Abstract
The early immune events in response to infective larvae of the parasitic helminth Schistosoma mansoni are poorly understood, but here for the first time we report on the potential of products released by schistosome larvae (material released in the first 3 h after transformation [0-3hRP]) to stimulate the maturation of dendritic cells (DC) and alter their T-cell-polarizing function. This was performed in comparison with lipopolysaccharide (LPS) and zymosan A, which classically activate DC to prime for Th1- and Th2-type responses, respectively. In our study, immature bone marrow-derived DC stimulated in vitro with 0-3hRP exhibited up-regulated expression of major histocompatibility complex class II, CD40, and CD86 and increased production of interleukin 12p40 (IL-12p40) and IL-6, albeit at lower levels than in response to LPS or zymosan A. Using an in vitro ovalbumin peptide-restricted priming assay, DC matured with 0-3hRP exhibited a potent capacity to drive Th2 polarization of CD4+ cells from DO11.10 transgenic mice. This was characterized by increased IL-4 production (but not gamma interferon) of a magnitude similar to that primed by DC matured with zymosan A. Inoculation of DO11.10 mice with 0-3hRP-activated DC pulsed with ovalbumin peptide also led to the development of a Th2-type polarized response in the skin-draining lymph nodes and spleen. However, ligation of CD40 on DC by anti-CD40 antibody treatment reversed the ability of 0-3hRP-activated DC to prime for Th2-type responses and instead caused the induction of a more Th1-type response.
Dendritic cells (DC) are involved in the initiation of both innate and acquired immune responses and reside in an immature state in peripheral sites, such as the skin and lungs, where they are optimally located to act as sentinels against potential pathogens (3). Studies of both human and murine DC demonstrate that they become activated and mature upon contact with a variety of different microbe-derived stimuli, including lipopolysaccharide (LPS) (34), zymosan A (10), and unmethylated CpG DNA (32). The process of DC maturation is conventionally associated with up-regulation of major histocompatibility complex (MHC) class II and costimulatory molecule surface expression (10, 32, 34), but it also depends upon the type of microbial stimulus and can result in the differentiation of DC to distinct phenotypic states (9, 6, 19, 24, 30, 34). The maturation response is so specific that DC can even discriminate between different cellular states of the same pathogen (9). Associated with this differential maturation is the acquisition by DC of the capacity to prime polarized T helper (Th) cell responses. Thus, depending upon the initial maturation signal, DC can prime Th cells to differentiate toward the Th1, Th2, or T-regulatory pole (4, 9, 6, 19, 23, 24, 30, 34). Although some of the factors that promote Th1 development (e.g., polarizing cytokines) are well defined, those that promote Th2 responses are less well understood. Feedback from Th cells also plays an important role in the phenotypic state of DC and is likely to be critical in determining the characteristics of the ensuing immune response. In this respect, the CD40-CD154 partnership is thought to be a major factor in the activation and function of DC, which can result in increased interleukin 12 (IL-12) production with the potential to drive Th1 polarization (5, 10, 16).
Chronic diseases caused by helminth infections, such as filariasis and schistosomiasis, are generally associated with strong Th2-type responses (22). In keeping with this, the filarial secretory product (ES-62) and the soluble fraction of schistosome eggs clearly activate DC to drive the differentiation of Th cell responses toward the Th2 pole (6, 19, 34). Both of these helminth products are derived from parasite stages associated with chronic or long-term exposure to the host. In contrast, it is not known whether the initial and early infectious stages of these parasites activate antigen-presenting cells (APCs), enabling them to drive polarized Th immune responses. In this context, schistosome larvae in the skin site of exposure induce a vigorous dermal inflammatory reaction and cellular influx that includes DC and macrophages with putative APC functions (11, 26, 28). In addition, there is evidence for the activation and maturation of DC in the skin, as determined by up-regulation of MHC class II and CD86 expression (2, 11, 12, 28). However, it is not known what larval components are responsible for activating these DC populations and how early immune events affect the phenotype of the ensuing acquired response.
In the absence of readily available quantities of immature epidermally derived Langerhans cells and based upon the acceptance that all subsets of DC are functionally plastic, we decided to use murine bone marrow-derived DC (BM-DC) to investigate the ability to activate potential APCs from material released by infectious schistosome cercariae within the first 3 h after transformation (0-3hRP). This was judged by cytokine production, costimulatory molecule expression, and T-cell-polarizing function. We show that 0-3hRP activates DC, which strongly drive Th cells toward the Th2 pole. However, ligation of CD40 on DC activated by 0-3hRP instead favors differentiation toward a more Th1-type response.
MATERIALS AND METHODS
Parasite material.
Cercariae of Schistosoma mansoni were concentrated by sedimentation on ice for 1 h and washed three times with sterile water. The parasites were mechanically transformed by vortexing them for 90 s in RPMI 1640 containing 200 U of penicillin/ml and 100 μg of streptomycin (Invitrogen, Paisley, United Kingdom)/ml (RPMI-0) and cultured in vitro for 3 h at 37°C and 5% CO2. The culture supernatant was concentrated 50-fold using centrifugal filter units (Ultrafree-MC with a 5-kDa cutoff; Millipore, Watford, United Kingdom); the preparation was designated 0-3hRP. As a control, an equivalent volume of RPMI-0 containing no parasite material was concentrated as described above (RPMIc). The preparations were sonicated (21kHz at 6.5-μm amplitude) for 3 min and centrifuged (100,000 × g) for 1 h to yield the soluble material. The protein content was determined using Coomasie Plus-200 (Perbio Science UK Ltd., Tattenhall, United Kingdom), while the endotoxin concentration was determined using a Pyrogent Plus Limulus Amoebocyte Lysate test kit (BioWhittaker, Wokingham, United Kingdom).
Animals and cell lines.
All mice were maintained at the University of York. BALB/c mice were obtained from Harlan UK Ltd. (Bicester, United Kingdom), and DO11.10 αβ TCR transgenic mice (on a BALB/c background) were a gift from Paul Garside (University of Glasgow, Glasgow, United Kingdom). All experiments were carried out in accordance with the guidelines of the United Kingdom Animals (Scientific Procedures) Act 1986. The fibroblast cell lines 3T3-CD154 and 3T3-SAMEN (control) were gifts from P. Hwu (National Cancer Institute, Bethesda, Md.) and were derived from NIH 3T3 cells by stable transfection with murine CD154 or empty vector, respectively.
Generation of DC from bone marrow.
BM-DC were prepared using a method adapted from that of Son et al. (31). Briefly, bone marrow cells were removed from the femur and resuspended in RPMI 1640 containing 2 mM l-glutamine (Invitrogen), 200 U of penicillin/ml, 100 μg of streptomycin/ml, 50 μM 2-mercaptoethanol (Invitrogen), and 10% heat-inactivated low-endotoxin fetal calf serum (Harlan Seralab, Loughborough, United Kingdom) (RPMI-10), and the red blood cells were lysed with ammonium chloride buffer (0.15 M NH4Cl, 10 mM KHCO3, and 0.1 mM Na2EDTA). The bone marrow cells were then washed three times with RPMI-10 before being cultured in six-well plates (Nalge Nunc International Corp., Naperville, Ill.) at 1.8 × 106/well in 3 ml of RPMI-10 plus 20 ng of granulocyte-macrophage colony-stimulating factor (GM-CSF) (Peprotech, London, United Kingdom)/ml. On day 4, a further 3 ml of RPMI-10 plus 40 ng of GM-CSF/ml was added, and the cells were cultured for a further 2 days (i.e., to day 6). This protocol results in pluripotent DC that can elicit mixed Th responses in the absence of added microbial stimuli and can drive Th1 or Th2 responses following maturation with different microbial agents.
DC stimulation and maturation.
DC were seeded into 24- or 96-well plates (Nalge Nunc) at 106/ml in RPMI-10 plus 20 ng of GM-CSF/ml. The cells were cultured alone for 24 h or in the presence of 0-3hRP (40 μg/ml), an equivalent volume of RPMIc, LPS (1 or 100 ng/ml; from Escherichia coli strain 0111:B4; Sigma-Aldrich, Poole, United Kingdom), or zymosan A (2 or 5 μg/ml; from Saccharomyces cerevisae; Sigma-Aldrich). In pilot experiments, the concentrations of 0-3hRP were compared over 3 log units (0.05 to 100 μg/ml), and 40 μg/ml was found to be optimal in terms of the amount of cytokine produced (unpublished observations). In order to control for low levels of naturally occurring endotoxin in 0-3hRP (4.9 ± 0.9 endotoxin units/40 μg of protein), DC and 0-3hRP were cultured in the presence of polymyxin B (PMB; 3 μg/ml; Sigma-Aldrich). After culture with different stimuli, the mature DC were analyzed for the expression of surface markers and intracellular IL-12p40, while the culture supernatants were stored at −20°C prior to cytokine detection by enzyme-linked immunosorbent assay (ELISA). As negative controls, DC were cultured with PMB alone, with PMB plus RPMIc, or with PMB plus LPS (1 to 10 ng/ml) containing a concentration of endotoxin equivalent to that of 0-3hRP. However, no significant difference was observed in the cytokine production, or the expression of surface markers, by these control groups compared to DC-medium. Therefore, only control data for DC-medium and/or DC-RPMIc are shown. In some experiments, DC plus 0-3hRP and DC-medium were cultured either on a monolayer of CD154+ (or control) fibroblasts or in the presence of low-endotoxin anti-CD40 (αCD40) monoclonal antibody (MAb) (5 μg/ml; clone HM40-3; BD PharMingen, Oxford, United Kingdom).
Phenotypic characterization of cell populations.
Flow cytometric analysis of various surface markers using specific MAbs or an irrelevant isotype-matched MAb was performed as previously described (11, 12). The antibodies were fluorescein isothiocyanate- or phycoerythrin-conjugated CD11c (HL3; BD PharMingen), CD40 (3/23; BD PharMingen), CD80 (RMMP-1; Caltag-Medsystems, Towcester, Ltd.), and CD86 (RMMP-2; Caltag) or biotin-conjugated I-Ab,d (28-16-8S; Caltag) or OX40L (RM134L; BD PharMingen), followed by streptavidin-conjugated Quantum Red (Sigma). Analysis was performed using a Coulter Epics XL (Beckman Coulter, High Wycombe, United Kingdom).
In vitro T-cell-priming assay.
In order to determine the T-cell-polarizing function of differentially activated BM-DC, an antigen-restricted assay was used incorporating CD4+ T cells from DO11.10 transgenic mice, which express a T-cell receptor (TCR) specific for OVA peptide (323-ISQAVHAAHAEINEAGR-339) in complex with I-Ad MHC class II. CD4+ cells were purified from spleens of naïve DO11.10 mice using magnetic-activated cell sorting; briefly, splenocytes were incubated with αCD4 microbeads for 15 min at 4°C, and positive cells were selected by passage through a MACS MS+ column (Miltenyi Biotec, Bisley, United Kingdom). Following overnight exposure to microbe-derived stimuli, BM-DC were washed three times and irradiated (1,500 krads) before being cultured in vitro (2.5 × 104 cells/ml) with CD4+ splenocytes (2.5 × 105/ml) in the presence or absence of OVA peptide (10 nM; Albachem, University of Edinburgh, United Kingdom). This dose of OVA has previously been shown to permit DC to prime for both Th1 and Th2 responses (4, 34). A DC/T-cell ratio of 1:10 was employed, since previous studies had demonstrated that under these culture conditions, DC-media prime for a mixed Th response (4, 34). After 72 h, phorbol myristate acetate (50 ng/ml) and ionomycin (500 ng/ml; Sigma-Aldrich) were added to the cells, and the culture supernatants were harvested 24 h later.
In vivo T-cell-priming assay.
For assays of in vivo T-cell priming, DC were activated with different stimuli in the presence of OVA peptide (100 nM). Culture of DC with OVA alone did not result in increased cytokine production, confirming that the OVA was endotoxin free (data not shown). After being thoroughly washed, differentially activated BM-DC (3 × 105 to 4.5 × 105 cells in 100 μl) were delivered to recipient naïve DO11.10 mice by subcutaneous injection over the sternum. After 7 days, single-cell suspensions from the skin-draining lymph nodes (sdLN) and spleens were prepared and cultured in 96-well plates (sdLN cells, 2 × 105/well; splenocytes, 4 × 105/well) alone, with OVA peptide (100 to 10,000 nM), or with plate-bound anti-CD3ɛ MAb (0.25 μg/well; clone 145-2C11; BD PharMingen). The cells were cultured in RPMI-0 supplemented with 2 mM l-glutamine, 50 μM 2-mercaptoethanol, and 3% heat-inactivated mouse serum, and 3 days later the culture supernatants were harvested.
Cytokine detection by ELISA.
Culture supernatants were tested as appropriate for the production of IL-1β, IL-4, IL-5, IL-6, IL-10, IL-12p40, IL-12p70, tumor necrosis factor alpha, and gamma interferon (IFN-γ), using ELISA (11, 12). IL-12p70 production was determined as for IL-12p40 but incorporated an IL-12p70-specific capture antibody (clone 9A5; BD PharMingen).
Statistics.
Comparisons of data within an individual experiment were tested for significance using Student's t test. Alternatively, data expressed as the mean log increase (n-fold) in cytokine production from several experiments were tested for difference from a theoretical value of 0 (equivalent to no increase or decrease) using the one-sample Student t test. The following nomenclature was used to indicate significant values: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
RESULTS
0-3hRP induces partial up-regulation of MHC class II and costimulatory-molecule expression on DC.
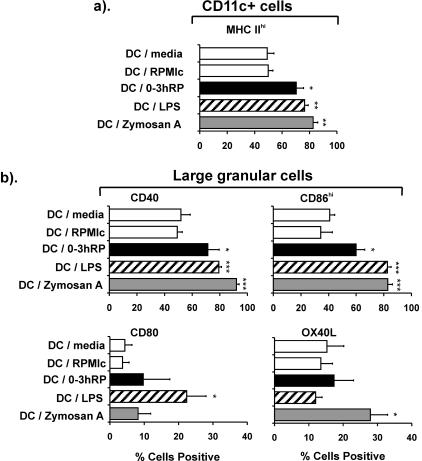
The ability of 0-3hRP to up-regulate the expression of MHC class II and costimulatory molecules on BM-DC was tested compared to the microbe-derived stimuli LPS and zymosan A, chosen for their known ability to activate DC to prime for Th1- and Th2-type responses, respectively (10, 19, 23, 34). DC were cultured in medium alone (DC-medium) or exposed to 0-3hRP (DC plus 0-3hRP), LPS (DC-LPS), zymosan A (DC-zymosan A), or the concentrated medium RPMIc (DC-RPMIc) for 24 h prior to analysis of the phenotype. The proportions of CD11c+ DC plus 0-3hRP, DC-LPS, and DC-zymosan that expressed high levels of MHC class II were all greater (P < 0.05 to 0.01) than the negative controls (DC-medium and DC-RPMIc), although fewer DC plus 0-3hRP were MHChi (70%) than DC-LPS (76%) or DC-zymosan A (82%) (Fig. 1a). CD11c+ cells within the DC population comprised >95% of all MHC class II+ events and were characterized by their large size and granularity (data not shown). Analysis of this DC population revealed that 0-3hRP stimulated significant increases in the expression of CD40 and CD86hi (P < 0.05), although LPS and zymosan A stimulated greater up-regulation (Fig. 1b). Neither CD80 nor OX40L expression was significantly upregulated by activation with 0-3hRP, whereas LPS stimulated up-regulation of CD80 (P < 0.05), but not OX40L (P > 0.05) and zymosan A, stimulated up-regulation of OX40L (P < 0.05), but not CD80 (P > 0.05) (Fig. 1b).
FIG. 1.
0-3hRP stimulates up-regulation of surface maturation markers upon DC. The percentages of CD11c+ cells expressing MHC class IIhi were determined in DC activated with medium only, RPMIc, 0-3hRP (40 μg/ml), LPS (100 ng/ml), or zymosan A (2 μg/ml) (a). Alternatively, the percentage of large granular cells known to be CD11c+ expressing CD40, CD80, CD86hi, or OX40L is shown (b). Data are the mean plus standard error of the mean of three experiments, and levels of significance are between DC-RPMIc and the DC test groups.
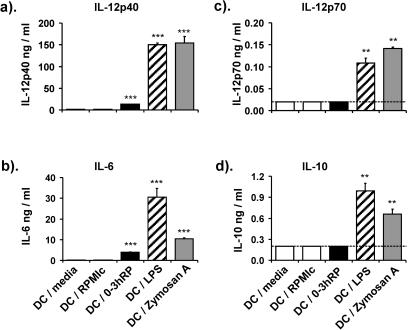
0-3hRP induces lower levels of cytokine production than other microbial stimuli.
0-3hRP stimulated increased IL-12p40 production (9-fold) than the two negative controls, although this increase was lower than those stimulated by LPS (96-fold) and zymosan A (99-fold) (Fig. 2a) (all P < 0.001). Consistent with this, the percentage of CD11c+ cells that were IL-12p40+ increased, and they had a higher intensity of cytokine staining, as shown by flow cytometric analysis; DC plus 0-3hRP were 13% IL-12p40+ with a mean fluorescence value of 1.5 compared to DC-RPMIc (4.6%; 1.1), DC-LPS (58%; 4.8), and DC-zymosan (56%; 5.2) (data not shown). 0-3hRP also stimulated an increase in IL-6 (28-fold), but both LPS (218-fold) and zymosan A (75-fold) stimulated secretion of greater amounts (Fig. 2b) (all P < 0.001). Detectable quantities of IL-12p70 and IL-10 were secreted only in response to LPS and zymosan A and not 0-3hRP (Fig. 2c and d).
FIG. 2.
Cytokine production by DC following activation with 0-3hRP, LPS, and zymosan A. DC were cultured with medium only or with different stimuli (as given in the legend to Fig. 1), and the production of IL-12p40, IL-12p70, IL-6, and IL-10 was determined by ELISA. Data are the mean plus standard error of the mean of four individual wells and are representative of at least three experiments. Levels of significance are between DC-RPMIc control and test groups. The dotted line represents the lower limit of ELISA detection.
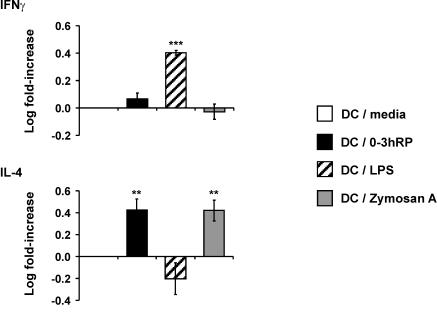
0-3hRP instructs DC to prime for Th2 polarization in vitro.
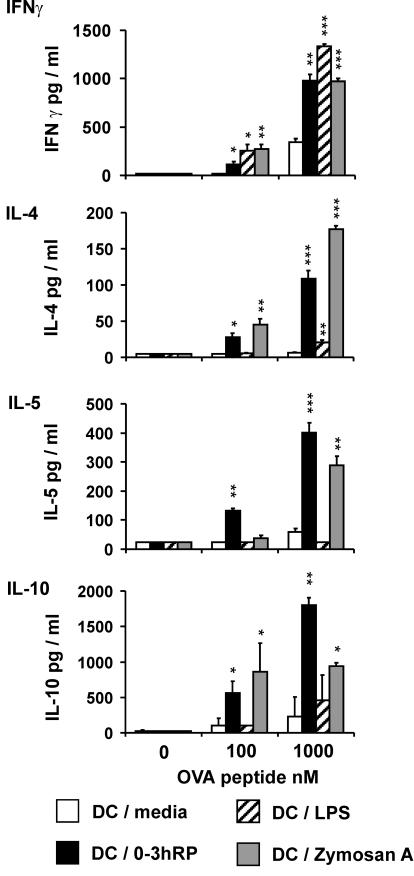
An in vitro antigen-restricted assay was used to determine whether DC plus 0-3hRP would prime for a distinct Th phenotype compared to DC-medium, DC-LPS, and DC-zymosan A. In this assay, DC activated with the different stimuli were cultured with CD4+ splenocytes from DO11.10 αβ TCR transgenic mice in the presence of their specific antigen (OVA peptide), and the resulting profiles of Th1 (IFN-γ) and Th2 (IL-4) signature cytokines were determined. IFN-γ production by CD4+ cells, expressed as a log (n-fold) increase in cytokine over that primed by DC-medium, was dramatically increased following priming by DC-LPS (P < 0.001), but not zymosan A (Fig. 3). DC plus 0-3hRP appeared to prime for a slight increase in IFN-γ, but this was not significant (P > 0.05) (Fig. 3). In contrast, DC plus 0-3hRP primed CD4+ cells to secrete increased IL-4 (P < 0.01), similar to that primed by DC-zymosan A, whereas DC-LPS appeared to cause a decreased log (n-fold) change in IL-4 (Fig. 3). Cultures of DC alone or CD4+ cells alone did not produce detectable levels of either cytokine (data not shown).
FIG. 3.
DC activated with 0-3hRP polarize naïve CD4+ cells toward a Th2 phenotype in vitro. Differentially activated mDC (stimuli as given in the legend to Fig. 1) were cocultured with CD4+ cells from DO11.10 mice in the presence of OVA peptide (10 nM). Culture supernatants were analyzed by ELISA for IFN-γ and IL-4. Data are the mean ± standard error of the mean of the log increase (n-fold) in cytokine production primed by test groups relative to DC-medium and are derived from three experiments. The peaks of cytokine production varied between experiments but were detected in the following ranges: IFN-γ, 2,500 to 8,000 pg/ml, and IL-4, 800 to 1,600 pg/ml. Levels of significance are between DC-medium and the DC test groups.
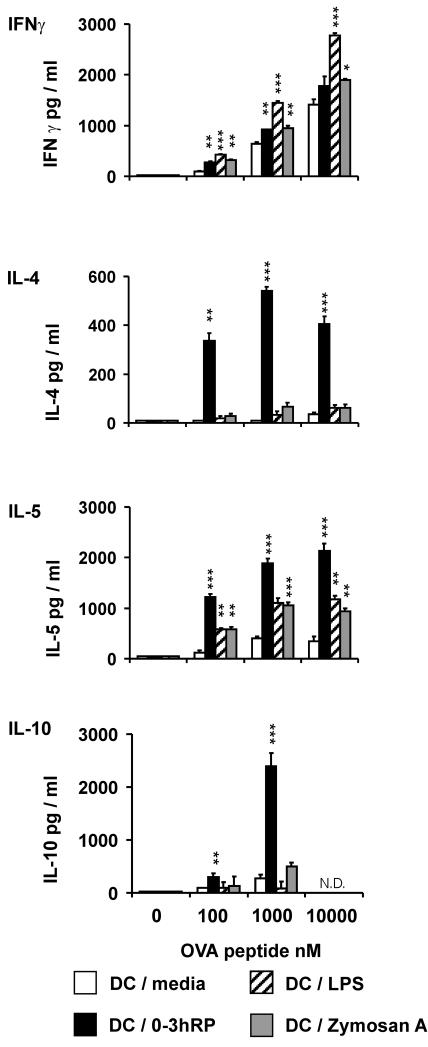
DC activated with 0-3hRP drive T-helper cell differentiation toward the Th2 pole.
The ability of differentially activated DC pulsed with OVA to prime Th cell responses in the sdLN of DO11.10 mice was examined following their subcutaneous delivery. The location was employed to approximate events that might follow percutaneous infection with schistosomes (11). The outcome of in vivo T-cell priming was determined 7 days after delivery of DC by restimulation of sdLN cells in vitro with OVA peptide. Following inoculation with DC-LPS, sdLN cells produced substantially more IFN-γ than cells from mice injected with DC-medium (Fig. 4). This ranged from a two-fold increase, using 10,000 nM OVA, up to a five-fold increase using only 100 nM OVA. Both DC plus 0-3hRP and DC-zymosan A also primed for increased IFN-γ production by sdLN cells (1.3- to 3-fold), but the levels were much lower than those primed by DC-LPS. Analysis of Th2 cytokines revealed that sdLN cells from recipients of DC plus 0-3hRP produced dramatically increased levels of IL-4 (11- to 54-fold), IL-5 (5- to 10-fold), and IL-10 (3- to 9-fold) compared to cells from recipients of DC-medium (Fig. 4). In contrast, sdLN cells from recipients of DC-LPS and DC-zymosan A did not secrete abundant IL-4 or IL-10 (Fig. 4), although they both caused a limited increase in IL-5 production (three- to five-fold) that was much less than that primed by DC plus 0-3hRP (Fig. 4).
FIG. 4.
DC activated with 0-3hRP drive polarization toward a Th2 phenotype in vivo. Naïve DO11.10 mice were inoculated subcutaneously with differentially activated, OVA-pulsed DC (stimuli as given in the legend to Fig. 1). After 7 days, sdLN cells were restimulated in vitro with OVA (0 to 10,000 nM) for 72 h. Culture supernatants were analyzed by ELISA for IFN-γ, IL-4, IL-5, and IL-10. Data are the mean plus standard error of the mean of three individual wells and are representative of four experiments. Levels of significance are between DC-medium and the DC test groups. N.D., not done.
To determine if subcutaneous injection of differentially matured DC also results in polarized immune responses in other sites, splenocytes from recipient mice were restimulated in vitro as for sdLN cells. Splenocytes from mice injected with DC plus 0-3hRP, DC-LPS, and DC-zymosan A all produced higher levels of IFN-γ than cells from recipients of DC-medium, although the greatest amounts were primed by DC-zymosan and/or DC-LPS (Fig. 5). Analysis of Th2 cytokines demonstrated that splenocytes from mice injected with DC plus 0-3hRP again produced dramatically increased levels of IL-4 compared to DC-medium and DC-LPS (Fig. 5). However, in contrast to the situation in the sdLN, DC-zymosan A also primed splenocytes for the production of high levels of IL-4. Splenocytes from recipients of DC plus 0-3hRP and DC-zymosan A produced abundant levels of IL-5 and IL-10, whereas cells from recipients of DC-medium and DC-LPS did not (Fig. 5). Splenocytes cultured with αCD3 antibody (in place of OVA) secreted profiles of cytokines that were comparable to those of their OVA-restimulated counterparts (data not shown).
FIG. 5.
DC activated with 0-3hRP drive systemic Th2 polarization. DO11.10 mice were inoculated subcutaneously with differentially activated, OVA-pulsed DC (stimuli as given in the legend to Fig. 1). After 7 days, splenocytes were restimulated in vitro with OVA (0 to 1,000 nM) for 72 h. Supernatants were analyzed by ELISA for IFN-γ, IL-4, IL-5, and IL-10. Data are the mean plus standard error of the mean of three individual wells and are representative of three experiments. Levels of significance are between DC-medium and the DC test groups.
These data sets clearly show that DC plus 0-3hRP drive both local and systemic immune responses strongly toward the Th2 pole in vivo, while DC-LPS induce more Th1-type responses. In contrast, DC-zymosan A consistently drive a nonpolarized response in the sdLN but a Th2-type response in the spleen.
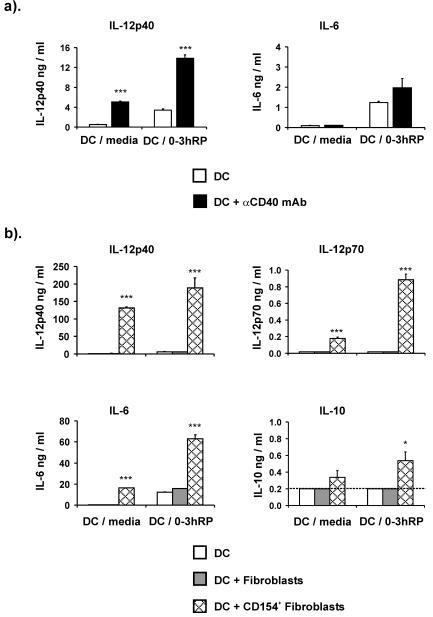
Ligation of CD40 on DC enhances cytokine production.
The effect of ligating CD40 upon the DC activation phenotype was assessed by culturing cells with stimulatory αCD40 MAb. The presence of the αCD40 MAb substantially increased IL-12p40 production by both DC-medium (10-fold) and DC plus 0-3hRP (4-fold) but had little effect upon IL-6 production by DC-medium (1.3-fold) or DC plus 0-3hRP (1.6-fold) (Fig. 6a). No IL-12p70 or IL-10 was detected in any supernatants (data not shown).
FIG. 6.
Ligation of CD40 increases IL-12 production by DC stimulated with 0-3hRP. DC-medium or DC plus 0-3hRP were matured in the presence or absence of αCD40 MAb (5 μg/ml) (a) or on a monolayer of CD154+ or control fibroblasts (b). Culture supernatants were tested by ELISA for IL-12p40, IL-12p70, IL-6, and IL-10; are shown as the mean plus standard error of the mean of three or more wells; and are representative of four experiments. Levels of significance are between untreated DC-medium or DC plus 0-3hRP and the respective DCs cultured with CD154+ fibroblasts or αCD40 MAb. The dotted line represents the lower limit of ELISA detection.
We also investigated the effect of CD40 ligation by coculturing DC with a fibroblast cell line transfected to express CD154. As expected, culture of DC-medium matured alone or with control fibroblasts did not result in appreciable levels of cytokine production, but the presence of CD154+ fibroblasts resulted in a dramatic increase in the production of IL-12p40, IL-12p70, and IL-6 (Fig. 6b) (all P < 0.001). DC plus 0-3hRP matured alone or with control fibroblasts both released increased levels of IL-12p40 and IL-6 compared to their DC-medium counterparts. However, coculture of DC plus 0-3hRP with CD154+ fibroblasts also caused a dramatic increase in the amounts of IL-12p40, IL-12p70, and IL-6 (all P < 0.001) and a slight increase in IL-10 (P < 0.05) (Fig. 6b).
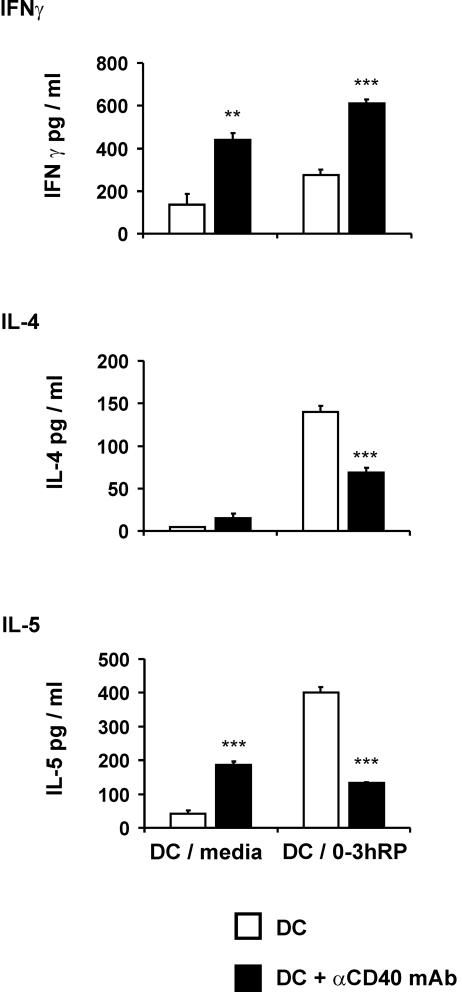
Culture of DC plus 0-3hRP with αCD40 antibody impairs their ability to drive Th2 responses.
The in vivo method of lymphocyte priming in DO11.10 TCR transgenic mice was used to examine the effect of CD40 ligation upon the T-cell-polarizing functions of DC-medium and DC plus 0-3hRP. For this purpose αCD40 MAb was used to ligate CD40 during initial DC maturation. Upon restimulation with OVA in vitro, sdLN cells from mice injected with αCD40-treated DC-medium and αCD40-treated DC plus 0-3hRP produced more IFN-γ than cells from mice injected with their respective untreated DC counterparts (Fig. 7). DC-medium treated with αCD40 MAb also primed sdLN cells to produce increased levels of IL-4 and IL-5 relative to sdLN cells from mice injected with untreated DC-medium (Fig. 7). However, although very high levels of IL-4 and IL-5 were primed for by untreated DC plus 0-3hRP, sdLN cells from recipients of αCD40-treated DC plus 0-3hRP produced significantly smaller quantities of these Th2 cytokines (Fig. 7). Therefore, αCD40 MAb treatment of DC-medium increases their ability to prime in vivo for both Th1 and Th2 cytokine production, whereas the same treatment reverses the ability of DC plus 0-3hRP to prime for Th2 responses, instead resulting in a more Th1-type profile of cytokine production.
FIG. 7.
Ligation of CD40 reverses the ability of DC activated with 0-3hRP to drive Th2 polarization. DO11.10 mice were inoculated subcutaneously with OVA peptide-pulsed DC-medium or DC plus 0-3hRP in the presence or absence of αCD40 MAb. After 7 days, sdLN cells were restimulated in vitro with OVA peptide (0 to 1,000 nM) for 72 h. Culture supernatants were tested by ELISA for IFN-γ, IL-4, and IL-5. Data are presented as the mean plus standard error of the mean of three individual wells and are representative of two experiments. Levels of significance are between DC-medium or DC plus 0-3hRP cultured alone and the respective cultures treated with αCD40 MAb.
DISCUSSION
It is widely accepted that various types of microbe-derived stimuli can differentially activate DC, resulting in contrasting maturation phenotypes capable of driving polarized Th responses (4, 6, 19, 24, 30, 34). In this paper, we are the first to report that material released by transforming schistosome larvae (0-3hRP) activates BM-DC and instructs them to drive acquired immune responses toward the Th2 pole both in vitro and in vivo. In vitro priming of CD4+ cells from DO11.10 TCR transgenic mice with DC plus 0-3hRP resulted in the secretion of IL-4 (but not IFN-γ) in quantities at least equal to that primed by the Th2 inducer zymosan A. In contrast, DC activated with the classical Th1 inducer LPS (19, 33) promoted greater levels of IFN-γ, but not IL-4. Furthermore, DC plus 0-3hRP also exhibited a strong Th2-driving function in vivo following their adoptive transfer into naïve DO11.10 mice. In this respect, DC plus 0-3hRP primed for a substantial increase in IL-4, IL-5, and IL-10 production by sdLN cells and splenocytes compared to DC-medium and DC-LPS.
While the priming of Th cells in vitro by differentially activated DC in our study clearly leads to the development of polarized Th1 and Th2 responses, it is evident that priming in vivo also results in the limited up-regulation of cytokines characteristic of the opposite pole. For example, DC plus 0-3hRP and Th2-inducing DC-zymosan A stimulated limited IFN-γ secretion in addition to abundant IL-4 and IL-5. Therefore, differentially activated DC appear to prime for mixed, albeit polarized, Th responses in vivo along a continuum between the extreme Th1 and Th2 poles. The position of the immune response along this continuum is likely to depend upon the maturation status, or phenotype, of the DC population. In this respect, the ability of DC plus 0-3hRP to prime toward the Th2 pole occurs in the absence of high-level cytokine production and suboptimal levels of CD40 and CD86 expression. This phenotype partially resembles the limited activation state of DC stimulated with schistosome egg antigen and filarial ES-62 (19, 34). Consequently, our data on DC plus 0-3hRP support the view that Th2 differentiation represents a T-cell default pathway that occurs in the absence of both strong cytokine production and costimulatory molecule expression (15, 19, 30, 34).
However, in contrast to the default hypothesis, our data also show that DC-zymosan A “instruct ” for Th2 polarization, despite producing abundant Th1-polarizing IL-12 (both bioactive IL-12p70 and potentially inhibitory IL-12p40 homodimers). This lends support to an alternative theory that DC actively drive Th2 polarization, possibly through expression of specific factors (e.g., surface molecules, such as Jagged1) (1). Nevertheless, our data do not allow the identification of these factors. The greatest levels of IL-6 were made by Th1-inducing DC-LPS, yet this cytokine has been associated with the development of Th2-type responses (7, 8). In contrast, abundant IL-12, which is known to drive Th1-type responses (6), and IL-10, a cytokine thought to contribute to Th2 polarization (23), were produced by both DC-LPS and Th2-inducing DC-zymosan A. The role of costimulatory molecules in T-cell polarization is also unclear. Although in our study only LPS stimulated DC to up-regulate CD80 expression, supporting a role in Th1 polarization (18), other studies suggest that the expression of CD80 and CD86 on DC acts purely as a costimulatory rather than polarizing signal (34). On the other hand, the interaction of OX40 with OX40L has been implicated in Th2 polarization (6, 13), and we observed that OX40L was up-regulated on DC-zymosan A. Nevertheless, OX40L was not increased on DC plus 0-3hRP, calling into question its necessity for the development of all Th2-type responses. Furthermore, our data do not support a role for low-density MHC class II expression in Th2 polarization (29), since although DC plus 0-3hRP stimulated the least up-regulation of MHC class II, DC-zymosan A contained the greatest proportion of MHC class IIhi cells. Although we have been unable to identify a shared feature of DC plus 0-3hRP and DC-zymosan A that explains their ability to induce Th2-type responses, an essential Th2-driving factor common to both cannot be ruled out. However, it is likely that there are multiple mechanisms through which DC prime for Th2-type responses that depend upon their overall level of activation.
Despite zymosan A being described as a Th2-driving stimulus (23), we found that in the sdLN DC-zymosan A failed to prime a strong Th2-type response, although the production of Th2-associated cytokines was evident in the spleen and following in vitro priming. One explanation is that the kinetics of immune priming induced by DC-zymosan A differs from that induced by DC plus 0-3hRP, so that recall responses were no longer detected in the sdLN 7 days postdelivery. Alternatively, the lack of recall responsiveness in the sdLN may be evidence for the induction of T-cell regulation. In this respect, zymosan A is thought to signal through TLR-2, a receptor associated with the instruction of DC to prime regulatory T-cell responses (14, 33). Ultimately, our data suggest that DC plus 0-3hRP and DC-zymosan A have overlapping yet unique phenotypes and highlight the fact that the site of analysis of recall responses is critical when assessing the outcome of T-cell priming by antigen-pulsed DC in vivo.
One of the most critical factors in the activation of DC and the priming and polarization of Th cells is the interaction of CD40 with CD154. In addition to recording elevated expression of CD40 on DC after activation with 0-3hRP, we found that ligation of CD40 on DC plus 0-3hRP (using either αCD40 MAb or CD154+ fibroblasts) led to a dramatic increase in the production of IL-12 by DC. Treatment of DC plus 0-3hRP with αCD40 MAb caused them to prime Th cells in vivo to secrete high levels of IFN-γ, thereby effectively reversing their ability to prime for Th2 polarization. Similar effects were observed following αCD40 MAb treatment of DC-zymosan A (data not shown). Ligation of CD40 following MAb treatment appears to exert this pro-Th1 effect by preferentially increasing the production of IL-12 (Fig. 6b) (5, 29). However, since the effect of MAb treatment would likely mimic that of the T-cell-DC interaction in vivo that results in a Th2 response, it is possible that the αCD40 MAb masks CD40, thus blocking signals received by the T cell through CD154, which may be important for Th2 development (20, 27). Either way, our data imply that manipulation of the CD40-CD154 interaction favors the development of Th1 responses at the expense of Th2 and underline the importance of signals received through CD40 on the maturation status of activated DC.
Our observations have relevance for understanding how cercariae first stimulate innate immune cells in the skin (including Langerhans cells and dermal DC) following natural infection and how these cells prime the acquired immune response. We show that secretions from invading cercariae (i.e., 0-3hRP) favor the activation of APCs that prime for Th2-type responses. Indeed, early responses (day 4) in the sdLN following normal infection show evidence of transient Th2 cytokine production in addition to Th1-associated IFN-γ (11), although multiple exposures may further promote Th2 cytokine production, as has been observed to occur following multiple exposures to a related schistosome species, Trichobilharzia regentii (17). In addition to possessing this instructional effect on APCs, proteases contained within 0-3hRP directly stimulate IL-4 production and histamine release by mast cells (21), suggesting a general propensity to propagate Th2 responses. This further supports a previous report (25) that 0-3hRP is less prone to induce Th1-type responses in vitro than larval antigens when used to restimulate sdLN cells taken from mice 4 to 6 days after larval exposure. The identity of the Th2-inducing ligand(s) in 0-3hRP is not known, but it is a relatively heterogeneous mixture of molecules. A few DC-activating ligands of helminth origin have been identified so far (see references 14, 22, and 30 for reviews), illustrating the diversity of molecular structures which can stimulate DC to prime for Th2-type responses. One recently described product of schistosome eggs that is a ligand of TLR2 is lyso-phosphatidylserine (33), but we do not yet know if it is also present in 0-3hRP. Parasite-derived prostaglandins are also a possible component of 0-3hRP and may affect DC polarization (2, 14), but since these eicosanoids are up-regulated by contact with fatty acids of skin origin and our 0-3hRP was obtained without skin contact, we are of the opinion that parasite-derived prostaglandins are unlikely to be a significant factor in our experiments.
Finally, it is known that protective immunity induced by radiation-attenuated S. mansoni larvae is associated with the development of IL-12-dependent Th1-type responses initiated in the skin (11, 12, 26). In this context, our observations that ligation of CD40 on DC activated with 0-3hRP causes the up-regulation of IL-12 production and the ability to prime for Th1-type responses may provide a route by which we can favor the development of protective Th1-associated immunity.
Acknowledgments
This work was supported by a Wellcome Trust University Fellowship to A.P.M. (grant 056213), and S. J. J. was supported by a Ph.D. studentship from the Biotechnology and Biological Sciences Research Council of the United Kingdom.
We thank the staff of the University of York Animal Unit; Ann Bamford (University of York) for maintenance of the parasite life cycle; Melanie Leech and Richard Grencis (University of Manchester); and Ayako Wakatsuki and Kevin Rigley (Edward Jenner Institute for Vaccine Research) for providing advice on DC culture.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Amsen, D., J. M. Blander, G. R. Lee, K. Tanigaki, T. Honjo, and R. A. Flavell. 2004. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell 117:515-526. [DOI] [PubMed] [Google Scholar]
- 2.Angeli, V., C. Faveeuw, O. Roye, J. Fontaine, E. Teissier, A. Capron, I. Wolowczuk, M. Capron, and F. Trottein. 2001. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J. Exp. Med. 193:1135-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 4.Boonstra, A., C. Asselin-Paturel, M. Gilliet, C. Crain, G. Trinchieri, Y. J. Liu, and A. O'Garra. 2003. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J. Exp. Med. 197:101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cella, M., D. Scheidegger, K. Palmer-Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong, E. C., P. L. Vieira, P. Kalinski, J. H. Schuitemaker, Y. Tanaka, E. A. Wierenga, M. Yazdanbakhsh, and M. L. Kapsenberg. 2002. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J. Immunol. 168:1704-1709. [DOI] [PubMed] [Google Scholar]
- 7.Diehl, S., and M. Rincon. 2002. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 39:531-536. [DOI] [PubMed] [Google Scholar]
- 8.Dodge, I. L., M. W. Carr, M. Cernadas, and M. B. Brenner. 2003. IL-6 production by pulmonary dendritic cells impedes Th1 immune responses. J. Immunol. 170:4457-4464. [DOI] [PubMed] [Google Scholar]
- 9.d'Ostiani, C. F., G. Del Sero, A. Bacci, C. Montagnoli, A. Spreca, A. Mencacci, P. Ricciardi-Castagnoli, and L. Romani. 2000. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 191:1661-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards, A. D., S. P. Manickasingham, R. Sporri, S. S. Diebold, O. Schulz, A. Sher, T. Kaisho, S. Akira, and C. Reis e Sousa. 2002. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J. Immunol. 169:3652-3660. [DOI] [PubMed] [Google Scholar]
- 11.Hogg, K. G., S. Kumkate, S. Anderson, and A. P. Mountford. 2003. Interleukin-12 p40 secretion by cutaneous CD11c+ and F4/80+ cells is a major feature of the innate immune response in mice that develop Th1-mediated protective immunity to Schistosoma mansoni. Infect. Immun. 71:3563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogg, K. G., S. Kumkate, and A. P. Mountford. 2003. IL-10 regulates early IL-12-mediated immune-responses induced by the radiation attenuated schistosome vaccine. Int. Immunol. 15:1451-1459. [DOI] [PubMed] [Google Scholar]
- 13.Ishii, N., L. C. Ndhlovu, K. Murata, T. Sato, M. Kamanaka, and K. Sugamura. 2003. OX40 (CD134) and OX40 ligand interaction plays an adjuvant role during in vivo Th2 responses. Eur. J. Immunol. 33:2372-2381. [DOI] [PubMed] [Google Scholar]
- 14.Kapsenberg, M. L. 2003. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 3:984-993. [DOI] [PubMed] [Google Scholar]
- 15.Kelsall, B. L., C. A. Biron, O. Sharma, and P. M. Kaye. 2002. Dendritic cells at the host-pathogen interface. Nat. Immunol. 3:699-702. [DOI] [PubMed] [Google Scholar]
- 16.Koch, F., U. Stanzl, P. Jennewein, K. Janke, C. Heufler, E. Kampgen, N. Romani, and G. Schuler. 1996. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 184:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kourilova, P., K. G. Hogg, L. Kolarova, and A. P. Mountford. 2004. Cercarial dermatitis caused by bird schistosomes comprises both immediate and late phase cutaneous hypersensitivity reactions. J. Immunol. 172:3766-3774. [DOI] [PubMed] [Google Scholar]
- 18.Kuchroo, V. K., M. P. Das, J. A. Brown, A. M. Ranger, S. S. Zamvil, R. A. Sobel, H. L. Weiner, N. Nabavi, and L. H. Glimcher. 1995. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell 80:707-718. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald, A. S., A. D. Straw, B. Bauman, and E. J. Pearce. 2001. CD8− dendritic cell activation status plays an integral role in influencing Th2 response development. J. Immunol. 167:1982-1988. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald, A. S., A. D. Straw, N. M. Dalton, and E. J. Pearce. 2002. Cutting edge: Th2 response induction by dendritic cells: a role for CD40. J. Immunol. 168:537-540. [DOI] [PubMed] [Google Scholar]
- 21.Machado, D. C., D. Horton, R. Harrop, P. T. Peachell, and B. A. Helm. 1996. Potential allergens stimulate the release of mediators of the allergic response from cells of mast cell lineage in the absence of sensitization with antigen-specific IgE. Eur. J. Immunol. 26:2972-2980. [DOI] [PubMed] [Google Scholar]
- 22.Maizels, R. M., and M. Yazdanbakhsh. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3:733-744. [DOI] [PubMed] [Google Scholar]
- 23.Manickasingham, S. P., A. D. Edwards, O. Schulz, and C. Reis e Sousa. 2003. The ability of murine dendritic cell subsets to direct T helper cell differentiation is dependent on microbial signals. Eur. J. Immunol. 33:101-107. [DOI] [PubMed] [Google Scholar]
- 24.McGuirk, P., C. McCann, and K. H. Mills. 2002. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J. Exp. Med. 195:221-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mountford, A. P., R. Harrop, and R. A. Wilson. 1995. Antigens derived from lung-stage larvae of Schistsosoma mansoni are efficient stimulators of proliferation and gamma interferon production by lymphocytes from mice vaccinated with attenuated larvae. Infect. Immun. 63:1980-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mountford, A. P., and F. Trottein. 2004. Schistosomes in the skin: a balance between immune priming and regulation. Trends Parasitol. 20:221-226. [DOI] [PubMed] [Google Scholar]
- 27.Poudrier, J., D. van Essen, S. Morales-Alcelay, T. Leanderson, S. Bergthorsdottir, and D. Gray. 1998. CD40 ligand signals optimize T helper cell cytokine production: role in Th2 development and induction of germinal centers. Eur. J. Immunol. 28:3371-3383. [DOI] [PubMed] [Google Scholar]
- 28.Riengrojpitak, S., S. Anderson, and R. A. Wilson. 1998. Induction of immunity to Schistosoma mansoni: interaction of schistosomula with accessory leucocytes in murine skin and draining lymph nodes. Parasitology 117:301-309. [DOI] [PubMed] [Google Scholar]
- 29.Ruedl, C., M. F. Bachmann, and M. Kopf. 2000. The antigen dose determines T helper subset development by regulation of CD40 ligand. Eur. J. Immunol. 30:2056-2064. [DOI] [PubMed] [Google Scholar]
- 30.Sher, A., E. Pearce, and P. Kaye. 2003. Shaping the immune response to parasites: role of dendritic cells. Curr. Opin. Immunol. 15:421-429. [DOI] [PubMed] [Google Scholar]
- 31.Son, Y. I., S. Egawa, T. Tatsumi, R. E. Redlinger, Jr., P. Kalinski, and T. Kanto. 2002. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J. Immunol. Methods 262:145-157. [DOI] [PubMed] [Google Scholar]
- 32.Sparwasser, T., E. S. Koch, R. M. Vabulas, K. Heeg, G. B. Lipford, J. W. Ellwart, and H. Wagner. 1998. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur. J. Immunol. 28:2045-2054. [DOI] [PubMed] [Google Scholar]
- 33.van der Kleij, D., E. Latz, J. F. Brouwers, Y. C. Kruize, M. Schmitz, E. A. Kurt-Jones, T. Espevik, E. C. de Jong, M. L. Kapsenberg, D. T. Golenbock, A. G. Tielens, and M. Yazdanbakhsh. 2002. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J. Biol. Chem. 277:48122-48129. [DOI] [PubMed] [Google Scholar]
- 34.Whelan, M., M. M. Harnett, K. M. Houston, V. Patel, W. Harnett, and K. P. Rigley. 2000. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J. Immunol. 164:6453-6460. [DOI] [PubMed] [Google Scholar]