Abstract
Background
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) act by increasing insulin secretion, decreasing glucagon secretion, slowing gastric emptying, and increasing satiety.
Objective
Published evidence directly comparing GLP-1RAs with other approved treatments for type 2 diabetes (T2D) was systematically reviewed.
Methods
A literature search was performed using MEDLINE and Embase databases to identify papers comparing GLP-1RAs with other classes of glucose-lowering therapy in patients with T2D.
Results
Of the 1303 papers identified, 57 met the prespecified criteria for a high-quality clinical trial or retrospective study. The efficacy and tolerability of approved GLP-1RAs (exenatide twice daily or once weekly, dulaglutide, liraglutide, lixisenatide, and albiglutide) were compared with insulin products (23 prospective studies + seven retrospective studies), dipeptidyl peptidase-4 inhibitors (11 prospective studies + three retrospective studies), sulfonylureas (nine prospective studies + one retrospective study), thiazolidinediones (five prospective studies), and metformin (two prospective studies). GLP-1RAs are effective as a second-line therapy in improving glycemic parameters in patients with T2D. Reductions in glycated hemoglobin from baseline with GLP-1RAs tended to be greater or similar compared with insulin therapy. GLP-1RAs were consistently more effective in reducing body weight than most oral glucose-lowering drugs and insulin and were associated with lower hypoglycemia risk versus insulin or sulfonylureas. GLP-1RAs improved cardiovascular risk factors, and preliminary data suggest they improve cardiovascular outcomes in patients with T2D compared with oral glucose-lowering drugs. However, results from ongoing studies are awaited to confirm these early findings.
Conclusion
This systematic review found that GLP-1RAs are an effective class of glucose-lowering drugs for T2D.
Keywords: antidiabetic drugs, randomized controlled trials, retrospective, type 2 diabetes
Introduction
Diabetes mellitus is a chronic disease affecting a substantial proportion of the population.1 In adults (age 20–79 years), the 2015 prevalence of diabetes worldwide was estimated at 8.8%, with type 2 diabetes (T2D) comprising 91% of cases.2 By 2030, diabetes is expected to be the seventh leading cause of death.3
Several classes of glucose-lowering agents are currently available for the treatment of T2D, each with different mechanisms of action and therapeutic effects. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are a class of glucose-lowering drugs that act on the glucagon-like peptide-1 (GLP-1) receptor on pancreatic beta cells and increase insulin secretion, decrease glucagon secretion, slow gastric emptying, and increase satiety; clinical trials have shown that GLP-1RAs decrease body weight, postprandial glucose excursions, and some cardiovascular risk factors, without increasing the risk of hypoglycemia.4 Clinical trials have also studied the effects of GLP-1RA therapy on cardiovascular outcomes in patients with T2D.5,6
The purpose of this systematic review was to compare the efficacy of GLP-1RAs with other glucose-lowering therapies, using comparative data from clinical trials or observational cohort studies.
Literature search strategy and filtering
Embase and MEDLINE databases were searched on April 8, 2016, using the following search string: (glucagon like peptide 1 receptor agonist or “Glucagon-Like Peptide 1”) OR ([“Glucagon-like peptide-1” or “Glucagon-like peptide 1” or GLP1R or GLP-1 or GLP-1-R] AND [agonist or suppress* or block* or inhibit* or anti* or mimetic]) OR (lixisenatide or exenatide or liraglutide or albiglutide or dulaglutide) AND ([diabetes or diabetic or DM] and [“Type 2” or “Type-2” or “Type2” or “Type II” or “Type-II” or “Noninsulin-dependent” or “Noninsulin dependent” or “Non insulin-dependent” or “Non insulin dependent”]).
The search string was limited to keywords in the abstract or title. Articles indexed as animal studies, case reports, books, and conference/symposium presentations were excluded. The limits applied were English language and articles published between January 2005 and April 2016. Duplicates were removed, leaving 1303 articles. The articles identified were filtered manually and only those describing a head-to-head comparison between a GLP-1RA and another class of glucose-lowering therapy in ≥100 patients, regardless of study design, follow-up duration, or medication doses, were included in the final total.
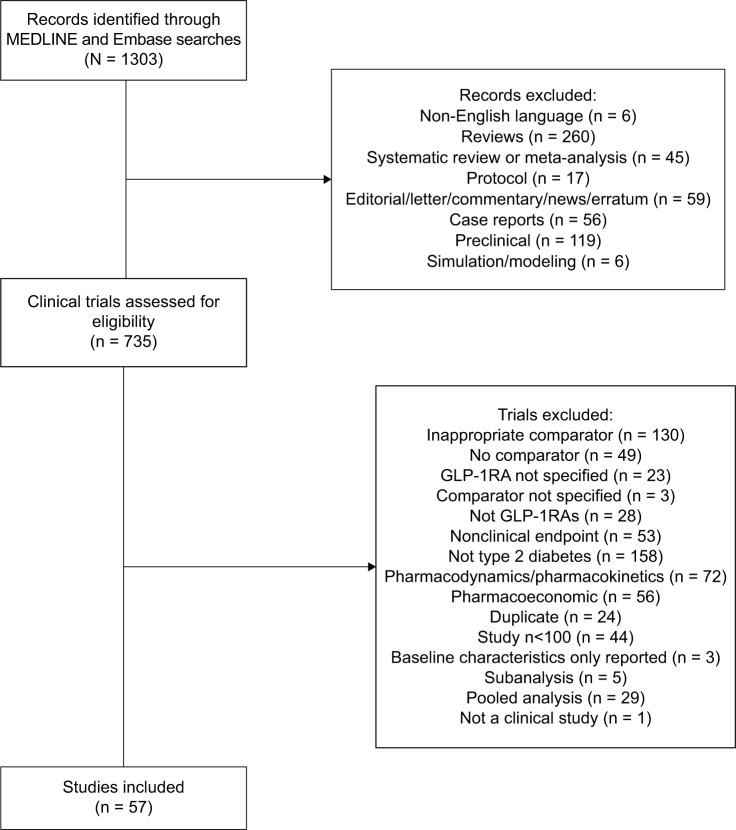
A total of 57 articles were included in the analysis (Figure 1); of these, 37 were randomized controlled trials (RCTs), seven were open-label extensions of RCTs, 11 were retrospective analyses, and two were prospective observational studies.
Figure 1.
PRISMA flow diagram.
Abbreviation: GLP-1RA, glucagon-like peptide-1 receptor agonist.
Efficacy of GLP-1RAs versus other glucose-lowering therapies
GLP-1RAs versus dipeptidyl peptidase-4 inhibitors (DPP-4is)
Eleven prospective studies7–17 and three retrospective studies compared GLP-1RAs with DPP-4is.18–20
Prospective studies
Exenatide once weekly (QW) was associated with significantly greater (P < 0.001) reductions in glycated hemoglobin (HbA1c) and fasting glucose (FG) compared with sitagliptin after 26 weeks in the Diabetes Therapy Utilization: Researching Changes in A1C, Weight and Other Factors Through Intervention with Exenatide Once Weekly (DURATION)-2 and DURATION-4 studies (Table 1).8,9 Although both exenatide QW and sitagliptin recipients lost weight, patients receiving exenatide QW had significantly greater weight loss from baseline.8,9 One study comparing exenatide twice daily (BID) with sitagliptin found that exenatide BID recipients had a reduction in FG similar to sitagliptin recipients, but a significantly greater reduction in weight.7
Table 1.
Study details and efficacy results of comparative trials of GLP-1RAs and oral glucose-lowering therapies
| Publications (acronym/ClinicalTrials.gov record) | Study design (study duration) | Treatment (patient number) | Background therapy | Change from baseline at study end, GLP-1RAs versus comparator
|
||
|---|---|---|---|---|---|---|
| HbA1c (%) | FG (mmol/L) | BW (kg) | ||||
| Dipeptidyl peptidase-4 inhibitors | ||||||
| Berg et al7 | CO, DB, DD, R in pts with T2D (2 × 4 weeks) | ExBID 10 µg/SITA 100 mg QD (41) SITA 100 mg QD/ExBID 10 µg (42) |
MET (n = 82) TZD (n = 1) |
NR | ExBID: −1.6 SITA: −1.6 |
ExBID: −1.4* SITA: −0.9 |
| Bergenstal et al8 (DURATION-2; NCT00637273) | DB, DD, MC, PG, R in pts with T2D (26 weeks) | ExQW 2 mg (160) SITA 100 mg QD (166) PIO 45 mg QD (165) |
MET | ExQW: −1.5*** SITA: −0.9 |
ExQW: −1.8** SITA: −0.9 |
ExQW: −2.3*** SITA: −0.8 |
| Russell-Jones et al9 (DURATION-4; NCT00676338) | DB, MC, PG, R in treatment-naive pts with T2D (26 weeks) | ExQW 2 mg (248) MET 2000 mg/day (246) PIO 45 mg QD (163) SITA 100 mg QD (163) |
None | ExQW: −1.5*** SITA: −1.2 |
ExQW: −2.3*** SITA: −1.1 |
ExQW: −2.0*** SITA: −0.8 |
| Nauck et al10 (AWARD-5; NCT00734474) | DB, MC, PG, R in pts with T2D (52 weeks) | DULA 0.75 mg QW (302) DULA 1.5 mg QW (304) SITA 100 mg QD (315) PBO (177) |
MET | DULA 0.75: −0.9*** DULA 1.5: −1.1*** SITA: −0.4 |
DULA 0.75: −1.6*** DULA 1.5: –2.4*** SITA: −0.9 |
DULA 0.75: −2.6*** DULA 1.5: −3.0*** SITA: −1.5 |
| Weinstock et al11 (AWARD-5; NCT00734474) | DB, MC, PG, R in pts with T2D (104 weeks) | DULA 0.75 mg QW (302) DULA 1.5 mg QW (304) SITA 100 mg QD (315) PBO (177) |
MET | DULA 0.75: −0.7*** DULA 1.5: −1.0*** SITA: −0.3 |
DULA 0.75: −1.4*** DULA 1.5: −2.0*** SITA: −0.5 |
DULA 0.75: −2.4 DULA 1.5: −2.9*** SITA: −1.8 |
| Charbonnel et al12 (NCT01296412) | OL, MC, PG, R in pts with T2D (26 weeks) | LIRA 1.2 mg/daya (253) SITA 100 mg ODb (269) |
MET | LIRA: −1.4 SITA: −1.3 |
LIRA: −2.2 SITA: −1.9 |
LIRA: −2.8 SITA: −0.4 |
| Pratley et al13 (NCT00700817) | OL, MC, PG, R in pts with T2D (52 weeks) | LIRA 1.2 mg/day (225) LIRA 1.8 mg/day (221) SITA 100 mg QD (219) |
MET | LIRA 1.2: −1.3*** LIRA 1.8: −1.5*** SITA: −0.9 |
LIRA 1.2: −1.7*** LIRA 1.8: −2.0*** SITA: −0.6 |
LIRA 1.2: −2.8*** LIRA 1.8: −3.7*** SITA: −1.2 |
| Pratley et al15 (NCT00700817) | OL extension of pts with T2D completing the 1860-LIRA-DPP-4 core study (26 weeks) | SITA 100 mg to LIRA 1.2 mg (67) SITA 100 mg to LIRA 1.8 mg (68) |
MET | LIRA 1.2: −0.2† LIRA 1.8: −0.5†† |
LIRA 1.2: −0.8† LIRA 1.8: −1.4†† |
LIRA 1.2: −1.6†† LIRA 1.8: −2.5†† |
| Takeshita et al16 | OL, PG, R in Japanese pts with T2D not adequately controlled by SITA-based therapy (12 weeks) | LIRA 0.9 mg QD (54) VILD 50 mg BID (58) |
None | LIRA: −0.7* VILD: −0.4 |
LIRA: −1.0 VILD: −0.8 |
LIRA: −1.6*** VILD: +0.1 |
| Van Gaal et al17 (NCT00976937) | DB, DD, MC, PG, R in young, obese pts with T2D (24 weeks) | LIXI 20 µg QD (158) SITA 100 mg QD (161) |
MET | LIXI: −0.7 SITA: −0.7 |
LIXI: −0.5 SITA: −0.7 |
LIXI: −2.5*** SITA: −1.2 |
| Russell-Jones et al9 (DURATION-4; NCT00676338) | DB, MC, PG, R in treatment-naive pts with T2D (26 weeks) | ExQW 2 mg (248) MET 2000 mg/day (246) PIO 45 mg QD (163) SITA 100 mg QD (163) |
None | ExQW: −1.5 MET: −1.5 |
ExQW: −2.3 MET: −2.0 |
ExQW: −2.0 MET: −2.0 |
| Umpierrez et al21 (AWARD-3; NCT01126580) | DB, DD, MC, PG, R in pts with T2D (52 weeksc) | DULA 0.75 mg QW (270) DULA 1.5 mg QW (269) MET ≥ 1500 mg/day (268) |
None | At 26 weeks DULA 0.75: −0.7* DULA 1.5: −0.8** MET: −0.6 At 52 weeks DULA 0.75: −0.6 DULA 1.5: −0.7* MET: −0.5 |
At 26 weeks DULA 0.75: −1.4 DULA 1.5: −1.6 MET: −1.3 At 52 weeks DULA 0.75: −1.0 DULA 1.5: −1.6* MET: −1.2 |
At 26 weeks DULA 0.75: −1.4** DULA 1.5: −2.3 MET: −2.2 At 52 weeks DULA 0.75: −1.1*** DULA 1.5: −1.9 MET: −2.2 |
| Sulfonylureas | ||||||
| Derosa et al22 | MC, R, SB in overweight pts with T2D poorly controlled on MET (12 months) | ExBID 10 µgd (63) GLYB 5 mg TIDd (65) |
MET | ExBID: −1.5 GLYB: −1.8 |
ExBID: −1.5 GLYB: −1.8 |
ExBID: −8.0*** GLYB: +4.3 |
| Derosa et al23 | MC, R, SB in overweight pts with T2D poorly controlled on MET (12 months) | ExBID 10 µgd (57) GLIM 2 mg TIDd (54) |
MET | ExBID: −1.2 GLIM: −1.4 |
ExBID: −1.5 GLIM: −1.6 |
ExBID: −5.1 GLIM: −0.9 |
| Gallwitz et al24 (EUREXA; NCT00359762) | OL, R, MC in overweight pts with T2D poorly controlled on MET (~3 years) | ExBID 10 µgd (490) GLIM 1 mg TIDe (487) |
MET | ExBID: −0.4** GLIM: −0.2 |
ExBID: −0.9* GLIM: −0.4 |
ExBID: −3.3*** GLIM: +1.2 |
| Nauck et al32 (LEAD-2; NCT00318461) | DB, DD, MC, PG, R in pts with T2D (26 weeks) | LIRA 0.6 mg QD (242) LIRA 1.2 mg QD (240) LIRA 1.8 mg QD (242) GLIM 4 mg QD (242) PBO (121) |
MET | LIRA 0.6: −0.7 LIRA 1.2: −1.0 LIRA 1.8: −1.0 GLIM 4: −1.0 |
LIRA 0.6: −1.1 LIRA 1.2: −1.6 LIRA 1.8: −1.7 GLIM 4: −1.3 |
LIRA 0.6: −1.8*** LIRA 1.2: −2.6*** LIRA 1.8: −2.8*** GLIM 4: +1.0 |
| Nauck et al28 (LEAD-2; NCT00318461) | OL extension of pts with T2D completing the LEAD-2 core study (18 months) | LIRA 0.6 mg QD (184) LIRA 1.2 mg QD (178) LIRA 1.8 mg QD (174) GLIM 4 mg QD (183) PBO (61) |
MET | LIRA 0.6: −0.4 LIRA 1.2: −0.6 LIRA 1.8: −0.6 GLIM 4: −0.5 |
LIRA 0.6: −0.8 LIRA 1.2: −1.2* LIRA 1.8: −1.2 GLIM 4: −0.6 |
LIRA 0.6: −2.1*** LIRA 1.2: −3.0*** LIRA 1.8: −2.9*** GLIM 4: +0.7 |
| Garber et al25 (LEAD-3 Mono; NCT00294723) | DB, DD, MC, PG, R in pts with early T2D (52 weeks) | LIRA 1.2 mg QD (251) LIRA 1.8 mg QD (247) GLIM 8 mg QD (248) |
None | LIRA 1.2: −0.8** LIRA 1.8: −1.1***‡ GLIM: −0.5 |
LIRA 1.2: −0.8* LIRA 1.8: −1.4***‡ GLIM: −0.3 |
LIRA 1.2: −2.0*** LIRA 1.8: −2.5*** GLIM: +1.1 |
| Garber et al26 (LEAD-3 Mono; NCT00294723) | OL extension of pts with T2D completing the LEAD-3 core study (52 weeks) | LIRA 1.2 mg QD (110) LIRA 1.8 mg QD (114) GLIM 8 mg QD (97) |
None | LIRA 1.2: −0.9* LIRA 1.8: −1.1** GLIM: −0.6 |
LIRA 1.2: −1.3** LIRA 1.8: −1.5*** GLIM: −0.3 |
LIRA 1.2: −2.1*** LIRA 1.8: −2.7*** GLIM: +1.1 |
| Seino et al30 (NCT00393718) | DB, DD, MC, PG, R in Japanese pts with T2D (24 weeks) | LIRA 0.9 mg QD (272) GLYB 2.5 mg/dayd (139) |
None | LIRA: −1.9*** GLYB: −1.4 |
LIRA: −3.6*** GLYB: −2.9 |
LIRA: −0.9*** GLYB: +1.0 |
| Kaku et al27 (NCT00393718) | OL extension of Seino study in Japanese pts with T2D (52 weeks) | LIRA 0.9 mg QD (268) GLYB 2.5 mg/dayd (132) |
None | LIRA: −1.5 GLYB: −1.0 |
LIRA: −3.2 GLYB: −2.5 |
LIRA: −0.8 GLYB: +1.0 |
| Thiazolidinediones | ||||||
| DeFronzo et al34 (NCT00135330) | OL, MC, PG, R in MET-treated pts with T2D (20 weeks) | ExBID 10 µgd (45) ROSI 4 mg BIDd (45) ExBID 10 µg + ROSI 4 mg EIDd (47) |
MET | ExBID: −0.9 ROSI: −1.0 |
ExBID: −1.5 ROSI: −1.8 |
ExBID: −2.8*** ROSI: +1.5 |
| Xu et al35 (CONFIDENCE; NCT01147627) | OL, MC, PG, R in treatment-naive pts with newly diagnosed T2D (48 weeks) | ExBID 10 µgd (142) PIO 30–45 mg QD (136) ILis 0.2 IU/kg BIDf (138) |
None | ExBID: −1.8** PIO: −1.5 |
ExBID: −1.9 PIO: −2.0 |
ExBID: −3.5*** PIO: 0 |
| Bergenstal et al8 (DURATION-2; NCT00637273) | DB, DD, MC, PG, R in pts with T2D (26 weeks) | ExQW 2 mg (160) SITA 100 mg QD (166) PIO 45 mg QD (165) |
MET | ExQW: −1.5* PIO: −1.2 |
ExQW: −1.8 PIO: −1.5 |
ExQW: −2.3*** PIO: +2.8 |
| Russell-Jones et al9 (DURATION-4; NCT00676338) | DB, MC, PG, R in treatment-naive pts with T2D (26 weeks) | ExQW 2 mg (248) MET 2000 mg/day (246) PIO 45 mg QD (163) SITA 100 mg QD (163) |
None | ExQW: −1.5 PIO: −1.6 |
ExQW: −2.3 PIO: −2.6 |
ExQW: −2.0*** PIO: +1.5 |
| Marre et al33 (LEAD-1 SU; NCT00318422) | DB, DD, MC, PG, R in pts with T2D treated with oral GLT for ≥ 3 months (26 weeks) | LIRA 0.6 mg/day (233) LIRA 1.2 mg/day (228) LIRA 1.8 mg/day (234) ROSI 4 mg/day (232) PBO (114) |
GLIM | LIRA 0.6: −0.6 LIRA 1.2: −1.1*** LIRA 1.8: −1.1*** ROSI 4: −0.4 |
LIRA 0.6: −0.7 LIRA 1.2: −1.6** LIRA 1.8: −1.6** ROSI 4: −0.9 |
LIRA 0.6: +0.7*** LIRA 1.2: +0.3*** LIRA 1.8: −0.2*** ROSI 4: +2.1 |
Notes:
After 12 weeks, LIRA up-titrated to 1.8 mg/day in patients with HbA1c ≥ 7.0% (53 mmol/mol);
after 12 weeks, GLIM 1 or 2 mg/day added to regimen of patients with HbA1c ≥ 7.0% (53 mmol/mol) and FG > 6.1 mmol/L;
primary end point was the change from baseline in HbA1c at 26 weeks. Secondary end points included the change from baseline in HbA1c at 52 weeks, and the change from baseline in FG and BW at 26 and 52 weeks;
administered at half the named dosage for the first month of the study;
dosage adjusted every 4 weeks up to the maximum tolerated dosage according to country-specific labeling information;
dosage was titrated according to self-monitored blood glucose levels.
P < 0.05;
P < 0.01;
P ≤ 0.001, GLP-1RA versus comparator;
P < 0.01,
P ≤ 0.0001 versus baseline (start of extension);
P < 0.05 versus LIRA 1.2 mg.
Abbreviations: AWARD, Assessment of Weekly Administration of Dulaglutide in Diabetes; BID, twice daily; BW, body weight; CO, crossover; CONFIDENCE, Comparison of Glycaemic Control and β-Cell Function Amongst Newly Diagnosed Patients With Type 2 Diabetes Treated With Exenatide, Insulin or Pioglitazone: A Multicentre Randomized Parallel-Group Study; DB, double blind; DD, double dummy; DPP-4, dipeptidyl peptidase 4; DULA, dulaglutide; DURATION, Diabetes Therapy Utilization: Researching Changes in A1C, Weight and Other Factors Through Intervention With Exenatide Once Weekly; EUREXA, European Exenatide Study; ExBID, exenatide twice daily; ExQW, exenatide once weekly; FG, fasting glucose; GLIM, glimepiride; GLP-1RA, glucagon-like peptide-1 receptor agonist; GLT, glucose-lowering therapy; GLYB, glyburide; HbA1c, glycated hemoglobin; ILis, insulin lispro; LEAD, Liraglutide Effect and Action in Diabetes; LIRA, liraglutide; LIXI, lixisenatide; MC, multicenter; MET, metformin; NR, not reported; OL, open label; PBO, placebo; PG, parallel group; PIO, pioglitazone; pts, patients; QD, once daily; QW, once weekly; R, randomized; ROSI, rosiglitazone; SB, single blind; SITA, sitagliptin; T2D, type 2 diabetes; TID, three times a day; TZD, thiazolidinedione; VILD, vildagliptin.
In the Assessment of Weekly Administration of LY2189265 in Diabetes (AWARD)-5 study, after 52 weeks of treatment, reductions from baseline in HbA1c, FG, and weight were significantly greater with dulaglutide than with sitagliptin (Table 1).10 These benefits were sustained over 104 weeks of treatment.11
Generally, liraglutide-treated patients had greater or similar reductions from baseline in HbA1c, FG, and weight compared with sitagliptin- or vildagliptin-treated patients (Table 1).12–14,16 In an open-label extension study, patients switching from sitagliptin to liraglutide had further reductions in these parameters.15
When administered for 24 weeks, lixisenatide produced reductions from baseline in HbA1c and FG that were similar to sitagliptin (Table 1);17 however, weight loss was significantly greater among lixisenatide versus sitagliptin recipients.
Hypoglycemia rates in patients receiving GLP-1RAs or DPP-4is were low, with only one instance of major/severe hypoglycemia reported across all studies (in a patient receiving liraglutide 1.2 mg).13,14 In studies of exenatide QW, minor hypoglycemia rates ranged from 1% to 3.6% with exenatide QW, with the highest hypoglycemia rates for concomitant sulfonylurea use, and from 0% to 3.0% with DPP-4is.7–9 Patients receiving dulaglutide 1.5 mg generally had numerically higher hypoglycemia rates than patients receiving dulaglutide 0.75 mg or sitagliptin (10.2% vs 5.3% and 4.8%; 12.8% vs 8.6% and 8.6%, respectively).10,11 Rates of hypoglycemia were similar for all liraglutide doses investigated versus DPP-4i comparators.12–14,16 Furthermore, the incidence of gastrointestinal adverse events (AEs; nausea, diarrhea, and vomiting) was higher7–11,13–15,17 or similar16 in patients receiving GLP-1RAs versus DPP-4is; gastrointestinal AEs led to treatment withdrawal in a higher percentage of patients receiving GLP-1RA therapy in these studies.8,10,11 Some studies reported that gastrointestinal AEs peaked on treatment initiation in the GLP-1RA group and then stabilized over the study period.10,11,13
Retrospective studies
Retrospective studies had similar results to the prospective studies (Table 2), with GLP-1RAs demonstrating greater or similar reductions in HbA1c, FG, and weight versus DPP-4is.18–20 Generally, hypoglycemia rates were not reported, although in one study the rate was similar between GLP-1RAs and DPP-4is.19 Nausea/vomiting was more frequent with exenatide compared with sitagliptin (incidence rate, 0.39 vs 0.032) in one study.19
Table 2.
Study details and efficacy results of retrospective studies of GLP-1RAs and oral glucose-lowering therapies or insulin
| Publications | Study design (study duration) | Treatment (patient number) | Background therapy | Change from baseline at study end, GLP-1RAs versus comparator
|
||
|---|---|---|---|---|---|---|
| HbA1c (%) | FG (mmol/L) | BW (kg) | ||||
| Dipeptidyl peptidase-4 inhibitors | ||||||
| Horton et al18 | Retrospective analysis of adult pts with T2D using data from the GEC database (3–12 months) | EXE (6280) INS (32,398) SITA (5861) |
OADs | EXE: −0.5 SITA: −0.6 |
EXE: −0.6 SITA: −0.8 |
EXE: −3.0 SITA: −1.1 |
| Montilla et al19 | Retrospective database analysis of adult pts with T2D in primary care in Italy (3–12 months) | EXE (21,064) SITA (38,811) VILD (17,989) |
OADs | EXE: −1.0 SITA: −0.9 VILD: −0.9 |
NR | EXE: ↓3.5% SITA: ↓ ~1.0%–1.5% VILD: ↓ ~1.0%–1.5% |
| Nyeland et al20 | Retrospective database analysis of adult pts with T2D in primary care in UK (6 months) | LIRA (287) SITA (2781) |
OADs | LIRA: −0.9** SITA: −0.6 |
NR | LIRA: −3.8*** SITA: −1.1 |
| Insulins | ||||||
| Baser et al58 | Retrospective analysis of adult pts with T2D using data from IMPACT national managed care database (1 year) | EXE (1958) IG (381) |
OADs | EXE: −0.9 IG: −1.2* |
NR | NR |
| Bounthavong et al59 | Retrospective analysis of adult pts with T2D using data from the VHA (2 years) | ExBID (446) IG or IDet (51,531) |
NR | ExBID: −0.6 LAI: −0.7 |
NR | NR |
| Dalal et al60 | Retrospective analysis of adult pts with T2D using data from IMPACT national managed care database (1 year) | EXE or LIRA (1705) RAI (5013) |
Basal INS | GLP-1RA: −0.6 RAI: −0.6 |
NR | NR |
| Horton et al18 | Retrospective analysis of adult pts with T2D using data from the GEC database (3–12 months) | EXE (6280) INS (32,398) SITA (5861) |
OADs | EXE: −0.5 INS: −1.0 |
EXE: −0.6 INS: −1.4 |
EXE: −3.0 INS: +0.6 |
| Pawaskar et al61 | Retrospective analysis of adult pts with T2D using data from the GEC database (1 year) | EXE (4494) IG (5424) |
OADs | EXE: −0.6* IG: −0.4 |
NR | EXE: −2.6* IG: −0.2 |
| Pawaskar et al62 | Retrospective analysis of elderly pts (≥ 65 years) with T2D using data from the GEC database (1 year) | EXE (1023) IG (2238) |
OADs | EXE: −0.5* IG: −0.2 |
NR | EXE: −2.8* IG: −0.2 |
| Sudhakaran et al63 | Retrospective analysis of adult pts with T2D in India (24 weeks) | ExBID 5–10 µg (47) IG QD (54) NPH QD or BID (23) |
OADs | ExBID: −1.0 IG: −0.8 NPH: −0.7 |
ExBID: −0.5 IG: −0.8 NPH: −0.8 |
ExBID: −1.6 IG: +1.8 NPH: +2.3 |
| Sulfonylureas | ||||||
| Chiefari et al31 | Retrospective analysis in adult out-pts with T2D in Italy (18 months) | LIRA 1.8 mg/day (76) GLIM 4 mg/day (103) |
MET | LIRA: −1.4*** GLIM: −0.4 |
LIRA: −2.1*** GLIM: −0.8 |
LIRA: −4.0*** GLIM: 0.0 |
Notes:
P < 0.05;
P < 0.01;
P ≤ 0.001, GLP-1RA versus comparator.
Abbreviations: BID, twice daily; BW, body weight; ExBID, exenatide twice daily; EXE, exenatide; FG, fasting glucose; GEC, general electric centricity; GLP-1RA, glucagon-like peptide-1 receptor agonist; GLIM, glimepiride; HbA1c, glycated hemoglobin; IDet, insulin detemir; IG, insulin glargine; INS, insulin; LAI, long-acting insulin; LIRA, liraglutide; MET, metformin; OAD, oral antidiabetes drug; NPH, neutral protamine Hagedorn insulin; NR, not reported; pts, patients; QD, once daily; RAI, rapid-acting insulin; SITA, sitagliptin; T2D, type 2 diabetes; VHA, Veterans Health Administration; VILD, vildagliptin.
GLP-1RAs versus metformin
Two prospective studies comparing GLP-1RAs and metfor-min were identified.9,21
Prospective studies
Both studies (RCTs) reported reductions from baseline in HbA1c, FG, and weight (Table 1).9,21 In DURATION-4, exenatide QW and metformin reduced HbA1c from baseline by ~1.5%, with no significant difference between groups at 26 weeks. In AWARD-3, dulaglutide 0.75 mg and 1.5 mg QW reduced HbA1c significantly more than metformin (P < 0.05) after 26 weeks. After 52 weeks, significant differences were observed in the reduction from baseline in HbA1c between dulaglutide 1.5 mg and metformin, but not between dulaglutide 0.75 mg and metformin.
Two RCTs investigated the effects of exenatide QW and dulaglutide on FG and weight, with no significant differences between these drugs in the reductions of these endpoints at 26 weeks.9,21 Treatment with dulaglutide at both dosages resulted in similar reductions in FG versus metformin at 26 weeks; at 52 weeks, recipients of dulaglutide 1.5 mg had a significantly greater reduction in FG than metformin recipients (P < 0.05; Table 1). The magnitude of weight loss achieved in AWARD-3 was similar between the dulaglutide 1.5 mg and metformin groups at 26 and 52 weeks; dulaglutide 0.75 mg recipients lost significantly less weight than patients receiving metformin at both time points.
Minor hypoglycemia was reported in 2.0% of exenatide QW recipients versus 0.0% of metformin recipients, while dulaglutide and metformin recipients had similar total hypoglycemia rates (12.3%, 11.1%, and 12.7% for dulaglutide 1.5 mg, dulaglutide 0.75 mg, and metformin, respectively).9,21 The incidence of gastrointestinal AEs was similar between treatment groups in AWARD-3,21 whereas in DURATION-4, nausea was reported in 11.3% and vomiting in 4.8% of exenatide-treated patients compared with 3.3% who experienced vomiting on metformin.9
GLP-1RAs versus sulfonylureas
Nine prospective studies investigated the comparative efficacy of GLP-1RAs and sulfonylureas.22–30 One retrospective study was identified: a comparison of liraglutide and glimepiride in outpatients in Italy.31
Prospective studies
Generally, exenatide BID recipients had greater or similar reductions from baseline in HbA1c, FG, and weight compared with glyburide or glimepiride recipients (Table 1).22–24 Furthermore, glycemic control was maintained for a longer duration with exenatide BID compared with glimepiride in the European Exenatide Study (EUREXA).24 Compared with glimepiride, liraglutide resulted in greater or similar reductions in HbA1c and FG, and consistently greater reductions in weight.25,26,28,32 Similar results were seen when liraglutide was compared with glyburide, with greater or similar reductions from baseline in HbA1c and FG; however, weight loss with liraglutide was not consistently greater versus glyburide.27,30
Patients receiving GLP-1RAs generally had significantly (P < 0.05) lower rates of hypoglycemia than sulfonylurea-treated patients;22–30 in cases where the P value was not stated, the rates were numerically different in favor of the GLP-1RA. The incidence of gastrointestinal AEs was generally higher with GLP-1RAs compared with sulfonylureas;25–28,30 however, one study reported similar incidence of gastrointestinal AEs with exenatide and glibenclamide.22 The incidence of treatment withdrawal due to gastrointestinal AEs was also higher with GLP-1RAs compared with sulfonylureas;22–24,32 differences in rates of gastrointestinal AEs were seen within 4 weeks in three studies29,30 and within 6 months in another study.24
Retrospective studies
In the retrospective analysis, liraglutide produced significantly greater reductions in HbA1c and FG from baseline compared with glimepiride (Table 2; P < 0.001).31 Liraglutide recipients also lost significantly more weight than glimepiride-treated patients (P < 0.001). As in the prospective studies, rates of hypoglycemia were lower with liraglutide than glimepiride.31 The incidence of gastrointestinal AEs was higher in the liraglutide group compared with the glimepiride group (P < 0.001).31
GLP-1RAs versus thiazolidinediones
Five prospective studies compared GLP-1RAs with thiazolidinediones.8,9,33–35
Prospective studies
Exenatide BID produced similar reductions in HbA1c to rosiglitazone and significantly greater reductions than pioglitazone (P < 0.01; Table 1).34,35 Similar reductions in FG were seen between exenatide BID and rosiglitazone and pioglitazone. Patients receiving exenatide BID had significantly greater weight loss than those receiving thiazolidinediones (P < 0.0001); in both studies, exenatide BID recipients had weight loss of ~3 kg, while patients receiving rosiglitazone gained weight and patients receiving pioglitazone had no change in weight.
Studies of exenatide QW showed that reductions from baseline in HbA1c, FG, and weight were either greater or similar than with thiazolidinedione comparators (Table 1).8,9 In the DURATION-2 study, exenatide QW recipients had significantly greater reductions in HbA1c versus pioglitazone, while in DURATION-4, reductions in HbA1c with exenatide QW and pioglitazone were similar; reductions in FG with exenatide QW and pioglitazone were similar. Significant reductions in weight were seen with exenatide QW in DURATION-2 and -4, whereas patients receiving pioglitazone gained weight (as expected in the presence of a sulfonylurea) in both studies (P < 0.0001 between groups).
The Liraglutide Effect and Action in Diabetes (LEAD)-1 SU study showed that liraglutide (0.6, 1.2, and 1.8 mg/day) was generally more effective as a second-line therapy in improving glycemic parameters and weight than rosiglitazone (Table 1).33 Liraglutide 1.2 mg and 1.8 mg was associated with significantly greater reductions in HbA1c (P < 0.0001) and FG (P < 0.001) than rosiglitazone. Recipients of lira-glutide 0.6 mg or 1.2 mg and rosiglitazone gained weight, whereas recipients of liraglutide 1.8 mg lost weight (Table 1); however, the weight gain with the lower dosages of liraglutide was significantly less than that with rosiglitazone, so the differences from baseline in weight were significant between rosiglitazone and all doses investigated (P < 0.0001).
Overall, the rate of hypoglycemia seen with GLP-1RAs was slightly greater than or similar to that seen with thiazolidinediones.8,9,33–35 The incidence of gastrointestinal AEs was higher with GLP-1RAs compared with thiazolidinediones.8,9,33–35 Gastrointestinal AEs led to treatment withdrawal in a higher number of patients in the exenatide group compared with thiazolidinediones in one study.35
GLP-1RAs versus insulin products
Twenty-three prospective studies35–57 and seven retrospective studies18,58–63 compared GLP-1RAs with insulin products.
Prospective studies
Four trials compared GLP-1RAs and insulin aspart (Table 3).38,47,54,55 Exenatide studies showed that exenatide BID produced a similar or lesser reduction in HbA1c and FG than insulin aspart.38,47,55 In contrast, exenatide BID recipients lost weight, while those using insulin aspart gained weight. In two of the three exenatide studies, the between-group difference in weight was significant (P < 0.001).
Table 3.
Study details and efficacy results of prospective (randomized controlled and noninterventional) trials of GLP-1RAs versus insulin products
| Publications (acronym/ClinicalTrials.gov record) | Study design (study duration) | Treatment (patient number) | Background therapy | Change from baseline at study end, GLP-1RAs versus insulin
|
||
|---|---|---|---|---|---|---|
| HbA1c (%) | FG (mmol/L) | BW (kg) | ||||
| Insulin | ||||||
| Ostenson et al56 (CHOICE; NCT00635492) |
MC, O study in pts with T2D (12 months) | ExBID (1096) INS (1239) |
OADs | ExBID: −1.0 INS: −1.8 |
NR | ExBID: −3.3 INS: +1.9 |
| Mathieu et al53 (CHOICE; NCT00635492) | MC, O study in pts with T2D (24 months) | ExBID (1114) INS (1274) |
OADs | ExBID: −1.0 INS: −1.7 |
NR | ExBID: −3.2 INS: +2.2 |
| Insulin aspart | ||||||
| Bergenstal et al38 (NCT00097877) | MC, OL, PG, R in pts with T2D (24 weeks) | ExBID 5–10 µg (124) Biphasic IAsp 30 QD (124) Biphasic IAsp 30 BID (124) |
MET SU |
ExBID: −1.8 IAsp QD: −2.3†† IAsp BID: −2.8†† |
ExBID: −1.2 IAsp QD: −2.9†† IAsp BID: −3.5†† |
ExBID: −1.9 IAsp QD: +2.8 IAsp BID: +4.1 |
| Gallwitz et al47 (NCT00434954) | MC, OL, PG, R in pts with T2D (26 weeks) | ExBID 5–10 µg (182) Biphasic IAsp 30 TID (181) |
MET | ExBID: −1.0 IAsp: −1.1 |
NR | ExBID: −4.1*** IAsp: +1.0 |
| Nauck et al55 (NCT00082407) | MC, OL, PG, R in pts with T2D (52 weeks) | ExBID 5–10 µg (253) Biphasic IAsp BID (248) |
SU | ExBID: −1.0 IAsp: −0.9 |
ExBID: −1.8 IAsp: −1.7 |
ExBID: −2.5*** IAsp: +2.9 |
| Mathieu et al54 (BEGIN: VICTOZA ADD-ON; NCT01388361) | MC, OL, PG, R in insulin-naive pts with T2D (26 weeks) | LIRA 0.6–1.8 mg/day (88) IAsp QD (89)a |
IDeg MET |
LIRA: −0.7** IAsp: −0.4 |
LIRA: −0.1 IAsp: −0.04 |
LIRA: −2.8*** IAsp: +0.9 |
| Insulin degludec | ||||||
| Gough et al50 (DUAL-1; NCT01336023) | MC, OL, PG, R in pts with T2D (26 weeks) | IDeg/LIRA QD (834) LIRA 0.6–1.8 mg QD (415) IDeg QD (414) |
MET ± PIO |
LIRA: −1.3 IDeg: −1.4 |
LIRA: −1.8 IDeg: −3.6 |
LIRA: −3.0 IDeg: +1.6 |
| Gough et al49 (DUAL-1; NCT01336023) | Extension of DUAL-I in pts with T2D (52 weeks) | IDeg/LIRA QD (665) LIRA 0.6–1.8 mg QD (313) IDeg QD (333) |
MET ± PIO |
LIRA: −1.2 IDeg: −1.4 |
LIRA: −1.7 IDeg: −3.4 |
LIRA: −3.0 IDeg: +2.3 |
| Insulin detemir | ||||||
| Davies et al41 (NCT01003184) | MC, OL, PG, R in pts with T2D (26 weeks) | ExQW 2 mg (111) IDet QD or BID (105) |
MET ± SU |
ExQW: −1.3*** IDet: −0.9 |
ExQW: −2.3 IDet: −2.4 |
ExQW: −2.7*** IDet: +0.8 |
| Insulin glargine | ||||||
| Barnett et al37 (NCT00099619) | CO, MC, OL, R in pts with T2D (2 × 16 weeks) | ExBID 5–10 µg/IG QD (68) IG QD/ExBID 5–10 µg (70) |
MET (55.1%) SU (44.9%) |
ExBID: −1.4 IG: −1.4 |
ExBID: −2.9 IG: −4.1†† |
ExBID: −1.6*** IG: +0.6 |
| Davies et al42 (HEELA) | MC, OL, PG, R in pts with T2D (26 weeks) | ExBID 5–10 µg (118) IG QD (117) |
MET SU TZD |
ExBID: −1.3 IG: −1.3 |
ExBID: −2.1 IG: −3.6†† |
ExBID: −2.7*** IG: +3.0 |
| Diamant et al46 (DURATION-3; NCT00641056) | MC, OL, PG, R in pts with T2D (26 weeks) | ExQW 2 mg (233) IG QD (223) |
MET ± SU |
ExQW: −1.5* IG: −1.3 |
ExQW: −2.1 IG: −2.8†† |
ExQW: −2.6*** IG: +1.4 |
| Diamant et al45 (DURATION-3 extension; NCT00641056) | OL extension of DURATION-3 in pts with T2D (84 weeks) | ExQW 2 mg (233) IG QD (223) |
MET ± SU |
ExQW: −1.2* IG: −1.0 |
ExQW: −2.4 IG: −3.0† |
ExQW: −2.1*** IG: +2.4 |
| Diamant et al44 (DURATION-3 extension; NCT00641056) | OL extension of DURATION-3 in pts with T2D (156 weeks) | ExQW 2 mg (194) IG QD (196) |
MET ± SU |
ExQW: −1.0* IG: −0.8 |
ExQW: −1.7 IG: −2.7†† |
ExQW: −2.5*** IG: +2.0 |
| Heine et al51 (NCT00082381) | MC, OL, PG, R in pts with T2D (26 weeks) | ExBID 5–10 µg (282) IG QD (267) |
MET SU |
ExBID: −1.1 IG: −1.1 |
ExBID: −1.4 IG: −2.9†† |
ExBID: −2.3 IG: +1.8 |
| Inagaki et al52 (NCT00935532) |
MC, OL, PG, R in Japanese pts with T2D (26 weeks) | ExQW 2 mg (215) IG QD (212) |
BG ± TZD |
ExQW: −1.1*** IG: −0.7 |
ExQW: −2.6 IG: −2.3 |
ExQW: −1.7*** IG: +0.3 |
| Araki et al36 (NCT01584232) | MC, OL, PG, R in Japanese pts with T2D (26 weeks) | DULA 0.75 mg QW (181) IG QD (180) |
BG ± SU |
DULA 0.75: −1.4*** IG: −0.9 |
DULA 0.75: −1.9 IG: −2.1 |
DULA 0.75: −0.5*** IG: +0.9 |
| Blonde et al39 (AWARD-4; NCT01191268) |
MC, OL, PG, R in pts with T2D (52 weeksb) | DULA 0.75 mg QW (293) DULA 1.5 mg QW (295) IG QD (296) |
ILis MET |
At 26 weeks DULA 0.75: −1.6* DULA 1.5: −1.6** IG: −1.4 |
At 26 weeks DULA 0.75: −0.2 DULA 1.5: −0.3 IG: −1.6†† |
At 26 weeks DULA 0.75: +0.2*** DULA 1.5: −0.9*** IG: +2.3 |
| D’Alessio et al40 (EAGLE) | MC, OL, PG, R in pts with T2D (24 weeks) | LIRA 0.6–1.8 mg QD (489) IG QD (489) |
MET ± SU |
LIRA: −1.8 IG: −1.9* |
LIRA: −2.4 IG: −2.8†† |
LIRA: −3.0*** IG: +2.0 |
| Giorgino et al48 (AWARD-2; NCT01075282) | MC, OL, PG, R in pts with T2D (78 weeksc) | DULA 0.75 mg QW (272) DULA 1.5 mg QW (273) IG QD (262) |
MET GLIM |
At 52 weeks DULA 0.75: −0.8*** DULA 1.5: −1.1*** IG: −0.6 |
At 52 weeks DULA 0.75: −0.9 DULA 1.5: −1.5 IG: −1.8††d |
At 52 weeks DULA 0.75: −1.3*** DULA 1.5: −1.9*** IG: +1.4 |
| Russell-Jones et al57 (LEAD-5; NCT00331851) | MC, OL, PG, R in pts with T2D (26 weeks) | LIRA 1.8 mg QD (230) IG QD (232) PBO (114) |
MET GLIM |
LIRA: −1.3** IG: −1.1 |
LIRA: −1.6 IG: −1.8 |
LIRA: −1.8*** IG: +1.6 |
| Insulin lispro | ||||||
| Diamant et al43 (NCT00960661) | MC, OL, PG, R in pts with T2D (30 weeks) | ExBID 5–10 µg (315) ILis TID (312) |
MET IG |
ExBID: −1.1 ILis: −1.1 |
ExBID: −0.5** ILis: +0.2 |
ExBID: −2.5*** ILis: +2.1 |
| Xu et al35 (CONFIDENCE; NCT01147627) |
MC, OL, PG, R in treatment-naive pts with newly diagnosed T2D (48 weeks) | ExBID 5–10 µg (142) PIO 30–45 mg QD (136) ILis BID (138) |
None | ExBID: −1.8 ILis: −1.7 |
ExBID: −1.9 ILis: −1.6 |
ExBID: −3.5*** ILis: +1.0 |
Notes:
Treatment arms were also compared with a nonrandomized group of patients who received IDeg alone (n = 236);
primary end point was change from baseline in HbA1c at 26 weeks;
primary end point was change from baseline in HbA1c at 52 weeks;
significant difference applies to IG QD versus DULA 0.75 mg QW comparison only.
P < 0.05;
P < 0.01;
P ≤ 0.001, GLP-1RA versus INS comparator;
P < 0.01;
P ≤ 0.001, INS comparator versus GLP-1RA.
Abbreviations: AWARD, Assessment of Weekly Administration of Dulaglutide in Diabetes; BG, biguanide; BID, twice daily; BW, body weight; CHOICE, Changes to Treatment and Outcomes in Patients With Type 2 Diabetes Initiating Injectable Therapy; CO, crossover; CONFIDENCE, Comparison of Glycaemic Control and β-Cell Function Amongst Newly Diagnosed Patients With Type 2 Diabetes Treated With Exenatide, Insulin or Pioglitazone: A Multicentre Randomized Parallel-Group Study; DUAL, Dual Action of Liraglutide and Insulin Degludec in Type 2 Diabetes; DULA, dulaglutide; DURATION, Diabetes Therapy Utilization: Researching Changes in A1C, Weight and Other Factors Through Intervention With Exenatide Once Weekly; EAGLE, Efficacy Assessment of Insulin Glargine versus Liraglutide After Oral Agent Failure; ExBID, exenatide twice daily; ExQW, exenatide once weekly; FG, fasting glucose; GLIM, glimepiride; GLP-1RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycated hemoglobin; HEELA, Helping Evaluate Exenatide in Patients With Diabetes Compared With Long-Acting Insulin; IAsp, insulin aspart; IDeg, insulin degludec; IDet, insulin detemir; IG, insulin glargine; ILis, insulin lispro; INS, insulin; LEAD, Liraglutide Effect and Action in Diabetes; LIRA, liraglutide; MC, multicenter; MET, metformin; NR, not reported; O, observational; OAD, oral antidiabetes drug; OL, open label; PBO, placebo; PG, parallel group; PIO, pioglitazone; pts, patients; QD, once daily; QW, once weekly; R, randomized; SU, sulfonylurea; T2D, type 2 diabetes; TID, three times daily; TZD, thiazolidinedione.
In the BEGIN™: VICTOZA® ADD-ON study, liraglutide significantly reduced HbA1c and weight versus insulin aspart, while reductions in FG were similar between treatments (Table 3).54 When liraglutide 1.8 mg was compared with insulin degludec, the reductions in HbA1c and FG were similar between groups; patients receiving liraglutide lost weight while patients receiving insulin gained weight.50
After 26 weeks of treatment, exenatide QW recipients had a significantly greater reduction from baseline in HbA1c than insulin detemir recipients and similar reductions in FG. Similar to liraglutide, those receiving exenatide lost weight, while insulin detemir recipients gained weight (between-group difference, P < 0.0001; Table 3).41
Twelve studies compared GLP-1RAs with insulin glargine (Table 3).36,37,39,40,42,44–46,48,51,52,57 In the exenatide (BID and QW) studies, reductions from baseline in HbA1c were greater or similar to those with insulin glargine, whereas reductions in FG were similar or smaller (Table 3). Furthermore, a significant difference was generally observed between the weight loss with exenatide and the weight gain with insulin glargine (Table 3).
In the AWARD-4 study, compared with insulin glargine, treatment with dulaglutide for 26 weeks resulted in significantly greater reductions in HbA1c in patients also receiving insulin lispro and significantly smaller reductions in FG.39 Patients receiving dulaglutide 1.5 mg lost weight versus baseline, while recipients of dulaglutide 0.75 mg and insulin glargine gained weight; however, the differences between the dulaglutide and insulin glargine groups were significant, irrespective of dulaglutide dose (Table 3).
The comparisons between liraglutide and insulin glargine in the Efficacy Assessment of Insulin Glargine Versus Liraglutide After Oral Agents Failure (EAGLE) and LEAD-5 studies were inconsistent with regard to HbA1c and FG; in the EAGLE study, liraglutide resulted in a lesser reduction in HbA1c and FG than insulin glargine, whereas in the LEAD-5 study, the reduction in HbA1c was greater with liraglutide versus insulin glargine and the reduction in FG was similar between groups.40,57 The change in weight was consistent between the two studies, with liraglutide recipients losing weight and insulin glargine recipients gaining weight (Table 3).
Two studies compared exenatide BID with insulin lispro (Table 3).35,43 In both studies, exenatide BID and insulin lispro reduced HbA1c from baseline to a similar degree. Reductions from baseline in FG were greater or similar with exenatide BID versus insulin lispro; exenatide BID treatment consistently resulted in weight loss, while insulin lispro recipients consistently gained weight.
Generally, the rate of hypoglycemia was lower with GLP-1RAs versus insulin products,35,38–40,43,46,47 or similar between groups.41,55,57 Overall, the incidence of gastrointestinal AEs among GLP-1RA-treated patients was higher than in insulin-treated patients;37–42,44–47,50–52,54,55,57 gastrointestinal AEs were generally observed on initiation of treatment with GLP-1RAs39,44,45,47,50,57 and sometimes led to treatment withdrawal.37,39,42,47,52
Retrospective studies
Seven retrospective studies compared GLP-1RAs and insulin products (Table 2),18,58–63 with similar results to the prospective studies; the reduction in HbA1c was greater with GLP-1RAs than insulin in most studies. Where reported, reductions from baseline in FG were not statistically different between groups. In studies in which the change in weight was reported, GLP-1RA treatment resulted in weight loss, while insulin treatment resulted in weight gain.
Where reported, the incidence of hypoglycemia was either similar between GLP-1RA and insulin treatment groups or lower in those receiving GLP-1RAs.58,60–63 Treatment-related nausea was reported in 10 patients in the exenatide group in one study and did not lead to treatment discontinuation.63
Effect of GLP-1RAs versus other glucose-lowering therapies on cardiovascular risk factors
The comparative studies identified by the literature search reported only limited information on cardiovascular risk factors and did not assess cardiovascular outcomes.
GLP-1RAs versus DPP-4is
Of the 10 prospective trials of GLP-1RAs and DPP-4is, all reported effects on blood pressure (BP) and/or lipid cardiovascular risk factors (Table S1).7–16 Two retrospective studies comparing GLP-1RAs with DPP-4is also reported changes in cardiovascular risk factors (Table S2).18,20 Changes in both diastolic BP and systolic BP (SBP) were variable for GLP-1RAs and DPP-4is, and changes in lipids were generally not significantly different between treatment groups. However, in AWARD-5, dulaglutide 1.5 mg resulted in a significantly greater reduction in low-density lipoprotein cholesterol compared with sitagliptin10 (Table S1).
GLP-1RAs versus metformin
Two studies reported data on cardiovascular risk factors after treatment with GLP-1RAs compared with metformin, neither of which showed any clinically significant between-group differences in cardiovascular risk factors (Table S1).9,21
GLP-1RAs versus sulfonylureas
Seven of the nine studies comparing GLP-1RAs with sulfonylureas reported data on cardiovascular risk factors (Table S1),24–28,30,32 along with one retrospective study (Table S2).31 In general, results indicated that the GLP-1RAs were associated with greater reductions in SBP than the sulfo-nylureas, with numerical reductions in various lipids observed in several studies comparing GLP-1RAs with sulfonylureas.
GLP-1RAs versus thiazolidinediones
All five prospective studies comparing GLP-1RAs with thiazolidinediones reported reductions from baseline in BP or fasting lipids (Table S1).8,9,33–35 Exenatide significantly reduced measures of cholesterol versus thiazolidinediones in three of these studies.8,34,35
GLP-1RAs versus insulin
Two of four trials comparing GLP-1RAs and insulin aspart reported changes in cardiovascular risk factors (Table S3).54,55 One trial reported a significantly smaller increase in high-density lipoprotein cholesterol with exenatide BID versus insulin aspart.55
Eight of ten prospective studies and two extension studies comparing GLP-1RAs with insulin glargine reported changes in cardiovascular risk factors (Table S3).36,39,40,42,44–46,48,52,57 Overall, numerical reductions in SBP were observed in most of these studies, with greater reductions generally seen with GLP-1RAs than for insulin glargine; significant differences between treatments for fasting lipids also generally favored GLP-1RAs.
Exenatide BID was compared with insulin lispro in two studies (Table S3).35,43 No significant differences between treatment groups were reported for changes in BP or lipids.
Of the seven retrospective studies comparing GLP-1RAs and insulin products, four reported data on cardiovascular risk factors (Table S2).18,61–63 Numerical reductions in BP and lipid measures were observed across exenatide and insulin therapies.
Discussion
GLP-1RAs represent an effective therapeutic option for patients with T2D. Currently, multiple studies provide data on the efficacy of approved GLP-1RAs in T2D (Figure 2). Although their glycemic efficacy is well established versus multiple classes of agents, including insulin, albeit with a notable lack of comparative studies versus sodium-glucose cotransporter 2 inhibitors, GLP-1RAs have additional benefits of modest weight loss and a favorable tolerability profile (Figure 3).64 These beneficial effects of GLP-1RAs should be considered in context with the increased frequency of nausea with GLP-1RAs, which may limit adherence, and the need for self-injection on a once- or twice-daily or weekly basis.
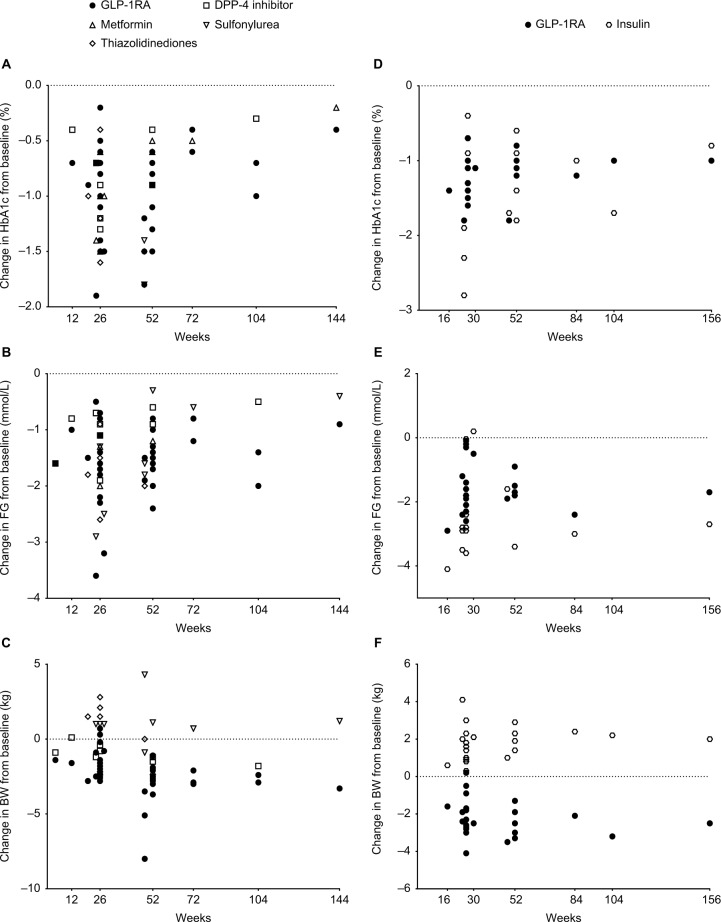
Figure 2.
Efficacy of glucose-lowering treatments in type 2 diabetes. Changes from baseline in (A) glycated hemoglobin (HbA1c), (B) fasting glucose (FG), and (C) body weight (BW) in prospective studies comparing glucagon-like peptide-1 receptor agonists (GLP-1RAs) with other oral glucose-lowering therapies and changes from baseline in (D) HbA1c, (E) FG, and (F) BW in prospective studies comparing GLP-1RAs with insulin products.
Abbreviation: DPP-4, dipeptidyl peptidase-4.
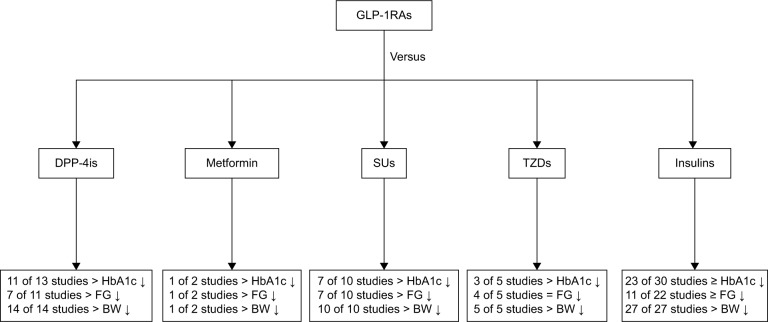
Figure 3.
Comparison of efficacy of GLP-1RAs with other glucose-lowering treatments in type 2 diabetes. General trends in glycemic parameters and body weight (BW) in comparative trials of glucagon-like peptide-1 receptor agonists (GLP-1RAs) and other glucose-lowering therapies. The total number of studies includes studies that reported these parameters.
Abbreviations: DPP-4i, dipeptidyl peptidase-4 inhibitor; FG, fasting glucose; HbA1c, glycated hemoglobin; SU, sulfonylurea; TZD, thiazolidinedione.
The efficacy of GLP-1RAs is generally greater than DPP-4is, owing to the supraphysiological concentrations of GLP-1 after administration of the former.65 This effect on incretin also accounts for the greater weight loss experienced by patients who receive GLP-1RAs versus the neutral weight effects produced by DPP-4is.65,66 Glycemic control was also mostly similar to that with insulin, largely driven by improvements in postprandial glucose with the short-acting GLP-1RAs, with similar effects on FG as insulin. However, real-world evidence suggests similar glycemic reductions between GLP-1RAs and insulin and within the GLP-1RA class. A real-world study in patients with T2D reported that addition of exenatide BID to basal insulin was as effective as addition of mealtime insulin in reducing HbA1c levels in these patients, with significant reductions in weight (P < 0.01) and hypoglycemia (P < 0.03) compared with mealtime insulin.67
Data comparing the effects of GLP-1RAs and other classes of glucose-lowering therapy on cardiovascular risk factors were limited but showed favorable effects on BP and lipid levels. Increases in heart rate have been observed with GLP-1RAs in clinical trials, although the underlying mechanisms and clinical relevance of these increases have yet to be established.68 The size and duration of increases in mean 24-hour heart rate vary between GLP-1RAs, ranging from transient (1–12 hours) increases of 1–3 beats per minute (bpm) with the short-acting GLP-1RAs exenatide BID and lixisenatide to more prolonged increases during treatment with longer-acting GLP-1RAs (3–4 bpm with exenatide QW or dulaglutide and 6–10 bpm with liraglutide or albiglutide).68 Data from cardiovascular outcome trials indicate that the observed increases in heart rate were not associated with an adverse effect on cardiovascular outcomes in patients with T2D.5,6,68,69
Limited clinical data are available regarding the effects of the various GLP-1RAs on cardiovascular outcomes, and none of these studies compared a GLP-1RA with another class of glucose-lowering therapy. The first published study with prospective outcomes data was the Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial,70 which found no significant difference in rates of cardiovascular events with lixisenatide versus placebo.6 A similar study investigating the effects of liraglutide on cardiovascular outcomes, the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial, showed improved effects of liraglutide on cardiovascular outcomes versus placebo.5 Finally, the Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6), which evaluated the effect of semaglutide (added on to standard care) on cardiovascular outcomes, demonstrated the noninferiority of semaglutide to placebo, with a significant reduction in cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction in the semaglutide group.69
Results of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial, evaluating the effect of exenatide QW on major adverse cardiovascular events (MACE) when given in addition to usual care;71 the Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND) study, evaluating the effects of dulaglutide on MACE;72 and the HARMONY Outcomes study, evaluating the effects of albiglutide on MACE,73 are all awaited and are expected to help contribute to the existing evidence regarding the impact of the GLP-1RAs on cardiovascular outcomes.
With the limited availability of prospective data, we can glean some information from meta-analyses and pooled analyses that have been conducted in an attempt to elucidate the effects of GLP-1RAs on cardiovascular outcomes. For example, a prespecified meta-analysis, which evaluated cardiovascular risk in the dulaglutide clinical development program,74 indicated that there were no significant differences between dulaglutide and placebo groups with regard to the risk of MACE. Similarly, a meta-analysis of cardiovascular events occurring during treatment with albiglutide, placebo, or active comparators in the HARMONY clinical trial program75 determined there was no significant difference between albiglutide and comparator for the risk of MACE or hospital admission for unstable angina.
The effect of exenatide BID on cardiovascular outcomes has also been assessed in a pooled meta-analysis of cardiovascular safety data from 12 long-term, randomized, placebo- or insulin comparator-controlled trials.76 The pooled relative risk for primary MACE for users of exenatide BID versus the comparator favored exenatide, suggesting no increased cardiovascular risk associated with exenatide use versus insulin or placebo. Two additional meta-analyses did not find any increase in the incidence of MACE in users of GLP-1RAs compared with comparators.77,78 Both studies showed that the GLP-1RAs were associated with a significantly lower risk of MACE compared with placebo, but not with active comparators (with the exception of pioglitazone).77,78 Consistent with these two studies, a sequential analysis of long-term trials comparing the effect of GLP-1RAs with other glucose-lowering drugs or placebo79 as well as a large comparative safety study of GLP-1RAs versus other glucose-lowering agents80 both demonstrated no significant difference in the risk of cardiovascular events between drug classes.
Real-world data can also provide some insight into the impact of the GLP-1RAs on cardiovascular risk in patients with T2D. Retrospective studies have suggested that exena-tide BID, with or without concomitant insulin, significantly reduced the risk of cardiovascular events compared with insulin81 and that exenatide BID is associated with a lower risk of cardiovascular events and hospitalizations versus other glucose-lowering therapies.82
The present review included prospective and retrospective studies that compared the efficacy and safety of approved GLP-1RAs with other currently available glucose-lowering therapies in the treatment of patients with T2D, with data showing clinical benefit of GLP-1RAs over other glucose-lowering therapies. However, it is critical to consider the patient selection criteria, background medications, properties of the individual GLP-1RAs, drug exposure across dose intervals, and outcome definitions of each study. Furthermore, the patient characteristics in these studies need to be taken into account to assess the generalizability of the results to the T2D population at large; overly strict criteria limit the applicability of the data to everyday clinical practice.
Conclusion
This systematic analysis shows that GLP-1RAs were generally as effective as, or more effective than, oral glucose-lowering therapies in improving glycemic parameters such as HbA1c and FG in patients with T2D. The reduction in HbA1c with GLP-1RAs tended to be similar or smaller compared with the reductions achieved with insulin therapy, with less hypoglycemia. GLP-1RAs were consistently more effective at reducing weight than oral glucose-lowering therapies and insulin. Additionally, the GLP-1RAs appeared to have favorable effects on cardiovascular risk factors such as BP and lipid levels. In summary, GLP-1RAs are an effective, safe, and well-tolerated treatment option for T2D, with minimal risk of hypoglycemia and providing modest weight loss.
Acknowledgments
Sheridan Henness, PhD, of inScience Communications, Springer Healthcare, provided medical writing support funded by AstraZeneca. The development of this manuscript was supported by AstraZeneca.
Footnotes
Author contributions
All authors have contributed sufficiently to the project to be included as authors. The authors contributed equally to the conception and design of the systematic review and literature search, interpretation of the results, and drafting of the manuscript.
Disclosure
PA Levin has received grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk, and Sanofi. He is a consultant for AstraZeneca, Novo Nordisk, and Sanofi, and a speaker for AstraZeneca, Boehringer Ingelheim, Eli Lily, GlaxoSmithKline, Janssen, Novo Nordisk, and Sanofi. H Nguyen was an employee of AstraZeneca at the time this review was conceived and drafted. ET Wittbrodt is an employee of AstraZeneca. SC Kim has received research grants to the Brigham and Women’s Hospital from Astra-Zeneca, Bristol-Myers Squibb, Genentech, Lilly, and Pfizer. The authors report no other conflicts of interest in this work.
References
- 1.American Diabetes Association Standards of medical care in diabetes-2015. Diabetes Care. 2015;38(Suppl 1):S1–S93. [PubMed] [Google Scholar]
- 2.International Diabetes Federation IDF Diabetes Atlas. 7th Ed. 2015. [Accessed January 31, 2017]. Available from: http://www.diabetesatlas.org/resources/2015-atlas.html.
- 3.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 5.Marso SP, Daniels GH, Brown-Frandsen K, et al. LEADER Steering Committee; LEADER Trial Investigators Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfeffer MA, Claggett B, Diaz R, et al. ELIXA Investigators Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 7.Berg JK, Shenouda SK, Heilmann CR, Gray AL, Holcombe JH. Effects of exenatide twice daily versus sitagliptin on 24-h glucose, glucoregulatory and hormonal measures: a randomized, double-blind, crossover study. Diabetes Obes Metab. 2011;13(11):982–989. doi: 10.1111/j.1463-1326.2011.01428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergenstal RM, Wysham C, Macconell L, et al. DURATION-2 Study Group Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376(9739):431–439. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- 9.Russell-Jones D, Cuddihy RM, Hanefeld M, et al. DURATION-4 Study Group Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study. Diabetes Care. 2012;35(2):252–258. doi: 10.2337/dc11-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD-5) Diabetes Care. 2014;37(8):2149–2158. doi: 10.2337/dc13-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab. 2015;17(9):849–858. doi: 10.1111/dom.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charbonnel B, Steinberg H, Eymard E, et al. Efficacy and safety over 26 weeks of an oral treatment strategy including sitagliptin compared with an injectable treatment strategy with liraglutide in patients with type 2 diabetes mellitus inadequately controlled on metformin: a randomised clinical trial. Diabetologia. 2013;56(7):1503–1511. doi: 10.1007/s00125-013-2905-1. [DOI] [PubMed] [Google Scholar]
- 13.Pratley R, Nauck M, Bailey T, et al. 1860-LIRA-DPP-4 Study Group One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract. 2011;65(4):397–407. doi: 10.1111/j.1742-1241.2011.02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pratley RE, Nauck M, Bailey T, et al. 1860-LIRA-DPP-4 Study Group Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375(9724):1447–1456. doi: 10.1016/S0140-6736(10)60307-8. [DOI] [PubMed] [Google Scholar]
- 15.Pratley RE, Nauck MA, Bailey T, et al. 1860-LIRA-DPP-4 Study Group Efficacy and safety of switching from the DPP-4 inhibitor sitagliptin to the human GLP-1 analog liraglutide after 52 weeks in metformin-treated patients with type 2 diabetes: a randomized, open-label trial. Diabetes Care. 2012;35(10):1986–1993. doi: 10.2337/dc11-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeshita Y, Takamura T, Kita Y, et al. Establishment of Rationale for Antiaging Diabetic Medicine (ERA-DM) Study Chapter 2 Group Vildagliptin vs liraglutide as a second-line therapy switched from sitagliptin-based regimens in patients with type 2 diabetes: a randomized, parallel-group study. J Diabetes Investig. 2015;6(2):192–200. doi: 10.1111/jdi.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Gaal L, Souhami E, Zhou T, Aronson R. Efficacy and safety of the glucagon-like peptide-1 receptor agonist lixisenatide versus the dipeptidyl peptidase-4 inhibitor sitagliptin in young (<50 years) obese patients with type 2 diabetes mellitus. J Clin Transl Endocrinol. 2014;1(2):31–37. doi: 10.1016/j.jcte.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horton ES, Silberman C, Davis KL, Berria R. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care. 2010;33(8):1759–1765. doi: 10.2337/dc09-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montilla S, Marchesini G, Sammarco A, et al. AIFA Anti-diabetics Monitoring Group Drug utilization, safety, and effectiveness of exena-tide, sitagliptin, and vildagliptin for type 2 diabetes in the real world: data from the Italian AIFA Anti-diabetics Monitoring Registry. Nutr Metab Cardiovasc Dis. 2014;24(12):1346–1353. doi: 10.1016/j.numecd.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Nyeland ME, Ploug UJ, Richards A, et al. Evaluation of the effectiveness of liraglutide and sitagliptin in type 2 diabetes: a retrospective study in UK primary care. Int J Clin Pract. 2015;69(3):281–291. doi: 10.1111/ijcp.12575. [DOI] [PubMed] [Google Scholar]
- 21.Umpierrez G, Tofé Povedano S, Pérez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3) Diabetes Care. 2014;37(8):2168–2176. doi: 10.2337/dc13-2759. [DOI] [PubMed] [Google Scholar]
- 22.Derosa G, Maffioli P, Salvadeo SA, et al. Exenatide versus glibenclamide in patients with diabetes. Diabetes Technol Ther. 2010;12(3):233–240. doi: 10.1089/dia.2009.0141. [DOI] [PubMed] [Google Scholar]
- 23.Derosa G, Putignano P, Bossi AC, et al. Exenatide or glimepiride added to metformin on metabolic control and on insulin resistance in type 2 diabetic patients. Eur J Pharmacol. 2011;666(1–3):251–256. doi: 10.1016/j.ejphar.2011.05.051. [DOI] [PubMed] [Google Scholar]
- 24.Gallwitz B, Guzman J, Dotta F, et al. Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial. Lancet. 2012;379(9833):2270–2278. doi: 10.1016/S0140-6736(12)60479-6. [DOI] [PubMed] [Google Scholar]
- 25.Garber A, Henry R, Ratner R, et al. LEAD-3 (Mono) Study Group Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473–481. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 26.Garber A, Henry RR, Ratner R, Hale P, Chang CT, Bode B, LEAD-3 (Mono) Study Group Liraglutide, a once-daily human glucagon-like peptide 1 analogue, provides sustained improvements in glycae-mic control and weight for 2 years as monotherapy compared with glimepiride in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13(4):348–356. doi: 10.1111/j.1463-1326.2010.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaku K, Rasmussen MF, Nishida T, Seino Y. Fifty-two-week, randomized, multicenter trial to compare the safety and efficacy of the novel glucagon-like peptide-1 analog liraglutide vs glibenclamide in patients with type 2 diabetes. J Diabetes Investig. 2011;2(6):441–447. doi: 10.1111/j.2040-1124.2011.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nauck M, Frid A, Hermansen K, et al. Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2 diabetes: 2-year results from the LEAD-2 study. Diabetes Obes Metab. 2013;15(3):204–212. doi: 10.1111/dom.12012. [DOI] [PubMed] [Google Scholar]
- 29.Nauck M, Marre M. Adding liraglutide to oral antidiabetic drug mono-therapy: efficacy and weight benefits. Postgrad Med. 2009;121(3):5–15. doi: 10.3810/pgm.2009.05.1997. [DOI] [PubMed] [Google Scholar]
- 30.Seino Y, Rasmussen MF, Nishida T, Kaku K. Efficacy and safety of the once-daily human GLP-1 analogue, liraglutide, vs glibenclamide monotherapy in Japanese patients with type 2 diabetes. Curr Med Res Opin. 2010;26(5):1013–1022. doi: 10.1185/03007991003672551. [DOI] [PubMed] [Google Scholar]
- 31.Chiefari E, Capula C, Vero A, et al. Add-on treatment with liraglutide improves glycemic control in patients with type 2 diabetes on metformin therapy. Diabetes Technol Ther. 2015;17(7):468–474. doi: 10.1089/dia.2014.0412. [DOI] [PubMed] [Google Scholar]
- 32.Nauck M, Frid A, Hermansen K, et al. LEAD-2 Study Group Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglu-tide effect and action in diabetes)-2 study. Diabetes Care. 2009;32(1):84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marre M, Shaw J, Brändle M, et al. LEAD-1 SU study group Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26(3):268–278. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeFronzo RA, Triplitt C, Qu Y, Lewis MS, Maggs D, Glass LC. Effects of exenatide plus rosiglitazone on beta-cell function and insulin sensitivity in subjects with type 2 diabetes on metformin. Diabetes Care. 2010;33(5):951–957. doi: 10.2337/dc09-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu W, Bi Y, Sun Z, et al. Comparison of the effects on glycaemic control and β-cell function in newly diagnosed type 2 diabetes patients of treatment with exenatide, insulin or pioglitazone: a multicentre randomized parallel-group trial (the CONFIDENCE study) J Intern Med. 2015;277(1):137–150. doi: 10.1111/joim.12293. [DOI] [PubMed] [Google Scholar]
- 36.Araki E, Inagaki N, Tanizawa Y, Oura T, Takeuchi M, Imaoka T. Efficacy and safety of once-weekly dulaglutide in combination with sulpho-nylurea and/or biguanide compared with once-daily insulin glargine in Japanese patients with type 2 diabetes: a randomized, open-label, phase III, non-inferiority study. Diabetes Obes Metab. 2015;17(10):994–1002. doi: 10.1111/dom.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exena-tide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther. 2007;29(11):2333–2348. doi: 10.1016/j.clinthera.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Bergenstal R, Lewin A, Bailey T, Chang D, Gylvin T, Roberts V, Novo-Log Mix-vs.-Exenatide Study Group Efficacy and safety of biphasic insulin aspart 70/30 versus exenatide in subjects with type 2 diabetes failing to achieve glycemic control with metformin and a sulfonylurea. Curr Med Res Opin. 2009;25(1):65–75. doi: 10.1185/03007990802597951. [DOI] [PubMed] [Google Scholar]
- 39.Blonde L, Jendle J, Gross J, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet. 2015;385(9982):2057–2066. doi: 10.1016/S0140-6736(15)60936-9. [DOI] [PubMed] [Google Scholar]
- 40.D’Alessio D, Häring HU, Charbonnel B, et al. EAGLE Investigators Comparison of insulin glargine and liraglutide added to oral agents in patients with poorly controlled type 2 diabetes. Diabetes Obes Metab. 2015;17(2):170–178. doi: 10.1111/dom.12406. [DOI] [PubMed] [Google Scholar]
- 41.Davies M, Heller S, Sreenan S, et al. Once-weekly exenatide versus once- or twice-daily insulin detemir: randomized, open-label, clinical trial of efficacy and safety in patients with type 2 diabetes treated with metformin alone or in combination with sulfonylureas. Diabetes Care. 2013;36(5):1368–1376. doi: 10.2337/dc12-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies MJ, Donnelly R, Barnett AH, Jones S, Nicolay C, Kilcoyne A. Exenatide compared with long-acting insulin to achieve glycaemic control with minimal weight gain in patients with type 2 diabetes: results of the Helping Evaluate Exenatide in patients with diabetes compared with Long-Acting insulin (HEELA) study. Diabetes Obes Metab. 2009;11(12):1153–1162. doi: 10.1111/j.1463-1326.2009.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diamant M, Nauck MA, Shaginian R, et al. 4B Study Group Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care. 2014;37(10):2763–2773. doi: 10.2337/dc14-0876. [DOI] [PubMed] [Google Scholar]
- 44.Diamant M, Van Gaal L, Guerci B, et al. Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trial. Lancet Diabetes Endocrinol. 2014;2(6):464–473. doi: 10.1016/S2213-8587(14)70029-4. [DOI] [PubMed] [Google Scholar]
- 45.Diamant M, Van Gaal L, Stranks S, et al. Safety and efficacy of once-weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeks. Diabetes Care. 2012;35(4):683–689. doi: 10.2337/dc11-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diamant M, Van Gaal L, Stranks S, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010;375(9733):2234–2243. doi: 10.1016/S0140-6736(10)60406-0. [DOI] [PubMed] [Google Scholar]
- 47.Gallwitz B, Böhmer M, Segiet T, et al. Exenatide twice daily versus premixed insulin aspart 70/30 in metformin-treated patients with type 2 diabetes: a randomized 26-week study on glycemic control and hypoglycemia. Diabetes Care. 2011;34(3):604–606. doi: 10.2337/dc10-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD-2) Diabetes Care. 2015;38(12):2241–2249. doi: 10.2337/dc14-1625. [DOI] [PubMed] [Google Scholar]
- 49.Gough SC, Bode BW, Woo VC, et al. One-year efficacy and safety of a fixed combination of insulin degludec and liraglutide in patients with type 2 diabetes: results of a 26-week extension to a 26-week main trial. Diabetes Obes Metab. 2015;17(10):965–973. doi: 10.1111/dom.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gough SCL, Bode B, Woo V, et al. NN9068-3697 (DUAL-I) trial investigators Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885–893. doi: 10.1016/S2213-8587(14)70174-3. [DOI] [PubMed] [Google Scholar]
- 51.Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG, GWAA Study Group Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143(8):559–569. doi: 10.7326/0003-4819-143-8-200510180-00006. [DOI] [PubMed] [Google Scholar]
- 52.Inagaki N, Atsumi Y, Oura T, Saito H, Imaoka T. Efficacy and safety profile of exenatide once weekly compared with insulin once daily in Japanese patients with type 2 diabetes treated with oral anti-diabetes drug(s): results from a 26-week, randomized, open-label, parallel-group, multicenter, noninferiority study. Clin Ther. 2012;34(9):1892–1908. doi: 10.1016/j.clinthera.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Mathieu C, Ostenson CG, Matthaei S, et al. Using exenatide twice daily or insulin in clinical practice: results from CHOICE. Diabetes Ther. 2013;4(2):285–308. doi: 10.1007/s13300-013-0037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathieu C, Rodbard HW, Cariou B, et al. BEGIN: VICTOZA ADD-ON (NN1250-3948) study group A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD-ON) Diabetes Obes Metab. 2014;16(7):636–644. doi: 10.1111/dom.12262. [DOI] [PubMed] [Google Scholar]
- 55.Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50(2):259–267. doi: 10.1007/s00125-006-0510-2. [DOI] [PubMed] [Google Scholar]
- 56.Ostenson CG, Matthaei S, Reaney M, et al. Treatment outcomes after initiation of exenatide twice daily or insulin in clinical practice: 12-month results from CHOICE in six European countries. Diabetes Metab Syndr Obes. 2013;6:171–185. doi: 10.2147/DMSO.S41827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046–2055. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baser O, Wei W, Baser E, Xie L. Clinical and economic outcomes in patients with type 2 diabetes initiating insulin glargine disposable pen versus exenatide BID. J Med Econ. 2011;14(6):673–680. doi: 10.3111/13696998.2011.605818. [DOI] [PubMed] [Google Scholar]
- 59.Bounthavong M, Tran JN, Golshan S, et al. Retrospective cohort study evaluating exenatide twice daily and long-acting insulin analogs in a Veterans Health Administration population with type 2 diabetes. Diabetes Metab. 2014;40(4):284–291. doi: 10.1016/j.diabet.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 60.Dalal MR, Xie L, Baser O, DiGenio A. Adding rapid-acting insulin or GLP-1 receptor agonist to basal insulin: outcomes in a community setting. Endocr Pract. 2015;21(1):68–76. doi: 10.4158/EP14290.OR. [DOI] [PubMed] [Google Scholar]
- 61.Pawaskar M, Li Q, Hoogwerf BJ, et al. Metabolic outcomes of matched patient populations initiating exenatide BID vs. insulin glargine in an ambulatory care setting. Diabetes Obes Metab. 2012;14(7):626–633. doi: 10.1111/j.1463-1326.2012.01581.x. [DOI] [PubMed] [Google Scholar]
- 62.Pawaskar M, Li Q, Reynolds MW. Metabolic outcomes of elderly patient populations initiating exenatide BID versus insulin glargine in an ambulatory care setting. Curr Med Res Opin. 2012;28(6):991–997. doi: 10.1185/03007995.2012.686901. [DOI] [PubMed] [Google Scholar]
- 63.Sudhakaran C, Fathima M, Anjana RM, Unnikrishnan RI, Mohan V. Effectiveness of exenatide in Asian Indians in a clinical care setting. Diabetes Technol Ther. 2010;12(8):613–618. doi: 10.1089/dia.2010.0033. [DOI] [PubMed] [Google Scholar]
- 64.Reid T. Choosing GLP-1 receptor agonists or DPP-4 inhibitors: weighing the clinical trial evidence. Clin Diabetes. 2012;30(1):3–12. [Google Scholar]
- 65.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60(4):470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smilowitz NR, Donnino R, Schwartzbard A. Glucagon-like peptide-1 receptor agonists for diabetes mellitus: a role in cardiovascular disease. Circulation. 2014;129(22):2305–2312. doi: 10.1161/CIRCULATIONAHA.113.006985. [DOI] [PubMed] [Google Scholar]
- 67.Lang K, Nguyen H, Huang H, Kaufman E, Levin P. Real-world treatment responses from electronic medical record (EMR) data among patients with type 2 diabetes (T2D) receiving basal insulin either with mealtime insulin or exenatide BID, by A1c attainment level and baseline A1c [abstract 1540-P] Diabetes. 2016;65(Suppl 1):A360–A431. [Google Scholar]
- 68.Lorenz M, Lawson F, Owens D, et al. Differential effects of glucagon-like peptide-1 receptor agonists on heart rate. Cardiovasc Diabetol. 2017;16(1):6. doi: 10.1186/s12933-016-0490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marso SP, Bain SC, Consoli A, et al. SUSTAIN-6 Investigators Sema-glutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 70.Bentley-Lewis R, Aguilar D, Riddle MC, et al. ELIXA Investigators Rationale, design, and baseline characteristics in Evaluation of LIX-isenatide in Acute Coronary Syndrome, a long-term cardiovascular end point trial of lixisenatide versus placebo. Am Heart J. 2015;169(5):631–638. doi: 10.1016/j.ahj.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 71.Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103–110. doi: 10.1016/j.ahj.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 72.Eli Lilly and Company The effect of dulaglutide on major cardiovascular events in patients with type 2 diabetes: Researching Cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) [Accessed October 11, 2016]. ClinicalTrials.gov record: NCT01394952. Available from: https://clinicaltrials.gov/ct2/show/NCT01394952.
- 73.GlaxoSmithKline A long term, randomised, double blind, placebo-controlled study to determine the effect of albiglutide, when added to standard blood glucose lowering therapies, on major cardiovascular events in patients with type 2 diabetes mellitus (HARMONY Outcomes) [Accessed October 11, 2016]. ClinicalTrials.gov identifier: NCT02465515. Available from: https://clinicaltrials.gov/ct2/show/NCT02465515.
- 74.Ferdinand KC, Botros FT, Atisso CM, Sager PT. Cardiovascular safety for once-weekly dulaglutide in type 2 diabetes: a pre-specified meta-analysis of prospectively adjudicated cardiovascular events. Cardiovasc Diabetol. 2016;15:38. doi: 10.1186/s12933-016-0355-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fisher M, Petrie MC, Ambery PD, Donaldson J, Ye J, McMurray JJ. Cardiovascular safety of albiglutide in the Harmony programme: a meta-analysis. Lancet Diabetes Endocrinol. 2015;3(9):697–703. doi: 10.1016/S2213-8587(15)00233-8. [DOI] [PubMed] [Google Scholar]
- 76.Ratner R, Han J, Nicewarner D, Yushmanova I, Hoogwerf BJ, Shen L. Cardiovascular safety of exenatide BID: an integrated analysis from controlled clinical trials in participants with type 2 diabetes. Cardiovasc Diabetol. 2011;10:22. doi: 10.1186/1475-2840-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Monami M, Cremasco F, Lamanna C, et al. Glucagon-like peptide-1 receptor agonists and cardiovascular events: a meta-analysis of randomized clinical trials. Exp Diabetes Res. 2011;2011:215764. doi: 10.1155/2011/215764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16(1):38–47. doi: 10.1111/dom.12175. [DOI] [PubMed] [Google Scholar]
- 79.Wu S, Sun F, Zhang Y, et al. The cardiovascular effects of glucagon-like peptide-1 receptor agonists: a trial sequential analysis of randomized controlled trials. J Clin Pharm Ther. 2014;39(1):7–13. doi: 10.1111/jcpt.12102. [DOI] [PubMed] [Google Scholar]
- 80.Patorno E, Everett BM, Goldfine AB, et al. Comparative cardiovascular safety of glucagon-like peptide-1 receptor agonists versus other anti-diabetic drugs in routine care: a cohort study. Diabetes Obes Metab. 2016;18(8):755–765. doi: 10.1111/dom.12665. [DOI] [PubMed] [Google Scholar]
- 81.Paul SK, Klein K, Maggs D, Best JH. The association of the treatment with glucagon-like peptide-1 receptor agonist exenatide or insulin with cardiovascular outcomes in patients with type 2 diabetes: a retrospective observational study. Cardiovasc Diabetol. 2015;14:10. doi: 10.1186/s12933-015-0178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care. 2011;34(1):90–95. doi: 10.2337/dc10-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]