Abstract
Bacteriophages and large dsDNA viruses encode sophisticated machinery to translocate their DNA into a preformed empty capsid. An essential part of this machine, the large terminase protein, processes viral DNA into constituent units utilizing its nuclease activity. Crystal structures of the large terminase nuclease from the thermophilic bacteriophage G20c show that it is most similar to the RuvC family of the RNase H-like endonucleases. Like RuvC proteins, the nuclease requires either Mn2+, Mg2+ or Co2+ ions for activity, but is inactive with Zn2+ and Ca2+. High resolution crystal structures of complexes with different metals reveal that in the absence of DNA, only one catalytic metal ion is accommodated in the active site. Binding of the second metal ion may be facilitated by conformational variability, which enables the two catalytic aspartic acids to be brought closer to each other. Structural comparison indicates that in common with the RuvC family, the location of the two catalytic metals differs from other members of the RNase H family. In contrast to a recently proposed mechanism, the available data do not support binding of the two metals at an ultra-short interatomic distance. Thus we postulate that viral terminases cleave DNA by the canonical RuvC-like mechanism.
INTRODUCTION
The large terminase protein is a key component of the DNA packaging machinery in tailed bacteriophages and evolutionarily related herpes viruses (1,2). Typically, in addition to an ATPase domain which powers DNA translocation (3,4), the large terminase contains a nuclease domain which cuts concatemeric DNA, generated by rolling circle replication (1,5,6). The nuclease cleaves the DNA concatemer first in the initiation phase and later in the completion stage of the DNA packaging process (7). After the first cut, the nascent genome end, in complex with the large terminase motor assembly, is docked onto the portal vertex of the empty procapsid (7) to enable DNA translocation into this protective container. Unlike phage λ where the nuclease cuts at the specific cosN site (8), in other phages such as T4, SPP1 and P22, only the first cut is made at a specific sequence close to the packaging (pac) site while the second cut is non-sequence specific (9–11). This second, or headful, cleavage event is made after around 102 to 110% of a genome length DNA has been packaged into the procapsid (12).
It has been assumed that the large terminase nuclease utilizes the two-metal catalysis mechanism proposed for other members of the RNase H-like endonucleases (13) such as RNase H, transposases, retroviral integrases and RuvC Holliday junction resolvases. This assumption is supported by two observations: firstly, the large terminase nuclease domain resembles the RNase-H fold (14–19). Secondly, simultaneous binding of two metals, occupying positions A and B, has been observed in crystal structures of human cytomegalovirus (HCMV) UL89 nuclease and in the structure of the Sf6 gp2 nuclease in complex with β-thujaplicinol (14,18). In general, the catalytic mechanism involving two metal ions was previously proposed for phosphoryl transfer reactions catalyzed by DNA polymerase I 3΄, 5΄-exonuclease, alkaline phosphatase, RNase P, group I and group II self-splicing introns and spliceosome (20–22). During the catalysis, the two metal ions form inner-sphere complexes with the scissile phosphate, the active site carboxylates and coordinated water molecules (Supplementary Figure S1). Metal A activates a coordinated water or sugar hydroxyl for nucleophilic attack, while metal B stabilizes the oxyanion leaving group in the transition state (20). More recent studies suggested that metal B is driving the reaction forward via energetically favorable transformation from an irregular dehydrated five-ligand coordination into a hydrated octahedral coordination (23,24).
A structural study on the Bacillus halodurans RNase H (Bh-RNase H) complex with an RNA/DNA hybrid suggested that during catalysis, the two metal ions, initially separated by ∼4.0 Å, are likely to move closer together, to ∼3.5 Å distance, neutralizing the developing negative charge of the pentavalent transition state (24) (Supplementary Figure S1). Recently, two different metal binding modes were reported for the Sf6 gp2 nuclease (14). In the first metal binding mode, the two Mg2+ or Mn2+ ions were modeled at ultra-short metal-metal distances of 2.42 and 2.64 Å, respectively, whereas in the second mode the two Mn2+ ions are separated by 3.75 Å. It was argued that binding two metals at the ultra-short metal-metal distance generates a highly positive electrostatic niche, driving the formation of the transition state (14).
Here, we present high resolution structures of the large terminase nuclease domain from Thermus thermophilus (Tth) bacteriophage G20c, a close relative of bacteriophages P74-26 and P23-45 (25). Structure comparison reveals plasticity in loop L1, which we propose plays an important role in facilitating nuclease activity during interaction with DNA. Structures of nuclease complexes with different divalent metal ions and their comparison with structural information on other members of the RNase H-like endonucleases, along with mutational and nuclease activity data, allow re-examination of the catalytic mechanism. This analysis supports a canonical RuvC-like mechanism for G20c and other viral large terminase nucleases, that does not involve bringing the two metals to an ultra-short distance.
MATERIALS AND METHODS
Phage isolation and sequencing
G20c was isolated from a natural hot water source with a temperature of ∼65 °C and pH 7.5 (Geyzer Valley, Kamchatka peninsula) using Tth HB8 strain as a host. Phage infection, isolation of individual plaques, preparation of phage lysate and phage genomic DNA purification and sequencing were performed as described for phages P74-26 and P23-45 (25). The percentage of G20c synteny to P74-26 and P23-45 (by total genome alignment) is 95 and 94%, respectively. Blastn analysis of ORFs of G20c reveals that 105 out of 111 ORFs are highly similar (e-value less than 1E-33) to those of P23-45 and/or P74-26.
Cloning, expression and purification
The DNA fragment encoding either the full length G20c large terminase (residues 1–485) or the nuclease domain (residues 257–443) were amplified by PCR and cloned into the vector pET-YSBLIC3C by using ligation-independent cloning (26). In this vector, the protein coding sequence is joined to a sequence encoding for an N-terminal 6-histidine tag fused to the human rhinovirus 3C protease cleavage site. Site directed mutagenesis was used to introduce codon changes for all the mutants using the CloneAmp™ HiFi PCR Premix (Takara Bio USA, Inc). The full-length terminase and the nuclease domain together with all the mutants were expressed using the same protocol in E. coli Rosetta (DE3) pLysS (Novagen EMD Millipore, USA) in LB medium containing 30 μg/ml kanamycin and 34 μg/ml chloramphenicol. Cells were grown at 37°C until OD600 reached 0.6–0.8 followed by induction with 1mM isopropyl 1-thio-β-D-galactopyranoside and further growth for 2 h. Cells were harvested by centrifugation for 20 min at 5000 × g at 4°C and frozen at −80°C before purification.
Before sonication, cell pellets were resuspended in buffer A (20 mM Tris pH 7.5, 1 M NaCl) containing 1 mM AEBSF, 0.5 μg/ml leupeptin, 0.7 μg/ml pepstatin and 0.1 mg/ml lysozyme. The lysate was clarified by centrifugation at 19 000 × g for 1 h and filtration using a 0.45 μm filter. Proteins were first purified by nickel affinity chromatography with a His-Trap column (GE Healthcare) equilibrated with buffer A containing 10 mM imidazole, and eluted with a 10–500 mM imidazole linear gradient in buffer A. The eluted target protein fractions were collected and dialyzed into 20 mM Tris pH 7.5, 250 mM NaCl, 0.5 mM DTT at 4°C overnight. During the dialysis, HRV 3C protease was added to the protein in a 1:50 (w/w) ratio to remove the N-terminal 6-His-tag. Protein samples after digestion were applied to the His-Trap column as before. A concentrated flow through was applied to a Superdex 200 Hiload 16/60 column pre-equilibrated in 20 mM Tris-HCl, pH 7.5, and 250 mM NaCl (buffer B). The final protein samples were concentrated to 20–100 mg/ml.
Crystallization, data collection, structure determination and refinement
Crystals of the nuclease domain were first obtained from an in-drop proteolysis of the full-length large terminase in 0.1 M MES pH 6.0, 20% (w/v) PEG 6000, 10 mM ZnCl2 (Table 1, Crystal form 1). However, these crystals were difficult to reproduce. A nuclease domain construct containing residues 257–443 was then cloned, expressed and purified for crystallization. Before crystallization, the protein was diluted to 10 mg/ml using 20 mM Tris pH 7.5, 50 mM NaCl solution. Crystallization was performed at 20°C using sitting drop vapor diffusion by mixing 0.5 μl of the protein solution with 0.5 μl of reservoir solution, before equilibrating against 100 μl of the reservoir solution. Crystals for form 2 (Table 1), space group P3221, grew from 0.2 M lithium sulphate, 0.1 M Bis-Tris pH 5.5, 25% (w/v) PEG 3350. Crystals were soaked in a cryo-protectant solution containing 0.2 M lithium sulphate, 0.1 M Bis-Tris pH 5.5, 30% (w/v) PEG 3350 and 1 mM CoCl2 for 20 seconds before vitrification in liquid nitrogen. As Co2+ was not observed in the electron density, we refer to this form as "Apo". Crystal form 3 (Table 1), space group P21, was obtained using 0.2 M ammonium tartrate, 0.1 M Bis-Tris pH 5.5, 20% (w/v) PEG 3350. To produce crystals with bound divalent metal ions, crystals belonging to crystal form 3 were soaked in a cryo-protectant solution containing 0.2 M ammonium sulphate, 0.1 M Bis-Tris pH 5.5, 30% (w/v) PEG 3350 with 50 mM MnCl2 / CaCl2 or 10 mM MgCl2/CoCl2 for 3 min before flash cooling in liquid nitrogen.
Table 1. X-ray data collection and refinement statistics.
| Crystal form | 1 Zn2+-SAD | 1 Zn2+ | 2 Apo | 3 Mg2+ | 3 Mn2+ | 3 Co2+ | 3 Ca2+ |
|---|---|---|---|---|---|---|---|
| Wavelength (Å) | 1.2841 | 0.9763 | 0.9795 | 0.9796 | 0.9795 | 0.9796 | 0.9795 |
| Space group | P65 | P65 | P3221 | P21 | P21 | P21 | P21 |
| Unit-cell a, c (Å) | 60.8, 90.4 | 60.9, 90.4 | 55.1, 95.7 | 43.8, 69.9 | 43.8, 70.0 | 42.9, 69.7 | 43.4, 69.8 |
| Unit-cell b (Å) | 54.0 | 53.9 | 53.9 | 53.6 | |||
| Unit-cell β (°) | 91.9 | 92.1 | 91.6 | 91.8 | |||
| Resolution (Å) | 45.53-1.60 (1.63-1.60) | 45.60-1.45 (1.47-1.45) | 47.72-2.15 (2.21-2.15) | 43.78-1.20 (1.22-1.20) | 43.78-1.20 (1.22-1.20) | 42.92-1.60 (1.63-1.60) | 43.43-1.10 (1.11-1.10) |
| Rmerge (%) | 7.9 (147.6) | 8.6 (129.1) | 4.3 (80.4) | 7.3 (76.5) | 4.2 (34.6) | 7.2 (71.9) | 7.8 (97.7) |
| I/σ<I> | 13.4 (1.7) | 8.2 (1.0) | 10.3 (1.0) | 7.4 (1.6) | 17.6 (4.0) | 6.7 (1.1) | 6.0 (1.0) |
| Completeness (%) | 100.0(100.0) | 99.7(99.5) | 95.6 (90.6) | 97.0 (97.6) | 95.0 (89.1) | 95.5 (84.6) | 98.8 (87.3) |
| Multiplicity | 8.8 (8.8) | 4.5 (3.9) | 2.2 (2.2) | 2.5 (2.5) | 4.3 (4.3) | 2.1 (2.0) | 3.2 (2.8) |
| CC1/2 | 0.998 (0.746) | 0.997 (0.420) | 0.999 (0.365) | 0.996 (0.522) | 0.999 (0.906) | 0.997 (0.611) | 0.996 (0.385) |
| Refinement | |||||||
| Rwork/Rfree | 0.201/0.211 | 0.200/0.280 | 0.183/0.209 | 0.169/0.182 | 0.185/0.224 | 0.222/0.235 | |
| Mean B factor (A2) | 23 | 56 | 14 | 11 | 22 | 12 | |
| R.M.S.D. | |||||||
| Bond lengths (Å) | 0.008 | 0.009 | 0.007 | 0.006 | 0.007 | 0.006 | |
| Bond angles (°) | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | |
| Ramachandran (%) | |||||||
| Favored | 98 | 97 | 99 | 98 | 99 | 98 | |
| Allowed | 2 | 3 | 1 | 2 | 1 | 2 | |
| Outliers | 0 | 0 | 0 | 0 | 0 | 0 | |
Values in the parentheses are for the outermost resolution shell.
SAD, single-wavelength anomalous dispersion.
Diffraction data were collected at Diamond Light Source beamlines I02, I03 and I04 (Table 1) and processed using XDS (27). The structure of the crystal form 1, containing bound Zn2+, was determined by single-wavelength anomalous dispersion (SAD) using SHELXD (28). Density modification was performed by SHELXE (29), followed by model building by ARP/wARP (30). Structures of the apo form and metal complexes were determined by molecular replacement, using Phaser (31). Refinement was carried out using REFMAC5 (32), accompanied by iterative model building with Coot (33). Chimera (34) and CCP4mg (35) were used for figure generation.
Examination of Sf6 structure
The mFo-DFc maps for the Sf6 gp2 nuclease with two modeled Mg2+ ions (PDB code: 5C12) or Mn2+ ions (PDB code: 5C15) were generated using phenix.maps by omitting the two modeled metal ions and surrounding water molecules in the active site (water molecules 908, 910, 972, 995 and 1027 for the 5C12 structure and water molecules 999, 976, 923 and 1096 for the 5C15 structure). To avoid any differences resulting from software versions, we used phenix.maps from the same (1.8.1_1168) version of Phenix (36) as phenix.refine (37) used by Zhao et al. (14).
Metal ion removal
Residual metal ion contaminants co-purified with either the protein samples or DNA substrate were removed using Chelex® 100 resin (Bio-Rad Laboratories, Inc.). Approximately 50 μl of the resin slurry was used for a 100 μl protein sample. The beads were first dried by filter centrifugation and the pellet then added directly into the protein sample. This was left to shake gently for 1 h before the protein was collected using a 0.22 μm benchtop Corning® Costar® Spin-X® centrifuge tube filter (Sigma-Aldrich, Inc.)
In vitro nuclease assays
The G20c nuclease is active in the temperature range 20–60°C. 37°C was chosen for incubation, as at this temperature the nuclease fully digested the DNA substrate in 20 min (Supplementary Figure S2). A total of 120 ng of supercoiled or EcoRI-linearized pUC18 DNA containing the SPP1 pacL site were used as generic DNA substrates, and incubated with the purified G20c large terminase protein (1 μM) in a 20 μl reaction mixture containing 7 mM HEPES pH 7.5, 7 mM potassium glutamate with various concentrations of divalent metal ions at 37°C for 30 min, unless otherwise stated. The reaction was stopped by the addition of EDTA (50 mM), SDS (0.5%) and proteinase K (50 μg/ml) with a further incubation at 37°C for 30 min. The resultant cleavage products were then separated on a 0.8 or 1.0% agarose gel (1 × TAE running buffer) followed by ethidium bromide staining.
RESULTS
Structure of the G20c large terminase nuclease
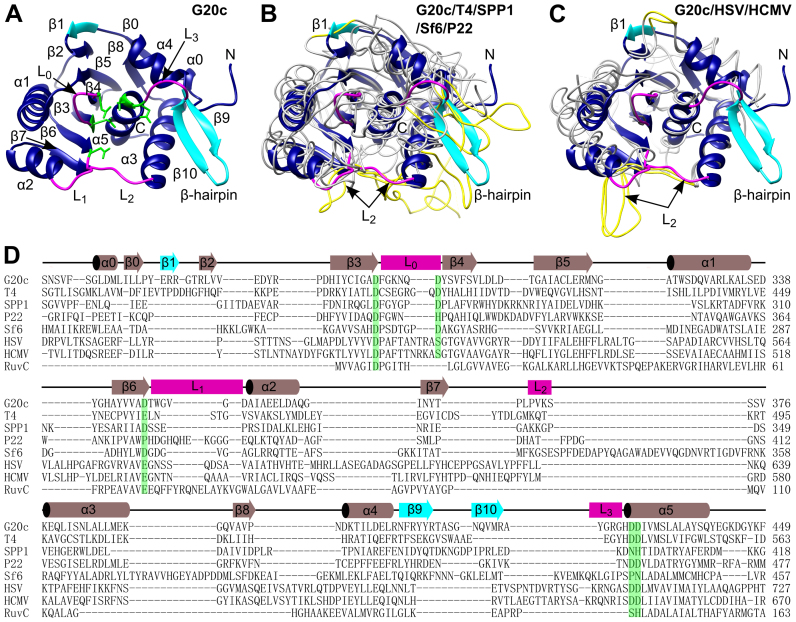
Initial crystals were obtained from a proteolytically cleaved C-terminal fragment of the full-length protein (Crystal form 1, Table 1). A bound Zn2+ ion from the crystallization solution enabled the structure to be determined by SAD. Subsequently a recombinant protein construct, residues 257 to 443, corresponding to the nuclease domain, crystallized in two different crystal forms, 2 and 3 (Table 1). Crystal forms 1 and 2 contain a single molecule, whereas crystal form 3 contains two protein molecules per asymmetric unit. The overall structure adopts the RNase-H fold (Figure 1A). As in other members of the RNase H-like endonucleases, a cluster of carboxylic acids is contributed to the active site by strands β3, β4 and β6, helix α5 and loops L0–L3. These residues were shown to be critical for bacteriophage function, DNA packaging or nuclease activities in bacteriophages T4 (17,38) and SPP1 (16,39). Loops L1 and L2, defined earlier for the P22 large terminase nuclease (15), correspond to residues 347–352 and 369–372, respectively, in the G20c large terminase. The two other loops, L0 and L3, residues 295–301 and 423–427, respectively, also contribute to the active site. The β-hairpin (β9 and β10 strands on Figure 1A), a unique feature of viral large terminases not observed in other members of the RNase H-like endonucleases (16), is well ordered in crystal forms 2 and 3, but is invisible in the structure of the proteolytic fragment (crystal form 1).
Figure 1.
Comparison of large terminase nuclease domains from different viruses. (A) Ribbon diagram of the G20c large terminase nuclease. β1 and β-hairpin (β9 and β10) are highlighted in cyan. Loop L0, L1, L2 and L3 are colored in magenta. Active site carboxylates are shown in green. (B) Superposition of the G20c nuclease (blue/cyan ribbon, as in panel A) with large terminase nucleases from T4, SPP1, Sf6 and P22 (gray chain traces). Segments equivalent to L2, β1 and the β-hairpin of the G20c nuclease are in yellow. (C) The same superposition as in (B) but with the herpesviruses HSV and HCMV nucleases. (D) Structure based sequence alignment of large terminase nucleases from bacteriophages G20c, T4, SPP1, P22, Sf6; herpesviruses HSV, HCMV and Thermus thermophilus RuvC resolvase. Secondary structure elements of the G20c nuclease are shown above the alignment. Conserved/equivalent active site residues are highlighted in green.
Comparison with other viral nucleases
Superposition of the G20c large terminase nuclease with bacteriophage (14–16,40) and herpes virus (18,19) nucleases reveals highly similar three-dimensional structures (Figure 1B and C), despite low sequence identity (Figure 1D). The highest similarity with phage nucleases is for T4 gp17 (40) (Cα rmsd of 1.9 Å for 176 residues that exhibit 25.4% sequence identity), while the lowest similarity is with the SPP1 G2P (16) (Cα rmsd of 2.9 Å for 143 residues with 12.6% sequence identity). Cα rmsd with the HSV pUL15 (19) (135 residues, 20.5% sequence identity) and HCMV UL89 (18) (141 residues, 19.2% sequence identity) nucleases is 2.4 and 2.5 Å, respectively.
The G20c nuclease has three major structural differences compared with nucleases from other viruses. Firstly, an additional β-strand (β1 on Figure 1A) extends the central β-sheet. Secondly, the uniquely viral terminase β-hairpin (strands β9 and β10 in Figure 1A), is more extended and better ordered (Figure 1B). Thirdly, loop L2 (‘hairpin’ in (19)) implicated in interaction with DNA in the HSV pUL15 nuclease is much shorter (Figure 1C).
Metal dependence of nuclease activity
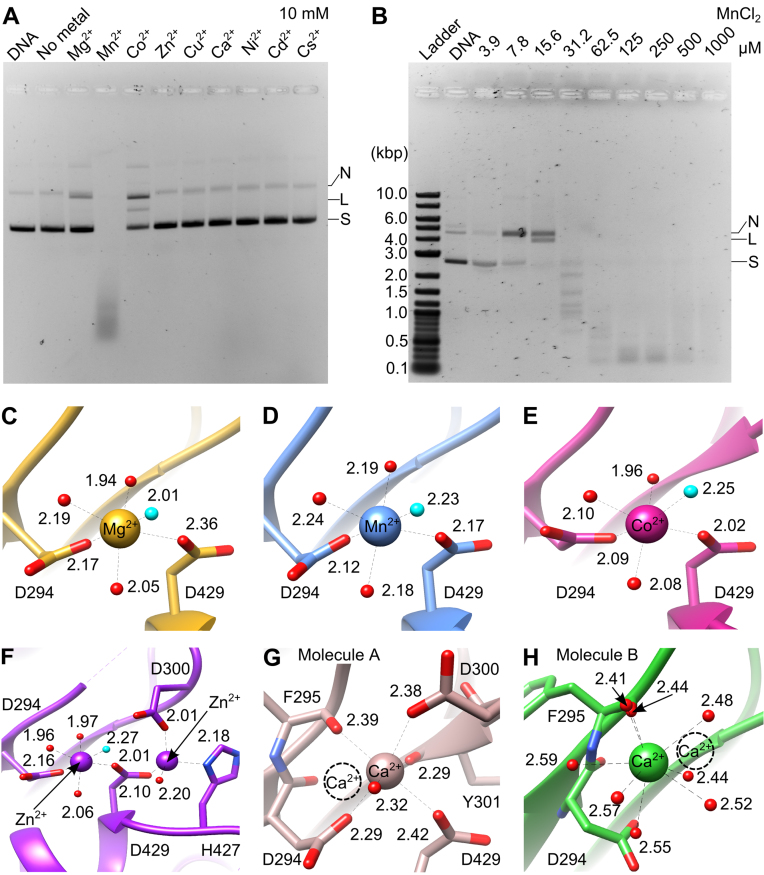
In order to easily detect the catalytic activity, assays were performed in low salt conditions, to facilitate binding of DNA and divalent metal ions. Similar to T4 and other headful phages, the nuclease activity appears to be non-sequence specific under these conditions. The nuclease was active in the presence of Mn2+, Mg2+ and Co2+ but inactive with Ni2+, Zn2+ or Ca2+ (Figure 2A and Supplementary Figure S2), consistent with observations for T4 gp17 (41), SPP1 G2P (39) and HCMV UL89 (18). Addition of Cu2+, Cd2+ and Cs2+ also did not support catalysis. Similar to G2P and UL89, addition of Mg2+ resulted only in limited activity (18,39), leading to production of nicked or linearized DNA when supercoiled DNA was used as substrate. However, G20c nuclease had minimal activity with Co2+, in contrast to the high non-specific in vitro nuclease activity observed for SPP1 G2P nuclease (comparable with Mn2+ (39)) or the absence of activity for the T4 gp17 nuclease (41). Significantly, in our assay conditions, Mn2+ supported the nuclease activity of the G20c large terminase even at very low (μM) concentrations, producing DNA segments with defined length (Figure 2B), suggesting some sequence preference for cleavage.
Figure 2.
Dependence of nuclease activity on metals and metal coordination in crystal structures. (A) Effect of divalent metal ions on the nuclease activity. N, nicked; L, linear; S, supercoiled DNA. (B) Effect of MnCl2 concentration on the nuclease activity: Ladder: 2-log DNA ladder (New England Biolabs). Metal coordination in crystal structures of the G20c nuclease in complex with (C) Mg2+, (D) Mn2+, (E) Co2+, (F) Zn2+, (G and H) Ca2+. The Ca2+ ion from the second molecule in the asymmetric unit is shown as dashed circle. The nucleophilic water molecule is highlighted in cyan.
Structures of nuclease-metal complexes
Structures for complexes with Mn2+, Mg2+, Co2+ and Ca2+ were determined by soaking crystals of the apo protein, whereas the structure of the Zn2+ complex was obtained by co-crystallization (Supplementary Figure S3). Only one metal ion, bound to the site A, was identified for conditions containing Mg2+, Mn2+ and Co2+. This metal ion is coordinated by the side chains of D294 and D429 and four water molecules in the canonical octahedral geometry (42) (Figure 2C–E). A similar coordination is observed for Zn2+, although there is also a second Zn2+ ion bound at an additional satellite site, at a distance of 4.6 Å from the site A Zn2+ ion (Figure 2F). Finally, two different Ca2+ binding modes are observed in each of the two protein molecules present in the asymmetric unit (Figure 2G and H).
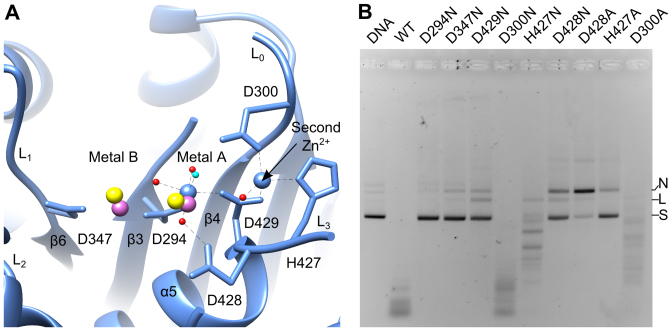
Superposition of the G20c nuclease structure containing two Zn2+ ions (Crystal form 1, Table 1) with SPP1 G2P and HCMV UL89 nucleases containing two bound Mn2+ ions (16,18) shows that while one Zn2+ ion is bound at site A, the second Zn2+ ion is bound on the opposite side of site B (Figure 3A). This Zn2+ ion is in a tetrahedral coordination (42) with D429, H427, D300 and a solvent molecule.
Figure 3.
Metal binding sites. (A) Active site of the G20c nuclease complex with Zn2+. The two Mn2+ ions, taken from structure superposition with SPP1 and HCMV nuclease-Mn2+ complexes are in magenta and yellow, respectively. The water nucleophile is shown in cyan. (B) In vitro nuclease assays. Activity is shown for the wild-type and active site mutant proteins. N, nicked; L, linear; S, supercoiled DNA.
Effect of active site residues on nuclease activity
The functional importance of the metal binding sites A, B and the satellite Zn2+ binding site was investigated using full-length protein containing both ATPase and nuclease domains. Aspartic acids coordinated by metals A and B were replaced by asparagine: D294N, D429N, D347N. Nuclease assays for all mutant proteins were performed in vitro in the presence of 0.1 or 1 mM MnCl2 (Figure 3B and Supplementary Figure S4). Wild-type large terminase converted the entire supercoiled DNA substrate into a smear of shorter DNA fragments at 1 mM MnCl2, while at 0.1 mM longer fragments with a somewhat defined length were observed. The lowest nuclease activity was observed for the D294N, D347N and D429N mutant proteins at both concentrations of MnCl2 (Figure 3B and Supplementary Figure S4). In contrast, D300N showed nuclease activity comparable to that of the wild-type protein whereas a modest decrease in nuclease activity was observed for D300A. A reduction of the nuclease activity was also observed for the H427N mutation. However, this mutant protein retained the ability to process longer DNA into smaller fragments, even at low (0.1 mM) MnCl2 concentrations (Figure 3B). Replacing this residue with alanine (H427A) resulted in deficiency in digestion of the supercoiled DNA at 0.1 mM MnCl2 concentration, like for the D347N mutation. This activity was partially recovered at 1 mM MnCl2 concentration where the H427A mutant protein could convert the entire supercoiled DNA into nicked and linearized DNA (Supplementary Figure S4). Both the D428N and D428A mutant proteins showed a significant drop in nuclease activity and were deficient for production of shorter DNA fragments (Figure 3B). These mutant proteins, like H427A, exhibited a slight increase in nuclease activity at 1 mM MnCl2 (Supplementary Figure S4).
DISCUSSION
Structural features
The structure of the nuclease from the thermophilic G20c bacteriophage is very similar to P74-26 large terminase (43), but differs from its mesophilic counterparts by shortened surface loops, notably a shorter loop L2 than that present in other bacteriophage and herpes virus nucleases (Figure 1B and C). There is also an increased number of salt bridges (44): 9 versus 5 to 7 found in nucleases of mesophilic viruses (14–16,18,19,40). In addition, the β-hairpin, present only in viral nucleases, is more extended and ordered (Figure 1B). These differences are expected to increase the protein stability at the higher environmental temperatures encountered by the G20c bacteriophage (45).
Role of active site residues
Mutational analysis indicated that metal coordinating residues D294, D347 and D429 are indispensable for catalysis (Figure 3B). Similar observations were made for SPP1 G2P nuclease (16,39) and for other members (46–48) of the RNase H-like nucleases. Taken together, the data support the two-metal catalysis mechanism, proposed earlier by Nowotny and Yang for the RNase H-like proteins (13,49).
Of the residues that co-ordinate the second Zn2+ ion, D300 is conserved among the large terminases of bacteriophages T4, RB49, SPP1 and Sf6, with the equivalent residue in T4 gp17 (D409) reported to be crucial for bacteriophage function (38). Comparison of the catalytic activity for mutants D300N and D300A, indicates that the negative charge of D300 is not essential for catalysis (Figure 3B and Supplementary Figure S4). The slight reduction in activity observed for D300A may be caused by disturbance of the hydrogen bonding network affecting D429 and/or bound DNA.
Histidine and glutamic acid residues adjacent to the metal A site in RNase H proteins have been suggested to play important roles in catalysis by affecting product release (50,51) and/or binding to a third Mg2+ during catalysis (52). This can explain the significant reduction in nuclease activity observed for the H427A mutant (Figure 3B and Supplementary Figure S4). Intriguingly, an equivalent serine residue found in the Sf6 gp2 nuclease forms a hydrogen bond with an oxygen atom on the bound metal chelator, occupying the position where the water nucleophile is normally coordinated by metal A (Supplementary Figure S5). Likewise, H427 may be involved in orienting the water nucleophile during the catalysis. This can be facilitated by the conformational flexibility of loop L3 and the β-hairpin.
The catalytic deficiency of D428 mutants (Figure 3B and Supplementary Figure S4) suggests that this residue may be responsible for stabilization of metal A binding, since it is proximal to metal A and forms a hydrogen bond with a coordinating inner shell water molecule (Figure 3A).
Structural basis for metal dependence of nuclease activity
RNase H-like endonucleases require divalent metal ions such as Mg2+ or Mn2+ for catalysis (46,53–55). The lack of activity in the presence of Ca2+ can be explained by the different coordination observed for this ion (Figure 2G and H), induced by its larger atomic radius and longer coordination distances, as compared to Mg2+ or Mn2+. A similar effect was observed for other RNase H-like enzymes in the presence of Ca2+ (50,56).
Due to the similarity in atomic radius, it was suggested that Zn2+ can substitute Mg2+ in catalysis (23). The activity was shown to be abrogated (56–58) or significantly reduced (51,59) by Zn2+ for the RNase H-like endonucleases. However, the structural basis for the reduction in activity remained unclear. In our structure, complexed with two Zn2+ ions, the Zn2+ ion bound at catalytic site A adopts an octahedral geometry (Figures 2F and 3A), resembling the canonical coordination of Mg2+ (Figure 2C) that would support catalysis. However, the second Zn2+ bound at an adjacent binding site, not previously reported for the RNase H-like endonucleases, is coordinated by catalytically important residues D429 and H427 (Figures 2F and 3A). Binding of this second Zn2+ ion perturbs charge distribution in the active site and may affect DNA and metal B binding as well as water nucleophile formation and coordination.
Re-examination of metal binding in Sf6 large terminase nuclease
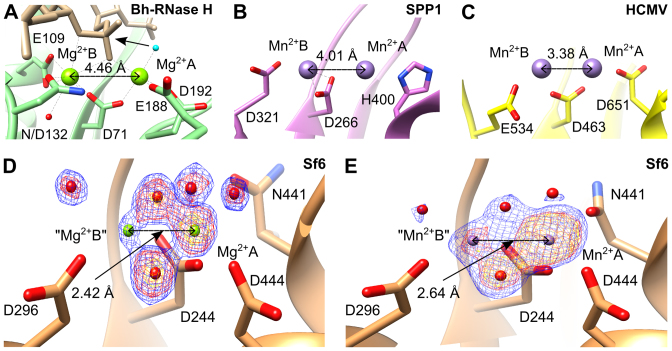
Structural observations for RNase H-like nucleases utilising the two-metal catalysis mechanism, show that the two metal ions, in the presence of the scissile phosphate, jointly coordinated by a conserved aspartic acid, are separated by 3.4–4.5 Å (13,24,50,60) (Figure 4A). Additionally, in the absence of bound DNA substrate the two manganese ions are separated by 4.0 Å (Figure 4B) and 3.4–3.6 Å (Figure 4C), respectively in SPP1 (16) and HCMV (18) large terminase nucleases. Comparable distances were observed for other enzymes catalysing phosphoryl-transfer by the two-metal catalysis mechanism (61).
Figure 4.
Metal–metal distances observed in crystal structures. (A) Active site of Bacillus halodurans RNase H complex with RNA/DNA hybrid and Mg2+ ions. The RNA is in beige and the nucleophile water molecule is in cyan. (B–E) Large terminase nucleases from (B) SPP1, (C) HCMV, (D) Sf6 with bound Mg2+ and (E) Sf6 with bound Mn2+. Models in panels (D) and (E) are overlaid with mFo-DFc electron density maps calculated after omitting metals and coordinated water molecules, contoured at 12 σ (yellow), 8 σ (red) and 6 σ (blue) in panel (D) and at 12 σ (yellow), 10 σ (red) and 6 σ (blue) in panel (E).
However for the Sf6 large terminase, in the absence of the scissile phosphate, the two metal ions were modeled at unusually ultra-short distances of 2.42 Å (Mg2+-Mg2+) (Figure 4D) and 2.64 Å (Mn2+-Mn2+) (14) (Figure 4E). We observed that in difference maps generated after omitting the two modeled metal ions and coordinating water molecules, the electron density for the metal at site A is clear whereas only ambiguous density, weaker than that for the coordinating solvent molecules, was observed at site B (Figure 4D and E). In both structures, the refined B-factor of the metal modeled at site B is around twice that of the metal at site A and the coordinating atoms, further indicating inconsistencies with experimental data. Therefore, we suggest that the observed weak electron density at the modeled metal site B presumably results from a low occupancy alternative metal binding position, as observed for another DNA processing protein (62), rather than the presence of two metals at the same time at an ultra-short distance which has not been observed before (13,61). These observations indicate that Sf6 nuclease uses a classical two-metal dependent catalysis mechanism, as described originally for RNase H (24,63) and below for G20c nuclease.
RuvC is the closest structural homologue of large terminase nuclease
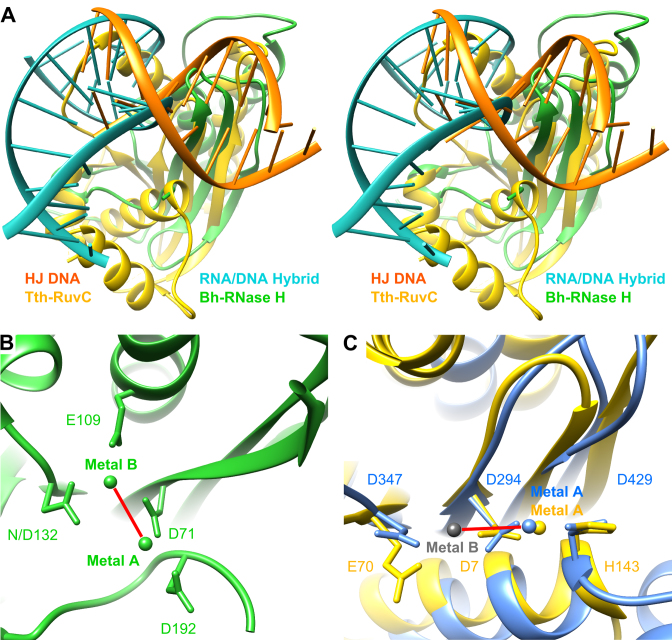
A DALI search (64) identified RuvC resolvases as the closest structural homologs of the G20c nuclease. Subsequent pairwise secondary-structure matching (SSM) analysis (65) using PDBeFold showed significantly higher Z-scores for Tth-RuvC (Z = 7.0 for 106 aligned residues) compared with the Bh-RNase H (Z = 1.9 for 93 aligned residues), Supplementary Table S1. Interestingly, structural comparison of the Bh-RNase H and Tth-RuvC with bound RNA/DNA hybrid or dsDNA respectively, reveals significant differences (Figure 5A). Notably, a different position of the metal A coordinating residue, D192 in Bh-RNase H versus H143 in Tth-RuvC, was observed (Figure 5B and C). These differences are due to different conformations, i.e. replacement of the extend strand (Bh-RNase H) by an α-helix (Tth-RuvC) which runs in the opposite direction (49,66). Moreover, the additional catalytic residue, E109, coordinated to metal B, is absent in RuvC (46,49,67). These differences result in distinctly different orientations of the active site metals and the bound nucleic acid duplex. It appears that the RuvC family evolved to adjust the position of their metal coordinating residues (and hence metal binding sites) to adapt to different nucleic acid substrates, while maintaining the classic RNase H fold. Structural superposition of the G20c large terminase nuclease with Tth-RuvC, unlike for Bh-RNase H, results in good alignment of the three catalytically important residues (Figure 5C), indicating that RuvC and viral large terminase nucleases utilize a highly similar catalytic mechanism.
Figure 5.
Comparison of metal location and DNA orientation. (A) Stereo view showing the superposition of nucleic acid complexes of Bacillus halodurans RNase H (green; RNA/DNA hybrid, cyan; PDB accession code 1ZBI) and Thermus thermophilus RuvC (yellow; Holliday junction DNA, orange; 4LD0). (B) Active site of Bh-RNase H nuclease with bound metal ions. (C) Close-up view at the active site in superposed G20c (blue, 5M1N) and Tth-RuvC (yellow, 4EP4) nucleases shown in the same orientation as (B). Bound metal ions (in both structures only at site A) are in corresponding colors. The gray sphere indicates the site B position (as in Lactococcus phage bIL67 RuvC, 4KTZ). Note the different relative orientation of the two metals in (B) and (C), indicated by red line.
DNA binding surface plasticity
Superposition of crystal forms 1, 2 and 3 (Table 1) shows that four loops (L0, L1, L2, L3) surrounding the catalytic site and the β-hairpin are flexible and adopt different conformations (Supplementary Figure S6). Importantly, all of these flexible structural segments are conserved both in phage (14–16,40) and herpesvirus (18,19) terminases. We note that D347, implicated in facilitating metal binding at site B during the catalysis (see below), is located at the N-terminus of L1. The position of this residue differs significantly between crystal forms 2 and 3 (Supplementary Figure S6), bringing the carboxyl group of D347 1.4 Å closer to the catalytic D294, which is expected to coordinate to both site A and B metals. We also note that a shorter distance between these two residues was observed earlier in two metal bound complexes of SPP1 (16) and HCMV (18) nucleases, and in the Lactococcus phage bIL67 RuvC complex with Mg2+ (68) (Supplementary Figure S6). A model of the DNA bound to the G20c nuclease was generated by superposition with the structure of the Tth–Ruvc complex with DNA (69) (Figure 6A). In the model, conserved loops L0, L1, L2, that form direct contacts with DNA in the Tth–RuvC resolvase, are in proximity to the DNA (Figure 6A and Supplementary Figure S7). Additionally, Loop L3 and the β-hairpin, absent in the Tth–RuvC resolvase, are also in close contact with the modeled DNA, indicating their potential involvement in DNA binding. This is consistent with previous suggestions for the involvement of β-hairpin in interaction with DNA (14–16). Furthermore, the DNA binding region predicted by the modeled G20c–DNA complex presented here is supported by the mutagenesis data for the P74-26 nuclease, reported in the accompanying paper (43).
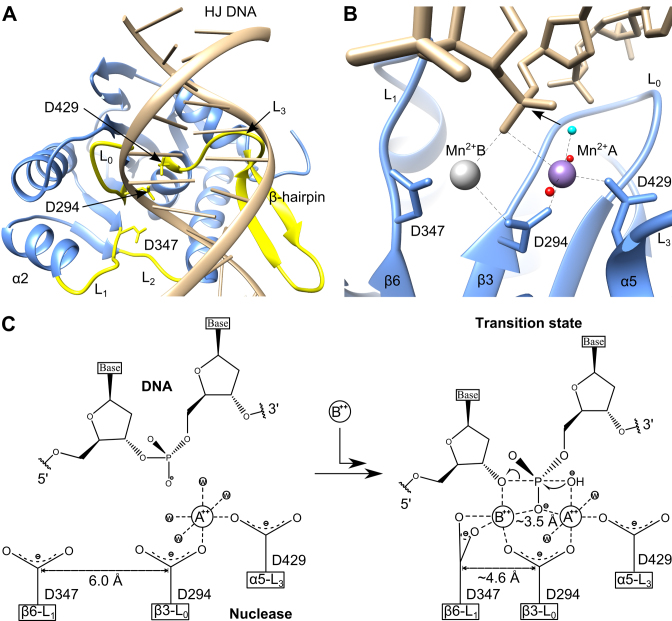
Figure 6.
Mechanism of DNA cleavage. (A) Model of G20c nuclease complex with DNA. The DNA model was obtained from nuclease superposition with the structure of Thermus thermophilus RuvC-DNA complex. Structural segments that are in proximity to the modeled DNA are in yellow. (B) Close-up view of the active site of the model (shown in panel A), with Mn2+ position at site B (gray sphere) corresponding to its observed position in the crystal structure of SPP1 nuclease. The nucleophile water molecule which is expected to perform the nucleophilic attack on the scissile phosphate is colored in cyan. (C) Schematic of the catalytic mechanism showing the proposed movement of D347 toward D294, concomitant with metal B and DNA binding, leading to transition state formation.
RuvC-like, canonical two-metal dependent catalysis
In the model of the G20c nuclease–DNA complex, the site B Mn2+ position (Figure 6B) corresponds to the position of the equivalent metal B in the crystal structure of SPP1 G2P (16), with the scissile phosphate of the Holliday junction DNA placed between the two metals. The water nucleophile coordinated by Mn2+ A (cyan in Figure 6B) is in proximity to the scissile phosphate, in a position favorable for nucleophilic attack (70).
It has been suggested that in addition to stabilizing the 3΄-leaving group, metal B serves to reduce to the energy barrier between the substrate/product states (23,24). This is facilitated by transformation from the fully dehydrated and irregular coordination in the substrate-bound complex, involving five ligands, into a hydrated octahedral geometry adopted after DNA cleavage. Unlike RNase H family proteins in which the metal site B is surrounded by three carboxylate side chains (Figures 4A and 5B; Supplementary Figure S1), only two conserved carboxylates are present in the large terminase nucleases of bacteriophages T4, SPP1, Sf6, G20c and herpes viruses HCMV and HSV (Figures 4B and C, and 6B; Supplementary Figure S5). Therefore, we suggest that D347 would coordinate metal B in a bidentate conformation (Figure 6C), to allow formation of a similarly dehydrated and irregular coordination at metal B (23,24). Binding of metal B can be facilitated by flexibility in the position of D347, observed in the crystal structures presented here, allowing D347 to move closer to D294. In accordance, structure superposition of the large terminase nucleases from G20c, SPP1, HCMV and other RuvC proteins, show that in the absence of metal B the two aspartate residues are more distant than in its presence (Supplementary Figure S6).
In summary, the following nuclease mechanism can be proposed for viral large terminases. In the absence of DNA, site A is occupied by a divalent metal ion, as in structures of Canarypox virus (71) and Tth–RuvC resolvases (72). Upon DNA binding, the negative charge provided by the scissile phosphate facilitates the recruitment of the second metal ion which binds at site B (Figure 6C). Binding of this metal is accompanied by change in the conformation of loop L1, bringing D347 closer to metal B, leading to formation of the transition state.
Insights into headful DNA packaging
The nuclease activity of the large terminase needs to be coupled to- and regulated by- DNA packaging for efficient production of infectious virions. This idea is supported by observations that ATP analogs stimulate the nuclease activity of T4 and P74-26 terminases (41,43). However, recent evidence suggests that this may be indirectly mediated through increased affinity of the ATPase domain of the full-length terminase towards DNA, thereby increasing nuclease activity (3,43). This explanation is consistent with observations that an isolated C-terminal nuclease domain is not as active as full-length large terminase (16) or even completely inactive (15), as observed for SPP1 and P22, respectively.
However, local DNA conformation is also likely to be essential for catalysis, given the similarity with RuvC, which binds branched and distorted DNA. During initiation of DNA packaging, when bacteriophage DNA is recognized by the small terminase protein, the DNA is expected to adopt a bent conformation, which may favor its binding within the active site leading to DNA cleavage (73,74). Finally, when the capsid is filled with DNA, the counteracting forces of the internal pressure of the capsid and the tight grip on the DNA by the stalled ATPase may induce DNA bending, facilitating the headful cleavage.
While this model only describes the cleavage of one strand of the dsDNA substrate, producing a nicked DNA product, cleavage of the second strand may be achieved by a major reorientation of the terminase–DNA complex. Alternatively, cleavage of the second DNA strand can result from binding of a second large terminase, either recruited to the initiation complex, or present as a subunit within the pentameric motor (3) for the headful cleavage event. Further work will ascertain the validity of either of these models.
ACCESSION NUMBERS
The genomic sequence for G20c has been deposited with the NCBI Genbank database, accession number KX987127. Structures of the G20c large terminase nuclease have been deposited with the Protein Data Bank, accession codes 5M1F (Apo), 5M1K (Mg2+), 5M1N (Mn2+), 5M1O (Co2+), 5M1P (Ca2+) and 5M1Q (Zn2+).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Johan Turkenburg and Sam Hart for X-ray data collection at Diamond Light Source, UK. We thank Diamond Light Source for access to beamlines I02 I03 and I04 (Proposal numbers mx-7864, mx-9948 and mx-13587) that contributed to the results presented here.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Wellcome Trust [098230, 101528 to A.A.A.]; RFBR [16-54-10074 КО_а] and NIH [R01 GM59295] (to K.S.); China Scholarship Council [201307040007] and Wild Fund (to R.G.X.). Funding for open access charge: Wellcome Trust.
Conflict of interest statement. None declared.
REFERENCES
- 1. Casjens S.R. The DNA-packaging nanomotor of tailed bacteriophages. Nat. Rev. Microbiol. 2011; 9:647–657. [DOI] [PubMed] [Google Scholar]
- 2. Baines J.D. Herpes simplex virus capsid assembly and DNA packaging:a present and future antiviral drug target. Trends Microbiol. 2011; 19:606–613. [DOI] [PubMed] [Google Scholar]
- 3. Hilbert B.J., Hayes J.A., Stone N.P., Duffy C.M., Sankaran B., Kelch B.A.. Structure and mechanism of the ATPase that powers viral genome packaging. Proc. Natl. Acad. Sci. U.S.A. 2015; 112:E3792–E3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun S., Kondabagil K., Gentz P.M., Rossmann M.G., Rao V.B.. The structure of the ATPase that powers DNA packaging into bacteriophage T4 procapsids. Mol. Cell. 2007; 25:943–949. [DOI] [PubMed] [Google Scholar]
- 5. Morris C.F., Sinha N.K., Alberts B.M.. Reconstruction of bacteriophage T4 DNA replication apparatus from purified components: rolling circle replication following de novo chain initiation on a single-stranded circular DNA template. Proc. Natl. Acad. Sci. U.S.A. 1975; 72:4800–4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roizman B., Sears A.. Herpes simplex viruses and their replication. Fields Virol. 2001; 2:2399–2459. [Google Scholar]
- 7. Black L.W. DNA packaging in dsDNA bacteriophages. Annu. Rev. Microbiol. 1989; 43:267–292. [DOI] [PubMed] [Google Scholar]
- 8. Catalano C.E., Cue D., Feiss M.. Virus DNA packaging: the strategy used by phage λ. Mol. Microbiol. 1995; 16:1075–1086. [DOI] [PubMed] [Google Scholar]
- 9. Rao V.B., Black L.W.. Catalano CE. DNA Packaging in Bacteriophage T4. Viral Genome Packaging Machines: Genetics, Structure, and Mechanism. 2005; NY: Springer; 40–58. [Google Scholar]
- 10. Kufer B., Backhaus H., Schmieger H.. The packaging initiation site of phage P22. Mol. Gen. Genet. 1982; 187:510–515. [DOI] [PubMed] [Google Scholar]
- 11. Bravo A., Alonso J.C., Trautner T.A.. Functional analysis of the Bacillus subtilis bacteriophage SPP1 pac site. Nucleic Acids Res. 1990; 18:2881–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rao V.B., Feiss M.. The bacteriophage DNA packaging motor. Annu. Rev. Genet. 2008; 42:647–681. [DOI] [PubMed] [Google Scholar]
- 13. Yang W. Nucleases: diversity of structure, function and mechanism. Q. Rev. Biophys. 2011; 44:1–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao H., Lin Z., Lynn A.Y., Varnado B., Beutler J.A., Murelli R.P., Le Grice S.F., Tang L.. Two distinct modes of metal ion binding in the nuclease active site of a viral DNA-packaging terminase: insight into the two-metal-ion catalytic mechanism. Nucleic Acids Res. 2015; 44:776–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roy A., Cingolani G.. Structure of p22 headful packaging nuclease. J. Biol. Chem. 2012; 287:28196–28205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smits C., Chechik M., Kovalevskiy O.V., Shevtsov M.B., Foster A.W., Alonso J.C., Antson A.A.. Structural basis for the nuclease activity of a bacteriophage large terminase. EMBO Rep. 2009; 10:592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alam T.I., Draper B., Kondabagil K., Rentas F.J., Ghosh‐Kumar M., Sun S., Rossmann M.G., Rao V.B.. The headful packaging nuclease of bacteriophage T4. Mol. Microbiol. 2008; 69:1180–1190. [DOI] [PubMed] [Google Scholar]
- 18. Nadal M., Mas P.J., Blanco A.G., Arnan C., Solà M., Hart D.J., Coll M.. Structure and inhibition of herpesvirus DNA packaging terminase nuclease domain. Proc. Natl. Acad. Sci. U.S.A. 2010; 107:16078–16083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sigamani S.S., Zhao H., Kamau Y.N., Baines J.D., Tang L.. The structure of the herpes simplex virus DNA-packaging terminase pUL15 nuclease domain suggests an evolutionary lineage among eukaryotic and prokaryotic viruses. J. Virol. 2013; 87:7140–7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steitz T.A., Steitz J.A.. A general two-metal-ion mechanism for catalytic RNA. Proc. Natl. Acad. Sci. 1993; 90:6498–6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beese L.S., Steitz T.A.. Structural basis for the 3΄-5΄exonuclease activity of Escherichia coli DNA polymerase I: a two metal ion mechanism. EMBO J. 1991; 10:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim E.E., Wyckoff H.W.. Reaction mechanism of alkaline phosphatase based on crystal structures: two-metal ion catalysis. J. Mol. Biol. 1991; 218:449–464. [DOI] [PubMed] [Google Scholar]
- 23. Yang W., Lee J.Y., Nowotny M.. Making and Breaking Nucleic Acids: Two-Mg2+-Ion Catalysis and Substrate Specificity. Mol. Cell. 2006; 22:5–13. [DOI] [PubMed] [Google Scholar]
- 24. Nowotny M., Yang W.. Stepwise analyses of metal ions in RNase H catalysis from substrate destabilization to product release. EMBO J. 2006; 25:1924–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Minakhin L., Goel M., Berdygulova Z., Ramanculov E., Florens L., Glazko G., Karamychev V.N., Slesarev A.I., Kozyavkin S.A., Khromov I. et al. . Genome comparison and proteomic characterization of thermus thermophilus bacteriophages P23-45 and P74-26: Siphoviruses with triplex-forming sequences and the longest known tails. J. Mol. Biol. 2008; 378:468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fogg M.J., Wilkinson A.J.. Higher-throughput approaches to crystallization and crystal structure determination. Biochem. Soc. Trans. 2008; 36:771–775. [DOI] [PubMed] [Google Scholar]
- 27. Kabsch W. XDS. Acta Crystallogr. D. 2010; 66:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schneider T.R., Sheldrick G.M.. Substructure solution with SHELXD. Acta Crystallogr. D. 2002; 58:1772–1779. [DOI] [PubMed] [Google Scholar]
- 29. Sheldrick G.M. Experimental phasing with SHELXC/D/E: combining chain tracing with density modification. Acta Crystallogr. D Biol. Crystallogr. 2010; 66:479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langer G., Cohen S.X., Lamzin V.S., Perrakis A.. Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nat. Protoc. 2008; 3:1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCoy A.J., Grosse-Kunstleve R.W., Adams P.D., Winn M.D., Storoni L.C., Read R.J.. Phaser crystallographic software. J. Appl. Crystallogr. 2007; 40:658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murshudov G.N., Vagin A.A., Dodson E.J.. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D. 1997; 53:240–255. [DOI] [PubMed] [Google Scholar]
- 33. Emsley P., Cowtan K.. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. 2004; 60:2126–2132. [DOI] [PubMed] [Google Scholar]
- 34. Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E.. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004; 25:1605–1612. [DOI] [PubMed] [Google Scholar]
- 35. McNicholas S., Potterton E., Wilson K., Noble M.. Presenting your structures: the CCP4mg molecular-graphics software. Acta Crystallogr. D Biol. Crystallogr. 2011; 67:386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adams P.D., Afonine P.V., Bunkóczi G., Chen V.B., Davis I.W., Echols N., Headd J.J., Hung L.W., Kapral G.J., Grosse-Kunstleve R.W.. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010; 66:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Afonine P.V., Grosse-Kunstleve R.W., Echols N., Headd J.J., Moriarty N.W., Mustyakimov M., Terwilliger T.C., Urzhumtsev A., Zwart P.H., Adams P.D.. Towards automated crystallographic structure refinement with phenix. refine. Acta Crystallogr. D Biol. Crystallogr. 2012; 68:352–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rentas F.J., Rao V.B.. Defining the bacteriophage T4 DNA packaging machine: evidence for a C-terminal DNA cleavage domain in the large terminase/packaging protein gp17. J. Mol. Biol. 2003; 334:37–52. [DOI] [PubMed] [Google Scholar]
- 39. Cornilleau C., Atmane N., Jacquet E., Smits C., Alonso J.C., Tavares P., Oliveira L.. The nuclease domain of the SPP1 packaging motor coordinates DNA cleavage and encapsidation. Nucleic Acids Res. 2013; 41:340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun S., Kondabagil K., Draper B., Alam T.I., Bowman V.D., Zhang Z., Hegde S., Fokine A., Rossmann M.G., Rao V.B.. The structure of the phage T4 DNA packaging motor suggests a mechanism dependent on electrostatic forces. Cell. 2008; 135:1251–1262. [DOI] [PubMed] [Google Scholar]
- 41. Ghosh-Kumar M., Alam T.I., Draper B., Stack J.D., Rao V.B.. Regulation by interdomain communication of a headful packaging nuclease from bacteriophage T4. Nucleic Acids Res. 2011; 39:2742–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harding M.M. Geometry of metal–ligand interactions in proteins. Acta Crystallogr. D Biol. Crystallogr. 2001; 57:401–411. [DOI] [PubMed] [Google Scholar]
- 43. Hilbert B.J., Hayes J.A., Stone N.P., Xu R.G., Kelch B.A.. The large terminase DNA packaging motor grips DNA with its ATPase domain for cleavage by the flexible nuclease domain. Nucleic Acids Res. 2016; doi:10.1093/nar/gkw1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sarakatsannis J.N., Duan Y.. Statistical characterization of salt bridges in proteins. Proteins. 2005; 60:732–739. [DOI] [PubMed] [Google Scholar]
- 45. Kumar S., Nussinov R.. How do thermophilic proteins deal with heat?. Cell. Mol. Life Sci. 2001; 58:1216–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nowotny M., Gaidamakov S.A., Crouch R.J., Yang W.. Crystal structures of RNase H bound to an RNA/DNA hybrid: substrate specificity and metal-dependent catalysis. Cell. 2005; 121:1005–1016. [DOI] [PubMed] [Google Scholar]
- 47. Derbyshire V., Grindley N., Joyce C.. The 3΄-5΄exonuclease of DNA polymerase I of Escherichia coli: contribution of each amino acid at the active site to the reaction. EMBO J. 1991; 10:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saito A., Iwasaki H., Ariyoshi M., Morikawa K., Shinagawa H.. Identification of four acidic amino acids that constitute the catalytic center of the RuvC Holliday junction resolvase. Proc. Natl. Acad. Sci. U.S.A. 1995; 92:7470–7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nowotny M. Retroviral integrase superfamily: the structural perspective. EMBO Rep. 2009; 10:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nowotny M., Gaidamakov S.A., Ghirlando R., Cerritelli S.M., Crouch R.J., Yang W.. Structure of human RNase H1 complexed with an RNA/DNA hybrid: insight into HIV reverse transcription. Mol. Cell. 2007; 28:264–276. [DOI] [PubMed] [Google Scholar]
- 51. Alla N.R., Nicholson A.W.. Evidence for a dual functional role of a conserved histidine in RNA.DNA heteroduplex cleavage by human RNase H1. FEBS J. 2012; 279:4492–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ho M.-H., De Vivo M., Dal Peraro M., Klein M.L.. Understanding the effect of magnesium ion concentration on the catalytic activity of ribonuclease H through computation: does a third metal binding site modulate endonuclease catalysis?. J. Am. Chem. Soc. 2010; 132:13702–13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Starnes M.C., Cheng Y.. Human immunodeficiency virus reverse transcriptase-associated RNase H activity. J. Biol. Chem. 1989; 264:7073–7077. [PubMed] [Google Scholar]
- 54. Berkower I., Leis J., Hurwitz J.. Isolation and characterization of an endonuclease from Escherichia coli specific for ribonucleic acid in ribonucleic acid· deoxyribonucleic acid hybrid structures. J. Biol. Chem. 1973; 248:5914–5921. [PubMed] [Google Scholar]
- 55. Tan C.K., Rigal C., Mian A.M., So A.G., Downey K.M.. Inhibition of the RNase H activity of HIV reverse transcriptase by azidothymidylate. Biochemistry. 1991; 30:4831–4835. [DOI] [PubMed] [Google Scholar]
- 56. Domanico P.L., Tse-Dinh Y.C.. Mechanistic studies on E. coli DNA topoisomerase I: divalent ion effects. J. Inorg. Biochem. 1991; 42:87–96. [DOI] [PubMed] [Google Scholar]
- 57. Jongruja N., You D.J., Kanaya E., Koga Y., Takano K., Kanaya S.. The N‐terminal hybrid binding domain of RNase HI from Thermotoga maritima is important for substrate binding and Mg2+‐dependent activity. FEBS J. 2010; 277:4474–4489. [DOI] [PubMed] [Google Scholar]
- 58. Shah R., Bennett R.J., West S.C.. Activation of RuvC Holliday junction resolvase in vitro. Nucleic Acids Res. 1994; 22:2490–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Takahagi M., Iwasaki H., Shinagawa H.. Structural requirements of substrate DNA for binding to and cleavage by RuvC, a Holliday junction resolvase. J. Biol. Chem. 1994; 269:15132–15139. [PubMed] [Google Scholar]
- 60. Steiniger-White M., Rayment I., Reznikoff W.S.. Structure/function insights into Tn5 transposition. Curr. Opin. Struct. Biol. 2004; 14:50–57. [DOI] [PubMed] [Google Scholar]
- 61. Sträter N., Lipscomb W.N., Klabunde T., Krebs B.. Two‐metal ion catalysis in enzymatic Acyl‐and phosphoryl‐transfer reactions. Angew. Chem. Int. Ed. Engl. 1996; 35:2024–2055. [Google Scholar]
- 62. He H., Chen Q., Georgiadis M.M.. High-resolution crystal structures reveal plasticity in the metal binding site of apurinic/apyrimidinic endonuclease I. Biochemistry. 2014; 53:6520–6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yang W., Hendrickson W.A., Crouch R.J., Satow Y.. Structure of ribonuclease H phased at 2 A resolution by MAD analysis of the selenomethionyl protein. Science. 1990; 249:1398–1405. [DOI] [PubMed] [Google Scholar]
- 64. Holm L., Sander C.. Dali: a network tool for protein structure comparison. Trends Biochem. Sci. 1995; 20:478–480. [DOI] [PubMed] [Google Scholar]
- 65. Krissinel E., Henrick K.. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 2004; 60:2256–2268. [DOI] [PubMed] [Google Scholar]
- 66. Majorek K.A., Dunin-Horkawicz S., Steczkiewicz K., Muszewska A., Nowotny M., Ginalski K., Bujnicki J.M.. The RNase H-like superfamily: new members, comparative structural analysis and evolutionary classification. Nucleic Acids Res. 2014; 42:4160–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yang W., Steitz T.A.. Recombining the structures of HIV integrase, RuvC and RNase H. Structure. 1995; 3:131–134. [DOI] [PubMed] [Google Scholar]
- 68. Green V., Curtis F.A., Sedelnikova S., Rafferty J.B., Sharples G.J.. Mutants of phage bIL67 RuvC with enhanced Holliday junction binding selectivity and resolution symmetry. Mol. Microbiol. 2013; 89:1240–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Górecka K.M., Komorowska W., Nowotny M.. Crystal structure of RuvC resolvase in complex with Holliday junction substrate. Nucleic Acids Res. 2013; 41:9945–9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Torres R.A., Bruice T.C.. The mechanism of phosphodiester hydrolysis: near in-line attack conformations in the hammerhead ribozyme. J. Am. Chem. Soc. 2000; 122:781–791. [Google Scholar]
- 71. Li H., Hwang Y., Perry K., Bushman F., Van Duyne G.D.. Structure and metal binding properties of a poxvirus resolvase. J. Biol. Chem. 2016; 291:11094–11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen L., Shi K., Yin Z., Aihara H.. Structural asymmetry in the thermus thermophilus RuvC dimer suggests a basis for sequential strand cleavages during Holliday junction resolution. Nucleic Acids Res. 2012; 41:648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gual A., Camacho A.G., Alonso J.C.. Functional analysis of the terminase large subunit, G2P, of Bacillus subtilis bacteriophage SPP1. J. Biol. Chem. 2000; 275:35311–35319. [DOI] [PubMed] [Google Scholar]
- 74. Greive S.J., Fung H.K., Chechik M., Jenkins H.T., Weitzel S.E., Aguiar P.M., Brentnall A.S., Glousieau M., Gladyshev G.V., Potts J.R.. DNA recognition for virus assembly through multiple sequence-independent interactions with a helix-turn-helix motif. Nucleic Acids Res. 2016; 44:776–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.