Abstract
Regulation of proliferation of normal human T lymphocytes (T cells) by glutathione (GSH) was explored with T-cell activation models that do not require accessory cell signals. L-Buthionine-(S,R)-sulfoximine (BSO), which inactivates gamma-glutamylcysteine synthetase and therefore inhibits GSH synthesis, inhibited proliferation elicited by monoclonal antibodies directed at cluster designation 2 (CD2) and CD3 antigens, or by sn-1,2-dioctanoylglycerol and ionomycin. L-Buthionine-(R)-sulfoximine, which does not inactivate gamma-glutamylcysteine synthetase, did not affect proliferation. BSO-induced inhibition of accessory cell-independent T-cell proliferation was not reversed by recombinant human interleukin 2, despite activation-dependent expression of interleukin 2 receptor alpha by T cells treated with BSO. However, BSO-associated inhibition of T-cell proliferation was reversed by GSH or GSH ester. These studies, which show that GSH can directly modulate proliferation of highly purified T cells, suggest that GSH is essential for steps close to or at DNA synthesis. The availability of methods for decreasing and for increasing GSH levels suggest therapies to produce (i) immunosuppression (of value in organ transplantation), and (ii) immunopotentiation (of potential value in treatment of immunodeficiency states such as AIDS).
Full text
PDF
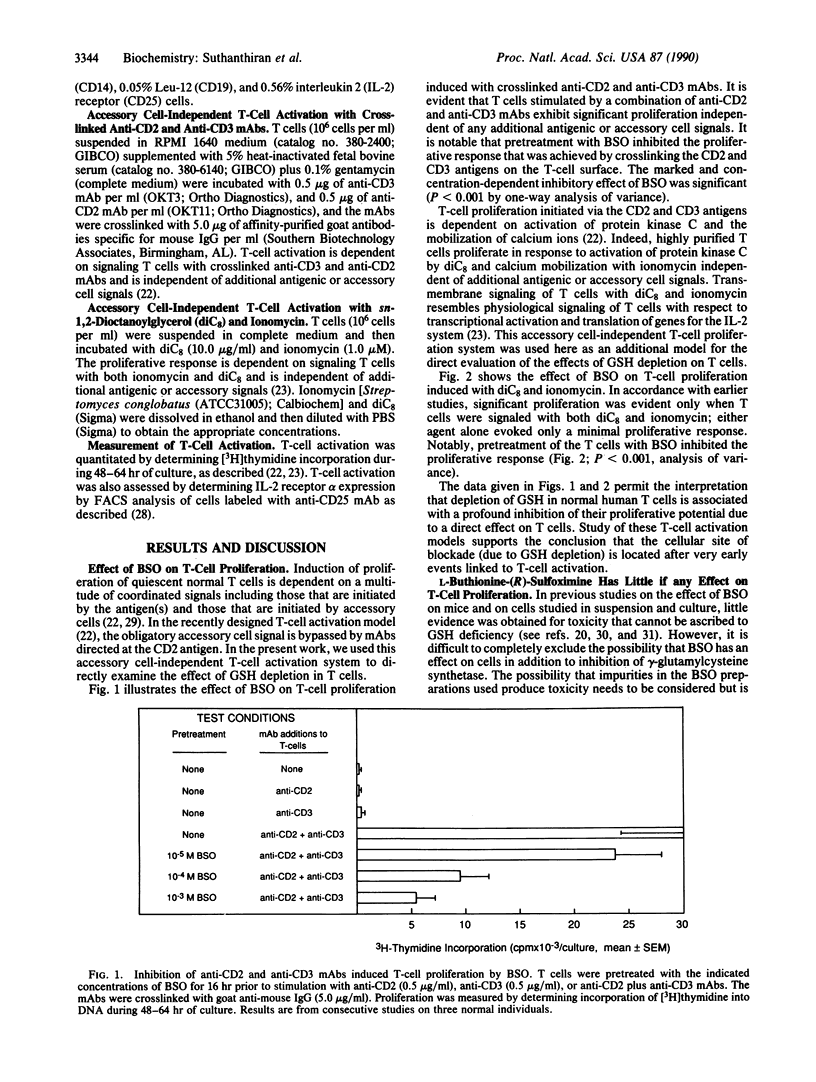
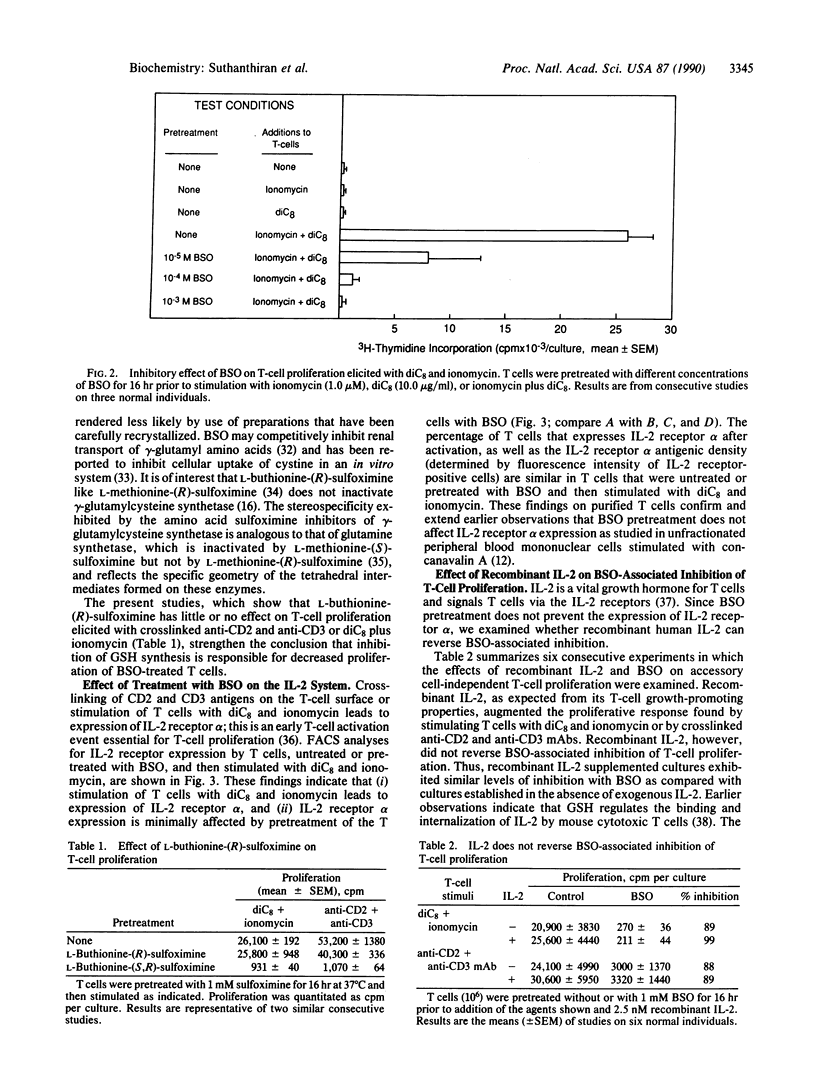
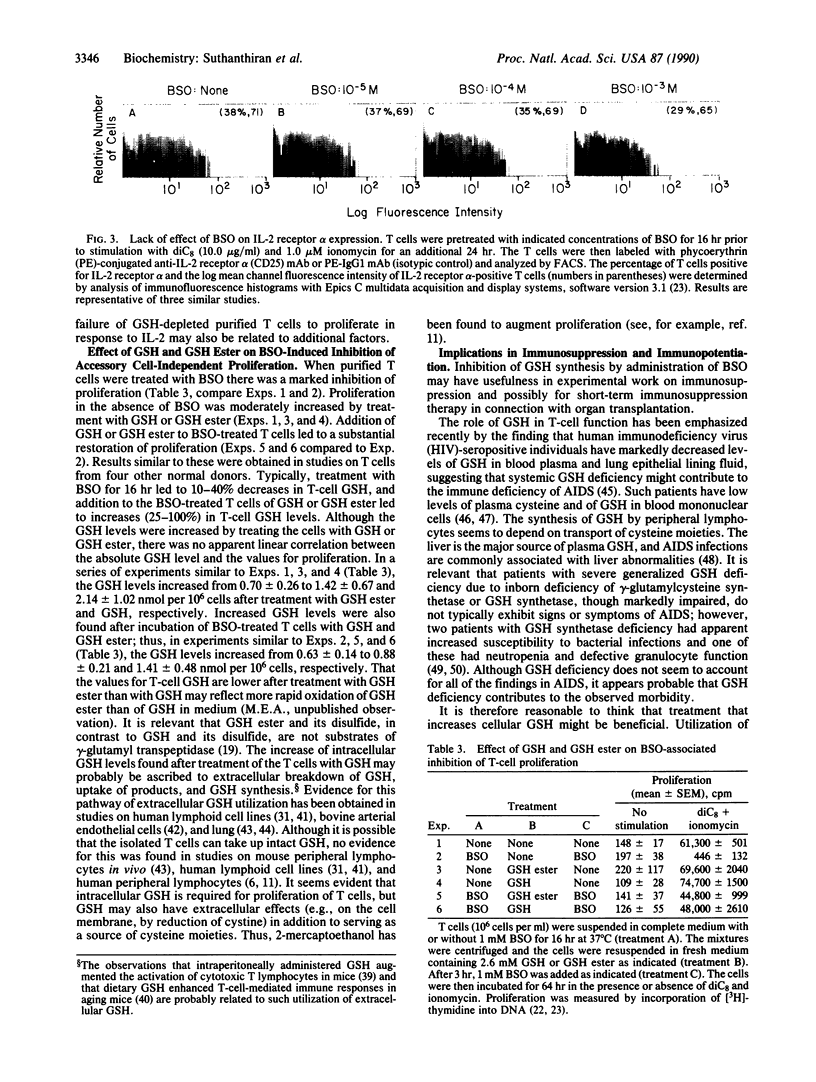

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Meister A. Glutathione monoesters. Anal Biochem. 1989 Nov 15;183(1):16–20. doi: 10.1016/0003-2697(89)90164-4. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Meister A. Marked increase of cysteine levels in many regions of the brain after administration of 2-oxothiazolidine-4-carboxylate. FASEB J. 1989 Mar;3(5):1632–1636. doi: 10.1096/fasebj.3.5.2920877. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Meister A. Transport and direct utilization of gamma-glutamylcyst(e)ine for glutathione synthesis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):707–711. doi: 10.1073/pnas.80.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. E., Powrie F., Puri R. N., Meister A. Glutathione monoethyl ester: preparation, uptake by tissues, and conversion to glutathione. Arch Biochem Biophys. 1985 Jun;239(2):538–548. doi: 10.1016/0003-9861(85)90723-4. [DOI] [PubMed] [Google Scholar]
- Berggren M., Dawson J., Moldéus P. Glutathione biosynthesis in the isolated perfused rat lung: utilization of extracellular glutathione. FEBS Lett. 1984 Oct 15;176(1):189–192. doi: 10.1016/0014-5793(84)80938-2. [DOI] [PubMed] [Google Scholar]
- Boxer L. A., Oliver J. M., Spielberg S. P., Allen J. M., Schulman J. D. Protection of granulocytes by vitamin E in glutathione synthetase deficiency. N Engl J Med. 1979 Oct 25;301(17):901–905. doi: 10.1056/NEJM197910253011702. [DOI] [PubMed] [Google Scholar]
- Boyland E., Chasseaud L. F. Enzyme-catalysed conjugations of glutathione with unsaturated compounds. Biochem J. 1967 Jul;104(1):95–102. doi: 10.1042/bj1040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie A. E., Reed D. J. Buthionine sulfoximine inhibition of cystine uptake and glutathione biosynthesis in human lung carcinoma cells. Toxicol Appl Pharmacol. 1985 Mar 15;77(3):381–387. doi: 10.1016/0041-008x(85)90177-2. [DOI] [PubMed] [Google Scholar]
- Buhl R., Jaffe H. A., Holroyd K. J., Wells F. B., Mastrangeli A., Saltini C., Cantin A. M., Crystal R. G. Systemic glutathione deficiency in symptom-free HIV-seropositive individuals. Lancet. 1989 Dec 2;2(8675):1294–1298. doi: 10.1016/s0140-6736(89)91909-0. [DOI] [PubMed] [Google Scholar]
- Chaplin D. D., Wedner J. H. Inhibition of lectin-induced lymphocyte activation by diamide and other sulfhydryl reagents. Cell Immunol. 1978 Mar 15;36(2):303–311. doi: 10.1016/0008-8749(78)90274-5. [DOI] [PubMed] [Google Scholar]
- Dethmers J. K., Meister A. Glutathione export by human lymphoid cells: depletion of glutathione by inhibition of its synthesis decreases export and increases sensitivity to irradiation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7492–7496. doi: 10.1073/pnas.78.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge W., Eck H. P., Näher H., Pekar U., Daniel V. Abnormal amino-acid concentrations in the blood of patients with acquired immunodeficiency syndrome (AIDS) may contribute to the immunological defect. Biol Chem Hoppe Seyler. 1988 Mar;369(3):143–148. doi: 10.1515/bchm3.1988.369.1.143. [DOI] [PubMed] [Google Scholar]
- Dröge W., Pottmeyer-Gerber C., Schmidt H., Nick S. Glutathione augments the activation of cytotoxic T lymphocytes in vivo. Immunobiology. 1986 Aug;172(1-2):151–156. doi: 10.1016/s0171-2985(86)80061-4. [DOI] [PubMed] [Google Scholar]
- Eck H. P., Gmünder H., Hartmann M., Petzoldt D., Daniel V., Dröge W. Low concentrations of acid-soluble thiol (cysteine) in the blood plasma of HIV-1-infected patients. Biol Chem Hoppe Seyler. 1989 Feb;370(2):101–108. doi: 10.1515/bchm3.1989.370.1.101. [DOI] [PubMed] [Google Scholar]
- Fidelus R. K., Ginouves P., Lawrence D., Tsan M. F. Modulation of intracellular glutathione concentrations alters lymphocyte activation and proliferation. Exp Cell Res. 1987 Jun;170(2):269–275. doi: 10.1016/0014-4827(87)90305-3. [DOI] [PubMed] [Google Scholar]
- Fischman C. M., Udey M. C., Kurtz M., Wedner H. J. Inhibition of lectin-induced lymphocyte activation by 2-cyclohexene-1-one: decreased intracellular glutathione inhibits an early event in the activation sequence. J Immunol. 1981 Dec;127(6):2257–2262. [PubMed] [Google Scholar]
- Furukawa T., Meydani S. N., Blumberg J. B. Reversal of age-associated decline in immune responsiveness by dietary glutathione supplementation in mice. Mech Ageing Dev. 1987 Apr;38(2):107–117. doi: 10.1016/0047-6374(87)90071-6. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Anderson M. E., Meister A. Inhibition of glutathione biosynthesis by prothionine sulfoximine (S-n-propyl homocysteine sulfoximine), a selective inhibitor of gamma-glutamylcysteine synthetase. J Biol Chem. 1979 Feb 25;254(4):1205–1210. [PubMed] [Google Scholar]
- Griffith O. W. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem. 1982 Nov 25;257(22):13704–13712. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Hamilos D. L., Wedner H. J. The role of glutathione in lymphocyte activation. I. Comparison of inhibitory effects of buthionine sulfoximine and 2-cyclohexene-1-one by nuclear size transformation. J Immunol. 1985 Oct;135(4):2740–2747. [PubMed] [Google Scholar]
- Hamilos D. L., Zelarney P., Mascali J. J. Lymphocyte proliferation in glutathione-depleted lymphocytes: direct relationship between glutathione availability and the proliferative response. Immunopharmacology. 1989 Nov-Dec;18(3):223–235. doi: 10.1016/0162-3109(89)90020-9. [DOI] [PubMed] [Google Scholar]
- Jensen G. L., Meister A. Radioprotection of human lymphoid cells by exogenously supplied glutathione is mediated by gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4714–4717. doi: 10.1073/pnas.80.15.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower E. M., Kosower N. S. Lest I forget thee, glutathione. Nature. 1969 Oct 11;224(5215):117–120. doi: 10.1038/224117a0. [DOI] [PubMed] [Google Scholar]
- Lebovics E., Dworkin B. M., Heier S. K., Rosenthal W. S. The hepatobiliary manifestations of human immunodeficiency virus infection. Am J Gastroenterol. 1988 Jan;83(1):1–7. [PubMed] [Google Scholar]
- Liang C. M., Lee N., Cattell D., Liang S. M. Glutathione regulates interleukin-2 activity on cytotoxic T-cells. J Biol Chem. 1989 Aug 15;264(23):13519–13523. [PubMed] [Google Scholar]
- Manning J. M., Moore S., Rowe W. B., Meister A. Identification of L-methionine S-sulfoximine as the diastereoisomer of L-methionine SR-sulfoximine that inhibits glutamine synthetase. Biochemistry. 1969 Jun;8(6):2681–2685. doi: 10.1021/bi00834a066. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Messina J. P., Lawrence D. A. Cell cycle progression of glutathione-depleted human peripheral blood mononuclear cells is inhibited at S phase. J Immunol. 1989 Sep 15;143(6):1974–1981. [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Davis D. C., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973 Oct;187(1):185–194. [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Frayer W., Meister A. Glutathione metabolism in the lung: inhibition of its synthesis leads to lamellar body and mitochondrial defects. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5296–5300. doi: 10.1073/pnas.86.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Steinherz R., Jain A., Meister A. Glutathione ester prevents buthionine sulfoximine-induced cataracts and lens epithelial cell damage. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8727–8731. doi: 10.1073/pnas.86.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri R. N., Meister A. Transport of glutathione, as gamma-glutamylcysteinylglycyl ester, into liver and kidney. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5258–5260. doi: 10.1073/pnas.80.17.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman P. G., Orlowski M., Meister A. Inhibition of gamma-glutamylcysteine synthetase by L-methionine-S-sulfoximine. J Biol Chem. 1973 Oct 10;248(19):6684–6690. [PubMed] [Google Scholar]
- Sehajpal P., Subramaniam A., Murthi V. K., Sharma V. K., Suthanthiran M. Demonstration of a direct inhibitory effect of cyclosporine on normal human T-cells with two novel models of T-cell activation as probes. Cell Immunol. 1989 Apr 15;120(1):195–204. doi: 10.1016/0008-8749(89)90187-1. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Subramaniam A., Sehajpal P., Murthi V. K., Stenzel K. H., Suthanthiran M. Activation of human T cells with the physiological regulator of protein kinase C. Cell Immunol. 1988 Oct 15;116(2):439–449. doi: 10.1016/0008-8749(88)90243-2. [DOI] [PubMed] [Google Scholar]
- Suthanthiran M. Oxidative mitogenesis: participation of CD2 antigen in the generation and/or transduction of obligatory accessory signals. Cell Immunol. 1988 Jun;114(1):117–125. doi: 10.1016/0008-8749(88)90259-6. [DOI] [PubMed] [Google Scholar]
- Suthanthiran M. T-cell differentiation antigen cluster 2 (CD2) is a receptor for accessory cells and can generate and/or transduce accessory signals. Cell Immunol. 1988 Mar;112(1):112–122. doi: 10.1016/0008-8749(88)90280-8. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., White J. E., Rosano C. L. Modulation of endothelial GSH concentrations: effect of exogenous GSH and GSH monoethyl ester. J Appl Physiol (1985) 1989 Mar;66(3):1029–1034. doi: 10.1152/jappl.1989.66.3.1029. [DOI] [PubMed] [Google Scholar]
- Waldmann T. A. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986 May 9;232(4751):727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]
- Wellner V. P., Anderson M. E., Puri R. N., Jensen G. L., Meister A. Radioprotection by glutathione ester: transport of glutathione ester into human lymphoid cells and fibroblasts. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4732–4735. doi: 10.1073/pnas.81.15.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. M., Boettcher B., Meister A. Intracellular cysteine delivery system that protects against toxicity by promoting glutathione synthesis. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6246–6249. doi: 10.1073/pnas.79.20.6246. [DOI] [PMC free article] [PubMed] [Google Scholar]