Abstract
Advanced timing of both seasonal migration and reproduction in birds has been strongly associated with a warming climate for many bird species. Phenological responses to climate linking these stages may ultimately impact fitness. We analyzed five decades of banding data from 17 migratory bird species to investigate 1) how spring arrival related to timing of breeding, 2) if the interval between arrival and breeding has changed with increasing spring temperatures, and 3) whether arrival timing or breeding timing best predicted local productivity. Four of 17 species, all mid- to long-distance migrants, hatched young earlier in years when migrants arrived earlier to the breeding grounds (~1:1 day advancement). The interval between arrival on breeding grounds and appearance of juveniles shortened with warmer spring temperatures for 12 species (1–6 days for every 1°C increase) and over time for seven species (1–8 days per decade), suggesting that some migratory passerines adapt to climate change by laying more quickly after arrival or reducing the time from laying to fledging. We found more support for the former, that the rate of reproductive advancement was higher than that for arrival in warm years. Timing of spring arrival and breeding were both poor predictors of avian productivity for most migrants analyzed. Nevertheless, we found evidence that fitness benefits may occur from shifts to earlier spring arrival for the multi-brooded Song Sparrow. Our results uniquely demonstrate that co-occurring avian species are phenologically plastic in their response to climate change on their breeding grounds. If migrants continue to show a weaker response to temperatures during migration than breeding, and the window between arrival and optimal breeding shortens further, biological constraints to plasticity may limit the ability of species to adapt successfully to future warming.
Introduction
Migratory birds living in seasonal environments, such as temperate forests where food supplies are restricted temporally, must optimize migration and reproductive timing to reduce fitness costs. Global climate is changing at an unprecedented rate [1], presenting a novel challenge for migrants by inducing phenological shifts in food availability. As such, individual birds must demonstrate plasticity to adapt to climate change in order to avoid negative fitness and survival repercussions. Fortunately, high plasticity in spring migration and reproductive timing [2–5] among songbirds has allowed many species to maximize productivity in the face of yearly climate variation.
Breeding phenology is highly variable interannually for bird populations and is determined by the interaction between photoperiod and environmental signals such as temperature, leaf emergence, and food abundance [6–9]. Early arrival during spring migration [10–15] and early laying dates [16–22] have resulted from warming temperatures. Early breeding may result from early arrival when emergence of vegetative and invertebrate resources advance concurrently [23–27], thereby leading to earlier hatching and fledging dates [21,28]. Breeding is constrained by arrival timing for migratory birds; thus, we expect a strong linkage between migratory and breeding phenology [29,30].
Timing of migration and breeding ultimately influences fitness in a variety of ways [31] and can carry over into the following breeding season [32]. Although the earliest birds may face inhospitable conditions, severe weather, and low food resources, individuals arriving late may fail to acquire a mate or miss peak food availability during migration or breeding [33–36]. In fact, early arriving birds tend to occupy better territories, have higher quality mates, and raise more broods [16,34,37,38]. For example, individual American Redstarts (Setophaga ruticilla) that arrived early hatched nestlings with a greater mass and sooner than later arriving individuals [35]. Early breeding can also result in higher reproductive success as was found for populations of Song Sparrows (Melospiza melodia; [39]) and Pied Flycatchers (Ficedula hypoleuca; [40]). Thus, increased reproductive success is possible if mismatches in timing of peak resource abundance do not occur [41].
There is ample evidence that many bird species are modifying migratory timing and subsequent breeding in response to climate change with individuals responding to annual climate variation (above). Despite this evidence, rigorous examination of the link between long-term migratory and breeding phenology is lacking. Furthermore, the fitness consequences migratory and breeding period variation have yet to be analyzed with a long-term, multi-species dataset. In this study, we used five decades of banding data from a constant effort mist-netting station in western Pennsylvania to investigate these linkages with climate change and productivity for 17 migratory bird species. Our dataset is uniquely consistent and precise, having been collected year round in a standardized effort across 53 years by only a few highly skilled bird banders. Specifically, we aim to address three main questions regarding shifts in breeding phenology. First, does earlier arrival lead to earlier breeding? Second, is the interval between arrival on breeding grounds and breeding shorter when spring temperatures are warmer; i.e., is either phenological event more sensitive to warming? We explored rates of change using time interval metrics between adult spring arrival date, breeding initiation, and juvenile appearance. As a subquestion, we investigated whether breeding advancement resulted from earlier nesting initiation and/or reduced nesting periods using a subset of the data. Third, focusing on population-level productivity, we asked whether arrival timing or breeding timing best predict reproductive success for 17 migratory bird species.
Materials and methods
Bird data
Bird banding data were collected from 1961–2014 at Powdermill Nature Reserve (PNR; 400 masl, 40°10’N, 79°16’W) in the Laurel Highlands of Westmoreland Co., PA, USA. The 10-ha banding area is comprised of scrub, wetland, and old field habitats. The station is operated year-round, typically 3–5 days per week during the breeding (June– 15 August) season and 6 days per week during spring migration (April—May). Number of mist-nets (20–60) and number of hours opened (~6 h) were recorded daily. Captured birds were fitted with individually numbered USGS aluminum leg bands. For each individual captured we recorded age, sex, wing length, fat score, presence of brood patch, and presence of gravidity prior to release.
Three timing metrics were derived from capture data using Julian date. We calculated spring arrival date as the 10% quantile of captures each year during spring migration. June 7 was used as a cut off for the terminus of spring migration because passage migrants have departed the study area by that date (11). Observations prior to March 1 were removed to eliminate birds that may have overwintered at PNR. We included between-year recaptures, but each individual was only used once per year in the 10% calculation (first capture date of the season). Migration arrival dates represent mostly passage migrants. Thus, we made the assumption that local breeders’ arrival was directly related to passage migrant arrival as a whole.
Secondly, we calculated juvenile appearance date to quantify reproductive timing. To determine breeding seasonal cut-off dates for reproductive data, capture frequencies by Julian day for each species were examined in addition to records on timing of reproductive events from the Second Pennsylvania Breeding Bird Atlas [42]. We used conservative end of breeding season dates to eliminate most autumn migrants but still include locally hatched birds and dispersers. Juvenile appearance date was estimated yearly for each species at which point 10% of juveniles had been captured during the breeding season within these pre-defined windows. Juveniles were only counted once, recaptures were omitted from the dataset. Years with <10 captures of juveniles were dropped, and species were only analyzed if >10 juveniles were captured for at least 10 years.
Lastly, for a subset of species we were able to calculate a surrogate for breeding initiation, a second indicator of reproductive timing. We determined the Julian date for each species at which point 10% of the breeding females (gravid or with brood patch) were captured by year. Only definitive brood patch scores were used; i.e., stages from the initiation (defeathering) of brood patch, complete vascularization, and subsequent patch refeathering [43]. The stages of brood patch and egg development, hormonally controlled, are tightly linked to breeding and thus represent excellent indicators of breeding phenology. For this breeding condition metric, we used species having >10 years of data with >10 recorded individuals with presence of breeding condition per season.
The 10% quantile was used in all metrics to reduce the effects of outliers. Temporal distributions such as quantiles are preferable to distributional extremes (e.g., first appearance/arrival) for reflecting phenology of populations, especially in cases of low sample size and imperfect detectability [12,44].
An index of breeding productivity was calculated on a yearly basis as (juvenile capture rate)/(adult capture rate) within the breeding window for all 17 migratory species (Table 1). Number of captures per 100 mist-net hours (capture rate) was determined for each species, age class, and year. Although not comparable among species due to interspecific differences in capture probability, this index has been useful to track changes in productivity [45–47].
Table 1. Species specific first day of 3-week period used for climate variables based on greatest correlation to each of three response variables.
Spring Temp = mean temperature at breeding initiation. Only dates for variables included in analyses are presented. The three response variables are the interval in days between spring arrival and juvenile appearance (10% quantile for both; "Arrival-Juvenile Appearance" the interval between arrival and breeding initiation as indicated by female breeding condition ("Arrival-Breeding"), and the interval between breeding initiation and juvenile appearance ("Breeding-Juvenile Appearance").
| Species | Miga | Habb | Broodsc | Arrival-Juvenile Appearance Spring Temp |
Arrival-Breeding Spring Temp |
Breeding-Juvenile Appearance Spring Temp |
|
|---|---|---|---|---|---|---|---|
| Ruby-throated Hummingbird | Archilochus colubris | long | forest | 2 | 17-May | ||
| Eastern Phoebe | Sayornis phoebe | short | open | 2 | 5-Apr | ||
| Red-eyed Vireo | Vireo olivaceus | long | forest | 2 | 16-Apr | 16-Apr | 15-May |
| House Wren | Troglodytes aedon | short | open | 2 | 24-Apr | 9-Apr | 28-Apr |
| Wood Thrush | Hylocichla mustelina | long | forest | 2 | 27-Apr | 26-Apr | 4-Apr |
| American Robin | Turdus migratorius | short | open | 3 | 31-Mar | ||
| Gray Catbird | Dumetella carolinensis | long | open | 3 | 25-Apr | 24-Mar | 24-Mar |
| Cedar Waxwing | Bombycilla cedrorum | short | forest | 2 | 28-Apr | 28-Apr | 28-Apr |
| Ovenbird | Seiurus aurocapilla | long | forest | 2 | 7-May | ||
| Common Yellowthroat | Geothlypis trichas | long | open | 2 | 28-May | 2-May | 2-May |
| Hooded Warbler | Setophaga citrina | long | forest | 2 | 30-Apr | ||
| American Redstart | Setophaga ruticilla | long | forest | 1 | 1-Apr | 1-Apr | 15-Apr |
| Yellow Warbler | Setophaga petechia | long | open | 1 | 12-Apr | 6-May | 16-Apr |
| Field Sparrow | Spizella pusilla | short | open | 2 | 26-Apr | 9-Mar | |
| Song Sparrow | Melospiza melodia | short | open | 3 | 2-Apr | 4-Apr | 29-Mar |
| Indigo Bunting | Passerina cyanea | long | open | 2 | 8-May | 1-May | |
| American Goldfinch | Carduelis tristis | short | open | 1 | 7-Jun | 27-Apr | 27-Apr |
a General migration distance
b Breeding habitat guild. Open = generalist and early-successional. Forest = mid to late-successional forest breeders
c Number of broods that are typically raised in a breeding season for the study area
Climate data
Data were accumulated in three-week intervals from February-August for the study period from weather stations (N = 21) within 40 km of PNR to account for climatic conditions near the breeding grounds. We retrieved average daily temperature from the National Oceanic and Atmospheric Administration (NOAA) climate database (http://www.ncdc.noaa.gov/cdo-web/search). Temperature was averaged over all possible three-week intervals. We decided a priori to restrict our analysis to three-week temperature intervals because prior research has demonstrated that migrants can adjust their migration and nesting phenology in response to short-term (i.e. two to four week windows) proximate climatic cues [11, 20, 30, 48, 49].
We used a sliding window approach (e.g., [20,21,30,48,49]) to identify the three week time period for which our climate variable (mean spring temperature) and response variable (below) were most closely correlated for each species (Table 1). Spring climate variables were chosen from months preceding and at the initiation of breeding season for each species studied, when they would be most influenced by resource availability due to plant and arthropod phenology.
Data analyses
First, we compared timing of spring arrival (10% quantile) to juvenile capture date (10% quantile) for 17 species (Table 1) using generalized linear regression models and estimated best fit lines to describe the relationship between these two variables.
Next, we modeled the effects of spring temperature and year (predictors) on time interval between arrival and juvenile appearance (response variable) using linear regression. Seventeen migratory species met the requirements for this analysis of the arrival-juvenile appearance interval, derived from our 10% capture timing metrics. This interval was defined as the difference between arrival on breeding grounds and appearance of young (juvenile appearance date—arrival date). To further investigate the potential mechanisms behind changes in phenology, we regressed two additional time intervals on spring temperature and year. A subset of years with adequate data on female breeding condition was used to split the time between the spring arrival of adults and appearance of juveniles into two distinct intervals: time interval between arrival and breeding initiation (10% female breeding condition date), and time interval between breeding initiation and appearance of juveniles.
To assess possible causal factors influencing fitness, we used an information-theoretic approach to examine whether arrival time or reproductive timing best explained yearly productivity index for 17 species [50]. The decision to include arrival time and breeding time in separate models was based on results from the first analysis. The candidate model sets included the following: 1.) a null model (no effects), 2.) a model with spring arrival timing, 3.) a model with juvenile appearance timing, and 4.) a global model which included both the timing of spring arrival and juvenile appearance. We selected the most parsimonious model using AIC adjusted for small sample sizes (AICc), ranked according to the top model as ΔAICc, and we evaluated models based on Akaike weight. Models with a ΔAICc < 2 were considered equally plausible [50]. All statistical analyses were performed using R version 3.0.3 [51]. Model-averaged parameter estimates were obtained for each candidate set (weighted averages of all competing models) using the ‘AICcmodavg’ package [52]. This package computes unconditional standard errors and 95% confidence intervals, which we used to evaluate relationships between variables.
Ethics statement
This research involved mist-netting and banding passerines, but no experiments on or collection of birds. Procedures were followed in accordance with state and federal legislation. This research was conducted using ethical guidelines following the North American Banding Council code of ethics (http://www.nabanding.net/banders-code-of-ethics/) and the Ornithological Council's guidelines to the use of wild birds in research (http://naturalhistory.si.edu/BIRDNET/documents/guidlines/Guidelines_August2010.pdf). We conducted mist-netting and banding under a Pennsylvania state permit and federal (U.S. Geological Survey) permit number 08231.
Results
Relationship between spring arrival and timing of breeding
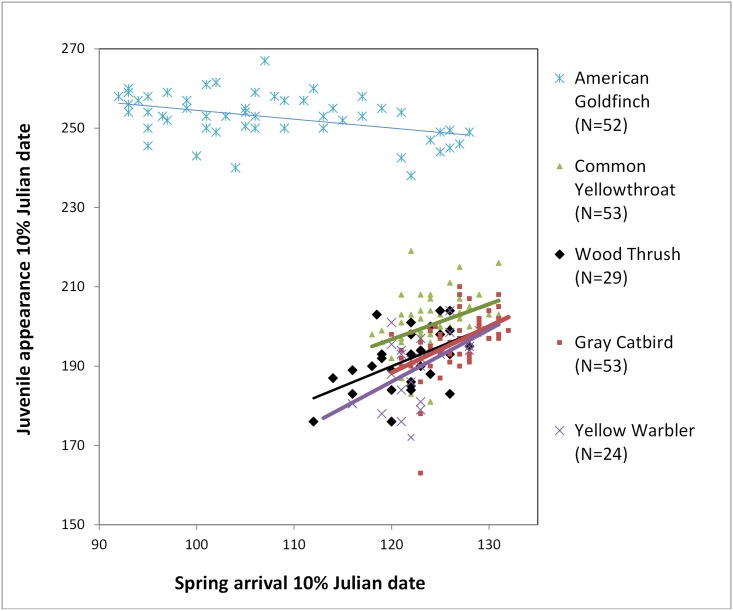
Most species showed no significant linear pattern (12 species), four species of mid to long-distance migrants hatched young earlier in years when migrants arrived earlier to the breeding grounds: Wood Thrush (scientific names in Table 1; F1,27 = 9.38, P = 0.005), Gray Catbird (F1,51 = 15.06, P<0.001), Common Yellowthroat (F1,51 = 8.88, P = 0.004), and Yellow Warbler (F1,22 = 7.33, P = 0.013). The relationship ranged from 0.9 days (Common Yellowthroat) to 1.3 days (Yellow Warbler) advancement in appearance of young to each day of advancing spring arrival (Fig 1). One species, the late-breeding American Goldfinch, showed a negative relationship (F1,50 = 10.73, P = 0.002; Fig 1), exhibiting later appearance of young in years of earlier arrival (Fig 1).
Fig 1. Seasonal timing of juvenile appearance (day at which 10% of young were captured) as related to spring arrival (day at which 10% of spring adult migrants were captured).
Bird data were collected from 1961–2014 at a constant effort mist-netting station in Pennsylvania.
Timing between phenological events changing with spring temperatures
The time interval between spring arrival on breeding grounds and appearance of juveniles decreased with warmer spring temperatures for 71% of migratory species analyzed (12 of 17 species; Table 2), and was evident for both single and multiple brooders (Table 1). The interval was reduced by 1–6 days for every 1°C increase in spring temperature. Seven species also reduced the interval between spring arrival and juvenile appearance over time (1–8 days per decade); despite a nonsignificant increase in spring temperatures over the study period in this region of Pennsylvania [28]. Conversely, American Goldfinches showed increasing intervals across years analyzed (4 days increase per decade; Table 2). The decrease in lag time between spring arrival and juvenile appearance for the majority of species analyzed met our a priori expectations and led us to examine changes within this interval.
Table 2. The relationship of three time interval variables with spring temperature (Table 1) and year effects.
Arrival-Juvenile = the interval between spring arrival and juvenile appearance (10% quantiles). Arrival-Breeding = the interval between spring arrival and breeding initiation as indicated by presence of female breeding condition (10% quantiles). Breeding-Juvenile = the interval between breeding initiation and juvenile appearance date. Parameter estimates for slopes are listed for significant effects (§ = P<0.10, * = P<0.05, ** = P<0.01, *** = P<0.001). N = years with >10 captures. Temp = days per 1°C increase.
| Species | Arrival-Juvenile Appearance | Arrival-Breeding | Breeding-Juvenile Appearance | Arrival capta |
Breeding captb |
Juvenile captc |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Temp | Year | N | Temp | Year | N | Temp | Year | ||||
| Ruby-throated Hummingbird | 45 | *-1.23 | NS | 61.8 | 39.4 | |||||||
| Eastern Phoebe | 36 | NS | NS | 15.2 | 38.5 | |||||||
| Red-eyed Vireo | 49 | *-1.28 | NS | 24 | § -3.89 | § 0.68 | 23 | NS | NS | 71.8 | 29.6 | 36.7 |
| House Wren | 44 | **-4.10 | NS | 14 | NS | NS | 12 | *8.40 | NS | 24 | 12.7 | 20.7 |
| Wood Thrush | 29 | ** -1.99 | **-0.29 | 13 | *-3.29 | NS | 13 | ***-3.60 | NS | 25.5 | 14.6 | 25.4 |
| American Robin | 27 | NS | *-0.75 | 23.3 | 19.7 | |||||||
| Gray Catbird | 53 | NS | *-0.12 | 25 | *-2.03 | NS | 25 | NS | NS | 118.1 | 31.7 | 90.5 |
| Cedar Waxwing | 50 | *-1.72 | **-0.27 | 34 | *-3.67 | NS | 33 | NS | NS | 103.7 | 36.5 | 105.3 |
| Ovenbird | 20 | **-3.42 | NS | 18.7 | 22.2 | |||||||
| Common Yellowthroat | 53 | * -1.64 | **-0.17 | 30 | *-3.02 | NS | 30 | *2.84 | NS | 92.8 | 16.3 | 56.8 |
| Hooded Warbler | 31 | *** -1.69 | **-0.25 | 28.9 | 46.3 | |||||||
| American Redstart | 41 | § -1.35 | NS | 21 | **-2.42 | NS | 22 | *-2.10 | NS | 31.8 | 21.8 | 59 |
| Yellow Warbler | 24 | NS | **-0.40 | 16 | NS | NS | 10 | NS | NS | 53.2 | 13.9 | 16.1 |
| Field Sparrow | 22 | *-2.00 | NS | 74.3 | 40.9 | |||||||
| Song Sparrow | 53 | *-2.06 | NS | 20 | NS | NS | 20 | NS | NS | 199.1 | 21.4 | 84.9 |
| Indigo Bunting | 38 | * -1.93 | NS | 11 | **-7.70 | NS | 43.2 | 12.2 | 21.8 | |||
| American Goldfinch | 52 | *-2.70 | ***0.46 | 41 | NS | NS | 40 | NS | NS | 189.1 | 28.3 | 56.9 |
a Mean captures per year used for timing of spring arrival in years with >10 captures.
b Mean captures per year used for timing of breeding initiation in years with >10 captures.
c Mean captures per year used for timing of juvenile appearance in years with >10 captures.
In warmer springs the interval between arrival and breeding shortened for six species (55%) and lengthened for none of the 11 species analyzed (Table 2). Year was nearly significant for one species; Red-eyed Vireos showed a longer delay in onset of breeding over time based on female breeding condition.
Of the of the 10 migrant species analyzed, the interval between breeding initiation and appearance of juveniles in warmer springs shortened for two species (Wood Thrush and American Redstart) while this interval lengthened for two species (House Wren and Common Yellowthroat; Table 2). No species showed a significant year effect.
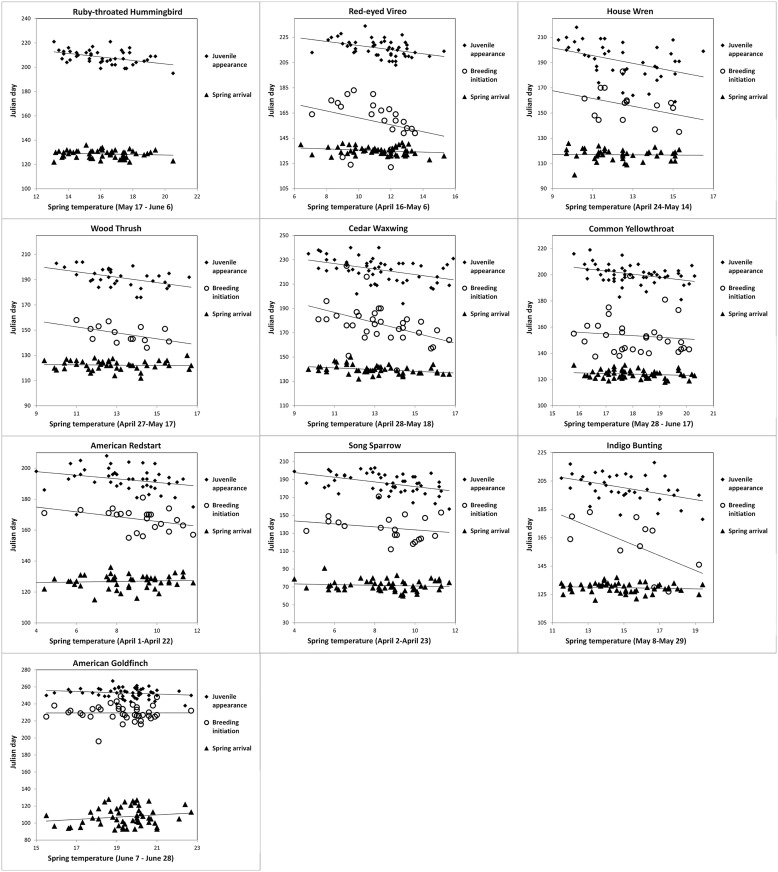
In general, the magnitude of change as a function of spring temperature was greater for these two within-period intervals (arrival-breeding and breeding-juvenile appearance) than the entire arrival to juvenile appearance period (Table 2). In most species the shortening of the overall interval with increasing temperatures can be attributed to advancing appearance of juveniles accompanied by a fairly steady or slightly advancing arrival on the breeding grounds (Fig 2). From our analyses there is evidence that rates of breeding initiation and juvenile appearance are changing similarly with temperature (i.e., comparable slopes), implying that onset of breeding occurs more quickly in warmer springs at the population level for many species we studied (Fig 2).
Fig 2. Phenology comparison for three events as a function of spring temperature for 10 migratory bird species.
The events are timing of juvenile appearance (Julian day at which 10% of young were captured), breeding initiation (Julian day at which 10% of females in breeding condition were captured), and spring arrival (Julian day at which 10% of spring adult migrants were captured). Mean spring temperature for each species was calculated from the three-week time period most correlated with the time interval between spring arrival and juvenile appearance each year (Table 1). Bird data were collected from 1961–2014 at a constant effort mist-netting station in Pennsylvania.
Effects of phenology on productivity
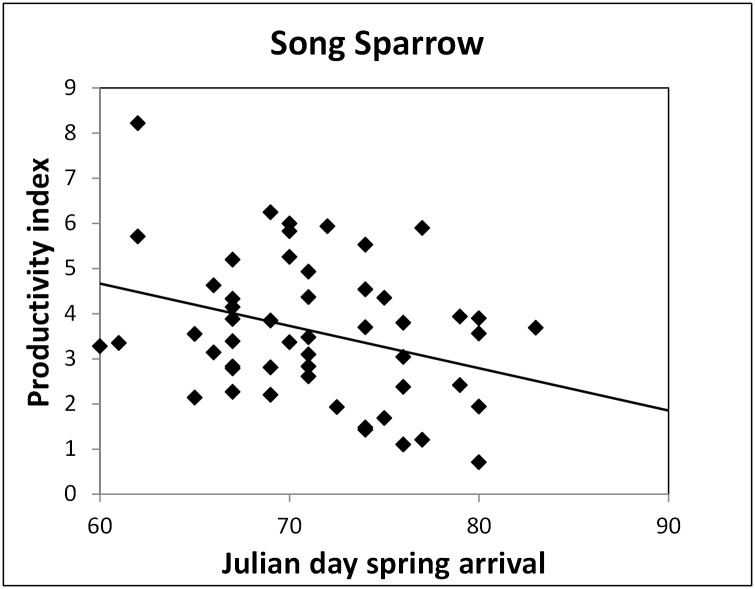
Timing of adult spring arrival and appearance of young were in general poor predictors of local bird productivity (Table 3). Timing of juvenile appearance was only associated with productivity for Hooded Warblers and American Redstarts; the parameter estimate was nonsignificant in all other species where it appeared in top models (Table 3). Later juvenile appearance was associated with higher productivity (capture rate of young) for both species. The slope for spring arrival timing was significant for two species for which it was in top weighted models (ΔAICc < 2). Of these, Song Sparrows produced more young in years with early arrival (Table 3; Fig 3), while Common Yellowthroats produced fewer young in years of early arrival.
Table 3. Candidate model weights (model probabilities) comparing 4 models for productivity index, when ΔAICc <2.
Model-averaged parameter estimates and unconditional standard errors (SE) are presented except when the 95% confidence interval overlapped zero (termed NS = non-significant). N = years with >10 captures.
| Species | N | Candidate Model Akaike weights | Parameter estimates (SE) | ||||
|---|---|---|---|---|---|---|---|
| Null | SA | JA | Global | Spring arrival | Juvenile appearance | ||
| Ruby-throated Hummingbird | 45 | 0.25 | 0.48 | . | . | NS | NS |
| Eastern Phoebe | 30 | 0.39 | 0.19 | 0.31 | . | NS | NS |
| Red-eyed Vireo | 49 | 0.51 | 0.19 | 0.21 | . | NS | NS |
| House Wren | 36 | 0.52 | . | 0.22 | . | NS | NS |
| Wood Thrush | 29 | 0.56 | . | . | . | NS | NS |
| American Robin | 27 | 0.56 | 0.22 | . | . | NS | NS |
| Gray Catbird | 53 | 0.5 | 0.21 | 0.22 | . | NS | NS |
| Cedar Waxwing | 50 | 0.31 | . | 0.44 | . | NS | NS |
| Ovenbird | 20 | 0.29 | 0.34 | 0.16 | 0.21 | NS | NS |
| Common Yellowthroat | 50 | . | 0.52 | . | 0.22 | 0.38 (0.18) | NS |
| Hooded Warbler | 31 | . | . | 0.59 | . | NS | 0.23 (0.10) |
| American Redstart | 41 | . | . | 0.63 | . | NS | 0.14 (0.06) |
| Yellow Warbler | 24 | 0.38 | . | 0.41 | . | NS | NS |
| Field Sparrow | 22 | 0.42 | . | 0.39 | . | NS | NS |
| Song Sparrow | 53 | . | 0.60 | . | 0.33 | -0.09 (0.03) | NS |
| Indigo Bunting | 38 | 0.44 | . | 0.30 | . | NS | NS |
| American Goldfinch | 52 | 0.27 | 0.26 | 0.32 | 0.15 | NS | NS |
SA = Model with spring arrival date (10% quantile). JA = Model with juvenile appearance date (10% quantile).
Fig 3. Productivity index, (juvenile capture rate)/(adult capture rate), as a function of adult spring arrival date (Julian day at which 10% of spring adult migrants were captured) for the Song Sparrow (N = 53 years).
This is the only species showing a significant increase in productivity with earlier arrival.
Discussion
Dissecting components of phenology allows us to better understand the relative importance of climate and carry-over effects of timing on reproductive performance. Individual phases within the avian lifecycle might be accelerated either due to a linkage with warmer temperatures or because preceding phenology was accelerated. For example, a species might respond to warmer temperatures by arriving earlier, but delay breeding a fixed number of days, and earlier fledging would entirely result from early arrival. Our results disentangle the interaction between climate, phenology and fitness for various species, and show that not all species respond to climatic shifts equally.
Arrival timing had little effect on timing of juvenile appearance, yet for most species, juveniles appeared earlier at PNR in warm springs (Fig 2). The earlier appearance of young may have resulted from either early onset of breeding or a shortened time interval between nesting to fledging. Warmer temperatures appeared to drive advanced onset of breeding for seven of the 17 species (Ruby-throated Hummingbird, Red-eyed Vireo, Wood Thrush, Gray Catbird, Cedar Waxwing, Common Yellowthroat, Indigo Bunting; Table 2). Only four species showed a link between early arrival and early breeding; Wood Thrushes, Gray Catbirds, Common Yellowthroats, and Yellow Warblers all advanced breeding by one day for every one day of earlier arrival. Changes in resource abundance along migratory routes may allow these mid-distance migrants to arrive earlier in warm springs, despite not having the same climate cues on their wintering grounds [53].
However, 12 of 17 species showed no relationship between early arrival and early breeding onset within a given year, consistent with the results of [54]. Incubation periods were shorter in warmer years in only two species (Breeding-Juvenile in Table 2; Wood Thrush and American Redstart). Seven species also showed advancing juvenile appearance across years, indicating some other force is driving early appearance of young that is neither strictly related to temperature nor arrival timing (1–8 days per decade: Wood Thrush, American Robin, Gray Catbird, Cedar Waxwing, Common Yellowthroat, Hooded Warbler, and Yellow Warbler). One prominent exception to the trend of early breeding lies with the American Goldfinch, a late summer breeder. We found the interval between arrival and juvenile appearance shortened in warmer springs with delayed arrival resulting in later breeding over time (see [28], for more detail).
The differences between the aforementioned species suggest that plasticity in breeding biology is partitioned and limited. Other studies showing plasticity in arrival and breeding phenology [2–5] have not dissected phenological components to the degree we have done here. For example, the decrease in time from arrival to juvenile appearance with rising temperature is significant in 12 of 17 species. Partitioning the period is informative: for arrival to breeding, seven of 14 species were significant, and for the breeding to juvenile appearance interval only two of 12 were significant. However, when advancement was significant within the partitions, the magnitude (i.e., number of days/°C) is greater than for the whole period considered together. Thus, even though both onset of breeding and time to fledging may contribute to a species’ phenological response, there is a tighter linkage with temperature and an individual component (either onset of breeding or time to fledging). The fact that 12 of 17 species here show no relationship between early arrival per se and early breeding onset implies that these species, when they arrive early, are not simply following a schedule, but apparently are responding to other climatic or environmental variables.
One remaining question is what information is available to long distance migrants that might accelerate their arrival time? It stands to reason that long-distance migrants are more likely to arrive on breeding grounds to find that the vegetative phenology is more advanced, with the migrants effectively arriving “late.” In hardwood forests such as those found at our site, the flexibility of many migratory species buffers them from resource timing mismatches; many long-distance migrants have a diverse prey base that is seasonally stable and have exhibited plastic responses in reproductive timing [41,55]. Breeding could advance at a greater rate than migration because breeding phenology is more sensitive to temperature for long-distance migrants [56]. We found that warmer temperature appears to drive advanced onset of breeding for seven species. (Red-eyed Vireo, Wood Thrush, Gray Catbird, Cedar Waxwing, Common Yellowthroat, American Redstart, Indigo Bunting, Table 2). Even short-distance migrants like House Wren and Cedar Waxwing bred much earlier in warmer springs in absence of strong shifts in arrival timing (Fig 2). This might suggest a linkage with earlier timing of spring leaf expansion, food abundance, or proximate cues such as temperature [8,9,20,27,57–61]. Thus, these migrants may be adjusting the onset of breeding based on plant phenology, which has shifted with temperature at a rate three times that of spring arrival timing [11], similar to the rate of reproductive advancement for many species [28].
Birds can modify the interval between laying and hatching by adjusting clutch size, varying onset of incubation, increasing the intensity of incubation to accelerate hatching, and investing differentially on eggs [62,63]. Total incubation time can be decreased to an extent under favorable conditions, but there are biological limits to the speed in which eggs hatch and nestlings fledge. Fledging times may be reduced in warmer years via an abundance of resources or reduced incubation costs [21,64–67]. Yet, we found little evidence for these effects. For long-distance migrants especially, it will not be sustainable to advance breeding faster than arrival with long-term warming. With a minimum 2–3.5°C increase in temperature predicted by the end of the current century (1), we expect the migratory species we studied to further advance arrival by >2–7 days [11] and breeding by >2–10 days [28]. In extremely warm springs (mean April/May temperatures of 15–20°C; Fig 2), some species may arrive too late to take advantage of optimal timing. For example, Red-eyed Vireos, Indigo Buntings, Cedar Waxwings and Wood Thrush might only need a 5°C increase before mismatches occur (Fig 2). Furthermore, despite strong patterns of advanced timing of breeding accompanying warmer seasons, there is no apparent fitness advantage to most species in our study. Of 17 species studied, only the Song Sparrow shows any increase in fitness associated with accelerated arrival (Fig 3, and below), indicating that the disruption of historical phenological patterns carries more risk than reward.
Effects of phenology on productivity
We found little evidence that timing of either arrival on breeding grounds or timing of breeding contributed to increased productivity at the population level. However, there was evidence that more young may be produced given shifts to earlier spring arrival for the multi-brooded Song Sparrow. Contrarily, Common Yellowthroats were more productive with later arrival, and American Redstarts and Hooded Warblers were more productive in years when juveniles appeared later.
Many studies have found that early arrival and breeding result in increased reproductive success in passerines when food availability allows, e.g., by raising multiple broods [55,68–71] or larger clutches [23,35,72,73]. Early arrival on breeding grounds has been associated with increased fledged young [40,74] including in Song Sparrows as we found [39] resulting in directional selection towards early arrival [27,35,36,75–78].
On the other hand, productivity or reproductive success may not be affected by timing of arrival or laying, as 13 of 17 species we studied demonstrated. Advanced laying dates may not impact productivity (i.e., a neutral effect; [79]), and some species lay smaller clutches earlier in the season [80]. If mismatches with timing of peak resource abundance increase with warming, it may be less effective to raise a second brood [81]. Other fitness components, such as nest success of individual pairs and adult survival (e.g., [82]) may be impacted by phenological shifts. Consequently, we hesitate to say that shifts in timing will not have fitness impacts in the species we studied. Rather, our results suggest that shifts in timing may have little effect on local productivity either because advancing phenology does not convey improved fitness or because our local breeding populations may track resource phenology by necessity to avoid mismatches and maintain consistent levels of productivity. In fact, changes in phenology were related to changes in productivity for only 24% of the species we studied, while prior research in the same study system demonstrated that temperature and precipitation explained changes in productivity for 57% and 75% of the species studied, respectively [28]. Given that nearly 3 out of 4 migratory birds in our study area advanced or delayed the window between arrival and breeding, changes in climate appear to be ultimate drivers of phenological shifts and productivity.
Conclusions
This study provides novel insight into linkages between phenological events in migratory birds uniquely capitalizing on five decades of bird banding data from a long-term monitoring program. We observed considerable phenological plasticity in most species studied, finding that even the farthest migrating species reproduced earlier in warm springs. Arrival timing, however, had no effect on breeding phenology or productivity for most species at PNR. Instead, breeding phenology advanced 1 to 6 days more quickly than arrival timing per 1°C increase, indicating that migratory birds are likely tracking resource phenology upon arrival. If migrants persistently show a weaker response to temperatures during migration than breeding, and the window between arrival and optimal breeding continues to shorten, biological constraints to plasticity may limit the ability of species to adapt successfully in the long run.
Acknowledgments
We are grateful to R. Leberman, R. Mulvihill, A. Leppold, A. Vitz, M. Shidel, and countless seasonal technicians and volunteers for collecting field data, and to M. Niedermeier for maintaining the database for many years. Thanks to J. Wenzel, A. Kautz, and J. Slyder for providing constructive comments on earlier drafts of the manuscript. Funding for this research was generously provided by the Colcom Foundation, Laurel Foundation, and numerous private donors who have supported the Powdermill Avian Research Center (PARC) since its inception. This manuscript is a contribution of the PARC, a long-term bird monitoring program located at Powdermill Nature Reserve, operated and supported by the Carnegie Museum of Natural History.
Data Availability
The minimal underlying data set used for analysis is available within Dryad. See: http://datadryad.org/reviewdoi=doi:10.5061/dryad.md712. Raw data may be made available by contacting Powdermill Avian Research Center at Carnegie Museum of Natural History. Contact Luke DeGroote at DegrooteL@carnegieMNH.org.
Funding Statement
This research was supported by grants from Colcom Foundation, Laurel Foundation, and private donors who have supported the Powdermill Avian Research Center since its inception. This manuscript is a contribution of the Powdermill Avian Research Center, a long-term bird monitoring program located at Powdermill Nature Reserve, operated and supported by the Carnegie Museum of Natural History. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pachauri RK, Allen MR, Barros VR, Broome J, Cramer W, Christ R, et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. 2014; http://epic.awi.de/37530/
- 2.Saino N, Szep T, Romano M, Rubolini D, Spina F, Møller AP. Ecological conditions during winter predict arrival date at the breeding quarters in a trans-Saharan migratory bird. Ecology letters. 2004;7: 21–25. [Google Scholar]
- 3.Dunn PO, Winkler DW, Møller AP, Fiedler W, Berthold P. Effects of climate change on timing of breeding and reproductive success in birds. Effects of climate change on birds. 2010; 113–128. [Google Scholar]
- 4.Studds CE, Marra PP. Rainfall-induced changes in food availability modify the spring departure programme of a migratory bird. Proceedings of the Royal Society of London B: Biological Sciences. 2011; rspb20110332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKellar AE, Marra PP, Hannon SJ, Studds CE, Ratcliffe LM. Winter rainfall predicts phenology in widely separated populations of a migrant songbird. Oecologia. 2013;172: 595–605. 10.1007/s00442-012-2520-8 [DOI] [PubMed] [Google Scholar]
- 6.Dawson A. Control of the annual cycle in birds: endocrine constraints and plasticity in response to ecological variability. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363: 1621–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visser ME, Holleman LJM, Caro SP. Temperature has a causal effect on avian timing of reproduction. Proceedings of the Royal Society B: Biological Sciences. 2009;276: 2323–2331. 10.1098/rspb.2009.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn PO, Winkler DW, Whittingham LA, Hannon SJ, Robertson RJ. A test of the mismatch hypothesis: How is timing of reproduction related to food abundance in an aerial insectivore? Ecology. 2011;92: 450–461. [DOI] [PubMed] [Google Scholar]
- 9.Schaper SV, Dawson A, Sharp PJ, Gienapp P, Caro SP, Visser ME. Increasing temperature, not mean temperature, is a cue for avian timing of reproduction. The American Naturalist. 2012;179: E55–E69. 10.1086/663675 [DOI] [PubMed] [Google Scholar]
- 10.Cotton PA. Avian migration phenology and global climate change. Proceedings of the National Academy of Sciences. 2003;100: 12219–12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marra PP, Francis CM, Mulvihill RS, Moore FR. The influence of climate on the timing and rate of spring bird migration. Oecologia. 2005;142: 307–315. 10.1007/s00442-004-1725-x [DOI] [PubMed] [Google Scholar]
- 12.Mills AM. Changes in the timing of spring and autumn migration in North American migrant passerines during a period of global warming. Ibis. 2005;147: 259–269. [Google Scholar]
- 13.Sparks TH, Bairlein F, Bojarinova JG, Hüppop O, Lehikoinen EA, Rainio K, et al. Examining the total arrival distribution of migratory birds. Global Change Biology. 2005;11: 22–30. [Google Scholar]
- 14.Gordo O. Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Climate Research. 2007;35: 37–58. [Google Scholar]
- 15.Lehikoinen E, Sparks TH. Changes in migration Effects of Climate Change on Birds. Oxford, UK: Oxford University Press; 2010. pp. 89–112. [Google Scholar]
- 16.Dunn P. Breeding Dates and Reproductive Performance Advances in Ecological Research. Elsevier; 2004. pp. 69–87. http://linkinghub.elsevier.com/retrieve/pii/S006525040435004X [Google Scholar]
- 17.Laaksonen T, Ahola M, Eeva T, Väisänen RA, Lehikoinen E. Climate change, migratory connectivity and changes in laying date and clutch size of the pied flycatcher. Oikos. 2006;114: 277–290. [Google Scholar]
- 18.Møller AP, Flensted-Jensen E, Mardal W. Rapidly advancing laying date in a seabird and the changing advantage of early reproduction. Journal of Animal Ecology. 2006;75: 657–665. 10.1111/j.1365-2656.2006.01086.x [DOI] [PubMed] [Google Scholar]
- 19.Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LE, Sheldon BC. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science. 2008;320: 800–803. 10.1126/science.1157174 [DOI] [PubMed] [Google Scholar]
- 20.Wiebe KL, Gerstmar H. Influence of Spring Temperatures and Individual Traits on Reproductive Timing and Success in a Migratory Woodpecker. The Auk. 2010;127: 917–925. [Google Scholar]
- 21.Matthysen E, Adriaensen F, Dhondt AA. Multiple responses to increasing spring temperatures in the breeding cycle of blue and great tits (Cyanistes caeruleus, Parus major). Global Change Biology. 2011;17: 1–16. [Google Scholar]
- 22.Shiao M-T, Chuang M-C, Yuan H-W, Wang Y. Effects of weather variation on the timing and success of breeding in two cavity-nesting species in a subtropical montane forest in Taiwan. The Auk. 2015;132: 671–684. [Google Scholar]
- 23.Winkel W, Hudde H. Long-term trends in reproductive traits of tits (Parus major, P. caeruleus) and pied flycatchers Ficedula hypoleuca. Journal of Avian Biology. 1997;28: 187–190. [Google Scholar]
- 24.McCleery RH, Perrins CM. Temperature and egg-laying trends. Nature. 1998;391: 30–31. [Google Scholar]
- 25.Visser ME, Van Noordwijk AJ, Tinbergen JM, Lessells CM. Warmer springs lead to mistimed reproduction in great tits (Parus major). Proceedings of the Royal Society of London B: Biological Sciences. 1998;265: 1867–1870. [Google Scholar]
- 26.Crick HQP, Sparks TH. Climate change related to egg-laying trends. Nature. 1999;399: 423–423. [Google Scholar]
- 27.Lany NK, Ayres MP, Stange EE, Sillett TS, Rodenhouse NL, Holmes RT. Breeding timed to maximize reproductive success for a migratory songbird: the importance of phenological asynchrony. Oikos. 2015; http://onlinelibrary.wiley.com/doi/10.1111/oik.02412/full [Google Scholar]
- 28.McDermott ME, DeGroote LW. Long-term climate impacts on breeding bird phenology in Pennsylvania, USA. Global Change Biology. 2016;22: 3304–3319. 10.1111/gcb.13363 [DOI] [PubMed] [Google Scholar]
- 29.Both C, Visser ME. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature. 2001;411: 296–298. 10.1038/35077063 [DOI] [PubMed] [Google Scholar]
- 30.Ahola M, Laaksonen T, Sippola K, Eeva T, Rainio K, Lehikoinen E. Variation in climate warming along the migration route uncouples arrival and breeding dates. Global Change Biology. 2004;10: 1610–1617. [Google Scholar]
- 31.Öberg M, Arlt D, Pärt T, Laugen AT, Eggers S, Low M. Rainfall during parental care reduces reproductive and survival components of fitness in a passerine bird. Ecology and Evolution. 2015;5: 345–356. 10.1002/ece3.1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Low M, Arlt D, Pärt T, Öberg M. Delayed timing of breeding as a cost of reproduction. Journal of Avian Biology. 2015;46: 325–331. [Google Scholar]
- 33.Brown CR, Brown MB. Breeding time in a migratory songbird is predicted by drought severity and group size. Ecology. 2014;95: 2736–2744. [Google Scholar]
- 34.Newton I. Weather-related mass-mortality events in migrants. Ibis. 2007;149: 453–467. [Google Scholar]
- 35.Smith RJ, Moore FR. Arrival timing and seasonal reproductive performance in a long-distance migratory landbird. Behavioral Ecology and Sociobiology. 2005;57: 231–239. [Google Scholar]
- 36.Gienapp P, Bregnballe T. Fitness consequences of timing of migration and breeding in cormorants. PLoS One. 2012;7: e46165 10.1371/journal.pone.0046165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forstmeier W. Benefits of early arrival at breeding grounds vary between males. Journal of Animal Ecology. 2002;71: 1–9. [Google Scholar]
- 38.Johansson J, Jonzén N. Effects of Territory Competition and Climate Change on Timing of Arrival to Breeding Grounds: A Game-Theory Approach. The American Naturalist. 2012;179: 463–474. 10.1086/664624 [DOI] [PubMed] [Google Scholar]
- 39.Wilson S, Arcese P. El Nino drives timing of breeding but not population growth in the song sparrow (Melospiza melodia). Proceedings of the National Academy of Sciences. 2003;100: 11139–11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velmala W, Helle S, Ahola MP, Klaassen M, Lehikoinen E, Rainio K, et al. Natural selection for earlier male arrival to breeding grounds through direct and indirect effects in a migratory songbird. Ecology and Evolution. 2015;5: 1205–1213. 10.1002/ece3.1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Townsend AK, Cooch EG, Sillett TS, Rodenhouse NL, Holmes RT, Webster MS. The interacting effects of food, spring temperature, and global climate cycles on population dynamics of a migratory songbird. Global Change Biology. 2015; http://onlinelibrary.wiley.com/doi/10.1111/gcb.13053/full [DOI] [PubMed] [Google Scholar]
- 42.Wilson AM, Brauning DW, Mulvihill RS, editors. Second atlas of breeding birds in Pennsylvania [Internet]. 2012. http://cupola.gettysburg.edu/esfac/11/ [Google Scholar]
- 43.Redfern CP. Brood-patch development and female body mass in passerines. Ringing & Migration. 2010;25: 33–41. [Google Scholar]
- 44.Moussus J-P, Julliard R, Jiguet F. Featuring 10 phenological estimators using simulated data. Methods in Ecology and Evolution. 2010;1: 140–150. [Google Scholar]
- 45.DeSante DF, Geupel GR. Landbird Productivity in Central Coastal California: The Relationship to Annual Rainfall, and a Reproductive Failure in 1986. The Condor. 1987;89: 636. [Google Scholar]
- 46.Desante DF, Burton KM, Saracco JF, Walker BL. Productivity indices and survival rate estimates from MAPS, a continent-wide programme of constant-effort mist-netting in North America. Journal of Applied Statistics. 1995;22: 935–948. [Google Scholar]
- 47.Nur N, Geupel GR, Ballard G. The use of constant-effort mist-netting to monitor demographic processes in passerine birds: annual variation in survival, productivity, and floaters. 3rd Partners in Flight workshop, US Department of Agriculture, Forest Service, Rocky Mountain Research Center, Ogden, UT. 2000. pp. 185–194. http://birds.cornell.edu/pifcapemay/nur.htm
- 48.Williams TD, Bourgeon S, Cornell A, Ferguson L, Fowler M, Fronstin RB, et al. Mid-winter temperatures, not spring temperatures, predict breeding phenology in the European starling Sturnus vulgaris. Open Science. 2015;2: 140301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pearce-Higgins JW, Eglington SM, Martay B, Chamberlain DE. Drivers of climate change impacts on bird communities. J Anim Ecol. 2015;84: 943–954. 10.1111/1365-2656.12364 [DOI] [PubMed] [Google Scholar]
- 50.Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach [Internet]. Springer; 2002. http://books.google.com/books?hl=en&lr=&id=fT1Iu-h6E-oC&oi=fnd&pg=PR7&dq=Burnham+and+Anderson+2002&ots=tdAq65ZIo2&sig=Pm12l9Eo_L_lPFWidM6Dt6n_naM [Google Scholar]
- 51.R Core Team. R: A language and environment for statistical computing. [Internet]. R Foundation for Statistical Computing, Vienna, Austria; 2015. https://www.R-project.org/. [Google Scholar]
- 52.Mazerolle. AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). [Internet]. 2013. http://CRAN.R-project.org/package=AICcmodavg
- 53.Cohen EB, Németh Z, Zenzal TJ Jr, Paxton KL, Diehl R, Paxton EH, et al. Spring resource phenology and timing of songbird migration across the Gulf of Mexico. Phenological Synchrony and Bird Migration: Changing Climate and Seasonal Resources in North America. 2015;47: 63. [Google Scholar]
- 54.Dunn PO, Møller AP. Changes in breeding phenology and population size of birds. Griffith S, editor. Journal of Animal Ecology. 2014;83: 729–739. 10.1111/1365-2656.12162 [DOI] [PubMed] [Google Scholar]
- 55.Townsend AK, Sillett TS, Lany NK, Kaiser SA, Rodenhouse NL, Webster MS, et al. Warm Springs, Early Lay Dates, and Double Brooding in a North American Migratory Songbird, the Black-Throated Blue Warbler. Festa-Bianchet M, editor. PLoS ONE. 2013;8: e59467 10.1371/journal.pone.0059467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pearce-Higgins JW, Green RE. Birds and climate change: impacts and conservation responses [Internet]. Cambridge University Press; 2014. https://books.google.com/books?hl=en&lr=&id=ySOjAwAAQBAJ&oi=fnd&pg=PA25&dq=Birds+and+Climate+Change:+Impacts+and+Conservation+Responses&ots=M3TS9tTAJl&sig=920YxL5TQzfr-FCP9BZAhFRfKFo [Google Scholar]
- 57.Both C, Piersma T, Roodbergen SP. Climatic change explains much of the 20th century advance in laying date of Northern Lapwing Vanellus vanellus in The Netherlands. Ardea. 2005;93: 79–88. [Google Scholar]
- 58.Schaefer T, Ledebur G, Beier J, Leisler B. Reproductive responses of two related coexisting songbird species to environmental changes: global warming, competition, and population sizes. Journal of Ornithology. 2006;147: 47–56. [Google Scholar]
- 59.Halupka L, Dyrcz A, Borowiec M. Climate change affects breeding of reed warblers Acrocephalus scirpaceus. Journal of Avian Biology. 2008;39: 95–100. [Google Scholar]
- 60.Bourgault P, Thomas D, Perret P, Blondel J. Spring vegetation phenology is a robust predictor of breeding date across broad landscapes: a multi-site approach using the Corsican blue tit (Cyanistes caeruleus). Oecologia. 2010;162: 885–892. 10.1007/s00442-009-1545-0 [DOI] [PubMed] [Google Scholar]
- 61.Borgman CC, Wolf BO. The indirect effects of climate variability on the reproductive dynamics and productivity of an avian predator in the arid Southwest. Oecologia. 2016;180: 279–291. 10.1007/s00442-015-3456-6 [DOI] [PubMed] [Google Scholar]
- 62.Haftorn S. Incubating female passerines do not let the egg temperature fall below the’physiological zero temperature’during their absences from the nest. Ornis Scandinavica. 1988; 97–110. [Google Scholar]
- 63.Tomás G. Hatching date vs laying date: what should we look at to study avian optimal timing of reproduction? Journal of Avian Biology. 2015;46: 107–112. [Google Scholar]
- 64.Cresswell W, Mccleery R. How great tits maintain synchronization of their hatch date with food supply in response to long-term variability in temperature. Journal of Animal Ecology. 2003;72: 356–366. [Google Scholar]
- 65.Both C, Visser ME. The effect of climate change on the correlation between avian life-history traits. Global Change Biology. 2005;11: 1606–1613. [Google Scholar]
- 66.Vedder O. Individual birds advance offspring hatching in response to increased temperature after the start of laying. Oecologia. 2012;170: 619–628. 10.1007/s00442-012-2335-7 [DOI] [PubMed] [Google Scholar]
- 67.Irons R. Changes in Tree Swallow Phenology Due to Climate Change in Alaska. 2015; http://scholar.colorado.edu/honr_theses/793/
- 68.Arcese P, Smith JN. Effects of population density and supplemental food on reproduction in song sparrows. The Journal of Animal Ecology. 1988; 119–136. [Google Scholar]
- 69.Nagy LR, Holmes RT. Food limits annual fecundity of a migratory songbird: an experimental study. Ecology. 2005;86: 675–681. [Google Scholar]
- 70.Bulluck L, Huber S, Viverette C, Blem C. Age-specific responses to spring temperature in a migratory songbird: older females attempt more broods in warmer springs. Ecology and Evolution. 2013; n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seward AM, Beale CM, Gilbert L, Jones TH, Thomas RJ. The Impact of Increased Food Availability on Reproduction in a Long-Distance Migratory Songbird: Implications for Environmental Change? Chapman M (Gee) G, editor. PLoS ONE. 2014;9: e111180 10.1371/journal.pone.0111180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Järvinen A. Clutch-size variation in the Pied Flycatcher Ficedula hypoleuca. Ibis. 1989;131: 572–577. [Google Scholar]
- 73.Møller AP. North Atlantic Oscillation (NAO) effects of climate on the relative importance of first and second clutches in a migratory passerine bird. Journal of Animal Ecology. 2002;71: 201–210. [Google Scholar]
- 74.Tarka M, Hansson B, Hasselquist D. Selection and evolutionary potential of spring arrival phenology in males and females of a migratory songbird. Journal of Evolutionary Biology. 2015;28: 1024–1038. 10.1111/jeb.12638 [DOI] [PubMed] [Google Scholar]
- 75.Møller AP. Phenotype-dependent arrival time and its consequences in a migratory bird. Behavioral Ecology and Sociobiology. 1994;35: 115–122. [Google Scholar]
- 76.Lozano GA, Perreault S, Lemon RE. Age, arrival date and reproductive success of male American redstarts Setophaga ruticilla. Journal of Avian Biology. 1996; 164–170. [Google Scholar]
- 77.Hötker H. Arrival of pied avocets Recurvirostra avosetta at the breeding site: effects of winter quarters and consequences for reproductive success. Ardea. 2003;90: 379–387. [Google Scholar]
- 78.Doxa A, Robert A, Crivelli A, Catsadorakis G, Naziridis T, Nikolaou H, et al. Shifts in breeding phenology as a response to population size and climatic change: a comparison between short-and long-distance migrant species. The Auk. 2012;129: 753–762. [Google Scholar]
- 79.Morrison CA, Robinson RA, Clark JA, Leech DI, Gill JA. Season-long consequences of shifts in timing of breeding for productivity in Willow Warblers, Phylloscopus trochilus. Bird Study. 2015;62: 161–169. [Google Scholar]
- 80.Sanz JJ. Large-scale effect of climate change on breeding parameters of pied flycatchers in Western Europe. Ecography. 2003;26: 45–50. [Google Scholar]
- 81.Husby A, Kruuk LEB, Visser ME. Decline in the frequency and benefits of multiple brooding in great tits as a consequence of a changing environment. Proceedings of the Royal Society B: Biological Sciences. 2009;276: 1845–1854. 10.1098/rspb.2008.1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dybala KE, Eadie JM, Gardali T, Seavy NE, Herzog MP. Projecting demographic responses to climate change: adult and juvenile survival respond differently to direct and indirect effects of weather in a passerine population. Global Change Biology. 2013;19: 2688–2697. 10.1111/gcb.12228 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The minimal underlying data set used for analysis is available within Dryad. See: http://datadryad.org/reviewdoi=doi:10.5061/dryad.md712. Raw data may be made available by contacting Powdermill Avian Research Center at Carnegie Museum of Natural History. Contact Luke DeGroote at DegrooteL@carnegieMNH.org.