Abstract
Gliotoxin was measured in the lungs (mean, 3,976 ± 1,662 ng/g of tissue) and sera (mean, 36.5 ± 30.28 ng/ml) of mice with experimentally induced invasive aspergillosis (IA), and levels decreased with antifungal therapy. Gliotoxin could also be detected in the sera of cancer patients with documented (proven or probable) IA.
Aspergillus fumigatus, the saprophytic mold associated with the majority of cases of human invasive aspergillosis (IA), is known to produce a variety of secondary metabolites during invasive (hyphal) growth (12). One of the most abundantly produced metabolites is the epipolythiodioxopiperazine metabolite gliotoxin, which has a broad spectrum of immunosuppressive activity in animals and humans (15). Gliotoxin has been shown in vitro to inhibit activation of the transcription factor NF-κB (9), neutrophil and macrophage oxidative killing (7, 8, 14), macrophage phagocytosis (7, 8), antigen-mediated lymphocyte stimulation and cytotoxic-T-cell activation (6, 15), and gamma interferon production by CD4+ lymphocytes (17). At higher concentrations (>250 ng/ml), gliotoxin induces apoptosis in macrophages and lymphocytes by a mechanism distinct from its antiphagocytic effects (16). Theoretically, the release of gliotoxin by Aspergillus during growth in tissues could aid in the evasion by the fungus of innate and professional effector immune cells.
(This work was presented in part at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 14 to 17 September 2003, abstr. M-1015 and M-1243.)
We hypothesized that gliotoxin production is involved in the pathogenesis of IA and could be a marker of invasive infection with A. fumigatus. To this end, we used a liquid chromatography-tandem mass spectrometry (LC-MS-MS) assay to measure gliotoxin concentrations in the lungs and sera of mice with experimentally induced invasive pulmonary aspergillosis (IPA). Additionally, we screened unselected banked serum samples for gliotoxin from a cohort of cancer patients with or without documented evidence of IA.
Serum samples were collected under institutional review board protocol LAB 02-200 at The University of Texas M. D. Anderson Cancer Center (MDACC). All animal studies were performed under the guidance and institutional standards of The University of Texas MDACC and The University of Houston Institutional Care and Animal Use Committees.
Serum or tissue samples were prepared for analysis by methanol extraction and precipitation of proteins followed by centrifugation at 10,000 × g. LC-MS-MS in negative mode was run using pairs of ions at an m/z of 324.9/260.8 for gliotoxin and 137.9/107.9 for the internal standard 3-nitrophenol. Concentrations of gliotoxin were assessed by interpolation of unknown samples to a seven-point standard curve (0.25 to 25 ng/ml) for purified gliotoxin (Sigma, St. Louis, Mo.) prepared in mouse sera or uninfected mouse lung homogenate. Off-scale unknown samples were diluted 100- to 1,000-fold and reassayed against the standard curve. Mean inter- and intraassay coefficients of variation over the ranges of the standard curve were less than 10%. The overall recovery of gliotoxin from samples was 72.9%.
An established murine model of acute IPA was used to characterize gliotoxin concentrations in lung tissue and sera during infection (4, 5). Briefly, 20- to 25-g female Swiss-Webster mice were rendered neutropenic with intraperitoneal injections of cyclophosphamide (150 mg/kg on day −4 and day −1) and subcutaneous cortisone acetate (250 mg/kg on day −1) prior to intransal inoculation (50 μl) of a suspension (1 × 108 conidia/ml) of A. fumigatus prepared in sterile water plus 0.2% Tween 80. Beginning 12 hours later, animals were treated with daily intraperitoneal injections of either 5% dextrose-water or amphotericin B (AMB) deoxycholate (0.25, 0.5, or 1 mg/kg/day) until 96 h after inoculation. Animals were housed in filter top cages and allowed access to food and water ad libitum. Animals that appeared moribund prior to 96 h were euthanized by CO2 narcosis, and death was recorded as occurring 8 h later. All animals surviving to 96 h were euthanized with CO2. Lungs were aseptically harvested, weighed, and analyzed for Aspergillus tissue burden, using a quantitative real-time PCR assay as described by Bowman et al. (3). Serum was collected by cardiac puncture. Each treatment arm consisted of ≥10 animals per group. Animal treatment groups were compared by the Kruskal-Wallis test (nonparametric analysis of variance) or the Mann-Whitney U test, as appropriate. Animal survival curves for each treatment group were plotted using Kaplan-Meier analysis. Differences in the survival rates in treatment groups and among untreated controls were analyzed by the chi-square log-rank test. The Fischer exact test was used to compare frequency of gliotoxin positivity between groups. All results were considered significant at a P ≤ 0.05.
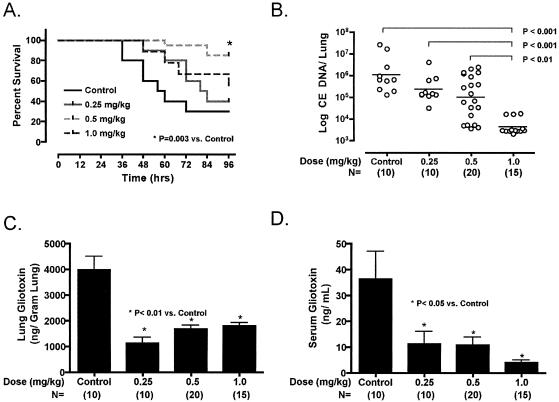
Mortality rates at 96 h exceeded 70% in the control animals (Fig. 1A). AMB administered 12 h after inoculation and daily thereafter until 96 h significantly improved animal survival compared to controls and reduced Aspergillus lung tissue fungal burden by ≥2 log10 units of conidial equivalent DNA at the highest tested dose (Fig. 1B). High concentrations of gliotoxin (mean, 3,976 ± 1,662 ng/g of tissue) could be detected in the lung tissues of control mice and were reduced (mean, 1,536 ± 596 ng/ml; P < 0.05) with AMB. These high concentrations of gliotoxin in lung tissue are consistent with tissue concentrations of gliotoxin previously found in livestock or avian Aspergillus infections (2, 11). The reduction in lung gliotoxin concentrations was maximized at the lowest daily dosage of AMB (Fig. 1C), with somewhat higher concentrations in the lungs at higher AMB dosages. This pattern of gliotoxin reduction is similar to observations by Reeves et al., who found significantly higher degrees of gliotoxin release into culture media by A. fumigatus following exposure to AMB at fungicidal versus fungistatic concentrations (10). Concordant gliotoxin concentrations in serum (Fig. 1D) were substantially lower than the lung tissue concentrations (mean, 37 ± 30 ng/ml) and decreased in a dose-dependent fashion with AMB therapy compared to controls (mean concentration in AMB-treated animals, 9 ± 8 ng/ml; P < 0.05).
FIG. 1.
(A) Survival curves for mice with IA treated with AMB doses ranging from 0.25 to 1 mg/kg/day and for the control. (B) Lung fungal burden of mice with IA treated with AMB. Data are expressed as conidial equivalent (CE) A. fumigatus DNA as determined by quantitative PCR. Each datum point represents one mouse; n ≥ 10 mice per treatment group. Lung (C) and serum (D) gliotoxin concentrations in mice with IPA treated with AMB deoxycholate. n ≥ 10 per treatment group.
Our ability to detect gliotoxin in the sera of mice with pulmonary aspergillosis suggested that this mycotoxin could similarly be found in the sera of humans with IA. To this end, single unselected serum samples from 16 high-risk patients with underlying risk factors for the development of IA submitted to the clinical microbiology laboratory for PCR detection of Aspergillus nucleic acid were analyzed for gliotoxin by using the LC-MS-MS assay. Investigators involved in the analysis of gliotoxin were unaware of the patient's clinical history or diagnostic status of IA. Five of the 16 patients (31%) were classified as having probable or proven IA according to EORTC/NIAID/MSG criteria (Table 1) (1). All patients were receiving mold-active antifungal therapy (e.g., lipid AMB formulation, voriconazole, caspofungin, or combinations) at the time of serum sample collection. Gliotoxin was detected in 2 of 11 (18%) patients with no documented IA versus 4 of 5 patients (80%) with documented IA (P = 0.04; Fisher exact test). Gliotoxin was detected in patients who were culture positive or had histopathological evidence of Aspergillus infection. Aspergillus terreus was the only species isolated by culture from gliotoxin-positive patients with proven IA, reflecting the unusual predominance of non-A. fumigatus Aspergillus species at our institution, which stems from either local environmental factors or extensive antifungal use in our heavily immunocompromised population (13). The one patient with documented IA that was not positive for gliotoxin had a solitary brain lesion from which A. fumigatus was cultured.
TABLE 1.
Serum gliotoxin concentrations in unselected patients at risk for IA
| Classification and patient | Underlying malignancyb | Neutropeniac | Transplantationd | Computer tomography findings of IA | Culture | Serum gliotoxin (ng/ml) concn |
|---|---|---|---|---|---|---|
| No evidence of IAa | ||||||
| 1 | AML | No | Allo-MUD | NDg | Negative | ND |
| 2 | CLL | No | No | ND | Negative | ND |
| 3 | AML | No | Allo-MUD | ND | Negative | ND |
| 4 | Lung cancer | No | No | ND | Negative | ND |
| 5 | MDS | No | No | ND | Negative | ND |
| 6 | ALL | No | No | ND | Negative | 65 |
| 7 | AML | Yes | Allo-MUD | ND | Negative | ND |
| 8 | ALL | Yes | No | Lung-unilateral | Negative | ND |
| 9 | AML | No | No | ND | Negative | 154 |
| 10 | CML | No | Allo-MUD | Lung-aspergillomae | Negative | ND |
| 11 | AML | Yes | Allo-cord | Lung-unilateral | Negative | ND |
| Documented IAa | ||||||
| 12 | AML | No | No | Lung-bilateral | A. fumigatus | 785 |
| 13 | HL | No | Allo-MUD | Brain-single lesion | A. fumigatus | ND |
| 14 | AML | No | No | Lung-unilateral | A. terreus | 203 |
| 15 | ALL | No | Allo-MUD | Lung-bilateral | Histopathologyf | 166 |
| 16 | NHL | No | Allo-MRD | Lung-bilateral | Histopathologyf | 372 |
According to EORTC/NIAID/MSG criteria (1).
AML, acute myelogenous leukemia; ALL, acute lymphocytic leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; HL, Hodgkin's lymphoma; MDS, myelodysplastic syndrome; NHL, non-Hodgkin's lymphoma.
Neutropenia = absolute neutrophil count ≤ 500 at time of sampling.
Allo-MUD, allogeneic matched unrelated donor; Allo-MRD, allogeneic matched related donor; Allo-cord, Allogeneic umbilical cord stem cell source.
Diagnosed by computer tomography and patient history.
Histopathology, septal branching (45°) hyphae seen in tissue histopathology consistent with Aspergillus or Fusarium.
ND, not detected.
This is the first report of gliotoxin in the sera of cancer patients with IA and suggests a possible role for detection of mycotoxins as a novel diagnostic approach for IA. Moreover, the very presence of a circulating immunosuppressive toxin produced by Aspergillus in animals and humans with IA raises intriguing questions concerning the role of secreted metabolites such as gliotoxin in the pathogenesis and persistence of this opportunistic infection. Studies are currently under way to further evaluate the role of gliotoxin in sera and other clinically relevant samples (e.g., bronchial alveolar lavage fluid and cerebral spinal fluid) as a diagnostic and prognostic marker for IA.
Acknowledgments
Financial support was provided by The Society of Infectious Diseases Pharmacists to R.E.L. The MDACC animal care unit is supported by NIH-NCI CORE support grant 16672.
Editor: T. R. Kozel
REFERENCES
- 1.Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, J., M. Gareis, A. Bott, and B. Gedek. 1989. Isolation of a mycotoxin (gliotoxin) from a bovine udder infected with Aspergillus fumigatus. J. Med. Vet. Mycol. 27:45-50. [DOI] [PubMed] [Google Scholar]
- 3.Bowman, J. C., G. K. Abruzzo, J. W. Anderson, A. M. Flattery, C. J. Gill, V. B. Pikounis, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon, D. M., A. Polak, and T. J. Walsh. 1989. Fungus dose-dependent primary pulmonary aspergillosis in immunosuppressed mice. Infect. Immun. 57:1452-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis, R. E., R. A. Prince, J. Chi, and D. P. Kontoyiannis. 2002. Itraconazole preexposure attenuates the efficacy of subsequent amphotericin B therapy in a murine model of acute invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 46:3208-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullbacher, A., and R. D. Eichner. 1984. Immunosuppression in vitro by a metabolite of a human pathogenic fungus. Proc. Natl. Acad. Sci. USA 81:3835-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullbacher, A., P. Waring, and R. D. Eichner. 1985. Identification of an agent in cultures of Aspergillus fumigatus displaying anti-phagocytic and immunomodulating activity in vitro. J. Gen. Microbiol. 131:1251-1258. [DOI] [PubMed] [Google Scholar]
- 8.Murayama, T., R. Amitani, Y. Ikegami, R. Nawada, W. J. Lee, and F. Kuze. 1996. Suppressive effects of Aspergillus fumigatus culture filtrates on human alveolar macrophages and polymorphonuclear leucocytes. Eur. Respir. J. 9:293-300. [DOI] [PubMed] [Google Scholar]
- 9.Pahl, H. L., B. Krauss, K. Schulze-Osthoff, T. Decker, E. B. Traenckner, M. Vogt, C. Myers, T. Parks, P. Warring, A. Muhlbacher, A. P. Czernilofsky, and P. A. Baeuerle. 1996. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-kappa B. J. Exp. Med. 183:1829-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeves, E. P., T. Murphy, P. Daly, and K. Kavanagh. 2004. Amphotericin B enhances the synthesis and release of the immunosuppressive agent gliotoxin from the pulmonary pathogen Aspergillus fumigatus. J. Med. Microbiol. 53:719-725. [DOI] [PubMed] [Google Scholar]
- 11.Richard, J. L., T. J. Dvorak, and P. F. Ross. 1996. Natural occurrence of gliotoxin in turkeys infected with Aspergillus fumigatus, Fresenius. Mycopathologia 134:167-170. [DOI] [PubMed] [Google Scholar]
- 12.Tomee, J. F., and H. F. Kauffman. 2000. Putative virulence factors of Aspergillus fumigatus. Clin. Exp. Allergy 30:476-484. [DOI] [PubMed] [Google Scholar]
- 13.Torres, H. A., G. A. Rivero, R. E. Lewis, R. Hachem, I. I. Raad, and D. P. Kontoyiannis. 2003. Aspergillosis caused by non-fumigatus Aspergillus species: risk factors and in vitro susceptibility compared with Aspergillus fumigatus. Diagn. Microbiol. Infect. Dis. 46:25-28. [DOI] [PubMed] [Google Scholar]
- 14.Tsunawaki, S., L. S. Yoshida, S. Nishida, T. Kobayashi, and T. Shimoyama. 2004. Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase. Infect. Immun. 72:3373-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waring, P., R. D. Eichner, and A. Mullbacher. 1988. The chemistry and biology of the immunomodulating agent gliotoxin and related epipolythiodioxopiperazines. Med. Res. Rev. 8:499-524. [DOI] [PubMed] [Google Scholar]
- 16.Waring, P., R. D. Eichner, A. Mullbacher, and A. Sjaarda. 1988. Gliotoxin induces apoptosis in macrophages unrelated to its antiphagocytic properties. J. Biol. Chem. 263:18493-18499. [PubMed] [Google Scholar]
- 17.Wichmann, G., O. Herbarth, and I. Lehmann. 2002. The mycotoxins citrinin, gliotoxin, and patulin affect interferon-gamma rather than interleukin-4 production in human blood cells. Environ. Toxicol. 17:211-218. [DOI] [PubMed] [Google Scholar]