Abstract
The licensure of new pneumococcal conjugate vaccines (PCVs) relies on immunogenicity data. When defining correlates of protection, vaccine efficacy data must be included. In the FinOM Vaccine Efficacy Trial, the PncOMPC vaccine showed an efficacy profile similar to that of the licensed PncCRM vaccine despite different antibody responses after primary and booster vaccinations. We determined antibody kinetics and avidities in a subgroup of infants participating in the FinOM trial. A total of 166 infants in three vaccine groups were immunized at 2, 4, 6, and 12 months of age with 7-valent PCV, PncCRM or PncOMPC, or hepatitis B vaccine. Concentrations of serum immunoglobulin G (IgG) against pneumococcal capsular polysaccharides were determined at 2, 4, 6, 7, 12, 13, and 24 months of age, and the avidity index (AI) to serotypes 6B, 19F, and 23F were determined at 7, 12, 13, and 24 months of age by enzyme immunoassay. Both PCVs were highly immunogenic, but they demonstrated different kinetics of antibody response; the concentration of IgG against serotypes 6B, 19F, and 23F declined faster after the third and fourth doses of vaccine in the PncCRM group than in the PncOMPC group. For both PCVs, the mean AI of anti-6B and -23F, but not of anti-19F, increased during the follow-up, which is in line with serotype-specific protection in the FinOM trial. Our data suggest that the kinetics and avidities of antibodies should be considered, in addition to antibody responses, when defining correlates of protection.
Several pneumococcal conjugate vaccines (PCVs) have been tested in phase II clinical trials (1, 2, 14, 31, 40, 42, 49, 54, 59), and two 7- or 9-valent vaccines have been tested in phase III trials (6, 17, 32, 33, 44). Efficacy trials with 9- to 11-valent vaccines are ongoing (11, 26). One of the vaccines, PncCRM, has been licensed in many industrialized countries based on its efficacy in the United States against invasive disease caused by the seven vaccine serotypes (6). It has proven effective against invasive disease in infants in high-risk populations (33, 44). PncCRM also has had an important impact on the incidence of pneumonia (7, 33). Two of the PCVs, PncCRM and PncOMPC, were tested in the Finnish Otitis Media (FinOM) Vaccine Efficacy Trial. The aggregate efficacies of the vaccines against pneumococcal acute otitis media (AOM) were 34% (95% confidence interval [CI], 21 to 45%) for PncCRM and 25% (95% CI, 11 to 37%) for PncOMPC, and the efficacies against vaccine-type AOM were 57% (95% CI, 44 to 67%) for PncCRM and 56% (95% CI, 44 to 66%) for PncOMPC (17, 32).
In the future, the licensure of new PCVs or PCV formulations will rely on immunogenicity data, since new placebo-controlled efficacy trials may not be possible to accomplish after the licensure of PncCRM. When new PCVs are compared to the licensed vaccine, the evaluation of their immunogenicities typically relies on the measurement of concentrations of serum antibody to the capsular polysaccharides 1 month after the third dose of vaccine (27). However, the existing antibody concentration in a child primed for memory responses may not be the only correlate of a protective immunity. Studies of different Haemophilus influenzae type b (Hib) conjugate vaccines suggest that differences in the kinetics of immune responses might be relevant to vaccine efficacy (10, 16, 19, 21). In addition, markers associated with the development of B-cell memory should be considered. Demonstration of B-cell memory has largely been based on the kinetics of antibody development and a rapid and strong antibody response to a dose of plain polysaccharide vaccine after priming with a conjugate vaccine (2, 14, 22, 29, 43, 45). It has also been suggested that B-cell memory can be demonstrated by showing avidity maturation of the antibodies (3, 20, 28, 36, 50).
Antibody avidity can also be considered as a measure of the protective functional activity of antibodies (52, 57). The host defense against Streptococcus pneumoniae depends largely on the binding of antibodies and complement to the surfaces of pneumococci, leading to phagocytosis of the bacteria (8, 9). Therefore, the efficiency of binding is crucial for the elimination of the bacteria.
We report here in detail the immunogenicities of the two PCVs, PncCRM and PncOMPC, used in parallel in the FinOM Vaccine Efficacy Trial. Immunogenicity and the ability to induce B-cell memory were evaluated by two parameters, the kinetics of antibody response and the development of antibody avidity. Antibody responses were evaluated for all vaccine serotypes, and the development of antibody avidity was evaluated for the most frequent pneumococcal causes of AOM in the FinOM trial: serotypes 6B, 19F, and 23F (17, 32). The persistence of antibodies and their quality were followed until the age of 24 months, and the quality of PCV-induced human antibodies was for the first time compared to that of natural antibodies.
MATERIALS AND METHODS
Study vaccines.
The PncCRM vaccine (Wyeth Lederle Vaccines, Pearl River, N.Y.) consisted of 2 μg of capsular polysaccharides (PSs) 4, 9V, 14, 19F, and 23F; 4 μg of PS 6B; and 2 μg of serotype 18C oligosaccharide, each individually conjugated to the CRM197 protein. The PncOMPC vaccine (Merck & Co., Inc., West Point, Pa.) contained 1 μg of pneumococcal capsular PSs 4 and 14, 1.5 μg of PS 9V, 2 μg of PSs 18C and 19F, 3 μg of PS 23F, and 5 μg of PS 6B, each individually conjugated to the outer membrane protein complex (OMPC) of Neisseria meningitidis serogroup B. The hepatitis B vaccine (Recombivax HB; Merck & Co., Inc.), used as a control vaccine for both study arms, contained 5 μg of recombinant hepatitis B virus surface protein.
Vaccinees, vaccinations, and sampling.
The subjects of this study formed a subcohort of 166 infants participating in the FinOM Vaccine Efficacy Trial (17, 32). Fifty-six infants were vaccinated with PncCRM and 46 were vaccinated with PncOMPC at 2, 4, 6, and 12 months of age. Six infants in the cohort of this study received three doses of PncOMPC followed by a dose of a 23-valent pneumococcal polysaccharide vaccine (Pneumovax23; Merck & Co., Inc.) at 12 months of age. The results for this group after the fourth dose of vaccine have been reported elsewhere (32). The control group of 58 infants received hepatitis B vaccine at the same ages as the PCVs were administered. A diphtheria-tetanus-whole-cell pertussis (DTwP) vaccine combined with a Hib conjugate vaccine (Tetramune; Wyeth Lederle Vaccines) was administered concomitantly with the first three doses of the study vaccines and at 24 months of age. Inactivated poliovirus vaccine (Imovax; Aventis Pasteur) was given at 7 months of age and concomitantly with the fourth dose of the study vaccines at 12 months of age. Measles-mumps-rubella vaccine (MMR II; Merck & Co., Inc.) was administered at 18 months of age. All vaccines were administered intramuscularly. Blood samples were obtained at the ages of 2, 4, 6, 7, 12, 13, and 24 months. Sera were separated by centrifugation and stored at −20°C.
Serological determinations.
All serological determinations were performed blinded at the Finnish National Public Health Institute. The laboratory personnel were not aware of the vaccination status or the age of the child at the time of sampling.
The concentrations of immunoglobulin G (IgG) antibodies to pneumococcal capsular PSs 4, 6B, 9V, 14, 18C, 19F, and 23F were measured from sera taken at 2, 4, 6, 7, 12, 13, and 24 months of age by enzyme immunoassay according to the method described by Koskela (34) with minor modifications (31). The results are expressed as micrograms per milliliter and were calculated on the basis of the officially assigned IgG values of the 89-SF reference serum (48). The detection limits of the assay were 0.06, 0.09, 0.08, 0.19, 0.07, 0.16, and 0.08 μg/ml for serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, respectively. Two control sera were included in each plate to follow the assay-to-assay variation; the coefficient of variation remained <15%.
The relative avidity of IgG antibodies to pneumococcal capsular PSs 6B, 19F, and 23F was determined from sera taken at 7, 12, 13, and 24 months of age in the PCV groups and at 24 months of age in the control group by the enzyme immunoassay method described by Anttila et al. (3). The assay was based on dissociation of antibody-antigen complexes by sodium thiocyanate (NaSCN). Serotype-specific concentrations of NaSCN (0.5 M for antibodies to 6B and 23F and 0.65 M for antibodies to 19F) were used to produce the widest range in avidity indices (AIs). The results are expressed as AI and assigned as percentage of antibodies that remained bound to the antigens after thiocyanate treatment. The AI could be reliably determined only for sera having at least 0.2 μg of specific IgG antibody/ml. Therefore, the number of samples available for avidity measurements varied according to the concentrations of IgG antibodies detected at different time points. For the same reason, the AIs in the control group were determined only for the sera taken at 24 months of age when a sufficient number of samples contained ≥0.2 μg of antibodies/ml. The reproducibility of the assay was followed by including two control sera in each plate. The coefficient of variation for interassay variation for both control sera remained <15%.
Statistical methods.
This is a descriptive study of the antibody responses to two PCVs. Antibody concentrations are presented as geometric mean concentrations (GMCs), and the relative avidities are presented as mean AIs (MAIs). The proportions of children with antibody concentrations of ≥0.35 μg/ml were calculated. This threshold of antibody concentration has recently been recommended by a group of experts in consultations that the World Health Organization undertook to be used as a surrogate for vaccine efficacy against invasive disease to establish the noninferiority of a new vaccine to a licensed vaccine (http://www.who.int/biologicals/Guidelines/vaccines.html. It was stressed that this threshold should not be used to evaluate PCVs against other clinical endpoints, e.g., AOM. We have, however, used this threshold, since no threshold has been suggested for AOM. Reverse cumulative distribution curves are used to display the distribution of antibody concentrations at different time points.
RESULTS
Kinetics of the IgG antibody response.
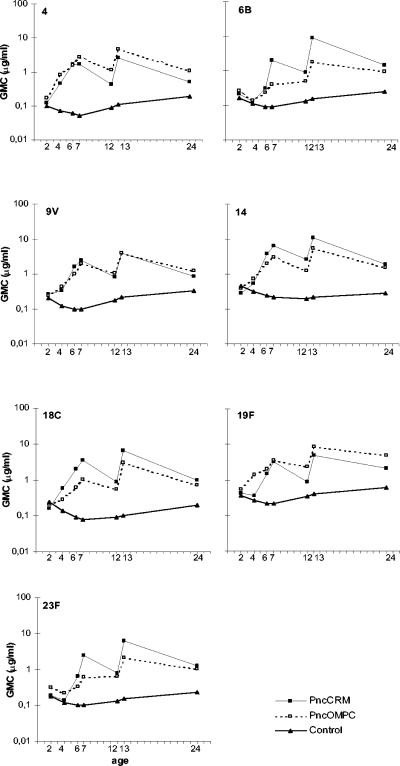
The GMCs of antibodies to the seven serotypes attained by the recipients of the study vaccines are shown in Table 1 and Fig. 1. The PncCRM, PncOMPC, and control vaccine groups had similar antibody concentrations at 2 months of age. In the control group, the mean antibody concentrations decreased for all serotypes during the first months of life and started to increase slightly thereafter.
TABLE 1.
GMCs of serum IgG antibodies to pneumococcal capsular PSs in infants immunized with a 7-valent pneumococcal conjugate vaccine or control vaccine at 2, 4, and 6 months of age and boosted with a homologous vaccine at 12 months of age
| Serotype | Vaccine | GMC [μg/ml (95% CI)] in infants at age (mo):
|
||||
|---|---|---|---|---|---|---|
| 2 (n = 52-58) | 7 (n = 53-55) | 12 (n = 52-56) | 13 (n = 46-55) | 24 (n = 44-54) | ||
| 4 | PncCRM | 0.12 (0.09-0.17) | 1.71 (1.32-2.20) | 0.42 (0.34-0.53) | 2.55 (1.99-3.27) | 0.50 (0.40-0.62) |
| PncOMPC | 0.17 (0.13-0.24) | 2.74 (1.92-3.91) | 1.12 (0.85-1.48) | 4.63 (3.09-6.95) | 1.11 (0.85-1.45) | |
| Control | 0.10 (0.08-0.14) | 0.05 (0.04-0.07) | 0.09 (0.07-0.12) | 0.11 (0.08-0.14) | 0.19 (0.15-0.25) | |
| 6B | PncCRM | 0.21 (0.15-0.30) | 1.99 (1.35-2.95) | 0.92 (0.64-1.32) | 9.02 (6.48-12.54) | 1.48 (1.11-1.99) |
| PncOMPC | 0.26 (0.18-0.36) | 0.40 (0.29-0.54) | 0.50 (0.37-0.67) | 1.76 (1.15-2.67) | 0.95 (0.65-1.39) | |
| Control | 0.16 (0.11-0.22) | 0.09 (0.07-0.12) | 0.13 (0.10-0.18) | 0.15 (0.12-0.20) | 0.25 (0.19-0.32) | |
| 9V | PncCRM | 0.26 (0.19-0.35) | 2.47 (1.97-3.11) | 0.81 (0.66-1.00) | 3.97 (3.20-4.91) | 0.87 (0.67-1.12) |
| PncOMPC | 0.24 (0.18-0.33) | 1.98 (1.55-2.54) | 1.03 (0.8-1.33) | 4.05 (3.14-5.22) | 1.21 (0.87-1.69) | |
| Control | 0.20 (0.15-0.27) | 0.10 (0.07-0.13) | 0.17 (0.13-0.23) | 0.21 (0.16-0.27) | 0.33 (0.25-0.45) | |
| 14 | PncCRM | 0.28 (0.19-0.41) | 6.26 (4.77-8.20) | 2.63 (2.12-3.26) | 10.80 (8.29-14.06) | 1.86 (1.47-2.36) |
| PncOMPC | 0.38 (0.26-0.56) | 2.96 (2.05-4.25) | 1.25 (0.93-1.68) | 5.31 (3.68-7.66) | 1.50 (1.06-2.13) | |
| Control | 0.46 (0.31-0.68) | 0.21 (0.16-0.27) | 0.19 (0.15-0.24) | 0.21 (0.17-0.27) | 0.28 (0.21-0.37) | |
| 18C | PncCRM | 0.16 (0.11-0.23) | 3.55 (2.80-4.49) | 0.88 (0.73-1.07) | 6.51 (5.04-8.42) | 0.99 (0.77-1.28) |
| PncOMPC | 0.21 (0.15-0.30) | 1.03 (0.76-1.40) | 0.56 (0.44-0.72) | 3.06 (2.27-4.12) | 0.70 (0.51-0.97) | |
| Control | 0.24 (0.16-0.34) | 0.08 (0.06-0.11) | 0.09 (0.07-0.11) | 0.10 (0.08-0.13) | 0.20 (0.13-0.29) | |
| 19F | PncCRM | 0.42 (0.30-0.60) | 3.28 (2.56-4.20) | 0.86 (0.65-1.13) | 4.96 (3.86-6.36) | 2.14 (1.47-3.12) |
| PncOMPC | 0.54 (0.39-0.75) | 3.54 (2.74-4.57) | 2.37 (1.65-3.40) | 8.58 (6.10-12.08) | 4.80 (2.95-7.82) | |
| Control | 0.37 (0.26-0.52) | 0.22 (0.17-0.29) | 0.34 (0.24-0.46) | 0.40 (0.31-0.53) | 0.62 (0.47-0.82) | |
| 23F | PncCRM | 0.19 (0.13-0.28) | 2.51 (1.84-3.43) | 0.79 (0.59-1.05) | 6.20 (4.51-8.52) | 1.28 (0.98-1.68) |
| PncOMPC | 0.32 (0.23-0.45) | 0.62 (0.46-0.82) | 0.64 (0.47-0.86) | 2.14 (1.54-2.99) | 1.05 (0.76-1.44) | |
| Control | 0.18 (0.13-0.25) | 0.10 (0.07-0.12) | 0.13 (0.10-0.18) | 0.15 (0.12-0.20) | 0.23 (0.17-0.31) | |
FIG. 1.
GMC against each of the serotypes of a 7-valent pneumococcal conjugate vaccine at different time points in infants immunized at 2, 4, 6, and 12 months of age with PncCRM (n = 54 to 56), PncOMPC (n = 44 to 55), or a control vaccine (n = 53 or 54).
After the first dose of study vaccines, the GMCs of antibodies to serotypes 4, 9V, 14, and 18C were already higher in the PCV groups than in the control group (Fig. 1). At the same time point, the GMCs for serotypes 6B, 19F, and 23F in the PncCRM group did not differ from the respective GMCs in the control group; the same was true for the GMC for serotype 6B in the PncOMPC group (Fig. 1). After the second dose of study vaccines, the GMCs for all seven serotypes were higher in both PCV groups than in the control group. However, the GMCs for serotype 6B in both PCV groups and for serotype 23F in the PncOMPC group remained low compared to the GMCs for other serotypes (Fig. 1).
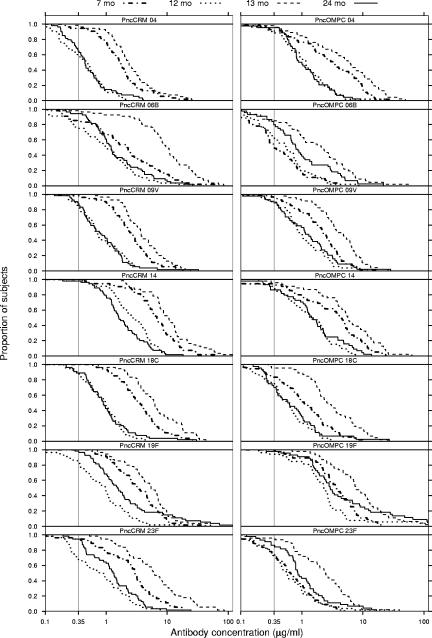
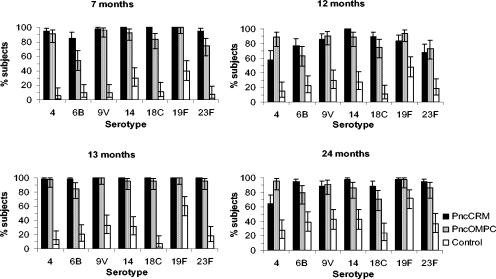
After the third dose of study vaccines, the GMCs of antibodies increased further in the PCV groups, except for serotype 4 in the PncCRM group (Fig. 1). At 7 months, the GMCs of antibodies to all serotypes were higher in the PCV groups than in the control group (Table 1 and Fig. 1). The percentages of children achieving an antibody concentration of ≥0.35 μg/ml after the third dose of the study vaccines ranged between 85 and 100% in the PncCRM group and between 55 and 100% in the PncOMPC group (Fig. 2 and 3). At the same time point, the respective percentages in the control group ranged between 6 and 40% (Fig. 3).
FIG. 2.
Reverse cumulative distribution curves demonstrating the percentages of children achieving various serum IgG antibody concentrations against each of the vaccine serotypes at various ages. The children were immunized at 2, 4, 6, and 12 months of age with a pneumococcal conjugate vaccine, PncCRM (n = 54 to 56) or PncOMPC (n = 44 to 55).
FIG. 3.
Percentages and 95% confidence intervals of infants with an antibody concentrations of ≥0.35 μg/ml at 7, 12, 13, and 24 months of age. The infants were immunized at 2, 4, 6, and 12 months of age with a 7-valent pneumococcal conjugate vaccine, PncCRM (n = 54 to 56) or PncOMPC (n = 44 to 55), or a control vaccine (n = 53 or 54).
In general the antibody concentrations declined in the PCV groups between the ages of 7 and 12 months (Table 1 and Fig. 1). In the PncCRM group, the GMCs to all serotypes declined. However, in the PncOMPC group, no or minor decreases were seen in the GMCs of antibodies to serotypes 6B, 19F, and 23F. Instead, 53, 20, and 41% of the children in the PncOMPC group experienced an increase in concentrations of antibodies to 6B, 19F, and 23F, respectively. The corresponding percentages in the PncCRM group were 21, 13, and 6%. For serotypes 4, 9V, 14, and 18C, the corresponding percentages were lower, ranging between 2 and 15% in the PncCRM group and between 10 and 18% in the PncOMPC group. The GMCs of antibodies for each serotype at 12 months of age were higher in both PCV groups than in the control group (Fig. 1 and Table 1). The difference in the decline of antibody concentrations is also seen in the reverse cumulative distribution curves for sera taken at 7 and 12 months (Fig. 2). In general, the curves lie closer to each other in the PncOMPC group than in the PncCRM group. Depending on the serotype, the percentages of infants having antibody concentrations of ≥0.35 μg/ml at 12 months of age ranged between 57 and 100% in the PncCRM group, 63 and 94% in the PncOMPC group, and 11 and 48% in the control group (Fig. 2 and 3).
At 13 months of age, 1 month after the fourth dose of the study vaccines, the GMCs of antibodies in the PCV groups were higher than at 7 and 12 months of age (Table 1 and Fig. 1). The percentages of infants achieving antibody concentration of ≥0.35 μg/ml at the age of 13 months ranged between 98 and 100% in the PncCRM group, 85 and 100% in the PncOMPC group (Fig. 2 and 3), and 7 and 61% in the control group (Fig. 3).
At 24 months of age, 12 months after the fourth dose of the study vaccines, the antibody concentrations had decreased in the PCV groups (Table 1 and Fig. 1). However, the decrease was less marked for serotypes 6B, 19F, and 23F in the PncOMPC group (Fig. 1 and 2). Nevertheless, at 24 months, the GMCs of antibodies of all serotypes in the PCV groups remained higher than in the control group. The percentages of children with antibody concentrations of ≥0.35 μg/ml were 65 to 98% in the PncCRM group, 70 to 98% in the PncOMPC group, and 24 to 72% in the control group (Fig. 2 and 3).
Relative avidity of IgG.
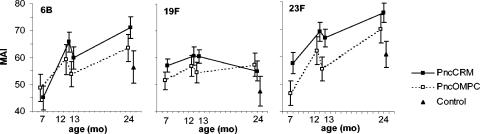
The mean MAI of antibodies increased between the ages of 7 and 12 months (i.e., 1 and 6 months after the third dose of study vaccines) in both PCV groups for all three serotypes studied (Fig. 4). The difference between the MAIs at 7 and 12 months of age was most notable for serotypes 6B and 23F. At 13 months, 1 month after the fourth dose of the PCVs, the MAIs of antibodies did not differ from those at the age of 12 months (Fig. 4)
FIG. 4.
MAIs and 95% confidence intervals of anti-pneumococcal type 6B, 19F, and 23F capsular polysaccharide IgG antibodies in infants immunized at 2, 4, 6, and 12 months of age with 7-valent pneumococcal conjugate vaccine, PncCRM (n = 53 to 56) or PncOMPC (n = 44 to 53), or a control vaccine (n = 40 to 47).
By the age of 24 months, the relative avidities of antibodies to serotypes 6B and 23F, but not to 19F, had increased further in both PCV groups (Fig. 4). The MAIs of antibodies were higher in the PCV groups than in the control group, with the exception of antibodies for 6B in the PncOMPC group.
Over the whole follow-up period (between 7 and 24 months of age), the antibody avidities to serotypes 6B and 23F increased notably, and that to 19F stayed unchanged in both PCV groups (Fig. 4). In general, the kinetics of avidity development were similar in the two PCV groups, but the MAIs of antibodies tended to be higher in the PncCRM than in the PncOMPC vaccine group.
DISCUSSION
This study was carried out to characterize in detail the immune responses to the two 7-valent PCVs, PncCRM and PncOMPC, used in parallel in the FinOM Vaccine Efficacy Trial. The results indicate that both vaccines are highly immunogenic. In addition, the overall kinetics of antibody production and avidity and the higher relative avidity of antibodies at 24 months of age in the pneumococcal vaccine than in the control vaccine recipients imply that the PCVs are able to induce immunological memory.
The kinetics of antibodies were clearly serotype dependent. For four of the serotypes included in the PCVs, the antibody concentrations were already notably higher in both PCV groups than in the control group after the first vaccine dose. For serotypes 6B and 23F, at least two doses of vaccine given in early infancy were required for an increase in the mean antibody concentration. This holds true for both PCVs in this study and has been demonstrated in previous studies with different PCVs (1, 25, 31, 42, 49, 54). Two doses of vaccine given during the first months of life, however, have also been shown to induce good immunological memory against serotypes 6B and 23F (30). The currently recommended schedule for PncCRM (Prevnar or Prevenar) includes three doses of vaccine in early infancy, followed by a booster dose during the second year of life (http://www.cdc.gov/nip/recs/child-schedule.pdf). However, data from different immunogenicity studies suggest that even fewer doses of PCV would induce a good immune response (25, 32, 35, 38). If fewer doses provided equal protection, the more affordable one- or two-dose schedule of PCVs could greatly benefit countries in which cost of the vaccine is a major hurdle to its introduction into the national immunization program.
In addition to the above-mentioned similarities in the antibody responses to the two PCVs, there were also differences. First, PncCRM tended to induce higher antibody concentrations than PncOMPC. For most of the serotypes, however, this was seen only after the third dose. Second, the persistence of antibodies varied by vaccine. The slopes of decline after the third and fourth doses of vaccine were sharper in the PncCRM group than in the PncOMPC group for all serotypes except type 14. In fact, for serotypes 6B, 19F, and 23F, the mean antibody concentrations induced by PncOMPC did not differ between 7 and 12 months of age. A substantial percentage of infants in the PncOMPC group even had increases in antibody concentrations during this period. The response to the fourth dose was notable for both PCVs. Again, the GMCs of antibodies to 6B and 23F were lower in the PncOMPC group than in the PncCRM group. Due to the different declines of antibodies, however, this no longer held true at 24 months. In spite of the different antibody responses after the third or the fourth vaccine dose, the protection levels against 6B- and 23F-specific AOM evoked by the two vaccines were very similar (17, 32). This suggests that the persistence of antibodies, in addition to the peak antibody response, should be taken into consideration when defining correlates of protection. The importance of antibody kinetics in relation to the vaccine efficacy is also supported by studies with different Hib conjugate vaccines. Although PRP-OMPC vaccine has been shown to induce lower antibody concentrations after a complete regimen than the other Hib vaccines, it has a different immunokinetic profile that provides the earliest antibody levels thought to be protective against invasive disease and thus better vaccine efficacy after one dose and comparable efficacy after two or three doses of vaccine in early infancy (19, 51).
One possible explanation for the vaccine-related differences in antibody kinetics could lie in the different immunogenic properties of the carriers used in the two PCVs. Unlike CRM and many other commonly used conjugate vaccine carriers, OMPC is not a single carrier protein. It is an outer membrane complex that contains many proteins and lipids and a small amount of lipopolysaccharide. The components of this complex can act as adjuvants and/or mitogens and thus enhance both T-cell-dependent and -independent antibody responses in infants (37, 46). Previous studies with Hib conjugate vaccines suggest that the carrier properties of OMPC could indeed be different from those of the other carriers (23, 52). The OMPC antigen may induce longer-lasting stimulation than the CRM antigen due to its adjuvancy and mitogenic capacity. It may be more efficient in stimulating the maturation and survival of long-lived plasma cells, which have been shown to be able to continue antibody secretion for extended periods (39, 55).
Another explanation for the different kinetics of the responses to the two PCVs could be the different capabilities of the immune system primed with different vaccines to respond to natural encounters with the bacteria. The fact that the serotype-specific antibody concentrations indeed increase after the primary series (between 7 and 12 months of age) for serotypes 6B, 19F, and 23F in 20 to 53% of infants in the PncOMPC group and in 6 to 21% of infants in the PncCRM group suggests that natural boosting takes place more frequently for children receiving PncOMPC. The natural boosting is supported by the fact that serotypes 6B, 19F, and 23F were not only the most common serotypes carried in the nasopharynx in the FinOM trial (56) but also the serotypes against which the highest percentages of infants developed increases in antibody concentrations from 7 to 12 or 13 to 24 months of age. Moreover, concentrations of antibodies to the less frequently carried serotypes, such as 4, 9V, and 18C, increased less often between immunizations.
In this study, all the infants received all vaccines of the Finnish national immunization program, including a diphtheria-tetanus-whole-cell pertussis vaccine combined with a Hib conjugate vaccine. Various DTaP-Hib vaccines, containing acellular pertussis components, have been associated with reduced Hib immunogenicity compared to DTwP vaccines (15, 18), but the clinical importance of this finding is unclear. The whole-cell pertussis vaccine might also serve as an adjuvant when PCVs are administered. However, recent studies have provided evidence that simultaneous administration of PncCRM and DTaP or DTwP result in immunogenicities comparable to those with PCV (30, 53), while an 11-valent PCV with different carriers seems to be more influenced by the concomitant administration of acellular pertussis vaccine (13).
The determination of the avidities of antibodies after PCV immunization can give information on both the development of B-cell memory (20) and the functional activities of antibodies (23, 57). Our study showed a strong increase in avidity between 7 and 24 months for serotypes 6B and 23F. A smaller increase was seen for serotype 19F. The weaker avidity maturation of antibodies to 19F was true for both PCVs in this study and is in accordance with previous studies (4, 58). Our findings suggest that B-cell maturation is induced and immunological memory is primed successfully by serotypes 6B and 23F, but to a lesser extent by serotype 19F.
This study compared, for the first time, the avidities of natural and PCV-induced antibodies for pneumococcal capsular polysaccharides of serotypes 6B, 19F, and 23F. The comparison was done at 24 months of age, when children in the control group started to have notable concentrations of natural antibodies. The vaccine-induced antibodies had higher relative avidities than the natural antibodies. This suggests that when vaccine-induced antibody concentrations decline, the good functional activity persists. In fact, the protective efficacy of the PncCRM vaccination against AOM can be sustained even up to the age of 4 to 5 years (A. A. Palmu, J. Verho, P. H. Mäkelä, and T. M. Kilpi, Abstr. 3rd Int. Symp. Pneumococci Pneumococcal Dis., p. 72, 2002), which suggests long persistence of antibodies of good quality.
Earlier studies with PCVs and Hib conjugate vaccines suggest that there are differences in the avidities of antibodies induced by different conjugate vaccines: OMPC conjugates have tended to induce lower avidities than the others (4, 12, 41, 52). Concordantly, PncCRM tended to induce higher-avidity antibodies than PncOMPC in this study. The most likely explanation for differences in antibody avidities is the greater T-cell dependency of the PncCRM conjugate vaccine, favoring greater affinity maturation of the antibodies by induction of somatic mutations in the variable region during the secondary immune response.
We could not show any evidence that the vaccine-specific differences in the avidities of antibodies was associated with their efficacies against AOM. This is supported by studies of Hib conjugate vaccines, which have shown similar efficacy of the PRP-OMPC vaccine (51), despite the lower immunogenicity of the vaccine and the lower mean avidity of antibodies compared to the other Hib conjugate vaccines (12, 22, 52).
In the FinOM Vaccine Efficacy Trial, the aggregate vaccine efficacy of serotypes contained in the vaccine, and also the serotype-specific efficacies against AOM of both PCVs, were evaluated in parallel (17, 32). This enabled us to look at the serotype-specific antibody formation in light of the serotype-specific efficacies of the vaccines. We could not show any relationship between the serotype-specific efficacy and the mean antibody concentrations after three or four doses of PCV. The point estimates for protection were high in both study arms for serotype 6B (84% [95% CI, 62 to 93%] for PncCRM and 79% [95% CI, 58 to 89%] for PncOMPC) and low for 19F AOM (25% [95% CI, 14 to 51%] for PncCRM and 37% [95% CI, 1 to 59%] for PncOMPC), despite the lower (or equal) mean antibody concentrations for 6B than for 19F after three or four doses of the PCVs (17, 32). On the contrary, there seemed to be a relationship between the change in the mean relative antibody avidity between 7 and 24 months of age and protection; serotype 6B was associated with high protection and demonstrated remarkable antibody avidity maturation, whereas serotype 19F, associated with poor protection, did not show antibody avidity maturation. This indicates that antibody avidity maturation, believed to be evidence of the development of memory cells, could be used as a correlate of protection.
The differences in specific clinical protection between serotypes may be due to bacterial factors, in addition to the characteristics of the immune response. In the in vitro standard opsonophagocytosis assay, more antibodies are needed for 50% killing of type 19F than type 6B strains (5, 47). This may be caused by serotypic variations in the deposition and degradation of the opsonic complement protein C3b on the surface of the pneumococcus, resulting in different resistances to phagocytosis and stimulation of antibody production (24).
Serotype-specific differences in efficacy have not been reported for invasive pneumococcal disease, indicating that requirements for protective immunity against AOM are different from those against invasive disease. Furthermore, in the FinOM trial, the levels of protection against AOM caused by serotype 6B were similar for the two PCVs, in spite of lower antibody responses after three or four doses of PncOMPC than for PncCRM. This raises a question as to whether the antibody concentration 1 month after the primary series alone is a sufficient correlate of protection and suggests that other parameters, like persistence of antibodies, antibody avidity maturation, and functionality of antibodies, should also be considered when defining correlates of protection.
Acknowledgments
We thank Hannele Lehtonen, Piia Pihlajamaa, Kaisa Jousimies, and Leena Saarinen for excellent technical assistance; Jaason Haapakoski and Esa Ruokokoski for experienced data management; Virva Jäntti for statistical advice; P. Helena Mäkelä for critical reading of the manuscript; and the personnel of the Kangasala Health Center and all the children and their parents who volunteered to participate in the FinOM study.
The FinOM studies were supported by Aventis Pasteur, Merck & Co., Inc., and Wyeth Vaccines.
T. Kilpi and H. Käyhty have served as consultants to Aventis Pasteur, and T. Kilpi has served as a consultant to Wyeth Vaccines.
The members of the FinOM Study Group are Juhani Eskola, Mervi Eerola, Tapani Hovi, Pekka Karma, Terhi Kilpi, Helena Käyhty, Maija Leinonen, P. Helena Mäkelä, Arto Palmu, Esa Ruokokoski, Aino K Takala, Terhi Kilpi, Kari S. Lankinen, Aino K. Takala, Petri Mattila, Arto Palmu, Pirjo-Riitta Saranpää, Anna-Stina Leinonen, Terhi Hulkko, Wilhelm Bredenberg, Kaisu Hattela, Tuija-Leena Huupponen, Marja-Leena Hyypiä, Elina Hyödynmaa, Päivi Leinonen, Päivi Limnell, Merja Mölsä, Hanna Rautio, Auli Räsänen, Päivi Savikurki-Heikkilä, Heljä Savolainen, Anneli Siro, Ritva Syrjänen, Sirpa Vesa, Sari Vikström, Hannele Holli, Marja-Leena Hotti, Helena Jokinen, Marjo-Riitta Kauppinen, Eija Lahtinen, Johanna Laitinen, Ella Lehto, Taina Nissinen, Sirkka Oikarinen, Sirkka-Liisa Piirto, Minna Ranta, Päivi Sirén, Terttu Suikkanen, Päivi Tervonen, Eija Kujanne, Hannamari Salonen, Marjo Virkki, Arja Katila, Maire Selin, Elja Herva, Aili Hökkä, Tarja Kaijalainen, Eeva-Liisa Korhonen, Maija Leinonen, Hilkka Ohukainen, Maijastiina Karpala, Minna Koivuniemi, Helena Käyhty, Hannele Lehtonen, Piia Pihlajamaa, Satu Rapola, Leena Saarinen, Merja Väkeväinen, Heidi Åhman, Soile Blomqvist, Tapani Hovi, Marjaana Kleemola, Pekka Karma, Marko Grönholm, Jaason Haapakoski, Eeva Koskenniemi, Satu Nahkuri, Esa Ruokokoski, Matti Sarjakoski, Mervi Eerola, Jukka Jokinen, Mika Lahdenkari, Jouko Verho, Ulla Johansson, Paula Solukko, Olli Ruuskanen, Paul Fine, Jussi Mertsola, Richard Moxon, Patrick Olin, and Karin Prellner.
Editor: D. L. Burns
REFERENCES
- 1.Åhman, H., H. Käyhty, P. Tamminen, A. Vuorela, F. Malinoski, and J. Eskola. 1996. Pentavalent pneumococcal oligosaccharide conjugate vaccine PncCRM is well-tolerated and able to induce an antibody response in infants. Pediatr. Infect. Dis. J. 15:134-139. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, E. L., D. J. Kennedy, K. M. Geldmacher, J. Donnelly, and P. M. Mendelman. 1996. Immunogenicity of heptavalent pneumococcal conjugate vaccine in infants. J. Pediatr. 128:649-653. [DOI] [PubMed] [Google Scholar]
- 3.Anttila, M., J. Eskola, H. Åhman, and H. Käyhty. 1998. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J. Infect. Dis. 177:1614-1621. [DOI] [PubMed] [Google Scholar]
- 4.Anttila, M., J. Eskola, H. Åhman, and H. Käyhty. 1999. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine 17:1970-1977. [DOI] [PubMed] [Google Scholar]
- 5.Anttila, M., M. Voutilainen, V. Jäntti, J. Eskola, and H. Käyhty. 1999. Contribution of serotype-specific IgG concentration, IgG subclasses and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae. Clin. Exp. Immunol. 118:402-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 7.Black, S. B., H. R. Shinefield, S. Ling, J. Hansen, B. Fireman, D. Spring, J. Noyes, E. Lewis, P. Ray, J. Lee, and J. Hackell. 2002. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr. Infect. Dis. J. 21:810-815. [DOI] [PubMed] [Google Scholar]
- 8.Brown, E. J., S. W. Hosea, and M. M. Frank. 1983. The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream. Rev. Infect. Dis. 5:S797-S805. [DOI] [PubMed] [Google Scholar]
- 9.Brown, E. J., S. W. Hosea, C. H. Hammer, C. G. Burch, and M. M. Frank. 1982. A quantitative analysis of the interactions of antipneumococcal antibody and complement in experimental pneumococcal bacteremia. J. Clin. Investig. 69:85-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulkow, L. R., R. B. Wainwright, G. W. Letson, S. J. Chang, and J. I. Ward. 1993. Comparative immunogenicity of four Haemophilus influenzae type b conjugate vaccines in Alaska Native infants. Pediatr. Infect. Dis. J. 12:484-492. [DOI] [PubMed] [Google Scholar]
- 11.Capeding, M. Z., T. Puumalainen, C. P. Gepanayao, H. Käyhty, M. G. Lucero, and H. Nohynek. 2003. Safety and immunogenicity of three doses of an eleven-valent diphtheria toxoid and tetanus protein-conjugated pneumococcal vaccine in Filipino infants. BMC Infect. Dis. 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung, G. H., K. H. Kim, R. S. Daum, R. A. Insel, G. R. Siber, S. Sood, R. K. Gupta, C. Marchant, and M. H. Nahm. 1995. The V-region repertoire of Haemophilus influenzae type b polysaccharide antibodies induced by immunization of infants. Infect. Immun. 63:4219-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dagan, R., D. Goldblatt, J. R. Maleckar, M. Yaich, and J. Eskola. 2004. Reduction of antibody response to an 11-valent pneumococcal vaccine coadministered with a vaccine containing acellular pertussis components. Infect. Immun. 72:5383-5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dagan, R., R. Melamed, O. Zamir, and O. Leroy. 1997. Safety and immunogenicity of tetravalent pneumococcal vaccines containing 6B, 14, 19F and 23F polysaccharides conjugated to either tetanus toxoid or diphtheria toxoid in young infants and their boosterability by native polysaccharide antigens. Pediatr. Infect. Dis. J. 16:1053-1059. [DOI] [PubMed] [Google Scholar]
- 15.Daum, R. S., C. E. Zenko, G. Z. Given, G. A. Ballanco, H. Parikh, and K. Germino. 2001. Magnitude of interference after diphtheria-tetanus toxoids-acellular pertussis/Haemophilus influenzae type b capsular polysaccharide-tetanus vaccination is related to the number of doses administered. J. Infect. Dis. 184:1293-1299. [DOI] [PubMed] [Google Scholar]
- 16.Decker, M. D., K. M. Edwards, R. Bradley, and P. Palmer. 1992. Comparative trial in infants of four conjugate Haemophilus influenzae type b vaccines. J. Pediatr. 120:184-189. [DOI] [PubMed] [Google Scholar]
- 17.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Käyhty, P. Karma, R. Kohberger, G. Siber, and P. H. Mäkelä. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 18.Eskola, J., J. Ward, R. Dagan, D. Goldblatt, F. Zepp, and C. A. Siegrist. 1999. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet 354:2063-2068. [DOI] [PubMed] [Google Scholar]
- 19.Galil, K., R. Singleton, O. S. Levine, M. A. Fitzgerald, L. Bulkow, M. Getty, B. A. Perkins, and A. Parkinson. 1999. Reemergence of invasive Haemophilus influenzae type b disease in a well-vaccinated population in remote Alaska. J. Infect. Dis. 179:101-106. [DOI] [PubMed] [Google Scholar]
- 20.Goldblatt, D., A. R. Vaz, and E. Miller. 1998. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J. Infect. Dis. 177:1112-1115. [DOI] [PubMed] [Google Scholar]
- 21.Granoff, D. M., E. L. Anderson, M. T. Osterholm, S. J. Holmes, J. E. McHugh, R. B. Belshe, F. Medley, and T. V. Murphy. 1992. Differences in the immunogenicity of three Haemophilus influenzae type b conjugate vaccines in infants. J. Pediatr. 121:187-194. [DOI] [PubMed] [Google Scholar]
- 22.Granoff, D. M., S. J. Holmes, M. T. Osterholm, J. E. McHugh, A. H. Lucas, E. L. Anderson, R. B. Belshe, J. L. Jacobs, F. Medley, and T. V. Murphy. 1993. Induction of immunologic memory in infants primed with Haemophilus influenzae type b conjugate vaccines. J. Infect. Dis. 168:663-671. [DOI] [PubMed] [Google Scholar]
- 23.Granoff, D. M., and A. H. Lucas. 1995. Laboratory correlates of protection against Haemophilus influenzae type b disease. Importance of assessment of antibody avidity and immunologic memory. Ann. N. Y. Acad. Sci. 754:278-288. [DOI] [PubMed] [Google Scholar]
- 24.Hostetter, M. K. 1986. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J. Infect. Dis. 153:682-693. [DOI] [PubMed] [Google Scholar]
- 25.Huebner, R. E., N. Mbelle, B. Forrest, D. V. Madore, and K. P. Klugman. 2002. Immunogenicity after one, two or three doses and impact on the antibody response to coadministered antigens of a nonavalent pneumococcal conjugate vaccine in infants of Soweto, South Africa. Pediatr. Infect. Dis. J. 21:1004-1007. [DOI] [PubMed] [Google Scholar]
- 26.Jaffar, S., A. Leach, P. G. Smith, F. Cutts, and B. Greenwood. 2003. Effects of misclassification of causes of death on the power of a trial to assess the efficacy of a pneumococcal conjugate vaccine in The Gambia. Int. J. Epidemiol. 32:430-436. [DOI] [PubMed] [Google Scholar]
- 27.Jodar, L., J. Butler, G. Carlone, R. Dagan, D. Goldblatt, H. Käyhty, K. Klugman, B. Plikaytis, G. Siber, R. Kohberger, I. Chang, and T. Cherian. 2003. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine 21:3265-3272. [DOI] [PubMed] [Google Scholar]
- 28.Joseph, H., E. Miller, M. Dawson, N. Andrews, I. Feavers, and R. Borrow. 2001. Meningococcal serogroup A avidity indices as a surrogate marker of priming for the induction of immunologic memory after vaccination with a meningococcal A/C conjugate vaccine in infants in the United Kingdom. J. Infect. Dis. 184:661-662. [DOI] [PubMed] [Google Scholar]
- 29.Käyhty, H., J. Eskola, H. Peltola, L. Saarinen, and P. H. Mäkelä. 1992. High antibody responses to booster doses of either Haemophilus influenzae capsular polysaccharide or conjugate vaccine after primary immunization with conjugate vaccines. J. Infect. Dis. 165(Suppl. 1):S165-S166. [DOI] [PubMed] [Google Scholar]
- 30.Käyhty, H., H. Åhman, K. Eriksson, M. Sörberg, and L. Nilsson. Immunogenicity and tolerability of a heptavalent pneumococcal conjugate vaccine administered at 3, 5 and 12 months of age. Pediatr. Infect. Dis. J., in press. [DOI] [PubMed]
- 31.Käyhty, H., H. Åhman, P. R. Rönnberg, R. Tillikainen, and J. Eskola. 1995. Pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine is immunogenic in infants and children. J. Infect. Dis. 172:1273-1278. [DOI] [PubMed] [Google Scholar]
- 32.Kilpi, T., H. Åhman, J. Jokinen, K. S. Lankinen, A. Palmu, H. Savolainen, M. Grönholm, M. Leinonen, T. Hovi, J. Eskola, H. Käyhty, N. Bohidar, J. C. Sadoff, and P. H. Mäkelä. 2003. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin. Infect. Dis. 37:1155-1164. [DOI] [PubMed] [Google Scholar]
- 33.Klugman, K. P., S. A. Madhi, R. E. Huebner, R. Kohberger, N. Mbelle, and N. Pierce. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 349:1341-1348. [DOI] [PubMed] [Google Scholar]
- 34.Koskela, M. 1987. Serum antibodies to pneumococcal C polysaccharide in children: response to acute pneumococcal otitis media or to vaccination. Pediatr. Infect. Dis. J. 6:519-526. [DOI] [PubMed] [Google Scholar]
- 35.Leach, A., S. J. Ceesay, W. A. Banya, and B. M. Greenwood. 1996. Pilot trial of a pentavalent pneumococcal polysaccharide/protein conjugate vaccine in Gambian infants. Pediatr. Infect. Dis. J. 15:333-339. [DOI] [PubMed] [Google Scholar]
- 36.Lee, L. H., C. E. Frasch, L. A. Falk, D. L. Klein, and C. D. Deal. 2003. Correlates of immunity for pneumococcal conjugate vaccines. Vaccine 21:2199-2205. [DOI] [PubMed] [Google Scholar]
- 37.Liu, M. A., A. Friedman, A. I. Oliff, J. Tai, D. Martinez, R. R. Deck, J. T. Shieh, T. D. Jenkins, J. J. Donnelly, and L. A. Hawe. 1992. A vaccine carrier derived from Neisseria meningitidis with mitogenic activity for lymphocytes. Proc. Natl. Acad. Sci. USA 89:4633-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucero, M. G., T. Puumalainen, J. M. Ugpo, G. Williams, H. Kayhty, and H. Nohynek. 2004. Similar antibody concentrations in Filipino infants at age 9 months, after 1 or 3 doses of an adjuvanted, 11-valent pneumococcal diphtheria/tetanus-conjugated vaccine: a randomized controlled trial. J. Infect. Dis. 189:2077-2084. [DOI] [PubMed] [Google Scholar]
- 39.Manz, R. A., A. Thiel, and A. Radbruch. 1997. Lifetime of plasma cells in the bone marrow. Nature 388:133-134. [DOI] [PubMed] [Google Scholar]
- 40.Mbelle, N., R. E. Huebner, A. D. Wasas, A. Kimura, I. Chang, and K. P. Klugman. 1999. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J. Infect. Dis. 180:1171-1176. [DOI] [PubMed] [Google Scholar]
- 41.Nahm, M. H., K. H. Kim, P. Anderson, S. V. Hetherington, and M. K. Park. 1995. Functional capacities of clonal antibodies to Haemophilus influenzae type b polysaccharide. Infect. Immun. 63:2989-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nurkka, A., H. Åhman, M. Yaich, J. Eskola, and H. Käyhty. 2001. Serum and salivary anti-capsular antibodies in infants and children vaccinated with octavalent pneumococcal conjugate vaccines, PncD and PncT. Vaccine 20:194-201. [DOI] [PubMed] [Google Scholar]
- 43.Obaro, S. K., Z. Huo, W. A. Banya, D. C. Henderson, M. A. Monteil, A. Leach, and B. M. Greenwood. 1997. A glycoprotein pneumococcal conjugate vaccine primes for antibody responses to a pneumococcal polysaccharide vaccine in Gambian children. Pediatr. Infect. Dis. J. 16:1135-1140. [DOI] [PubMed] [Google Scholar]
- 44.O'Brien, K. L., L. H. Moulton, R. Reid, R. Weatherholtz, J. Oski, L. Brown, G. Kumar, A. Parkinson, D. Hu, J. Hackell, I. Chang, R. Kohberger, G. Siber, and M. Santosham. 2003. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet 362:355-361. [DOI] [PubMed] [Google Scholar]
- 45.O'Brien, K. L., M. C. Steinhoff, K. Edwards, H. Keyserling, M. L. Thoms, and D. Madore. 1996. Immunologic priming of young children by pneumococcal glycoprotein conjugate, but not polysaccharide, vaccines. Pediatr. Infect. Dis. J. 15:425-430. [DOI] [PubMed] [Google Scholar]
- 46.Perez-Melgosa, M., H. D. Ochs, P. S. Linsley, J. D. Laman, M. van Meurs, R. A. Flavell, R. K. Ernst, S. I. Miller, and C. B. Wilson. 2001. Carrier-mediated enhancement of cognate T cell help: the basis for enhanced immunogenicity of meningococcal outer membrane protein polysaccharide conjugate vaccine. Eur. J. Immunol. 31:2373-2381. [DOI] [PubMed] [Google Scholar]
- 47.Puumalainen, T., N. Ekström, R. Zeta-Capeding, J. Ollgren, K. Jousimies, M. Lucero, H. Nohynek, and H. Käyhty. 2003. Functional antibodies elicited by an 11-valent diphtheria-tetanus toxoid-conjugated pneumococcal vaccine. J. Infect. Dis. 187:1704-1708. [DOI] [PubMed] [Google Scholar]
- 48.Quataert, S. A., C. S. Kirch, L. J. Wiedl, D. C. Phipps, S. Strohmeyer, C. O. Cimino, J. Skuse, and D. V. Madore. 1995. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin. Diagn. Lab. Immunol. 2:590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rennels, M. B., K. M. Edwards, H. L. Keyserling, K. S. Reisinger, D. A. Hogerman, D. V. Madore, I. Chang, P. R. Paradiso, F. J. Malinoski, and A. Kimura. 1998. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics 101:604-611. [DOI] [PubMed] [Google Scholar]
- 50.Richmond, P., R. Borrow, D. Goldblatt, J. Findlow, S. Martin, R. Morris, K. Cartwright, and E. Miller. 2001. Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J. Infect. Dis. 183:160-163. [DOI] [PubMed] [Google Scholar]
- 51.Santosham, M., M. Wolff, R. Reid, M. Hohenboken, M. Bateman, J. Goepp, M. Cortese, D. Sack, J. Hill, W. Newcomer, et al. 1991. The efficacy in Navajo infants of a conjugate vaccine consisting of Haemophilus influenzae type b polysaccharide and Neisseria meningitidis outer-membrane protein complex. N. Engl. J. Med. 324:1767-1772. [DOI] [PubMed] [Google Scholar]
- 52.Schlesinger, Y., D. M. Granoff, et al. 1992. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. JAMA 267:1489-1494. [PubMed] [Google Scholar]
- 53.Schmitt, H. J., J. Faber, I. Lorenz, B. Schmole-Thoma, and N. Ahlers. 2003. The safety, reactogenicity and immunogenicity of a 7-valent pneumococcal conjugate vaccine (7VPnC) concurrently administered with a combination DTaP-IPV-Hib vaccine. Vaccine 21:3653-3662. [DOI] [PubMed] [Google Scholar]
- 54.Shinefield, H. R., S. Black, P. Ray, I. Chang, N. Lewis, B. Fireman, J. Hackell, P. R. Paradiso, G. Siber, R. Kohberger, D. V. Madore, F. J. Malinowski, A. Kimura, C. Le, I. Landaw, J. Aguilar, and J. Hansen. 1999. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr. Infect. Dis. J. 18:757-763. [DOI] [PubMed] [Google Scholar]
- 55.Slifka, M. K., R. Antia, J. K. Whitmire, and R. Ahmed. 1998. Humoral immunity due to long-lived plasma cells. Immunity 8:363-372. [DOI] [PubMed] [Google Scholar]
- 56.Syrjänen, R. K., T. M. Kilpi, T. H. Kaijalainen, E. E. Herva, and A. K. Takala. 2001. Nasopharyngeal carriage of Streptococcus pneumoniae in Finnish children younger than 2 years old. J. Infect. Dis. 184:451-459. [DOI] [PubMed] [Google Scholar]
- 57.Usinger, W. R., and A. H. Lucas. 1999. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect. Immun. 67:2366-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wuorimaa, T., R. Dagan, M. Väkeväinen, F. Bailleux, R. Haikala, M. Yaich, J. Eskola, and H. Käyhty. 2001. Avidity and subclasses of IgG after immunization of infants with an 11-valent pneumococcal conjugate vaccine with or without aluminum adjuvant. J. Infect. Dis. 184:1211-1215. [DOI] [PubMed] [Google Scholar]
- 59.Zangwill, K. M., D. P. Greenberg, C. Y. Chiu, P. Mendelman, V. K. Wong, S. J. Chang, S. Partridge, and J. I. Ward. 2003. Safety and immunogenicity of a heptavalent pneumococcal conjugate vaccine in infants. Vaccine 21:1894-1900. [DOI] [PubMed] [Google Scholar]