Supplemental Digital Content is available in the text.
Keywords: acetaminophen, antipyretics, fever, mortality, sepsis
Abstract
Objective:
This meta-analysis aimed to examine the impact of antipyretic therapy on mortality in critically ill septic adults.
Data Sources:
Literature searches were implemented in Ovid Medline, Embase, Scopus, Cumulative Index of Nursing and Allied Health Literature, Cochrane Central Register of Controlled Trials, NHS Economic Evaluation Database, and ClinicalTrials.gov through February 2016.
Study Selection:
Inclusion criteria were observational or randomized studies of septic patients, evaluation of antipyretic treatment, mortality reported, and English-language version available. Studies were excluded if they enrolled pediatric patients, patients with neurologic injury, or healthy volunteers. Criteria were applied by two independent reviewers.
Data Extraction:
Two reviewers independently extracted data and evaluated methodologic quality. Outcomes included mortality, frequency of shock reversal, acquisition of nosocomial infections, and changes in body temperature, heart rate, and minute ventilation. Randomized and observational studies were analyzed separately.
Data Synthesis:
Eight randomized studies (1,507 patients) and eight observational studies (17,432 patients) were analyzed. Antipyretic therapy did not reduce 28-day/hospital mortality in the randomized studies (relative risk, 0.93; 95% CI, 0.77–1.13; I2 = 0.0%) or observational studies (odds ratio, 0.90; 95% CI, 0.54–1.51; I2 = 76.1%). Shock reversal (relative risk, 1.13; 95% CI, 0.68–1.90; I2 = 51.6%) and acquisition of nosocomial infections (relative risk, 1.13; 95% CI, 0.61–2.09; I2 = 61.0%) were also unchanged. Antipyretic therapy decreased body temperature (mean difference, –0.38°C; 95% CI, –0.63 to –0.13; I2 = 84.0%), but not heart rate or minute ventilation.
Conclusions:
Antipyretic treatment does not significantly improve 28-day/hospital mortality in adult patients with sepsis.
Over 1 million patients are hospitalized with sepsis annually in the United States, and sepsis is the leading cause of death in critically ill patients (1). Fever, a common sign of infection, occurs in approximately 40% of critically ill septic patients at some point during their ICU stay (2, 3). It is an extremely complex physiologic response with potentially beneficial and harmful effects in septic patients. Fever boosts several aspects of innate and adaptive immunity, inhibits microorganism growth, slows viral replication, and augments antibiotic efficacy (4–8). In animal models, artificially raising core body temperature leads to improved survival and lower infectious burden (9, 10). However, fever generation also raises the metabolic rate, increases oxygen consumption, and can adversely affect cardiac function (11–13). In septic patients, who are vulnerable to malperfusion and tissue hypoxia, this physiologic expense could be particularly detrimental.
Despite the potential benefits of fever in patients with sepsis, treatment with antipyretic therapies is common in the ICU. In a recent international survey of ICU practitioners in 23 countries, greater than 80% of respondents reported controlling fever in critically ill patients most or all of the time (14). Data supporting this practice, however, remain inconclusive because of limited sample sizes and lack of reproducibility of study results. In fact, some studies have suggested that antipyresis in critically ill septic patients may be harmful (15–17). The majority of prior meta-analyses of the effect of antipyretic therapy in the critically ill have not focused on septic patients (18–20). Because antipyretic therapy may impact infected and noninfected patients differently (16), conclusions from these studies are difficult to interpret. Furthermore, methodologic limitations in previous evaluations of antipyretic therapy in sepsis render the question of optimal fever management in this population unclear (21).
The objective of this meta-analysis was to evaluate the effect of antipyretic therapy on mortality in critically ill septic patients. Secondary aims included assessing the impact of fever control on the acquisition of nosocomial infections, shock reversal, and physiologic variables such as body temperature, heart rate, and minute ventilation. The primary hypothesis was that antipyretic therapy would not improve mortality in septic patients.
MATERIALS AND METHODS
This meta-analysis was conducted and reported in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis and Meta-Analysis of Observational Studies in Epidemiology guidelines (Supplemental Digital Content 1, http://links.lww.com/CCM/C421) (22, 23). The study protocol (Supplemental Digital Content 2, http://links.lww.com/CCM/C422) was developed prior to initiation of the search strategy and has been registered on PROSPERO (registration number: CRD42016037622). Ethical approval from the human research protection office was not required.
Literature Search and Study Selection
Published literature was electronically searched by two medical librarians (A.C.H., S.A.F.) for the concepts of sepsis, fever, antipyretics, and physical cooling in adults. These strategies were implemented in Ovid Medline, Embase, Scopus, Cumulative Index of Nursing and Allied Health Literature, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, NHS Economic Evaluation Database, and ClinicalTrials.gov between January 1946 and February 2016. Full search strategies are provided in Supplemental Digital Content 3 (http://links.lww.com/CCM/C423).
Two authors (A.M.D., E.T.M.) independently screened titles and abstracts for potentially eligible studies. These included observational or randomized studies evaluating mortality in septic patients treated with and without antipyretic therapy. The full list of inclusion and exclusion criteria is available in Supplemental Digital Content 4 (http://links.lww.com/CCM/C424). Studies of antipyretic therapy that included both septic and nonseptic patients were included if mortality data were provided for the subgroup of septic patients. If these data were not reported, authors were contacted via electronic mail to request it. The authors (A.M.D., E.T.M.) also reviewed bibliographies of included articles and performed a hand search of critical care–related journals to identify additional studies. Abstracts from critical care–related meetings (full list provided in the protocol) from 2008 to 2015 were searched to identify any unpublished literature. Any article identified by either screener as being potentially eligible was reviewed in full.
Following the initial screening, full articles were independently reviewed by two authors (A.M.D., B.M.F.) with application of the same inclusion and exclusion criteria. Disagreements regarding study inclusion were resolved by consensus. Studies excluded after full-text review are listed in Supplemental Table 1 (Supplemental Digital Content 5, http://links.lww.com/CCM/C425).
Data Extraction
Data on study characteristics, patient characteristics, study interventions, and outcomes were independently extracted from each study by two study members (A.M.D., E.A.A.) using standardized forms created in an online data management system (24). A full list of variables collected is provided in Supplemental Digital Content 2 (http://links.lww.com/CCM/C422). Primary data reported solely in graphical form were extracted using an online plot data extraction tool (25). When necessary, authors were contacted to provide missing data. Following extraction, data were compared and disagreements were resolved by consensus.
Outcomes
The primary outcome was 28-day mortality. Studies reporting hospital mortality were pooled with those reporting 28-day mortality. Secondary outcomes included “early” mortality (defined as mortality on or prior to day 14 after enrollment or within the ICU), frequency of acquisition of nosocomial infections, frequency of shock reversal, and mean changes in body temperature, heart rate, and minute ventilation with antipyretic treatment. A priori, the decision was made to analyze 28-day and 14-day mortality separately based on observations of different mortality rates for these different follow-up periods (26, 27). For randomized trials, postintervention physiologic values were pooled for meta-analysis rather than the pre- to postchange in those values because no study provided measures of dispersion for the pre- to postintervention changes. This was considered to be a valid approach based on the assumption that in randomized trials, the differences in mean final values are similar to the differences in changes of these values (28).
Quality Assessment
For randomized trials, study quality was assessed independently by two reviewers (A.M.D., E.A.A.) using the Cochrane Collaboration Risk of Bias Tool with standardized criteria for evaluating bias in seven domains (29). Quality of observational studies was evaluated with the Newcastle-Ottawa Scale (NOS), a 9-point scale assessing bias in the areas of patient selection, comparability, exposure, and outcome (30). Disagreements were resolved by a third reviewer (B.M.F.). A priori, it was decided that randomized studies with a high or unclear risk of bias in less than two domains or observational studies with an NOS score greater than 7 would be considered to be high quality.
Data Analysis
Observational and randomized studies were analyzed separately, as recommended by expert opinion (31), using STATA/IC 14.1 (StataCorp, College Station, TX). For categorical outcomes, a relative risk (RR) with 95% CI (for randomized studies) or odds ratio (OR) with 95% CI (for observational studies) was calculated for each study. Data were combined using the DerSimonian and Laird (32) random effects model and plotted as forest plots. Higgins I2 tests were used to assess heterogeneity. A random effects model was used even if no heterogeneity was observed due to limitations of statistical tests for heterogeneity. For observational studies, adjusted ORs, if available, were preferentially used in the meta-analysis. For studies evaluating multiple methods of antipyresis, the overall OR for any type of antipyresis was used in the meta-analysis. However, if an overall OR was not reported, ORs for each method of antipyresis were included separately. For continuous outcomes, weighted mean differences were calculated using a random effects model for continuous outcomes. For continuous data reported as median and interquartile range, mean and sd were estimated using previously published methods (33). A p value less than 0.05 was considered statistically significant.
Publication bias was assessed using funnel plots and Egger test. Extended funnel plots were created to graphically display the effect size and se combinations needed for an additional randomized trial to change the results of the meta-analysis (34, 35). Simulation methods were used to create a graph demonstrating the power achieved by an additional randomized trial to change the results of the meta-analysis at different sample sizes up to a maximum of 30,000 patients (36, 37).
Stratified analyses were conducted for the primary outcome by the type of intervention, duration of treatment, and primary goal of the study (evaluation of anti-inflammatory treatment or evaluation of fever treatment). Predefined subgroup analyses for the primary outcome were performed for the subset of studies with a low risk of bias and for the subset of patients with fever and septic shock.
RESULTS
Details regarding the literature search and study selection are shown in Figure 1. A total of 16 studies (eight randomized studies and eight observational studies) met eligibility criteria (15, 16, 26, 27, 38–49). Study characteristics are shown in Supplemental Table 2 (Supplemental Digital Content 5, http://links.lww.com/CCM/C425).
Figure 1.
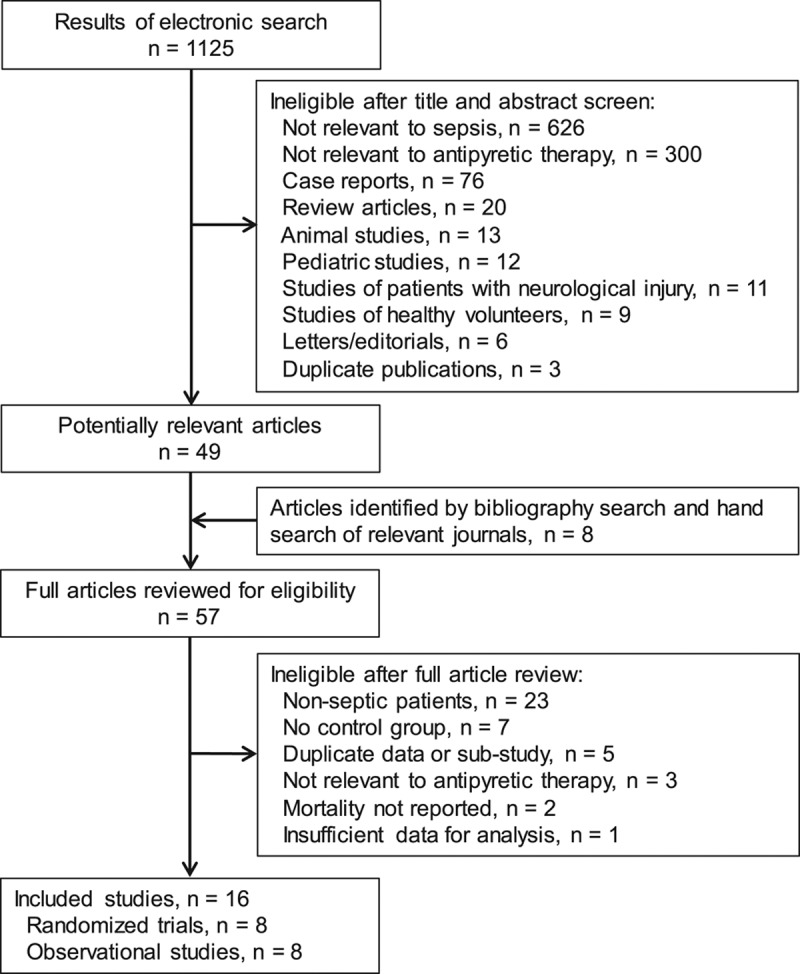
Flowchart of study selection.
Randomized Trials
The randomized studies enrolled a total of 1,531 patients (1,507 patients included in analysis of the primary outcome). Patient characteristics and outcome data for the individual trials are shown in Supplemental Tables 3 and 4 (Supplemental Digital Content 5, http://links.lww.com/CCM/C425). Risk of bias assessments are shown in Supplemental Table 5 (Supplemental Digital Content 5, http://links.lww.com/CCM/C425). Five studies had a low risk of bias.
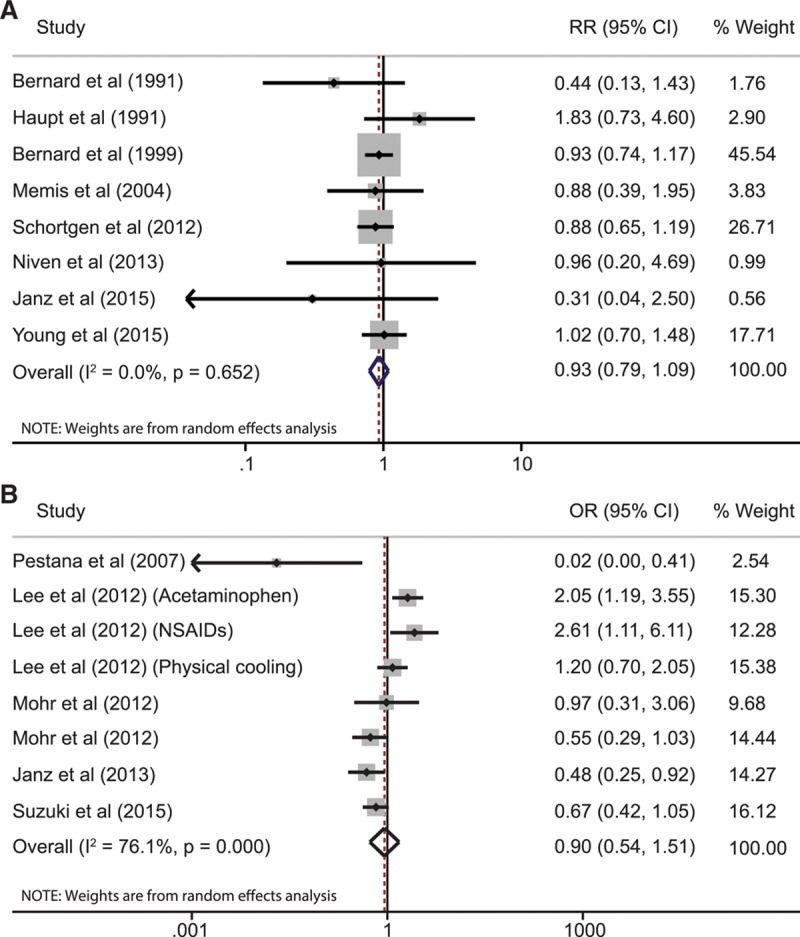
Results of the meta-analyses for the primary and secondary outcomes are listed in Table 1. Four studies (1,198 patients) reported 28-day mortality with a pooled RR of 0.93 (95% CI, 0.77–1.13; I2 = 0.0%) comparing antipyretic therapy to control. The remaining four studies reported hospital mortality; adding this data to the analysis (1,507 total patients) resulted in a pooled RR of 0.93 (95% CI, 0.79–1.09; I2 = 0.0%) (Fig. 2). Subgroup analyses of 28-day/hospital mortality in febrile patients (RR, 0.96; 95% CI, 0.80–1.14; I2 = 0.0%) and patients with shock (RR, 0.91; 95% CI, 0.74–1.11; I2 = 0.0) yielded similar results. Stratified analyses by type of therapy and treatment goal also did not differ significantly from that of the aggregate data (Table 1).
TABLE 1.
Summary of Meta-Analysis Results
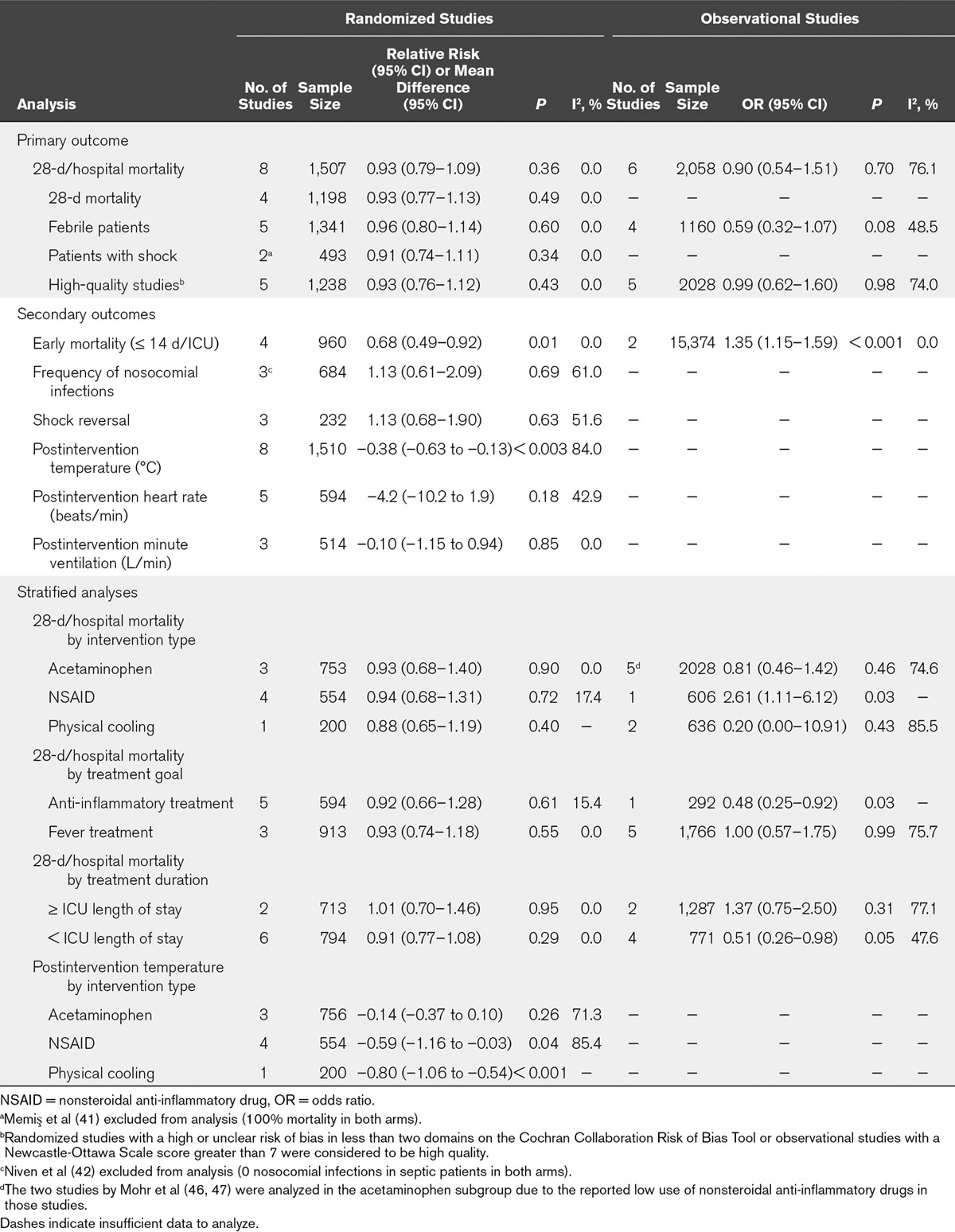
Figure 2.
Results of meta-analysis for 28 days per hospital mortality in (A) randomized studies and (B) observational studies. A relative risk (RR) or odds ratio (OR) less than 1 favors antipyretic therapy. The size of the grey box corresponds to weight in the random effects analysis. NSAID = nonsteroidal anti-inflammatory drug.
Analyses of secondary outcomes (Table 1) showed a significant decrease in early mortality (RR, 0.68; 95% CI, 0.49–0.92; I2 = 0.0%) with antipyretic therapy. Postintervention body temperature was also significantly lower (mean difference, –0.38°C; 95% CI, –0.63 to –0.13; I2 = 84.0%) in patients treated with antipyretics. Stratified analysis of postintervention body temperature by type of intervention showed that physical cooling and nonsteroidal anti-inflammatory drugs (NSAIDs) lowered body temperature (mean difference, –0.80°C; 95% CI, –1.06 to –0.54 and mean difference, –0.59°C; 95% CI, –1.16 to –0.03; I2 = 85.4%) more effectively than acetaminophen (mean difference, –0.14°C; 95% CI, –0.37 to 0.10; I2 71.3%). However, only one study used physical cooling as the primary antipyretic intervention. Postintervention heart rate and minute ventilation were not significantly different between the groups. The frequency of nosocomial infections and shock reversal were also unchanged (forest plots shown in Supplemental Figs. 1–3, Supplemental Digital Content 5, http://links.lww.com/CCM/C425).
Publication bias was not evident (Egger test, p = 0.60) (Supplemental Fig. 4, Supplemental Digital Content 5, http://links.lww.com/CCM/C425). The extended funnel plot, which graphically demonstrates the combinations of effect size and se that would be required for an additional study to change the results of this meta-analysis to support a 28-day/hospital mortality benefit with antipyretic therapy, is shown in Figure 3. Supplemental Figure 5 (Supplemental Digital Content 5, http://links.lww.com/CCM/C425) shows the power curve generated from simulation-based sample size calculations. To achieve a power of 80% to change the results of this meta-analysis, an additional study would require a total sample size of approximately 29,000 patients.
Figure 3.
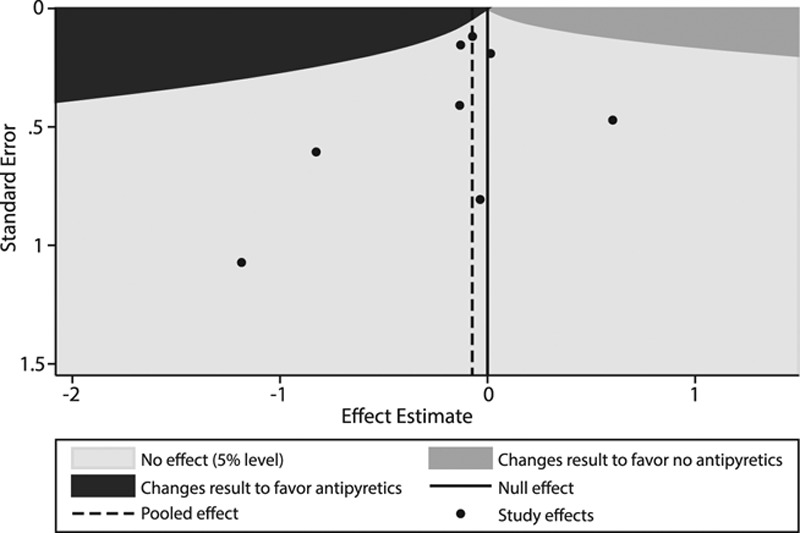
Extended funnel plot demonstrating the effect size and se combinations required of an additional randomized study to change the results of this meta-analysis using a fixed effects model with an alpha level of 0.05. Black dots represent the effect size and ses of the included randomized studies. To change the results of the meta-analysis to favor antipyretics, an additional study would need to have an effect estimate and se combination that falls in the dark grey shaded area. Note that none of the current studies have an effect size-se in the dark grey region; this suggests that the results of the meta-analysis would be robust to an additional study.
Observational Studies
Eight observational studies were deemed eligible. Supplemental Tables 6 and 7 (Supplemental Digital Content 5, http://links.lww.com/CCM/C425) describe the patient characteristics and results of the quality assessments. Six studies were high quality; two, low quality.
A total of 2,058 septic patients (six studies) were included in the analysis of 28-day/hospital mortality; 15,374 septic patients (two studies) were included in the analysis of early mortality. Outcome data for the individual studies, including adjusted and unadjusted ORs for mortality, are shown in Supplemental Table 8 (Supplemental Digital Content 5, http://links.lww.com/CCM/C425). The pooled OR for 28-day/hospital mortality was 0.90 (95% CI, 0.54–1.51; I2 = 76.1%) (Fig. 2). The pooled OR for early mortality was 0.22 (95% CI, 0.004–13.14; I2 = 86.7%) (Supplemental Fig. 1, Supplemental Digital Content 5, http://links.lww.com/CCM/C425). Other secondary outcomes were not reported in a sufficient number of studies to be analyzed. No specific antipyretic method was significantly associated with mortality benefit, and stratification by study quality did not yield results that differed from the overall pooled OR (Table 1). Publication bias was not evident (Egger test, p = 0.54). (Supplemental Fig. 4, Supplemental Digital Content 5, http://links.lww.com/CCM/C425).
DISCUSSION
Despite lack of evidence showing benefit of antipyretic therapy in septic patients, treatment of fever is ubiquitous in the ICU (14). This meta-analysis was undertaken to inform clinical practice by assessing outcomes associated with antipyretic therapy. The results demonstrate that, while associated with a reduction in body temperature, antipyretic therapy does not confer a 28-day/hospital mortality benefit in septic patients. Secondary outcomes, including shock reversal and acquisition of nosocomial infections, were also unaffected by antipyretic treatments. Consistency in results was demonstrated across study design as well as in a priori subgroup and stratified analyses. Furthermore, the extended funnel plot analysis suggests that these results are likely to be robust to the impact of an additional trial in the future; none of the existing studies generated an effect size-se combination that could change the pooled RR to favor antipyretic treatment. Additionally, simulation analysis showed that to achieve a reasonable power to change the meta-analysis results, an additional trial would need to enroll tens of thousands of patients. Based on the sample sizes and enrollment durations of the existing multicenter studies, a trial of this size seems unfeasible.
Interestingly, early mortality (occurring within 14 d or during ICU stay) was significantly lower in patients treated with antipyretic therapy in the randomized studies. This outcome was analyzed separately from 28-day/hospital mortality because several studies reported 14-day/ICU mortality rates that differed from those at later time points (26, 27). The importance of improved early mortality, though, is questionable as a patient-centered outcome, and this finding should not influence clinical practice. One hypothesis for the decrease in early, but not later, deaths is that fever treatment blunts the immunologic benefit of hyperthermia leading to increased nosocomial infections later in the hospital course. The results of this meta-analysis demonstrated no significant differences in the acquisition of nosocomial infections among patients who did and did not receive antipyretic therapy. Analysis of this outcome, however, included only three studies, so evidence is limited. This may be an area for future study.
Proponents of fever treatment advocate that the chief benefit of antipyretic therapy in critically ill patients is a reduction of the metabolic burden typically associated with elevated body temperature (50). This meta-analysis shows that although antipyretic therapy is effective in decreasing body temperature, heart rate and minute ventilation are less affected. Also, antipyresis did not improve mortality in the subgroup of patients with septic shock, who presumably would be the most likely to benefit from a reduction in metabolic burden. These results suggest that the potential physiologic benefit of antipyretic therapies may be overstated and does not translate into improvement in outcomes.
Definitions of fever ranged from a body temperature of 38.0°C to 38.4°C in the randomized studies and from 37.3°C to 39.5°C in the observational studies. The larger range of fever definitions in the observational studies may have contributed (along with other factors such as variations in study design, patient population, and analysis techniques) to the greater heterogeneity observed in the meta-analysis results. Of note, the observational study with the highest threshold for fever treatment (39.5°C) demonstrated the most substantial improvement in 28-day/hospital mortality with antipyretic therapy (44). The implication of this finding is unclear, though, because of this study’s small sample size, methodologic limitations, and unique method of physical cooling (continuous venovenous hemofiltration).
This meta-analysis has important limitations. Many of studies included in the analysis were not designed primarily to evaluate the clinical effect of fever treatment but rather the effect of specific anti-inflammatory actions of the interventions being studied. Thus, both febrile and afebrile patients were enrolled, and administration of other antipyretics beyond the specific therapy being studied was not controlled. To address this limitation, analysis of the primary outcome was stratified by the goal of the study (evaluation of anti-inflammatory treatment vs evaluation of fever treatment) and a subgroup analysis including only febrile patients was performed. The results of these analyses were virtually identical to the pooled RR and 95% CI reported for the entire cohort indicating that this limitation should not significantly affect the overall conclusions of this meta-analysis.
The studies included in this meta-analysis also varied considerably in terms of the specific antipyretic interventions being evaluated (NSAIDs, acetaminophen, physical cooling) and the duration of antipyretic treatment. Each intervention is associated with distinct effects beyond fever control which could potentially affect patient outcomes. Despite this, study heterogeneity was low (I2 = 0.0%) in the randomized studies, and stratified analysis did not show significant differences in mortality among different intervention types or intervention durations. However, since fewer studies were analyzed in each stratum as compared to the overall meta-analysis, it is possible that future studies of specific treatments would have more power to change these stratified results at any given sample size than indicated by our sample size simulation analysis.
Additionally, although the search was the most rigorous and comprehensive one on this topic to date, several studies that included mixed populations of infected and noninfected patients were excluded because mortality data could not be obtained for the subset of infected patients. Based on the extended funnel plot and the power curve generated from simulated sample size calculations, the inclusion of these studies would not have changed the results of the primary outcome.
Finally, sepsis is a heterogeneous syndrome by definition. Although our results strongly suggest that antipyretic therapy, across a broad cohort of septic patients, does not improve outcome, it is unable to inform on the individual septic patient who may accrue benefit from fever control.
CONCLUSIONS
Antipyretic treatment does not significantly improve 28-day/hospital mortality in adult patients with sepsis. Additional studies are unlikely to be powered sufficiently to change this conclusion.
Supplementary Material
Footnotes
This work was performed at Washington University School of Medicine, St. Louis, MO.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Drs. Drewry and Fuller were supported by the Washington University Institute of Clinical and Translational Sciences grants UL1 TR000448 and KL2 TR000450 from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). Dr. Drewry was also supported by the Foundation for Anesthesia Education and Research and by a grant from the Division of Clinical and Translational Research of the Department of Anesthesiology at Washington University School of Medicine. She received funding from the NIH. Ms. Murry was supported by The Foundation for Anesthesia Education and Research. Ms. Dalton disclosed work for hire. Dr. Fuller received support for article research from the NIH. His institution received funding from KL2 career development award from the NIH Clinical and Translational Science Awards Program and from Barnes Jewish Hospital Foundation. Dr. Colditz received funding from legal team in litigation talc and ovarian cancer (expert on general causation). He received support for article research from Foundation for Barnes Jewish Hospital. Dr. Ablordeppey, Dr. Stoll, Ms. Izadi, Dr. Fuller, and Dr. Colditz were supported by the Foundation for Barnes-Jewish Hospital. Dr. Ablordeppey was supported by the Washington University School of Medicine Faculty Scholars grant. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Lagu T, Rothberg MB, Shieh MS, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012; 40:754–761 [DOI] [PubMed] [Google Scholar]
- 2.Kushimoto S, Gando S, Saitoh D, et al. JAAM Sepsis Registry Study Group: The impact of body temperature abnormalities on the disease severity and outcome in patients with severe sepsis: An analysis from a multicenter, prospective survey of severe sepsis. Crit Care 2013; 17:R271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young PJ, Saxena M, Beasley R, et al. Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Med 2012; 38:437–444. [DOI] [PubMed] [Google Scholar]
- 4.Evans SS, Repasky EA, Fisher DT.Fever and the thermal regulation of immunity: The immune system feels the heat. Nat Rev Immunol 2015; 15:335–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CT, Zhong L, Mace TA, et al. Elevation in body temperature to fever range enhances and prolongs subsequent responsiveness of macrophages to endotoxin challenge. PLoS One 2012; 7:e30077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Small PM, Täuber MG, Hackbarth CJ, et al. Influence of body temperature on bacterial growth rates in experimental pneumococcal meningitis in rabbits. Infect Immun 1986; 52:484–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu CM, Tian SF, Ren GF, et al. Occurrence of temperature-sensitive influenza A viruses in nature. J Virol 1982; 41:353–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackowiak PA, Marling-Cason M, Cohen RL.Effects of temperature on antimicrobial susceptibility of bacteria. J Infect Dis 1982; 145:550–553 [DOI] [PubMed] [Google Scholar]
- 9.Jiang Q, Cross AS, Singh IS, et al. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun 2000; 68:1265–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kluger MJ, Ringler DH, Anver MR.Fever and survival. Science 1975; 188:166–168 [PubMed] [Google Scholar]
- 11.Frankenfield DC, Smith JS, Jr, Cooney RN, et al. Relative association of fever and injury with hypermetabolism in critically ill patients. Injury 1997; 28:617–621 [DOI] [PubMed] [Google Scholar]
- 12.Manthous CA, Hall JB, Olson D, et al. Effect of cooling on oxygen consumption in febrile critically ill patients. Am J Respir Crit Care Med 1995; 151:10–14 [DOI] [PubMed] [Google Scholar]
- 13.Haupt MT, Rackow EC.Adverse effects of febrile state on cardiac performance. Am Heart J 1983; 105:763–768 [DOI] [PubMed] [Google Scholar]
- 14.Niven DJ, Laupland KB, Tabah A, et al. EUROBACT Investigators: Diagnosis and management of temperature abnormality in ICUs: A EUROBACT investigators’ survey. Crit Care 2013; 17:R289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Chen L, Ni H.Antipyretic therapy in critically ill patients with sepsis: An interaction with body temperature. PLoS One 2015; 10:e0121919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee BH, Inui D, Suh GY, et al. Fever and Antipyretic in Critically ill patients Evaluation (FACE) Study Group: Association of body temperature and antipyretic treatments with mortality of critically ill patients with and without sepsis: Multi-centered prospective observational study. Crit Care 2012; 16:R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang YL, Liu DW, Wang XT, et al. Body temperature control in patients with refractory septic shock: Too much may be harmful. Chin Med J (Engl) 2013; 126:1809–1813 [PubMed] [Google Scholar]
- 18.Jefferies S, Weatherall M, Young P, et al. The effect of antipyretic medications on mortality in critically ill patients with infection: A systematic review and meta-analysis. Crit Care Resusc 2011; 13:125–131 [PubMed] [Google Scholar]
- 19.Niven DJ, Stelfox HT, Laupland KB.Antipyretic therapy in febrile critically ill adults: A systematic review and meta-analysis. J Crit Care 2013; 28:303–310 [DOI] [PubMed] [Google Scholar]
- 20.Serpa Neto A, Pereira VG, Colombo G, et al. Should we treat fever in critically ill patients? A summary of the current evidence from three randomized controlled trials. Einstein (Sao Paulo) 2014; 12:518–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Z.Antipyretic therapy in critically ill patients with established sepsis: A trial sequential analysis. PLoS One 2015; 10:e0117279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009; 339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012 [DOI] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohatgi AWebPlotDigitizer Version 3.9. October 2015. Available at: http://arohatgi.info/WebPlotDigitizer. Accessed February 10, 2016
- 26.Schortgen F, Clabault K, Katsahian S, et al. Fever control using external cooling in septic shock: A randomized controlled trial. Am J Respir Crit Care Med 2012; 185:1088–1095 [DOI] [PubMed] [Google Scholar]
- 27.Young P, Saxena M, Bellomo R, et al. HEAT Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group: Acetaminophen for fever in critically ill patients with suspected infection. N Engl J Med 2015; 373:2215–2224 [DOI] [PubMed] [Google Scholar]
- 28.Deeks JJ, Higgins JPT, Altman DG, et al. Higgins JPT, Green S.Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions 2011. Available at: The Cochrane Collaboration, handbook.cochrane.org. Accessed Februrary 10, 2016 [Google Scholar]
- 29.Higgins JPT, Altman DG, Sterne JAC, et al. Higgins JPT, Green S.Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions 2011. The Cochrane Collaboration, Available at: handbook.cochrane.org. Accessed February 10, 2016 [Google Scholar]
- 30.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed February 10, 2016.
- 31.Reeves BC, Deeks JJ, Higgins JPT, et al. Higgins JPT, Green S.Including non-randomised studies. Cochrane Handbook for Systematic Reviews of Interventions 2011. The Cochrane Collaboration, Available at: handbook.cochrane.org. Accessed February 10, 2016 [Google Scholar]
- 32.DerSimonian R, Laird N.Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188 [DOI] [PubMed] [Google Scholar]
- 33.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crowther MJ, Langan D, Sutton AJ.Graphical augmentations to the funnel plot to assess the impact of a new study on an existing meta-analysis. The Stata Journal 2012; 12:606–622 [DOI] [PubMed] [Google Scholar]
- 35.Langan D, Higgins JP, Gregory W, et al. Graphical augmentations to the funnel plot assess the impact of additional evidence on a meta-analysis. J Clin Epidemiol 2012; 65:511–519 [DOI] [PubMed] [Google Scholar]
- 36.Crowther MJ, Hinchliffe SR, Donald A, et al. Simulation-based sample-size calculation for designing new clinical trials and diagnositic test accuracy studies to update an existing meta-analysis. Stata J 2013; 13:451–473 [Google Scholar]
- 37.Sutton AJ, Cooper NJ, Jones DR, et al. Evidence-based sample size calculations based upon updated meta-analysis. Stat Med 2007; 26:2479–2500 [DOI] [PubMed] [Google Scholar]
- 38.Bernard GR, Reines HD, Halushka PV, et al. Prostacyclin and thromboxane A2 formation is increased in human sepsis syndrome. Effects of cyclooxygenase inhibition. Am Rev Respir Dis 1991; 144:1095–1101 [DOI] [PubMed] [Google Scholar]
- 39.Haupt MT, Jastremski MS, Clemmer TP, et al. Effect of ibuprofen in patients with severe sepsis: A randomized, double-blind, multicenter study. The ibuprofen study group. Crit Care Med 1991; 19:1339–1347 [DOI] [PubMed] [Google Scholar]
- 40.Bernard GR, Wheeler AP, Russell JA, et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. The ibuprofen in sepsis study group. N Engl J Med 1997; 336:912–918 [DOI] [PubMed] [Google Scholar]
- 41.Memiş D, Karamanlioğlu B, Turan A, et al. Effects of lornoxicam on the physiology of severe sepsis. Crit Care 2004; 8:R474–R482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niven DJ, Stelfox HT, Léger C, et al. Assessment of the safety and feasibility of administering antipyretic therapy in critically ill adults: A pilot randomized clinical trial. J Crit Care 2013; 28:296–302 [DOI] [PubMed] [Google Scholar]
- 43.Janz DR, Bastarache JA, Rice TW, et al. Acetaminophen for the Reduction of Oxidative Injury in Severe Sepsis Study Group: Randomized, placebo-controlled trial of acetaminophen for the reduction of oxidative injury in severe sepsis: The acetaminophen for the reduction of oxidative injury in severe sepsis trial. Crit Care Med 2015; 43:534–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pestaña D, Casanova E, Villagrán MJ, et al. Continuous hemofiltration in hyperthermic septic shock patients. J Trauma 2007; 63:751–756 [DOI] [PubMed] [Google Scholar]
- 45.Selladurai S, Eastwood GM, Bailey M, et al. Paracetamol therapy for septic critically ill patients: A retrospective observational study. Crit Care Resusc 2011; 13:181–186 [PubMed] [Google Scholar]
- 46.Mohr NM, Fuller BM, McCammon CA, et al. Antipyretic use does not increase mortality in emergency department patients with severe sepsis. Acad Emerg Med 2012; 19:S161–S162 [Google Scholar]
- 47.Mohr N, Skrupky L, Fuller B, et al. Early antipyretic exposure does not increase mortality in patients with gram-negative severe sepsis: A retrospective cohort study. Intern Emerg Med 2012; 7:463–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janz DR, Bastarache JA, Peterson JF, et al. Association between cell-free hemoglobin, acetaminophen, and mortality in patients with sepsis: An observational study. Crit Care Med 2013; 41:784–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki S, Eastwood GM, Bailey M, et al. Paracetamol therapy and outcome of critically ill patients: A multicenter retrospective observational study. Crit Care 2015; 19:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohr NM, Doerschug KC.Point: Should antipyretic therapy be given routinely to febrile patients in septic shock? Yes. Chest 2013; 144:1096–1098; discussion 1101 [DOI] [PubMed] [Google Scholar]


