Abstract
Trypanosoma cruzi infects 15 to 20 million people in Latin America and causes Chagas disease, a chronic inflammatory disease with fatal cardiac and gastrointestinal sequelae. How the immune response causes Chagas disease is not clear, but during the persistent infection both proinflammatory and anti-inflammatory responses are critical. Natural killer T (NKT) cells have been shown to regulate immune responses during infections and autoimmune diseases. We report here that during acute T. cruzi infection NKT-cell subsets provide distinct functions. CD1d−/− mice, which lack both invariant NKT (iNKT) cells and variant NKT (vNKT) cells, develop a mild phenotype displaying an increase in spleen and liver mononuclear cells, anti-T. cruzi antibody response, and muscle inflammation. In contrast, Jα18−/− mice, which lack iNKT cells but have vNKT cells, develop a robust phenotype involving prominent spleen, liver, and skeletal muscle inflammatory infiltrates comprised of NK, dendritic, B and T cells. The inflammatory cells display activation markers; produce more gamma interferon, tumor necrosis factor alpha, and nitric oxide; and show a diminished antibody response. Strikingly, most Jα18−/− mice die. Thus, in response to the same infection, vNKT cells appear to augment a robust proinflammatory response, whereas the iNKT cells dampen this response, possibly by regulating vNKT cells.
Trypanosoma cruzi, the causative agent of Chagas disease, infects approximately 18 million individuals in Latin America and is the major cause of heart disease in areas of endemicity (71). Most infected individuals survive the acute phase and then remain infected throughout their lifetime. Thirty to forty percent of those infected develop Chagas disease, a chronic inflammatory disease that commonly results in cardiomyopathy and gastrointestinal tract dysfunction (71). The etiology of Chagas disease appears complicated (10). Why some infected individuals develop Chagas disease and others do not and why the clinical manifestations of Chagas disease are highly heterogeneous are unclear. A critical factor might be an individual's ability to regulate the anti-T. cruzi immune response that controls the persistent parasites, which can also contribute to the inflammatory damage of tissues that causes Chagas disease.
Natural killer T (NKT) cells are a class of T cells that regulate innate and adaptive immune responses. Unlike conventional T cells, NKT cells are stimulated by glycolipids presented by the major histocompatibility complex-like CD1d molecular complex that is composed of β2-microglobulin and the nonpolymorphic CD1d chain (8). NKT-cell selection is dependent on the CD1d molecular complex (8). Thus, mice lacking the CD1d gene (CD1d−/− mice) are deficient in all CD1d-restricted NKT cells (61). Many NKT cells express an invariant Vα14-Jα18 T-cell receptor (TCR) (48). Thus, mice lacking the Jα18 gene (Jα18−/− mice) are deficient in the invariant NKT (iNKT) cells, but the T-cell repertoire of these mice includes CD1d-restricted NKT cells that utilize diverse αβ TCR and γδ TCR genes (variant NKT [vNKT] cells) (15). The regulatory functions of NKT cells have been shown to prevent autoimmune diseases and to contribute to protective responses against pathogens (28, 32, 46).
NKT cells have been shown to suppress several mouse autoimmune diseases. In the mouse models of diabetes and systemic lupus erythematosus, NKT-cell populations are diminished, and increasing the number of NKT cells prevents disease (6, 31, 49, 50, 70, 76). Similarly, in several human autoimmune diseases, including diabetes, scleroderma, systemic lupus erythematosus, and rheumatoid arthritis, the NKT-cell population appears to be diminished and unable to prevent self-damaging responses (64, 69, 73). During infections, NKT cells have been shown to secrete proinflammatory cytokines that stimulate the innate and adaptive responses that eliminate the pathogen (22, 43, 44). In contrast, during other infections, NKT cells have been shown to secrete anti-inflammatory cytokines that limit the infection-induced pathology (19). It remains unclear how NKT cells during some infections augment proinflammatory responses to control pathogens, whereas in other infections they inhibit inflammatory responses to prevent infection-induced tissue damage.
We previously demonstrated that during T. cruzi infection NKT cells contribute to the control of the acute-phase parasitemia (21). We report here that during acute infection NKT cells both augment and limit aspects of the antiparasite response. Furthermore, our data indicate that Jα18−/− mice (deficient in only iNKT cells) suffer much greater morbidity and mortality than CD1d−/− mice (deficient in both iNKT and vNKT cells). These data argue that during the same infection vNKT cells augment the antiparasite response, whereas iNKT cells function to limit the antiparasite response. Thus, the interaction of the two NKT-cell subsets during T. cruzi infection appears to modulate the level of parasite persistence and inflammatory tissue damage.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free, 7- to 10-week-old mice with similar preinfection weights were used. Female mice were used except where noted. Wild-type C57BL/6 mice were purchased from Charles River, Wilmington, Mass. C57BL/6 CD1d−/− and Jα18−/− mice were bred in the animal facilities of the Corixa Corp., Seattle, Wash. (15, 61). The CD1d−/− mice were back-crossed to C57BL/6 mice a minimum of seven generations, and the Jα18−/− mice were back-crossed to C57BL/6 mice a minimum of nine generations. All of the animal procedures were approved by the institutional animal care and use committee.
T. cruzi.
The CL strain of T. cruzi was used (77). Trypomastigotes were obtained from culture supernatants of infected 3T3 cells grown in Dulbecco modified Eagle medium (BioWhittaker, Walkersville, Md.) supplemented with 10% heat-inactivated calf serum (BioWhittaker) and 50,000 U of penicillin-streptomycin (BioWhittaker). Mice were inoculated intraperitoneally with T. cruzi trypomastigotes.
Histology.
Livers and muscles were fixed in formalin (Sigma, St. Louis, Mo.), sectioned, and either stained with hematoxylin and eosin (Sigma) or processed for T-cell detection. For T-cell detection, 4-μm sections were placed on charged slides (VWR International, South Plainfield, N.J.), air dried, and heated for 30 min at 59°C. Paraffin was removed by incubation in Histo-Clear (National Diagnostics, Atlanta, Ga.) and aqueous ethanol, and the slides were boiled in sodium citrate buffer to “retrieve” the epitopes. The slides were washed in phosphate-buffered saline and incubated for 30 min in 0.3% H2O2 in methanol, followed by incubation with a polyclonal rabbit anti-human CD3 antibody that cross-reacts with mouse CD3 or a polyclonal rabbit anti-human control antibody (both antibodies from DakoCytomation, Glostrup, Denmark) diluted in 3% normal goat serum and the stain developed by using a Vectastain ABC kit (Vector Laboratories, Burlingame, Calif.).
Cell enumeration and flow cytometry.
Liver and spleen mononuclear cells were prepared as previously described (21). Cells were incubated with combinations of the following fluorescent-labeled antibodies: anti-B220 (RA3-6B2), anti-CD11c (HL3), anti-CD69 (H1.2F3), anti-NK1.1 (PK136), and anti-TCRβ (H57-597) (all from BD Pharmingen, San Diego, Calif.). Flow cytometry was performed by using FACScan (BD Biosciences, San Jose, Calif.), and the data were analyzed with WinMDI 2.7 (Joseph Trotter [http://facs.scripps.edu/software.html]).
IFN-γ, TNF-α, and NO production.
Mononuclear cells were incubated in 24-well plates (Corning, Inc., Corning, N.Y.) at 2 × 106 cells/ml in RPMI 1640 supplemented with 5% heat-inactivated fetal calf serum (BioWhittaker) and 50,000 of U penicillin-streptomycin for 48 h. Supernatants were analyzed by using an gamma interferon (IFN-γ) enzyme-linked immunosorbent assay (ELISA; BD Pharmingen), a tumor necrosis factor alpha (TNF-α) ELISA (DuoSet; R&D Systems, Minneapolis, Minn.), and the Greiss reaction for nitric oxide (NO) (52).
Antibody analyses.
Individual mouse sera were analyzed by antibody capture ELISA with a previously described anti-T. cruzi ELISA (21). Briefly, ELISA plates (Nunc, Rochester, N.Y.) were coated with heat-killed trypomastigotes and blocked with 1% bovine serum albumin-phosphate-buffered saline. Then, in consecutive order, serum samples, 1 μg of biotinylated detection antibodies for immunoglobulin G2a (IgG2a; R19-15; BD Pharmingen), streptavidin-horseradish peroxidase (Genzyme, Cambridge, Mass.), and ABTS [2,2′azinobis(3-ethylbenzthiazolinesulfonic acid)]-H2O2 (Kirkegaard and Perry Laboratories, Gaithersburg, Md.)/ml were added to the plates. Plates were analyzed at 405 nm (ELX808; Bio-Tek Instruments, Inc., Winooski, Vt.). The antibody titers were defined as the greatest dilution that provided an optical density at 405 nm greater than the mean optical density at 405 nm and three standard deviations (SD) of uninfected sera at the same dilution.
Inflammatory score.
Blinded investigators obtained five random ×10 images of the left and right quadriceps muscle (10 images per mouse; Eclipse E200 microscope and Coolpix 4500 camera; Nikon, Tokyo, Japan). Blinded investigators overlaid the printed images with a grid of 49 evenly dispersed points, and the percentage of points intersected by nuclei was determined. The percentage of points intersected by nuclei of uninfected quadriceps images (background) was subtracted. Three mice per group were analyzed to provide 30 scores per strain for statistical analysis.
Statistics.
The P values for Kaplan-Meier survival analysis were determined by using a log-rank statistic method (Epi Info 2002 [http://www.cdc.gov/epiinfo/]). All other P values were determined by using the Student t test (Microsoft Excel; Microsoft Corp., Redmond, Wash.).
RESULTS
During T. cruzi infection two NKT-cell-deficient mouse strains display differences in survival.
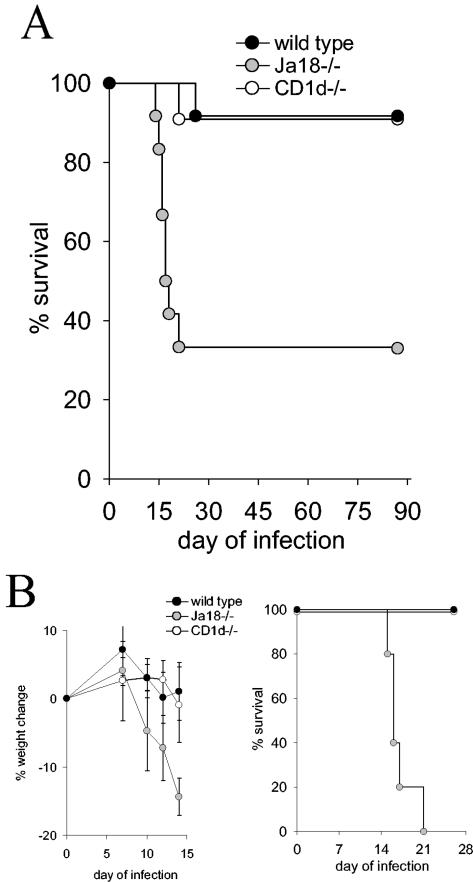
We previously demonstrated that two NKT-cell-deficient mouse strains (the CD1d−/− strain that is deficient in all CD1d-restricted NKT cells [iNKT and vNKT cells], and the Jα18−/− strain that is deficient in only iNKT cells) are more susceptible to T. cruzi infection (21). In these previous experiments female mice were inoculated with a sublethal dose of trypomastigotes (105) and compared to wild-type mice, both NKT-cell-deficient strains exhibited greater parasitemia (21). To further examine the role of NKT cells during T. cruzi infection, wild-type, CD1d−/−, and Jα18−/− female mice were inoculated with a larger dose of trypomastigotes (2 × 105). The combined results of three independent experiments demonstrate a striking increase in mortality of the Jα18−/− mice compared to either the wild-type or the CD1d−/− mice (Fig. 1A). A total of 67% of the Jα18−/− mice died compared to 8% of the wild-type mice (P < 0.005) or 9% of the CD1d−/− mice (P < 0.005) (Fig. 1A). In Fig. 1B, the data of one of these independent experiments are presented. The Jα18−/− mice, compared to CD1d−/− and wild-type mice, demonstrate more severe infection-induced weight loss and increased mortality (P < 0.005) (Fig. 1B). In an additional experiment, Jα18−/− and CD1d−/− male mice were inoculated with 5 × 105 trypomastigotes, and again the Jα18−/− mice suffered increased infection-induced weight loss and death (data not shown). These data indicate that during acute T. cruzi infection Jα18−/− mice, compared to wild-type and CD1d−/− mice, suffer more infection-induced weight loss and death.
FIG. 1.
T. cruzi infection of Jα18−/− mice, compared to CD1d−/− mice, causes increased mortality. (A and B) Female mice were inoculated with 2 × 105 trypomastigotes. In panel A, the combined survival data of three separate experiments that used a total of 12 Jα18−/− mice, 11 CD1d−/− mice, and 12 wild-type mice are presented. The log-rank survival statistic was calculated, and the P values were derived as follows: Jα18−/− mice compared to wild-type mice, P < 0.005; and Jα18−/− mice compared to CD1d−/− mice, P < 0.005. In panel B, a single experiment from panel A that used five mice per group is presented. On the indicated days each mouse was weighed, and the mean and the standard error (SE) of the percentage weight change compared to day 0 for each group is displayed. The log-rank survival statistic was calculated, and the P value was derived as follows: Jα18−/− mice compared to wild-type mice or CD1d−/− mice, P < 0.005.
During T. cruzi infection Jα18−/− mice, compared to wild-type and CD1d−/− mice, exhibit altered immune responses of liver, spleen, and skeletal muscle.
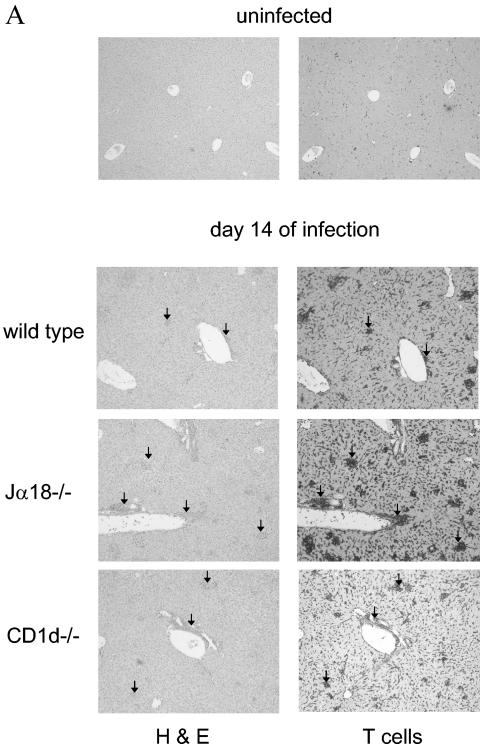
Since our previous data indicated that both Jα18−/− and CD1d−/− mouse strains develop similarly increased parasitemia compared to wild-type mice, we were surprised that the larger inoculum of trypomastigotes resulted in a marked increase in mortality of the Jα18−/− mice (Fig. 1) (21). These data suggest that the increased mortality in the Jα18−/− strain is not related to the lack of parasite control but rather is related to improper regulation of the proinflammatory response that is elicited by T. cruzi infection (30, 39). To begin to examine the possibility that poor immune regulation causes increased mortality of the Jα18−/− mice, we decided to analyze aspects of the liver and spleen immune responses. These organs both contain significant NKT-cell populations and are critically involved in the immune response against the parasite. Wild-type, CD1d−/−, and Jα18−/− mice were infected with T. cruzi, and we examined sections of liver stained with hematoxylin and eosin or a T-cell-specific antibody 7, 11, and 14 days after inoculation. At day 7 of the infection, no differences were detected between strains, but by day 11 more inflammatory foci, including T-cell-rich infiltrates, were evident in the Jα18−/− mice (data not shown). At day 14 of the infection this difference was maximal (Fig. 2A). The Jα18−/− livers contained the greatest density of inflammatory foci, the CD1d−/− livers contained an intermediate value, and the wild-type livers contained the fewest foci. Comparisons of adjacent sections stained with hematoxylin and eosin or T-cell-specific antibody indicate that all of the foci included abundant T cells (Fig. 2A). At higher magnification, the T cells of the infected livers, compared to the uninfected livers, appeared larger, suggesting that they were activated (data not shown). These differences in T-cell-rich, inflammatory responses indicate that the immune response to the parasite is regulated differently in wild type, CD1d−/−, and Jα18−/− mice.
FIG. 2.
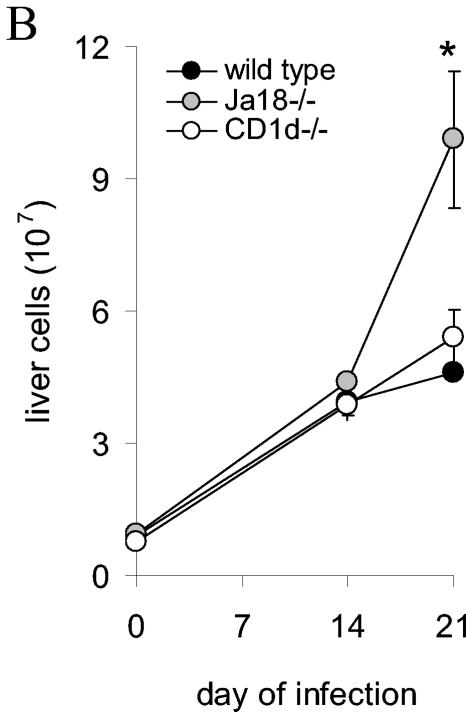
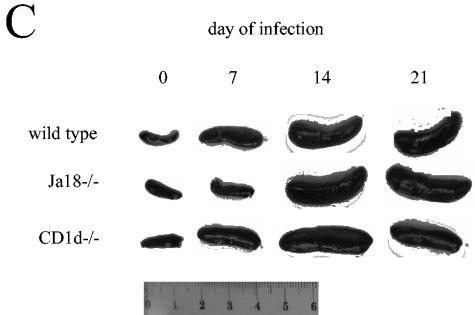
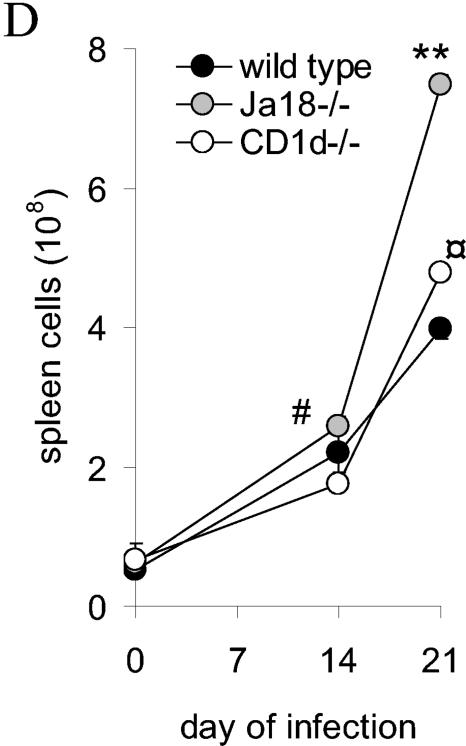
During T. cruzi infection Jα18−/−, CD1d−/− and wild-type mice display differences in their liver and spleen immune responses. Mice were inoculated with trypomastigotes, and on different days of the infection the livers and spleens were removed for analyses. (A) Representative images of livers stained with hematoxylin and eosin or antibody to T cells from mice infected for 14 days (there was no staining with a control antibody). Arrows indicate inflammatory foci. Original magnification, ×40. (B) Liver mononuclear cells were quantified. (C) Spleens were removed from mice at the indicated time of infection and photographed. The images are representative of three independent experiments and a total of six mice per strain. (D) Splenocytes were quantified. In panels A and C, mice were inoculated with 2 × 105 trypomastigotes. In panels B and D, mice were inoculated with 105 trypomastigotes, and each datum point represents the mean and SD of two mice. Significance: in panel B, comparing Jα18−/− to wild type, “*” indicates P < 0.05; in panel D, comparing Jα18−/− to CD1d−/−, “#” indicates P < 0.05; comparing Jα18−/− to wild type or CD1d−/−, “**” indicates P < 0.01; and comparing CD1d−/− to wild type, “ ” indicates P < 0.05. Similar results were obtained in four independent experiments.
To confirm our subjective histopathologic findings, liver mononuclear cell density (combination of normal hepatic cell types and inflammatory cells) was quantified on different days of the infection. Consistent with the estimated densities of T-cell-rich infiltrates described above, on day 21 more mononuclear cells were detected in the Jα18−/− mice compared to both the wild-type mice (P < 0.05) and the CD1d−/− mice (P = 0.06) (Fig. 2B). Taken together, these data argue that during the acute infection Jα18−/− mice, compared to wild-type and CD1d−/− mice, have altered regulation of their immune response and reinforce the possibility that this altered regulation might contribute to the increased morbidity and mortality suffered by the Jα18−/− mice.
When the spleens of T. cruzi-infected wild type, CD1d−/−, and Jα18−/− mice were investigated at different times of the infection we observed strain-specific differences in spleen enlargement (Fig. 2C). On day 7 of infection the spleens of wild-type and CD1d−/− mice were reproducibly larger than the spleens of the Jα18−/− mice (Fig. 2C). On days 14 and 21, all three strains continued to develop increasing splenomegaly, but the Jα18−/− spleens appeared the largest (Fig. 2C). These data suggest the possibility that in Jα18−/− mice the immune response is initially delayed causing a lag in spleen enlargement but that, once the immune response becomes established, it is less well controlled and results in greater splenomegaly.
To further examine the differences, splenocytes were quantified. Because an inoculum of 2 × 105 trypomastigotes killed most Jα18−/− mice by day 15 of infection (Fig. 1), additional mice were inoculated with 105 trypomastigotes so that later time points could be analyzed. Again, in these experiments, on day 14 of the infection the Jα18−/− mice had an increased number of splenocytes compared to CD1d−/− mice (P < 0.05) (Fig. 2D). On day 21 of the infection this increase in Jα18−/− splenocytes was more pronounced (P < 0.005 for Jα18−/− mice compared to either CD1d−/− or wild-type mice) (Fig. 2D). Furthermore, the CD1d−/− mice have more splenocytes than the wild-type mice (P < 0.05) (Fig. 2D). These data indicate that during the late acute phase of T. cruzi infection, Jα18−/− mice, compared to wild-type and CD1d−/− mice, have increased splenocytes. Our data indicate that Jα18−/− and CD1d−/− mice have different immune responses and suggest that the immune response in the Jα18−/− mice is less well controlled.
During acute infection, activated cells of the innate and adaptive immune responses increase in Jα18−/− mice.
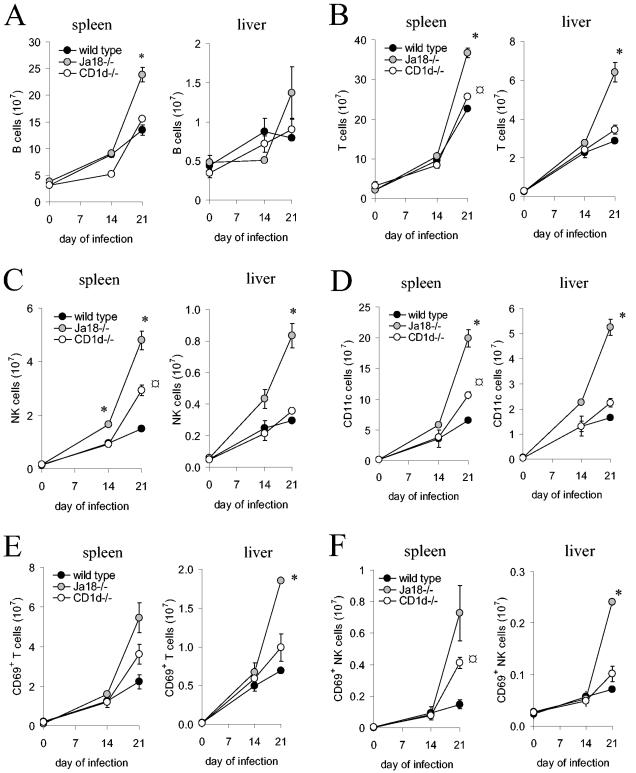
In Jα18−/− mice the large increase in spleen and liver mononuclear cells that occurs during T. cruzi infection might reflect alterations in cells of the innate and adaptive immune responses, which are known to be regulated by NKT cells. Therefore, during the acute infection spleen and liver mononuclear cells were isolated and labeled with antibodies to quantify cell types (anti-NK1.1 to detect NK cells, anti-TCRβ to detect T cells, anti-CD11c to detect dendritic cells, and anti-B220 to detect B cells). In the spleens and livers of all strains of mice, the number of B cells, T cells, NK cells, and dendritic cells were increased on day 21 of the infection (Fig. 3). Interestingly, for all cell types analyzed, the wild-type mice demonstrated the smallest increase, the CD1d−/− mice an intermediate increase, and the Jα18−/− mice the largest increase (Fig. 3). On day 21 of the infection, in the spleen and liver, the Jα18−/− mice compared to the wild-type mice, demonstrated an approximate twofold increase in B cells and T cells (Fig. 3A and B). More striking was that on day 21 of the infection, comparing either spleen or liver cells of Jα18−/− mice to wild-type mice, there was an ∼3-fold increase in NK cells and dendritic cells (Fig. 3C and D). These data indicate that during T. cruzi infection CD1d-restricted NKT cells regulate cells of both the innate and adaptive immune responses. These data again demonstrate that the Jα18−/− mice, which lack only iNKT cells, have a much greater increase in cells and apparent dysregulation than the CD1d−/− mice, which lack both iNKT and vNKT cells. Thus, it appears that during T. cruzi infection the iNKT cells have a distinct regulatory function from that of the vNKT cells. The increase in innate and adaptive immune response cells in the spleen and liver could be contributing to a more robust inflammatory immune response that causes the increased morbidity and mortality of the Jα18−/− mice.
FIG. 3.
During T. cruzi infection Jα18−/− mice have increased numbers of cells of the innate and adaptive immune response. Wild-type, CD1d−/−, and Jα18−/− mice were inoculated with 105 trypomastigotes and splenocytes, and liver mononuclear cells were prepared from two mice of the three strains on days 14 and 21 of the infection. Uninfected (day 0) mice were also analyzed. The mononuclear cells were stained with directly conjugated fluorescent monoclonal antibodies to NK1.1, TCRβ, CD11c, B220, and CD69 and analyzed by flow cytometry. The mean and SD of the number of spleen and liver B cells (A), T cells (B), NK cells (C), CD11c+ cells (D), CD69+ T cells (E), and CD69+ NK cells (F) are shown. Significance: comparing Jα18−/− to wild type or CD1d−/−, “* indicates P < 0.05; comparing CD1d−/− to wild type, “ ” indicates P < 0.05. Two mice were analyzed at each time, and the results are representative of three independent experiments.
If the innate and adaptive cells that are increased in number are contributing to an increase in the inflammatory response, then they are likely to be activated. The histological analyses of the livers argued that the T cells of the infected mice were enlarged and therefore activated (Fig. 2A and data not shown). To confirm our histological observations, CD69 expression, which is increased on activated T cells and NK cells, was examined. These data indicate that as the number of spleen and liver T and NK cells increased, so did the number of activated T and NK cells (Fig. 3E and F). Again, these results are consistent with the possibility that in the Jα18−/− mice, the absence of iNKT cells and their regulatory function results in more activated T and NK cells with associated increased morbidity and mortality.
Jα18−/− mice produce more IFN-γ, TNF-α, and NO.
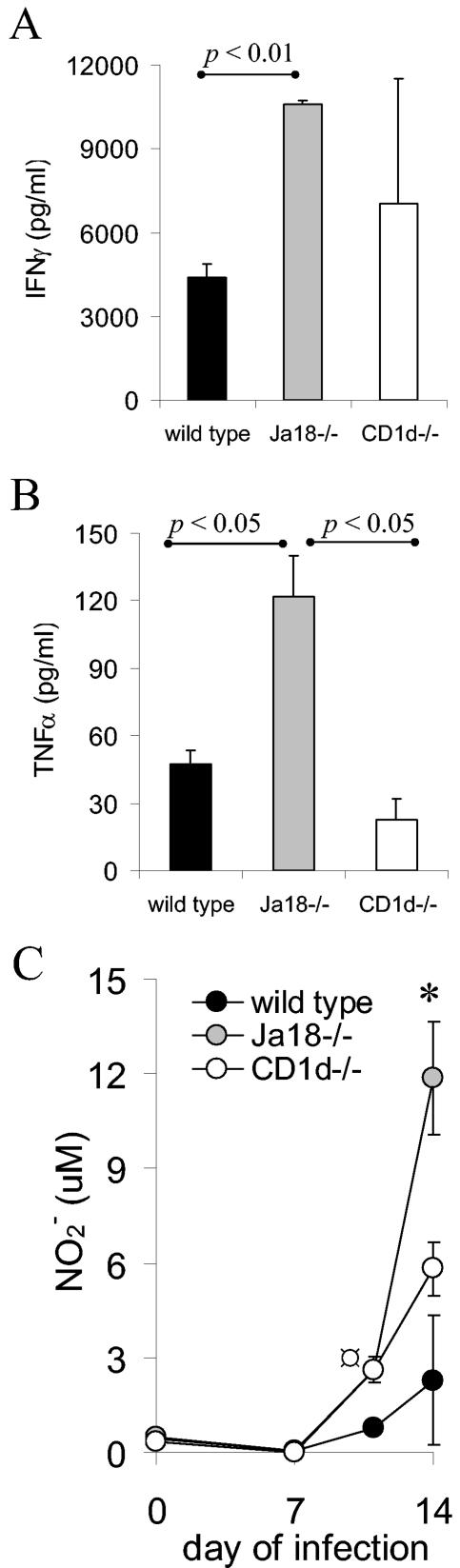
The increased number of spleen and liver inflammatory cells in Jα18−/− mice suggested that these cells might be secreting effector molecules that were contributing to the increased morbidity and mortality. To investigate this possibility, wild-type, CD1d−/−, and Jα18−/− mice were infected with T. cruzi and on days 7, 11, 14, and 21 of the infection splenocytes were isolated and cultured ex vivo for 48 h without exogenous stimulation. The supernatants were then analyzed for IFN-γ, TNF-α, and NO. The splenocytes of Jα18−/− mice isolated on day 14 of the infection produced more IFN-γ and TNF-α (Fig. 4A and B). Furthermore, on days 11 and 14 of the infection the Jα18−/− splenocytes secreted more NO (Fig. 4C, P < 0.05). Taken together, these data argue that the Jα18−/− mice lack iNKT cells that control the inflammatory response and, compared to the wild-type and CD1d−/− mice, the Jα18−/− mice produce increased amounts of IFN-γ, TNF-α, and NO that might contribute to increased morbidity and mortality.
FIG. 4.
Jα18−/− mouse splenocytes produce increased amounts of proinflammatory mediators. Wild type, CD1d−/−, and Jα18−/− mice were inoculated with 105 trypomastigotes and, on the indicated day of the infection, the splenocytes were isolated and placed in culture without exogenous stimulation. After 48 h the culture supernatants were assayed for IFN-γ (day 14) (A), TNF-α (day 14) (B), or NO (on the indicated days) (C). Two individual mice were analyzed at eachtime, and the means and SD are shown. Cells of uninfected mice did not produce detectable amounts. Significance: in panel C, comparing Jα18−/− to wild type or CD1d−/−, “*” indicates P < 0.05; comparing wild type to CD1d−/−, “ ” indicates P < 0.05. Similar results were obtained in four independent experiments.
Diminished antibody response in Ja18−/− mice.
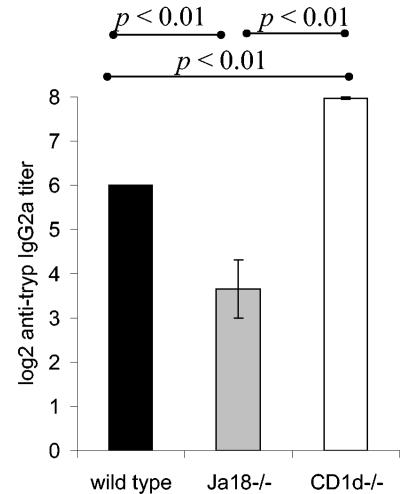
Previous studies have demonstrated that NKT cells can influence the antibody responses (25, 26, 34). The data in Fig. 4 argue that the Jα18−/− mice have an increased proinflammatory response and suggested that they may have a diminished humoral response. Wild-type, CD1d−/−, and Jα18−/− mice were infected with T. cruzi, and titers were determined in sera from day 21-infected mice by serial dilutions for anti-T. cruzi antibodies. These data demonstrated a marked decrease in the anti-T. cruzi IgG2a antibody titer of the Jα18−/− mice compared to the wild-type mice (Fig. 5). Interestingly, the CD1d−/− mice, compared to the wild-type mice, demonstrated a slight increase in the anti-T. cruzi IgG2a response (Fig. 5). In addition, we examined the anti-T. cruzi IgG1 response. In each strain of mice the IgG1 titer was much lower than the IgG2a titer, and there was no significant difference in IgG1 titers between the strains (data not shown). These data further support the observations that, during T. cruzi infection, wild-type, CD1d−/−, and Jα18−/− mice have different immune responses and further argue that the iNKT cells and vNKT cells perform different regulatory functions. The data suggest that the increased proinflammatory response in Jα18−/− mice blunts their anti-T. cruzi antibody response, whereas the CD1d−/− mice develop a more tempered acute proinflammatory response that supports a larger antibody response.
FIG. 5.
Jα18−/− mice have a diminished anti-T. cruzi antibody response. Wild-type, CD1d−/−, and Jα18−/− mice (five animals per group) were inoculated with 105 trypomastigotes, and the IgG2a titers of individual mice on day 21 of the infection were determined by assaying twofold serial dilutions by using a T. cruzi ELISA. The results are expressed as the mean and SE of the titer for each strain.
NKT-cell-deficient mice develop increased infection-induced muscle inflammation.
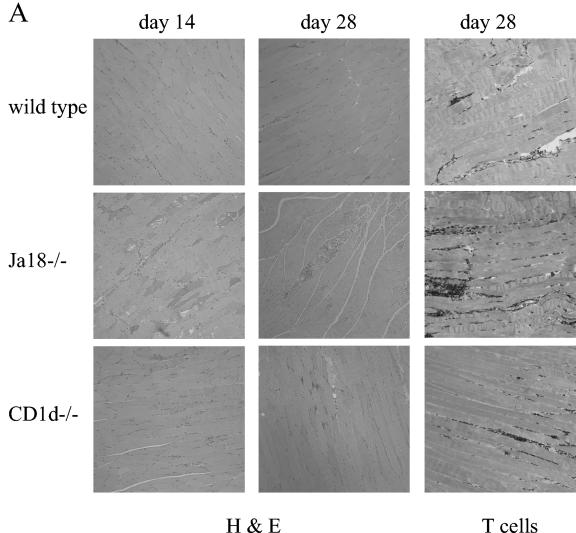
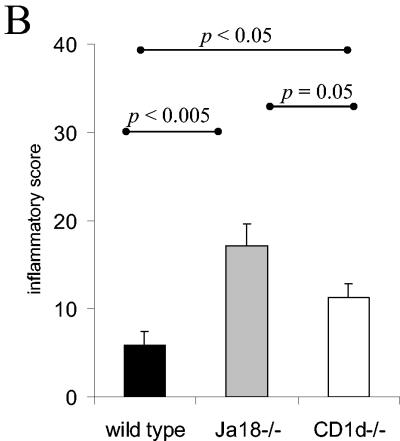
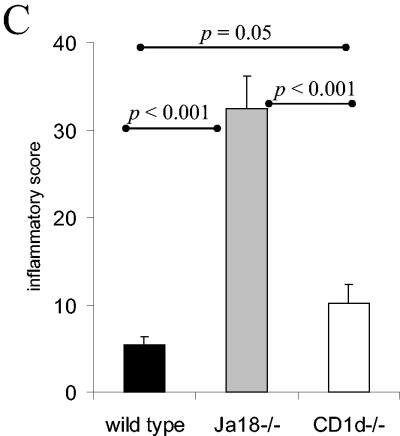
During T. cruzi infection parasites invade and replicate in muscle tissue and trigger an inflammatory response that is often clinically significant. Therefore, we analyzed the muscles of wild-type, CD1d−/−, and Jα18−/− mice that had been infected for 14 or 28 days. At day 14 of the infection, the skeletal muscles of the Jα18−/− mice exhibited significantly greater inflammation than the muscles of both CD1d−/− and wild-type mice (Fig. 6A and B). In addition, the muscles of CD1d−/− mice were more inflamed than the muscles of the wild-type mice (Fig. 6B). In this particular experiment, mice survived to day 28 of the infection, and the Jα18−/− mice, compared to the wild-type and CD1d−/− mice, had dramatically increased muscle inflammation (Fig. 6A and C). In addition, at day 28 of the infection, the CD1d−/− mice developed more inflammation than the wild-type mice (Fig. 6C). Finally, immunohistochemistry analysis with an anti-T-cell antibody demonstrated, as in the liver, that in wild-type, CD1d−/−, and Jα18−/− muscle the infiltrating cells appear to be rich in T cells (Fig. 6A). Together, these data indicate that during acute T. cruzi infection both NKT-cell-deficient mouse strains suffer increased peripheral muscle inflammation compared to wild-type mice. These data argue that during T. cruzi infection, NKT cells absent in Jα18−/− mice and CD1d−/− mice help regulate the immune response and help control the development of inflammation and pathology. Furthermore, the Jα18−/− mice suffered significantly more muscle inflammation than the CD1d−/− mice. Taken together, these data suggest that during T. cruzi infection the iNKT cells (absent in both the Jα18−/− mice and the CD1d−/− mice) are critical for downregulation and control of the acute immune response, whereas the vNKT cells (which are present in the Jα18−/− mice but absent in the CD1d−/− mice) upregulate aspects of the inflammatory immune response.
FIG. 6.
NKT-cell-deficient mice suffer greater muscle inflammation. Mice were inoculated with 105 trypomastigotes, and 14 or 28 days later skeletal muscles were prepared for histological analyses. (A) Representative images of muscle stained with either hematoxylin-eosin or antibody to T cells. Original magnification, ×100. (B and C) At day 14 (B) and day 28 (C) of the infection, the muscle inflammatory scores of the different strains are presented as the mean and SE.
DISCUSSION
We used two different NKT-cell-deficient mouse strains (Jα18−/− mice and CD1d−/− mice) to investigate the function of NKT cells during acute T. cruzi infection and found that these two strains not only display different phenotypes than wild-type mice but also display strikingly different phenotypes compared to each other. During the acute infection, compared to wild-type mice, both NKT-cell-deficient mouse strains demonstrated a greater increase in mononuclear cells in the spleen and liver. The increased mononuclear cells included NK cells, dendritic cells, B cells, and T cells. The inflammatory infiltrates in tissues of the NKT-cell-deficient mice appeared activated and produced greater amounts of NO. In addition, the anti-T. cruzi antibody responses of both NKT-cell-deficient strains deviated from those of wild-type mice, albeit in opposite directions. Strikingly, the Jα18−/− mice, compared to the CD1d−/− mice, demonstrated greater increases in spleen and liver mononuclear cells; greater increases in IFN-γ, TNF-α, and NO production; and greater muscle inflammation. These data argue that the Jα18−/− mice, compared to the wild-type and CD1d−/− mice, are less able to control the immune response against the parasite. It appears that the poor control of the immune response by the Jα18−/− mice leads to greater morbidity and mortality. Taken together, these results indicate that during T. cruzi infection iNKT cells and vNKT cells have distinct functions: the vNKT cells, which are absent in the CD1d−/− mice, appear to promote the acute-phase proinflammatory response, whereas the iNKT cells, which are absent in both the Jα18−/− and CD1d−/− mice, appear to downregulate the acute-phase proinflammatory response.
The data presented here are consistent with our previous results that NKT cells contribute to the immune response against T. cruzi (21). In contrast to our results, other studies have not demonstrated a role for NKT cells in experimental T. cruzi infection (53, 56). This discrepancy may relate to the different experimental systems used. Since we are interested in understanding how the immune response against T. cruzi permits parasite persistence and the evolution of Chagas disease in people, we use the CL strain, a well-defined strain that causes Chagas disease and permits the investigation of chronic T. cruzi infection in mice (20, 77). In a study by Procopio et al., a large inoculum of Y strain parasites that resulted in death in <3 weeks was given to wild-type and CD1d−/− mice; Jα18−/− mice were not investigated (56). In these studies wild-type and CD1d−/− mice suffered similar mortality, in agreement with our data (56). In their study, however, no difference in parasitemia was detected, possibly because they used male mice, which have increased variability in parasitemia (56). Furthermore, the large inoculum of parasites might have overwhelmed the contribution of the NKT cells (56). In the study by Miyahira et al., the highly virulent Tulahuen strain was used (53). This parasite strain does not appear to cause Chagas disease in humans, and in mice inocula of <100 parasites causes unremitting parasitemia and death (53). Again, CD1d−/− mice, but not Jα18−/− mice, were investigated, and in agreement with our data, their results also demonstrated that wild-type and CD1d−/− mice have similar mortality (53). Miyahira et al. did not detect differences in parasitemia between the wild-type and CD1d−/− strains, and again this might be because the virulence of the Tulahuen strain overwhelms the protective NKT-cell response. Our data clearly indicate that during T. cruzi infection NKT cells contribute significantly to the immune response.
In the present study, the acute-phase immune response to T. cruzi of wild-type, CD1d−/−, and Jα18−/− mice are compared. The T. cruzi antibody response of the Jα18−/− mice, compared to wild-type mice, is decreased as might be expected given that Jα18−/− mice have an increased proinflammatory response that would diminish the antibody response. Interestingly, the CD1d−/− mice, compared to wild-type mice, have an increased T. cruzi antibody response, suggesting that the lack of both iNKT cells and vNKT cells better supports the acute phase antibody response. The antigens or epitopes of these acute phase antibody responses are not known. Because T. cruzi trans-sialidase superfamily glycoproteins are known to stimulate acute-phase antibody responses, we investigated the acute-phase antibody response to the SA85-1.1 protein, a member of the trans-sialidase superfamily; we did not find a difference in the antibody response between wild-type or either NKT-cell-deficient mice strain (data not shown) (12, 42). Interestingly, in our previous investigations, during the chronic phase of the infection both NKT-cell-deficient mouse strains had a decreased antibody response to the SA85-1.1 glycoprotein, but a similar response to the whole parasite (21). Thus, NKT cells appear to affect the antibody responses during acute and chronic T. cruzi infection differently. Moreover, our data and the studies of several other investigators indicate that NKT cells can affect antibody responses (16, 25, 34, 47).
Our data indicate that during T. cruzi infection NKT-cell-deficient mice accumulate more liver and spleen inflammatory cells. This increase could be caused either by increased proliferation or homing of cells to the spleen and liver or by decreased removal of cells. CD1d can function to delete activated T cells and in the CD1d−/− mice this might account for the increased T cells (17). Clearly, other mechanisms are required to account for the increase in the other cell types, as well as the increase of cells in the CD1d-sufficient Jα18−/− mice. Several reports indicate that iNKT cells can localize to inflamed tissues and regulate immune responses (3, 40, 45). Thus, our data support the possibility that during T. cruzi infection iNKT and vNKT cells of the spleen and liver can regulate the homing or proliferation of a wide variety of cells. Similarly, during the infection NKT cells in the muscle might regulate the local inflammatory response.
Many studies indicate that NKT cells function in the immune response against various pathogens (32). During some infections NKT cells augment the response that kills the pathogen (44, 55). In contrast, during some infections, NKT cells limit the response to prevent tissue damage (19, 34, 57). These studies suggest that functionally distinct NKT-cell subsets exist and that during different infections different subsets are activated. NKT subsets have been defined by their TCR usage (invariant or variant), tissue distribution, or cell surface antigens (e.g., CD4, CD8, DX5, and CD69) (13, 14, 29). Our data suggest that during T. cruzi infection different NKT-cell subsets are activated. The Jα18−/− mice lack the iNKT subset, a well-defined subset that utilizes the Vα14-Jα18 TCR gene, whereas the CD1d−/− mice lack both the iNKT and the vNKT subsets. The cells of the vNKT subset are poorly defined. Although it is possible that differences in tissue parasite burden might contribute to differences in the muscle inflammatory response of the different mouse strains, our data argue that the phenotype of the Jα18−/− mice is caused by a lack of iNKT cells that limit the inflammatory response and prevent tissue damage, whereas the vNKT cells increase the inflammatory response that contributes to morbidity and mortality. The data suggest that in wild-type mice a balanced immune response is achieved, since the iNKT cells and vNKT cells provide complementary functions. To the best of our knowledge, the present study demonstrates for the first time that during the same infection CD1d-resticted NKT cells contribute to both the proinflammatory antipathogen responses and the anti-inflammatory, self-protective responses.
During T. cruzi infection iNKT cells appear to downregulate the immune response, but how they do so is unclear. Anti-inflammatory functions of NKT cells have been attributed to their secretion of IL-4, IL-10, IL-13, and transforming growth factor β (23, 54, 62, 66-68). These cytokines are all produced during T. cruzi infection and could be produced or regulated by iNKT cells (1, 2, 27, 39, 59, 60). During T. cruzi infection Jα18−/− mice develop a phenotype similar to mice deficient in the cytokine receptor subunit WSX-1 (30). Furthermore, it has been shown that NKT cells of WSX-1−/− mice produce increased amounts of proinflammatory cytokines (75). Thus, during T. cruzi infection the anti-inflammatory NKT cells might require WSX-1.
Other investigations support our observation that vNKT cells augment the proinflammatory response. Stenstrom et al. identified vNKT cells as an aggressive, proinflammatory subset of NKT cells (63). Also, during experimental hepatitis B virus and coxsackievirus infections, the vNKT cells appear to cause inflammatory-mediated tissue damage (5, 37). In the coxsackievirus studies, CD1d-restricted γδ T cells (vNKT cells) cause a fatal myocarditis (38). Interestingly, during T. cruzi infection γδ T cells cause significant tissue damage, suggesting that during T. cruzi infection CD1d-restricted γδ T cells might contribute to the morbidity and mortality (58).
Our data argue that during T. cruzi infection CD1d-restricted NKT cells both augment and limit the antiparasite response (21). It is unclear how NKT cells provide these opposing affects during the same infection. It is possible that self-antigens derived from different cellular compartments at different times of the infection stimulate distinct NKT-cell subsets (9, 18, 25, 41, 74). Alternatively, during the infection, as NKT cells are stimulated, their phenotype might evolve from proinflammatory to anti-inflammatory. For example, ligation of NK1.1 on NKT cells is associated with IFN-γ secretion, and therefore during the infection decreased expression of NK1.1 might diminish IFN-γ secretion (4, 33). We have observed that during T. cruzi infection NK1.1 expression on NKT cells decreases, with a nadir occurring as parasitemia is controlled (21). Thus, as parasitemia is controlled there is a decrease in NK1.1+ NKT cells, and this decrease might contribute to the evolution of a proinflammatory NKT-cell response to an anti-inflammatory response (11, 51, 72). Finally, we have also found that during T. cruzi infection CD4+ CD25+ Treg cells help limit the acute-phase inflammatory response (unpublished observations), and it is possible that NKT cells interact with CD4+ CD25+ Treg cells to limit the acute-phase response (7, 35, 36, 65).
In summary, our data indicate that during T. cruzi infection iNKT cells and vNKT cells perform complementary functions. The vNKT cells appear to promote the antiparasite proinflammatory response and, in contrast, the iNKT cells appear to limit the T. cruzi-induced response. In Jα18−/− mice, the absence of iNKT cells leads to a striking phenotype with increased inflammatory morbidity and death. Interestingly, in CD1d−/− mice, the absence of both iNKT cells and vNKT cells leads to a less severe phenotype, with a mild increase in inflammatory morbidity. Together, the data suggest the possibility that during T. cruzi infection the iNKT cells control the vNKT cells. The further definition of NKT-cell subsets and functions will better enable the development of therapeutics for inflammatory diseases.
Acknowledgments
We thank Karen Krause, Lori Lager, and Sally Norton at Children's Hospital and Regional Medical Center, Seattle, Wash., for advice and assistance with histological analyses.
This study was supported by National Institutes of Health grant AI49455.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abrahamsohn, I. A., A. P. da Silva, and R. L. Coffman. 2000. Effects of interleukin-4 deprivation and treatment on resistance to Trypanosoma cruzi. Infect. Immun. 68:1975-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antunez, M. I., and R. L. Cardoni. 2001. Early IFN-gamma production is related to the presence of interleukin (IL)-18 and the absence of IL-13 in experimental Trypanosoma cruzi infections. Immunol. Lett. 79:189-196. [DOI] [PubMed] [Google Scholar]
- 3.Apostolou, I., Y. Takahama, C. Belmant, T. Kawano, M. Huerre, G. Marchal, J. Cui, M. Taniguchi, H. Nakauchi, J. J. Fournie, P. Kourilsky, and G. Gachelin. 1999. Murine natural killer T (NKT) cells contribute to the granulomatous reaction caused by mycobacterial cell walls. Proc. Natl. Acad. Sci. USA 96:5141-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arase, H., N. Arase, and T. Saito. 1996. Interferon gamma production by natural killer (NK) cells and NK1.1+ T cells upon NKR-P1 cross-linking. J. Exp. Med. 183:2391-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron, J. L., L. Gardiner, S. Nishimura, K. Shinkai, R. Locksley, and D. Ganem. 2002. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity 16:583-594. [DOI] [PubMed] [Google Scholar]
- 6.Baxter, A. G., S. J. Kinder, K. J. Hammond, R. Scollay, and D. I. Godfrey. 1997. Association between αβTCR+ CD4− CD8− T-cell deficiency and IDDM in NOD/Lt mice. Diabetes 46:572-582. [DOI] [PubMed] [Google Scholar]
- 7.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+ CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. [DOI] [PubMed] [Google Scholar]
- 8.Bendelac, A., O. Lantz, M. E. Quimby, J. W. Yewdell, J. R. Bennink, and R. R. Brutkiewicz. 1995. CD1 recognition by mouse NK1+ T lymphocytes. Science 268:863-865. [DOI] [PubMed] [Google Scholar]
- 9.Bendelac, A., M. N. Rivera, S. H. Park, and J. H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535-562. [DOI] [PubMed] [Google Scholar]
- 10.Buckner, F. S., and W. C. Van Voorhis. 2000. Immune response to Trypanosoma cruzi: control of infection and pathogenesis of Chagas' disease. Lippincott-Raven Press, Philadelphia, Pa.
- 11.Burdin, N., L. Brossay, and M. Kronenberg. 1999. Immunization with α-galactosylceramide polarizes CD1-reactive NK T cells toward Th2 cytokine synthesis. Eur. J. Immunol. 29:2014-2025. [DOI] [PubMed] [Google Scholar]
- 12.Campetella, O., D. Sanchez, J. J. Cazzulo, and A. C. C. Frasch. 1992. A Superfamily of Trypanosoma cruzi surface antigens. Parasitol. Today 8:378-381. [DOI] [PubMed] [Google Scholar]
- 13.Cardell, S., S. Tangri, S. Chan, M. Kronenberg, C. Benoist, and D. Mathis. 1995. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J. Exp. Med. 182:993-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, H., and W. E. Paul. 1998. A population of CD62Llow Nk1.1− CD4+ T cells that resembles NK1.1+ CD4+ T cells. Eur. J. Immunol. 28:3172-3182. [DOI] [PubMed] [Google Scholar]
- 15.Cui, J., T. Shin, T. Kawano, H. Sato, E. Kondo, I. Toura, Y. Kaneko, H. Koseki, M. Kanno, and M. Taniguchi. 1997. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science 278:1623-1626. [DOI] [PubMed] [Google Scholar]
- 16.Cui, J., N. Watanabe, T. Kawano, M. Yamashita, T. Kamata, C. Shimizu, M. Kimura, E. Shimizu, J. Koike, H. Koseki, Y. Tanaka, M. Taniguchi, and T. Nakayama. 1999. Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Vα14 natural killer T cells. J. Exp. Med. 190:783-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dao, T., M. Exley, W. Z. Mehal, S. M. Tahir, S. Snapper, M. Taniguchi, S. P. Balk, and I. N. Crispe. 2001. Involvement of CD1 in peripheral deletion of T lymphocytes is independent of NK T cells. J. Immunol. 166:3090-3097. [DOI] [PubMed] [Google Scholar]
- 18.Dellabona, P., E. Padovan, G. Casorati, M. Brockhaus, and A. Lanzavecchia. 1994. An invariant Vα24-JαQ/Vβ11 T-cell receptor is expressed in all individuals by clonally expanded CD4−8− T cells. J. Exp. Med. 180:1171-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieli, F., M. Taniguchi, M. Kronenberg, S. Sidobre, J. Ivanyi, L. Fattorini, E. Iona, G. Orefici, G. De Leo, D. Russo, N. Caccamo, G. Sireci, C. Di Sano, and A. Salerno. 2003. An anti-inflammatory role for Vα14 NK T cells in Mycobacterium bovis bacillus Calmette-Guerin-infected mice. J. Immunol. 171:1961-1968. [DOI] [PubMed] [Google Scholar]
- 20.Di Noia, J. M., C. A. Buscaglia, C. R. De Marchi, I. C. Almeida, and A. C. Frasch. 2002. A Trypanosoma cruzi small surface molecule provides the first immunological evidence that Chagas' disease is due to a single parasite lineage. J. Exp. Med. 195:401-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duthie, M. S., M. Wleklinski-Lee, S. Smith, T. Nakayama, M. Taniguchi, and S. J. Kahn. 2002. During Trypanosoma cruzi infection CD1d-restricted NK T cells limit parasitemia and augment the antibody response to a glycophosphoinositol-modified surface protein. Infect. Immun. 70:36-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emoto, M., M. Miyamoto, Y. Emoto, I. Yoshizawa, V. Brinkmann, N. van Rooijen, and S. H. Kaufmann. 2003. Highly biased type 1 immune responses in mice deficient in LFA-1 in Listeria monocytogenes infection are caused by elevated IL-12 production by granulocytes. J. Immunol. 171:3970-3976. [DOI] [PubMed] [Google Scholar]
- 23.Faunce, D. E., and J. Stein-Streilein. 2002. NKT cell-derived RANTES recruits APCs and CD8+ T cells to the spleen during the generation of regulatory T cells in tolerance. J. Immunol. 169:31-38. [DOI] [PubMed] [Google Scholar]
- 24.Faveeuw, C., V. Angeli, J. Fontaine, C. Maliszewski, A. Capron, L. Van Kaer, M. Moser, M. Capron, and F. Trottein. 2002. Antigen presentation by CD1d contributes to the amplification of Th2 responses to Schistosoma mansoni glycoconjugates in mice. J. Immunol. 169:906-912. [DOI] [PubMed] [Google Scholar]
- 25.Galli, G., S. Nuti, S. Tavarini, L. Galli-Stampino, C. De Lalla, G. Casorati, P. Dellabona, and S. Abrignani. 2003. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J. Exp. Med. 197:1051-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galli, G., S. Nuti, S. Tavarini, L. Galli-Stampino, C. De Lalla, G. Casorati, P. Dellabona, and S. Abrignani. 2003. Innate immune responses support adaptive immunity: NKT cells induce B-cell activation. Vaccine 21(Suppl. 2):S48-S54. [DOI] [PubMed] [Google Scholar]
- 27.Gazzinelli, R. T., I. P. Oswald, S. Hieny, S. L. James, and A. Sher. 1992. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur. J. Immunol. 22:2501-2506. [DOI] [PubMed] [Google Scholar]
- 28.Godfrey, D. I., K. J. Hammond, L. D. Poulton, M. J. Smyth, and A. G. Baxter. 2000. NKT cells: facts, functions and fallacies. Immunol. Today 21:573-583. [DOI] [PubMed] [Google Scholar]
- 29.Gumperz, J. E., S. Miyake, T. Yamamura, and M. B. Brenner. 2002. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 195:625-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamano, S., K. Himeno, Y. Miyazaki, K. Ishii, A. Yamanaka, A. Takeda, M. Zhang, H. Hisaeda, T. W. Mak, A. Yoshimura, and H. Yoshida. 2003. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity 19:657-667. [DOI] [PubMed] [Google Scholar]
- 31.Hammond, K. J. L., L. D. Poulton, L. J. Palmisano, P. A. Silveira, D. I. Godfrey, and A. G. Baxter. 1998. α/β-T cell receptor (Tcr)+ CD4− CD8− (Nkt) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (Nod)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J. Exp. Med. 187:1047-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen, D. S., and L. Schofield. 2004. Regulation of immunity and pathogenesis in infectious diseases by CD1d-restricted NKT cells. Int. J. Parasitol. 34:15-25. [DOI] [PubMed] [Google Scholar]
- 33.Hansen, D. S., M. A. Siomos, L. Buckingham, A. A. Scalzo, and L. Schofield. 2003. Regulation of murine cerebral malaria pathogenesis by CD1d-restricted NKT cells and the natural killer complex. Immunity 18:391-402. [DOI] [PubMed] [Google Scholar]
- 34.Hansen, D. S., M. A. Siomos, T. De Koning-Ward, L. Buckingham, B. S. Crabb, and L. Schofield. 2003. CD1d-restricted NKT cells contribute to malarial splenomegaly and enhance parasite-specific antibody responses. Eur. J. Immunol. 33:2588-2598. [DOI] [PubMed] [Google Scholar]
- 35.Hesse, M., C. A. Piccirillo, Y. Belkaid, J. Prufer, M. Mentink-Kane, M. Leusink, A. W. Cheever, E. M. Shevach, and T. A. Wynn. 2004. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J. Immunol. 172:3157-3166. [DOI] [PubMed] [Google Scholar]
- 36.Hisaeda, H., Y. Maekawa, D. Iwakawa, H. Okada, K. Himeno, K. Kishihara, S. Tsukumo, and K. Yasutomo. 2004. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat. Med. 10:29-30. [DOI] [PubMed] [Google Scholar]
- 37.Huber, S., D. Sartini, and M. Exley. 2003. Role of CD1d in coxsackievirus B3-induced myocarditis. J. Immunol. 170:3147-3153. [DOI] [PubMed] [Google Scholar]
- 38.Huber, S. A., D. Sartini, and M. Exley. 2002. Vγ4+ T cells promote autoimmune CD8+ cytolytic T-lymphocyte activation in coxsackievirus B3-induced myocarditis in mice: role for CD4+ Th1 cells. J. Virol. 76:10785-10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunter, C. A., L. A. Ellis-Neyes, T. Slifer, S. Kanaly, G. Grunig, M. Fort, D. Rennick, and F. G. Araujo. 1997. IL-10 is required to prevent immune hyperactivity during infection with Trypanosoma cruzi. J. Immunol. 158:3311-3316. [PubMed] [Google Scholar]
- 40.Ito, K., M. Karasawa, T. Kawano, T. Akasaka, H. Koseki, Y. Akutsu, E. Kondo, S. Sekiya, K. Sekikawa, M. Harada, M. Yamashita, T. Nakayama, and M. Taniguchi. 2000. Involvement of decidual Vα14 NKT cells in abortion. Proc. Natl. Acad. Sci. USA 97:740-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joyce, S., A. S. Woods, J. W. Yewdell, J. R. Bennink, A. D. De Silva, A. Boesteanu, S. P. Balk, R. J. Cotter, and R. R. Brutkiewicz. 1998. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science 279:1541-1544. [DOI] [PubMed] [Google Scholar]
- 42.Kahn, S., W. C. Van Voorhis, and H. Eisen. 1990. The major 85-kD surface antigen of the mammalian form of Trypanosoma cruzi is encoded by a large heterogeneous family of simultaneously expressed genes. J. Exp. Med. 172:589-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawakami, K., Y. Kinjo, K. Uezu, S. Yara, K. Miyagi, Y. Koguchi, T. Nakayama, M. Taniguchi, and A. Saito. 2001. Monocyte chemoattractant protein-1-dependent increase of Vα14 NKT cells in lungs and their roles in Th1 response and host defense in cryptococcal infection. J. Immunol. 167:6525-6532. [DOI] [PubMed] [Google Scholar]
- 44.Kawakami, K., N. Yamamoto, Y. Kinjo, K. Miyagi, C. Nakasone, K. Uezu, T. Kinjo, T. Nakayama, M. Taniguchi, and A. Saito. 2003. Critical role of Vα14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur. J. Immunol. 33:3322-3330. [DOI] [PubMed] [Google Scholar]
- 45.Kim, C. H., B. Johnston, and E. C. Butcher. 2002. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among Vα24+Vβ11+ NKT cell subsets with distinct cytokine-producing capacity. Blood 100:11-16. [DOI] [PubMed] [Google Scholar]
- 46.Kronenberg, M., and L. Gapin. 2002. The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2:557-568. [DOI] [PubMed] [Google Scholar]
- 47.Kumar, H., A. Belperron, S. W. Barthold, and L. K. Bockenstedt. 2000. Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi. J. Immunol. 165:4797-4801. [DOI] [PubMed] [Google Scholar]
- 48.Lantz, O., and A. Bendelac. 1994. An invariant T-cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 180:1097-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lehuen, A., O. Lantz, L. Beaudoin, V. Laloux, C. Carnaud, A. Bendelac, J. F. Bach, and R. C. Monteiro. 1998. Overexpression of natural killer T cells protects Vα14-Jα281 transgenic nonobese diabetic mice against diabetes. J. Exp. Med. 188:1831-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mars, L. T., V. Laloux, K. Goude, S. Desbois, A. Saoudi, L. Van Kaer, H. Lassmann, A. Herbelin, A. Lehuen, and R. S. Liblau. 2002. Cutting edge: Vα14-Jα281 NKT cells naturally regulate experimental autoimmune encephalomyelitis in nonobese diabetic mice. J. Immunol. 168:6007-6011. [DOI] [PubMed] [Google Scholar]
- 51.Matsuda, J. L., L. Gapin, J. L. Baron, S. Sidobre, D. B. Stetson, M. Mohrs, R. M. Locksley, and M. Kronenberg. 2003. Mouse Vα14i natural killer T cells are resistant to cytokine polarization in vivo. Proc. Natl. Acad. Sci. USA 100:8395-8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Millar, A. E., and S. J. Kahn. 2000. Trypanosoma cruzi: the effect of nitric oxide synthesis inhibition on the CD4 T cell response to the trans-sialidase superfamily. Exp. Parasitol. 94:84-91. [DOI] [PubMed] [Google Scholar]
- 53.Miyahira, Y., M. Katae, K. Takeda, H. Yagita, K. Okumura, S. Kobayashi, T. Takeuchi, T. Kamiyama, Y. Fukuchi, and T. Aoki. 2003. Activation of natural killer T cells by α-galactosylceramide impairs DNA vaccine-induced protective immunity against Trypanosoma cruzi. Infect. Immun. 71:1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakamura, T., K. H. Sonoda, D. E. Faunce, J. Gumperz, T. Yamamura, S. Miyake, and J. Stein-Streilein. 2003. CD4+ NKT cells, but not conventional CD4+ T cells, are required to generate efferent CD8+ T regulatory cells following antigen inoculation in an immune-privileged site. J. Immunol. 171:1266-1271. [DOI] [PubMed] [Google Scholar]
- 55.Nieuwenhuis, E. E., T. Matsumoto, M. Exley, R. A. Schleipman, J. Glickman, D. T. Bailey, N. Corazza, S. P. Colgan, A. B. Onderdonk, and R. S. Blumberg. 2002. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat. Med. 8:588-593. [DOI] [PubMed] [Google Scholar]
- 56.Procopio, D. O., I. C. Almeida, A. C. Torrecilhas, J. E. Cardoso, L. Teyton, L. R. Travassos, A. Bendelac, and R. T. Gazzinelli. 2002. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins from Trypanosoma cruzi bind to CD1d but do not elicit dominant innate or adaptive immune responses via the CD1d/NKT cell pathway. J. Immunol. 169:3926-3933. [DOI] [PubMed] [Google Scholar]
- 57.Roberts, T. J., Y. Lin, P. M. Spence, L. Van Kaer, and R. R. Brutkiewicz. 2004. CD1d1-dependent control of the magnitude of an acute antiviral immune response. J. Immunol. 172:3454-3461. [DOI] [PubMed] [Google Scholar]
- 58.Santos, L.-E. C., and P. Minoprio. 1996. Chagas' disease is attenuated in mice lacking gamma delta T cells. Infect. Immun. 64:215-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silva, J. S., P. J. Morrissey, K. H. Grabstein, K. M. Mohler, D. Anderson, and S. G. Reed. 1992. Interleukin 10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J. Exp. Med. 175:169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silva, J. S., D. R. Twardzik, and S. G. Reed. 1991. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor beta (TGF-β). J. Exp. Med. 174:539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smiley, S. T., M. H. Kaplan, and M. J. Grusby. 1997. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science 275:977-979. [DOI] [PubMed] [Google Scholar]
- 62.Sonoda, K. H., M. Exley, S. Snapper, S. P. Balk, and J. Stein-Streilein. 1999. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J. Exp. Med. 190:1215-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stenstrom, M., M. Skold, A. Ericsson, L. Beaudoin, S. Sidobre, M. Kronenberg, A. Lehuen, and S. Cardell. 2004. Surface receptors identify mouse NK1.1+ T cell subsets distinguished by function and T cell receptor type. Eur. J. Immunol. 34:56-65. [DOI] [PubMed] [Google Scholar]
- 64.Sumida, T., A. Sakamoto, H. Murata, Y. Makino, H. Takahashi, S. Yoshida, K. Nishioka, I. Iwamoto, and M. Taniguchi. 1995. Selective reduction of T cells bearing invariant Vα24JαQ antigen receptor in patients with systemic sclerosis. J. Exp. Med. 182:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suvas, S., U. Kumaraguru, C. D. Pack, S. Lee, and B. T. Rouse. 2003. CD4+ CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198:889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szalay, G., C. H. Ladel, C. Blum, L. Brossay, M. Kronenberg, and S. H. Kaufmann. 1999. Cutting edge: anti-CD1 monoclonal antibody treatment reverses the production patterns of TGF-β2 and Th1 cytokines and ameliorates listeriosis in mice. J. Immunol. 162:6955-6958. [PubMed] [Google Scholar]
- 67.Teige, A., I. Teige, S. Lavasani, R. Bockermann, E. Mondoc, R. Holmdahl, and S. Issazadeh-Navikas. 2004. CD1-dependent regulation of chronic central nervous system inflammation in experimental autoimmune encephalomyelitis. J. Immunol. 172:186-194. [DOI] [PubMed] [Google Scholar]
- 68.Terabe, M., S. Matsui, J. M. Park, M. Mamura, N. Noben-Trauth, D. D. Donaldson, W. Chen, S. M. Wahl, S. Ledbetter, B. Pratt, J. J. Letterio, W. E. Paul, and J. A. Berzofsky. 2003. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J. Exp. Med. 198:1741-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Vliet, H. J., B. M. von Blomberg, N. Nishi, M. Reijm, A. E. Voskuyl, A. A. van Bodegraven, C. H. Polman, T. Rustemeyer, P. Lips, A. J. van den Eertwegh, G. Giaccone, R. J. Scheper, and H. M. Pinedo. 2001. Circulating V(α24+) Vβ11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin. Immunol. 100:144-148. [DOI] [PubMed] [Google Scholar]
- 70.Wang, B., Y. B. Geng, and C. R. Wang. 2001. CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes. J. Exp. Med. 194:313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.WHO. 2002. Control of Chagas disease. World Health Organization, Geneva, Switzerland.
- 72.Wilson, M. T., C. Johansson, D. Olivares-Villagomez, A. K. Singh, A. K. Stanic, C. R. Wang, S. Joyce, M. J. Wick, and L. Van Kaer. 2003. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc. Natl. Acad. Sci. USA 100:10913-10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilson, S. B., S. C. Kent, K. T. Patton, T. Orban, R. A. Jackson, M. Exley, S. Porcelli, D. A. Schatz, M. A. Atkinson, S. P. Balk, J. L. Strominger, and D. A. Hafler. 1998. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature 391:177-181. [DOI] [PubMed] [Google Scholar]
- 74.Wu, D. Y., N. H. Segal, S. Sidobre, M. Kronenberg, and P. B. Chapman. 2003. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J. Exp. Med. 198:173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamanaka, A., S. Hamano, Y. Miyazaki, K. Ishii, A. Takeda, T. W. Mak, K. Himeno, A. Yoshimura, and H. Yoshida. 2004. Hyperproduction of proinflammatory cytokines by WSX-1-deficient NKT cells in concanavalin A-induced hepatitis. J. Immunol. 172:3590-3596. [DOI] [PubMed] [Google Scholar]
- 76.Yang, J. Q., V. Saxena, H. Xu, L. Van Kaer, C. R. Wang, and R. R. Singh. 2003. Repeated α-galactosylceramide administration results in expansion of NK T cells and alleviates inflammatory dermatitis in MRL-lpr/lpr mice. J. Immunol. 171:4439-4446. [DOI] [PubMed] [Google Scholar]
- 77.Zingales, B., M. E. Pereira, R. P. Oliveira, K. A. Almeida, E. S. Umezawa, R. P. Souto, N. Vargas, M. I. Cano, S.-J. F. da, N. S. Nehme, C. M. Morel, Z. Brener, and A. Macedo. 1997. Trypanosoma cruzi genome project: biological characteristics and molecular typing of clone CL Brener. Acta Trop. 68:159-173. [DOI] [PubMed] [Google Scholar]