Abstract
Recently, cytolethal distending toxin V (CDT-V), a new member of the CDT family, was identified in Shiga toxin-producing Escherichia coli (STEC) O157 and particular non-O157 serotypes. Here we investigated the biological effects of CDT-V from STEC O157:H− (strain 493/89) on human endothelial cells, which are believed to be major pathogenetic targets in severe STEC-mediated diseases. CDT-V caused dose-dependent G2/M cell cycle arrest leading to distension, inhibition of proliferation, and death in primary human umbilical vein endothelial cells (HUVEC) and two endothelial cell lines, EA.hy 926 cells (HUVEC derived) and human brain microvascular endothelial cells (HBMEC). The cell cycle effects of CDT-V were cell type specific. In HUVEC and EA.hy 926 cells, CDT-V caused a slowly developing but persistent G2/M block which resulted in delayed nonapoptotic cell death. In contrast, in HBMEC, CDT-V induced a rapidly evolving but transient G2/M block which was followed by progressive, mostly apoptotic cell death. In both HBMEC and EA.hy 926 cells, G2/M arrest was preceded by the early accumulation of a phosphorylated inactive form of cdc2 kinase. Significant G2/M arrest and inhibition of proliferation in both HUVEC and each of the endothelial cell lines were induced by 2 to 15 min of exposure to CDT-V, indicating that the effects of the toxin are irreversible. CDT-V-treated HBMEC and EA.hy 926 cells displayed fragmented nuclei and expressed phosphorylated histone protein H2AX, indicative of DNA damage followed by a DNA repair response. Our data demonstrate that CDT-V causes irreversible damage to human endothelial cells and thus may contribute to the pathogenesis of STEC-mediated diseases.
Cytolethal distending toxins (CDTs), a family of proteins which interfere with the cell cycle control machinery through their genotoxic activity (12, 22, 47), are produced by multiple pathogens, such as Escherichia coli (30, 44, 45, 51, 57), Campylobacter spp. (31, 46), Shigella spp. (41), Salmonella enterica serovar Typhi (24), Haemophilus ducreyi (11), Actinobacillus actinomycetemcomitans (37), and Helicobacter spp. (9, 59). CDTs are tripartite toxins encoded by three adjacent or slightly overlapping genes, cdtA, cdtB, and cdtC (11, 30, 35, 44, 45, 51). All cdt genes are required for arrest of the eukaryotic cell cycle in the G1 phase (12, 13, 26) and/or the G2 phase (12, 13, 35, 44, 45, 58); this arrest characteristically distends cells and eventually causes cell death (12, 34, 35, 44, 47). Recent studies suggest that within the CDT holotoxin, CdtB is the enzymatically active (A) subunit (34, 35), which is transported into the nucleus (40). In the nucleus, CdtB damages through DNase I-like activity (18, 19, 34) host cell DNA (18, 34), thereby triggering DNA damage checkpoint responses (12, 22) that arrest the cell cycle (12, 18, 19, 34, 35). The CdtA and CdtC polypeptides constitute the heterodimeric (B) subunit (15, 35, 36), which is required for CDT binding to target cells (15, 36) and for the intracellular delivery of CdtB (15, 35). This mechanism of action is supported by the crystal structure of CDT holotoxin from H. ducreyi (HdCDT) (38). The cellular response to CDT is associated with the accumulation of serine-20-phosphorylated (stabilized) tumor supressor protein p53, which is the key regulator of G1 arrest (12, 13, 22), or of a tyrosine-15- and threonine-14-phosphorylated, inactive form of cyclin-dependent kinase cdc2, which accounts for G2 arrest (10). Some cells, such as B lymphocytes (13) and T lymphocytes (52), undergo apoptosis after exposure to CDT.
Shiga toxin (Stx)-producing E. coli (STEC) strains, including E. coli O157:H7 (56) and several non-O157 serotypes (3, 20, 42), cause diarrhea and hemolytic-uremic syndrome (HUS) worldwide. Stx is considered to be the cardinal virulence factor of STEC, but several other bacterial products, including toxins (49), adhesins (8, 29, 43, 55, 60), and proteases (7, 50), have been implicated as putative virulence factors of these strains. Recently, a new member of the CDT family, CDT-V, was identified in sorbitol-fermenting (SF) STEC O157:H− strain 493/89 (30). This toxin was shown to be produced by the majority (87%) of SF STEC O157:H− strains and a subset (6%) of STEC O157:H7 strains (30). Subsequently, it was demonstrated that CDT-V is also frequently found in non-O157 STEC strains of serotypes O91:H21 and O113:H21 (4), which cause serious human diseases, including HUS (4, 27, 42), despite the fact that these strains lack the eae gene encoding intimin (4, 42), a STEC factor associated with virulence and HUS (6). Thus, we have speculated that CDT-V may contribute to the pathogenicity of these organisms (4).
Injury to microvascular endothelial cells in the renal glomeruli, large intestine, and brain is a key histopathological event underlying the development of severe STEC-mediated diseases, such as HUS (48). Recently, Svensson and colleagues (54) reported that HdCDT, another member of the CDT family, has antiproliferative effects on microvascular endothelial cells from human skin and hypothesized that these effects may play a role in the pathogenesis of the disease caused by H. ducreyi (54). The earlier finding of CDT-V in STEC of serotypes frequently associated with HUS, combined with the results of that report (54), prompted us to investigate the effects of CDT-V on human endothelial cells in vitro.
MATERIALS AND METHODS
Reagents.
Cell culture materials and their sources were as follows: medium 199 and Dulbecco minimal essential medium (MEM)-F-12 medium (1:1) containing GlutaMAX-I, Gibco Invitrogen (Paisley, United Kingdom); RPMI 1640, fetal calf serum (FCS), l-glutamine, sodium pyruvate, MEM nonessential amino acids, MEM vitamins, penicillin-streptomycin, trypsin, and phosphate-buffered saline (PBS), Cambrex Bioscience (Verviers, Belgium); Nu-Serum, Becton Dickinson Biosciences (Bedford, Mass.); human serum and endothelial cell growth supplement, PromoCell (Heidelberg, Germany); heparin, Roche (Mannheim, Germany); collagenase and fibronectin, Sigma-Aldrich (Taufkirchen, Germany); and cell culture flasks (25 and 75 cm2) and cell culture plates (6, 12, and 96 wells), Corning Inc. (Corning, N.Y.). The antibodies used were as follows: anti-p34cdc2 mouse monoclonal antibody (clone A17), Sigma-Aldrich; anti-phospho-histone H2AX (serine-139) mouse monoclonal antibody (clone JBW301), Biomol (Hamburg, Germany); and horseradish peroxidase-conjugated affinity-purified goat anti-mouse immunoglobulin G antibody, Dianova (Hamburg, Germany). Other reagents and their sources were as follows: irreversible broad-spectrum caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp(methyl ester)-fluoromethylketone (zVAD-fmk), Enzyme Systems (Livermore, Calif.); propidium iodide (PI), nocodazole, RNase A, diamidinophenylindole (DAPI), Triton X-100, sodium citrate, and Tween 20, Sigma-Aldrich; skim milk powder, Roth (Karlsruhe, Germany); and mounting medium for fluorescence microscopy, DakoCytomation (Hamburg, Germany).
Cell cultures.
Human umbilical vein endothelial cells (HUVEC) were isolated from umbilical cords by collagenase treatment (28). These cells were grown on fibronectin-coated (50 μg/ml) dishes with medium 199 containing 10% FCS, 10% human serum, 0.2% endothelial cell growth supplement, 0.1% heparin, penicillin (100 U/ml), and streptomycin (100 μg/ml). EA.hy 926 cells, a permanent endothelial cell line derived from HUVEC by fusion with the lung carcinoma cell line A549 (17), were kindly provided by Volker Gerke (University Hospital of Münster, Münster, Germany). These cells were maintained in Dulbecco MEM-F-12 (1:1) containing GlutaMAX-I and supplemented with 10% FCS, penicillin (100 U/ml), and streptomycin (100 μg/ml). Human brain microvascular endothelial cells (HBMEC) (53) were a generous gift from Kwang Sik Kim (School of Medicine, Johns Hopkins University, Baltimore, Md.). These cells were grown in RPMI 1640 supplemented with 10% FCS, 10% Nu-Serum, 2 mM l-glutamine, 1 mM sodium pyruvate, 1% MEM nonessential amino acids, 1% MEM vitamins, penicillin (100 U/ml), and streptomycin (100 μg/ml).
CDT-V preparation.
CDT-V was produced from E. coli XL1-Blue MR hosting a cosmid (SuperCos I; Stratagene, Heidelberg, Germany) which contains the three CDT-V open reading frames (cdtA, cdtB, and cdtC) cloned from SF STEC O157:H− strain 493/89 (32) as described previously (30). The CDT-V preparation was a filter-sterilized (0.22-μm-pore size; Schleicher & Schuell GmbH, Dassel, Germany) supernatant of a 24-h-aerated (180 rpm) culture of the CDT-V open reading frame-containing cosmid clone (clone 13-18) in cell culture medium. The protein concentration was 3.7 mg/ml. The 50% cytotoxic doses (CD50) for HUVEC, EA.hy 926 cells, and HBMEC, which were defined as the highest dilutions of the CDT-V preparation that caused distension in 50% of the cells after 5 days of incubation, corresponded to dilutions of 1:64, 1:512, and 1:512, respectively, and to protein concentrations of 57.8, 7.2, and 7.2 μg/ml, respectively. In the experiments, 1, 2, or 8 CD50/ml were used. The control preparation was a sterilely filtered supernatant of E. coli XL1-Blue MR hosting the SuperCos I cosmid without the CDT-V insert. In all experiments, it was used at a dilution corresponding to that of the CDT-V preparation which contained 8 CD50/ml. Cells treated with the control preparation did not display any significant changes in their morphology, cell cycle, viability, or proliferation compared to untreated cells. Therefore, in this article the term “control cells” refers to cells exposed to the control preparation. Both the CDT-V and the control preparations were stored in aliquots at −20°C until use.
CDT bioassay.
The morphological effect of CDT-V on human endothelial cells was assayed by adding 1-ml portions of twofold dilutions of the CDT-V preparation to HUVEC, EA.hy 926 cells, and HBMEC freshly seeded in six-well tissue culture plates in amounts of 1 × 106, 1.5 × 105, and 1 × 105 cells/well, respectively. The assay mixtures were incubated for 5 days at 37°C in 5% CO2 and examined daily by using a light microscope (Axiovert 100; Zeiss, Jena, Germany) at a final magnification of ×100.
Cell cycle analysis and apoptosis assay.
HUVEC, EA.hy 926 cells, and HBMEC were seeded in 12-well plates in amounts of 1 × 105, 1.4 × 105, and 7 × 104 cells/well, respectively, and grown (37°C, 5% CO2) until 70 to 80% confluence. The CDT-V preparation (1, 2, or 8 CD50/ml) or the control preparation was added to the cells, and the mixtures were incubated for 24, 48, 72, 96, and 120 h. At each time point, detached cells that were free within the culture medium and adherent cells harvested by trypsinization were pelleted together and stained with Nicoletti buffer (0.1% Triton X-100, 0.1% sodium citrate, 50 μg of PI/ml, 20 μg of RNase/ml) (39). After incubation for 30 min on ice, the DNA content of the nuclei was determined by flow cytometry (FACScalibur; Becton Dickinson, Heidelberg, Germany) with red (PI) emission (FL-2 channel, 570 nm). After forward scatter-side scatter gating for the exclusion of debris, the data from at least 104 nuclei were collected and analyzed by using CellQuest software (Becton Dickinson). In a set of experiments in which the irreversible effect of CDT was studied, cells were exposed to CDT for various times, CDT was removed, and cells were washed twice with PBS, incubated in fresh medium for up to 24 h (HBMEC) or 120 h (EA.hy 926 cells), and then analyzed as described above. Apoptosis was measured by flow cytometric determination of the proportion of hypodiploid nuclei (39) as described earlier (2, 25). In some experiments, the polycaspase inhibitor zVAD-fmk (50 μM) was added to the cells 30 min before the other stimuli.
Cell viability and cell proliferation.
Cell viability and proliferation were tested by trypan blue (0.2%) exclusion and two different commercial assays (Roche). The WST-1 metabolic viability assay is based on the mitochondrial reduction of the tetrazolium salt WST-1 to formazan, which is quantified spectrophotometrically. In the DNA synthesis assay (cell proliferation enzyme-linked immunosorbent assay [ELISA] of bromodeoxyuridine [BrdU]), the incorporation of pyrimidine analog BrdU into newly synthesized DNA of replicating cells is measured by an ELISA. The WST-1 and BrdU assays were performed with 96-well plates seeded with EA.hy 926 cells at 2 × 103/well and 3 × 103/well, respectively, and with HBMEC at 6 × 102/well and 2 × 102/well, respectively. Overnight monolayers were treated with the CDT-V preparation (1, 2, or 8 CD50/ml) or the control preparation for 24, 48, 72, 96, and 120 h and subsequently processed according to the manufacturer's instructions. The absorbance was measured at 450 nm and reference wavelength 650 nm by using an ELISA reader (Emax precision microplate reader; MWG Biotech, Ebersberg, Germany). To avoid interference of starvation with cell proliferation, fresh medium was added to cells after 48 h. The irreversible effect of CDT-V on cell proliferation was tested by exposing cells to CDT-V for various times, washing them with PBS, incubating them in fresh medium for up to 96 h, and then processing them in a proliferation assay.
Immunoblot analysis.
EA.hy 926 cells and HBMEC (1.4 × 105 and 7 × 104/well, respectively) were grown in 12-well plates until 70 to 80% confluence and stimulated with the CDT-V preparation (8 CD50/ml), the control preparation, or nocodazole (100 nM) for various times. Subsequently, the cells were washed with PBS, harvested by trypsinization, resuspended in electrophoresis sample buffer (33), lysed by being heated to 99°C for 10 min, and subjected to sonication (Sonopuls; Bandelin Electronic, Berlin, Germany) for 90 s. Total cellular proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (33) in a mini-slab gel apparatus (Bio-Rad, Munich, Germany) with 13% separation gels. Separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon; Roth) by using a semidry blotting system (Roth). After blocking was done for 1 h in PBS containing 0.1% Tween 20 (PBS-T; pH 7.4) and 1% skim milk powder, membranes were incubated for 2 to 16 h with specific antibodies diluted 1:2,000 (anti-p34cdc2) or 1:10,000 (anti-phospho-histone H2AX) in PBS-T. Membranes then were washed and incubated with horseradish peroxidase-conjugated anti-mouse immunoglobulin G secondary antibody diluted 1:1,000 in PBS-T for 1 h. After washing was done, the membranes were developed by using a chemiluminescence enhancement kit (Perbio Science, Bonn, Germany), and signals were visualized on a chemiluminescence photoimager (Boehringer, Mannheim, Germany).
Fluorescence microscopy.
EA.hy 926 cells (1.5 × 105/well) and HBMEC (1 × 105/well) were cultured with 8 CD50 of the CDT-V preparation/ml or the control preparation on cover slides placed in six-well plates. After 5 days, the cells were stained with DAPI (20 μg/ml) by the procedure described by De Rycke et al. (16). The slides were mounted in DakoCytomation mounting medium and examined by using a fluorescence microscope (Axiophot; Zeiss) with a ×40 objective lens.
Statistical analysis.
Statistical analysis was performed by using the paired t test (two tailed) calculated with OpenStat2 software (W. Miller, Iowa State University; http://www.statpages.org/miller/openstat/OPENSTAT2.htm). P values of <0.05 were considered significant.
RESULTS
CDT-V interferes with the cell cycle of endothelial cells in a cell type-specific manner.
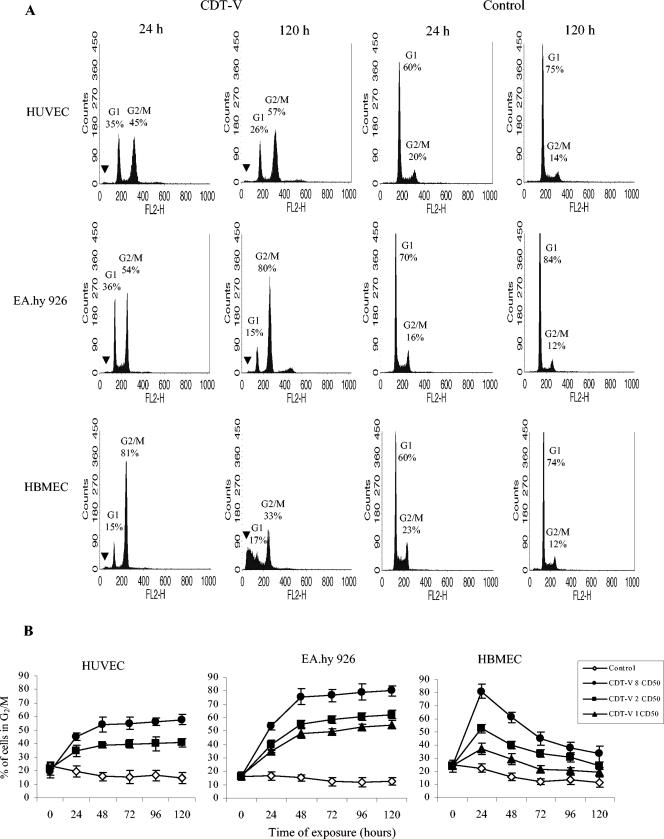
To investigate the effect of CDT-V on the cell cycle of human endothelial cells, HUVEC, EA.hy 926 cells, and HBMEC were treated with the toxin for 1 to 5 days, and the DNA content was analyzed by flow cytometry. CDT-V caused G2/M arrest, characterized by a 4n DNA content, in each of the cell cultures investigated (Fig. 1A). In contrast, control cells stimulated with the vector preparation demonstrated normal cycling, with the majority of cells being in the G1 phase (2n DNA content) (Fig. 1A). The G2/M arrest was dose and time dependent (Fig. 1B), and its time course differed among the cell types investigated. In HUVEC and EA.hy 926 cells, the proportion of cells arrested in G2/M increased from day 1 to day 5 posttreatment, reaching a plateau on day 2 (Fig. 1B). However, at each time point, the proportion of HUVEC in G2/M was lower than that of EA.hy 926 cells (Fig. 1), with a substantial proportion of the former cells remaining in G1 even after 5 days of exposure to CDT-V (Fig. 1A). The same pattern of G2/M arrest for HUVEC was observed after treatment with the G2/M cell cycle inhibitor nocodazole (100 nM), which blocked between 46 and 58% of HUVEC in G2/M between days 1 and 5, leaving between 36 and 26% of cells in G1 (data not shown). In contrast to the findings for HUVEC and EA.hy 926 cells, the G2/M block in HBMEC peaked 24 h after toxin exposure (81% of cells in G2/M) and then decreased steadily during the next 4 days to 33% of cells in G2/M on day 5 (Fig. 1).
FIG. 1.
CDT-V causes G2/M arrest in human endothelial cells. (A) Flow cytometric analysis of HUVEC, EA.hy 926 cells, and HBMEC after 1 and 5 days of treatment with 8 CD50 of CDT-V/ml or with the control preparation. The proportions of cells in G1 (2n DNA) and G2/M (4n DNA) are indicated. Black arrowheads indicate the sub-G1 population. Data represent one of three independent experiments. (B) HUVEC, EA.hy 926 cells, and HBMEC were treated with 1 (triangles), 2 (squares), or 8 (circles) CD50 of CDT-V/ml or with the control preparation (diamonds) for the indicated times. The proportions of cells in G2/M were determined by flow cytometry. Time zero indicates basal numbers of cells in G2/M, which were obtained by exposing the cells to CDT-V or the control preparation for 5 min, followed by immediate flow cytometric analysis. Data are means and standard deviations from three independent experiments.
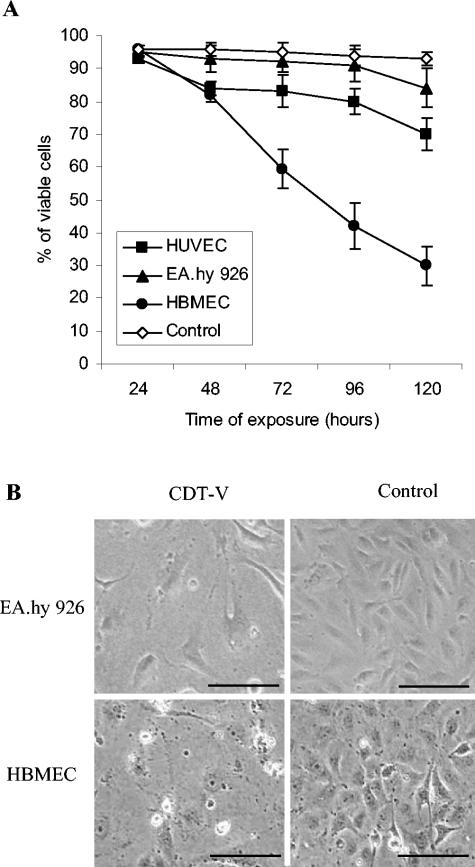
As determined by trypan blue exclusion (Fig. 2A), the majority of HUVEC and EA.hy 926 cells arrested in G2/M remained alive up to day 4, with cell death starting on day 5. In contrast, the number of viable HBMEC decreased sharply between day 1 and day 5, indicating that the observed decrease in the G2/M block was caused by cell death. The latter finding was consistent with the rapid decrease in the HBMEC G2/M block being accompanied by a progressive increase in the population of cells with hypodiploid nuclei (sub-G1) (Fig. 1A), which are considered apoptotic. The proportion of cells with hypodiploid nuclei rose from 28% on day 1 to 83% on day 5, when it was more than threefold larger in CDT-V-treated cells than in control cells (data not shown). Neither HUVEC nor EA.hy 926 cells contained a sub-G1 population (Fig. 1A).
FIG. 2.
G2/M arrest of endothelial cells results in cell distension and ultimately cell death. (A) Viability of cells arrested in G2/M. Cells analyzed for G2/M arrest were stained with trypan blue. Proportions of viable cells were determined at each time point microscopically. Numbers of viable control cells did not differ significantly in the three cell cultures and are shown combined as “Control.” Data are means and standard deviations from three independent experiments. (B) Photomicrographs of EA.hy 926 cell and HBMEC cultures exposed for 5 and 4 days, respectively, to 8 CD50 of CDT-V/ml or to the control preparation. CDT-V caused the formation of giant cells that were four- to eightfold larger than control cells and had nuclei that were two- to threefold larger. Magnification, ×100; bars, 200 μm.
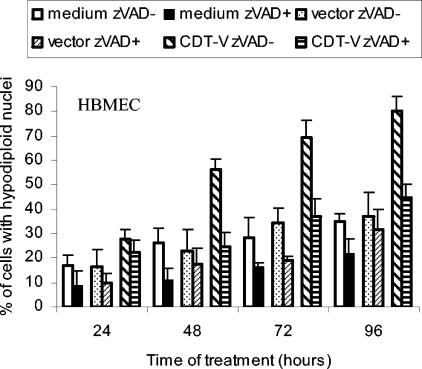
To determine whether HBMEC in the sub-G1 population underwent caspase-mediated apoptotic cell death, we determined the proportions of cells containing hypodiploid nuclei with and without pretreatment with the polycaspase inhibitor zVAD-fmk (Fig. 3). The proportion of CDT-V-exposed cells with hypodiploid nuclei was largely reduced by preincubation with zVAD-fmk to the level seen in cells exposed to the vector (Fig. 3). This result suggests that caspase-dependent apoptotic cell death accounted for most of the observed death of G2/M-arrested HBMEC.
FIG. 3.
CDT-V induces apoptotic cell death in HBMEC. Cells were incubated with 8 CD50 of CDT-V/ml, the vector preparation, or cell culture medium in the absence or the presence of zVAD-fmk (50 μM). Apoptosis was determined by flow cytometry as a proportion of hypodiploid nuclei. Data are means and ranges from two experiments.
Taken together, these data demonstrate that CDT-V displays cell type-specific patterns of interaction with the cell cycle of human endothelial cells. HUVEC and EA.hy 926 cells sustain a slowly developing but persistent G2/M arrest, resulting in delayed nonapoptotic cell death, whereas HBMEC experience a rapidly peaking but transient G2/M block, followed by progressive cell death caused mostly by caspase-dependent apoptosis. In each of the cell cultures, cell death was preceded by cellular distension which was apparent from day 3 after CDT-V exposure and which was more pronounced in EA.hy 926 cells and HBMEC (Fig. 2B) than in HUVEC (data not shown).
CDT-V-mediated G2/M arrest is preceded by the accumulation of the phosphorylated form of cdc2 kinase in endothelial cells.
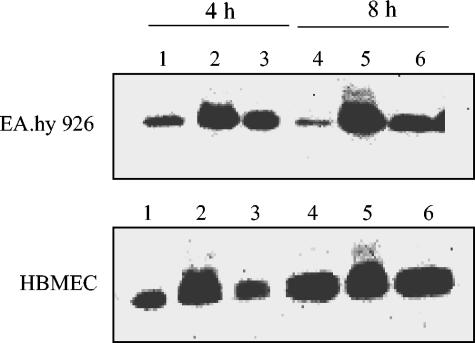
The phosphorylation status of cdc2 in EA.hy 926 cells and HBMEC treated with CDT-V or nocodazole (100 nM) for 4 or 8 h or in control cells was determined by immunoblot analysis of total cellular proteins with a monoclonal antibody against cdc2 (Fig. 4). At each time point, CDT-V-treated cells of both lines (Fig. 4, lanes 2 and 5) expressed two bands reacting with the anti-cdc2 antibody; these bands were consistent with the phosphorylated, inactive form of cdc2 (upper band) and the dephosphorylated, active form of cdc2 (lower band) (10). Dephosphorylation of cdc2 on threonine-14 and tyrosine-15 in late G2 is a prerequisite for its activation and for cells to enter mitosis (10). The phosphorylated form of cdc2 was absent from control cells (Fig. 4, lanes 1 and 4) and from cells treated with nocodazole (Fig. 4, lanes 3 and 6), both of which contained only the dephosphorylated form of the kinase. The absence of the phosphorylated form of cdc2 in nocodazole-treated cells, which progress from G2 to mitosis but are blocked in prometaphase (10), and its presence in cells treated with CDT-V suggest that the CDT-V-treated cells were unable to pass from G2 to mitosis; these cells were therefore arrested in G2 rather than in mitosis.
FIG. 4.
CDT-V induces the accumulation of phosphorylated, inactive cdc2 kinase. EA.hy 926 cells and HBMEC were treated with 8 CD50 of CDT-V/ml (lanes 2 and 5), the vector preparation (lanes 1 and 4), or nocodazole (lanes 3 and 6) for 4 h (lanes 1 to 3) or 8 h (lanes 4 to 6). Isolated total cellular proteins were analyzed by immunoblotting with anti-cdc2 antibody. The strong signals displayed by cells treated with CDT-V (lanes 2 and 5) were double bands which were not completely separated on the gels.
CDT-V inhibits the proliferation of human endothelial cells.
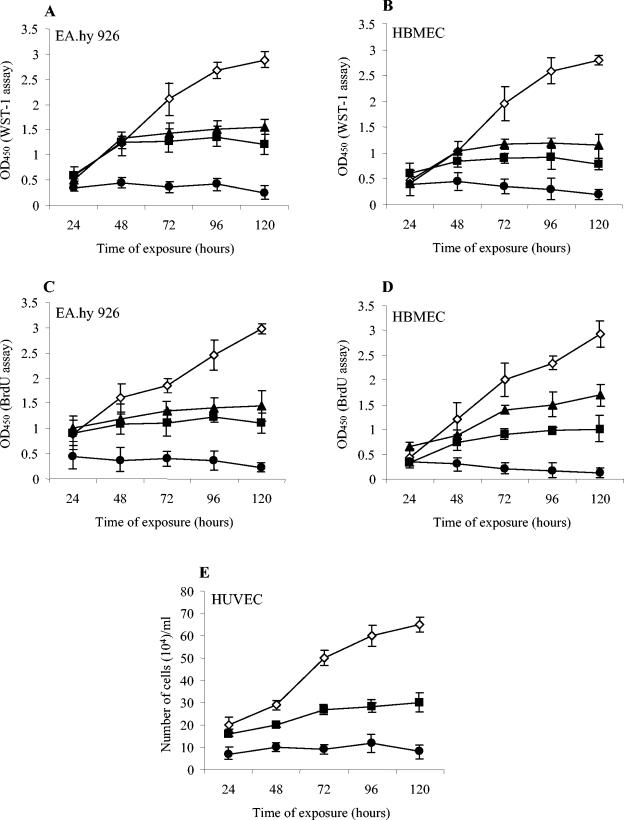
To investigate the effect of CDT-V on the proliferation of endothelial cells, cells were treated with 1, 2, or 8 CD50 of toxin/ml for 1 to 5 days. Proliferation was measured at 24-h intervals by quantifying cellular metabolic activity (WST-1 assay) and DNA synthesis (BrdU incorporation assay) and by light microscopy (trypan blue exclusion). Based on metabolic activity (Fig. 5A and B) and DNA synthesis (Fig. 5C and D), CDT-V inhibited the proliferation of EA.hy 926 cells (Fig. 5A and C) and HBMEC (Fig. 5B and D) in a dose- and time-dependent manner. In contrast, control cells proliferated linearly during the experimental period (Fig. 5A to D). The low levels of metabolic activity and DNA synthesis in EA.hy 926 cells and HBMEC treated with 8 CD50 of CDT-V/ml (Fig. 5A to D) corresponded to a nearly complete cessation of cell proliferation in each of the cell lines, as determined by trypan blue exclusion (data not shown). The latter test also demonstrated that the declines in metabolic activity and DNA synthesis observed on day 5 in EA.hy 926 cells (Fig. 5A and C) and throughout the entire experimental period in HBMEC (Fig. 5B and D) reflected cell death (data not shown). The effect of CDT-V on the proliferation of HUVEC, which did not grow reliably in the 96-well plates used for the WST-1 and BrdU assays, was determined by trypan blue exclusion performed on cells cultured in 12-well plates. The toxin inhibited the proliferation of these primary endothelial cells (Fig. 5E) in a manner similar to that observed in EA.hy 926 cells and HBMEC.
FIG. 5.
CDT-V inhibits the proliferation of human endothelial cells. EA.hy 926 cells (A and C), HBMEC (B and D), and HUVEC (E) were treated with 1 (triangles), 2 (squares), or 8 (circles) CD50 of CDT-V/ml or with the vector preparation (diamonds) for the indicated times. Cells then were processed in the WST-1 (A and B), BrdU (C and D), and trypan blue (E) assays. Data are means and standard deviations from three independent experiments.
CDT-V-mediated G2/M arrest and inhibition of proliferation are irreversible.
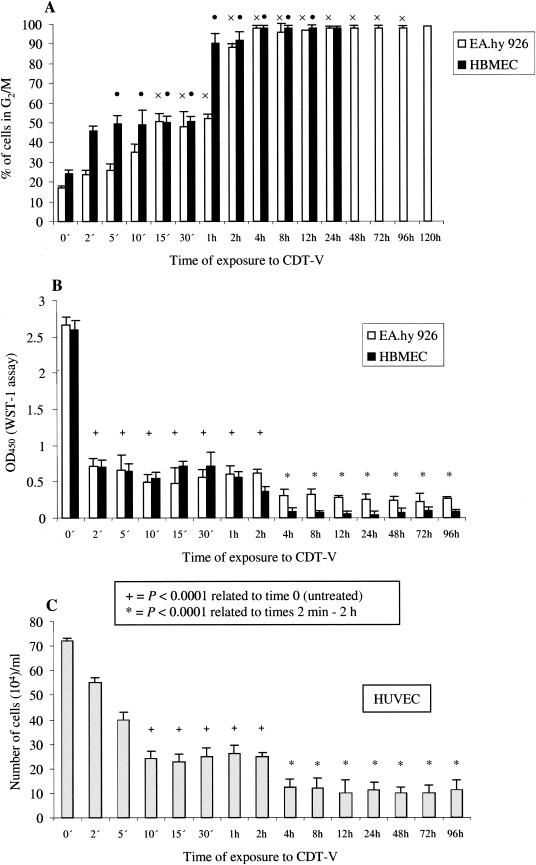
All experiments described so far were performed with cells that were continuously stimulated with CDT-V. Therefore, we further investigated the minimum length of exposure to CDT-V which is required to induce a G2/M block and inhibit cell proliferation. To this end, cells were exposed to CDT-V (8 CD50/ml) for various intervals (2 min to 120 h) and then washed and incubated in fresh medium without CDT-V until the end of each experiment. Exposures of 5 and 15 min (Fig. 6A) were sufficient for CDT-V to cause G2/M arrest in 50% of the total HBMEC and EA.hy 926 cells, respectively, which were arrested in G2/M at the time when the block peaked upon continuous stimulation (24 h for HBMEC and 120 h for EA.hy 926 cells). The plateau of G2/M arrest in HBMEC and EA.hy 926 cell cultures was induced by exposures of 1 and 2 h, respectively (Fig. 6A). Exposure to CDT-V for as little as 2 min resulted in an almost complete cessation of proliferation of both HBMEC and EA.hy 926 cells, as determined by the WST-1 assay (Fig. 6B). In HUVEC, CDT-V significantly inhibited cell proliferation, as measured by trypan blue exclusion, after 10 min of exposure (Fig. 6C). In every instance, the inhibition of proliferation was significantly higher (P value determined by the paired t test, <0.0001) in cells treated with CDT-V for ≥4 h than in those treated for ≤2 h (Fig. 6B and C).
FIG. 6.
Minimum length of exposure to CDT-V required for G2/M arrest and inhibition of proliferation in endothelial cells. Cells were exposed to CDT-V (8 CD50/ml) for the indicated times, the toxin then was removed, and cells were washed and incubated in medium without CDT-V. G2/M arrest (A) was analyzed by flow cytometry 24 h (HBMEC) or 120 h (EA.hy 926 cells) after the addition of CDT-V. The percentages of the total numbers of cells arrested in G2/M after 24 h (HBMEC) or 120 h (EA.hy 926 cells) are shown, and the exposure times which resulted in G2/M arrest in significant proportions (≥50%) of EA.hy 926 cells (×) and HBMEC (•) also are shown. Cell proliferation was measured by the WST-1 assay (EA.hy 926 cells and HBMEC) (B) or trypan blue exclusion (HUVEC) (C) 96 h after the addition of the toxin. Cells at 0 min were not exposed to CDT-V and were cultured in medium only. Differences in mean optical densities at 450 nm (OD450) (EA.hy 926 cells and HBMEC) and cell numbers (HUVEC) were compared with the paired t test, and the P values are shown. All data are means and standard deviations from three independent experiments.
CDT-V causes DNA damage in human endothelial cells.
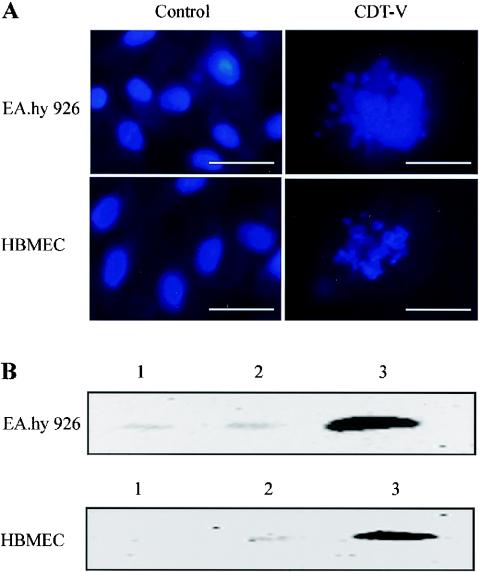
The ability of CDT-V to cause DNA damage in endothelial cells was investigated by detecting DNA fragmentation by DAPI staining and the expression of phosphorylated histone protein H2AX (γ-H2AX), an indicator of double-stranded DNA breaks (26), by immunoblot analysis. As evidenced by DAPI staining, 11 and 14% of EA.hy 926 cells and HBMEC, respectively, treated with 8 CD50 of CDT-V/ml for 5 days had fragmented nuclei; examples are shown in Fig. 7A. No cells with fragmented nuclei were visible in any of the cell cultures after 5 days of treatment with the vector preparation (Fig. 7A). Protein immunoblot analysis (Fig. 7B) demonstrated the expression of γ-H2AX in EA.hy 926 cells and HBMEC treated with CDT-V for 21 h but not in control cells. γ-H2AX was not detected in CDT-V-treated or control cells after 3 h of exposure (data not shown).
FIG. 7.
CDT-V induces nuclear fragmentation and the formation of γ-H2AX in endothelial cells. (A) DAPI staining of EA.hy 926 cells and HBMEC treated for 5 days with the vector preparation or with 8 CD50 of CDT-V/ml. A Zeiss fluorescence microscope with a ×40 objective lens was used; magnification, ×400; bars, 50 μm. (B) Immunoblot analysis with a γ-H2AX-specific monoclonal antibody of EA.hy 926 cells and HBMEC treated for 21 h with 8 CD50 of CDT-V/ml (lanes 3) or the vector preparation (lanes 2) or left untreated (lanes 1).
DISCUSSION
We demonstrate for the first time that CDT-V from STEC O157:H− strain 493/89 has the potential to act on a variety of endothelial cells in vitro. This ability was previously shown only for HdCDT (54) and not for other members of the CDT family, although the latter CDTs have the capacity to interact with many other cell types, including epithelial cells, keratinocytes, and fibroblasts (1, 10, 12, 13, 26, 34, 44, 45, 51, 58). Moreover, our finding that CDT-V affects, in addition to primary cells, endothelial cell lines, including HUVEC-derived EA.hy 926 cells and HBMEC, extends a previous report (54) that HdCDT affects primary endothelial cells (HUVEC and microvascular endothelial cells from human dermal tissue [HMVEC-d]). Specifically, CDT-V induced, in a dose-dependent manner, irreversible G2/M arrest, which led to cell distension, inhibition of proliferation, and ultimately lethality in HUVEC and in each of the cell lines. However, the responses to CDT-V differed among the cell cultures investigated. This phenomenon has not been reported for primary endothelial cells (54). Although both HUVEC and EA.hy 926 cells displayed slowly developing G2/M arrest, they substantially differed in the proportions of cells arrested in G2/M. In contrast to a nearly complete G2/M block in EA.hy 926 cells 5 days posttreatment (Fig. 1A), only 57% of HUVEC were arrested in G2/M at this time. The observation in HUVEC of the same pattern in the response to nocodazole, which synchronizes cells in mitosis (10, 13), suggests that this incomplete G2 arrest probably reflects a finite life span of these cells, which divide relatively slowly (54), rather than an additional block in G1; the latter scenario was previously reported for human foreskin and embryonic lung fibroblasts (13). The incomplete G2/M block in HUVEC and HMVEC-d was also observed after treatment with HdCDT (54) and might be a consequence of a relatively low sensitivity of heterogeneous primary cell populations to CDT (54). Accordingly, the CD50 of CDT-V in our study is approximately 10-fold higher for HUVEC than for each of the cell lines.
In contrast to the findings for HUVEC and EA.hy 926 cells, the G2/M arrest induced by CDT-V in HBMEC peaked 24 h after toxin exposure but rapidly declined during the next 4 days, apparently as a consequence of progressive cell death. The fact that the polycaspase inhibitor zVAD-fmk largely inhibited the formation of an HBMEC population with hypodiploid nuclei, which increased concomitantly with the decrease in the G2/M block (Fig. 1A), suggests that the death of HBMEC was mostly due to caspase-mediated apoptosis. In addition, caspase-independent apoptosis (14) or necrosis might have contributed to the death of these cells. Interestingly, the pattern of response of HBMEC to CDT-V differed from that of human T cells to CDT from A. actinomycetemcomitans, which is characterized by slowly developing G2 arrest followed by apoptotic cell death (52), and from that reported for B lymphocytes (13), which respond to HdCDT by the rapid development of apoptosis with only slight G2 arrest. Notably, apoptotic cell death was not observed in HUVEC and EA.hy 926 cells, as evidenced by the absence of populations with hypodiploid nuclei in these cultures (Fig. 1A). The absence of an apoptotic cell population in HUVEC cultures in our study, which is in contrast to findings reported by Svensson et al. (54), might reflect either qualitative differences between HdCDT and CDT-V or different toxin doses used by those authors (100 cytopathic units/ml) and in our study (8 CD50/ml).
Despite the observed differences in their cell cycle responses to CDT-V, the initial mechanisms underlying these responses appear to be similar in EA.hy 926 cells and HBMEC. This suggestion is based on the fact that the accumulation of phosphorylated cdc2 kinase, a mediator of G2 arrest, preceded the development of the G2/M block in both of these cell lines. Moreover, the mechanism which underlies the CDT-mediated G2/M block in these cell lines is similar to that described previously for primary endothelial cells, including HUVEC and HVMEC-d (54). Thus, our present data do not allow us to determine a basis for the observed cell type-specific effects of CDT-V on the cell cycle of the endothelial cells investigated. Cortes-Bratti et al. (13) recently reported that human cells of other types, such as epithelial cells, keratinocytes, fibroblasts, and B cells, respond to HdCDT in a cell type-specific manner (arrest exclusively in G2, arrest in both G1 and G2, or apoptosis). These responses were associated with different DNA damage checkpoint pathways and with different kinetics of expression of phosphorylated p53 protein (mediator of G1 arrest) and phosphorylated cdc2 kinase during 24 h of intoxication (13). Because we determined the expression of phosphorylated cdc2 in CDT-V-treated endothelial cells only at early (after 4 and 8 h of exposure) and not at later time points, it is possible that similar differences in the kinetics of the cdc2-dependent checkpoint response might be one of the factors contributing to the cell type-specific responses of endothelial cells to CDT-V. However, the basis for this phenomenon is likely to be more complex and needs to be investigated in further studies.
To our knowledge, the irreversible effect of CDT on endothelial cells, as observed in this study for CDT-V on HUVEC, EA.hy 926 cells, and HBMEC, was not reported previously. In our study, this effect was demonstrated by the finding that as little as 2 to 15 min of exposure to the toxin resulted in significant G2/M arrest and inhibition of proliferation in the endothelial cells investigated (Fig. 6). This finding of a rapid and irreversible interaction between CDT-V and endothelial cells extends earlier observations on similar interactions between CDT from Campylobacter jejuni (58) and E. coli CDT-II (1) and epithelial cells. Closer insight into an as-yet-unknown mechanism(s) underlying the irreversible interaction of CDT with host cells might result in a potentially therapeutically useful approach for preventing this CDT effect.
CDTs from several pathogens have been shown to degrade chromatin and to cause double-stranded DNA breaks with subsequent repair responses in various epithelial cells and fibroblasts (18, 22, 23, 34). Therefore, we investigated the ability of CDT-V to cause DNA fragmentation and to induce the expression of γ-H2AX, which plays a crucial role in the recruitment of DNA repair complexes to sites of double-stranded DNA breaks (26), in endothelial cells. The findings of nuclear fragmentation as well as γ-H2AX expression in HBMEC and EA.hy 926 cells exposed to CDT-V suggest that this toxin has the potential to induce DNA damage and DNA repair responses in these cells. To our knowledge, a similar effect of CDT was not reported for endothelial cells. The detection of γ-H2AX in EA.hy 926 cells and HBMEC at 21 h but not at 3 h postintoxication suggests that DNA lesions induced by CDT-V might be delayed but persistent. Accordingly, a persistent DNA lesion, as indicated by the expression of γ-H2AX 22 h posttreatment, was induced by CDT from C. jejuni in human fibroblasts (26). Because no apoptosis was detected in EA.hy 926 cells up to day 5 posttreatment, DNA fragmentation and γ-H2AX expression likely resulted from a direct effect of CDT-V. In contrast, because apoptosis was a prominent feature in HBMEC cultures after 5 days of CDT-V exposure, when DNA fragmentation was determined, our data do not allow us to differentiate between a direct DNA-damaging effect of CDT-V and DNA cleavage secondary to CDT-V-induced apoptosis.
Although Stxs are presumably the major virulence factors accounting for endothelial cell damage during STEC infection (5), several lines of evidence support a potential contribution of CDT-V to the pathogenesis of STEC-mediated diseases, in particular those caused by STEC lacking the intimin-encoding eae gene. These include (i) a significant association between CDT-V production by STEC isolates and clinical symptoms versus asymptomatic carriage in subjects infected with eae-negative STEC strains (4); (ii) the ability of eae-negative STEC of serotypes O91:H21 and O113:H21, which frequently produce CDT-V (4), to cause severe disease, including HUS (4, 27, 42), a feature which is otherwise rare among eae-negative STEC strains (6, 21); and (iii) the ability of recombinant CDT-V to irreversibly damage human endothelial cells in vitro, as demonstrated in this study. In any event, it is almost certain that non-Stx virulence factors contribute to host injury during STEC infection. On the basis of our data, CDT-V should be studied further in order to determine its role in augmenting or supporting the pathogenicity of STEC, particularly eae-negative strains.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG) program “Infections of the Endothelium” SPP 1130, grant KA 717/4-1, and in part by DFG grant SCHU 1180/2-1.
We thank K. S. Kim (School of Medicine, Johns Hopkins University, Baltimore, Md.) and V. Gerke (University Hospital of Münster, Münster, Germany) for providing us with HBMEC and EA.hy 926 cells, respectively. Moreover, we are grateful to the following colleagues from the University Hospital of Münster for their contributions: B. Haslinger-Löffler (Institut für Medizinische Mikrobiologie) for assistance with experiments on HUVEC, L. Greune (Institut für Infektiologie) for obtaining photomicrographs, and A. W. Friedrich (Institut für Hygiene) for statistical analysis. To P. I. Tarr (School of Medicine, Washington University, St. Louis, Mo.) we are indebted for critical reading of the manuscript and stimulating discussions. The skillful technical assistance of M. Hülsmann and K. Strangfeld is greatly appreciated.
Editor: A. D. O'Brien
REFERENCES
- 1.Aragon, V., K. Chao, and L. A. Dreyfus. 1997. Effect of cytolethal distending toxin on F-actin assembly and cell division in Chinese hamster ovary cells. Infect. Immun. 65:3774-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bantel, H., B. Sinha, W. Domschke, G. Peters, K. Schulze-Osthoff, and R. U. J.änicke. 2001. α-Toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J. Cell Biol. 155:637-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bielaszewska, M., and H. Karch. 2000. Non-O157:H7 Shiga toxin (verocytotoxin)-producing Escherichia coli strains: epidemiological significance and microbiological diagnosis. World J. Microbiol. Biotechnol. 16:711-718. [Google Scholar]
- 4.Bielaszewska, M., M. Fell, L. Greune, R. Prager, A. Fruth, H. Tschäpe, M. A. Schmidt, and H. Karch. 2004. Characterization of cytolethal distending toxin genes and expression in Shiga toxin-producing Escherichia coli strains of non-O157 serogroups. Infect. Immun. 72:1812-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bitzan, M., and D. M. W. M. Te Loo. 2003. Interaction of Shiga toxin with endothelial cells. Methods Mol. Med. 73:243-262. [DOI] [PubMed] [Google Scholar]
- 6.Boerlin, P., S. A. McEwen, F. Boerlin-Petzold, J. B. Wilson, B. J. Johnson, and C. L. Gyles. 1999. Association between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 37:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunder, W., H. Schmidt, and H. Karch. 1997. EspP, a novel extracellular serine protease of enterohaemorrhagic Escherichia coli O157:H7 cleaves human coagulation factor V. Mol. Microbiol. 24:767-778. [DOI] [PubMed] [Google Scholar]
- 8.Brunder, W., A. Salam Khan, J. Hacker, and H. Karch. 2001. A novel fimbrial gene cluster encoded by the large plasmid of sorbitol-fermenting enterohemorrhagic Escherichia coli O157:H−. Infect. Immun. 69:4447-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chien, C. C., N. S. Taylor, Z. Ge, D. B. Schauer, V. B. Young, and J. G. Fox. 2000. Identification of cdtB homologues and cytolethal distending toxin activity in enterohepatic Helicobacter spp. J. Med. Microbiol. 49:525-534. [DOI] [PubMed] [Google Scholar]
- 10.Comayras, C., C. Tasca, S. Y. Peres, B. Ducommun, E. Oswald, and J. De Rycke. 1997. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect. Immun. 65:5088-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cope, L. D., S. Lumbley, J. L. Latimer, J. Klesney-Tait, M. K. Stevens, L. S. Johnson, M. Purven, R. S. Munson, and E. J. Hansen. 1997. A diffusible cytotoxin of Haemophilus ducrey. Proc. Natl. Acad. Sci. USA 94:4056-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortes-Bratti, X., T. Frisan, and M. Thelestam. 2001. The cytolethal distending toxins induce DNA damage and cell cycle arrest. Toxicon 39:1729-1736. [DOI] [PubMed] [Google Scholar]
- 13.Cortes-Bratti, X., C. Karlsson, T. Lagergard, M. Thelestam, and T. Frisan. 2001. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via the DNA damage checkpoint pathways. J. Biol. Chem. 276:5296-5302. [DOI] [PubMed] [Google Scholar]
- 14.Cregan, S. P., V. L. Dawson, and R. S. Slack. 2004. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene 23:2785-2796. [DOI] [PubMed] [Google Scholar]
- 15.Deng, K., and E. J. Hansen. 2003. A CdtA-CdtC complex can block killing of HeLa cells by Haemophilus ducreyi cytolethal distending toxin. Infect. Immun. 71:6633-6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Rycke, J., P. Mazars, J. P. Nougayrede, C. Tasca, M. Boury, F. Herault, A. Valette, and E. Oswald. 1996. Mitotic block and delayed lethality in HeLa epithelial cells exposed to Escherichia coli BM2-1 producing cytotoxic necrotizing factor type 1. Infect. Immun. 64:1694-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edgell, C. J., C. C. McDonald, and J. B. Graham. 1983. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc. Natl. Acad. Sci. USA 80:3734-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elwell, C., K. Chao, K. Patel, and L. Dreyfus. 2001. Escherichia coli CdtB mediates cytolethal distending toxin cell cycle arrest. Infect. Immun. 69:3418-3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elwell, C. A., and L. A. Dreyfus. 2000. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol. Microbiol. 37:952-963. [DOI] [PubMed] [Google Scholar]
- 20.Friedrich, A. W., M. Bielaszewska, W.-L. Zhang, M. Pulz, T. Kuczius, A. Ammon, and H. Karch. 2002. Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J. Infect. Dis. 185:74-84. [DOI] [PubMed] [Google Scholar]
- 21.Friedrich, A. W., J. Borell, M. Bielaszewska, A. Fruth, H. Tschäpe, and H. Karch. 2003. Shiga toxin 1c-producing Escherichia coli strains: phenotypic and genetic characterization and association with human disease. J. Clin. Microbiol. 41:2448-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frisan, T., X. Cortes-Bratti, and M. Thelestam. 2002. Cytolethal distending toxins and activation of DNA damage-dependent checkpoint responses. Int. J. Med. Microbiol. 291:495-499. [DOI] [PubMed] [Google Scholar]
- 23.Frisan, T., X. Cortes-Bratti, E. Chaves-Olarte, B. Stenerlow, and M. Thelestam. 2003. The Haemophilus ducreyi cytolethal distending toxin induces DNA double-strand breaks and promotes ATM-dependent activation of RhoA. Cell. Microbiol. 5:695-707. [DOI] [PubMed] [Google Scholar]
- 24.Haghjoo, E., and J. E. Galan. 2004. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc. Natl. Acad. Sci. USA 101:4614-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haslinger, B., K. Strangfeld, G. Peters, K. Schulze-Osthoff, and B. Sinha. 2003. Staphylococcus aureus α-toxin induces apoptosis in peripheral blood mononuclear cells: role of endogenous tumour necrosis factor-α and the mitochondrial death pathway. Cell. Microbiol. 5:729-741. [DOI] [PubMed] [Google Scholar]
- 26.Hassane, D. C., R. B. Lee, and C. L. Pickett. 2003. Campylobacter jejuni cytolethal distending toxin promotes DNA repair responses in normal human cells. Infect. Immun. 71:541-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito, H., A. Terai, H. Kurazono, Y. Takeda, and M. Nishibuchi. 1990. Cloning and nucleotide sequencing of Vero toxin 2 variant genes from Escherichia coli O91:H21 isolated from a patient with the hemolytic uremic syndrome. Microb. Pathog. 8:47-60. [DOI] [PubMed] [Google Scholar]
- 28.Jaffe, E. A., R. L. Nachman, C. G. Becker, and C. R. Minick. 1973. Culture of human endothelial cells derived from umbilical veins. J. Clin. Investig. 52:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janka, A., M. Bielaszewska, U. Dobrindt, and H. Karch. 2002. Identification and distribution of the enterohemorrhagic Escherichia coli factor for adherence (efa1) gene in sorbitol-fermenting Escherichia coli O157:H−. Int. J. Med. Microbiol. 292:207-214. [DOI] [PubMed] [Google Scholar]
- 30.Janka, A., M. Bielaszewska, U. Dobrindt, L. Greune, M. A. Schmidt, and H. Karch. 2003. The cytolethal distending toxin (cdt) gene cluster in enterohemorrhagic Escherichia coli O157:H− and O157:H7: characterization and evolutionary considerations. Infect. Immun. 71:3634-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson, W. M., and H. Lior. 1988. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb. Pathog. 4:115-126. [DOI] [PubMed] [Google Scholar]
- 32.Karch, H., H. Bohm, H. Schmidt, F. Gunzer, S. Aleksic, and J. Heesemann. 1993. Clonal structure and pathogenicity of Shiga-like toxin-producing, sorbitol-fermenting Escherichia coli O157:H−. J. Clin. Microbiol. 31:1200-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 34.Lara-Tejero, M., and J. E. Galan. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290:354-357. [DOI] [PubMed] [Google Scholar]
- 35.Lara-Tejero, M., and J. E. Galan. 2001. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect. Immun. 69:4358-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, R. B., D. C. Hassane, D. L. Cottle, and C. L. Pickett. 2003. Interactions of Campylobacter jejuni cytolethal distending toxin subunits CdtA and CdtC with HeLa cells. Infect. Immun. 71:4883-4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayer, M. P., L. Bueno, E. J. Hansen, and J. M. DiRienzo. 1999. Identification of a cytolethal distending toxin gene locus and features of a virulence-associated region in Actinobacillus actinomycetemcomitans. Infect. Immun. 67:1227-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nešić D., Y. Hsu, and C. E. Stebbins. 2004. Assembly and function of a bacterial genotoxin. Nature 429:429-433. [DOI] [PubMed] [Google Scholar]
- 39.Nicoletti, I., G. Migliorati, M. C. Pagliacci, F. Grignani, and C. Riccardi. 1991. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide and flow cytometry. J. Immunol. Methods 139:271-279. [DOI] [PubMed] [Google Scholar]
- 40.Nishikubo, S., M. Ohara, Y. Ueno, M. Ikura, H. Kurihara, H. Komatsuzawa, E. Oswald, and M. Sugai. 2003. An N-terminal segment of the active component of the bacterial genotoxin cytolethal distending toxin B (CDTB) directs CDTB into the nucleus. J. Biol. Chem. 278:50671-50681. [DOI] [PubMed] [Google Scholar]
- 41.Okuda, J., H. Kurazono, and Y. Takeda. 1995. Distribution of the cytolethal distending toxin A gene (cdtA) among species of Shigella and Vibrio, and cloning and sequencing of the cdt gene from Shigella dysenteriae. Microb. Pathog. 18:167-172. [DOI] [PubMed] [Google Scholar]
- 42.Paton, A. W., M. C. Woodrow, R. M. Doyle, J. A. Lanser, and J. C. Paton. 1999. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J. Clin. Microbiol. 37:3357-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paton, A. W., P. Srimanote, M. C. Woodrow, and J. C. Paton. 2001. Characterization of Saa, a novel autoagglutinating adhesin produced by locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli strains that are virulent for humans. Infect. Immun. 69:6999-7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peres, S. P., O. Marches, F. Daigle, J. P. Nougayrede, F. Herault, C. Tasca, J. DeRycke, and E. Oswald. 1997. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol. Microbiol. 24:1095-1107. [DOI] [PubMed] [Google Scholar]
- 45.Pickett, C. L., D. L. Cottle, E. C. Pesci, and G. Bikah. 1994. Cloning, sequencing, and expression of the Escherichia coli cytolethal distending toxin genes. Infect. Immun. 62:1046-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pickett, C. L., E. C. Pesci, D. L. Cottle, G. Russell, A. N. Erdem, and H. Zeytin. 1996. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter sp. cdtB gene. Infect. Immun. 64:2070-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pickett, C. L., and C. A. Whitehouse. 1999. The cytolethal distending toxin family. Trends Microbiol. 7:292-297. [DOI] [PubMed] [Google Scholar]
- 48.Richardson, S. E., M. A. Karmali, L. E. Becker, and C. R. Smith. 1988. The histopathology of the hemolytic uremic syndrome associated with verocytotoxin-producing Escherichia coli infections. Hum. Pathol. 19:1102-1108. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt, H., L. Beutin, and H. Karch. 1995. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect. Immun. 63:1055-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt, H., W.-L. Zhang, U. Hemmrich, S. Jelacic, W. Brunder, P. I. Tarr, U. Dobrindt, J. Hacker, and H. Karch. 2001. Identification and characterization of a novel genomic island integrated at selC in locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infect. Immun. 69:6863-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scott, D. A., and J. B. Kaper. 1994. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect. Immun. 62:244-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shenker, B. J., R. H. Hoffmaster, A. Zekavat, N. Yamaguchi, E. T. Lally, and D. R. Demuth. 2001. Induction of apoptosis in human T cells by Actinobacillus actinomycetemcomitans cytolethal distending toxin is a consequence of G2 arrest of the cell cycle. J. Immunol. 167:435-441. [DOI] [PubMed] [Google Scholar]
- 53.Stins, M. F., F. Gilles, and K. S. Kim. 1997. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J. Neuroimmunol. 76:81-90. [DOI] [PubMed] [Google Scholar]
- 54.Svensson, L. A., P. Henning, and T. Lagergard. 2002. The cytolethal distending toxin of Haemophilus ducreyi inhibits endothelial cell proliferation. Infect. Immun. 70:2665-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tarr, P. I., S. S. Bilge, J. C. Vary, S. Jelacic, R. L. Habeeb, T. R. Ward, M. R. Baylor, and T. E. Besser. 2000. Iha: a novel Escherichia coli O157:H7 adherence-conferring molecule encoded on a recently acquired chromosomal island of conserved structure. Infect. Immun. 68:1400-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tarr, P. I., and M. A. Neill. 2001. Escherichia coli O157:H7. Gastroenterol. Clin. N. Am. 30:735-751. [DOI] [PubMed] [Google Scholar]
- 57.Toth, I., F. Herault, L. Beutin, and E. Oswald. 2003. Production of cytolethal distending toxins by pathogenic Escherichia coli isolated from human and animal sources: establishment of the existence of a new cdt variant (type IV). J. Clin. Microbiol. 41:4285-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitehouse, C. A., P. M. Balbo, E. C. Pesci, D. L. Cottle, P. M. Mirabito, and C. L. Pickett. 1998. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect. Immun. 66:1934-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young, V. B., C. C. Chien, K. A. Knox, N. S. Taylor, D. B. Schauer, and J. G. Fox. 2000. Cytolethal distending toxin in avian and human isolates of Helicobacter pullorum. J. Infect. Dis. 182:620-623. [DOI] [PubMed] [Google Scholar]
- 60.Yu, J., and J. B. Kaper. 1992. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 6:411-417. [DOI] [PubMed] [Google Scholar]