Abstract
Helicobacter pylori chronically colonizes the stomach and duodenum and causes peptic ulcers or gastric adenocarcinoma in 10 to 20% of infected individuals. We hypothesize that the inability of patients to clear H. pylori infections is a consequence of active suppression of the immune response. Here we show that H. pylori-infected individuals have increased frequencies of CD4+ CD25high T cells in both the stomach and duodenal mucosa compared to uninfected controls. These cells have the phenotype of regulatory T cells, as they express FOXP3, a key gene for the development and function of regulatory T cells, as well as high levels of the cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) protein. In contrast, mucosal CD4+ CD25low and CD4+ CD25− cells express little FOXP3 mRNA and low levels of the CTLA-4 protein. Mucosal CD4+ CD25high T cells are present in individuals with asymptomatic H. pylori infections as well as in duodenal ulcer patients. The frequencies of CD4+ CD25high cells are also increased in the stomachs of H. pylori-infected patients with gastric adenocarcinoma, particularly in cancer-affected tissues. These findings suggest that regulatory T cells may suppress mucosal immune responses and thereby contribute to the persistence of H. pylori infections.
The gastrointestinal mucosa is in constant contact with both harmless and harmful antigens. The immune system has to discriminate between these antigens to maintain a balance between active defense and the prevention of immunopathology. In mouse models, naturally occurring CD4+ CD25+ regulatory T cells (Treg cells) have been implicated in playing an important role in suppressing immune responses to the normal intestinal flora (28) as well as to pathogens (22, 25). However, little is currently known about the role of Treg cells in the human gastrointestinal mucosa.
Most studies of human Treg cells have been performed with cells isolated from peripheral blood (2, 17, 32), but CD4+ CD25+ cells with suppressor activity have also been demonstrated in the thymus (29), cord blood (36), synovial fluid (4), tonsils (32), and a few types of tumors (19, 37). Human T cells have a more variable expression of CD25 (the interleukin-2 receptor α-chain) than do mouse T cells, and only those that express CD25 with the highest intensities (CD25high) are suppressive (2, 4). T cells expressing intermediate levels of CD25 (CD25low) are instead activated effector or memory T cells and lack a regulatory function. Treg cells suppress the activity of other T cells via a contact-dependent mechanism, but the molecules directly mediating this suppression have still not been clearly identified (22). However, the Foxp3 gene (FOXP3 in humans), which encodes the transcription factor scurfin, has recently been demonstrated to be a key regulatory gene for the development and function of Treg cells (10, 15, 16). Humans with defects in the FOXP3 gene experience strong activation of the immune system, leading to multiorgan autoimmune disease, inflammatory bowel disease, allergies, and severe infections, collectively known as the IPEX syndrome (immune dysregulation, polyendocrinopathy, enteropathy, X-linked inheritance syndrome) (11). FOXP3/Foxp3 is expressed by CD4+ CD25+ Treg cells in humans and mice, and the transduction of CD25− cells from mice with this gene converts the cells into regulatory cells. Although recent data indicate that FOXP3 gene expression can be induced in CD25− cells under special conditions (5, 9, 33, 34), these induced FOXP3-expressing cells also have a suppressive capacity, suggesting that a tight link exists between FOXP3 expression and a regulatory function.
We are currently investigating the role of Treg cells in chronic Helicobacter pylori infection (20, 25). Although H. pylori colonization of the gastric and duodenal mucosa induces strong immune responses involving both innate immune cells and H. pylori-specific T and B cells, the infection is not cleared and a state of chronic active gastritis is established that can be life-long (8). We hypothesize that Treg cells may actively suppress the immune response to H. pylori, thereby limiting acute infection-induced immunopathology but at the same time promoting bacterial persistence and possibly also long-term pathology as a result of chronic infection. In agreement with this hypothesis, we recently showed that memory CD4+ T cells isolated from the peripheral blood of H. pylori-infected individuals respond poorly to H. pylori antigens in vitro but that this unresponsiveness can be counteracted by the depletion of CD4+ CD25high Treg cells (20). Furthermore, the numbers of cells expressing CD25, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) (31), and transforming growth factor beta (TGF-β) (18, 30) are increased in infected mucosae compared to uninfected mucosae, indicating that local suppression of immune responses to the bacteria may take place. Studies with a mouse model of H. pylori infection have also shown that Treg cells suppress H. pylori-induced gastritis at the expense of higher bacterial loads (25).
The majority of individuals who are infected with H. pylori remain asymptomatic, but 10 to 15% of those infected develop peptic ulcers and 1 to 2% develop gastric adenocarcinoma (8). However, the two types of disease are rarely seen in the same individual. Duodenal ulcer patients have a history of antral gastritis, an increased acid load, the development of gastric metaplasia in the duodenum, and subsequently, duodenal ulceration. In contrast, gastric adenocarcinoma develops from pangastritis that progresses into atrophic gastritis with intestinal metaplasia, which may lead to dysplasia and malignant transformation (27). The reasons for the different outcomes of H. pylori infection are not clear. However, alterations in Treg cell activity that affect the balance between bacterial colonization and inflammation may be involved in the development of both types of disease.
In the present study, we analyzed the presence of Treg cells at the sites of infection and inflammation for different categories of H. pylori-infected subjects in an attempt to elucidate the role of Treg cells in the clinical outcome of infection. We demonstrate increased proportions of CD4+ CD25high T cells with a regulatory phenotype in H. pylori-infected mucosae compared to uninfected mucosae, suggesting that CD4+ CD25high Treg cells may suppress mucosal immune responses and contribute to the persistence of H. pylori infections.
MATERIALS AND METHODS
Volunteers and collection of specimens.
Eleven adult Swedes who were infected with H. pylori and had no subjective symptoms (H. pylori positive; mean age, 48 years; age range, 31 to 60 years; two females) and eight healthy, uninfected volunteers (H. pylori negative; mean age, 34 years; age range, 24 to 55 years; three females) were recruited for this study from blood donors at Sahlgrenska University Hospital (SUH), Göteborg, Sweden. Ten biopsies each were collected from the antrum and the duodenum by gastroduodenal endoscopy. One biopsy from the antrum and one from the duodenum were embedded in optimal cutting temperature compound (Tissue-Tek; Miles Inc., Elkhart, Ind.), immediately snap-frozen, and used for immunohistochemistry (IHC). One-half of a biopsy from each site was used for histological examination. The remaining 8.5 biopsies from each site, corresponding to ∼16 mg of tissue, were pooled and used for the isolation of lymphocytes and subsequent flow cytometry (FCM).
Seven H. pylori-positive patients with noncardia gastric adenocarcinoma (mean age, 69 years; age range, 50 to 87 years; three females) who were undergoing gastrectomy at SUH were also included in the study. The patients had not undergone radiotherapy or chemotherapy. Cancer-affected tissues (from five of seven patients) as well as healthy tissues from the antrum and corpus (from all patients) were cut out from the resected stomachs. The cancer tissues and healthy tissues were identified based on their visual appearance, and their status was confirmed by histological examination by an experienced pathologist. Healthy tissues were collected at least 5 cm away from cancer-affected tissues. Pieces of tissue were snap-frozen and used for IHC, and lymphocytes were isolated from the remaining tissue and analyzed by FCM.
To determine the prevalence of cells with a Treg cell phenotype in duodenal ulcer patients, we reevaluated previously obtained IHC data (31) for 10 H. pylori-positive duodenal ulcer patients, using new criteria for Treg cell identification. Ten asymptomatic carriers of H. pylori and 10 uninfected individuals from the same study were used as controls.
Heparinized venous blood was collected at the time of endoscopy or gastrectomy. Leukocyte-enriched buffy coats were obtained from the blood bank at SUH. This study was approved by the Ethical Committee for Human Research, Göteborg University, and informed consent was obtained from each volunteer before participation.
Diagnosis of H. pylori infection. (i) H. pylori-positive asymptomatic carriers, duodenal ulcer patients, and H. pylori-negative controls.
Bacteria were cultured from biopsies on horse blood-Columbia Iso agar plates at 37°C under microaerobic conditions (10% CO2, 5% O2, and 85% N2) and were screened for H. pylori-like colonies after 3 days of culture. One-half of a biopsy from the antrum and of one from the duodenum was examined by an experienced histopathologist and evaluated for the presence of Helicobacter-like organisms, and inflammation was graded according to the Sydney system (24). Sera were screened for H. pylori-specific immunoglobulin G (IgG) antibodies by use of an in-house enzyme-linked immunosorbent assay (ELISA) (21). H. pylori-positive subjects were positive by at least two of these tests, and H. pylori-negative volunteers were negative by all three tests.
(ii) Adenocarcinoma patients.
Bacteria were cultured from tissues as described above, and sera were screened for H. pylori-specific IgG and IgA antibodies by the use of in-house ELISAs (21) and a Pyloriset EIA-G III ELISA (Orion Diagnostica, Espoo, Finland). Two of the seven patients included in the study were both culture positive and positive by at least one of the ELISAs, and the remaining patients were positive by at least two of the ELISAs.
Isolation of lymphocytes.
Tissues collected from H. pylori-positive cancer patients were cut into small pieces after the removal of the muscle and fat layers. The epithelium and the intraepithelial lymphocytes were removed from the biopsies or resected tissues by incubation in prewarmed (37°C) Hank's balanced salt solution without calcium or magnesium containing 1 mM EDTA and 1 mM dithiothreitol six times for 15 min each (stomach tissue) or four times for 15 min each (duodenal biopsies). Lamina propria lymphocytes (LPLs) were isolated by stirring the remaining tissue in a collagenase-DNase solution (100 U of collagenase/ml [Sigma] and 0.1 mg of DNase/ml [Sigma]) at 37°C for 2 h. The cell suspension was then filtered through mesh, and the number of lymphocytes was counted under a microscope. Initial experiments showed that this cell isolation protocol gave a maximal yield of cells, with little of the epithelium remaining in the lamina propria fraction, and that the isolation procedure had no or only marginal effects on the expression of different cell surface markers.
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood or buffy coats by density gradient centrifugation on Ficoll-Paque (Amersham Biosciences, Uppsala, Sweden). CD4+ and CD8+ T cells were isolated by positive selection from the PBMCs by the use of magnetic beads (Dynabeads; Dynal AS, Oslo, Norway).
IHC.
IHC was performed as previously described (31). Briefly, 8-μm-thick cryosections were stained with specific monoclonal antibodies against CD4 and CD25 (both from Dako, Roskilde, Denmark), and the number of positive mononuclear cells (MNC) in the lamina propria in each entire section was enumerated under a microscope with randomly coded slides. The total area of the sections was determined with Qwin software (Leica Microsystems, Cambridge, United Kingdom), and the number of positive cells was expressed as the number of MNC per square millimeter of total tissue area. For the preparation of cytospin slides, 5 × 105 CD4+ or CD8+ peripheral blood T cells were spun down onto each microscope slide. After fixation in acetone, the cells were stained for CD25 expression according to the same protocol as that for cryosections.
Flow cytometric staining, analysis, and sorting.
LPLs (5 × 104 to 5 × 105 cells/sample) or PBMCs (105 cells/sample) were stained for FCM analysis of various cell surface markers with combinations of the following antibodies: anti-CD3-fluorescein isothiocyanate (anti-CD3-FITC), anti-CD45RA-FITC, anti-CD62L-phycoerythrin (anti-CD62L-PE), anti-CD25-PE, anti-CD25-allophycocyanin (anti-CD25-APC), anti-CD69-APC, anti-CD4-peridinin chlorophyll protein (all from BD Pharmingen, San Diego, Calif.), and anti-CD8-APC (Diatec, Oslo, Norway). For intracellular staining, LPLs (1 × 105 to 2 × 105 cells/sample) or PBMCs (5 × 105 cells/sample) were permeabilized with Cytofix/Cytoperm solution (BD Pharmingen) and stained with an anti-CTLA-4-PE antibody (BD Pharmingen). All cells were fixed in formaldehyde before FCM analysis, which was performed in a FACSCalibur instrument equipped with blue and red lasers (BD Pharmingen).
The FCM data were analyzed with FlowJo software (Tree Star Inc.). Lymphocytes were gated via their forward and side scatter properties, and T cells were identified based on their expression of CD4 and/or CD8. Control samples showed that the contamination of CD3− cells in the CD4 and CD8 gates was negligible (<0.5%). The number of CD4+ T cells isolated from 8.5 pooled biopsies from each individual was estimated by multiplying the frequency of CD4+ T cells detected by FCM with the total number of isolated lymphocytes.
To discriminate between CD25high Treg and CD25low activated effector-memory T cells, we used CD25 expression on CD8+ cells as an internal control (Fig. 1). CD8+ cells only express intermediate levels of CD25 (CD25low), whereas CD4+ T cells express CD25 with high (CD25high) or intermediate (CD25low) intensities. Only CD4+ cells expressing CD25 with higher intensities than the CD8+ cells were included in the gate for CD25high cells. The gate for CD25low cells was set to include cells expressing CD25 at levels above those of the isotype control and unstained cells but at lower expression levels than the CD25high cells. For the other markers, unstained cells and cells stained with isotype-matched control antibodies served as controls.
FIG. 1.
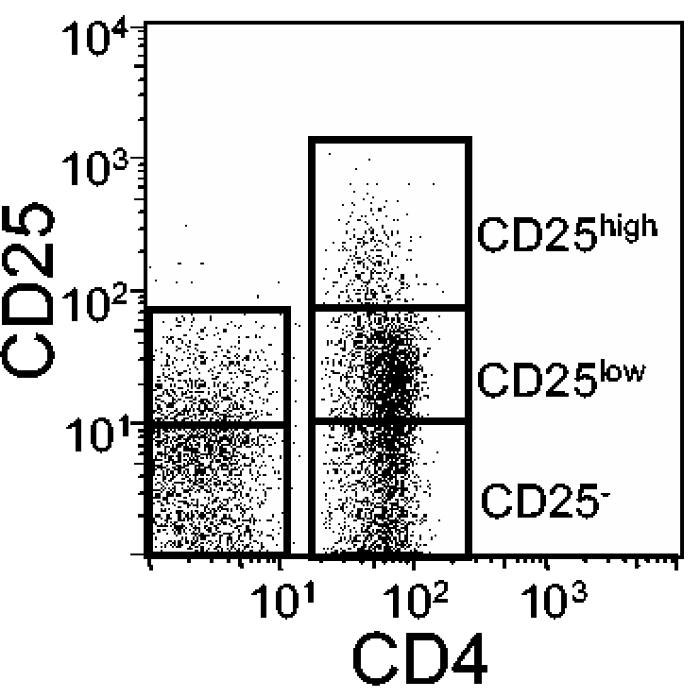
Gating approach for discrimination of CD25high, CD25low, and CD25− cells. Cells were labeled with antibodies against CD4, CD8, and CD25 and then analyzed by FCM. For this example, the CD25 expression on CD4+ and CD8+ T cells from the antral mucosa of an H. pylori-positive individual was analyzed in a single plot. The gates for the CD25high and CD25low populations were set by comparing the CD25 expression levels of CD4+ and CD8+ cells, as described in Materials and Methods.
To sort cells into CD4+ CD25high, CD4+ CD25low, and CD4+ CD25− fractions, we labeled LPLs and PBMCs with anti-CD25-APC, anti-CD4-FITC, anti-CD8-PE, and anti-CD19-PE (all from BD Pharmingen) and sorted them in a FACSVantage SE instrument (BD Pharmingen) operating at a sheath pressure of 22 lb/in2. After sorting, the CD25high fraction contained >87% CD25high cells (median purity, 93%) and the CD25low and CD25− fractions contained <3% CD25high cells (median contamination, 0.1%).
T-cell stimulation.
Sorted cells were stimulated (5 × 104 cells/well) with plate-bound anti-CD3 and soluble anti-CD28 antibodies as previously described (7), except for the anti-CD28 antibody concentration, which was 0.5 μg/ml in the present study. The cells were then cultured at 37°C in 5% CO2 in Iscove's medium supplemented with 3 μg of l-glutamine/ml, 50 μg of gentamicin/ml, and 5% human AB+ serum for 5 days.
Expression analysis of FOXP3.
Total RNAs were isolated from sorted cells by use of a total RNA extraction kit for mammalian RNA (Sigma Aldrich, St. Louis, Mo.), and cDNAs were synthesized by use of an oligo(dT) primer and a Sensiscript RT-PCR kit (Qiagen, Hilden, Germany).
Multiplex PCRs were performed with the FOXP3 primers 5′-CAGCACATTCCCAGAGTTCCTC-3′ and 5′-GCGTGTGAACCAGTGGTAGATC-3′ and the β-actin primers 5′-AGCACTGTGTTGGCGTACAG-3′ and 5′-GGACTTCGAGCAAGAGATG-3′ under standard PCR conditions (1.5 mM MgCl2 and 2 μl of cDNA). The PCRs were denatured at 94°C (5 min) and amplified by 30 to 35 cycles of 94°C (30 s), 55°C (30 s), and 72°C (30 s), followed by a final extension at 72°C (5 min). The PCR products were separated in polyacrylamide gels (Novex 4 to 20% TBE gels; Invitrogen) and then silver stained (Plus One DNA silver staining kit; Amersham Biosciences).
FOXP3 mRNA levels were quantified by real-time PCRs performed in a LightCycler instrument (Roche Diagnostics, Mannheim, Germany) by use of a LightCycler FastStart DNA master SYBR green I kit (Roche Diagnostics) and the FOXP3 primers 5′-CAGCACATTCCCAGAGTTCCT-3′ and 5′-GCGTGTGAACCAGTGGTAGAT-3′. GAPDH was used as an endogenous reference gene for relative quantification and was amplified with the primers 5′-GGCTGCTTTTAACTCTGG-3′ and 5′-GGAGGGATCTCGCTCC-3′. The PCR cycling conditions were as follows: 95°C for 10 min followed by 45 cycles of 95°C (15 s), 65°C (7 s), and 72°C (10 s). All samples were run in duplicate, and a melting curve analysis was performed to ensure the specificity of the primers. Data were collected with LightCycler data analysis software (Roche Diagnostics), and LightCycler relative quantification software (Roche Diagnostics) was used to calibrate normalized relative quantification. FOXP3-expressing CD4+ CD25+ T cells from blood were used as a positive control.
Statistical analysis.
The Mann-Whitney test was used to evaluate differences between the study groups. P values of <0.05 were considered significant.
RESULTS
Increased numbers of CD4+ T cells in H. pylori-infected antral mucosae.
The numbers of CD4+ T cells in H. pylori-infected mucosae were determined for biopsies from antra and duodena of asymptomatic H. pylori-positive subjects and H. pylori-negative control individuals. All H. pylori-positive individuals had moderate chronic gastritis, and an infiltration of neutrophils was observed for the majority of the individuals. No signs of gastritis were detected in the H. pylori-negative subjects. About threefold higher numbers of lymphocytes (P = 0.02) and CD4+ T cells (P = 0.008) were isolated from H. pylori-positive than from H. pylori-negative gastric mucosae (data not shown). In H. pylori-positive individuals, ∼60% of the CD3+ T cells were CD4+, whereas only ∼30% of these cells were CD4+ in H. pylori-negative individuals. In agreement with the FCM data, an IHC analysis of the antral lamina propria showed an approximately twofold average increase in the numbers of infiltrating CD4+ T cells in H. pylori-positive individuals compared to H. pylori-negative controls, although this difference was not statistically significant (P > 0.05; data not shown). Six of eight asymptomatic H. pylori-positive volunteers also showed signs of mild duodenitis. However, similar numbers of lymphocytes were isolated from the duodena of H. pylori-positive and -negative individuals, and the numbers of CD4+ T cells detected by FCM and IHC for this site were comparable for the two study groups.
Lamina propria T cells display the phenotype of activated memory cells.
The vast majority of T cells isolated from the mucosa were found to have a memory phenotype (CD45RA− L-selectin+/− or CD45RA+ L-selectin−) (23). The frequencies of memory cells were comparable for H. pylori-positive and -negative individuals and were similar in the antrum (>93%) and the duodenum (>99%). The mucosal T cells also expressed the early activation marker CD69 to a large extent (78% of CD4+ cells and 86% of CD8+ cells [median values]), with similar frequencies in the antrum and duodenum and for H. pylori-positive and -negative subjects. In contrast, in the blood of all individuals, <10% of the T cells were CD69+. Substantial proportions of both CD4+ (32%) and CD8+ (18%) lamina propria T cells were CD25low, i.e., they were activated memory or effector T cells, with comparable frequencies for H. pylori-positive and -negative individuals and in the antrum and the duodenum, whereas slightly smaller proportions of CD25low cells were found in peripheral blood (16% of CD4+ cells and 8% of CD8+ cells [median values]).
CD4+ CD25high CTLA-4-expressing cells in H. pylori-infected mucosa.
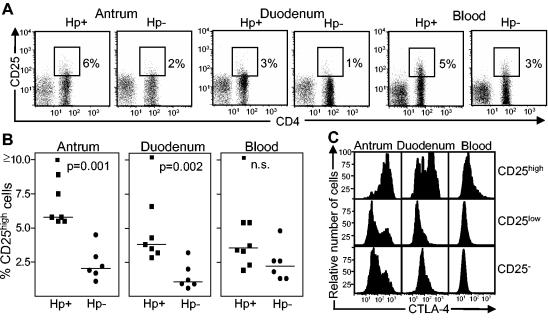
FCM showed that the frequencies of CD25high cells among CD4+ cells from both the antrum and the duodenum were increased three- to fourfold in H. pylori-positive individuals compared to H. pylori-negative controls (Fig. 2A and B), whereas the proportions of CD4+ CD25high cells were similar in the blood for the two study groups. To support the hypothesis that the mucosal CD4+ CD25high cells were Treg cells, we analyzed their intracellular expression of CTLA-4 by FCM (17, 35) (Fig. 2C). In antra and duodena of H. pylori-positive individuals, 93 and 61%, respectively, of the CD4+ CD25high cells expressed CTLA-4, in contrast to 47 and 12% of the CD4+ CD25low cells (median values). In the blood, only 25% of the CD25high and 4% of the CD25low CD4+ cells were CTLA-4+. Antral and duodenal CD4+ CD25high cells also expressed significantly lower levels of CD4 (exemplified in Fig. 2A) and CD69 (not shown) than did CD4+ CD25low cells, further distinguishing the CD4+ CD25high population from activated effector T cells.
FIG. 2.
Expression of CD25 and CTLA-4 on T cells isolated from the antral and duodenal lamina propriae and peripheral blood of H. pylori-positive asymptomatic individuals and H. pylori-negative controls. (A) CD25 expression detected by FCM on CD4+ and CD8+ T cells. CD25high cells were gated as indicated in the figure, and the frequencies of CD4+ cells expressing CD25high are shown by the numbers outside the gates. The results shown are for one representative experiment of seven experiments with H. pylori-positive individuals and six experiments with H. pylori-negative individuals. (B) Presence of CD4+ CD25high cells in the antral and duodenal lamina propriae and peripheral blood of H. pylori-positive and -negative individuals. Each symbol represents the percentage of CD25high cells among the CD4+ lymphocytes for one individual, and median values are indicated by horizontal bars. (C) Intracellular expression of CTLA-4 on CD4+ CD25high, CD25low, and CD25− cells from an H. pylori-positive individual. The results shown are from one representative experiment of three total experiments. Five thousand or more CD4+ cells and ≥250 CD4+ CD25high cells were analyzed for each sample.
FOXP3 expression is specific for CD4+ CD25high cells in both blood and mucosa.
Previous studies showed that CD4+ CD25high cells, but not CD4+ CD25low or CD4+ CD25− cells, have a suppressive activity (2, 4). However, after activation, some effector T cells may acquire the CD25high phenotype without gaining a suppressive function (17). To verify that the mucosal CD4+ CD25high cells were Treg cells, we used reverse transcriptase PCR (RT-PCR) to analyze the expression of the FOXP3 gene, which has been shown to be expressed by cells with regulatory functions in both humans and mice (9, 10, 15, 16, 34).
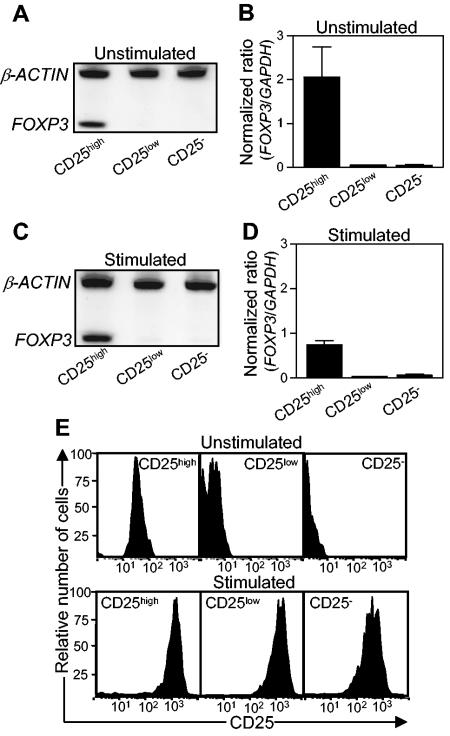
In initial experiments with sorted CD4+ cells from human blood, we demonstrated that CD25high cells expressed at least 10-fold higher levels of FOXP3 mRNA than did CD25low or CD25− cells (Fig. 3A and B). After 5 days of stimulation with anti-CD3 and anti-CD28 antibodies, CD25high cells continued to express FOXP3, although at somewhat lower levels (Fig. 3C and D). Little FOXP3 mRNA could be detected in stimulated CD25low or CD25− cells (Fig. 3C and D), although both cell fractions had up-regulated their CD25 expression (Fig. 3E). Thus, our results support the notion that FOXP3 is a specific marker for human CD4+ CD25high Treg cells.
FIG. 3.
Expression of FOXP3 and CD25 by CD4+ T cells from peripheral blood. Peripheral blood T cells were sorted into CD4+ CD25high, CD4+ CD25low, and CD4+ CD25− cell fractions, and the expression of FOXP3 and CD25 before and after stimulation with anti-CD3 and anti-CD28 antibodies was determined. (A and C) FOXP3 mRNA as well as mRNA for the housekeeping gene β-actin was detected by RT-PCR, and the PCR products were visualized in silver-stained polyacrylamide gels. Results for one representative experiment of three total experiments are shown. (B and D) Expression levels of FOXP3 were determined by real-time PCR and were related to the level of the housekeeping gene GAPDH. The values shown are arithmetic means plus standard errors of the means (SEM) (n = 3). (E) CD25 expression on sorted cells, as determined by FCM. The results shown are from one representative experiment of three total experiments.
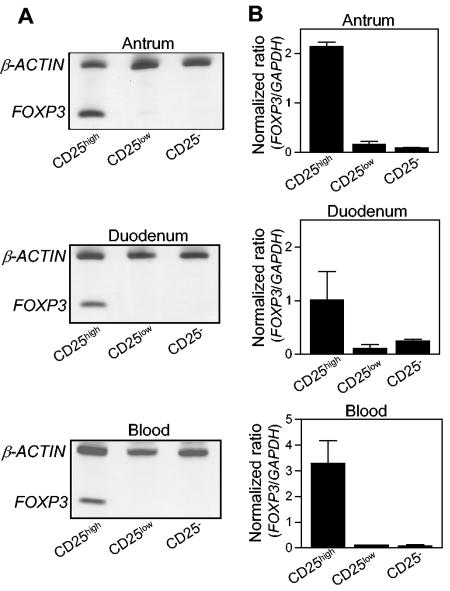
We then analyzed the expression of FOXP3 mRNA in CD4+ CD25high, CD4+ CD25low, and CD4+ CD25− cells that were isolated and sorted from the antral and duodenal mucosae and from the blood of H. pylori-positive individuals. FOXP3 mRNA was detected in CD4+ CD25high cells from the antrum, the duodenum, and blood (Fig. 4), but little FOXP3 expression could be detected in CD25low or CD25− cells in any of the tissues analyzed. Thus, the same pattern of FOXP3 expression was found among CD4+ CD25high cells in the gastrointestinal mucosa as that found in the blood, indicating that the CD4+ CD25high cell population is enriched in Treg cells in both the mucosa and the blood.
FIG. 4.
FOXP3 expression by CD4+ T cells isolated from antral and duodenal lamina propriae and peripheral blood from asymptomatic H. pylori-positive individuals. T cells were sorted into CD4+ CD25high, CD4+ CD25low, and CD4+ CD25− cell fractions, and the expression of FOXP3 mRNA was determined. (A) FOXP3 mRNA as well as mRNA for the housekeeping gene β-actin was detected by RT-PCR, and the PCR products were visualized in silver-stained polyacrylamide gels. Results for one experiment of two total experiments are shown. (B) Expression levels of FOXP3 were determined by real-time PCR and were related to the level of the housekeeping gene GAPDH. The values shown are arithmetic means plus SEM (n = 2).
CD4+ CD25high T cells are localized throughout the lamina propria.
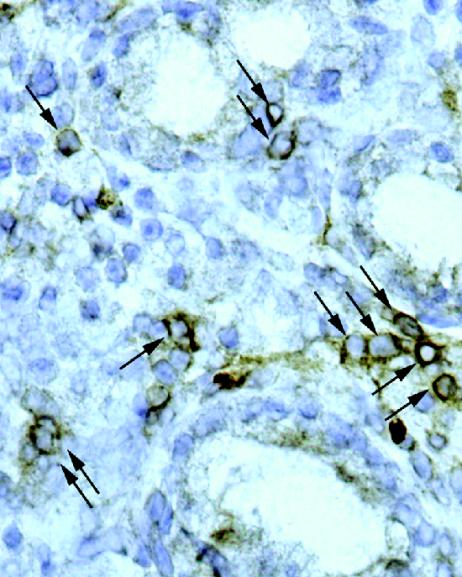
In an attempt to visualize the distribution of Treg cells within the H. pylori-positive mucosa, we stained mucosal cryosections for CD25 by IHC. CD25+ cells were detected throughout the mucosa (Fig. 5), but it was unclear whether these cells were only CD25high or both CD25high and CD25low. To address this question, we first compared the CD25 expression levels detected for isolated blood T cells by IHC and FCM. An IHC analysis of purified CD4+ T cells on cytospin slides showed CD25 staining on ∼4% of the cells, whereas CD8+ blood T cells were completely negative for CD25 by this method (Table 1). However, FCM analysis of isolated CD8+ cells demonstrated ∼5% CD25low cells within this population, but these cells were not detected by the IHC method used. Thus, these results suggest that most of the CD25+ cells detected in the mucosa by the IHC method were CD4+ CD25high cells.
FIG. 5.
CD25 expression in the antrum of an H. pylori-positive asymptomatic individual, as detected by IHC. Cryosections were stained for CD25 and analyzed under a microscope. Arrows indicate CD25+ MNCs. Original magnification, ×400.
TABLE 1.
Comparison of IHC and FCM for detection of CD25high cellsa
| Analysis method | Sample type | % CD25+ by IHC | % CD25high by FCM | Ratio of IHC/FCM data | % CD25total by FCM |
|---|---|---|---|---|---|
| Blood cytospin | CD4+ PBMCs | 4.0 (2.8-11.0) | 2.2 (1.4-5.0) | 2.0 (1.8-2.2) | 9.7 (8.2-29.7) |
| CD8+ PBMCs | 0.0 (0.0-0.0) | 0.1 (0.1-0.2) | 4.5 (4.2-5.9) | ||
| Tissue sections | Antrum (H. pylori positive) | 8.0 (3.9-10.4) | 5.9 (5.8-8.2) | 0.9 (0.7-1.3) | 39.1 (28.7-44.4) |
| Duodenum (H. pylori positive) | 3.8 (3.8-7.2) | 4.3 (3.7-5.5) | 1.0 (0.4-1.9) | 44.3 (24.9-53.1) |
IHC data are given as ratios (%) between the numbers of CD25+ and CD4+ cells per square millimeter of tissue. FCM data are given as frequencies (%) of CD25high cells among CD4+ T cells. For PBMCs, n = 3, and data are medians (and ranges). For antral and duodenal samples, n = 7, and data are medians (and quartiles).
We then compared CD25 analyses performed by FCM and IHC with tissue material from the same patients. Calculations of the ratios of CD25+ cells per total number of CD4+ cells detected by IHC in serial tissue sections showed that the frequencies of CD25+ cells obtained by IHC were comparable to the frequencies of CD25high cells detected by FCM for both the antrum and the duodenum (Table 1). In contrast, the total frequencies of CD25+ cells detected by FCM (CD25high and CD25low combined) were severalfold higher than the frequencies of CD25+ cells detected by IHC. Thus, these results confirmed that the majority of the CD25+ cells detected by the IHC method used were CD4+ CD25high cells.
Taken together, our IHC analyses of mucosal biopsies showed that CD25high T cells were present throughout the lamina propria in both antra (Fig. 5) and duodena of H. pylori-positive individuals, whereas very few mucosal CD25high cells were found in H. pylori-negative controls. In infected gastric mucosae, most of the CD25high cells were localized outside the gastric lymphoid follicles, although a few positive cells could also be detected inside follicles for a small number of volunteers.
Increased frequencies of CD4+ CD25high cells in cancer tissue from gastric adenocarcinoma patients.
The three methods used, i.e., FCM, IHC, and FOXP3 mRNA analysis, demonstrated the presence of T cells with a Treg phenotype in the gastrointestinal mucosae of asymptomatic H. pylori-positive individuals. These findings prompted us to also study the presence of such cells in H. pylori-positive patients with gastric adenocarcinoma or duodenal ulcers, two diseases that have been shown to be highly associated with H. pylori infection (8).
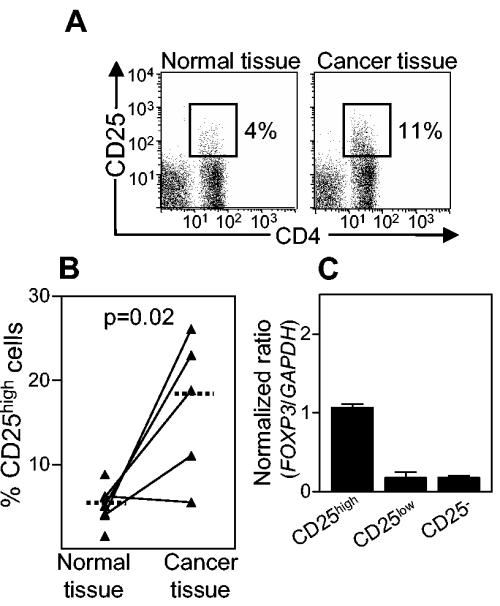
In the gastric mucosae of adenocarcinoma patients, histologically non-cancer-affected tissues contained comparable frequencies of CD4+ CD25high cells as the antral mucosae of asymptomatic H. pylori-positive individuals, as determined by FCM (Fig. 6A and B) and IHC (not shown). Furthermore, both methods showed that the cancer tissue was infiltrated by approximately threefold more CD4+ CD25high cells than the surrounding normal tissue. As seen for asymptomatic H. pylori-positive individuals, gastric CD4+ CD25high cells isolated from cancer patients expressed high levels of FOXP3 mRNA (Fig. 6C) and CTLA-4 (data not shown).
FIG. 6.
Expression of CD25 and FOXP3 by lamina propria T cells isolated from histologically normal and cancer-affected stomach mucosae of H. pylori-positive gastric adenocarcinoma patients. (A) CD25 expression on gated CD4+ and CD8+ T cells. The results shown are from one representative experiment each of seven experiments with healthy tissue and five experiments with cancer tissue. (B) Presence of CD4+ CD25high cells in healthy and cancer-affected stomach mucosae. Each symbol represents the percentage of CD25high cells among the CD4+ lymphocytes from one individual, and median values are indicated by horizontal bars. For FCM, ≥3,500 CD4+ cells and ≥150 CD4+ CD25high cells were analyzed for each sample. (C) Expression levels of FOXP3 in sorted CD4+ CD25high, CD4+ CD25low, and CD4+ CD25− cells isolated from histologically normal stomach mucosa were determined by real-time PCR and then related to the level of the housekeeping gene GAPDH. The values shown are arithmetic means plus SEM (n = 2).
Increased frequencies of mucosal CD4+ CD25high T cells in duodenal ulcer patients.
Using IHC, we have previously shown increased numbers of mucosal CD25+ cells in both asymptomatic individuals and duodenal ulcer patients compared to H. pylori-negative controls (31). Due to problems with recruiting duodenal ulcer patients for our new study, we could not perform FCM or FOXP3 mRNA analysis on freshly isolated cells from this patient group. However, our demonstration that mainly CD4+ CD25high cells are detected by our IHC method and that mucosal CD4+ CD25high cells have a Treg phenotype enables interpretation of the IHC data from duodenal ulcer patients in a new way (Table 2). Calculations of the ratios of CD25+ cells per total number of CD4+ cells detected in the lamina propria revealed increased proportions of CD4+ CD25high cells in both antra and duodena from duodenal ulcer patients compared to H. pylori-negative controls. No major differences in the frequencies of CD25high cells were detected between duodenal ulcer patients and individuals with asymptomatic H. pylori infections.
TABLE 2.
Frequencies of CD25+ cells among CD4+ cells from H. pylori-negative (Hp−) individuals, asymptomatic H. pylori-positive individuals (Hp+ AS), and H. pylori-positive duodenal ulcer patients (Hp+ DU), as detected by IHCa
| Sample site | Frequency of CD25+ cells
|
||
|---|---|---|---|
| Hp− | Hp+ AS | Hp+ DU | |
| Antrum | 0.0 (0.0, 2.1) | 3.1* (1.0, 14.6) | 5.5* (3.2, 13.1) |
| Duodenum | 0.6 (0.0, 1.1) | 3.6* (1.2, 10.8) | 6.7* (4.2, 13.9) |
Data are given as ratios (%) between the numbers of CD25+ and CD4+ cells per square millimeter of tissue. Data are medians (with quartiles). n = 10 for all study groups. *, P < 0.05 versus H. pylori-negative group.
DISCUSSION
CD4+ CD25+ Treg cells were first described to be important for the maintenance of self-tolerance (26), but recent studies suggest that Treg cells also play a role in immune responses to infections (22). We recently demonstrated a role for Treg cells in H. pylori infection in that impaired CD4+ memory T-cell responses to H. pylori antigens observed in the peripheral blood of H. pylori-positive individuals could be restored by the depletion of CD4+ CD25high Treg cells (20). The present study was designed to evaluate whether Treg cells are present at the site of infection and inflammation in H. pylori-positive individuals. Using FCM, IHC, and gene expression analysis, we were able to identify CD4+ CD25high T cells expressing the Treg-associated markers FOXP3 and CTLA-4 in both gastric and duodenal mucosae of H. pylori-positive individuals. The CD25high cells corresponded to about 5% of all CD4+ T cells at these sites, whereas only 1 to 2% of the CD4+ cells in uninfected gastric and duodenal mucosae were CD25high cells. For humans, experiments with sorted CD4+ CD25high and CD4+ CD25low cells have clearly demonstrated that the suppressor function resides within the CD25high cell fraction, whereas CD25low cells lack a regulatory capacity and are likely activated effector or memory T cells (2, 4, 35). However, when CD4+ CD25− cells are activated in vitro, their CD25 expression may increase, although the cells remain nonsuppressive (17). Little is known about the in vivo situation, but a recent study of Treg cells that were isolated from the synovial fluid of patients with rheumatoid arthritis suggested that even under inflammatory conditions, the CD25high fraction is still enriched in functionally suppressive Treg cells compared to the CD25low cell fraction (4). Since tissue-resident Treg cells are not readily accessible in large enough numbers to enable functional testing, more specific Treg markers are needed for the identification of such cells. The most Treg-specific marker identified so far is expression of the FOXP3 gene, which is a key regulator for the development and function of Treg cells (10, 15, 16). In the present study, we confirmed previous results (9, 34) showing that CD4+ CD25high cells from human blood express high levels of FOXP3 mRNA, whereas FOXP3 expression in CD25low or CD25− cells is considerably lower. We further demonstrated that T cells with induced CD25 expression as a result of in vitro activation do not express FOXP3 mRNA. Our results are in agreement with recent results by Fantini et al. (9), who showed that activation alone is not sufficient to induce FOXP3 expression. In contrast to these results, Walker et al. have shown that activated CD25− T cells that become CD25+ start to express FOXP3 (34). However, since the CD25+ FOXP3-expressing cells induced in the study by Walker et al. also gained suppressive activity, which has not been observed by others (17), it is likely that special cell-sorting criteria, stimulatory signals, and/or culture conditions may explain the disparate results obtained in that study. Importantly, both Fantini et al. and Walker et al. demonstrated a link between FOXP3 expression and suppressive activity, although the means by which the expression could be induced differed for the two reports. Thus, both studies support the use of FOXP3 expression as a marker for T cells with a regulatory function.
When analyzing mucosal T cells, we found that they had the same pattern of FOXP3 expression as peripheral blood CD4+ cells. Thus, high levels of FOXP3 mRNA were detected in CD4+ CD25high cells, whereas only low levels of FOXP3 expression were found in CD4+ CD25− and CD4+ CD25low cells. The mucosal CD4+ CD25high cells also expressed high levels of CTLA-4 and low levels of CD4 protein, which is characteristic of circulating Treg cells. It is interesting that the mucosal CD25low cells, which likely represent cells that have been activated in vivo, only expressed low levels of FOXP3 mRNA. This supports our in vitro data showing that FOXP3 expression is not induced in CD25low or CD25− cells upon activation and strengthens the association between Treg cells and this marker. We therefore believe that the majority of the cells within the mucosal CD4+ CD25high cell population are indeed Treg cells and that the infected antral and duodenal mucosa, which contains increased frequencies of CD4+ CD25high cells compared to uninfected mucosa, is thus enriched in Treg cells.
Previous studies of the mucosal T-cell response to H. pylori have shown a local production of gamma interferon, but not interleukin-4, suggesting that the response is of the Th1 type (18). Vaccination studies with mice indicate that a shift towards a Th2 response can result in reduced bacterial colonization (6), but it is clear that the T-cell response induced by the infection is unable to eliminate the bacteria. We believe that the inefficient immune response and the persistence of infection may be explained, at least in part, by immune suppression mediated by Treg cells. In support of this, studies with a mouse model of H. pylori infection recently showed that CD4+ CD25+ Treg cells suppress H. pylori-induced Th1-type T-cell responses and the development of gastritis, but at the expense of higher bacterial loads in the gastric mucosa (25). The role of Treg cells in promoting the persistence of chronic infections is further supported by studies of individuals with chronic viral, bacterial, or parasitic infections, for whom T cells with regulatory activities have been found in peripheral blood (1, 22). Furthermore, Treg cells can mediate the long-term persistence of Leishmania major (3) and Pneumocystis carinii (14) infections in mice and can contribute to immune suppression during malarial infections (13).
In asymptomatic individuals, Treg cells may help to maintain a balance between bacterial colonization and inflammation, preventing disease development, and any alteration in Treg cell activity may disturb this balance and contribute to disease. Previous observations that duodenal ulcer patients have lower epithelial cytokine expression levels (30), increased numbers of CTLA-4+ cells (31), and higher bacterial loads (12) in the duodenum than asymptomatic individuals suggest that duodenal immune suppression may occur in duodenal ulcer patients. We have previously shown by IHC that duodenal ulcer patients have an increased expression of CD25 in both the gastric and duodenal mucosae compared to uninfected controls (31). When comparing the CD25 expression levels detected by FCM and IHC in the present study, we observed that the majority of the cells detected with our IHC method were indeed CD4+ CD25high cells. Thus, our data suggest that duodenal ulcer patients have increased frequencies of mucosal Treg cells both in the antrum and in the duodenum compared to H. pylori-negative controls. However, we found no major differences in the frequencies of CD25high cells in duodenal ulcer patients and asymptomatic individuals, but further studies are needed to determine the functional activity of CD4+ CD25high cells and their influence on surrounding cells in these two study groups.
We also found increased frequencies of CD4+ CD25high cells in the stomachs of H. pylori-positive patients with gastric adenocarcinoma compared to uninfected controls and threefold-more CD4+ CD25high cells in the cancer tissue than in the surrounding healthy tissue or in gastric tissues from asymptomatic individuals. The CD4+ CD25high cells isolated from the gastric mucosae of cancer patients had the same phenotype as CD4+ CD25high cells from the mucosae of asymptomatic individuals, expressing high levels of FOXP3 and CTLA-4, suggesting that they have a regulatory function. This is interesting, as studies with mice support a role for Treg cell-mediated immunosuppression in cancer development. Thus, the elimination of CD4+ CD25+ Treg cells has been shown to evoke effective tumor immunity in otherwise nonresponding mice and to lead to an enhanced immunogenicity of tumor vaccines (26). Furthermore, large populations of CD4+ CD25+ cells with a functional regulatory capacity have been found in tumors from patients with pancreatic or breast adenocarcinoma (19) as well as from patients with lung cancer (37). Future studies will clarify whether the manipulation of Treg cells may be used for tumor immunotherapy in humans.
In conclusion, this study demonstrates the presence of increased populations of CD4+ CD25high T cells with a regulatory phenotype in the mucosae of individuals with asymptomatic H. pylori infections as well as in the mucosae of H. pylori-positive patients with duodenal ulcers or gastric adenocarcinoma. These cells have the potential to actively suppress immune responses against the bacteria and thereby contribute to the persistence of the infection. The identification of regulatory T cells at the site of a chronic infection in humans may also have important implications for the understanding of immune responses to other chronic infections.
Acknowledgments
This study was supported by grants from the Swedish Cancer Foundation, the Swedish Research Council, the Tore Nilsson's Foundation for Medical Research, the Nanna Svartz Foundation, the Ragnhild and Einar Lundströms' Memorial Foundation, the Adelbertska Research Foundation, and Wilhelm and Martina Lundgrens' Research Foundation.
We thank Gunilla Bogren for help with the recruitment of volunteers and Ann-Marie Hytönen for technical assistance with gel electrophoresis.
Editor: A. D. O'Brien
REFERENCES
- 1.Aandahl, E. M., J. Michaelsson, W. J. Moretto, F. M. Hecht, and D. F. Nixon. 2004. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 78:2454-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baecher-Allan, C., J. A. Brown, G. J. Freeman, and D. A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245-1253. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. [DOI] [PubMed] [Google Scholar]
- 4.Cao, D., V. Malmstrom, C. Baecher-Allan, D. Hafler, L. Klareskog, and C. Trollmo. 2003. Isolation and functional characterization of regulatory CD25bright CD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur. J. Immunol. 33:215-223. [DOI] [PubMed] [Google Scholar]
- 5.Chen, W., W. Jin, N. Hardegen, K. J. Lei, L. Li, N. Marinos, G. McGrady, and S. M. Wahl. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 198:1875-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Giudice, G., A. Covacci, J. L. Telford, C. Montecucco, and R. Rappuoli. 2001. The design of vaccines against Helicobacter pylori and their development. Annu. Rev. Immunol. 19:523-563. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson, K., I. Nordstrom, C. Czerkinsky, and J. Holmgren. 2000. Differential effect of cholera toxin on CD45RA+ and CD45RO+ T cells: specific inhibition of cytokine production but not proliferation of human naive T cells. Clin. Exp. Immunol. 121:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst, P. B., and B. D. Gold. 2000. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 54:615-640. [DOI] [PubMed] [Google Scholar]
- 9.Fantini, M. C., C. Becker, G. Monteleone, F. Pallone, P. R. Galle, and M. F. Neurath. 2004. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 172:5149-5153. [DOI] [PubMed] [Google Scholar]
- 10.Fontenot, J. D., M. A. Gavin, and A. Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330-336. [DOI] [PubMed] [Google Scholar]
- 11.Gambineri, E., T. R. Torgerson, and H. D. Ochs. 2003. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr. Opin. Rheumatol. 15:430-435. [DOI] [PubMed] [Google Scholar]
- 12.Hamlet, A., A. C. Thoreson, O. Nilsson, A. M. Svennerholm, and L. Olbe. 1999. Duodenal Helicobacter pylori infection differs in cagA genotype between asymptomatic subjects and patients with duodenal ulcers. Gastroenterology 116:259-268. [DOI] [PubMed] [Google Scholar]
- 13.Hisaeda, H., Y. Maekawa, D. Iwakawa, H. Okada, K. Himeno, K. Kishihara, S. Tsukumo, and K. Yasutomo. 2004. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat. Med. 10:29-30. [DOI] [PubMed] [Google Scholar]
- 14.Hori, S., T. L. Carvalho, and J. Demengeot. 2002. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 32:1282-1291. [DOI] [PubMed] [Google Scholar]
- 15.Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299:1057-1061. [DOI] [PubMed] [Google Scholar]
- 16.Khattri, R., T. Cox, S. A. Yasayko, and F. Ramsdell. 2003. An essential role for scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337-342. [DOI] [PubMed] [Google Scholar]
- 17.Levings, M. K., R. Sangregorio, and M. G. Roncarolo. 2001. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193:1295-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindholm, C., M. Quiding-Jarbrink, H. Lonroth, A. Hamlet, and A. M. Svennerholm. 1998. Local cytokine response in Helicobacter pylori-infected subjects. Infect. Immun. 66:5964-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liyanage, U. K., T. T. Moore, H. G. Joo, Y. Tanaka, V. Herrmann, G. Doherty, J. A. Drebin, S. M. Strasberg, T. J. Eberlein, P. S. Goedegebuure, and D. C. Linehan. 2002. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J. Immunol. 169:2756-2761. [DOI] [PubMed] [Google Scholar]
- 20.Lundgren, A., E. Suri-Payer, K. Enarsson, A. M. Svennerholm, and B. S. Lundin. 2003. Helicobacter pylori-specific CD4+ CD25high regulatory T cells suppress memory T-cell responses to H. pylori in infected individuals. Infect. Immun. 71:1755-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattsson, A., A. Tinnert, A. Hamlet, H. Lonroth, I. Bolin, and A. M. Svennerholm. 1998. Specific antibodies in sera and gastric aspirates of symptomatic and asymptomatic Helicobacter pylori-infected subjects. Clin. Diagn. Lab. Immunol. 5:288-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGuirk, P., and K. H. Mills. 2002. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 23:450-455. [DOI] [PubMed] [Google Scholar]
- 23.Mitra, D. K., S. C. De Rosa, A. Luke, A. Balamurugan, B. K. Khaitan, J. Tung, N. K. Mehra, A. I. Terr, A. O'Garra, L. A. Herzenberg, and M. Roederer. 1999. Differential representations of memory T cell subsets are characteristic of polarized immunity in leprosy and atopic diseases. Int. Immunol. 11:1801-1810. [DOI] [PubMed] [Google Scholar]
- 24.Price, A. B. 1991. The Sydney system: histological division. J. Gastroenterol. Hepatol. 6:209-222. [DOI] [PubMed] [Google Scholar]
- 25.Raghavan, S., M. Fredriksson, A. M. Svennerholm, J. Holmgren, and E. Suri-Payer. 2003. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clin. Exp. Immunol. 132:393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakaguchi, S., N. Sakaguchi, J. Shimizu, S. Yamazaki, T. Sakihama, M. Itoh, Y. Kuniyasu, T. Nomura, M. Toda, and T. Takahashi. 2001. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 182:18-32. [DOI] [PubMed] [Google Scholar]
- 27.Scheiman, J. M., and A. F. Cutler. 1999. Helicobacter pylori and gastric cancer. Am. J. Med. 106:222-226. [DOI] [PubMed] [Google Scholar]
- 28.Singh, B., S. Read, C. Asseman, V. Malmstrom, C. Mottet, L. A. Stephens, R. Stepankova, H. Tlaskalova, and F. Powrie. 2001. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 182:190-200. [DOI] [PubMed] [Google Scholar]
- 29.Stephens, L. A., C. Mottet, D. Mason, and F. Powrie. 2001. Human CD4+CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur. J. Immunol. 31:1247-1254. [DOI] [PubMed] [Google Scholar]
- 30.Stromberg, E., A. Edebo, A. M. Svennerholm, and C. Lindholm. 2003. Decreased epithelial cytokine responses in the duodenal mucosa of Helicobacter pylori-infected duodenal ulcer patients. Clin. Diagn. Lab. Immunol. 10:116-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stromberg, E., A. Lundgren, A. Edebo, S. Lundin, A. M. Svennerholm, and C. Lindholm. 2003. Increased frequency of activated T-cells in the Helicobacter pylori-infected antrum and duodenum. FEMS Immunol. Med. Microbiol. 36:159-168. [DOI] [PubMed] [Google Scholar]
- 32.Taams, L. S., J. Smith, M. H. Rustin, M. Salmon, L. W. Poulter, and A. N. Akbar. 2001. Human anergic/suppressive CD4+CD25+ T cells: a highly differentiated and apoptosis-prone population. Eur. J. Immunol. 31:1122-1131. [DOI] [PubMed] [Google Scholar]
- 33.Verhasselt, V., O. Vosters, C. Beuneu, C. Nicaise, P. Stordeur, and M. Goldman. 2004. Induction of FOXP3-expressing regulatory CD4pos T cells by human mature autologous dendritic cells. Eur. J. Immunol. 34:762-772. [DOI] [PubMed] [Google Scholar]
- 34.Walker, M. R., D. J. Kasprowicz, V. H. Gersuk, A. Benard, M. Van Landeghen, J. H. Buckner, and S. F. Ziegler. 2003. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25− T cells. J. Clin. Investig. 112:1437-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wing, K., A. Ekmark, H. Karlsson, A. Rudin, and E. Suri-Payer. 2002. Characterization of human CD25+ CD4+ T cells in thymus, cord and adult blood. Immunology 106:190-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wing, K., S. Lindgren, G. Kollberg, A. Lundgren, R. A. Harris, A. Rudin, S. Lundin, and E. Suri-Payer. 2003. CD4 T cell activation by myelin oligodendrocyte glycoprotein is suppressed by adult but not cord blood CD25+ T cells. Eur. J. Immunol. 33:579-587. [DOI] [PubMed] [Google Scholar]
- 37.Woo, E. Y., H. Yeh, C. S. Chu, K. Schlienger, R. G. Carroll, J. L. Riley, L. R. Kaiser, and C. H. June. 2002. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J. Immunol. 168:4272-4276. [DOI] [PubMed] [Google Scholar]