Abstract
Rad6 and Bre1, ubiquitin-conjugating E2 and E3 enzymes respectively, are responsible for histone H2B lysine 123 mono-ubiquitination (H2Bub1) in Saccharomyces cerevisiae. Previous studies have shown that Rad6 and Bre1 regulate telomere length and recombination. However, the underlying molecular mechanism remains largely unknown. Here we report that H2BK123 mutation results in telomere shortening, while inactivation of Ubp8 and/or Ubp10, deubiquitinases of H2Bub1, leads to telomere lengthening in Rad6–Bre1-dependent manner. In telomerase-deficient cells, inactivation of Rad6–Bre1 pathway retards telomere shortening rate and the onset of senescence, while deletion of UBP8 and/or UBP10 accelerates senescence. Thus, Rad6–Bre1 pathway regulates both telomere length and recombination through its role in H2Bub1. Additionally, inactivation of both Rad6–Bre1–H2Bub1 and Mre11–Rad50–Xrs2 (MRX) pathways causes synthetic growth defects and telomere shortening in telomerase-proficient cells, and significantly accelerates senescence and eliminates type II telomere recombination in telomerase-deficient cells. Furthermore, RAD6 or BRE1 deletion, or H2BK123R mutation decreases the accumulation of ssDNA at telomere ends. These results support the model that Rad6–Bre1–H2Bub1 cooperates with MRX to promote telomere-end resection and thus positively regulates both telomerase- and recombination-dependent telomere replication. This study provides a mechanistic link between histone H2B ubiquitination and telomere replication.
INTRODUCTION
Telomeres are specialized DNA–protein structures at the end of eukaryotic linear chromosomes. The telomere structure is essential for the maintenance of genome integrity and stability (1–3). In the budding yeast Saccharomyces cerevisiae, telomeric DNA is composed of ∼300 ± 75 bps of TG1–3/C1–3A repeats and a protruding single-stranded 3΄ G-overhang (or G-tail) (4,5). Telomeric DNA is usually elongated by telomerase, a specialized reverse transcriptase that consists of at least four subunits: a catalytic subunit Est2, an RNA template TLC1 and two accessory subunits Est1 and Est3 (6,7). Cdc13 is a telomeric single-stranded DNA binding protein, and its interaction with Est1 helps to recruit telomerase to telomere ends (8,9). Thus it is generally believed that the single-stranded G-overhang is essential for telomerase action (10). Tel1 kinase interacts with the C-terminus of Xrs2 subunit of Mre11–Rad50–Xrs2 (MRX) (11), and they function in the same pathway to positively regulate telomere length (12). Both Tel1 and MRX complex preferentially associate with short telomeres (13,14), which is thought to lengthen telomeres by promoting telomerase recruitment via the Cdc13–Est1 interaction (13,15). However, further study found that telomerase recruitment is independent of Tel1-mediated phosphorylation of Cdc13 (16). In a de novo telomere addition assay, MRX complex is required for C-strand resection and plays a critical role in generation of 3΄ G-overhang for the loading of Cdc13 (10,17). In addition, Tel1 regulates telomere-end resection by promoting MRX's resection activity (18,19). Furthermore, both MRX complex and Tel1 have been shown to be essential for the generation of proper constitutive G-overhangs at native telomeres (19,20). Therefore, it has been proposed that MRX complex and Tel1 are involved in the generation of a 3΄ ssDNA at the end of a telomere, an optimal substrate for telomerase action (16). In support of this model, the Tel1–hy909 mutant with increased telomeric ssDNA displays telomerase-dependent telomere over-elongation (19). Reversely, Rif2, a Rap1-interacting factor at double-stranded telomeric DNA, competes with Tel1 for the binding to MRX and thus inhibits MRX's resection activity at telomere ends (18,19,21), accounting for negative role of Rif2 in telomere length regulation (18,22).
Telomeric DNA can also be maintained by homologous recombination (HR) in telomerase-deficient yeast cells (23,24). In the absence of telomerase, yeast cells usually experience gradual telomere attrition and cellular senescence (25). A very small portion of cells can overcome the crisis by repairing their telomeres through Rad52-dependent HR, and these cells are termed survivors (23). The survivors can be categorized into type I and type II according to their telomeric DNA arrangements and growth characteristics (26). The type I survivors have highly amplified subtelomeric Y΄ elements separated by short tracts of TG1–3 repeats; while type II survivors exhibit long heterogeneous terminal TG1–3 sequence (26). Type I survivors occur more frequently on solid medium; type II survivors grow faster than type I survivors and dominate the culture in liquid medium. The generation of type I and type II survivors appears to have different genetic requirements. For examples, Rad51, Rad54, Rad55 and Rad57 are specifically required for generating type I survivors; while MRX complex, Rad59, Sgs1, Sae2, Exo1, Top3 and Sua5 are required for the formation of type II survivors (27–33). In addition, Rif1/2 proteins, especially Rif2, delay the onset of senescence and inhibit type II survivors (34–36). Recently, we screened telomere-length-maintenance genes and identified novel regulators of telomere recombination, such as Rad6–Bre1 ubiquitination enzymes, KEOPS complex, INO80 chromatin remodeling complex and Pif1 helicase (36). The mechanisms by which these factors regulate telomere recombination in survivors remain to be elucidated.
Rad6 encodes an E2 ubiquitin-conjugating enzyme in S. cerevisiae, and it interacts with various E3 ubiquitin ligases to affect different DNA repair pathways (37). Rad6–Bre1 pathway is responsible for mono-ubiquitination of lysine 123 of histone H2B (H2Bub1) (38). H2Bub1 is essential for methylation of lysines 4 and 79 of histone H3, contributing to transcriptional regulation (39,40). In mammalian cells, RNF20-dependent H2BK120 mono-ubiquitination affects DNA-end resection and regulates HR repair through chromatin reorganization (41). Recently, it is shown that CRL4Wdr70 mediated H2BK119 mono-ubiquitination facilitates Exo1-dependent resection and thus influences HR and genome stability in Schizosaccharomyces pombe (42). Several genome-wide studies have demonstrated that Rad6–Bre1 pathway participates in both telomerase- and recombination-dependent telomere replication in S. cerevisiae (36,43). However, it remains unclear whether or not the regulation of Rad6–Bre1 pathway on telomere replication depends on its downstream H2Bub1.
In the current study, we have investigated the functions of Rad6–Bre1–H2Bub1 pathway on both telomerase- and recombination-dependent telomere replication. Our results indicate that Rad6–Bre1–H2Bub1 cooperates with MRX in promoting telomere-end resection to regulate telomere replication.
MATERIALS AND METHODS
Yeast strains, plasmids and molecular manipulations
Yeast strains used in this study were mostly derived from BY4743 as listed in Supplementary Table S1. The plasmids used for gene knockout experiments were derived from pRS303, pRS305, pRS306 as described elsewhere (44).
Gene knockout experiments in yeast were performed using standard genetic procedures as described previously (44). Briefly, two fragments (∼500 bp in length) located immediately upstream and downstream of the target gene were amplified from the genomic DNA, and the products were digested with appropriate restriction enzymes and cloned into the pRS plasmid. The resulting plasmid was linearized and transformed into BY4743 to knock out the target gene by using one-step gene-replacement method. Following confirmation by polymerase chain reaction (PCR) analysis, the diploid strain heterozygous for the target gene(s) was sporulated and then tetrads were dissected.
PCR-based site-directed mutagenesis was used to generate H3K4A, H3K79A and H3K4AK79A mutations. The parental yeast strain was derived from YPH499. The genomic copies of H3-H4 genes were deleted, and the plasmid-derived H3-H4, H3K4A-H4, H3K79A-H4 and H3K4AK79A-H4 was introduced to maintain cell viability, respectively.
Single-colony re-streaking assay
For determination of telomere length, cells directly from spores were re-streaked three to five successive times on rich growth medium (YPD) plates. In particular, spore colonies were streaked onto YPD plates. After the emergency of single colonies (normally 2 days at 30°C), a single colony was re-streaked on YPD plates. This procedure was repeated three to five times every 2 days. For the mutants that have slow growth phenotypes, the incubation time between re-streaks was 3 to 4 days to ensure that they had undergone similar population doublings.
Assay of kinetics of senescence/survival
Assay of kinetics of senescence/survival was performed as described previously (27). In liquid assays, randomly selected colonies directly from dissected spores were inoculated into 5 ml of YPD medium and grown to saturation at 30°C. Then, cultures were diluted with fresh medium to a density of OD600 = 0.01–0.02 every 24 h (48 h for slow growing mutants). This procedure was repeated for at least 12 times. Each experiment was repeated at least twice, each in triplicate (three independent colonies). In streak assays, a single colony was re-streaked on fresh YPD plates until the occurrence of senescence and the emergency of survivors.
Telomere blot
Telomere blot was performed as described previously (36). Briefly, cells were harvested either from liquid cultures or from re-streaking plates. Genomic DNA was purified from these cells by the phenol/chloroform method, digested with XhoI or PstI, and fractionated by electrophoresis on 1.0% agarose gel. After transferring to a Hybond-N+ Nylon membrane (GE Healthcare), probe labeling, hybridization and immunological detection were performed using DIG-High Prime DNA Labeling and the Detection Starter Kit II (Roche, USA). A TG1–3 DNA fragment was chosen as a telomere-specific probe.
Telomere PCR
Telomere PCR was performed as described previously (45,46). Telomere ends of chromosome 1-L were amplified using primers o286S and G18 (47) (Supplementary Table S2). PCR products were cloned to pMD18-T vector (Takara, Japan) and then subjected to sequencing.
Determination of single-stranded DNA
Single colonies were inoculated into 3 ml of YPD and cultured overnight at 30°C. The cultures were then diluted to OD600 = 0.01 and grown to exponential phase (OD600 = 1.5–2.0) at 30°C. For yku70Δ cells, the cultures in exponential phase were placed at 37°C for additional 5 h and cultures were diluted to allow exponential growth when required. Genomic DNA was prepared using a Qiagen-based method as described for DNA preparation for 2D gel analysis (48). Single-stranded DNA (ssDNA) was measured using QAOS (Quantitative amplification of ssDNA) as described previously (49,50). Before QAOS assay, one copy of DNA samples was denatured to obtain total telomeric ssDNA. The amount of ssDNA was normalized by that of total ssDNA. The sequences of primers for QAOS were shown in Supplementary Table S2.
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed as described previously (51). Mre11 tagged with 13Myc epitope was introduced as described (52). Immunoprecipitation of cross-linked DNA was done with anti-Myc and protein G Sepharose beads (GE healthcare). The primers targeting VI-R telomere and ARO1 (a gene far from a telomere) was used as described (13) (Supplementary Table S2).
RESULTS
Rad6–Bre1–H2Bub1 positively regulates telomere length in telomerase-proficient cells
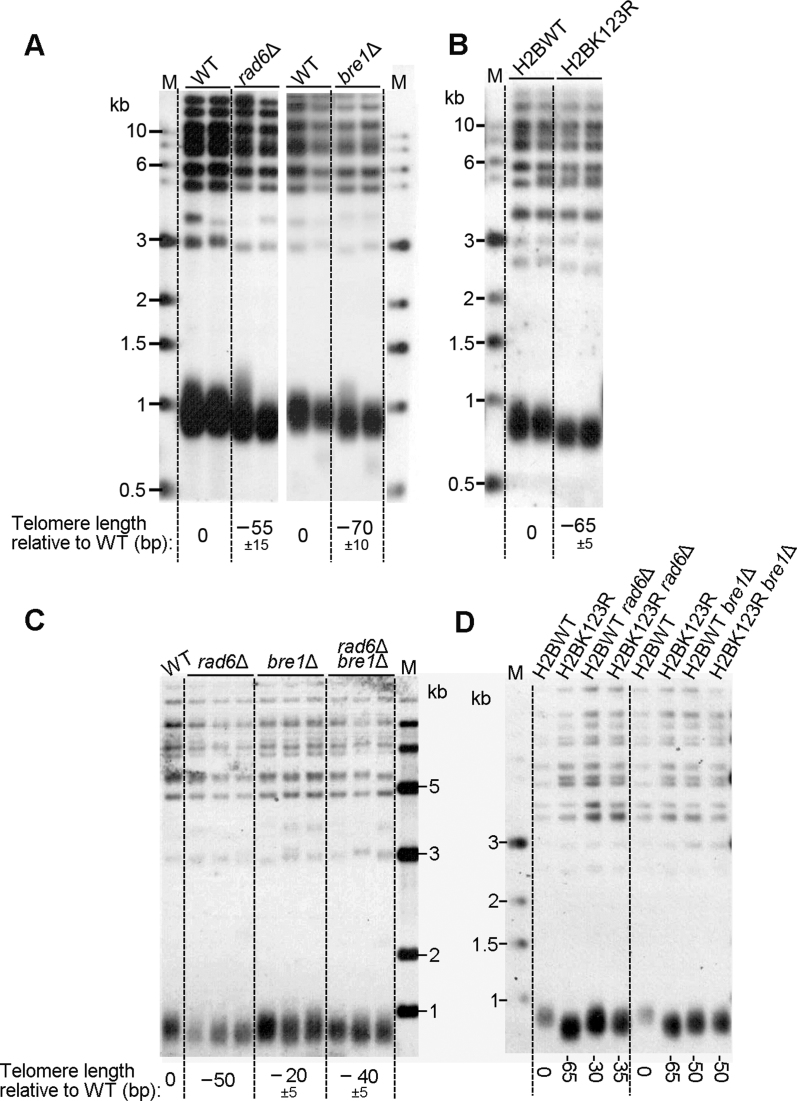
Previous genome-wide study has shown that deletion of either RAD6 or BRE1 leads to shortened telomeres (43); however, how Rad6–Bre1 pathway regulates telomeres remains unclear. In order to validate the role of Rad6–Bre1 pathway on telomere-length regulation, we generated rad6Δ and bre1Δ haploid strains by sporulating a RAD6/rad6Δ and BRE1/bre1Δ diploid strain, respectively. The genomic DNAs of the rad6Δ, bre1Δ and their isogenic strains were isolated for Southern blot analysis using a telomeric TG1–3 probe. Consistent with previous report (43), both the rad6Δ and bre1Δ cells had slightly shorter telomeres (∼60 bp shorter) than the wild-type cells (Figure 1A). Since Rad6–Bre1 catalyzes mono-ubiquitination on histone H2BK123 (38), we asked whether H2Bub1 also participates in telomere length regulation. We firstly examined the telomere length of YZS277 strain, an H2Bub1-deficient mutant (H2BK123R) described previously (39). Interestingly, the YZS277 mutant exhibited shorter telomeres (∼65 bp shorter) than the YZS276 wild-type (Figure 1B). In order to validate this observation, we constructed a diploid strain that was heterozygous for both hht1Δ and hht2Δ, and contained H2BK123R or H2BK123A mutation. Through sporulation and tetrad dissection, we obtained haploid cells that only contained H2BK123R or H2BK123A mutant allele of H2B. Southern blot analysis showed that cells carrying either H2BK123R or H2BK123A mutation had ∼60 bp shorter telomeres than cells containing wild-type H2B gene (H2BWT) (Supplementary Figure S1). To further test whether Rad6, Bre1 and H2Bub1 act in the same pathway in telomere length regulation, we performed epistasis analysis, comparing telomere length of double mutants (rad6Δ bre1Δ, rad6Δ H2BK123R and bre1Δ H2BK123R) with that of single mutants. As shown in Figure 1C and D, either rad6Δ bre1Δ, rad6Δ H2BK123R or bre1Δ H2BK123R did not exhibit further telomere shortening compared to single mutants. This result indicated that Rad6, Bre1 and H2Bub1 function in a single pathway to positively regulate telomere length; i.e. Rad6–Bre1 positively regulates telomere length through its role in H2Bub1. In addition to Bre1–H2Bub1 pathway, Rad6 functions in DNA repair through Rad18-mediated PCNA ubiquitination and Ubr1-mediated protein degradation (37). Unlike bre1Δ or H2BK123R, either rad18Δ, ubr1Δ or PCNA-K164R mutants displayed no effect on telomere length (Supplementary Figure S2).
Figure 1.
Mutation of H2BK123 results in shortened telomeres. (A) RAD6/rad6Δ and BRE1/bre1Δ diploid strains were respectively sporulated, and tetrads were dissected. The haploid strains were successively re-streaked for three times on YPD plates to equilibrate telomere length. Genomic DNA was extracted from each strain, digested with PstI and subjected to Southern blot analysis with a telomere-specific TG1–3 probe. For each strain, the telomere lengths were quantified for 4 spore colonies from two independent tetrads, and the ‘±’ signs indicated standard deviation. (B) Southern blot analysis of telomere length of YZS277 strain, an H2Bub-deficient mutant (H2BK123R) reported previously. (C and D) Epistasis analysis of effects of RAD6- and BRE1-deletion, and H2BK123 mutation on telomere length regulation. RAD6/rad6Δ BRE1/bre1Δ diploid strain was sporulated, and tetrads were dissected (C). The rad6Δ and bre1Δ mutants were constructed in H2B wild-type (YZS276) and H2BK123R mutant (YZS277) strains, respectively (D). Southern blot analysis of telomere length was performed as in A.
In S. cerevisiae, H2Bub1 is required for both histone H3K4 and H3K79 methylation (39,40). To test whether the function of H2Bub1 on telomere replication involves its downstream H3K4 and K79 methylation, we examined the telomere length in cells with H3K4A and/or H3K79A mutations. As shown in Supplementary Figure S3, compared to H3WT, H3K79A mutation caused little change on telomere length; while H3K4A mutation alone or in combination with H3K79A mutation resulted in ∼20 bp shorter telomeres. The H3K4A-induced telomere shortening echoed a previous study showing that loss of Set1, the methyltransferase responsible for histone H3K4 methylation, led to slightly shorter telomeres (53). The extent of telomere shortening in either H3K4A or set1Δ mutants was not as significant as that observed in H2BK123R mutant (Figure 1B and D), suggesting that the function of H2Bub1 on telomere-length regulation might not depend on its downstream H3 methylation.
Rad6–Bre1 pathway accelerates telomere shortening and senescence in telomerase-deficient cells
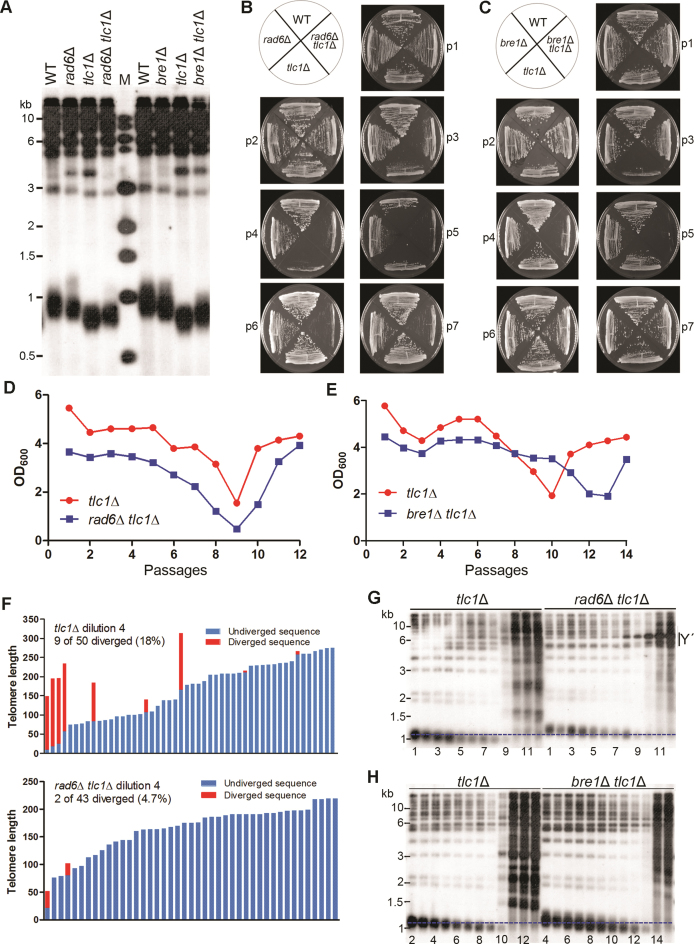
To determine whether Rad6–Bre1 regulates telomere length through telomerase pathway, we generated TLC1/tlc1Δ RAD6/rad6Δ and TLC1/tlc1Δ BRE1/bre1Δ diploid strains, and obtained isogenic haploid strains of different genotypes through tetrad dissections. If Rad6–Bre1 pathway functions independently of telomerase pathway, deletion of RAD6 or BRE1 is expected to further shorten telomere length and accelerate the senescence in tlc1Δ cells. Strikingly, telomeres in both rad6Δ tlc1Δ and bre1Δ tlc1Δ cells were longer than those in tlc1Δ cells (Figure 2A), indicating that RAD6 or BRE1 deletion retarded telomere shortening in telomerase-deficient cells.
Figure 2.
Deletion of RAD6 or BRE1 decreases telomere shortening rate and delays senescence and survivor formation in telomerase-deficient cells. (A) Southern blot analysis of telomere lengths of rad6Δ tlc1Δ and bre1Δ tlc1Δ cells. The spore colonies were directly streaked on YPD plates, and their genomic DNAs were extracted, digested with PstI and subjected to Southern blot analysis. (B and C) Senescence analysis of rad6Δ tlc1Δ (B) and bre1Δ tlc1Δ (C) cells on YPD plate. The isogenic spores were successively re-streaked for seven times (P1–P7) on YPD plates until survivors arose. (D and E) Cell viability assay of rad6Δ tlc1Δ (D) and bre1Δ tlc1Δ (E) in liquid medium. The isogenic spores from a single tetrad were directly inoculated in liquid culture for cell viability assays. (F) Telomere I-L sequencing in tlc1Δ and rad6Δ tlc1Δ cells. Telomere I-L was cloned and sequenced from the fourth passage of the tlc1Δ and rad6Δ tlc1Δ cells, respectively. The tlc1Δ and rad6Δ tlc1Δ haploids were obtained from the same tetrad. (G and H) Telomere Southern blot analysis of senescing and survival cells cultured in liquid medium. Their genomic DNAs were extracted from liquid cultures (D and E), digested with XhoI and subjected to Southern blot analysis.
Next we examined the effect of Rad6–Bre1 pathway on senescence/survival in tlc1Δ cells. The tlc1Δ, rad6Δ tlc1Δ and bre1Δ tlc1Δ cells directly from dissected spores were successively re-streaked on plates, and their growths were documented. The tlc1Δ and rad6Δ tlc1Δ cells senesced at the third passage, and reached crisis at the fourth and fifth passages, respectively (Figure 2B). The delayed onset of senescence was more significant in the bre1Δ tlc1Δ cells: the bre1Δ tlc1Δ cells largely lost viability at the fifth passage, compared to the third passage in the tlc1Δ cells (Figure 2C). These results indicated that deletion of RAD6 or BRE1 delayed the onset of senescence in telomerase-deficient cells. Notably, the recovery of the rad6Δ tlc1Δ cells was much slower than its corresponding tlc1Δ sibling mutants (Figure 2B), suggesting that the rad6Δ tlc1Δ cells encountered more difficulties in generating survivors.
The kinetics of senescence and survival of the rad6Δ tlc1Δ and bre1Δ tlc1Δ cells were also measured in liquid assays. As shown in Figure 2D, the tlc1Δ and rad6Δ tlc1Δ cells reached the peak of crisis at the same passage (the ninth passage). The growth nadir of the bre1Δ tlc1Δ cells was seen at the thirteenth passage, significantly later than that of the tlc1Δ cells (the tenth passage) (Figure 2E). The delay of senescence in bre1Δ tlc1Δ mutants was also observed in two additional independent clones from different tetrads (Supplementary Figure S4). We were puzzled why the rad6Δ tlc1Δ cells did not display a delay of senescence when cultured in liquid medium. It could be attributed to the reduced efficiency of Bre1-independent recombination in the absence of Rad6, since Rad6 is involved in different repair pathways through interaction with various E3 ubiquitin ligases (37). The defect in recombination accelerated the senescence, which counteracted the bre1Δ-induced delay of senescence. Indeed, deletion of RAD18 accelerated the onset of senescence in telomerase-deficient cells (Supplementary Figure S5). This hypothesis was further supported by telomere PCR-sequencing results. In the tlc1Δ cells, 18% telomeres were elongated, while in the rad6Δ tlc1Δ cells only 4.3% telomeres were elongated through recombination (Figure 2F). Therefore we concluded that inactivation of Rad6 downstream Bre1 pathway retarded the telomere shortening rate and the onset of senescence.
Telomere patterns in successive liquid cultures were determined by Southern blot analysis. Gradually shortening of telomeres was detected during liquid passages, and the shortening rate in both the rad6Δ tlc1Δ and bre1Δ tlc1Δ cells appeared to be significantly lower than that in the tlc1Δ cells (Figure 2G and H). In the tlc1Δ cells, type II telomere recombination took place at the ninth or tenth passage (Figure 2G and H), when the vast majority of cells were senescing (Figure 2D and E). In the rad6Δ tlc1Δ cells, Y΄ recombination occurred at the eighth passage, indicative of generation of type I survivors (Figure 2G). Notably, in the bre1Δ tlc1Δ cells, type II telomere recombination occurred at the fourteenth passage (Figure 2H). These results revealed that the senescence delay of the bre1Δ tlc1Δ cells was likely attributed to the lower rate of telomere shortening.
Taken together, these findings indicate that Rad6–Bre1 pathway promotes telomere shortening and accelerates senescence in telomerase-deficient cells.
Deubiquitinases Ubp8 and Ubp10 antagonize the function of Rad6–Bre1 pathway at telomeres
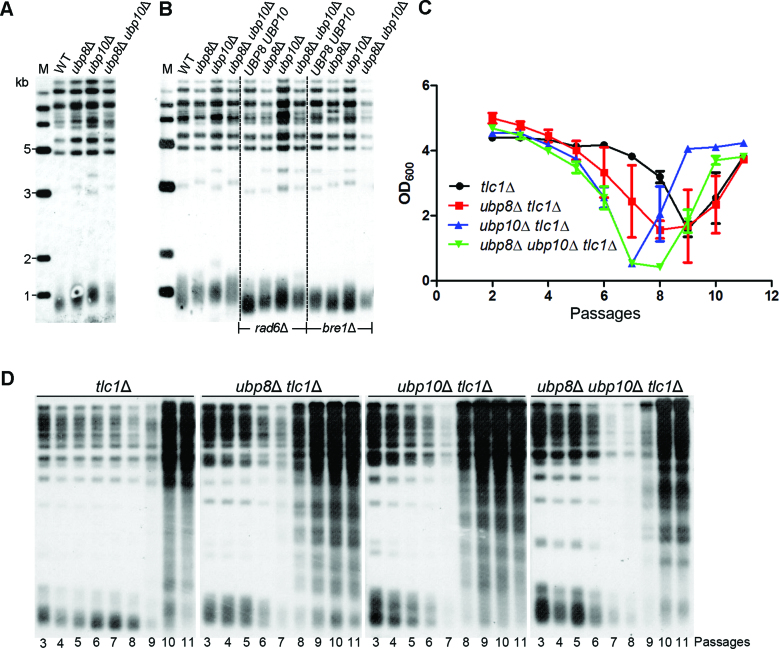
As described above, Rad6–Bre1 pathway functions on telomeres through its role in H2Bub1. Ubp8 and Ubp10 are deubiquitinases that target histone H2BK123 (54), raising the possibility that they affect telomeres in a way opposite to Rad6–Bre1 pathway. To test this possibility, we examined telomere lengths of ubp8Δ, ubp10Δ and ubp8Δ ubp10Δ haploid strains obtained through sporulating a diploid strain heterozygous for ubp8Δ and ubp10Δ. The spore clones for each mutant were re-streaked on plates for five times, and their telomere lengths were examined by Southern blot analysis. Both the ubp8Δ and ubp10Δ cells showed longer telomeres than wild-type cells (Figure 3A). The ubp10Δ mutation conferred stronger effects than the ubp8Δ mutation. This was in agreement with the previous studies showing that Ubp10 regulates H2Bub1 more effectively than Ubp8 at the telomere (55,56). To further ask whether telomere lengthening in ubp8Δ and/or ubp10Δ cells was a specific effect of H2Bub1, we knocked out RAD6 or BRE1 in ubp8Δ and/or ubp10Δ cells and examined telomere length. The results showed that ubp8Δ- and/or ubp10Δ-induced telomere extension was dependent on Rad6–Bre1 pathway (Figure 3B). These data indicated that the increase of H2Bub1 by inactivation of UBP8 and/or UBP10 promotes telomere extension.
Figure 3.
Inactivation of deubiquitinases Ubp8 and Ubp10 causes telomere lengthening and accelerates the onset of senescence in telomerase-deficient cells. (A) The UBP8/ubp8Δ UBP10/ubp10Δ diploid strain was sporulated and tetrads were dissected, respectively. The isogenic haploid spores with respective genotypes were re-streaked for five times on YPD plates, and then their telomere lengths were examined by Southern blot analysis. (B) The UBP8/ubp8Δ UBP10/ubp10Δ RAD6/rad6Δ and UBP8/ubp8Δ UBP10/ubp10Δ BRE1/bre1Δ diploid strains were sporulated and tetrads were dissected, respectively. Southern blot analysis of telomere length was performed as in A. (C) Cell viability assay of ubp8Δ tlc1Δ, ubp10Δ tlc1Δ and ubp8Δ ubp10Δ tlc1Δ cells. The TLC1/tlc1Δ UBP8/ubp8Δ UBP10/ubp10Δ diploid strain was sporulated and tetrads were dissected. Spores with indicated genotypes were directly inoculated in liquid culture for cell viability assays. (D) Telomere Southern blot analysis of senescing and survival cells. Their genomic DNAs were extracted from the corresponding liquid cultures (C), digested with XhoI and subjected to Southern blot analysis.
We also examined the senescence and survival of the ubp8Δ tlc1Δ, ubp10Δ tlc1Δ and ubp8Δ ubp10Δ tlc1Δ cells in liquid medium. As shown in Figure 3C, the tlc1Δ cells underwent senescence at the ninth passage, while the ubp8Δ tlc1Δ, ubp10Δ tlc1Δ and ubp8Δ ubp10Δ tlc1Δ cells senesced at the seventh or eighth passage, indicating that UBP8 and/or UBP10 deletion accelerated senescence in the tlc1Δ cells. Consistent with the effect on telomere length, the UBP10 deletion also conferred stronger effects on senescence than the UBP8 deletion in tlc1Δ cells. Consistently, Southern blot analysis revealed that massive telomere recombination took place at the eighth passage in both the ubp8Δ tlc1Δ and ubp10Δ tlc1Δ cells, earlier than the tenth passage in the tlc1Δ cells (Figure 3D). These observations indicated that the increase of H2Bub1 by Ubp8 and/or Ubp10 inactivation accelerates the onset of senescence of telomerase-deficient cells.
Taken together, these observations indicate that increased H2Bub1 via deletion of UBP8 and/or UBP10 facilitates telomere-length extension and accelerates the onset of senescence of telomerase-deficient cells. Consistently, it supports the conclusion that Rad6–Bre1–H2Bub1 plays a positive role in regulation of both telomere length and telomere recombination.
Bre1-mediated H2Bub1 is partially responsible for Rad6-dependent type II telomere recombination
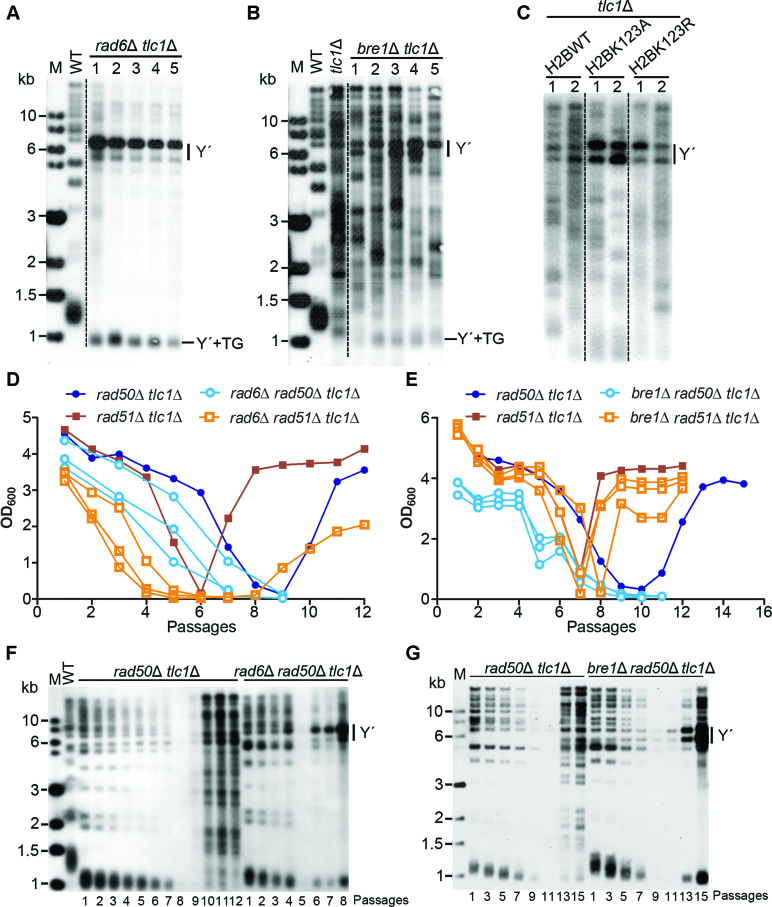
Since type II survivors were impeded in the rad6Δ tlc1Δ cells (36) (Figure 2G), we wanted to know the effect of Rad6–Bre1–H2Bub1 on telomere recombination type selection. Independent clones of rad6Δ tlc1Δ, bre1Δ tlc1Δ, H2BK123A tlc1Δ and H2BK123R tlc1Δ strains were grown in liquid cultures for about 120 generations. Their genomic DNAs were prepared for Southern blot assay. The results showed that typical Y΄-amplification, an indication of type I survivors, was seen in all of the rad6Δ tlc1Δ clones (Figure 4A), suggesting that type II survivors were significantly impeded in the rad6Δ tlc1Δ cells. To address whether Rad6 also affects the generation of type I telomere recombination, we blocked the type II pathway by deleting Sae2 (28,29) and compared the efficiency of type I survivor formation in sae2Δ tlc1Δ and sae2Δ rad6Δ tlc1Δ strains. Interestingly, the sae2Δ rad6Δ tlc1Δ mutant spent longer time in crisis, indicative of reduced efficiency of type I recombination (Supplementary Figure S6). Thus, loss of Rad6 affected both type I and type II telomere recombination, but exhibited more significant effect on type II recombination. The Y΄-amplification was detected in two of the bre1Δ tlc1Δ clones, and the appearance of TG1–3 recombination appeared to be decreased in all of the bre1Δ tlc1Δ clones (Figure 4B). Consistently, significant Y΄-amplification was also observed in the H2BK123A tlc1Δ and H2BK123R tlc1Δ mutant cells (Figure 4C and Supplementary Figure S7). Since Rad6 is involved in different repair pathways, our results suggested that Rad6 functions on telomere recombination partially through its downstream Bre1-mediated H2Bub1.
Figure 4.
Functional interaction of Rad6–Bre1 pathway and Rad50 in telomere recombination. (A–C) Southern blot analysis of telomere recombination of rad6Δ tlc1Δ, bre1Δ tlc1Δ, H2BK123A tlc1Δ and H2BK123R tlc1Δ cells cultured in liquid medium. Five clones of rad6Δ tlc1Δ (A) and bre1Δ tlc1Δ (B), and two clones of H2BK123A tlc1Δ and H2BK123R tlc1Δ (C) cells were grown in liquid cultures for about 120 generations, and genomic DNAs were extracted and digested with XhoI for Southern blot analysis. Y΄-amplification was indicated on right. (D) Cell viability assay of rad6Δ rad50Δ tlc1Δ and rad6Δ rad51Δ tlc1Δ cells in liquid cultures. TLC1/tlc1Δ RAD6/rad6Δ RAD50/rad50Δ and TLC1/tlc1Δ RAD6/rad6Δ RAD51/rad51Δ diploid strains were respectively sporulated, and tetrads were dissected. Spore colonies with indicated genotypes were subjected to cell viability assay in liquid cultures. The results of three clones of rad6Δ rad50Δ tlc1Δ and rad6Δ rad51Δ tlc1Δ were shown. (E) Cell viability assay of bre1Δ rad50Δ tlc1Δ and bre1Δ rad51Δ tlc1Δ cells in liquid cultures. The experimental procedures are the same as in D. (F and G) Southern blot analysis of telomere attrition and recombination of rad6Δ rad50Δ tlc1Δ (F), and bre1Δ rad50Δ tlc1Δ (G) cells. The strains were cultured and diluted every 48 h in liquid medium, and their genomic DNAs were extracted, digested with XhoI and subjected to Southern blot analysis.
Next, we tested the effect of H3K4A and H3K79A mutation, alone or in combination, on telomere recombination in the absence of telomerase. The results showed that TG1–3 amplification, but not Y΄-amplification, took place in either H3K4A tlc1Δ, H3K79A tlc1Δ or H3K4A K79A tlc1Δ mutants when the cells were cultured in liquid medium (Supplementary Figure S8). Thus, our results indicated that the function of H2Bub1 on telomere recombination control was likely independent of its downstream methylation of H3K4 and K79.
Rad6–Bre1 collaborates with Rad50 in telomere recombination
In telomerase-deficient cells, RAD50 deletion greatly reduces type II survivor formation and RAD51 deletion only generates type II survivors (26,27). Thus, Rad50- and Rad51-dependent HR pathways respectively define the two types of telomere recombination. To confirm the regulatory function of Rad6–Bre1 pathway on telomere recombination in telomerase-deficient cells, we constructed rad6Δ rad50Δ tlc1Δ, rad6Δ rad51Δ tlc1Δ, bre1Δ rad50Δ tlc1Δ and bre1Δ rad51Δ tlc1Δ cells, measured their senescence and survival rates in liquid cultures, and analyzed their telomere patterns. Expectedly, two of the three clones of the rad6Δ rad51Δ tlc1Δ mutant were unable to generate survivors (Figure 4D), reinforcing the conclusion that Rad6 is important for type II telomere recombination. However, the kinetics of senescence/survival rates of the bre1Δ rad51Δ tlc1Δ cells exhibited little difference compared to the rad51Δ tlc1Δ cells (Figure 4E), further suggesting that Bre1 pathway plays limited roles in type II telomere recombination. Unexpectedly, both the rad6Δ rad50Δ tlc1Δ and bre1Δ rad50Δ tlc1Δ cells displayed accelerated senescence and difficulties in survivor generation (Figure 4D and E), reflecting a functional collaboration between Rad6–Bre1 pathway and Rad50 on telomere recombination.
The rad6Δ rad50Δ tlc1Δ and bre1Δ rad50Δ tlc1Δ cells could not generate survivors when the cultures were diluted every 24 h. However, survivors were salvageable after eight to ten passages when the cultures of the triple mutant cells were diluted every 48 h. Telomere blot assay revealed that the rad6Δ rad50Δ tlc1Δ and bre1Δ rad50Δ tlc1Δ survivors only adopted the Y΄-recombination (type I), but not the TG1–3 recombination (type II) (Figure 4F and G). These results indicated that Rad6–Bre1 is required for type II telomere recombination in the absence of RAD50, and further suggested the cooperation between Rad50 and Rad6–Bre1 pathway in regulating telomere recombination.
Rad6–Bre1–H2Bub1 supports cell viability in the absence of MRX
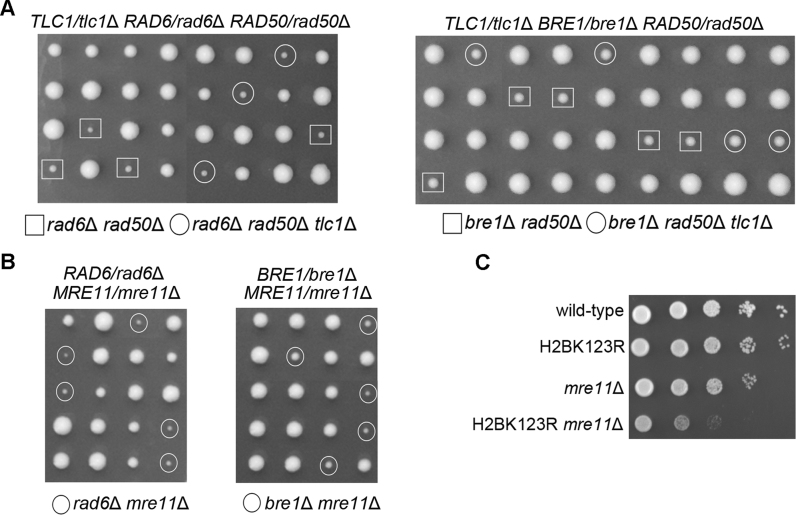
The cooperation between Rad6–Bre1 and Rad50 on HR appeared not to be limited at telomeres. We noticed that rad6Δ rad50Δ and bre1Δ rad50Δ spores formed extremely smaller colonies than each single-deletion spores (Figure 5A). The aggravated growth defects of the rad6Δ rad50Δ and bre1Δ rad50Δ mutants appeared to be independent of telomerase, because the rad6Δ rad50Δ tlc1Δ and bre1Δ rad50Δ tlc1Δ cells exhibited nearly the same extent of growth defects (Figure 5A). These data suggested a functional interaction between Rad6–Bre1 and Rad50 on general DNA repair by HR. The spores with double-deletion of RAD51 and RAD6 formed smaller colonies, but the spores with double-deletion of RAD51 and BRE1 formed normal colonies as each single-deletion spores (Supplementary Figure S9). This result is consistent with the notion that in addition to Bre1, Rad6 interacts with other E3 ligases to function in DNA repair (34).
Figure 5.
Functional interaction of Rad6–Bre1 pathway and MRX in cell viability. (A) Tetrad analysis of TLC1/tlc1Δ RAD6/rad6Δ RAD50/rad50Δ (left panel) and TLC1/tlc1Δ BRE1/bre1Δ RAD50/rad50Δ (right panel) on YPD plates. (B) Tetrad analysis of RAD6/rad6Δ MRE11/mre11Δ (left panel) and BRE1/bre1Δ MRE11/mre11Δ (right panel) on YPD plates. (C) Dotting assay for cell growth of H2BK123R- and mre11Δ-single and H2BK123R mre11Δ-double mutants. Equal amounts of serial dilutions of exponentially growing cells (OD600 = ∼2) were spotted on YPD plates and grown for 2 days at 30°C.
Rad50 functions as a subunit of MRX complex, raising the possibility that Rad6–Bre1 pathway participates in HR by collaborating with MRX in promoting DNA-end resection. As Mre11 is responsible for nuclease activity in MRX complex (10), we investigated the effect of deletion of RAD6 or BRE1 or mutation of H2BK123 on cell viability in mre11Δ cells. RAD6/rad6Δ MRE11/mre11Δ and BRE1/bre1Δ MRE11/mre11Δ diploid strains were respectively sporulated and tetrads were analyzed for spore viability. Consistently, both rad6Δ mre11Δ or bre1Δ mre11Δ spores formed smaller colonies than each single-deletion spores (Figure 5B), similar with the results of the rad6Δ rad50Δ and bre1Δ rad50Δ spores (Figure 5A). Indeed, both the rad6Δ mre11Δ and bre1Δ mre11Δ cells showed significant growth defects compared to each single mutant (Supplementary Figure S10). Importantly, H2BK123R mre11Δ cells also showed synthetic growth defects compared to H2BK123R or mre11Δ single-mutation cells (Figure 5C), consistent with the previous report showing that H2Bub1 is involved in the DNA damage response pathway in the absence of MRX complex (57). Exo1 and Sgs1 have been demonstrated to be required for extensive 5΄ resection (58). Deletion of RAD6 did not cause synthetic growth defects in either exo1Δ or sgs1Δ cells (Supplementary Figure S11). Together, these results indicated that Rad6–Bre1–H2Bub1 supports cell viability in the absence of MRX, and suggested that Rad6–Bre1–H2Bub1 cooperates with MRX to function in DNA-end resection process.
Rad6–Bre1–H2Bub facilitates accumulation of telomeric ssDNA
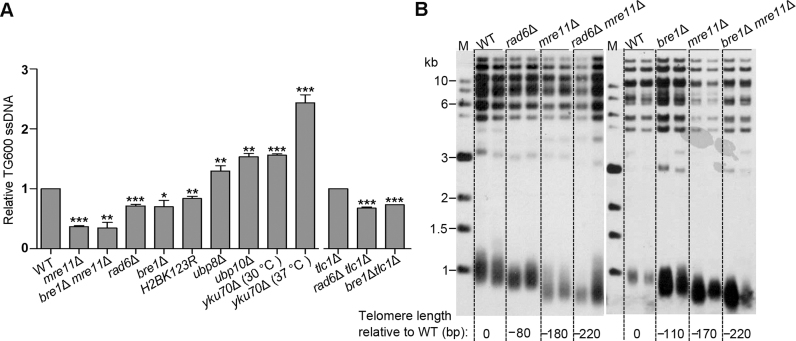
To directly determine whether Rad6–Bre1–H2Bub1 affects telomere-end resection, quantitative amplification of ssDNA (QAOS) was used to detect the accumulation of ssDNA at telomeres (49). As a positive control, telomeric ssDNA was accumulated in yku70Δ strains, especially when cells were cultured at non-permissive temperature (37°C) for 5 h (Figure 6A). As a negative control, telomeric ssDNA was greatly decreased in the mre11Δ cells (Figure 6A), consistent with the role of MRX complex in telomere-end resection (20). Notably, the rad6Δ, bre1Δ or H2BK123R mutant cells contained less telomeric ssDNA than the wild-type cells, but to a lesser extent compared to the mre11Δ cells (Figure 6A). This result was in accordance with the fact that Rad6–Bre1–H2Bub1 plays a relatively weaker role than MRX complex at telomeres. In addition, we also observed the decrease of telomeric ssDNA in both rad6Δ tlc1Δ and bre1Δ tlc1Δ cells compared to the tlc1Δ cells (Figure 6A). Accordingly, the amount of telomeric ssDNA in both the ubp8Δ and ubp10Δ cells was significantly higher than that in the wild-type cells (Figure 6A), consistent with the results that Ubp8/Ubp10 antagonizes the function of Rad6–Bre1 pathway in telomere replication (Figure 3).
Figure 6.
RAD6- or BRE1-deletion, or H2BK123R mutation decreases telomeric ssDNA. (A) Detection of ssDNA on the TG strand at Y΄ sequences. All strains were grown to exponential phase at 30°C, except a shift of exponential growing cultures to the 37°C for additional 5 h for yku70Δ cells. The ssDNA was quantified relative to that of wild-type, except that the ssDNA of rad6Δ tlc1Δ and bre1Δ tlc1Δ cells was quantified relative to that of tlc1Δ cells, and the results were quantified from three independent experiments. (B) Southern blot analysis of telomere lengths of rad6Δ mre11Δ and bre1Δ mre11Δ double mutants. Spore colonies with indicated genotypes (Figure 5B) were successively re-streaked for five times, and the genomic DNA was extracted, digested by PstI and subjected to Southern blot analysis.
If Rad6–Bre1–H2Bub1 functions in parallel with MRX on telomere-end resection, simultaneous inactivation of both Rad6–Bre1 and MRX pathways should lead to synthetic telomere shortening. Thus, we examined telomere lengths in the rad6Δ mre11Δ and bre1Δ mre11Δ double-deletion cells. As reported previously (12), telomeres in the mre11Δ cells were ∼180 bp shorter than those in the wild-type cells (Figure 6B). Telomeres in both the rad6Δ mre11Δ and bre1Δ mre11Δ double-deletion cells were ∼220 bp shorter than those in the wild-type cells, and ∼40 bp shorter than those in the mre11Δ cells (Figure 6B). In addition, we determined the association of Mre11-13myc protein with telomere VI-R in both wild-type and bre1Δ cells. As shown in Supplementary Figure S12, Mre11 association to telomere VI-R appeared to be indistinguishable in wild-type and bre1Δ cells. These results further support the conclusion that Rad6–Bre1–H2Bub1 functions in parallel with MRX complex to facilitate telomere-end resection.
Rad6–Bre1–H2Bub1 is important for rif2Δ-induced telomere elongation
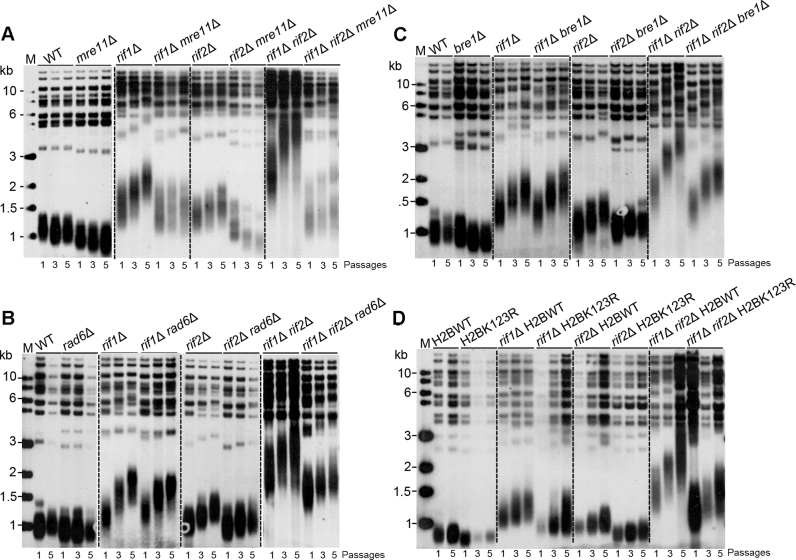
It has been demonstrated that Rif2 inhibits both telomere resection and elongation at de novo telomeres by blocking MRX's association (18,21). In order to further investigate the role of Rad6–Bre1–H2Bub1 in telomere-end resection, we examined the genetic interaction between Rad6–Bre1–H2Bub1 pathway and Rif2. As a control, we examined the effects of Mre11 on rif1Δ- and rif2Δ-induced telomere elongation. The results showed that telomeres in rif2Δ mre11Δ cells gradually shortened, and reached to a length comparable to that in mre11Δ cells (Figure 7A). In contrast, rif1Δ mre11Δ cells had more heterogeneous telomeres compared to rif1Δ and mre11Δ single mutants (Figure 7A). These data were consistent with the observation that telomere length was differently regulated for X and Y΄ telomeres in rif1Δ tel1Δ strains (59), and indicated that Mre11 (Tel1)-mediated resection cannot, at least not fully, explain the telomere elongation in the rif1Δ cells.
Figure 7.
RAD6- or BRE1-deletion, or H2BK123R mutation affects rif2Δ-induced telomere elongation. (A–C) MRE11/mre11Δ RIF1/rif1Δ RIF2/rif2Δ (A), RAD6/rad6Δ RIF1/rif1Δ RIF2/rif2Δ (B) and BRE1/bre1Δ RIF1/rif1Δ RIF2/rif2Δ (C) diploid strains were respectively sporulated, and tetrads were dissected. Spore colonies with indicated genotypes were re-streaked on plates for five times, and genomic DNAs extracted from the first, third and fifth cultures were digested with PstI and subjected to Southern blot analysis. (D) The rif1Δ- and rif2Δ-single and rif1Δ rif2Δ-double mutants were constructed in H2B wild-type (YZS276) and H2BK123R mutant (YZS277) strains. The telomere length was examined as in A–C.
We then performed epistasis analysis of telomere length in rad6Δ/bre1Δ/H2BK123R, rif1Δ and rif2Δ mutants. RAD6/rad6Δ RIF1/rif1Δ RIF2/rif2Δ and BRE1/bre1Δ RIF1/rif1Δ RIF2/rif2Δ diploid strains were sporulated respectively, and the tetrads were dissected. The spore colonies were re-streaked on plates for five successive times, and genomic DNAs prepared from the first, third and fifth cultures were subjected to Southern blot assay. Interestingly, the telomere elongation was affected in both rad6Δ rif2Δ and bre1Δ rif2Δ cells compared to rif2Δ cells, and dramatically impaired in rad6Δ rif1Δ rif2Δ and bre1Δ rif1Δ rif2Δ cells compared to rif1Δ rif2Δ cells (Figure 7B and C). Consistently, the H2BK123R mutation also decreased telomere lengthening in both the rif2Δ single and rif1Δ rif2Δ double mutants (Figure 7D). In contrast, the deletion of either RAD6 or BRE1 had little effect on rif1Δ-induced telomere elongation (Figure 7B and C). Given that excessive Mre11΄s binding (18) and ssDNA formation (60) was observed in the rif1Δ rif2Δ cells, Mre11-dependent extended resection might account for the telomere over-elongation in this strain. Consistently, the effect of RAD6- or BRE1-deletion, or H2BK123R mutation on telomere elongation was significantly stronger in the rif1Δ rif2Δ cells than that in the rif2Δ cells (Figure 7B–D). Together, these results support the role of Rad6–Bre1–H2Bub1 in telomere-end resection. Notably, the RAD6- or BRE1-deletion, or H2BK123R mutation conferred a weaker effect on rif2Δ-induced telomere elongation than the MRE11 deletion, reinforcing the notion that Rad6–Bre1–H2Bub1 plays a relatively weaker role in telomere-end resection than MRX complex.
DISCUSSION
The maintenance of telomere homeostasis involves telomerase and telomere associated regulators [for reviews, see (1,2,4)]. Several genome-wide screening studies have revealed that about three hundreds genes are involved in telomere maintenance in telomerase-proficient and/or telomerase-deficient yeast cells (36,43,61). However, as to how these genes regulate telomeres, particular attentions have been paid to those that are known to function in DNA repair pathways (62–64). In this work, we reported that Rad6–Bre1–H2Bub1 plays regulatory roles in both telomerase- and recombination-dependent telomere maintenance. We for the first time provided genetic evidence demonstrating that Rad6–Bre1–H2Bub1 cooperates with MRX complex to promote telomere-end resection, and thereby facilitates both telomerase- and recombination-dependent telomere replication.
It has been shown that rad6Δ or bre1Δ cells have shorter telomeres (43) and exhibit difficulties in implementing of type II telomere recombination in tlc1Δ cells (36). We found that H2BK123A or H2BK123R mutant strains have shorter telomeres and show typical type I telomere recombination in tlc1Δ cells (Figure 1B and 4C; Supplementary Figure S1 and 5). Furthermore, deletion of UBP8 and/or UBP10 results in longer telomeres, which is dependent on Rad6–Bre1 pathway (Figure 3A). These data indicate that Rad6–Bre1 pathway functions on telomeres through its downstream H2Bub1. Although Rad6–Bre1–H2Bub1 is essential for methylation of histone H3K4 and K79 which affects gene transcription globally (39,40), mutations of histone H3K4A and/or H3K79A have little effect on either telomere length or telomere recombination (Supplementary Figure S2 and 6). Thus, Rad6–Bre1–H2Bub1 appears to directly participate in telomere function, independently of its downstream H3 methylation.
Inactivation of Rad6–Bre1 pathway decreases the telomere shortening rate, and delays the onset of senescence in the telomerase-deficient cells (Figure 2). These phenotypes are similar to those seen in the mre11Δ tlc1Δ, rad50Δ tlc1Δ and exo1Δ tlc1Δ cells reported previously (27,31). These data suggested that, like MRX complex and Exo1, Rad6–Bre1 pathway participates in telomere-end resection. Consistently, inactivation of Rad6–Bre1–H2Bub1 decreases the telomere type II survivors in the tlc1Δ cells, in accordance with the notion that genes involved in resection, such as MRX, Sae2, Exo1, play critical roles in type II telomere recombination (27,29,31,65). Interestingly, deletion of RAD6 or BRE1 accelerated senescence and almost blocked type II telomere recombination in the rad50Δ tlc1Δ cells (Figure 4D–G). Additionally, in the experiments that aimed to dissect the pathway of Rad6–Bre1 in telomere recombination, we found that Rad6–Bre1–H2Bub1 supports cell viability in the absence of MRX complex, independently of telomerase pathway (Figure 5). These findings indicated the cooperation of Rad6–Bre1–H2Bub1 and MRX on DNA-end resection and that telomere functions of Rad6–Bre1–H2Bub1 are attributed to its roles in telomere-end resection.
In addition to resection genes, several recombination genes, such as Sgs1 and Mdt1 (30,62,66), have been found to be required for type II recombination. Our previous study showed that Rad6 is required for type II telomere recombination (36). In the current study, we found that loss of Rad6 also affects the type I telomere recombination (Supplementary Figure S4). In addition, we also showed that Bre1–H2Bub1 was partially responsible for Rad6-dependent type II telomere recombination (Figure 4A–E). As Rad6 is involved in different repair pathways through its interaction with various E3 ubiquitin ligases (37), Rad6 affects telomere recombination through both H2Bub1-facilitated resection and H2Bub1-independent roles. Thus, Bre1–H2Bub1 mediated telomere-end resection plays important roles on the onset of senescence, and is partially responsible for Rad6-dependent type II telomere recombination.
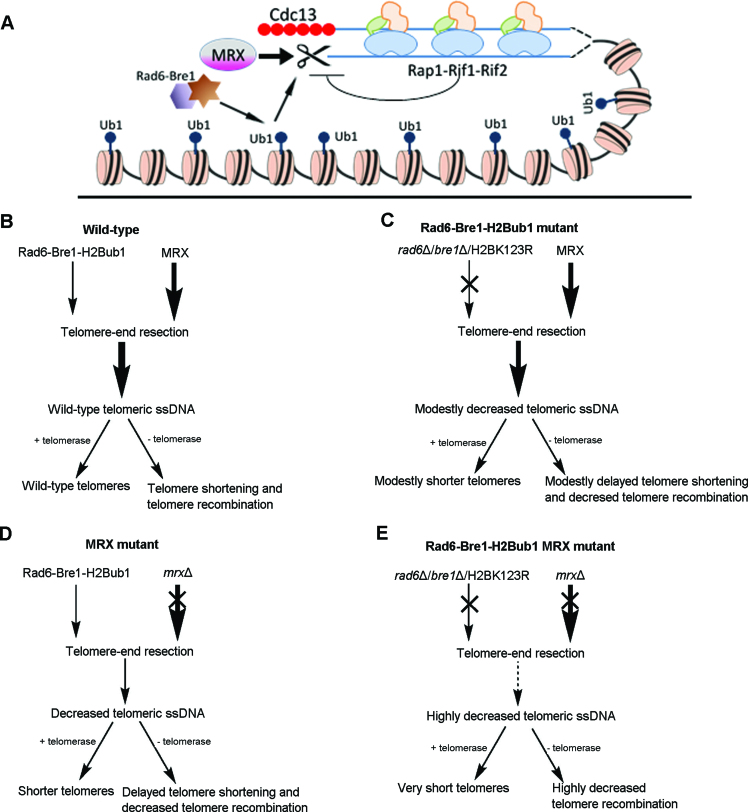
A model for functions of MRX on telomeres has been proposed, where MRX promotes the 5΄-end resection of telomeric C-strand and the generation of 3΄ G-tail, an optimal substrate for telomerase action and telomere recombination in the absence of telomerase (16,27). Here, we propose that Rad6–Bre1–H2Bub1 cooperates with MRX to promote telomere-end resection, and thereby contributes to telomere replication in both telomerase-positive and -negative cells (Figure 8). In this model, MRX plays a major role, while Rad6–Bre1–H2Bub1 plays a minor role in telomere-end resection (Figure 8A), and consistently MRX exhibits stronger effects on telomeres (Figure 8B–E). The model provides plausible explanation to most of the experimental results presented in the current study. First, deletion of RAD6 or BRE1, or mutation of H2BK123 results in a decrease of telomeric ssDNA (Figure 6A), an indication of the defect in telomere-end resection, which in turn causes shortened telomere length and impaired type II telomere recombination (Figure 1 and 4A–C). These phenotypical defects are similar but exacerbated in MRX mutants (12,27). Inactivation of Ubp8 and/or Ubp10 increases telomeric ssDNA (Figure 6A), providing more optimal substrate for telomerase and type II recombination activity (Figure 3). Second, the reduction of telomere-end resection in bre1Δ tlc1Δ cells decreases the telomere shortening rate, and thus delays the onset of senescence (Figure 2). These results are reminiscent of those reported previously in rad50Δ tlc1Δ, mre11Δ tlc1Δ and exo1Δ tlc1Δ cells (27). Third, telomere (DNA)-end resection is largely suppressed when the Rad6–Bre1–H2Bub1 and MRX pathways are simultaneously inactivated. Consistently, cells grow extremely slow (Figure 5) and telomerase activity is inhibited (Figure 6B), and cellular senescence is accelerated and survivor generation encounters difficulties in the absence of telomerase (Figure 4D and E). Fourth, Rif2 inhibits MRX's access to telomere ends, accounting for the negative effect of Rif2 on telomere addition (21). Consistently, MRE11 deletion dramatically suppressed rif2Δ-induced telomere elongation (Figure 7A). Analogous to MRE11 deletion, RAD6- or BRE1-deletion, or H2BK123R mutation impaired telomere elongation in rif2Δ cells (Figure 7B–D).
Figure 8.
A model for the function of Rad6–Bre1–H2Bub1 on telomere replication. (A) In this model, the MRX complex and Rad6–Bre1–H2Bub1 pathway collaborate to promote telomere-end resection, whereas MRX displays a stronger effect and Rad6–Bre1–H2Bub1 exhibits a weaker effect. (B–E) The level of telomeric ssDNA positively correlates with the regulation of telomere length (+telomerase) and telomere recombination efficiency (−telomerase) in the wild-type (B), rad6Δ/bre1Δ/H2BK123R mutant (C), mrxΔ mutant (D) and rad6Δ/bre1Δ/H2BK123R mrxΔ double mutant (E) cells.
It has been well documented that histone H2Bub1 plays roles in multiple chromatin associated processes such as replication, transcription and HR in both yeast and mammals (67–69). In both fission yeast and human cells, H2Bub1 has been demonstrated to facilitate DNA-end resection likely through chromatin reorganization, and thus influence HR and genome stability (41,42). In budding yeast, H2Bub1 has been shown to make the chromatin compact, and lack of H2Bub1 leads to a loose chromatin (70,71). Given this, a model is proposed for the positive role of H2Bub1 in transcription elongation, where H2Bub1 stabilizes the nucleosomes to maintain the chromatin structure for transcription events (69,71). The function of H2Bub1 in chromatin structure regulation has been seen at telomeres. For example, the absence of Rad6–Bre1–H2Bub1 results in loss of telomere silencing (39). Though it remains elusive how H2Bub1 affects telomere chromatin, we speculate that H2Bub1 may help chromatin reorganization, facilitating the access of nuclease(s) which functions in telomere-end resection.
In summary, we revealed that H2Bub1 participates in telomere-end resection to promote telomere replication. Importantly, our finding highlights the engagement and the importance of histone H2BK123 mono-ubiquitination in telomere replication. Given that the function of H2Bub1 on DNA-end resection appears to be conserved from yeast to mammalians, it will be intriguing to explore whether or not the mechanism by which H2Bub1 affects telomeres seen in budding yeast could be extended to other organisms.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr David Allis for providing YZS276 and YZS277 strains.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Ministry of Science and Technology [2013CB910403]; National Natural Science Foundation of China [NSFC 31230040/31461143003/31521061 to J-Q.Z.]; China Postdoctoral Science Foundation [2015M571611, 2016T90386]; NSFC [31500658 to Z.W.]. Funding for open access charge: Ministry of Science and Technology [2013CB910403].
Conflict of interest statement. None declared.
REFERENCES
- 1. Bianchi A., Shore D.. How telomerase reaches its end: mechanism of telomerase regulation by the telomeric complex. Mol. Cell. 2008; 31:153–165. [DOI] [PubMed] [Google Scholar]
- 2. Malyavko A.N., Parfenova Y.Y., Zvereva M.I., Dontsova O.A.. Telomere length regulation in budding yeasts. FEBS Lett. 2014; 588:2530–2536. [DOI] [PubMed] [Google Scholar]
- 3. McEachern M.J., Krauskopf A., Blackburn E.H.. Telomeres and their control. Annu. Rev. Genet. 2000; 34:331–358. [DOI] [PubMed] [Google Scholar]
- 4. Wellinger R.J., Zakian V.A.. Everything you ever wanted to know about Saccharomyces cerevisiae telomeres: beginning to end. Genetics. 2012; 191:1073–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kupiec M. Biology of telomeres: lessons from budding yeast. FEMS Microbiol. Rev. 2014; 38:144–171. [DOI] [PubMed] [Google Scholar]
- 6. Greider C.W., Blackburn E.H.. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985; 43:405–413. [DOI] [PubMed] [Google Scholar]
- 7. Kelleher C., Teixeira M.T., Forstemann K., Lingner J.. Telomerase: biochemical considerations for enzyme and substrate. Trends Biochem. Sci. 2002; 27:572–579. [DOI] [PubMed] [Google Scholar]
- 8. Pennock E., Buckley K., Lundblad V.. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell. 2001; 104:387–396. [DOI] [PubMed] [Google Scholar]
- 9. Nugent C.I., Hughes T.R., Lue N.F., Lundblad V.. Cdc13p: a single-strand telomeric DNA-binding protein with a dual role in yeast telomere maintenance. Science. 1996; 274:249–252. [DOI] [PubMed] [Google Scholar]
- 10. Diede S.J., Gottschling D.E.. Exonuclease activity is required for sequence addition and Cdc13p loading at a de novo telomere. Curr. Biol. 2001; 11:1336–1340. [DOI] [PubMed] [Google Scholar]
- 11. Nakada D., Matsumoto K., Sugimoto K.. ATM-related Tel1 associates with double-strand breaks through an Xrs2-dependent mechanism. Genes Dev. 2003; 17:1957–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ritchie K.B., Petes T.D.. The Mre11p/Rad50p/Xrs2p complex and the Tel1p function in a single pathway for telomere maintenance in yeast. Genetics. 2000; 155:475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sabourin M., Tuzon C.T., Zakian V.A.. Telomerase and Tel1p preferentially associate with short telomeres in S. cerevisiae. Mol. Cell. 2007; 27:550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McGee J.S., Phillips J.A., Chan A., Sabourin M., Paeschke K., Zakian V.A.. Reduced Rif2 and lack of Mec1 target short telomeres for elongation rather than double-strand break repair. Nat. Struct. Mol. Biol. 2010; 17:U1438–U1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goudsouzian L.K., Tuzon C.T., Zakian V.A.. S. cerevisiae Tel1p and Mre11p are required for normal levels of Est1p and Est2p telomere association. Mol. Cell. 2006; 24:603–610. [DOI] [PubMed] [Google Scholar]
- 16. Gao H., Toro T.B., Paschini M., Braunstein-Ballew B., Cervantes R.B., Lundblad V.. Telomerase recruitment in Saccharomyces cerevisiae is not dependent on Tel1-mediated phosphorylation of Cdc13. Genetics. 2010; 186:1147–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonetti D., Martina M., Clerici M., Lucchini G., Longhese M.P.. Multiple pathways regulate 3΄ overhang generation at S. cerevisiae telomeres. Mol. Cell. 2009; 35:70–81. [DOI] [PubMed] [Google Scholar]
- 18. Hirano Y., Fukunaga K., Sugimoto K.. Rif1 and Rif2 inhibit localization of Tel1 to DNA ends. Mol. Cell. 2009; 33:312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martina M., Clerici M., Baldo V., Bonetti D., Lucchini G., Longhese M.P.. A balance between Tel1 and Rif2 activities regulates nucleolytic processing and elongation at telomeres. Mol. Cell. Biol. 2012; 32:1604–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Larrivee M., LeBel C., Wellinger R.J.. The generation of proper constitutive G-tails on yeast telomeres is dependent on the MRX complex. Genes Dev. 2004; 18:1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bonetti D., Clerici M., Anbalagan S., Martina M., Lucchini G., Longhese M.P.. Shelterin-like proteins and Yku inhibit nucleolytic processing of Saccharomyces cerevisiae telomeres. PLoS Genet. 2010; 6:e1000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wotton D., Shore D.. A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev. 1997; 11:748–760. [DOI] [PubMed] [Google Scholar]
- 23. Lundblad V., Blackburn E.H.. An alternative pathway for yeast telomere maintenance rescues est1- senescence. Cell. 1993; 73:347–360. [DOI] [PubMed] [Google Scholar]
- 24. Teng S.C., Zakian V.A.. Telomere-telomere recombination is an efficient bypass pathway for telomere maintenance in Saccharomyces cerevisiae. Mol. Cell. Biol. 1999; 19:8083–8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lundblad V., Szostak J.W.. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989; 57:633–643. [DOI] [PubMed] [Google Scholar]
- 26. Chen Q., Ijpma A., Greider C.W.. Two survivor pathways that allow growth in the absence of telomerase are generated by distinct telomere recombination events. Mol. Cell. Biol. 2001; 21:1819–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Le S., Moore J.K., Haber J.E., Greider C.W.. RAD50 and RAD51 define two pathways that collaborate to maintain telomeres in the absence of telomerase. Genetics. 1999; 152:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Churikov D., Charifi F., Simon M.N., Geli V.. Rad59-facilitated acquisition of Y' elements by short telomeres delays the onset of senescence. PLoS Genet. 2014; 10:e1004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hardy J., Churikov D., Geli V., Simon M.N.. Sgs1 and Sae2 promote telomere replication by limiting accumulation of ssDNA. Nat. Commun. 2014; 5:5004. [DOI] [PubMed] [Google Scholar]
- 30. Johnson F.B., Marciniak R.A., McVey M., Stewart S.A., Hahn W.C., Guarente L.. The Saccharomyces cerevisiae WRN homolog Sgs1p participates in telomere maintenance in cells lacking telomerase. EMBO J. 2001; 20:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maringele L., Lydall D.. EXO1 plays a role in generating type I and type II survivors in budding yeast. Genetics. 2004; 166:1641–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meng F.L., Chen X.F., Hu Y., Tang H.B., Dang W., Zhou J.Q.. Sua5p is required for telomere recombination in Saccharomyces cerevisiae. Cell Res. 2010; 20:495–498. [DOI] [PubMed] [Google Scholar]
- 33. Tsai H.J., Huang W.H., Li T.K., Tsai Y.L., Wu K.J., Tseng S.F., Teng S.C.. Involvement of topoisomerase III in telomere-telomere recombination. J. Biol. Chem. 2006; 281:13717–13723. [DOI] [PubMed] [Google Scholar]
- 34. Teng S.C., Chang J., McCowan B., Zakian V.A.. Telomerase-independent lengthening of yeast telomeres occurs by an abrupt Rad50p-dependent, Rif-inhibited recombinational process. Mol. Cell. 2000; 6:947–952. [DOI] [PubMed] [Google Scholar]
- 35. Chang M., Dittmar J.C., Rothstein R.. Long telomeres are preferentially extended during recombination-mediated telomere maintenance. Nat. Struct. Mol. Biol. 2011; 18:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hu Y., Tang H.B., Liu N.N., Tong X.J., Dang W., Duan Y.M., Fu X.H., Zhang Y., Peng J., Meng F.L. et al. . Telomerase-null survivor screening identifies novel telomere recombination regulators. PLoS Genet. 2013; 9:e1003208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Game J.C., Chernikova S.B.. The role of RAD6 in recombinational repair, checkpoints and meiosis via histone modification. DNA Rep. 2009; 8:470–482. [DOI] [PubMed] [Google Scholar]
- 38. Robzyk K., Recht L., Osley M.A.. Rad6-dependent ubiquitination of histone H2B in yeast. Science. 2000; 287:501–504. [DOI] [PubMed] [Google Scholar]
- 39. Sun Z.W., Allis C.D.. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature. 2002; 418:104–108. [DOI] [PubMed] [Google Scholar]
- 40. Briggs S.D., Xiao T., Sun Z.W., Caldwell J.A., Shabanowitz J., Hunt D.F., Allis C.D., Strahl B.D.. Gene silencing: trans-histone regulatory pathway in chromatin. Nature. 2002; 418:498. [DOI] [PubMed] [Google Scholar]
- 41. Nakamura K., Kato A., Kobayashi J., Yanagihara H., Sakamoto S., Oliveira D.V., Shimada M., Tauchi H., Suzuki H., Tashiro S. et al. . Regulation of homologous recombination by RNF20-dependent H2B ubiquitination. Mol. Cell. 2011; 41:515–528. [DOI] [PubMed] [Google Scholar]
- 42. Zeng M., Ren L., Mizuno K., Nestoras K., Wang H., Tang Z., Guo L., Kong D., Hu Q., He Q. et al. . CRL4(Wdr70) regulates H2B monoubiquitination and facilitates Exo1-dependent resection. Nat. Commun. 2016; 7:11364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gatbonton T., Imbesi M., Nelson M., Akey J.M., Ruderfer D.M., Kruglyak L., Simon J.A., Bedalov A.. Telomere length as a quantitative trait: genome-wide survey and genetic mapping of telomere length-control genes in yeast. PLoS Genet. 2006; 2:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sikorski R.S., Hieter P.. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989; 122:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Forstemann K., Hoss M., Lingner J.. Telomerase-dependent repeat divergence at the 3΄ ends of yeast telomeres. Nucleic Acids Res. 2000; 28:2690–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peng J., He M.H., Duan Y.M., Liu Y.T., Zhou J.Q.. Inhibition of telomere recombination by inactivation of KEOPS subunit Cgi121 promotes cell longevity. PLoS Genet. 2015; 11:e1005071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lee J.Y., Kozak M., Martin J.D., Pennock E., Johnson F.B.. Evidence that a RecQ helicase slows senescence by resolving recombining telomeres. PLoS Biol. 2007; 5:1334–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu J.R., Gilbert D.M.. Rapid DNA preparation for 2D gel analysis of replication intermediates. Nucleic Acids Res. 1995; 23:3997–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Booth C., Griffith E., Brady G., Lydall D.. Quantitative amplification of single-stranded DNA (QAOS) demonstrates that cdc13-1 mutants generate ssDNA in a telomere to centromere direction. Nucleic Acids Res. 2001; 29:4414–4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tong X.J., Li Q.J., Duan Y.M., Liu N.N., Zhang M.L., Zhou J.Q.. Est1 protects telomeres and inhibits subtelomeric Y'-element recombination. Mol. Cell. Biol. 2011; 31:1263–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Duan Y.M., Zhou B.O., Peng J., Tong X.J., Zhang Q.D., Zhou J.Q.. Molecular dynamics of de novo telomere heterochromatin formation in budding yeast. J. Genet. Genomics. 2016; 43:451–465. [DOI] [PubMed] [Google Scholar]
- 52. Longtine M.S., McKenzie A., Demarini D.J., Shah N.G., Wach A., Brachat A., Philippsen P., Pringle J.R.. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998; 14:953–961. [DOI] [PubMed] [Google Scholar]
- 53. Corda Y., Schramke V., Longhese M.P., Smokvina T., Paciotti V., Brevet V., Gilson E., Geli V.. Interaction between Set1p and checkpoint protein Mec3p in DNA repair and telomere functions. Nat. Genet. 1999; 21:204–208. [DOI] [PubMed] [Google Scholar]
- 54. Schulze J.M., Hentrich T., Nakanishi S., Gupta A., Emberly E., Shilatifard A., Kobor M.S.. Splitting the task: Ubp8 and Ubp10 deubiquitinate different cellular pools of H2BK123. Genes Dev. 2011; 25:2242–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gardner R.G., Nelson Z.W., Gottschling D.E.. Ubp10/Dot4p regulates the persistence of ubiquitinated histone H2B: distinct roles in telomeric silencing and general chromatin. Mol. Cell. Biol. 2005; 25:6123–6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Emre N.C.T., Ingvarsdottir K., Wyce A., Wood A., Krogan N.J., Henry K.W., Li K.Q., Marmorstein R., Greenblatt J.F., Shilatifard A. et al. . Maintenance of low histone ubiquitylation by Ubp10 correlates with telomere-proximal Sir2 association and gene silencing. Mol. Cell. 2005; 17:585–594. [DOI] [PubMed] [Google Scholar]
- 57. Faucher D., Wellinger R.J.. Methylated H3K4, a transcription-associated histone modification, is involved in the DNA damage response pathway. PLoS Genet. 2010; 6:e1001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhu Z., Chung W.H., Shim E.Y., Lee S.E., Ira G.. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008; 134:981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Craven R.J., Petes T.D.. Dependence of the regulation of telomere length on the type of subtelomeric repeat in the yeast Saccharomyces cerevisiae. Genetics. 1999; 152:1531–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ribeyre C., Shore D.. Anticheckpoint pathways at telomeres in yeast. Nat. Struct. Mol. Biol. 2012; 19:307–313. [DOI] [PubMed] [Google Scholar]
- 61. Askree S.H., Yehuda T., Smolikov S., Gurevich R., Hawk J., Coker C., Krauskopf A., Kupiec M., McEachern M.J.. A genome-wide screen for Saccharomyces cerevisiae deletion mutants that affect telomere length. Proc. Natl. Acad. Sci. U.S.A. 2004; 101:8658–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pike B.L., Heierhorst J.. Mdt1 facilitates efficient repair of blocked DNA double-strand breaks and recombinational maintenance of telomeres. Mol. Cell. Biol. 2007; 27:6532–6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Grandin N., Charbonneau M.. Control of the yeast telomeric senescence survival pathways of recombination by the Mec1 and Mec3 DNA damage sensors and RPA. Nucleic Acids Res. 2007; 35:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Grandin N., Charbonneau M.. Mitotic cyclins regulate telomeric recombination in telomerase-deficient yeast cells. Mol. Cell. Biol. 2003; 23:9162–9177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bertuch A.A., Lundblad V.. EXO1 contributes to telomere maintenance in both telomerase-proficient and telomerase-deficient Saccharomyces cerevisiae. Genetics. 2004; 166:1651–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Azam M., Lee J.Y., Abraham V., Chanoux R., Schoenly K.A., Johnson F.B.. Evidence that the S.cerevisiae Sgs1 protein facilitates recombinational repair of telomeres during senescence. Nucleic Acids Res. 2006; 34:506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Trujillo K.M., Osley M.A.. A role for H2B Ubiquitylation in DNA replication. Mol. Cell. 2012; 48:734–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lin C.Y., Wu M.Y., Gay S., Marjavaara L., Lai M.S., Hsiao W.C., Hung S.H., Tseng H.Y., Wright D.E., Wang C.Y. et al. . H2B mono-ubiquitylation facilitates fork stalling and recovery during replication stress by coordinating Rad53 activation and chromatin assembly. PLoS Genet. 2014; 10:e1004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fleming A.B., Kao C.F., Hillyer C., Pikaart M., Osley M.A.. H2B ubiquitylation plays a role in nucleosome dynamics during transcription elongation. Mol. Cell. 2008; 31:57–66. [DOI] [PubMed] [Google Scholar]
- 70. Chandrasekharan M.B., Huang F., Sun Z.W.. Ubiquitination of histone H2B regulates chromatin dynamics by enhancing nucleosome stability. Proc. Natl. Acad. Sci. U.S.A. 2009; 106:16686–16691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chandrasekharan M.B., Huang F., Sun Z.W.. Histone H2B ubiquitination and beyond regulation of nucleosome stability, chromatin dynamics and the trans-histone H3 methylation. Epigenetics. 2010; 5:460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.