. Pooled prevalence estimates were calculated with random effects model of raw proportions. Statistical analysis was performed in R (version 3.2.2, R Studio Version 0.99.441), including plots of proportions with each pathogen using the metafor procedure.
RESULTS
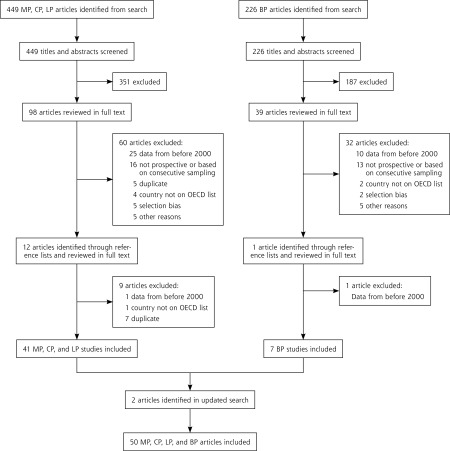
The search for M pneumoniae, C pneumoniae, and L pneumophila yielded 449 abstracts. A separate search for B pertussis returned 226. After screening titles and abstracts, 98 articles for M pneumoniae, C pneumoniae, and L pneumophila and 39 for B pertussis remained for full-text review. Thirteen articles were additionally identified through a review of the reference lists (12 for M pneumoniae, C pneumoniae, and L pneumophila, and 1 for B pertussis). Full-text review excluded 102 articles. The most common reasons for exclusion were that the majority of data was collected before 2000 or that the study did not use a cohort design with prospective data collection. An updated search before writing yielded 2 additional studies17,18 for a final of 50 included studies (Figure 1).
Figure 1.
PRISMA diagram.
BP = Bordetella pertussis; CP = Chlamydophila pneumoniae; LP = Legionella pneumophila; MP = Mycoplasma pneumonia; OECD = Organization for Economic Cooperation and Development.
To compare the prevalences given in the identified studies with the prevalences from surveillance systems, we abstracted surveillance data for reported cases of B pertussis and L pneumophila in 2012. Data, which were available for 31 of the 32 high-income member countries of the OECD, are summarized in Table 1 (Israel did not provide any publicly accessible data.)
Table 1.
Reported Bordetella pertussis and Legionella pneumophila Prevalence in 2012 by Case-Based Surveillance Systems of High-Income Countries Belonging to the OECD
| Countrya | BP Cases | LP Cases | Populationb | BP Rate per 100,000 | LP Rate per 100,000 |
|---|---|---|---|---|---|
| Australia | 24,069 | 382 | 22,918,688 | 105.0 | 1.67 |
| Austria | 425 | 101 | 8,428,915 | 5.0 | 1.20 |
| Belgium | ND | 106 | 10,787,788 | ND | 0.98 |
| Canada | 4,540 | 483 | 34,674,708 | 13.1 | 1.39 |
| Chile | 4,237 | ND | 17,423,214 | 24.3 | ND |
| Czech Republic | 707 | 56 | 10,565,678 | 6.7 | 0.53 |
| Denmark | 1,136 | 127 | 5,592,738 | 20.3 | 2.27 |
| Estonia | 149 | 3 | 1,339,762 | 11.1 | 0.22 |
| Finland | 541 | 10 | 5,402,627 | 10.0 | 0.19 |
| France | ND | 1,298 | 63,457,777 | ND | 2.05 |
| Germany | ND | 628 | 81,990,837 | ND | 0.33 |
| Greece | 40 | 27 | 11,418,878 | 0.35 | 0.77 |
| Hungary | 5 | 33 | 9,949,589 | 0.05 | 0.24 |
| Iceland | 36 | 2 | 328,290 | 11.0 | 0.61 |
| Ireland | 264 | 15 | 4,579,498 | 5.8 | 0.33 |
| Italy | 262 | 1,332 | 60,964,145 | 0.43 | 2.18 |
| Japan | ND | 903 | 126,434,653 | ND | 0.71 |
| Korea, Rep. | 126 | 25 | 48,588,326 | 0.26 | 0.05 |
| Luxembourg | 11 | 5 | 523,362 | 2.1 | 0.96 |
| Netherlands | 12,868 | 304 | 16,714,228 | 77.0 | 1.82 |
| New Zealand | 2,320 | 152 | 4,461,257 | 52.0 | 3.41 |
| Norway | 4,243 | 25 | 4,960,482 | 85.5 | 0.50 |
| Polandc | 1,824 | 8 | 38,317,090 | 4.8 | 0.02 |
| Portugal | 230 | 140 | 10,699,333 | 2.1 | 1.31 |
| Slovak Republic | 917 | 4 | 5,480,332 | 16.7 | 0.07 |
| Slovenia | 153 | 82 | 2,040,057 | 7.5 | 4.02 |
| Spain | 1,565 | 972 | 46,771,596 | 3.3 | 2.08 |
| Sweden | 279 | 12 | 9,495,392 | 2.9 | 0.13 |
| Switzerland | ND | 91 | 7,733,709 | ND | 1.18 |
| United Kingdom | 11,993 | 401 | 62,798,099 | 19.1 | 0.64 |
| United States | 48,277 | 3,688 | 315,791,284 | 15.3 | 1.17 |
BP = Bordetella pertussis; LP = Legionella pneumophila; ND = no data; OECD = Organisation for Economic Cooperation and Development.
No data available for Israel.
Population based on Gapminder 2012.19
Poland used aggregated instead of case-based data.
Prevalence of Mycoplasma pneumoniae, Chlamydophila pneumonia, and Legionella pneumophila
A total of 30 studies reported the prevalence of M pneumoniae, C pneumoniae, or L pneumophila in adults,18,20–48 and 10 studies reported the prevalence of these pathogens in children49–58 (Table 2). Only 2 studies were set in the United States.18,53
Table 2.
Characteristics of Studies of the Prevalence of Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila in Patients With Community-Acquired Pneumonia or Lower Respiratory Tract Infection
| Author, Year (Country) | Population | Total/Confirmed Casesa | Setting | Age | Pathogen | Data Collection Period | Diagnostic Method |
|---|---|---|---|---|---|---|---|
| CAP in Adults | |||||||
| Jain et al,18 2015b (United States) | Adults ≥18 y with CAP | 2,320/853 | Inpatient | Median 57 y, | MP, CP, LP | 2010–2012 | PCR, Culture, UA |
| Angeles et al,20 2006 (Spain) | Adults ≥15 y with CAP | 198/112 | Inpatient | Median 70 y | MP, CP, LP | 2003–2004 | Serology, UA |
| Beović et al,21 2003 (Slovenia) | Adults ≥15 y with CAP (PSI = I or II) | 113/68 | NR | Mean 44.9 y | MP, CP, LP | 1999–2001 | Serology |
| Charles et al,22 2008 (Australia) | Adults ≥18 y with CAP | 885/404 | Inpatient | Mean 65.1 y, range 18 y–100 y) | MP, CP, LP | 2004–2006 | Serology, UA |
| Cilloniz et al,23 2012 (Spain) | Adults ≥16 y with CAP | 568/188 | Outpatient | Mean 47.2 y | MP, CP, LP | 2000–2010 | Serology, UA |
| Diaz et al,24 2007 (Chile) | Adults ≥16 y with CAP | 176/98 | Inpatient | Mean 65.8 y, range 17 y–101 y | MP, CP, LP | 2003–2005 | Serology, UA |
| Espana et al,25 2012 (Spain) | Adults ≥18 y with CAP | 344/153 | 73 Inpatient, 271 outpatient | Mean 53.5 y | MP, CP, LP | 2006–2007 | Serology, UA |
| Falguera et al,26 2010 (Spain) | Adults ≥18 y or older with CAP (PSI IV or V) | 88/25 | Inpatient | Mean 64 y | LP | 2006–2008 | Serology, UA |
| Gutierrez et al,27 2006 (Spain) | Adults ≥15 y with CAP | 493/250 | 361 Inpatient, 132 outpatient | Mean 56.6 y, range 15 y–94 y | MP, CP, LP | 1999–2001 | Serology, UA |
| Herrera-Lara et al,28 2013 (Spain) | Adults ≥18 y with CAP | 243/139 | Inpatient | Mean 63.9 y | MP, CP, LP | 2006–2009 | Serology, UA |
| Holm et al,29 2007b (Denmark) | Adults ≥18 y with CAP | 48/21 | 9 Inpatient, 39 outpatient | Mean 61 y, range 22 y–88 y | MP, CP, LP | 2002–2003 | PCR |
| Huijskens et al,30 2013 (Netherlands) | Adults ≥20 y with CAP | 408/263 | NR | Mean 65 y, range 20 y–94 y | MP, CP, LP | 2008–2009 | Serology, PCR, UA |
| Johansson et al,31 2010 (Sweden) | Adults ≥18 y with CAP | 184/124 | Inpatient | Mean 61.3 y, range 18 y–93 y | MP, CP, LP | 2004–2005 | Serology, PCR, UA |
| Lee et al,32 2002 (South Korea) | Adults ≥16 y with CAP | 81/15 | Inpatient | Mean 66.3 y, range 17 y–92 y | MP, CP, LP | 1999–2000 | Serology |
| Luchsinger et al,33 2013 (Chile) | Adults ≥18 y with CAP | 356/232 | 330 Inpatient, 26 outpatient | Mean 59.3 yc | MP, CP, LP | 2005–2007 | Serology, PCR, UA |
| Marrie et al,34 2005 (Canada) | Adults ≥18 y with CAP | 507/245 | Outpatient | Mean 47.8 y | MP, CP | 2003 | Serology |
| Miyashita et al,35 2005 (Japan) | Adults >16 y with CAP | 506/318 | 400 Inpatient, 106 outpatient | Mean 58.3 y, range 16 y–97 y | MP, CP, LP | 1998–2003 | Serology, UA |
| Molinos et al,36 2009 (Spain) | Patients with CAPd | 710/274 | Inpatient | Mean 67.1 y | MP, CP, LP | 2003–2004 | Serology, UA |
| Prat et al,37 2006 (Spain) | Patients with CAPd | 217/116 | Inpatient | Mean 56.6 y | LP | 2005–2005 | UA |
| Saito et al,38 2006 (Japan) | Adults ≥17 y with CAP | 232/170 | 200 Inpatient, 32 outpatient | Mean 60.2 y, range 17 y–99 y | MP, CP, LP | 1999–2000 | Serology, PCR, UA, Culture |
| Sangil et al,39 2012 (Spain) | Adults ≥18 y with CAP | 131/92 | Inpatient | Mean 64.4 y, range 48 y–80 | MP, CP, LP | 2009–2010 | Serology, PCR, UA |
| Shibli et al,40 2010 (Israel) | Adults ≥18 y with CAP | 126/84 | Inpatient | Mean 58.3, range 18 y–93 y | MP, CP, LP | 2006–2007 | Serology, PCR |
| Stralin et al,41 2010 (Sweden) | Adults ≥18 y with CAP | 235/133 | Inpatient | Median 71 y, range 18 y–96 y | MP, CP, LP | 1999–2002 | Serology, PCR, UA |
| Templeton et al,42 2005 (Netherlands) | Adults ≥18 y with CAP | 105/80 | 92 inpatient, 13 outpatient | NR | MP, CP, LP | 2000–2002 | PCR |
| van de Garde et al,43 2008 (Netherlands) | Patients with CAPd | 201/128 | Inpatient | Mean 63 y | MP, LP | 2004–2006 | PCR |
| von Baum et al,44 2008 (Germany [CAPNETZ]) | Adults ≥18 y with CAP | 2,503/877 | 1,727 Inpatient, 776 outpatient | Mean 61 y | LP | 2002–2005 | PCR, UA, Culture |
| von Baum et al,45 2009 (Germany [CAPNETZ]) | Adults ≥18 y with CAP | 4,532/928 | 2,922 Inpatient, 1,610 outpatient | Mean 60 y | MP | 2002–2005 | Serology, PCR |
| Wellinghausen et al,46 2006 (Germany [CAPNETZ]) | Adults ≥18 y with CAP | 546/NR | 364 Inpatient, 182 outpatient | Median 62 y; | CP | 2002–2004 | PCR |
| Andreo et al,47 2006 (Spain) | Adults ≥16 y with CAP | 107/39 | Inpatient | Mean 58.6 y, range 16 y–86 y | MP, CP, LP | 2000–2001 | Serology |
| Capelastegui et al,48 2012 (Spain) | Adults ≥18 y with CAP | 700/390 | 276 Inpatient, 424 outpatient | Mean 59.7 y | MP, CP, LP | 2006–2007 | Serology, UA |
| CAP in Children | |||||||
| Cantais et al,49 2014 (France) | Children age 1 mo to 16.5 y with CAP | 85/81 | Inpatient | Median 2.8 y, range 1 mo to 16.5 y | MP, CP | 2012–2013 | PCR |
| Cevey-Macherel et al,50 2009 (Switzerland) | Children 2 mo to 5 y with CAP | 99/85 | Inpatient | Mean 29 mo, range 2 mo to 5 y | MP, CP | 2003–2005 | Serology, PCR |
| Don et al,51 2005 (Italy) | Children 4 mo to 16 y with CAP | 101/66 | Inpatient | Mean 4.7 y, range 0.3 y–16 y | MP, CP | 2001–2002 | Serology |
| Hamano-Hasegawa et al,52 2008 (Japan) | Children <19 y with CAP | 1,700/1,316 | NR | Median 6.1 y for MP; Median 5.4 y for CP, Range 0 y–19 y | MP, CP, LP | 2005–2006 | PCR |
| Jain et al,53 2015a (United States) | Children <18 y with CAP | 2,222/1,802 | Inpatient | Median 2 y, range 0 y–17 y | MP, CP | 2010–2012 | PCR |
| Kurz et al,54 2013 (Austria) | Children 2 mo to 17 y with CAP | 279/190 | Inpatient | Median 36 mo, range 2 mo to 17 y | MP, CP | 2005–2008 | PCR |
| Laundy et al,55 2003 (England) | Children <5 y with CAP | 51/25 | 42 Inpatient, 9 outpatient | Median 1.3 y, range 2 wk to 4,8 y | MP, CP | 2001–2002 | PCR |
| Maltezou et al,56 2004e (Greece) | Children 6 mo to 14 y with CAP (n = 60), cough >3 weeks (n = 1) or infectious asthma exacerbation (n = 4) | 65/19 | Inpatient | Mean 6 y, range 10 mo to 13 y | MP, CP, LP | 2001 | Serology |
| Numazaki et al,57 2004b (Japan) | Children <15 y with CAP | 398/383 | 362 Inpatient, 36 outpatient | NR | MP, CP | 2000–2001 | Serology, PCR |
| Tsolia et al,58 2004 (Greece) | Children 5y–14 y with CAP | 75/58 | Inpatient | Median 86.5 mo, range 5 y–14 y | MP, CP | 2003 | Serology, PCR |
| Nonpneumonia LRTI | |||||||
| Graffelman et al,59 2008f (Netherlands) | Adults ≥18 y consulting GP with LRTI; 26 of 129 had CAP | 129/84 | Outpatient | Mean 50 y | MP | 1998–2001 | Serology, PCR, Culture |
| Numazaki et al,57 2004b (Japan) | Children <15 y with non-pneumonia LRTI | 523/470 | 436 Inpatient, 87 outpatient | NR | MP, CP | 2000–2001 | Serology, PCR |
| Holm et al,29 2007b (Denmark) | Adults ≥18 y with non-pneumonia LRTI | 316/124 | 10 Inpatient, 306 outpatient | Median 48 y, range 18 y–94 y | MP, CP, LP | 2002–2003 | PCR |
| Various | |||||||
| Defilippi et al,60 2008 (Italy) | Children with LRTI (acute bronchitis, wheezy bronchitis, pneumonia, or bronchiolitis) admitted to the hospital | 886/NR | Mean 6.2 y, range 1 mo to 13.5 y | MP | 2005–2006 | PCR |
AP = community-acquired pneumonia; CP = Chlamydia pneumoniae; LP = Legionella pneumophila; LRTI = lower respiratory tract infection; MP = Mycoplasma pneumoniae; NR = not reported; PCR = polymerase chain reaction; PSI = pneumonia severity index; UA = urine antigen testing.
Total = number of patients included in study. Confirmed = number of patients with a pathogen identified.
Study findings reported separately for patients with CAP and those with non-pneumonic LRTI.
Estimated from median using method of Hozo.61
Age not reported but presumably adult based on hospital and mean age.
Classified as study of CAP if at least 85% of patients in the series were diagnosed with CAP.
In this study, LRTI was defined as abnormal lung sounds plus 2 of 3 of: (1) fever; (2) dyspnea or cough; (3) tachypnea, malaise or confusion.
Patients With Community-Acquired Pneumonia
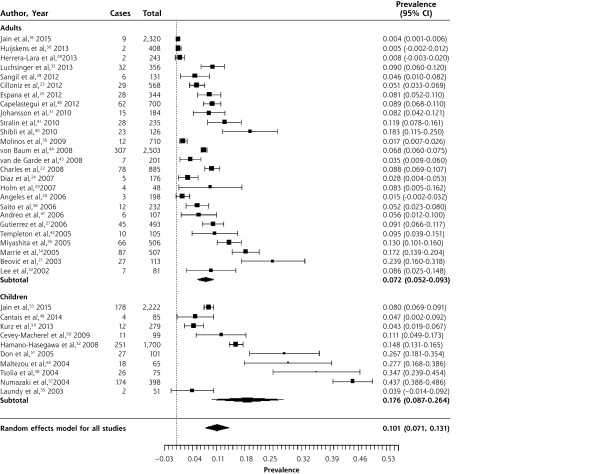
Figures 2–4 show the forest plots for M pneumoniae, C pneumoniae, and L pneumophila respectively in patients with CAP. The overall prevalence of M pneumoniae was 10.1% (95% CI, 7.1%–13.1%). The prevalence was higher in children (17.6%; 95% CI, 8.7%–26.4%) than in adults (7.2%; 95% CI, 5.2%–9.3%). There was significant heterogeneity, though, especially in studies of children. This is likely because outbreaks of M pneumoniae are thought to occur every 4 to 6 years, and inspection of the forest plot, which is sorted chronologically, does reveal peaks around 2004 and 2010.62,63
Figure 2.
Forest plot of the prevalence of Mycoplasma pneumoniae in adults and children with community-acquired pneumonia, sorted in reverse chronological order.
Heterogeneity (I2) = 99.27
Figure 4.
Forest plot of the prevalence of Legionella pneumophila in adults and children with community-acquired pneumonia, sorted by prevalence.
Heterogeneity (I2) = 91.18
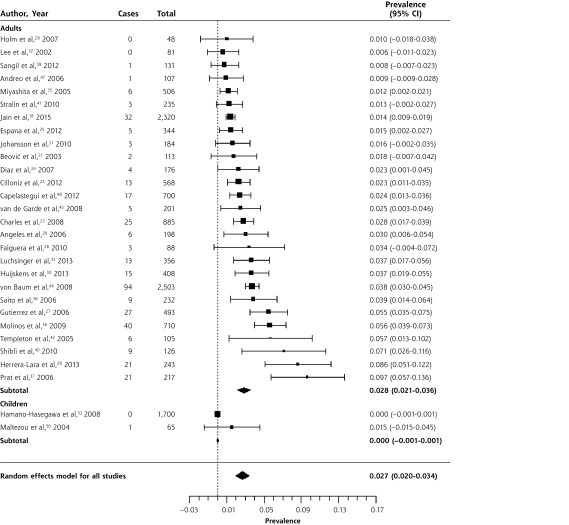
Figure 3.
Forest plot of the prevalence of Chlamydia pneumoniae in adults and children with community-acquired pneumonia, sorted by prevalence.
Heterogeneity (I2) = 98.4
The overall prevalence of C pneumoniae in patients with CAP was 3.5% (95% CI, 2.2%–4.9%). Infection with C pneumoniae was more common in adults (4.3%, 95% CI, 2.4%–6.2%) than in children (1.0%, 95% CI, 0.6%–1.5%). There was significant heterogeneity, although only 4 of 25 studies in adults had a prevalence greater than 10%, while the remainder had a prevalence between 0.3% and 7.7%. In children, only 2 of 10 studies had prevalences greater than 5%, while the remaining 8 had prevalences ranging from 0.5% to 2.7%. We reviewed the 6 identified outliers, but were unable to determine a reason for their high prevalence. There was also no clear pattern of variation by year of study.
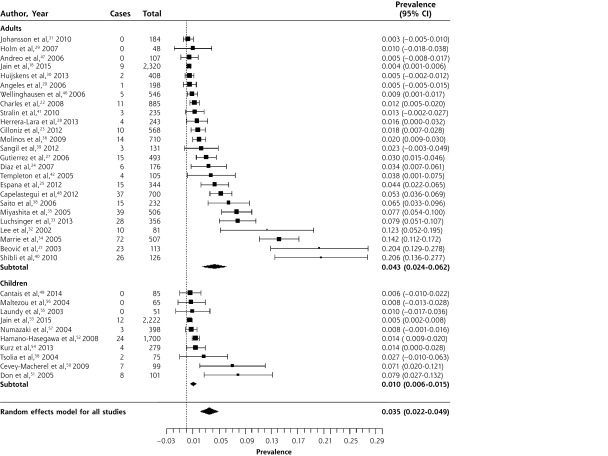
Legionella pneumophila was exceedingly rare in children, with only 1 case in 1,765 patients with CAP.52,56 The overall prevalence in adults was 2.8% (95% CI, 2.1%–3.6%), although in most studies it was between 1% and 3%. Again, there was significant heterogeneity. Of the studies reporting a prevalence of 5% or higher, 4 of 6 were in Spain,27,28,36,37 and a fifth, a study that also reported the highest prevalence of C pneumoniae, was set in another Mediterranean country, Israel.40 The largest series, set in Germany, found L pneumophila in 3.7% of patients treated in ambulatory care and 3.8% of inpatients.44 Clearly, it is not only found in severely ill patients.
Patients With Non-Pneumonia LRTI
Two studies reported the prevalence of atypical pathogens in patients with LRTI in whom pneumonia had been excluded by normal chest radiography,29,57 and a third enrolled predominantly patients with non-pneumonia LRTI.59 The prevalence of M pneumoniae was 7/316 (2.2%), 13/129 (10.0%), and 78/523 (14.9%) in these 3 studies,29,57,59 while the prevalence of C pneumoniae was 2/316 (0.6%) in 1 study29 and 3/523 (0.6%) in a second.57 A single study found no cases of L pneumophila in a primary care series of 316 adults with non-pneumonia LRTI.29 A fourth study did not provide adequate information to differentiate the number of children with acute bronchitis, pneumonia, or bronchiolitis.60
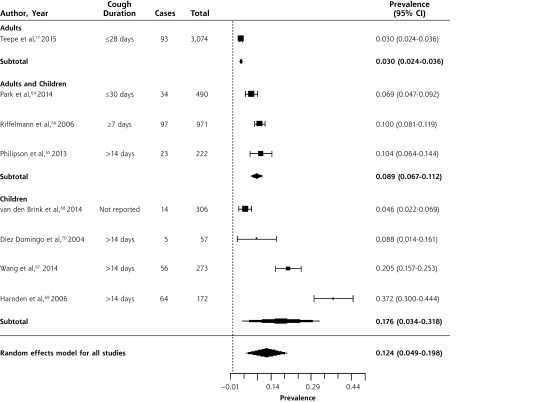
Prevalence of Bordetella pertussis in Outpatients
Table 3 summarizes data from 8 studies of the prevalence of B pertussis in outpatients with prolonged or bothersome cough, largely in primary care.17,64–70 Three studies enrolled adults and children; 4, children only; and 1, adults only. Data were collected between 2001 and 2012. One study assessed children referred from primary care due to suspicion for B pertussis, based on the duration of cough.68 The prevalence of B pertussis is summarized in the forest plot in Figure 5. While there was significant heterogeneity when including all studies, this was primarily due to heterogeneity in the 4 studies of children only.
Table 3.
Characteristics of Studies of the Prevalence of Bordetella pertussis in Outpatients With Prolonged Cough or Non-Pneumonia Lower Respiratory Tract Infection
| Author, Year | Population | Age | Year of Data Collection | Diagnostic Method |
|---|---|---|---|---|
| Adults and children | ||||
| Park et al,64 2014 (South Korea) | Adolescents and adults age 11 y and older presenting to GP with bothersome cough up to 30 days duration | Mean 44.3 y | 2011–2012 | PCR |
| Philipson et al,65 2013 (New Zealand) | Children and adults age 5 to 49 y with cough for 2 weeks or longer | Range 5–49 y | 2011 | Serology |
| Riffelmann et al,66 2006 (Germany) | Patients presenting to GP with at least 7 days cough | Not reported (all ages) | 2001–2004 | Serology or PCR |
| Children | ||||
| Wang et al,67 2014 (United Kingdom) | Children with cough of 2–8 weeks duration presenting to GP | Mean 9.6 y | 2010–2012 | Serology |
| van den Brink et al,68 2014 (Netherlands) | Children age 12 y and under with RTI referred for evaluation of suspected BP | <12 y | 2007–2009 | PCR |
| Harnden et al,69 2006 (England, United Kingdom) | Children 5–16 y presenting to their GP with cough for at least 2 weeks | Mean age 9.4 y, range 5–17 | 2001–2005 | Serology |
| Diez Domingo et al,70 2004 (Spain) | Children age 15 y and under presenting with cough for at least 2 weeks | Mean 6.2 y, range 0–15 y | 2001–2002 | Serology |
| Adults | ||||
| Teepe et al,17 2015 (12 European countries) | Adults with acute cough <28 days duration presenting to GP | Mean age 50 y | 2007–2010 | Serology or PCR |
BP = Bordetella pertussis; GP = general practitioner; PCR = polymerase chain reaction.
Figure 5.
Forest plot of the prevalence of Bordetella pertussis in outpatients with prolonged cough or non-pneumonia lower respiratory tract infection, sorted by prevalence.
Heterogeneity (I2) = 98.83
The overall prevalence was 12.4% (95% CI, 4.9%–19.8%). In a large, multi-country, European prospective study of adults presenting to primary care with cough of up to 28 days duration,17 prevalence was 3% (95% CI, 2.4%–3.6%). The prevalence was higher in studies of children (17.6%; 95% CI, 3.4%–31.8%) than in those of adults and children (8.9%; 95% CI, 6.7%–11.2%), but there was significant heterogeneity in the studies of children, with a range from 4.6% to 37.2%.67–70
Surveillance Data for Bordetella pertussis and Legionella pneumophila
Of the 26 countries to report data on B pertussis, Australia had the highest incidence rate of 105.0 cases per 100,000 persons per year. Hungary reported the lowest incidence rate of 0.05 cases per 100,000 persons per year. With 48,277 cases, the United States had the most reported cases of all countries, twice as many as the next country. Of the 30 countries reporting L pneumophila, the United States had the most cases at 3,688. Poland reported the lowest incidence of L pneumophila (0.02 per 100,000 persons per year) and Slovenia the highest (4.02 per 100,000 persons per year). It is likely that differences in surveillance systems and reporting account for much of this variability.
DISCUSSION
Among adults with CAP, 14% had an atypical pathogen: 7% had Mycoplasma pneumoniae, 4% had Chlamydophila pneumoniae, and 3% had Legionella pneumophila. Among children with CAP, 18% had Mycoplasma pneumoniae, only 1% had Chlamydophila pneumoniae, and Legionella pneumophila was extremely rare (1 case in 1,765 patients). Among patients with prolonged cough, 9% of adults and 18% of children had Bordetella pertussis.
Evidence for Underdiagnosis
CAP is diagnosed in an estimated 5.6 million patients annually in the United States, and 1.1 million hospitalizations result.71,72 Laboratory-based surveillance, however, identifies only 3,700 infections caused by L pneumophila each year, or 0.06% of all community-acquired pneumonias. Our systematic review found that when a consecutive series of patients with CAP are all tested for L pneumophila, it is detected in 3% of patients, with a range of 1% to 10%. This is consistent with the most recent US study,18 which found that 1.9% of episodes of CAP in a consecutive series of hospitalized adults were caused by L pneumophila. If 2% of all episodes of CAP are caused by L pneumophila, this would be 112,000 cases per year. Thus, the vast majority of cases of L pneumophila in the United States, approximately 100,000, may be undiagnosed. It is therefore important that physicians consider this pathogen when diagnosing CAP, and consider ordering urine antigen tests for L pneumophila more routinely, particularly when patients are non-responsive or slowly responsive to therapy with a beta-lactam. The recommended antibiotic for L pneumophila is a respiratory fluoroquinolone.73,74
Similarly, the annual incidence of acute bronchitis or non-pneumonia LRTI is approximately 440 episodes in 10,000 adults,75 and the annual incidence of B pertussis based on surveillance is 1.5 of 10,000 persons. Our systematic review found that 18% of episodes of non-pneumonia LRTI in children and 9% of those in adults were caused by B pertussis. Most of these studies limited inclusion to patients with a cough for at least 1 to 2 weeks, although 1 included adults and children with a shorter duration of cough and still found a prevalence of 7%.64 If one conservatively estimates based on these data that 3% of episodes of acute bronchitis or non-pneumonia LRTI are caused by B pertussis, that corresponds to 13 episodes per 10,000. Again, these data suggest that there is widespread underdiagnosis of B pertussis in the United States, with approximately 90% of episodes undiagnosed. This is important because family members and relatives are the source for 75% to 83% of pertussis cases in infants.76,77 Moreover, immunization with the pertussis vaccine wanes after five years.78–80 Current recommendations to vaccinate pregnant women with Tdap should be closely adhered to.
C pneumoniae infection has traditionally been described as being more common in children. We found that the mean prevalence, however, was 4% in studies of adults with CAP compared with 1% in children.
Diagnosis of these infections could be improved in several ways. One is to make better use of the history and physical examination. The best evidence regarding diagnosis of each pathogen is summarized in Table 4. Data regarding diagnosis are quite limited, and only in the case of L pneumophila has an attempt been made to develop and validate a clinical decision rule that combines several signs and symptoms.84 In general, individual signs and symptoms are of little value in the diagnosis of these atypical pathogens. Another approach would be to integrate signs and symptoms with a point-of-care test such as c-reactive protein (CRP), as has been done for pneumonia and influenza diagnosis.86,87 Greater use of urine antigen tests for L pneumophila should be encouraged for patients diagnosed with CAP, and the development of accurate, rapid point-of-care tests for C pneumoniae and B pertussis should be prioritized.
Table 4.
Accuracy of Signs and Symptoms for Respiratory Infections With Atypical Pathogens
| Symptom or Sign (number of studies) | Sensitivity (95%CI) | Specificity (95%CI) | Positive LR (95%CI) | Negative LR (95%CI) |
|---|---|---|---|---|
| Mycoplasma pneumoniaea | ||||
| Cough (5) | 0.89 (0.67–0.97) |
0.15 (0.05–0.37) |
1.04 (0.95–1.13) |
0.78 (0.44–1.39) |
| Wheeze (6) | 0.25 (0.17–0.36) |
0.67 (0.56–0.76) |
0.76 (0.60–0.97) |
1.12 (1.02–1.23) |
| Coryza (4) | 0.32 (0.08–0.72) |
0.66 (0.28–0.91) |
0.95 (0.71–1.26) |
1.03 (0.90–1.17) |
| Crepitations (5) | 0.84 (0.78–0.88) |
0.22 (0.14–0.32) |
1.06 (0.96–1.18) |
0.77 (0.52–1.12) |
| Fever (5) | 0.53–0.94 | 0.02–0.43 | ||
| Rhonchi (4) | 0.11–0.74 | 0.33–0.81 | ||
| Chest pain (2) | 0.08–0.19 | 0.93–0.97 | ||
| Diarrhea (2) | 0.14–0.21 | 0.79–0.85 | ||
| Chlamydophila pneumoniae | ||||
| Adultsb | ||||
| History of cough | 0.81 | |||
| History of sore throat | 0.52 | |||
| Abnormal breathing sounds | 0.38 | |||
| History of fever | 0.24 | |||
| Childrenc | ||||
| Rales | 0.85 | |||
| Fever | 0.80 | |||
| Cough | 0.50 | |||
| Rhinitis | 0.30 | |||
| Tachypnea | 0.25 | |||
| Wheezes | 0.20 | |||
| Rhonchi | 0.15 | |||
| Legionella pneumophilad | aOR (95% CI) | |||
| C-reactive protein >187 mg, L | 4.4 (2.0–9.6) | |||
| Sodium <133 mmo/L | 4.5 (2.2–9.0) | |||
| Temperature >39.4°C | 4.3 (1.9–9.8) | |||
| Platelet count <171 × 103/mL | 1.2 (0.6–2.5) | |||
| Lactate dehydrogenase >225 mmol/L | 1.7 (0.4–7.6) | |||
| Dry cough | 0.6 (0.3–1.4) | |||
| Bordetella pertussise | ||||
| Paroxysmal cough | 1.1 (1.1–1.2) | 0.52 (0.27–.0) | ||
| Posttussive emesis | 1.8 (1.4–2.2) | 0.58 (0.44–0.77) | ||
| Inspiratory whoop | 1.9 (1.4–2.6) | 0.78 (0.66–0.93) |
aOR = adjusted odds ratio from multivariate analysis; CAP = community-acquired pneumonia; LR = likelihood ratio.
Cochrane systematic review of 7 moderate quality studies with a total of 1,491 children, although each sign and symptom was only reported by a subset of studies. Pooled results from 4 to 6 studies are shown for cough, wheeze, coryza, and crepitations; for the other signs and symptoms, a range or the results of a single study are shown.81
Data from a study of 21 adult primary care patients diagnosed with Chlamydophila pneumoniae infection (7 primary infections and 14 with reinfection based on the antibody pattern).82
Data from a study of 20 children hospitalized for CAP and diagnosed with Chlamydophila pneumoniae.83
Data from 37 patients hospitalized with CAP due to Legionella pneumophila. A clinical rule that included 6 variables had an area under the receiver operating curve of 0.73.84
Systematic review of 3 studies with a total of 486 adults and children set in South Korea, United Kingdom, and United States.85
Limitations
As with any systematic review, our conclusions are limited by the quality of the published literature and the completeness and accuracy of reporting. We found considerable heterogeneity. For M pneumoniae this may be related to the cyclical nature of outbreaks, while for other pathogens the cause is less clear but may may lie in the differences in the populations studied, varying laboratory techniques, and varying sample collection methods across countries. It is noteworthy that the majority of studies found similar prevalences, with the heterogeneity for C pneumoniae and L pneumophila introduced by a small number of outliers, and for B pertussis limited to studies in children only. We limited our analysis to studies that gathered data within the past 15 years in highly developed economies, so our findings may not be generalizable to low- or middle-income countries. Many patients with acute cough do not seek care. It is possible that those seeking care have a different (and perhaps more severe) illness and a different prevalence of these pathogens. Finally, the literature regarding the prevalence of pathogens in patients with non-pneumonia lower respiratory tract infection is quite limited, with no studies in the United States or Canada.
We have demonstrated that atypical bacterial pathogens are relatively common causes of CAP in a range of populations including both adults and children, and that B pertussis is a common cause of prolonged cough. We do not feel that broader use of antibiotics for patients with acute cough is warranted. What is needed are studies to help clinicians more accurately diagnose these pathogens or to help them identify a large group of patients at low risk for such pathogens who do not require further testing or antibiotic therapy. Approaches that develop clinical decision rules integrating signs, symptoms, and point-of-care tests such as CRP are particularly promising.88 Finally, research is needed to determine if and when antibiotics are helpful, since data regarding treatment of B pertussis and M pneumoniae from well designed, adequately powered contemporary clinical trials are lacking.
Footnotes
Conflicts of interest: authors report none.
Supplementary materials: Available at http://www.AnnFamMed.org/content/14/6/552/suppl/DC1/
References
- 1.Hsiao CJ, Cherry D, Beatty PC, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2007 summary. Hyattsville, MD: National Center for Health Statistics; 2010. National Health Statistics Reports, No. 27. [PubMed] [Google Scholar]
- 2.van Vugt SF, Verheij TJ, de Jong PA, et al. ; GRACE Project Group. Diagnosing pneumonia in patients with acute cough: clinical judgment compared to chest radiography. Eur Respir J. 2013;42(4):1076–1082. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2014 Provisional Pertussis Surveillance Report. http://www.cdc.gov/pertussis/downloads/pertuss-surv-report-2014.pdf. Published Oct 2015.
- 4.Omori R, Nakata Y, Tessmer HL, Suzuki S, Shibayama K. The determinant of periodicity in Mycoplasma pneumoniae incidence: an insight from mathematical modelling. Sci Rep. 2015;5:14473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguipdop-Djomo P, Fine P, Halsby K, Chalker V, Vynnycky E. Cyclic epidemics of Mycoplasma pneumoniae infections in England and Wales from 1975 to 2009: time-series analysis and mathematical modelling. The Lancet. 2013;382(S78). [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC). Mycoplasma pneumoniae outbreak at a university - Georgia, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(30):603–606. [PMC free article] [PubMed] [Google Scholar]
- 7.Zhan P, Suo LJ, Qian Q, et al. Chlamydia pneumoniae infection and lung cancer risk: a meta-analysis. Eur J Cancer. 2011;47(5):742–747. [DOI] [PubMed] [Google Scholar]
- 8.Hahn DL, Schure A, Patel K, Childs T, Drizik E, Webley W. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7(4):e35945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston SL, Martin RJ. Chlamydophila pneumoniae and Mycoplasma pneumoniae: a role in asthma pathogenesis? Am J Respir Crit Care Med. 2005;172(9):1078–1089. [DOI] [PubMed] [Google Scholar]
- 10.Phin N, Parry-Ford F, Harrison T, et al. Epidemiology and clinical management of Legionnaires’ disease. Lancet Infect Dis. 2014;14(10):1011–1021. [DOI] [PubMed] [Google Scholar]
- 11.Fisman DN, Lim S, Wellenius GA, et al. It’s not the heat, it’s the humidity: wet weather increases legionellosis risk in the greater Philadelphia metropolitan area. J Infect Dis. 2005;192(12):2066–2073. [DOI] [PubMed] [Google Scholar]
- 12.Shaikh R, Guris D, Strebel PM, Wharton M. Underreporting of pertussis deaths in the United States: need for improved surveillance. Pediatrics. 1998;101(2):323. [DOI] [PubMed] [Google Scholar]
- 13.Organisation for Economic Co-operation and Development (OECD). List of high income OECD countries and high income Euro area countries. In: Country Classification 2011 – as of 26 July 2011. https://www.oecd.org/tad/xcred/48405330.pdf Accessed Oct 31, 2016.
- 14.The Cochrane Collaboration. Assessing risk of bias in included studies. In: Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. http://handbook.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm. Updated Mar 2011.
- 15.Whiting PF, Rutjes AW, Westwood ME, et al. ; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. [DOI] [PubMed] [Google Scholar]
- 16.Shamliyan T, Kane RL, Dickinson S. A systematic review of tools used to assess the quality of observational studies that examine incidence or prevalence and risk factors for diseases. J Clin Epidemiol. 2010;63(10):1061–1070. [DOI] [PubMed] [Google Scholar]
- 17.Teepe J, Broekhuizen BD, Ieven M, et al. ; GRACE consortium. Prevalence, diagnosis, and disease course of pertussis in adults with acute cough: a prospective, observational study in primary care. Br J Gen Pract. 2015;65(639):e662–e667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain S, Self WH, Wunderink RG, et al. ; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gapminder. Population, total. In: Data in Gapminder World. http://www.gapminder.org/data Accessed June 2015.
- 20.Angeles Marcos M, Camps M, Pumarola T, et al. The role of viruses in the aetiology of community-acquired pneumonia in adults. Antivir Ther. 2006;11(3):351–359. [PubMed] [Google Scholar]
- 21.Beović B, Bonac B, Kese D, et al. Aetiology and clinical presentation of mild community-acquired bacterial pneumonia. Eur J Clin Microbiol Infect Dis. 2003;22(10):584–591. [DOI] [PubMed] [Google Scholar]
- 22.Charles PG, Whitby M, Fuller AJ, et al. ; Australian CAP Study Collaboration. The etiology of community-acquired pneumonia in Australia: why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin Infect Dis. 2008;46(10):1513–1521. [DOI] [PubMed] [Google Scholar]
- 23.Cillóniz C, Ewig S, Polverino E, et al. Community-acquired pneumonia in outpatients: aetiology and outcomes. Eur Respir J. 2012;40(4):931–938. [DOI] [PubMed] [Google Scholar]
- 24.Díaz A, Barria P, Niederman M, et al. Etiology of community-acquired pneumonia in hospitalized patients in chile: the increasing prevalence of respiratory viruses among classic pathogens. Chest. 2007;131(3):779–787. [DOI] [PubMed] [Google Scholar]
- 25.España PP, Capelastegui A, Bilbao A, et al. ; Population Study of Pneumonia (PSoP) Group. Utility of two biomarkers for directing care among patients with non-severe community-acquired pneumonia. Eur J Clin Microbiol Infect Dis. 2012;31(12):3397–3405. [DOI] [PubMed] [Google Scholar]
- 26.Falguera M, Ruiz-González A, Schoenenberger JA, et al. Prospective, randomised study to compare empirical treatment versus targeted treatment on the basis of the urine antigen results in hospitalised patients with community-acquired pneumonia. Thorax. 2010;65(2):101–106. [DOI] [PubMed] [Google Scholar]
- 27.Gutiérrez F, Masiá M, Mirete C, et al. The influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogens. J Infect. 2006;53(3):166–174. [DOI] [PubMed] [Google Scholar]
- 28.Herrera-Lara S, Fernández-Fabrellas E, Cervera-Juan Á, Blanquer-Olivas R. Do seasonal changes and climate influence the etiology of community acquired pneumonia? Arch Bronconeumol. 2013;49(4):140–145. [DOI] [PubMed] [Google Scholar]
- 29.Holm A, Nexoe J, Bistrup LA, et al. Aetiology and prediction of pneumonia in lower respiratory tract infection in primary care. Br J Gen Pract. 2007;57(540):547–554. [PMC free article] [PubMed] [Google Scholar]
- 30.Huijskens EG, van Erkel AJ, Palmen FM, Buiting AG, Kluytmans JA, Rossen JW. Viral and bacterial aetiology of community-acquired pneumonia in adults. Influenza Other Respir Viruses. 2013;7(4):567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson N, Kalin M, Tiveljung-Lindell A, Giske CG, Hedlund J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis. 2010;50(2):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SJ, Lee MG, Jeon MJ, Jung KS, Lee HK, Kishimoto T. Atypical pathogens in adult patients admitted with community-acquired pneumonia in Korea. Jpn J Infect Dis. 2002;55(5):157–159. [PubMed] [Google Scholar]
- 33.Luchsinger V, Ruiz M, Zunino E, et al. Community-acquired pneumonia in Chile: the clinical relevance in the detection of viruses and atypical bacteria. Thorax. 2013;68(11):1000–1006. [DOI] [PubMed] [Google Scholar]
- 34.Marrie TJ, Poulin-Costello M, Beecroft MD, Herman-Gnjidic Z. Etiology of community-acquired pneumonia treated in an ambulatory setting. Respir Med. 2005;99(1):60–65. [DOI] [PubMed] [Google Scholar]
- 35.Miyashita N, Fukano H, Mouri K, et al. Community-acquired pneumonia in Japan: a prospective ambulatory and hospitalized patient study. J Med Microbiol. 2005;54(Pt 4):395–400. [DOI] [PubMed] [Google Scholar]
- 36.Molinos L, Clemente MG, Miranda B, et al. ; ASTURPAR Group. Community-acquired pneumonia in patients with and without chronic obstructive pulmonary disease. J Infect. 2009;58(6):417–424. [DOI] [PubMed] [Google Scholar]
- 37.Prat C, Domínguez J, Andreo F, et al. Procalcitonin and neopterin correlation with aetiology and severity of pneumonia. J Infect. 2006;52(3):169–177. [DOI] [PubMed] [Google Scholar]
- 38.Saito A, Kohno S, Matsushima T, et al. ; Study Group. Prospective multicenter study of the causative organisms of community-acquired pneumonia in adults in Japan. J Infect Chemother. 2006;12(2):63–69. [DOI] [PubMed] [Google Scholar]
- 39.Sangil A, Calbo E, Robles A, et al. Aetiology of community-acquired pneumonia among adults in an H1N1 pandemic year: the role of respiratory viruses. Eur J Clin Microbiol Infect Dis. 2012;31(10):2765–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibli F, Chazan B, Nitzan O, et al. Etiology of community-acquired pneumonia in hospitalized patients in northern Israel. Isr Med Assoc J. 2010;12(8):477–482. [PubMed] [Google Scholar]
- 41.Strålin K, Olcén P, Törnqvist E, Holmberg H. Definite, probable, and possible bacterial aetiologies of community-acquired pneumonia at different CRB-65 scores. Scand J Infect Dis. 2010;42(6–7):426–434. [DOI] [PubMed] [Google Scholar]
- 42.Templeton KE, Scheltinga SA, van den Eeden WC, Graffelman AW, van den Broek PJ, Claas EC. Improved diagnosis of the etiology of community-acquired pneumonia with real-time polymerase chain reaction. Clin Infect Dis. 2005;41(3):345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van de Garde EM, Endeman H, van Hemert RN, et al. Prior outpatient antibiotic use as predictor for microbial aetiology of community-acquired pneumonia: hospital-based study. Eur J Clin Pharmacol. 2008;64(4):405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Baum H, Ewig S, Marre R, et al. ; Competence Network for Community Acquired Pneumonia Study Group. Community-acquired Legionella pneumonia: new insights from the German competence network for community acquired pneumonia. Clin Infect Dis. 2008;46(9):1356–1364. [DOI] [PubMed] [Google Scholar]
- 45.von Baum H, Welte T, Marre R, Suttorp N, Lück C, Ewig S. Mycoplasma pneumoniae pneumonia revisited within the German Competence Network for Community-acquired pneumonia (CAPNETZ). BMC Infect Dis. 2009;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wellinghausen N, Straube E, Freidank H, von Baum H, Marre R, Essig A. Low prevalence of Chlamydia pneumoniae in adults with community-acquired pneumonia. Int J Med Microbiol. 2006;296(7):485–491. [DOI] [PubMed] [Google Scholar]
- 47.Andreo F, Domínguez J, Ruiz J, et al. Impact of rapid urine antigen tests to determine the etiology of community-acquired pneumonia in adults. Respir Med. 2006;100(5):884–891. [DOI] [PubMed] [Google Scholar]
- 48.Capelastegui A, España PP, Bilbao A, et al. ; Poblational Study of Pneumonia (PSoP) Group. Etiology of community-acquired pneumonia in a population-based study: link between etiology and patients characteristics, process-of-care, clinical evolution and outcomes. BMC Infect Dis. 2012;12:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cantais A, Mory O, Pillet S, et al. Epidemiology and microbiological investigations of community-acquired pneumonia in children admitted at the emergency department of a university hospital. J Clin Virol. 2014;60(4):402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cevey-Macherel M, Galetto-Lacour A, Gervaix A, et al. Etiology of community-acquired pneumonia in hospitalized children based on WHO clinical guidelines. Eur J Pediatr. 2009;168(12):1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Don M, Fasoli L, Paldanius M, et al. Aetiology of community-acquired pneumonia: serological results of a paediatric survey. Scand J Infect Dis. 2005;37(11–12):806–812. [DOI] [PubMed] [Google Scholar]
- 52.Hamano-Hasegawa K, Morozumi M, Nakayama E, et al. ; Acute Respiratory Diseases Study Group. Comprehensive detection of causative pathogens using real-time PCR to diagnose pediatric community-acquired pneumonia. J Infect Chemother. 2008;14(6):424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain S, Williams DJ, Arnold SR, et al. ; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurz H, Göpfrich H, Huber K, et al. Spectrum of pathogens of inpatient children and youths with community acquired pneumonia: a 3 year survey of a community hospital in Vienna, Austria. Wien Klin Wochenschr. 2013;125(21–22):674–679. [DOI] [PubMed] [Google Scholar]
- 55.Laundy M, Ajayi-Obe E, Hawrami K, Aitken C, Breuer J, Booy R. Influenza A community-acquired pneumonia in East London infants and young children. Pediatr Infect Dis J. 2003;22(10)(Suppl):S223–S227. [DOI] [PubMed] [Google Scholar]
- 56.Maltezou HC, La-Scola B, Astra H, et al. Mycoplasma pneumoniae and Legionella pneumophila in community-acquired lower respiratory tract infections among hospitalized children: diagnosis by real time PCR. Scand J Infect Dis. 2004;36(9):639–642. [DOI] [PubMed] [Google Scholar]
- 57.Numazaki K, Chiba S, Umetsu M, et al. Etiological agents of lower respiratory tract infections in Japanese children. In Vivo. 2004;18(1):67–71. [PubMed] [Google Scholar]
- 58.Tsolia MN, Psarras S, Bossios A, et al. Etiology of community-acquired pneumonia in hospitalized school-age children: evidence for high prevalence of viral infections. Clin Infect Dis. 2004;39(5):681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Graffelman AW, Willemssen FE, Zonderland HM, Neven AK, Kroes AC, van den Broek PJ. Limited value of chest radiography in predicting aetiology of lower respiratory tract infection in general practice. Br J Gen Pract. 2008;58(547):93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Defilippi A, Silvestri M, Tacchella A, et al. Epidemiology and clinical features of Mycoplasma pneumoniae infection in children. Respir Med. 2008;102(12):1762–1768. [DOI] [PubMed] [Google Scholar]
- 61.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noah ND. Epidemiology of Mycoplasma pneumoniae infection in the United Kingdom: an analysis of reports to the Public Health Laboratory Service of England and Wales. Infection. 1976;4(1)(Suppl):25–28. [DOI] [PubMed] [Google Scholar]
- 63.Lind K, Benzon MW, Jensen JS, Clyde WA., Jr A seroepidemiological study of Mycoplasma pneumoniae infections in Denmark over the 50-year period 1946–1995. Eur J Epidemiol. 1997;13(5):581–586. [DOI] [PubMed] [Google Scholar]
- 64.Park S, Lee SH, Seo KH, et al. Epidemiological aspects of pertussis among adults and adolescents in a Korean outpatient setting: a multicenter, PCR-based study. J Korean Med Sci. 2014;29(9):1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Philipson K, Goodyear-Smith F, Grant CC, Chong A, Turner N, Stewart J. When is acute persistent cough in school-age children and adults whooping cough? A prospective case series study. Br J Gen Pract. 2013;63(613):e573–e579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riffelmann M, Littmann M, Hülsse C, O’Brien J, Wirsing von König CH. [Pertussis: incidence, symptoms and costs]. Dtsch Med Wochenschr. 2006;131(50):2829–2834. [DOI] [PubMed] [Google Scholar]
- 67.Wang K, Fry NK, Campbell H, et al. Whooping cough in school age children presenting with persistent cough in UK primary care after introduction of the preschool pertussis booster vaccination: prospective cohort study. BMJ. 2014;348:g3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van den Brink G, Wishaupt JO, Douma JC, Hartwig NG, Versteegh FG. Bordetella pertussis: an underreported pathogen in pediatric respiratory infections, a prospective cohort study. BMC Infect Dis. 2014;14:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harnden A, Grant C, Harrison T, et al. Whooping cough in school age children with persistent cough: prospective cohort study in primary care. BMJ. 2006;333(7560):174–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diez-Domingo J, Ballester A, Baldó JM, et al. Incidence of pertussis in persons < or =15 years of age in Valencia, Spain: seroprevalence of antibodies to pertussis toxin (PT) in children, adolescents and adults. J Infect. 2004;49(3):242–247. [DOI] [PubMed] [Google Scholar]
- 71.Niederman MS, McCombs JS, Unger AN, Kumar A, Popovian R. The cost of treating community-acquired pneumonia. Clin Ther. 1998;20(4):820–837. [DOI] [PubMed] [Google Scholar]
- 72.Centers for Disease Control and Prevention, National Center for Health Statistics. Pneumonia. http://www.cdc.gov/nchs/fastats/pneumonia.htm. Updated Jul 6, 2016.
- 73.Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dunbar LM, Khashab MM, Kahn JB, Zadeikis N, Xiang JX, Tennenberg AM. Efficacy of 750-mg, 5-day levofloxacin in the treatment of community-acquired pneumonia caused by atypical pathogens. Curr Med Res Opin. 2004;20(4):555–563. [DOI] [PubMed] [Google Scholar]
- 75.Macfarlane J, Holmes W, Gard P, et al. Prospective study of the incidence, aetiology and outcome of adult lower respiratory tract illness in the community. Thorax. 2001;56(2):109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bisgard KM, Pascual FB, Ehresmann KR, et al. Infant pertussis: who was the source? Pediatr Infect Dis J. 2004;23(11):985–989. [DOI] [PubMed] [Google Scholar]
- 77.Wendelboe AM, Njamkepo E, Bourillon A, et al. ; Infant Pertussis Study Group. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J. 2007;26(4):293–299. [DOI] [PubMed] [Google Scholar]
- 78.Klein NP, Bartlett J, Fireman B, Rowhani-Rahbar A, Baxter R, et al. , Comparative effectiveness of acellular versus whole-cell pertussis vaccines in teenagers. Pediatrics. 2013;131(6):e1716–22. [DOI] [PubMed] [Google Scholar]
- 79.Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012;367(11):1012–1019. [DOI] [PubMed] [Google Scholar]
- 80.Koepke R, Eickhoff JC, Ayele RA, et al. Estimating the effectiveness of tetanus-diphtheria-acellular pertussis vaccine (Tdap) for preventing pertussis: evidence of rapidly waning immunity and difference in effectiveness by Tdap brand. J Infect Dis. 2014;210(6):942–953. [DOI] [PubMed] [Google Scholar]
- 81.Wang K, Gill P, Perera R, Thomson A, Mant D, Harnden A. Clinical symptoms and signs for the diagnosis of Mycoplasma pneumoniae in children and adolescents with community-acquired pneumonia. Cochrane Database Syst Rev. 2012;10:CD009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thom DH, Grayston JT, Campbell LA, Kuo CC, Diwan VK, Wang SP. Respiratory infection with Chlamydia pneumoniae in middle-aged and older adult outpatients. Eur J Clin Microbiol Infect Dis. 1994;13(10):785–792. [DOI] [PubMed] [Google Scholar]
- 83.Principi N, Esposito S. Emerging role of Mycoplasma pneumoniae and Chlamydia pneumoniae in paediatric respiratory-tract infections. Lancet Infect Dis. 2001;1(5):334–344. [DOI] [PubMed] [Google Scholar]
- 84.Haubitz S, Hitz F, Graedel L, Batschwaroff M, Wiemken T4, Peyrani P, et al. Ruling out Legionella in community-acquired pneumonia. Am J Med. 2014;127(10):1010e11–9. [DOI] [PubMed] [Google Scholar]
- 85.Cornia PB, Hersh AL, Lipsky BA, Newman TB, Gonzales R. Does this coughing adolescent or adult patient have pertussis? JAMA. 2010;304(8):890–896. [DOI] [PubMed] [Google Scholar]
- 86.van Vugt SF, Broekhuizen BD, Lammens C, et al. ; GRACE consortium. Use of serum C reactive protein and procalcitonin concentrations in addition to symptoms and signs to predict pneumonia in patients presenting to primary care with acute cough: diagnostic study. BMJ. 2013;346:f2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haran JP, Beaudoin FL, Suner S, Lu S. C-reactive protein as predictor of bacterial infection among patients with an influenza-like illness. Am J Emerg Med. 2013;31(1):137–144. [DOI] [PubMed] [Google Scholar]
- 88.van Vugt SF, Broekhuizen BD, Zuithoff NP, et al. ; GRACE Consortium. Validity of a clinical model to predict influenza in patients presenting with symptoms of lower respiratory tract infection in primary care. Fam Pract. 2015;32(4):408–414. [DOI] [PubMed] [Google Scholar]