Abstract
Pulmonary NEB, widely distributed within the airway mucosa of mammalian lungs, are presumed hypoxia sensitive airway O2 sensors responding to changes in airway gas concentration. NEB cell hyperplasia has been reported after exposure to chronic hypoxia and in a variety of paediatric and adult lung disorders. Prolyl hydroxylases (PHD 1–3) regulate the stability of hypoxia-inducible factors (HIF’s) in an O2-dependent manner and function as intrinsic oxygen sensors. To determine a possible role of PHD-1in NEB cells we have quantitated NEB’s in lungs of neonatal (P2) and adult (2 months) PHD-1-deficient mice and compared them to wild type (WT) control mice. Lung tissues fixed in formalin and embedded in paraffin were processed for immunoperoxidase method and frozen sections for multilabel immunoflourescence using antibodies for NEB markers synaptophysin, synaptic vesicle protein 2 and the peptide CGRP. The frequency and size of NEB in lungs of PHD-1 deficient neonatal mice (P2) and at 2 months was increased significantly compared to WT controls (p < 0.01). The present data suggests an important role for PHD enzymes in NEB cell biology deserving further studies. Since the PHD-1 deficient mouse appears to be the first animal model showing NEB cell hyperplasia it may be useful for studies of NEB physiology and pathobiology.
Keywords: Airway oxygen sensors, Neuroendocrine cells, Oxygen sensing mechanism, Cell proliferation and differentiation, Prolyl hydroxylases, Hypoxia-inducible factor -1
21.1. Introduction
Airway mucosa of human and animal lung contains amine (serotonin,5-HT) and peptide producing cells distributed as solitary cells referred to as pulmonary neuroendocrine cells (PNEC) and as innervated clusters called neuroepithelial bodies(NEB). NEB’s are widely distributed within the mucosa of intrapulmonary airways and are thought to function as polymodal airway sensors responding to a variety of intraluminal stimuli including gas concentration (pO2, p CO2) and mechanical stretch (Cutz et al. 2007a). PNEC/NEB’s appear more numerous in fetal/neonatal lungs and are less conspicuous in adult lungs suggesting developmental regulation and a critical role during the perinatal period. (Cutz et al. 1985, 2007a). NEB cells are excitable and exhibit hypoxia sensing properties owing to the expression of a membrane delimited O2 sensor complex composed of an O2 sensing protein(NAPDH oxidase/NOX2) coupled to an O 2 sensitive K+ channel (Youngson et al. 1993; Wang D et al. 1996; Fu et al. 2000). Although the precise function of NEB is at present unknown, they are considered as a part of a specialized homeostatic oxygen-sensing system of the body (Weir et al. 2005). Hyperplasia of NEB cells has been described in lungs of animals and human exposed to chronic hypoxia and in a variety of pediatric and adult lung disorders (Cutz et al. 2007a; Cutz et al. 2008). The mechanisms and clinical significance of NEB hyperplasia is currently unknown and requires further investigation. It is now well established that hypoxia inducible factor –1 (HIF-1 alpha) is a ubiquitous transcription factor that regulates expression of numerous genes involved in adaptive responses to O2 deprivation (Semenza 2009). Closely linked in mediation of hypoxia responses are prolyl hydroxylase domains enzymes (PHD 1–3)that regulate the stability of HIF’s in an O2- dependent manner and hence function as intrinsic O2 sensors (Kaelin and Ratcliffe 2008). Analysis in vivo of PHD expression patterns by immunohistochemistry shows that PHD1 expression is mostly confined to chromogranin A-positive neuroendocrine tissues such as the pancreatic islets, carotid body and adrenal medulla (unpublished observations). Further, PHD-1 was strongly expressed across a range of neuroendocrine tumours, for example, intestinal and lung carcinoids, carotid paragangliomas and phaemochromocytomas. Thus PHD1 is strongly expressed in neuroendocrine tissue, suggesting that it plays an important role in neuroendocrine function. To determine the possible role of PHD- 1 in NEB cells, we examined the frequency, number and the size of NEB’s in lungs of PHD-1 deficient mice using NEB cell specific immunomarkers and morphometric methods. Our studies demonstrate a striking hyperplasia of NEBs in lungs of PHD–1 deficient mice during both the perinatal period and in adults suggesting an important role for PHD enzymes in NEB cell biology and pathobiology.
21.2. Methods
Lung tissues were obtained from PHD-1 deficient mice (Egln2 −/−) and wild type (WT ) control mice (Aragones et al. 2008). Tissues from neonatal (P1-2, n= 12), and adult (2 month, n= 7) PHD –1 deficient and age matched WT control mice were fixed in 10% neutral buffered formalin ,embedded in paraffin and processed for immunoperoxidase (IP) labeling method using standard procedures (Cutz et al. 2007b). To identify NEB in formalin fixed paraffin section we used Invitrogen Polymer detection system (SuperPicTure™ polymer detection kit, Invitrogen Corp. Camarillo, CA). As primary antibodies we used mouse monoclonal antibody against synaptic vesicle protein 2(SV2 ,Hybridoma Bank, Iowa City, IA ; 1:20 dilution), rabbit monoclonal antibody against synaptophysin (NeoMarkers ,Freemont, CA; 1:100 dilution) and monoclonal antibody against calcitonin gene related peptide (CGRP, Abcam Inc, Cambridge, MA;1:150 dilution). As a secondary antibody detection procedure we used Invitrogen Polymer detection system with horse radish peroxidase (HRP) polymer-secondary antibody conjugate.
For immunofluorescence (IF)staining and confocal microscopy we used frozen sections of lung tissues(~100um) from PHD-deficient and age matched WT controls (P1 n= 4 and P15 n= 4 respectively) using tissue preparation and immunolabeling protocols using above primary antibodies as previously reported(Pan et al. 2006). Fluorescent images of PNEC/NEB cells, the airway nerves and smooth muscle actin-FITC (Sigma-Aldrich, St Louis, MI) in the double stained (Texas Red/FITC) whole mounts were obtained using a Leica confocal laser scanning microscope (model TCS-SPE) and LAS-AF software.
Morphometric analysis of NEB frequency and size was performed using methods as previously reported (Cutz et al. 2007b; Pan et al. 2006). The integrated surface area of airways of different sizes, expressed in square millimeters of the section (5 um IP/100 um IF section thickness) was measured by Nikon simple PCI imaging software (IP method) (Cutz et al. 2007b; Pan et al. 2006) and the NIH-Image J program (confocal IF images) standardized by an internal scale bar in each counted image (Pan et al. 2006). For statistical analysis we used Student’s t-test.
21.3. Results
21.3.1. Immunoperoxidase Method
The overall morphology of lungs from PHD – 1 deficient mice was similar to WT controls with no obvious abnormalities affecting the airways or alveolar compartment. The sections of lungs from neonatal mice (P2) ,both PHD- 1 deficient and age matched WT controls, immunostained for SV 2 showed clusters of positive cells within the airways corresponding to NEB (Fig. 21.1a, b). In addition peribronchial nerve fibers and fine nerve endings were also positive for SV2 (Fig. 21.1d, e). At higher magnification ,NEB in the lungs of WT mice usually consisted of between 5 and 7 cells (Fig. 21.1c, d), while in PHD-1 deficient mice they appeared much larger consisting of 15–30 cells (Fig. 21.1e, f). Some hyperplastic NEB in PHD – 1 deficient mice formed prominent cell aggregates at airway branch points protruding into the airway lumen (Fig. 21.1f). Morphometric analysis confirmed the increased frequency and size of NEB’s in lungs of PHD –1 deficient mice compared to their WT counterparts (Fig. 21.2a, b). The mean frequency of NEB (%immunostained area/mm2) in PHD- 1 deficient mice at P2 (2.24 +/−0.4))was increased significantly compared to WT controls(1.62 +/−0.3 ; p < 0.01) Similarly the size of NEB in PHD –1 deficient l mice (1494.6 +/− 175.3)was close to twice that of WT control mice (865.2 +/−162.6; p < 0.01). Immunostaining for CGRP, the principal peptide in NEBs of rodent lung, showed a pattern of expression similar to SV2 with almost twofold increase in the number and size of NEB’s in lungs of PHD- 1 deficient mice compared to age matched WT controls (Fig. 21.2c, d). In the lungs of 2 month old PHD-1 deficient mice the number and size of NEB assessed by immunostaining for either SV2 or CGRP was increased compared to WT controls, but the difference was at lower level of statistical significance(p < 0.05)(Fig. 21.2a, b, c, d).
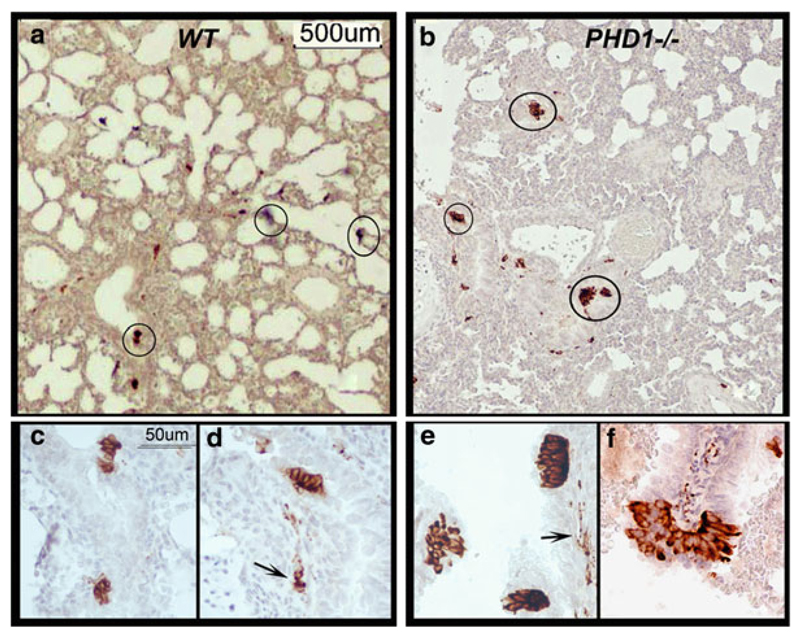
Fig. 21.1.
NEB in lungs of WT control and PHD-1 deficient mice visualized using immunoperoxidase method with anti-SV2 antibody. (a) Low magnification view of lung section from neonatal (P2) WT mice showing positive NEB in small airways (circled). (b) Section of lung from neonatal ( P2 ) PHD-1 deficient mouse lung at the same magnification as in (a) showing more prominent NEBs (circled) in similar distribution. (c) Close up of two NEBs consisting of small clusters of immunopositive cells in epithelium of small airway in lung from WT mouse. (d) Another small NEB in the same sample as in (c) with immunopositive nerve fibers (arrow) in submucosa. (e) Higher magnification view from sample (b) with three prominent NEBs forming compact intraepithelial corpuscles and immunoreactive nerve fibres in submucosa (arrow). (f) Close up of large, hyperplastic NEB from sample (b) situated at airway bifurcation and protruding into airway lumen
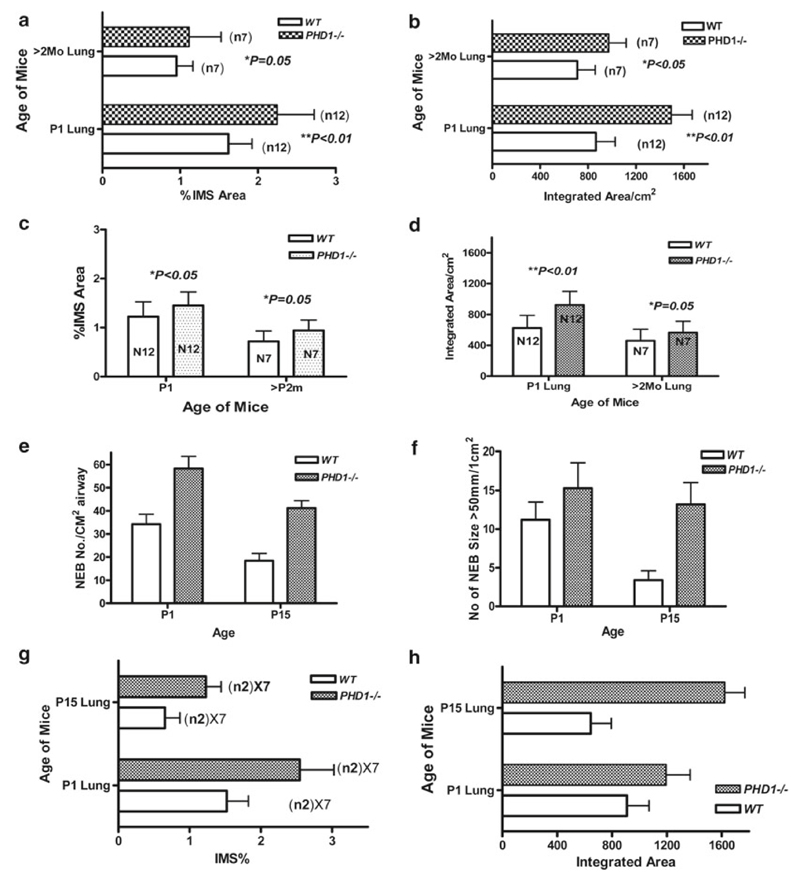
Fig. 21.2.
Morphometric assessment of NEB frequency, number and size in lungs of PHD-1deficient and WT control lungs using immunoperoxidase (a, b, c, d) and immunofluorescence (e, f, g, h) methods. (a) Comparison of mean values of %immunostained (SV2 antibody) area (IMS)per mm2 of section from PHD1- deficient and WT controls at different ages (P2 and 2 month). (b) Mean values for NEB size from same samples as in a). (c) Means of % IMS for NEB immunostained for CGRP as in samples shown in (a) & (b). (d) Means of NEB size in samples immunostained for CGRP as in samples shown in (a) & (b). (e) Comparison of mean number of synaptophysin immunoreactive NEBs/cm2 of airway in lungs of PHD-1 deficient mice and WT controls at P2 and P15. (f) Mean values for NEB size from same samples as in (e). (g) & (h) Same samples as in (e) & (f) with values expressed as IMS %of NEB (g) and NEB size /integrated area (h)
21.3.2. Multilabel Immunofluorescence Method
The studies using IF method confirmed the findings of IP study showing that NEB s in the lungs of PHD- 1deficient mice at either P2 or P15 were more numerous and larger compared to WT age matched controls ,and were frequently localized at airway branch points (Fig. 21.3a).At higher magnification, hyperplastic NEBs in lungs of PHD –1 deficient mice sometimes formed an intraepithelial row of up to 20 columnar cells with fine nerve branches entering at the base (Fig. 21.3b). In contrast, NEB in the lungs of WT controls were much smaller (~5–7 cells) but showed a similar pattern of innervation (Fig. 21.3c). Morphometric analysis of sections immunostained for synaptophysin confirmed up to twofold increase in the number(58.3 +/−5.2 vs.34.2 +/−4.3) and size(15.3 +/− 3.2 vs 11.2 +/−2.3) of NEB in PHD-1 deficient mice compared to age-matched WT controls (Fig. 21.2e, f, g, h). Although nerve fibers associated with NEB and airway smooth muscle were more clearly visualized, there were no obvious differences in nerve density between PHD-1 deficient and WT mice.
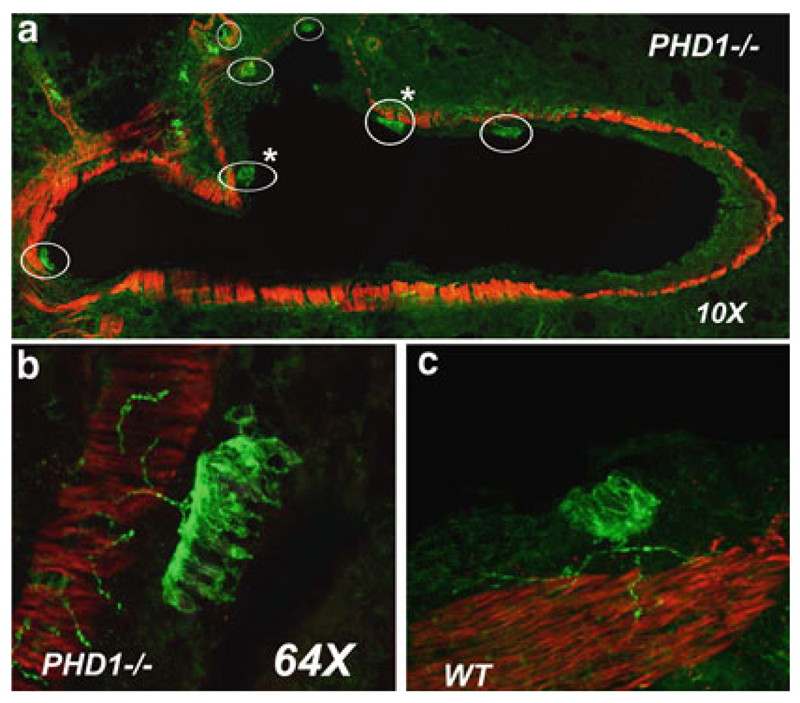
Fig. 21.3.
NEBs in lung sections from PHD-1 deficient and WT control mice visualized by multilabel immunoflourescence method and confocal microscopy. (a) Low magnification view of cross section of large airway in lung from PHD –1 deficient mouse (P2) immunostained for synaptophysin (green) to label NEB and nerve fibers and for smooth muscle actin to outline airway smooth muscle (red). Several large NEBs (circled) are present, some that are located at airway branch points (asterisks). (b) Close –up of large NEB from sample a) with closely packed immunoreactive cells and fine, beaded nerve fibres in adjacent smooth muscle and at the base of NEB cell cluster. (c) For comparison, NEB in lung of WT mouse is significantly smaller; however submucosal nerve fibres appear similar
21.4. Discussion
We report striking NEB cell hyperplasia in the lungs of PHD- 1deficient mice. The increased number and size of NEB was observed in lungs of neonates and was maintained into adulthood, suggesting persistence of NEB cell hyperplasia during post natal lung growth and development. Although the precise mechanisms are not known, NEB cell hyperplasia is likely the result of increased recruitment from precursor cells rather than due to increased cell proliferation since in vivo and in vitro labeling studies revealed that NEB cells are a slowly renewing cell population with low cell turnover compared to adjacent airway epithelial cells (Sorokin et al. 1997). In preliminary studies we observed that NEB cells of PHD1 –deficient mice did not show increased labeling with a proliferation marker MIB-1(Ki-67) indicating that the mitotic activity was not affected. During lung development the differentiation of both PNEC and NEB is governed by proneural genes such as a mammalian homolog of the achete-scute complex (Mash-1 in mice; HASH-1 in human). Mice deficient in Mash –1(Mash –1 k/o ) lack both PNEC and NEBs (Borges et al. 1997).
We have shown recently that during early stages of mouse lung development, pO2 concentration in concert with Mash-1 expression modulates neuroendocrine cell phenotype (McGovern et al. 2010). Organ cultures of fetal mouse lung at E12 maintained in hypoxia (5%O2) showed loss of Mash-1 expression and consequently markedly reduced numbers of PNEC, whereas cultures incubated in “normoxia” (20% O2) showed high levels of Mash-1 expression and numerous PNEC. Switching hypoxia grown cultures to normoxia resulted in a burst of Mash-1 expression followed by PNEC differentiation when cultures were kept under normoxia. In contrast, hypoxia had no effects on Mash-1 expression or PNEC numbers in cultures from later gestation (E16) indicating a locked-in developmental programming. Therefore it seems plausible that hypoxia promotes expansion of precursor cells and that oxygen promotes differentiation of PNEC via Mash-1.
Although the precise mechanisms regulating Mash-1 expression in NEB cells is at present unknown, hypoxia inducible factors (HIFs 1–3) are likely to be involved. Prolyl hydroxylase domain enzymes (PHD1-3) function as cellular O2 sensors via modulation of HIF’s expression and thus play a key role in oxygen homeostasis in both health and disease (Kaelin and Ratcliffe 2008; Appelhoff et al. 2004). In terms of hypoxia responses, studies using various tumor cell lines have shown that levels of PHD –1 mRNA were unchanged or decreased under hypoxia,whereas mRNA levels of PHD –2 and PHD –3 were increased, especially PHD- 3 (Appelhoff et al. 2004). The tissue and cell expression of PHD enzymes is variable (Kaelin and Ratcliffe 2008; Appelhoff et al. 2004). For example PHD-3 mRNA is highly expressed in cardiac muscle while PHD –1 mRNA levels are increased in the testis. At the protein level, however, PHD-2 was found to be abundantly expressed in most mouse organs examined (Appelhoff et al. 2004). The expression and distribution of PHD isozymes in the lung (or NEB cells) has not been investigated in detail. In terms of physiological importance, PHD-2 appears to be critical in oxygen sensing under basal conditions, since its inactivation during development is embryo lethal (Kaelin and Ratcliffe 2008). In contrast, PHD- 1 and PHD –3 deficient mice are viable and appear normal at birth (Kaelin and Ratcliffe 2008).
The precise role of PHD –1 in NEB cell hyperplasia and whether it involves the O2 sensing mechanism is at present unknown. In oxidase deficient mice(gp91 phox, NOX2 k/o mice), inactivation of a gene involved in acute O2 sensing, that is HIF-independent, NEB cell responses to acute hypoxia were abrogated without causing NEB cell hyperplasia (Fu et al. 2000; Kazemian et al. 2001).We postulate that in PHD-1 deficient mice, NEB cell hyperplasia could be mediated via a HIF driven up regulation of neurogenic gene Mash-1, which in turn could lead to increased recruitment and differentiation from precursor cells(see above). In this scenario, the lack of PHD –1 may be compensated by over expression of PHD’s 2 and 3, especially under conditions of hypoxia since the lung develops in a relatively hypoxic environment (fetal pO2 20–30 mmHg) (Appelhoff et al. 2004). This in turn could lead to increased production of HIF’s and downstream activation of the Mash-1 gene. Alternatively, other HIF-independent mechanisms of PHD’s function may be involved since in breast cancer cells PHD –1 is induced by estrogen and can stimulate cell proliferation in vitro (Seth et al. 2002).
Hyperplasia of PNEC/NEB cells, suggesting altered function, has been described in a number of pediatric lung disorders including bronchopulmonary dysplasia, cystic fibrosis and asthma (Cutz et al. 2007a).In adults it has been linked to the pathogenesis of tobacco induced lung disease, pulmonary fibrosis and lung carcinogenesis (Cutz et al. 2008). It is of interest to note that the PHD- 1 deficient mouse appears to be a first example of a transgenic animal model with NEB cell hyperplasia. Therefore further studies using the PHD- 1 deficient mouse model may provide mechanistic insights in to the function of NEB cells under normal conditions and in a variety of pulmonary diseases.
Acknowledgements
Supported by grants from the Canadian Institutes for Health Research (MOP 15270) and Canadian Cystic Fibrosis to E.C. and H.Y and Wellcome Trust (Programme grant #091857 ) to P.R. and T.B.
References
- Appelhoff RJ, Ya-Min Tian, Raval RR, et al. Differential function of the prolyl hydroxylases PHD 1, PHD 2, and PHD 3 in the regulation of hypoxia –inducible factor. J Biol Chem. 2004;279:38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- Aragones J, Schneider M, VanGeyte K, et al. Deficiency or inhibition of oxygen sensor PHD 1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–189. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- Borges MW, Linnoila RI, van de Velde HJ, et al. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature. 1997;386:852–855. doi: 10.1038/386852a0. [DOI] [PubMed] [Google Scholar]
- Cutz E, Gillan JE, Bryan AC. Neuroendocrine cells in the developing human lung: morphologic and functional considerations. Pediatr Pulmonol. 1985;1:S21–S29. [PubMed] [Google Scholar]
- Cutz E, Yeger H, Pan J. Pulmonary neuroendocrine cell system in pediatric lung disease-recent advances. Pediatr Dev Pathol. 2007a;10:419–435. doi: 10.2350/07-04-0267.1. [DOI] [PubMed] [Google Scholar]
- Cutz E, Perrin DG, Pan J, Haas EA, Krous FK. Pulmonary neuroendocrine cells and neuroepithelial bodies in sudden infant death syndrome: potential markers of airway chemoreceptor dysfunction. Ped Dev Pathol. 2007b;10:106–116. doi: 10.2350/06-06-0113.1. [DOI] [PubMed] [Google Scholar]
- Cutz E, Yeger H, Pan J, Ito T. Pulmonary neuroendocrine cell system in health and disease. Curr Respir Med Rev. 2008;4:174–183. [Google Scholar]
- Fu XW, Wang D, Nurse CA, Dinauer MC, Cutz E. NADPH oxidase in an O2 sensor in airway chemoreceptors: evidence from K+ current modulation in wild type and oxidase deficient mice. Proc Natl Acad Sci U S A. 2000;97:4374–4379. doi: 10.1073/pnas.97.8.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Kazemian P, Stephenson R, Yeger H, Cutz E. Respiratory control in neonatal mice with NADPH oxidase deficiency. Respir Physiol. 2001;126:89–100. doi: 10.1016/s0034-5687(01)00205-5. [DOI] [PubMed] [Google Scholar]
- McGovern S, Pan J, Oliver G, Cutz E, Yeger H. The role of hypoxia and neurogenic genes(Mash-1 and Prox-1) in the developmental programming and maturation of pulmonary neuroendocrine cells in fetal mouse lung. Lab Invest. 2010;90:180–195. doi: 10.1038/labinvest.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Luk C, Kent G, Cutz E, Yeger H. Pulmonary neuroendocrine cells, airway innervation and smooth muscle are altered in Cftr null mice. Am J Respir Cell Mol Biol. 2006;35(3):320–6. doi: 10.1165/rcmb.2005-0468OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. Regulation of oxygen homeostasis by hypoxia-inducible factor. Physiology. 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- Seth P, Krop L, Porter D, Polyak K. Novel estrogen and tamoxifen induced genes identified by SAGE (Serial analysis of gene expression) Oncogene. 2002;21:836–843. doi: 10.1038/sj.onc.1205113. [DOI] [PubMed] [Google Scholar]
- Sorokin SP, Hoyt RF, Jr, Shaffer MJ. Ontogeny of neuroepithelial bodies: correlations with mitogenesis and innervation. Microsc Res Tech. 1997;37:43–61. doi: 10.1002/(SICI)1097-0029(19970401)37:1<43::AID-JEMT5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Wang D, Youngson C, Wong V, et al. NADPH oxidase and hydrogen peroxide-sensitive K+ channel may function as an oxygen sensor complex in airway chemoreceptors and small cell carcinoma cell lines. Proc Natl Acad Sci U S A. 1996;93:13182–13187. doi: 10.1073/pnas.93.23.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen sensing mechanisms. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngson C, Nurse C, Yeger H, Cutz E. Oxygen sensing in airway chemoreceptors. Nature. 1993;365:153–156. doi: 10.1038/365153a0. [DOI] [PubMed] [Google Scholar]