Abstract
Background
At present, the treatment of coxsackievirus-induced myocarditis remains difficult. Berberine (BBR), an isoquinoline alkaloid isolated from traditional medicine herbs, exhibits significant anti-viral efficacy against various viruses. However, the underlying mechanism by which BBR controls CVB3 infection has not yet been reported. The purpose of this study was to investigate the anti-viral efficacy of BBR against CVB3 infection and its mechanism.
Material/Methods
In our experiments, the protein levels of VP1 and MAPKs signal pathway were measured by Western blot. The mRNA level of VP1 was measured by RT-PCR. The virus titers were determined by TCID50 assay.
Results
We found that BBR treatment significantly decreased CVB3 replication in HeLa cells. In addition, the BBR treatment reduced the phosphorylation levels of JNK and p38 MAPK upon CVB3 infection in both HeLa cells and primary rat myocardial cells.
Conclusions
Taken together, these results suggest that BBR inhibits CVB3 replication through the suppression of JNK and p38 MAPK activation, shedding new light on the investigation of therapeutic strategies against CVB3-induced viral myocarditis.
MeSH Keywords: Berberine, JNK Mitogen-Activated Protein Kinases, p38 Mitogen-Activated Protein Kinases, Virus Replication
Background
Myocarditis is an insidious cardiac disease associated with myocardial inflammation and injury [1]. As myocarditis progresses, the heart develops left ventricular dysfunction, dilated cardiomyopathy (DCM), or even heart failure [2]. Infectious agents such as coxsackievirus, adenovirus, influenza virus, and human immunodeficiency virus (HIV) play dominant roles in triggering myocarditis [3]. However, among those etiological agents, the coxsackievirus, particularly the coxsackie B virus serotype 3 (CVB3), is a predominant cause of viral myocarditis, especially in neonates and young children [2].
Coxsackievirus is a small, nonenveloped, positive-sense, single-stranded RNA (+ssRNA) virus, which belongs to the genus Enterovirus in the Picornaviridae family [4]. Even though the pathogenic mechanisms of coxsackievirus have been extensively documented, effective virus intervention strategies are still limited [2]. At present, the available therapeutic methods for the clinical treatment of coxsackievirus-induced myocarditis still rely on the application of broad-spectrum anti-viral drugs such as ribavirin and interferon alpha, or on conventional supportive therapy [5,6]. Due to the limited therapeutic methods and their adverse effects, many intervention strategies have arisen, such as the nasal treatment with cardiac myosin major histocompatibility class (MHC) II peptide [7], coxsackievirus and adenovirus receptor trap therapy [8], and traditional medicinal herb treatment [9].
Berberine (BBR), an isoquinoline alkaloid isolated from traditional medicine herbs including Rhizoma coptidis and Cortex phellodendri has exhibited significant anti-viral efficacy against various viruses including influenza virus, HIV, human cytomegalovirus, and herpes simplex virus (HSV) [10–13]. It has been shown that BBR can inhibit virus infection by directly affecting individual steps of the virus replication cycle [13], inhibiting viral protein translocation, or by regulating host signal pathways [10].
The MAPKs signal pathways, which contain extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK, are among the most widespread mechanisms of eukaryotic cell regulation. They control the fundamental cellular processes of growth, proliferation, differentiation, and cell death when activated by environmental stresses and inflammatory stimuli [14,15]. It has been reported that the MAPKs signal pathways also play a critical role in BBR-mediated regulation of virus replication [13,16]. The inhibition of JNK activation by BBR can result in the inhibition of the attachment/entry and genome DNA replication of herpes simplex virus [13], while the inhibition of p38 MAPK might impair the entrance of Zaire Ebola virus into the human dendritic cells [16].
Even though the roles of BBR in the inhibition of virus replication have been extensively documented, the underlying mechanism of BBR in controlling CVB3 replication has not yet been reported. In the present study, we investigated the effects of BBR on CVB3 replication and provide evidence that BBR suppresses CVB3 infection by the inhibition of virus replication via suppressing the cellular JNK and p38 MAPK activation, providing insight into its latent protective effect on viral myocarditis induced by CVB3.
Material and Methods
Virus strain, cells and pharmacological compounds
CVB3 (Nancy strain) was purchased from the Wuhan Institute of Virology, Chinese Academy of Sciences (Wuhan, China) and was propagated in human cervical carcinoma (HeLa) cells. The HeLa cells, purchased from ATCC, were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone) supplemented with 10% (vol/vol) fetal bovine serum (FBS, Hyclone) in the presence of 5% CO2 at 37°C. The primary myocardial cells were obtained from neonatal rat hearts and grown in α-MEM (Hyclone) containing 10% (vol/vol) FBS, as previously described [17].
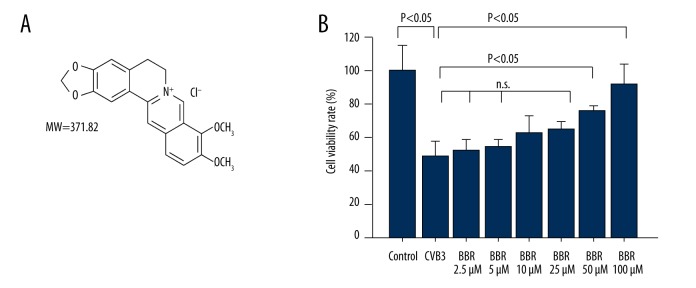
The BBR (molecular weight 371.82, purity >97%, molecular structure is shown in Figure 1A) dissolved in dimethyl sulfoxide (DMSO) was diluted in DMEM or α-MEM for cell treatment. The p38 MAPK inhibitor SB203580 (S1076) and JNK inhibitor SP600125 (S1460) (Selleckchem, USA) were dissolved in DMSO and diluted in DMEM for cell treatment.
Figure 1.
Effects of BBR on HeLa cell viability. (A) The chemical structure of BBR. (B) The cell viability in CVB3-infected or BBR-treated cells were determined by CCK-8 assay. Data show mean ±SD from 3 independent experiments. n.s. indicates no significance.
Viral infection and drug treatment
For virus infection assay, the HeLa cells or primary myocardial cells (2×105 cells/well) were cultured in a 12-well plate in the indicated culture media until they reached a confluence of 80%. Then, the supernatants of the cells were discarded, and the cells were rinsed with PBS and infected with CVB3 (6×105 PFU/ml) in DMEM or α-MEM at a multiplicity of infection (MOI) of 3 for 1 h. After virus absorption, the supernatants were discarded, and the cells were rinsed with PBS and cultured with 1 ml of DMEM or α-MEM containing 2% FBS for 20 h. The mock-infected controls were treated with an equal volume of DMEM or α-MEM. The CVB3 dissolved in the DMEM was exposed to UV light for 10 min at room temperature for a total dose of 15 J/cm2 to inactivate the virus, as previously described [18]. For the drug treatment experiment, the supernatants of the cells infected with CVB3 at a MOI of 3 for 1 h were discarded, and the cells were treated with BBR diluted in DMEM or α-MEM at the indicated concentrations for 20 h.
Cell viability assay
For analysis of cell viability after CVB3 and BBR treatment, the HeLa cells were cultured in 96-well plates, and the CVB3 or BBR treatment procedure was applied following the method previously mentioned. After being treated with BBR for 20 h, the cells viability was determined by using the Cell Counting Kit-8 (CCK-8) (Dojindo, Japan) according to the manufacturer’s protocol.
Immunofluorescence staining
The double-stranded RNAs (dsRNAs) were evaluated by immunofluorescence staining using mouse anti-dsRNA monoclonal antibody (1: 100, English & Scientific Consulting) as the primary antibody. Then, the cells were incubated with Dylight 649 conjugated goat anti-mouse IgG secondary antibody (1: 1000, Abbkine). The nuclei of the cells were stained with 4,6-diamidino-2-phenylindole (DAPI) (Beyotime, China). Images were acquired and analyzed using a Leica TCS SP5 laser confocal microscope.
Western blot assay
For Western blot analysis, 40 μg proteins from the cell lysates were loaded onto a 10% SDS polyacrylamide gel. Then, the proteins were transferred onto polyvinylidene difluoride (PVDF) membranes and hybridized with specific antibodies as follows: p-JNK (1: 1000, Fantibody), JNK (1: 1000, Fantibody), p-ERK (1: 2000, Fantibody), ERK (1: 1000, Fantibody), p-P38 (1: 1000, Fantibody), P38 (1: 1000, Fantibody), GAPDH (1: 1000, Fantibody), β-actin (1: 1000, Santa Cruz), and/or CVB3 capsid protein VP1 antibody, generated in our laboratory [19]. The signal was detected using an ECL Plus kit (Pierce, USA). The densitometric analysis of protein bands was calculated using Image J software.
Real-time PCR
The real-time PCR assay for the gene expression of CVB3 capsid VP1 was applied following the manufacturer’s instructions (Takara, Japan). The primers used in this study were as follows: VP1 forward: 5′-AAACTCAGGTGCCAAGCGGT-3′, reverse: 5′-TTGGTGTGTTAGGATCTGTGC-3′; GAPDH forward: 5′-CATCAAGAAGGTGGTGAAGCAG-3′, reverse: 5′-CGTCAAAGGTGGAGGAGTGG-3′. Relative gene expression was determined by the comparative C(t) (ΔΔCt) method with GAPDH as the endogenous control.
Measurement of virus titer
After CVB3/BBR treatment, HeLa cells, along with medium, were subjected to 3 freeze-thaw cycles and centrifuged. The supernatant was 10-fold sequentially diluted with DMEM medium to determine virus titers by theTCIDP50 assay [20]. Briefly, HeLa cells were seeded into 96-well plates until they reached 70% confluence. A series of diluted supernatants were inoculated into HeLa cells at 37°C and 5% CO2 for 72 h. Viral titer was measured by cytopathic effect using the Reed-Muench method [21].
Statistical analysis
The data are presented as mean ±SD. Statistical analysis was performed by one-way ANOVA followed by Tukey’s post hoc test using Origin 8.0. A P value of less than 0.05 was accepted as statistically significant.
Results
BBR inhibits CVB3-induced cytotoxicity
To investigate whether BBR protects against CVB3 infection, the cell viability of HeLa cells infected with CVB3 was determined by CCK8 assay. As shown in Figure 1B, CVB3 infection induced about 50% cell death in HeLa cells at 20 h after virus infection, whereas BBR treatment decreased CVB3-induced cytotoxicity in a dose-dependent manner.
BBR inhibits CVB3 replication in vitro
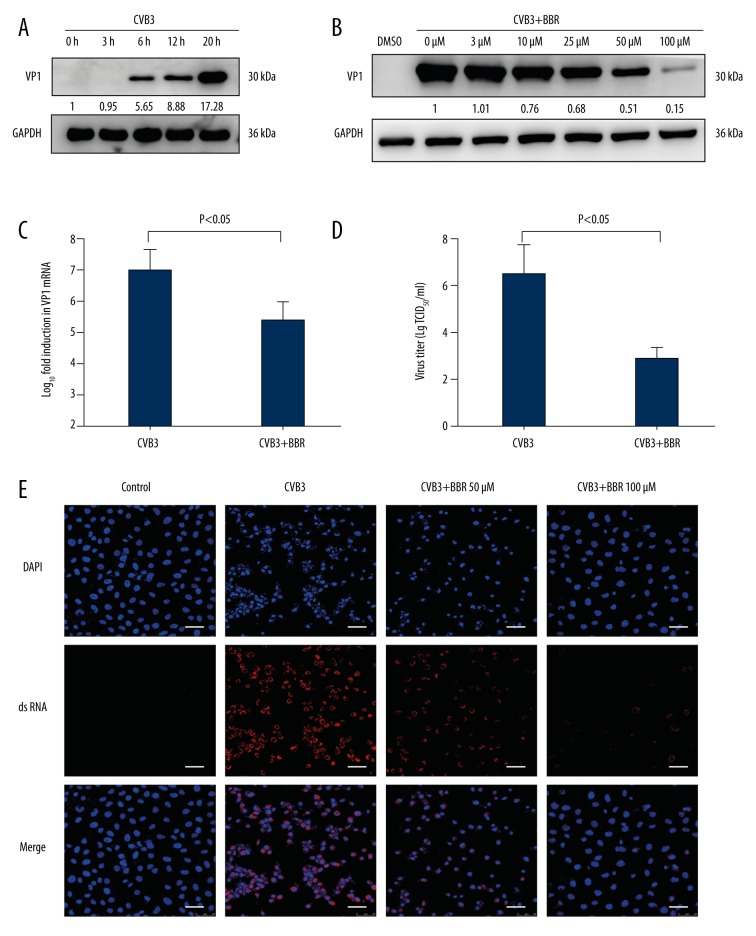
To determine whether BBR protects against CVB3 infection by suppressing virus replication, the viral capsid VP1 protein expression at various time points following viral infection in HeLa cells was analyzed. We found that the virus VP1 protein in CVB3-infected HeLa cells increased in a time-dependent manner, and this protein reached a high level at 20 h (Figure 2A). Then, the expression of CVB3 VP1 proteins was gradually inhibited by an increasing concentration of BBR, particularly at 100 μM (Figure 2B). Moreover, BBR exhibits no significant cytotoxicity to HeLa cells at 100 μM (data not shown). Further analysis of CVB3 vp1 gene expression demonstrated a similar inhibitory effect of BBR on VP1 expression at the RNA level (Figure 2C). In addition, the viral titer of the BBR-treated cells was evaluated in vitro. As shown in Figure 2D, the titers of progeny virus in HeLa cells were decreased with BBR treatment compared with the CVB3-infected group. Consistent with these results, the analysis of IF staining revealed that the BBR treatment significantly reduced the synthesis of virus dsRNAs in CVB3-infected cells (Figure 2E). Taken together, these results demonstrate that BBR can inhibit CVB3 replication in vitro.
Figure 2.
BBR inhibits CVB3 replication in vitro. (A) HeLa cells were infected with CVB3 at 3 MOI for 1 h. Cell lysates were collected at the indicated times (3 h, 6 h, 12 h, 20 h) post-infection. Western blot analysis was performed to detect the expression of virus VP1 protein. (B) At 1 h after HeLa cells were infected with CVB3 (MOI=3), they were treated with BBR at the indicated concentrations (3 μM, 10 μM, 25 μM, 50 μM, 100 μM) for 20 h. Cell lysates were harvested and subjected to further analyses. Western blot analysis was performed to evaluate the expression of virus VP1 protein. (C) At 1 h after HeLa cells were infected with CVB3 (MOI=3), they were treated with BBR (100 μM) for 20 h. Real-time PCR analysis was performed to evaluate the levels of virus vp1 mRNA. (D) The viral titers after BBR (100 μM) treatment were determined by the Reed-Muench method. (E) IF staining was performed to detect the production of virus dsRNA during virus replication (dsRNA, red; nuclei, blue). The scale bar is 25 μm. Representative data from triplicate experiments are shown. Data show mean ±SD from 3 independent experiments. GAPDH was used as a protein loading control or endogenous reference gene.
BBR suppresses CVB3 replication through the inhibition of JNK/p38 MAPK pathways
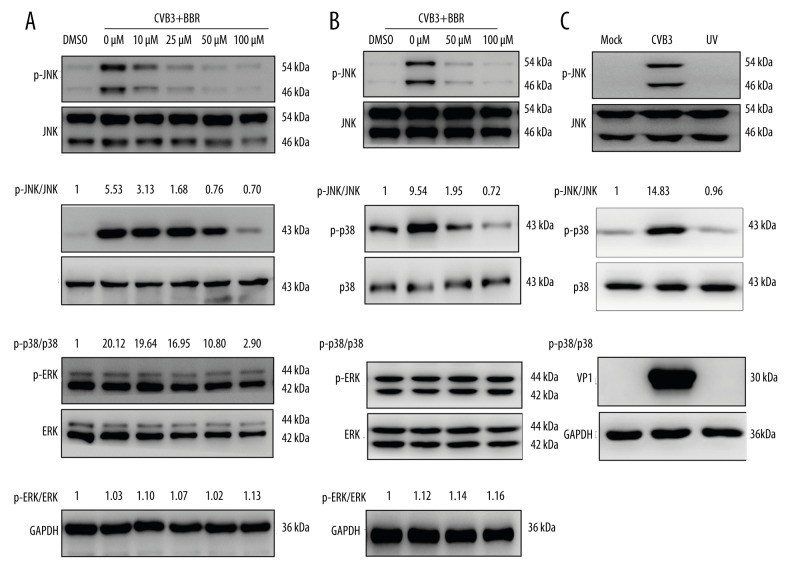
To address whether the inhibitory effect of BBR against CVB3 replication is associated with MAPKs signal pathways, we analyzed the phosphorylation levels of JNK, p38, and ERK upon CVB3 infection. Because the UV-irradiated virus is capable of attaching to the cell receptor and entering into the host cell, but is not capable of replicating in the cells, the UV-inactivated CVB3 was utilized to investigate the underlying mechanism of MAPKs action on CVB3 replication. The results showed that CVB3 infection led to increased phosphorylation levels of JNK and p38 in HeLa cells but failed to activate the ERK (Figure 3A). However, the UV-inactivated CVB3 did not affect the MAPK phosphorylation levels in HeLa cells (Figure 3C), suggesting that the JNK/p38 MAPK activation was due to post-entry viral replication in the host cells.
Figure 3.
BBR inhibited CVB3-induced activation of JNK and p38. HeLa cells and primary myocardial cells infected with CVB3 (MOI=3) for 1 h were treated with BBR at the indicated concentrations (10 μM, 25 μM, 50 μM, 100 μM) for 20 h. The phosphorylation levels of JNK, p38, and ERK in HeLa cells (A) and in primary myocardial cells (B) were analyzed by Western blot. The controls were treated with vehicle control (DMSO, 0.1%). (C) HeLa cells were infected with mock-control, CVB3, or UV-inactivated CVB3 (MOI=3). Representative data from triplicate experiments are shown. GAPDH was used as a loading control.
The fact that CVB3 infection activates MAPKs (Figure 3) suggests either the inhibition of the activation of JNK or that p38 might reduce the CVB3 replication. In accordance with this speculation, BBR treatment significantly inhibits the phosphorylation levels of JNK and p38 in HeLa cells (Figure 3A). Consistent with the results obtained from the HeLa cells, the inhibitory effect of BBR on JNK and p38 activation upon CVB3 infection was also observed in primary myocardial cells (Figure 3B).
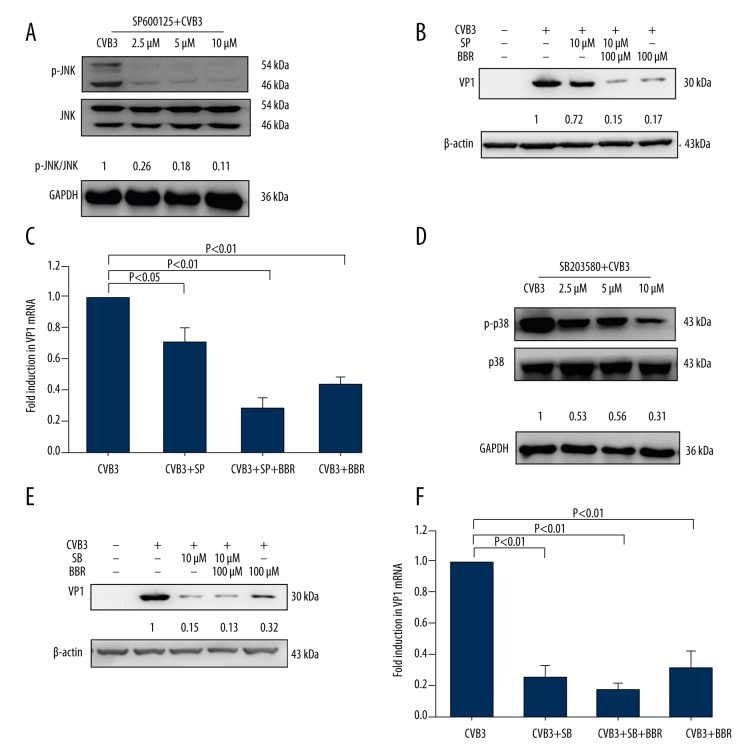
To further verify these results, the HeLa cells infected with CVB3 were subsequently treated with the JNK inhibitor SP600125 or the p38 inhibitor SB203580, and the virus VP1 protein and mRNA levels were analyzed. The CVB3-infected cells that were treated with a JNK inhibitor SP600125 showed reduced JNK phosphorylation level (Figure 4A), as well as reduced CVB3 VP1 protein and mRNA levels (Figure 4B, 4C). Similarly, when treated with p38 inhibitor SB203580, the CVB3-infected cells revealed a similar trend to that shown when treated with the JNK inhibitor (Figure 4D–4F). Taken together, these results suggest that BBR suppresses CVB3 replication through the inhibition of JNK and p38 activation.
Figure 4.
The inhibition of JNK or p38 activation by its corresponding inhibitor or BBR reduces CVB3 replication. HeLa cells infected with CVB3 (MOI=3) for 1 h were treated with JNK inhibitor SP600125 (SP, 2.5 μM, 5 μM, or 10 μM) or p38 inhibitor SB203580 (SB, 2.5 μM, 5 μM, or 10 μM) for 20 h. The phosphorylation levels of JNK or p38 were analyzed by Western blot (A, D). HeLa cells were infected with either mock-control or CVB3 (MOI=3). At 1 h after infection, the virus-infected cells treated with SP or SB (10 μM) were concurrently treated with or without BBR (100 μM) for 20 h. The expression of CVB3 VP1 was determined by Western blot (B, E) and real-time PCR (C, F). Representative data from triplicate experiments are shown in A, B, D, and E. GAPDH and β-actin were used as the loading control. Data in C and F show mean ±SD from 3 independent experiments.
Discussion
BBR, an active component extracted from medicinal herbs, is predominantly applied in the clinic for the treatment of bacterial diarrhea, intestinal parasite infection, and ocular trachoma infections [22]. In addition, BBR also has therapeutic potential against viral infections. Song et al. discovered that BBR inhibits HSV replication by precluding the expression of its immediate early genes ICP4 and ICP27 (infected cell polypeptide, ICP), 2 major transcriptional activators regulating the expression of viral early and late genes [13]. Cecil et al. showed that BBR exhibits anti-influenza virus activity through the inhibition of virus particle maturation by blocking the intracellular trafficking of virus hemagglutinin (HA) protein from the plasma membrane to the cell surface [10]. In the present study, we found that BBR exhibits anti-CVB3 activity in vitro by inhibiting CVB3 replication through the suppression of JNK and p38 MAPK activation (Figures 2, 3). Similar to our results, other researchers showed that the inhibition of p38 MAPK led to impaired CVB3 replication through the suppression of progeny release [23]. Interestingly, in our study, BBR treatment had no effects on ERK phosphorylation in HeLa cells, but several studies have demonstrated that BBR can activate ERK [24]. This discrepancy is probably due to the fact that ERK was fully phosphorylated in HeLa cells in the high-glucose medium used in the present study (Figure 3A), which is consistent with previous studies reporting that the activation of ERK is increased in high-glucose-induced cells [25,26]. These results indicate that the MAPKs play a critical role in the BBR-mediated inhibition of CVB3 replication. However, how MAPKs exert their effects during the process of BBR-mediated inhibition of CVB3 replication is still not fully understood.
Researchers have recently demonstrated that MAPKs inhibit virus replication by multiple mechanisms, such as blocking virus attachment/entry [27], inhibiting genome DNA replication, or restricting viral gene transcription and translation [28]. In the present study, we found that CVB3, but not the UV-inactivated one, activated JNK and p38 MAPK of HeLa cells (Figure 3C), suggesting that BBR restricts CVB3 replication by disturbing the viral post-entry steps. To confirm this conjecture, the virus RNA and protein synthesis levels in CVB3-infected cells were analyzed after BBR treatment. Results demonstrated that BBR treatment led to inhibited viral VP1 protein level and dsRNA synthesis upon CVB3 infection in HeLa cells (Figure 2B, 2E). Given that the maturation of CVB3 structural proteins (VP1, VP2, VP3, and VP4) and functional proteases/polymerases (2Apro, 2B, 2C, 3A, 3B/VPg, 3Cpro/3CDpro, and 3Dpol) originate from the splicing of viral precursor protein P1, P2, and P3, which are translated from virus positive-sense RNA template [2], the decreased VP1 level in the BBR-treated HeLa cells might be due to the inhibition of viral RNA synthesis. Consistently, the significantly decreased CVB3 dsRNA formation was observed in BBR-treated cells compared to the CVB3-infected group (Figure 2E). Nevertheless, the underlying mechanism by which BBR inhibits dsRNA synthesis remains elusive. The CVB3 RNA-dependent RNA polymerase 3Dpol, which is involved in launching the synthesis of negative-sense RNA strand intermediate from the viral positive-sense RNA template [29,30], plays a critical role in the formation of viral dsRNA. Whether BBR inhibits CVB3 replication by affecting 3Dpol function deserves further investigation. As shown in Figure 4B, 4C, the protein expression of VP1 was lower in the CVB3+SP+BBR group compared to the CVB3+SP group, indicating that the effect of BBR on inhibiting CVB3 replication is partially independent of the JNK signaling pathway. This difference can be explained by the effects of BBR on the p38 signaling pathway. These data suggest that JNK and p38 MAPKs are probably not the only pathways involved in BBR-mediated protection against CVB3 infection. We intend to perform further research on the possible pathways, including MAPKs and other signal pathways, involved in CVB3 RNA synthesis and BBR-mediated inhibition of CVB3 replication.
Conclusions
In summary, the present study demonstrated for the first time that BBR suppresses CVB3 replication by the inhibition of JNK and p38 activation, providing new clues to therapeutic strategies for the treatment of viral myocarditis.
Footnotes
Source of support: This work was financially supported by the National Natural Science Foundation of China (No. 81471067) and Key Project of the Science Committee of Chongqing, China (No. cstc2014yykfB10009)
References
- 1.Aretz HT, Billingham ME, Edwards WD, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 2.Esfandiarei M, McManus BM. Molecular biology and pathogenesis of viral myocarditis. Ann Rev Pathol. 2008;3:127–55. doi: 10.1146/annurev.pathmechdis.3.121806.151534. [DOI] [PubMed] [Google Scholar]
- 3.Grist NR, Reid D. Organisms in myocarditis/endocarditis viruses. J Infect. 1997;34:155. doi: 10.1016/s0163-4453(97)92547-0. [DOI] [PubMed] [Google Scholar]
- 4.Baboonian C, Davies MJ, Booth JC, McKenna WJ. Coxsackie B viruses and human heart disease. Curr Top Microbiol Immunol. 1997;223:31–52. doi: 10.1007/978-3-642-60687-8_3. [DOI] [PubMed] [Google Scholar]
- 5.Okada I, Matsumori A, Matoba Y, et al. Combination treatment with ribavirin and interferon for coxsackievirus B3 replication. J Lab Clin Med. 1992;120:569–73. [PubMed] [Google Scholar]
- 6.Schultz JC, Hilliard AA, Cooper LT, Jr, Rihal CS. Diagnosis and treatment of viral myocarditis. Mayo Clin Proc. 2009;84:1001–9. doi: 10.1016/S0025-6196(11)60670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fousteri G, Dave A, Morin B, et al. Nasal cardiac myosin peptide treatment and OX40 blockade protect mice from acute and chronic virally-induced myocarditis. J Autoimmun. 2011;36:210–20. doi: 10.1016/j.jaut.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein EA, Pinkert S, Becher PM, et al. Combination of RNA interference and virus receptor trap exerts additive antiviral activity in coxsackievirus B3-induced myocarditis in mice. J Infect Dis. 2015;211:613–22. doi: 10.1093/infdis/jiu504. [DOI] [PubMed] [Google Scholar]
- 9.Song JH, Choi HJ, Song HH, et al. Antiviral activity of ginsenosides against coxsackievirus B3, enterovirus 71, and human rhinovirus 3. J Ginseng Res. 2014;38:173–79. doi: 10.1016/j.jgr.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cecil CE, Davis JM, Cech NB, Laster SM. Inhibition of H1N1 influenza A virus growth and induction of inflammatory mediators by the isoquinoline alkaloid berberine and extracts of goldenseal (Hydrastis canadensis) Int Immunopharmacol. 2011;11:1706–14. doi: 10.1016/j.intimp.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Bodiwala HS, Sabde S, Mitra D, et al. Synthesis of 9-substituted derivatives of berberine as anti-HIV agents. Eur J Med Chem. 2011;46:1045–49. doi: 10.1016/j.ejmech.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi K, Minoda K, Nagaoka Y, et al. Antiviral activity of berberine and related compounds against human cytomegalovirus. Bioorg Med Chem Lett. 2007;17:1562–64. doi: 10.1016/j.bmcl.2006.12.085. [DOI] [PubMed] [Google Scholar]
- 13.Song S, Qiu M, Chu Y, et al. Downregulation of cellular c-Jun N-terminal protein kinase and NF-kappaB activation by berberine may result in inhibition of herpes simplex virus replication. Antimicrob Aagents Chemother. 2014;58:5068–78. doi: 10.1128/AAC.02427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol Rev. 2012;92:689–737. doi: 10.1152/physrev.00028.2011. [DOI] [PubMed] [Google Scholar]
- 15.Pu H, Wang X, Su L, et al. Heroin activates ATF3 and CytC via c-Jun N-terminal kinase pathways to mediate neuronal apoptosis. Med Sci Monit Basic Res. 2015;21:53–62. doi: 10.12659/MSMBR.893827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin HB, Choi MS, Yi CM, et al. Inhibition of respiratory syncytial virus replication and virus-induced p38 kinase activity by berberine. Int Immunopharmacol. 2015;27:65–68. doi: 10.1016/j.intimp.2015.04.045. [DOI] [PubMed] [Google Scholar]
- 17.Boltzen U, Eisenreich A, Antoniak S, et al. Alternatively spliced tissue factor and full-length tissue factor protect cardiomyocytes against TNF-alpha-induced apoptosis. J Mol Cell Cardiol. 2012;52:1056–65. doi: 10.1016/j.yjmcc.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie W, Wang L, Dai Q, et al. Activation of AMPK restricts coxsackievirus B3 replication by inhibiting lipid accumulation. J Mol Cell Cardiol. 2015;85:155–67. doi: 10.1016/j.yjmcc.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Zhang K, Yu H, Xie W, et al. Expression of coxsackievirus and adenovirus receptor (CAR)-Fc fusion protein in Pichia pastoris and characterization of its anti-coxsackievirus activity. J Biotechnol. 2013;164:461–68. doi: 10.1016/j.jbiotec.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Liu R, An L, Liu G, et al. Mouse lung slices: An ex vivo model for the evaluation of antiviral and anti-inflammatory agents against influenza viruses. Antiviral Res. 2015;120:101–11. doi: 10.1016/j.antiviral.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ennaciri J, Menezes J, Proulx F, Toledano BJ. Induction of apoptosis by herpes simplex virus-1 in neonatal, but not adult, neutrophils. Pediatr Res. 2006;59:7–12. doi: 10.1203/01.pdr.0000191816.57544.b4. [DOI] [PubMed] [Google Scholar]
- 22.Birdsall TC, Kelly GS. Berberine: Therapeutic potential of an alkaloid found in several medicinal plants. Altern Med Rev. 1997;2:94–103. [Google Scholar]
- 23.Si X, Luo H, Morgan A, et al. Stress-activated protein kinases are involved in coxsackievirus B3 viral progeny release. J Virol. 2005;79:13875–81. doi: 10.1128/JVI.79.22.13875-13881.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui G, Qin X, Zhang Y, et al. Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J Biol Chem. 2009;284:28420–29. doi: 10.1074/jbc.M109.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo R, Li W, Liu B, et al. Resveratrol protects vascular smooth muscle cells against high glucose-induced oxidative stress and cell proliferation in vitro. Med Sci Monit Basic Res. 2014;20:82–92. doi: 10.12659/MSMBR.890858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He T, Guan X, Wang S, et al. Resveratrol prevents high glucose-induced epithelial-mesenchymal transition in renal tubular epithelial cells by inhibiting NADPH oxidase/ROS/ERK pathway. Mol Cell Endocrinol. 2015;402:13–20. doi: 10.1016/j.mce.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Johnson JC, Martinez O, Honko AN, et al. Pyridinyl imidazole inhibitors of p38 MAP kinase impair viral entry and reduce cytokine induction by Zaire ebolavirus in human dendritic cells. Ant Res. 2014;107:102–9. doi: 10.1016/j.antiviral.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirasawa K, Kim A, Han H-S, et al. Effect of p38 mitogen-activated protein kinase on the replication of encephalomyocarditis virus. J Virol. 2003;77:5649–56. doi: 10.1128/JVI.77.10.5649-5656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Dyke TA, Flanegan JB. Identification of poliovirus polypeptide P63 as a soluble RNA-dependent RNA polymerase. J Virol. 1980;35:732–40. doi: 10.1128/jvi.35.3.732-740.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul AV, Boom JH, Filippov D, Wimmer W. Protein-primed RNA synthesis by purified poliovirus RNA polymerase. Nature. 1998;393:280–84. doi: 10.1038/30529. [DOI] [PubMed] [Google Scholar]