Abstract
Kidney lesions similar to those in humans with hemolytic-uremic syndrome were observed histologically in 82 of 122 piglets inoculated intragastrically with Shiga-toxigenic Escherichia coli but not in 29 controls. The locations of lesions matched locations where Stx-2 binding and Gb3 (globotriasylceramide receptors for Stx) were identified immunohistochemically.
Enterohemorrhagic Escherichia coli strains are Shiga toxin (Stx)-producing E. coli (STEC) strains that cause hemorrhagic colitis and hemolytic-uremic syndrome (HUS) in humans. STEC O157:H7 is the most frequently reported serotype associated with human disease worldwide and the major cause of bloody diarrhea and acute renal failure in children in North America. However, non-O157 STEC strains may be responsible for 20 to 50% of all human STEC infections (3, 10). About 10% of STEC-infected patients with hemorrhagic colitis develop HUS, and about 20% of HUS patients may develop end-stage kidney damage (1, 11).
STEC strains can be grouped by the array of virulence factors that they express. All STEC strains produce one or more Stx's (also called verotoxins). Stx1 and Stx2 are the two main groups of Stx. The Stx2 group contains variants, including Stx2c (24), Stx2d-activatable (15, 16, 27), Stx2d-nonactivatable (21), Stx2e (29), and Stx2f (19, 23). Many STEC strains, including O157:H7, produce intimin (encoded by the eae gene) and can cause attaching and effacing lesions in the intestines of animals or in tissue culture models (7). Many non-O157 STEC strains that lack intimin and do not cause attaching and effacing lesions do cause systemic disease in animals and humans (3, 4).
HUS is characterized by severe hemolytic anemia, thrombocytopenia, and thrombotic microangiopathy (TMA). In later stages of disease, victims develop uremia, somnolence, mental disturbances, and severe kidney damage. Kidney lesions are thought to occur secondarily to arteriolar and arterial vascular damage; tubular and glomerular dysfunctions are thought to result from glomerular capillary damage. However, several studies provide evidence of primary renal tubular cell damage in Stx-mediated HUS (17). Stx-induced kidney damage has been demonstrated in several different animal models, including piglets (4, 12), but the sequence of events from ingestion of STEC bacteria to the development of HUS is not yet understood (22).
The objectives of this retrospective histological examination of kidney samples from STEC-infected and control piglets from multiple experiments were (i) to describe the Stx-induced kidney lesions that occurred within the first few days after neonatal piglets were orally inoculated with STEC O157:H7 or non-O157:H7 STEC strains, (ii) to compare STEC-induced lesions in piglets with those seen in humans with HUS, and (iii) to determine if the sites where lesions were seen histologically in STEC-inoculated piglets matched those where Stx-2 binding and globotriasylceramide (Gb3, receptors for Stx) were demonstrated immunohistochemically.
Formalin-fixed, paraffin-embedded kidney tissues (at least two samples from each piglet) from 96 naturally farrowed, colostrum-fed (suckling; 1 to 11 days old) and 55 cesarean-derived, colostrum-deprived (CDCD; 1 to 3 days old) piglets used in earlier STEC infection studies at the National Animal Disease Center in Ames, Iowa (4, 5, 8)(unpublished data) were stained with hematoxylin and eosin (H&E), periodic acid-Schiff reagent (PAS), or trichrome. Glutaraldehyde-fixed samples from six STEC-infected and four control piglets were processed for transmission electron microscopy by routine methods (5). Piglets were orally inoculated by gavage within the first 8 h of life with 1010 or 106 CFU of different strains of STEC or Stx− E. coli (control) bacteria (Table 1).
TABLE 1.
E. coli strains and results of histological examination of kidneys from neonatal piglets at 1 to 11 days after inoculation with STEC or control Stx-negative E. coli
| Inoculum strain | Strain reference | Serotype | Shiga toxin genotype | eae gene | Dose (CFU) | No. tested (no. of litters) | Days p.i.a | No. of pigs with kidney lesions/no. of pigs tested (% positive)
|
|
|---|---|---|---|---|---|---|---|---|---|
| CDCD | Suckling | ||||||||
| Stx-negative strains | |||||||||
| 123 | 18 | O43:H28 | Negative | − | 1010 | 6 (5) | 2-3 | 0/6 | |
| 87-23 | 8 | O157:H7 | Negative | + | 1010 | 4 (1) | 2-3 | 0/4 | |
| 87-23 | 8 | O157:H7 | Negative | + | 106 | 19 (2) | 3-10 | 0/19 | |
| All Stx-negativeE. coli | 29 (8) | 2-10 | 0/6 | 0/23 | |||||
| STEC strains | |||||||||
| 933 | 6 | O157:H7 | stx1 stx2 | + | 1010 | 7 (1) | 1 | 7/7 | |
| 86-24 | 7 | O157:H7 | stx 2 | + | 1010 | 11 (1) | 1 | 10/11 | |
| 86-24 | 7 | O157:H7 | stx 2 | + | 106 | 17 (2) | 1-2 | 12/17 | |
| 86-24 | 7 | O157:H7 | stx 2 | + | 106 | 42 (6) | 1-10 | 24/42 | |
| 86-24 | 7 | O157:H7 | stx 2 | + | 1010 | 10 (4) | 8/10 | ||
| E32511/HSC/L | 4 | O157:H− | stx2c | + | 1010 | 5 (2) | 2-3 | 4/5 | |
| All O157 STEC | 92 (14) | 1-11 | 12/15 (80) | 53/77 (69) | |||||
| 3024-94 | 4 | O104:H21 | stx2d | − | 1010 | 2 (1) | 2 | 2/2 | |
| B2F1 | 4 | O91:H21 | stx2d1 stx2d2 | − | 1010 | 5 (4) | 2-3 | 1/5 | |
| B2F1 (plasmid cured) | 4 | O91:H21 | stx2d1 stx2d2 | − | 1010 | 4 (2) | 2 | 1/4 | |
| B2F1 Stx2d2-activatable+ | 4 | O91:H21 | stx2d2 | − | 1010 | 7 (2) | 1-2 | 3/7 | |
| B2F1 Stx2d1-activatable+ | 4 | O91:H21 | stx2d2 | − | 1010 | 7 (2) | 2 | 6/7 | |
| E2779910 | 16 | O91:H21 | stx2 | − | 1010 | 5 (1) | 2-3 | 4/5 | |
| All non-O157 STEC | 30 (6) | 1-3 | 17/30 (57) | ||||||
| All STEC | 82/122 (67) | 29/45 (64) | 53/77 (70) | ||||||
p.i., postinfection.
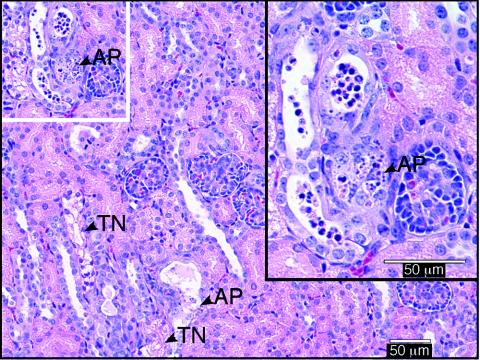
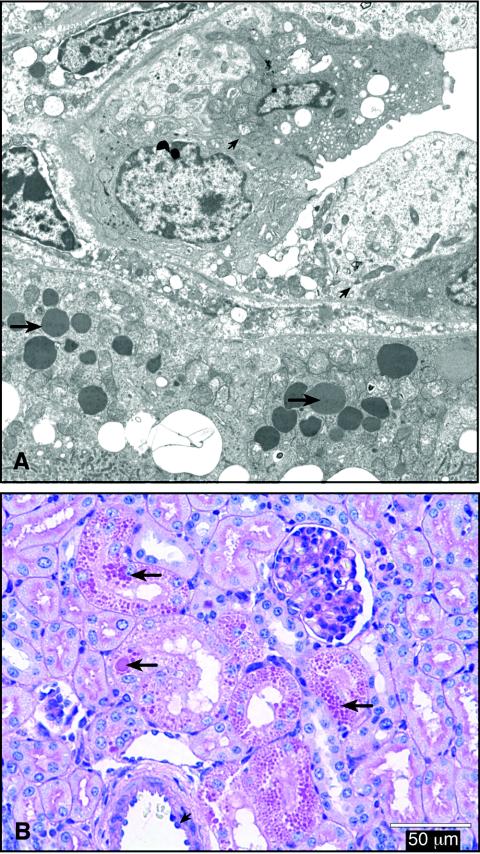
Kidney lesions were present in 82 of 122 STEC-inoculated piglets from 14 litters but not in any of the 29 controls. Lesions occurred in all groups of STEC-inoculated piglets, whether or not the inoculum strain produced intimin, and were seen as early as 24 to 48 h and as late as 11 days (latest time tissue had been collected) after inoculation. Lesions were characterized by focal to patchy tubular necrosis of varying severity and distribution and focal apoptosis in tubular epithelial cells (Fig. 1), comparable to those seen in humans with HUS (26). There also were marked tubular dilatation and intratubular accumulations of sloughed epithelial cells, predominantly in the outer cortex, where immature glomeruli (normally present in newborn piglets) were lost. Detachment of degenerate epithelial cells (Fig. 1 inset) was also demonstrated by electron microscopy (Fig. 2A). The presence of irregularly sized, electron-dense droplets (Fig. 2A), which stained positive with PAS (Fig. 2B), in vacuolated cells of convoluted tubules was interpreted as indicating plasma leakage and Stx-induced cell damage.
FIG. 1.
H&E-stained section of kidney from a piglet necropsied 2 days after inoculation with STEC, showing tubular necrosis (TN) and apoptosis (AP). Upper right inset shows a higher magnification of the white-framed segment at the upper left.
FIG. 2.
Lesions in sections of kidney from a piglet necropsied 2 days after inoculation with STEC. (A) Transmission electron micrographic demonstration of electron-dense, irregularly sized droplets in vacuolated cells of convoluted tubules (long arrows) and sloughing degenerate cells (short arrows in upper segment of image). Magnification, ×11,100. (B) PAS-stained section showing PAS-positive, irregularly sized protein droplets in convoluted tubules (long arrows) and endothelial swelling (short arrow).
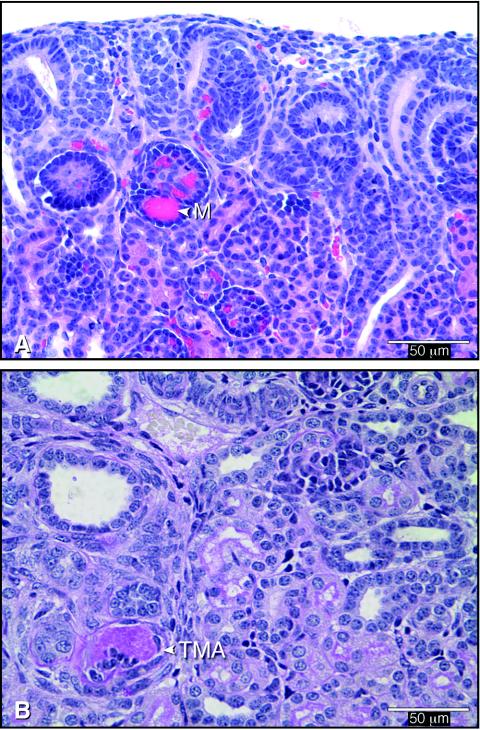
Other typical findings were endothelial swelling (Fig. 2B) and severe arteriolar constriction with extreme venous dilatation. In several cases, endothelial damage resulted in microthrombus formation (Fig. 3). TMA (Fig. 3B) occurred less often than glomerular microthrombi, which occurred in at least 60% of the cases. More-severe lesions, such as vascular and glomerular sclerosis, interpreted as resulting from early tissue lesions, were seen in a few piglets necropsied 11 days after inoculation. Similar vascular damage, but no tubular necrosis, was recently described for gnotobiotic piglets at 3 to 33 days after inoculation with STEC O157:H7 (5 of 6 positive) or non-O157 STEC (5 of 7 positive) (12). Differences in experimental procedures may explain why renal lesions were not found in gnotobiotic piglets inoculated with STEC or Stx in earlier studies (20).
FIG. 3.
Vascular lesions in a kidney from a piglet necropsied 2 days after inoculation with STEC. (A) H&E-stained section showing microthrombi in capillaries of a glomerulus (M) in the immature cortex. (B) PAS-stained section showing TMA in a small vessel of the inner cortex, which is occluded by accumulated PAS-positive fibrin admixed with cells.
All of the renal lesions (tubular necrosis, glomerular TMA, vascular endothelial swelling, and thrombus formation) in our piglets inoculated with Stx2-producing E. coli were consistent with those described for baboons that succumbed to severe disease within 52 to 72 h after they were intravenously inoculated with a high dose (100 ng/kg of body weight) of Stx1 (25). Tubular damage (and Stx binding to Gb3 receptors on cortical tubules) was described for mice after they were inoculated intraperitoneally with Stx1 (31), and tubular necrosis and damage to glomeruli were seen in 6-week-old ferrets following oral inoculation with STEC (32).
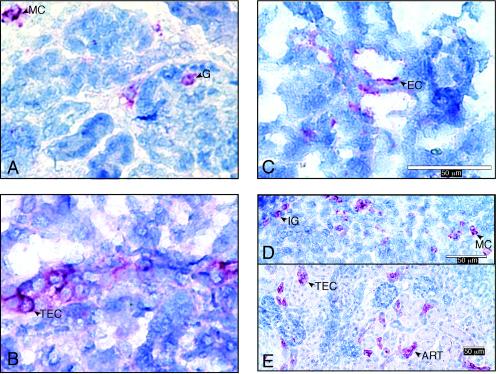
Stx binding sites and Gb3 were identified by immunohistochemical staining of frozen kidney sections from 45 and 37 piglets, respectively. Stx binding sites were identified by using a Stx2 overlay assay (30), and Gb3 was detected with anti-CD77/Gb3 antibodies (30). Negative controls, in which incubations with Stx2 or anti-CD77/Gb3, respectively, were omitted, were included in all assays. Stx binding sites and Gb3 were present in tubules, glomeruli, vessels, and single cells of the outer cortex (Fig. 4). These sites histologically matched sites where renal lesions were seen in STEC-inoculated piglets. These results extend the evidence that Gb3 receptors, which are present on several mammalian cell types, are receptors for Stx (17, 22, 28).
FIG. 4.
Immunohistochemically stained sections of kidney tissues from piglets necropsied 1 to 4 days after inoculation with STEC. Gb3 was identified by anti-CD77 staining (A to C), and Stx binding (D and E) was identified by a Stx overlay immunoassay. (A) Gb3 on glomerular cells (G) and mononuclear cells of outer cortex (MC). (B) Gb3 on tubular epithelial cells (TEC). (C) Gb3 on endothelial cells of small vessels (EC). (D) Stx binding to immature glomeruli (IG) of outer cortex. (E) Stx2 binding on TEC, or wall of an arteriole (ART).
Many details of the involvement of Gb3 in the pathogenesis of HUS are not yet fully understood. Stx binding to Gb3-containing cultured human epithelial cells stimulates cytokine production (interleukin-1 [IL-1], IL-6, and tumor necrosis factor alpha) (13). Tumor necrosis factor alpha increases Gb3 levels in cultured human brain endothelial cells and enhances their sensitivity to Stx (9). Urinary IL-6 levels are increased in baboons following intravenous inoculation with Stx2 (25) and in children with HUS (22). Unfortunately, measurements of these and other immunomodulators in urine and plasma were not included in the original studies from which the tissues for our study were obtained. There were, however, no clinical signs for the renal damage observed in these piglets.
Five major conclusions can be drawn from our investigation of kidney tissues from neonatal piglets experimentally inoculated with different strains of E. coli. First, STEC-inoculated piglets developed renal lesions. Second, tubular and vascular lesions in STEC-inoculated piglets were similar to the tubular necrosis and arterial and glomerular TMA seen in humans with HUS (2, 22, 26). To our knowledge, this is the first report of tubular necrosis in pigs experimentally inoculated with STEC. Third, epithelial and vascular damage occurred early (as early as 24 h after inoculation) at sites that contained Gb3. The coincident presence of early tubular and vascular lesions suggests that tubular necrosis is not dependent on prior glomerular damage. Fourth, STEC strains that produce different types of Stx caused similar types of kidney lesions in neonatal pigs. Fifth, intimin was not required for the pathogenesis of STEC-mediated kidney damage. This finding confirms that intimin is not required for the pathogenicity of STEC in neonatal piglets (5).
Based on our findings for STEC-infected piglets and in accordance with the literature, we propose the following chain of events in the pathogenesis of HUS: (i) Stx crosses the intestinal barrier shortly after STEC bacteria are ingested; (ii) STEC bacteria colonize the intestinal mucosa and release Stx; (iii) Stx attacks renal epithelial cells, resulting in necrosis of tubular epithelium, and attacks endothelial cells, resulting in early brain lesions; and (iv) continuous toxin absorption occurs in individuals who survive this attack but are not able to exclude the toxin-producing bacteria from their intestines (as happens in experimentally infected adult monkeys [14] and neonatal piglets [12]), resulting in the induction of Gb3 receptors, further uptake of toxins, toxin interactions with the hematopoietic system, and later signs associated with HUS in humans. Although we have not been able to prove this chain of events, we are convinced that neonatal piglets are an excellent model for further study of this life-threatening disease.
Acknowledgments
We thank B. K. Wheeler for technical assistance; NADC Visual Services and S. L. Johnson for preparation of the manuscript; and H. W. Moon for advice and critical evaluation of the manuscript.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Editor: J. T. Barbieri
REFERENCES
- 1.Andreoli, S. P., H. Trachtman, D. W. Acheson, R. L. Siegler, and T. G. Obrig. 2002. Hemolytic uremic syndrome: epidemiology, pathophysiology, and therapy. Pediatr. Nephrol. 17:293-298. [DOI] [PubMed] [Google Scholar]
- 2.Argyle, J. C., R. J. Hogg, T. J. Pysher, F. G. Silva, and R. L. Siegler. 1990. A clinicopathological study of 24 children with hemolytic uremic syndrome. A report of the Southwest Pediatric Nephrology Study Group. Pediatr. Nephrol. 4:52-58. [DOI] [PubMed] [Google Scholar]
- 3.Banatvala, N., P. M. Griffin, K. D. Greene, T. J. Barrett, W. F. Bibb, J. H. Green, and J. G. Wells. 2001. The United States National Prospective Hemolytic Uremic Syndrome Study: microbiologic, serologic, clinical, and epidemiologic findings. J. Infect. Dis. 183:1063-1070. [DOI] [PubMed] [Google Scholar]
- 4.Dean-Nystrom, E. A., A. R. Melton-Celsa, J. F. Pohlenz, H. W. Moon, and A. D. O'Brien. 2003. Comparative pathogenicity of Escherichia coli O157 and intimin-negative non-O157 Shiga toxin-producing E. coli strains in neonatal pigs. Infect. Immun. 71:6526-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dean-Nystrom, E. A., J. Pohlenz, H. W. Moon, and A. D. O'Brien. 2000. Escherichia coli O157:H7 causes more severe systemic disease in suckling piglets than in colostrum-deprived neonatal piglets. Infect. Immun. 68:2356-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean-Nystrom, E. A., B. T. Bosworth, W. C. Cray, Jr., and H. W. Moon. 1997. Pathogenicity of Escherichia coli O157:H7 in the intestines of neonatal calves. Infect. Immun. 65:1842-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean-Nystrom, E. A., B. T. Bosworth, H. W. Moon, and A. D. O'Brien. 1998. Escherichia coli O157:H7 requires intimin for enteropathogenicity in calves. Infect. Immun. 66:4560-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean-Nystrom, E. A., L. J. Gansheroff, M. Mills, H. W. Moon, and A. D. O'Brien. 2002. Vaccination of pregnant dams with intiminO157 protects suckling piglets from Escherichia coli O157:H7 infection. Infect. Immun. 70:2414-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenhauer, P. B., P. Chaturvedi, R. E. Fine, A. J. Ritchie, J. S. Pober, T. G. Cleary, and D. S. Newburg. 2001. Tumor necrosis factor alpha increases human cerebral endothelial cell Gb3 and sensitivity to Shiga toxin. Infect. Immun. 69:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fey, P. D., R. S. Wickert, M. E. Rupp, T. J. Safranek, and S. H. Hinrichs. 2000. Prevalence of non-O157:H7 Shiga toxin-producing Escherichia coli in diarrheal stool samples from Nebraska. Emerg. Infect. Dis. 6:530-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldwater, P. N., and K. A. Bettelheim. 1998. New perspectives on the role of Escherichia coli O157:H7 and other enterohemorrhagic E. coli serotypes in human disease. J. Med. Microbiol. 47:1039-1045. [DOI] [PubMed] [Google Scholar]
- 12.Gunzer, F., I. Hennig-Pauka, K. H. Waldmann, R. Sandhoff, H. J. Grone, H. H. Kreipe, A. Matussek, and M. Mengel. 2002. Gnotobiotic piglets develop thrombotic microangiopathy after oral infection with enterohemorrhagic Escherichia coli. Am. J. Clin. Pathol. 118:364-375. [DOI] [PubMed] [Google Scholar]
- 13.Hughes, A. K., P. K. Stricklett, and D. E. Kohan. 2001. Shiga toxin-1 regulation of cytokine production by human glomerular epithelial cells. Nephron 88:14-23. [DOI] [PubMed] [Google Scholar]
- 14.Kang, G., A. B. Pulimood, R. Koshi, A. Hull, D. Acheson, P. Rajan, G. T. Keusch, V. I. Mathan, and M. M. Mathan. 2001. A monkey model for enterohemorrhagic Escherichia coli infection. J. Infect. Dis. 184:206-210. [DOI] [PubMed] [Google Scholar]
- 15.Kokai-Kun, J. F., A. R. Melton-Celsa, and A. D. O'Brien. 2000. Elastase in intestinal mucus enhances the cytotoxicity of Shiga toxin type 2d. J. Biol. Chem. 275:3713-3721. [DOI] [PubMed] [Google Scholar]
- 16.Melton-Celsa, A. R., H. F. Kokai-Kun, and A. D. O'Brien. 2002. Activation of Shiga toxin type 2d (Stx2d) by elastase involves cleavage of the C-terminal two amino acids of the A2 peptide in the context of the appropriate B pentamer. Mol. Microbiol. 43:207-215. [DOI] [PubMed] [Google Scholar]
- 17.Meyers, K. E., and B. S. Kaplan. 2000. Many cell types are Shiga toxin targets. Kidney Int. 57:2650-2651. [DOI] [PubMed] [Google Scholar]
- 18.Moon, H. W., D. K. Sorensen, and J. H. Sautter. 1968. Experimental enteric colibacillosis in piglets. Can. J. Comp. Med. 32:493-497. [PMC free article] [PubMed] [Google Scholar]
- 19.Morabito, S., G. Dell'Omo, U. Agrimi, H. Schmidt, H. Karch, T. Cheasty, and A. Caprioli. 2001. Detection and characterization of Shiga toxin-producing Escherichia coli in feral pigeons. Vet. Microbiol. 82:275-283. [DOI] [PubMed] [Google Scholar]
- 20.Moxley, R. A., and D. H. Francis. 1998. Overview of animal models, p. 249-260. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press, Washington, D.C.
- 21.Pierard, D., G. Muyldermans, L. Moriau, D. Stevens, and S. Lauwers. 1998. Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal Escherichia coli isolates. J. Clin. Microbiol. 36:3317-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proulx, F., E. G. Seidman, and D. Karpman. 2001. Pathogenesis of Shiga toxin-associated hemolytic uremic syndrome. Pediatr. Res. 50:163-171. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt, H., J. Scheef, S. Morabito, A. Caprioli, L. H. Wieler, and H. Karch. 2000. A new Shiga toxin 2 variant (Stx2f) from Escherichia coli isolated from pigeons. Appl. Environ. Microbiol. 66:1205-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt, C. K., M. L. McKee, and A. D. O'Brien. 1991. Two copies of Shiga-like toxin II-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157:H− strain E32511. Infect. Immun. 59:1065-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegler, R. L., T. G. Obrig, T. J. Pysher, V. L. Tesh, N. D. Denkers, and F. B. Taylor. 2003. Response to Shiga toxin 1 and 2 in a baboon model of hemolytic uremic syndrome. Pediatr. Nephrol. 18:92-96. [DOI] [PubMed] [Google Scholar]
- 26.Taguchi, T., H. Uchida, N. Kiyokawa, T. Mori, N. Sato, H. Horie, T. Takeda, and J. Fujimoto. 1998. Verotoxins induce apoptosis in human renal tubular epithelium derived cells. Kidney Int. 53:1681-1688. [DOI] [PubMed] [Google Scholar]
- 27.Teel, L. D., C. K. Schmitt, A. R. Melton-Celsa, and A. D. O'Brien. 2002. One of two copies of the gene for the activatable Shiga toxin type 2d in Escherichia coli O91:H21 strain B2F1 is associated with an inducible bacteriophage. Infect. Immun. 70:4282-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tesh, V. L., J. A. Burris, J. W. Owens, V. M. Gordon, E. A. Wadolkowski, A. D. O'Brien, and J. E. Samuel. 1993. Comparison of the relative toxicities of Shiga-like toxins type I and type II for mice. Infect. Immun. 61:3392-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinstein, D. L., M. P. Jackson, J. A. Samuel, R. K. Holmes, and A. D. O'Brien. 1988. Cloning and sequencing of a Shiga-like toxin type II variant from an Escherichia coli strain responsible for edema disease of swine. J. Bacteriol. 170:4223-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winter, K. R. K., W. C. Stoffregen, and E. A. Dean-Nystrom. 2004. Shiga toxin binding to isolated porcine tissues and peripheral blood leukocytes. Infect. Immun. 72:6680-6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolski, V. M., A. M. Soltyk, and J. L. Brunton. 2002. Tumour necrosis factor alpha is not an essential component of verotoxin 1-induced toxicity in mice. Microb. Pathog. 32:263-271. [DOI] [PubMed] [Google Scholar]
- 32.Woods, J. F., C. K. Schmitt, S. C. Darnell, K. C. Meysick, and A. D. O'Brien. 2002. Ferrets as a model system for renal disease secondary to intestinal infection with Escherichia coli O157:H7 and other Shiga toxin-producing E. coli. J. Infect. Dis. 185:550-554. [DOI] [PubMed] [Google Scholar]