Abstract
Transfer RNA modifications play pivotal roles in protein synthesis. N6-threonylcarbamoyladenosine (t6A) and its derivatives are modifications found at position 37, 3΄-adjacent to the anticodon, in tRNAs responsible for ANN codons. These modifications are universally conserved in all domains of life. t6A and its derivatives have pleiotropic functions in protein synthesis including aminoacylation, decoding and translocation. We previously discovered a cyclic form of t6A (ct6A) as a chemically labile derivative of t6A in tRNAs from bacteria, fungi, plants and protists. Here, we report 2-methylthio cyclic t6A (ms2ct6A), a novel derivative of ct6A found in tRNAs from Bacillus subtilis, plants and Trypanosoma brucei. In B. subtilis and T. brucei, ms2ct6A disappeared and remained to be ms2t6A and ct6A by depletion of tcdA and mtaB homologs, respectively, demonstrating that TcdA and MtaB are responsible for biogenesis of ms2ct6A.
INTRODUCTION
RNA modifications are a type of qualitative information embedded in RNA molecules (1). To date, about 140 species of modified nucleosides have been identified in various RNAs from all domains of life (2). tRNAs contain a number of chemical modifications that are required for accurate translation of the genetic code and stabilization of the tRNA tertiary structure (3–5). In particular, a wide variety of modifications is present in the anticodon loop, especially at the first position of the anticodon (position 34) and position 37, which is 3΄-adjacent to the anticodon. These modifications play critical roles in modulating codon recognition and ensuring accurate translation (6).
N 6-threonylcarbamoyladenosine (t6A) (Supplementary Data) and its derivatives are evolutionarily conserved essential modified bases at position 37 of tRNAs responsible for recognition of adenosine-starting codons (ANN codons) (7). The bulky side chain of t6A stabilizes the anticodon loop, promoting accurate decoding of ANN codons during protein synthesis (8,9). In addition, t6A is required for efficient aminoacylation of tRNA and efficient translocation, and it also prevents leaky scanning of initiation codons and read-through of stop codons. The biogenesis of t6A has been studied extensively. In E. coli, four enzymes, TsaC (YrdC), TsaD (YgjD), TsaB (YeaZ), and TsaE (YjeE), are required to synthesize t6A; L-threonine, adenosine triphosphate (ATP) and bicarbonate as substrates (8). In yeast, the YrdC homolog Sua5 and several components of the EKC-KEOPS complex, including Kae1, Pcc1, Gon7 and Bud32, are involved in t6A formation (10–12).
The presence of t6A in tRNAs from E. coli and yeast was first documented more than four decades ago (13). In 2013, however, we showed that most fraction of t6A in E. coli tRNAs is a hydrolyzed artifact of cyclic t6A (ct6A) (Supplementary Data) (9). ct6A is an additional modification of t6A that enhances tRNA decoding activity. ct6A is present in tRNAs from certain groups of bacteria, fungi, plants and some protists, but not in tRNAs of mammals, archaea or other bacteria. In E. coli, little t6A is present in tRNAs because almost all t6A is converted to ct6A via an ATP-dependent dehydration catalyzed by TcdA. We also identified two additional factors, CsdA and CsdE, which are required for efficient ct6A formation. CsdA is a cysteine desulfurase, and CsdE is a sulfur acceptor protein, implying that sulfur relay is involved in efficient formation of ct6A.
Initially, mass spectrometric and nuclear magnetic resonance (NMR) analyses determined the chemical structure of ct6A to be a cyclized active ester of the oxazolone ring (Supplementary Data) (9,14). Very recently, however, X-ray crystallography of synthetic ct6A nucleoside revealed the existence of a distinct isoform with a hydantoin structure (Supplementary Data) (15). LC/MS co-injection analyses showed that chemically synthesized ct6A nucleoside and natural ct6A in Escherichia coli tRNAs co-elute as a single peak by both reverse-phase and hydrophilic interaction liquid chromatography. They also exhibit identical patterns of product ions in collision-induced dissociation (CID) and a characteristic UV spectrum with maximum absorption at 269 nm. These observations strongly suggest that the hydantoin isoform of ct6A is actually present in E. coli tRNAs.
Another derivative of t6A, N6-methyl-N6-threonylcarbamoyladenosine (m6t6A) (Supplementary Data), is present in tRNAs from bacteria, fly, plants and mammals. In E. coli, m6t6A is present at position 37 of two species of tRNAThr responsible for translating ACY codons. We identified TrmO, a member of a novel class of AdoMet-dependent RNA methyltransferase, as the enzyme responsible for N6 methylation of m6t6A37 of bacterial tRNAThr (16). Its human homolog, TRMO, is responsible for formation of m6t6A37 in cytoplasmic tRNASer. Lack of TrmO decreases attenuation activity of the thr operon, indicating that N6 methylation of m6t6A37 ensures efficient decoding of ACY.
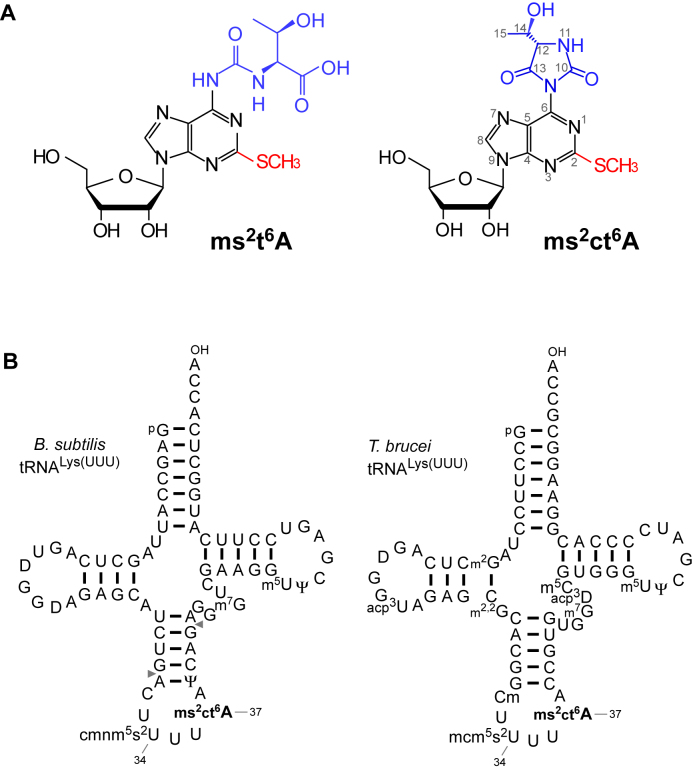
2-Methylthio-N6-threonylcarbamoyladenosine (ms2t6A) (Figure 1A) is another derivative of t6A found in tRNALys from Bacillus subtilis (Figure 1B), Trypanosoma (Figure 1B), plants and mammals (17–20). The methylthiolation of ms2t6A is required for the accurate decoding of Lys codons. B. subtilis MtaB (21,22) and human Cdkal1 (23) are the methylthiotransferases responsible for ms2t6A formation in tRNAs. Mutations in CDKAL1 are associated with risk of type 2 diabetes (24); consistent with this, pancreatic β-cell–specific knockout of mouse Cdkal1 results in a phenotype similar to that of type 2 diabetes (23). The presence of ct6A and TcdA homologs in B. subtilis, plants and Trypanosoma prompted us to speculate that the cyclic form of ms2t6A (ms2ct6A) (Figure 1A) would also be present in these organisms. As with ct6A, ms2ct6A must be hydrolyzed and converted to ms2t6A during tRNA preparation or conventional nucleoside analysis, which explains why it has not been previously detected. Indeed, a Tris-adduct of ms2t6A was detected in tRNALys (Figure 1B) from Trypanosoma brucei (19), indicating the presence of ms2ct6A; primary amines easily react with ct6A to form amine adducts of t6A (25).
Figure 1.
Detection of 2-methylthio-cyclic-N6-threonylcarbamoyladenosine (ms2ct6A). (A) The chemical structure of ms2t6A (left) and ms2ct6A (right). The 2-methylthio modification is shown in red, and the N6-threonylcarbamoyl group and its hydantoin form are shown in blue. (B) Secondary structures of B. subtilis tRNALys (left) and T. brucei tRNALys (right) with post-transcriptional modifications: dihydrouridine (D), 5-carboxymethylaminomethyl-2-thiouridine (cmnm5s2U), 2-methylthio cyclic N6-threonylcarbamoyladenosine (ms2ct6A), pseudouridine (Ψ), 7-methylguanosine (m7G), 5-methyluridine (m5U), N2-methylguanosine (m2G), 3-(3-amino-3-carboxypropyl)uridine (acp3U), N2, N2-dimethylguanosine (m2,2G), 2΄-O-methylcytidine (Cm), 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U), 3-(3-amino-3-carboxypropyl)dihydrouridine (acp3D), 5-methylcytidine (m5C). The position numbers of the residues are displayed according to the nucleotide numbering system (44). Pairs of gray triangles in B. subtilis tRNALys indicate the positions of cleavage by RNase T1 that generate RNA fragments containing the anticodon region.
Here, we describe ms2ct6A as a novel derivative of ct6A in tRNAs from B. subtilis, plants, and T. brucei. The chemical structure of ms2ct6A in natural tRNA was confirmed by LC/MS co-injection with the chemically synthesized authentic nucleoside. We also confirmed that orthologs of TcdA and MtaB are responsible for biogenesis of ms2ct6A in B. subtilis and T. brucei. In addition, we observed slower growth of T. brucei when the TcdA ortholog was down-regulated in the presence of cycloheximide, indicating that cyclic form of t6A is involved in integrity of protein synthesis.
MATERIALS AND METHODS
Bacterial strains and cultivation
B. subtilis str. 168 (WT) was kindly provided by Akiko Soma (Chiba University). B. subtilis strains were grown overnight in LB medium at 37°C. The ΔyqeV strain harboring the erythromycin resistance marker (Emr) was obtained from the National BioResource Project (National Institute of Genetics, Japan). The ΔyrvM strain was constructed from the WT strain, and the ΔyqeV/ΔyrvM double-deletion strain was constructed from the ΔyqeV strain by homologous recombination (26,27). The 5΄ upstream and 3΄ downstream regions (700–800 nt) of the yrvM gene were PCR-amplified from B. subtilis str. 168 genomic DNA with pairs of primers, 5΄-ggcacctattctgtatccattgatg-3΄ and 5΄-aagcgcagctcttgatgatcaaggctgtttttttg-3΄ and 5΄-tgaggatgaaggctgatccatgagcagccg-3΄ and 5΄-gagcatgatccggaagaaggc -3΄, respectively. Chloramphenicol resistant gene (Cmr) gene was PCR-amplified from pCBB31 (28) using a set of primers, 5΄-gatcatcaagagctgcgcttttttgtgtc-3΄ and 5΄-tggatcagccttcatcctcatattataaaagccag-3΄. These 3 products were ligated by PCR using primers, 5΄-ggcacctattctgtatccattgatg-3΄ and 5΄-gagcatgatccggaagaaggc-3΄, and subjected to the nested PCR amplification using a pair of primers, 5΄-tccatgatgatcaggcgatgga-3΄ and 5΄-ggaagaaggccgtttttacgca-3΄. The resultant PCR fragment was used for transformation.
Total RNA extraction
B. subtilis cells were suspended with 5 ml of RNA extraction buffer [50 mM NaOAc (pH 5.2) and 10 mM Mg(OAc)2 (pH 5.2)] and vigorously stirred for 10 min at room temperature. Next, 5 ml of water-saturated phenol was added and stirred for 10 min at room temperature. The mixture was frozen with liquid nitrogen and thawed in water; this process was repeated twice. The thawed solution was stirred for 50 min at room temperature. The aqueous phase was separated by centrifugation and washed once with chloroform, followed by re-extraction with 0.75 volumes of Trizol-LS (Life Technologies). Then, total RNA was precipitated with 2-propanol. The RNA pellet was dissolved in deionized water and subjected to ethanol precipitation; the resultant pellet was rinsed with 80% ethanol and dried. Thus, prepared RNA can be stored in pellet form for a long period of time without hydrolysis of ct6A and ms2ct6A. For use in all experiments, pellets were dissolved in ultrapure water.
Total RNA of spinach and Arabidopsis thaliana were extracted from plants as previously described (9). Nicotiana tabacum total RNA was obtained by the same procedure from tobacco BY-2 cells cultured for 1 week in modified Linsmaier and Skoog medium (29). Total RNA samples of spinach and tobacco were subjected to brief purification by weak anion exchange chromatography with DEAE Sepharose Fast Flow (GE Healthcare) to remove bulk contaminants and rRNA, as described (30).
Isolation of B. subtilis tRNALys
B. subtilis tRNALys was isolated from B. subtilis total RNA by reciprocal circulating chromatography, as described previously (9,31). The DNA probe, TGGTGAGCCATGAAGGACTCGAACCTTCGA with 5΄-terminal EC amino linker was covalently immobilized on NHS-activated Sepharose 4 Fast Flow (GE Healthcare). Fifty one micrograms of highly purified tRNALys was obtained from 1.7 mg total RNA.
Nucleoside preparation
Before digestion, total RNA was pre-cleared by gel filtration on a Centri-Sep spin column (Princeton Separations) with deionized water or trimethylamine (TMA)-HCl (pH 7.0) buffer to remove contaminants that could interfere with the ionization efficiency of nucleosides. For this experiment, enzymes including nuclease P1 (Wako Pure Chemical Industries), phosphodiesterase I (PDase I, Worthington Biochemical Corporation) and bacterial alkaline phosphatase (BAP from E. coli C75, Wako Pure Chemical Industries) were dialyzed with deionized water and stored at −30°C until use. Phosphodiesterase II (PDase II, from bovine spleen, Sigma) was dissolved in 10 mM TMA-AcOH (pH 5.3) buffer and centrifuged. The supernatant was filtered through a 0.22 μm Ultrafree-MC unit (Merck-Millipore) and stored at −30°C.
For conventional digestion (32), 40 μg of total RNA was digested at 37°C for 1 h in a 25–50 μl reaction mixture consisting of 0.1 U nuclease P1 and 25 mM NH4OAc (pH 5.3), followed by addition of 0.1 volume of 1 M ammonium bicarbonate (pH 8.2) and 0.08 U BAP, and then incubated at 37°C for 3 h.
For neutral digestion (9), 40 μg of total RNA was digested at 37°C for 1 h in a 25–50 μl reaction mixture consisting of 0.1 U nuclease P1 and 25 mM NH4OAc (pH 5.3), followed by addition of 0.1 volume of TMA-HCl (pH 7.0) and 0.127 U PDase I, and then incubated at 37°C for 1 h. The prepared nucleotides were dephosphorylated with 0.08 U BAP at 37°C for 3 h at neutral pH.
Digestion of plant and several other RNAs was carried out by one-step acidic digestion. Specifically, a solution (typically 40 μl) containing 1 μg/μl total RNA, 20 mM TMA acetate (pH 5.3), nuclease P1 (0.1 units for 40 μg of RNA), PDase II (0.1 units for 40 μg of RNA) and BAP (0.16 units for 40 μg of RNA) was incubated at 37°C for 1 h. In this procedure, PDase II was used for complete digestion of hypermodified adenosines under acidic conditions. We observed conversion of adenosine and N6-methyladenosine (m6A) to inosine, indicating contamination by adenosine deaminase activity in the Sigma PDase II product (P9041). t6A derivatives remained intact under these conditions.
LC/MS analyses of total nucleosides and isolated tRNA
LC/MS analyses of total nucleosides were performed essentially as described previously (9,33–34), using an LCQ Advantage ion-trap (IT) mass spectrometer (Thermo Fisher Scientific) equipped with an ESI source and an HP1100 liquid chromatography system (Agilent Technologies) or a Q Exactive hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific) equipped with an ESI source and an Ultimate 3000 liquid chromatography system (Dionex).
For RPC/ESI-MS with an LCQ Advantage instrument, nucleosides were separated on an Inertsil ODS-3 column (2.1 mm × 250 mm, GL sciences) and analyzed as described previously (34). For RPC/ESI-MS with a Q Exactive instrument, digests were separated on a Sunshell C18 column (2.6 μm core-shell silica particle, 2.1 × 150 mm, ChromaNik Technologies). The mobile phase consisted of 5 mM ammonium acetate (pH 5.3) (solvent A) and acetonitrile (ACN) (solvent B). The gradient program was as follows: 0–40% B from 0 to 30 min, 40% B for 5 min and then 0% B at a flow rate of 75 μl/min. Nucleoside digest (8–12 μg) or synthetic ms2ct6A (100–500 pmol) dissolved in LC/MS grade ultrapure water (Wako) was injected.
For HILIC/ESI-MS, a ZIC-cHILIC column (3 μm particle size, 2.1 × 150 mm, Merck-Millipore) was used on a Q Exactive instrument (33). The mobile phase consisted of 5 mM ammonium acetate (pH 5.3) (solvent A) and ACN (solvent B). Total nucleosides (8–12 μg) or synthetic ms2ct6A (450–500 fmol) dissolved in 90% ACN was injected and chromatographed at a flow rate of 100 μl/min in a multistep linear gradient: 90–40% B from 0 to 30 min, 40% B for 10 min and then 0% B. Proton adducts of nucleosides were scanned in a positive polarity mode over a range of m/z 110–700 or 110–900.
For RNA fragment analysis of isolated tRNA, B. subtilis tRNALys was digested by RNase T1, followed by subjected to capillary liquid chromatography (LC) coupled to nano electrospray (ESI)/mass spectrometry (MS) on a linear ion trap-Orbitrap hybrid mass spectrometer (LTQ Orbitrap XL; Thermo Fisher Scientific) as described (9,34).
Chemical synthesis of ms2ct6A
The substrate nucleoside, 2-methylthio-N6-threonylcarbamoyladenosine (ms2t6A) was synthesized according to the procedures described previously (35–38). Cyclization of ms2t6A to form ms2ct6A was performed on the basis of carbodiimide chemistry. ms2t6A (10 mg, 0.022 mmol) was dissolved in anhydrous DMF (1 ml) and mixed with EDC·HCl (42 mg, 0.22 mmol). The reaction mixture was stirred at room temperature. After 3 h, consumption of all substrate was confirmed by TLC analysis (nBuOH/H2O, 85/15, v/v, Rf values of ms2t6A and ms2ct6A are 0.15 and 0.43, respectively). The solvent was removed under reduced pressure, and the crude product was purified by reverse-phase chromatography (Ascentis C18 HPLC Column, 10 μm, 21.2 × 250 mm) at a flow rate of 7 ml/min with a linear gradient of acetonitrile in 0.1% acetic acid (B) and water (A) as follows: 2–30% B from 0 to 40 min, 30–50% from 40 to 45 min, 50–2% B from 45 to 47 min, 2% B for 3 min. The ms2ct6A fraction (22.35 min) was collected and evaporated to dryness. Yield of ms2ct6A nucleoside was 44% (4.2 mg).
The purity of ms2ct6A was checked by high performance liquid chromatography (HPLC) analysis using an XTerra® Waters column (MS C8, 5 μm, 4.6 × 150 mm, 100 Å) (Supplementary Data). The mobile phase consisted of 5 mM sodium acetate (pH 7) in water (solvent A) and ACN (solvent B). Chemically synthesized ms2t6A (A) and ms2ct6A (B) were chromatographed at a flow rate of 1 ml/min with a dual-step linear gradient: 0–20% B from 0 to 30 min, and 20–40% B from 30 to 40 min. Isolated ms2ct6A was characterized by UV spectroscopy (Supplementary Data), IR spectroscopy (Supplementary Data), 1H-NMR (Supplementary Data), 13C-NMR (Supplementary Data) and high resolution MS (Supplementary Data).
Cultivation and RNAi of T. brucei
Partial segments of the coding sequences of the Tb427tmp.02.2830 (TbTcdA) and Tb427.06.3510 (TbMtaB) from T. brucei were cloned into the tetracycline-inducible RNAi vector p2T7-177. These plasmids were then linearized by NotI digestion and introduced into procyclic T. brucei 29-13 cells for genomic integration; clonal lines were obtained by limiting dilution. The cell lines were grown in SDM-79 medium, and RNAi was induced by addition of 1 μg/ml tetracycline. Cell counts were taken every 24 h using a Beckman Z2 Coulter counter over the course of 12 days post-induction in the presence or absence of tetracycline (1 μg/ml) and cycloheximide (50 μg/ml).
Total RNA was extracted from uniduced and RNAi-induced T. brucei cells using a standard protocol (39). The steady-state levels of individual mRNAs were measured by RT-PCR. The cDNAs of TbMtaB (677 bps) and TbTcdA (443 bps) were amplified using the following primers: 5΄-attcacttaacttccctatttgc-3΄ and 5΄-gttctgacagcattcttcaaacc-3΄ for TbMtaB, and 5΄-gcctaccaaccgggaagccttcttg-3΄ and 5΄-ggattgcattgacagcgtcgagtgtaag-3΄ for TbTcdA.
PCR products were then analyzed by agarose gel electrophoresis. The same reaction performed without reverse transcriptase was used as a negative control (RT-).
RESULTS
Identification of ms2ct6A in B. subtilis tRNAs
We previously detected ct6A in total nucleosides of B. subtilis tRNAs digested under neutral conditions (9). Consistent with this finding, the B. subtilis YrvM is an ortholog gene of TcdA, which catalyzes ATP-dependent dehydration of t6A to form ct6A (9). In addition, ms2t6A is present in tRNALys from B. subtilis (21,22). These observations strongly suggest that, in B. subtilis, ms2t6A in tRNALys is converted to ms2ct6A by the TcdA homolog.
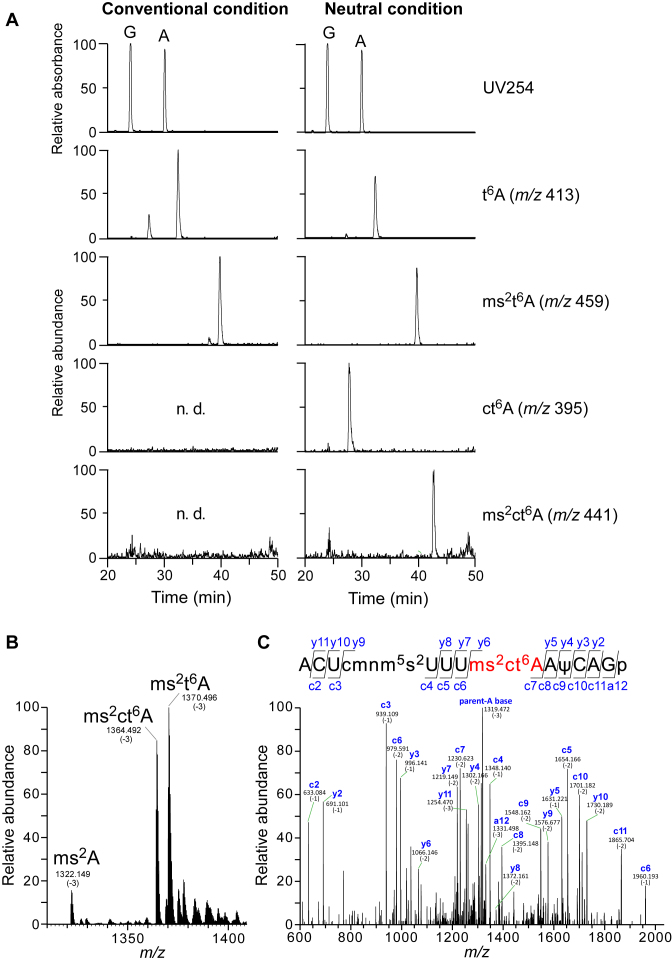
To confirm the presence of ms2ct6A, total RNA of B. subtilis was digested into nucleosides under conventional or neutral conditions, and then subjected to LC/MS analysis (Figure 2A). In the conventional conditions, we observed proton adducts of t6A and ms2t6A, but no ct6A, as reported previously (9). On the other hand, in the neutral conditions, ct6A was clearly detected. In addition, we detected a dehydrated form of ms2t6A (m/z 441). This modified species, tentatively named N440, was not detected in total nucleosides digested in the conventional conditions, indicating that it was susceptible to hydrolysis (Figure 2A). This observation strongly suggested that N440 is ms2ct6A.
Figure 2.
Mass spectrometric analyses of total RNA and isolated tRNALys from B. subtilis. (A) Nucleoside analyses of total RNA from B. subtilis. Total nucleosides were prepared under conventional conditions (left panels) and neutral conditions (right panels). The panels second from the bottom show mass chromatograms corresponding to the proton adducts of t6A (m/z 413), ms2t6A (m/z 459), ct6A (m/z 395) and ms2ct6A (m/z 441), respectively. n.d., not detected. (B) Mass spectrum of the 12 mer-fragments containing anticodon region. Three peaks for the triply-charged negative ions of the RNA fragments having ms2A, ms2t6A and ms2ct6A at position 37 are indicated. (C) A collision-induced dissociation (CID) spectrum of the 12 mer-fragment of B. subtilis tRNALys digested by RNase T1. The triply-charged negative ion of the ms2ct6A37-containing fragment (m/z 1364.492) was used as a precursor ion for CID. The product ions were assigned according to the literature (45). Sequences of parent ion and assigned product ions are described upper side in this panel.
Considering that B. subtilis tRNALys has ms2t6A at position 37 (Figure 1B) (17), ms2ct6A should be found in this tRNA. B. subtilis tRNALys was isolated by the reciprocal circulating chromatography (9,31), digested by RNase T1 and subjected to capillary LC coupled to ESI/MS. The 12 mer-fragments containing anticodon region were clearly detected. Judging from the m/z values of triply-charged negative ions of this fragment (Figure 2B), we clearly detected three fragments having different modifications at position 37, namely ms2A, ms2t6A and ms2ct6A. Although ms2t6A37 was present more abundant than ms2ct6A37 in the isolated tRNALys, it is likely that a certain population of ms2t6A37 in this tRNA originates from ms2ct6A37 hydrolyzed during tRNA isolation by RCC. The 12 mer-fragment with ms2ct6A was further probed by collision-induced dissociation to map the modified residues (Figure 2C). By assignment of product ions in the CID spectrum, we unequivocally mapped cmnm5s2U at position 34 and ms2ct6A at position 37 (Figure 2C).
To determine the structure of N440, we chemically synthesized ms2ct6A from ms2t6A (Supplementary Data). As reported in the accompanying paper (15), activation of the carboxyl group of t6A by water-soluble carbodiimide (EDC), which facilitates cyclization of the side chain, predominantly generates the hydantoin isoform of ct6A. Because we employed the same procedure as for ct6A synthesis to cyclize the side chain of ms2t6A, the hydantoin isoform should be the predominant form in chemically synthesized ms2ct6A. Detailed spectroscopic analyses of the synthesized ms2ct6A using UV (Supplementary Data), IR (Supplementary Data), 1H NMR (Supplementary Data) and 13C NMR (Supplementary Data) supported the hydantoin isoform as observed for ct6A in the accompanying paper (15). Especially, in the IR spectrum (Supplementary Data), two characteristic absorption bands at 1720 cm−1 and 1788 cm−1 are associated with C = O bond stretching in the hydantoin ring.
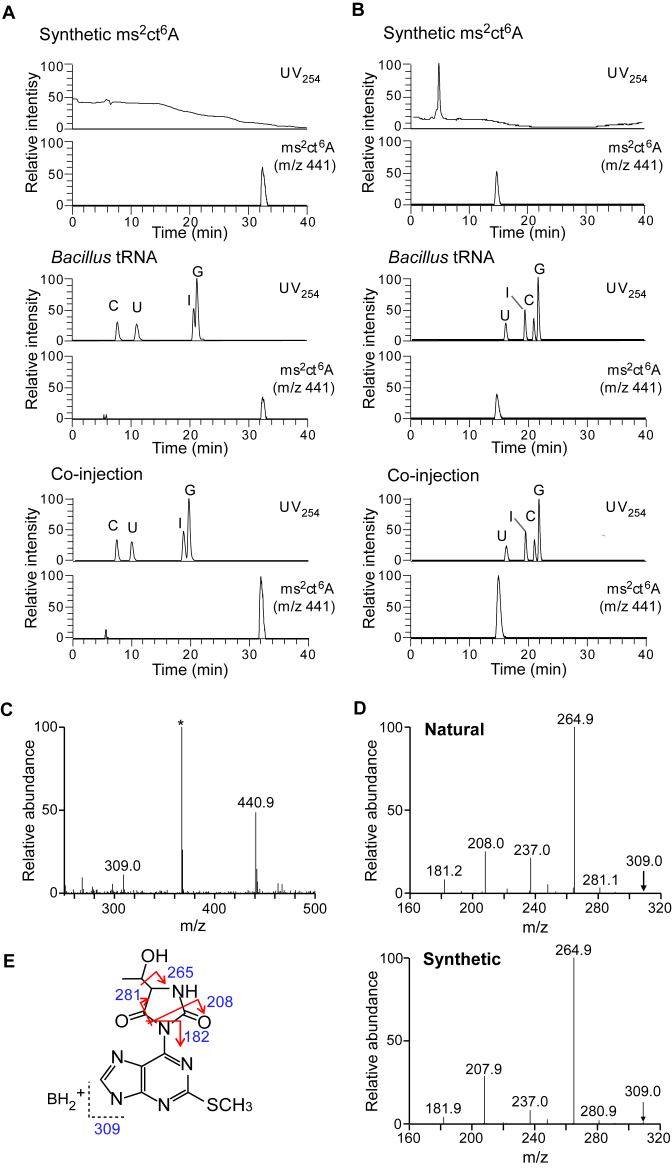
The synthetic ms2ct6A was mixed with total nucleosides of B. subtilis and subjected to LC/MS analyses using reverse-phase chromatography (Figure 3A), as well as hydrophilic interaction chromatography (Figure 3B). The synthetic ms2ct6A co-eluted with N440 as a single peak under both conditions. Next, we used CID to further probe the base-related ions (BH2+, m/z 309) of N440 and the synthetic ms2ct6A (Figure 3C). The product ions of these two compounds exhibited identical patterns (Figure 3D), and assigned in the chemical structures of the ms2ct6A base (Figure 3E). Collectively, these observations indicated that N440 is ms2ct6A.
Figure 3.
Structural confirmation of ms2ct6A from B. subtilis. Co-injection analyses of synthetic ms2ct6A and total RNA from B. subtilis by (A) RPC/ESI-MS and (B) HILIC/ESI-MS. UV trace at 254 nm and mass chromatograms of the synthetic ms2ct6A, total nucleosides of B. subtilis and co-injection are shown in the top, middle and bottom panels, respectively. Conversion of adenosine to inosine is due to the contamination of PDase II (Sigma P9041) with adenosine deaminase activity. (C) The mass spectrum for the proton adduct of natural ms2ct6A (MH+, m/z 440.9) from B. subtilis. The base-related ion (BH2+, m/z 309.0) was also detected. (D) The CID spectra for natural (upper panel) and synthetic (lower panel) BH2+ of ms2ct6A. Parent ions for CID are indicated by arrows. (E) Assignment of the product ions in the CID spectrum of the natural ms2ct6A BH2+ ion.
Biogenesis of ms2ct6A in B. subtilis
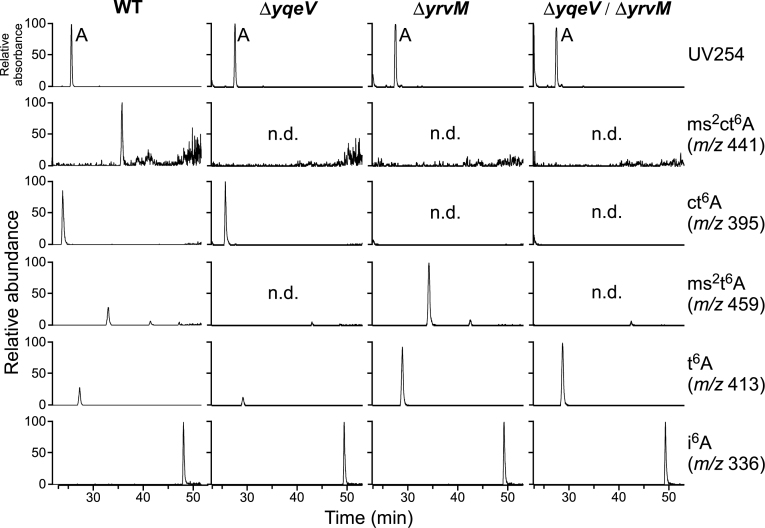
The yqeV and yrvM of B. subtilis are homologs of mtaB and tcdA, respectively (9,22). To confirm that these genes are involved in ms2ct6A formation, we constructed a knockout of yrvM (ΔyrvM) and the double-deletion strain (ΔyqeV/ΔyrvM) by homologous recombination. Total RNA from wild-type B. subtilis (WT) and a series of knockout strains were digested into nucleosides and subjected to LC/MS analysis (Figure 4). In the ΔyqeV strain, both ms2ct6A and ms2t6A disappeared as expected and probably remained to be ct6A and t6A, respectively. In fact, the level of ct6A increased slightly relative to that in the WT strain. In ΔyrvM, both ms2ct6A and ct6A disappeared and remained to be ms2t6A and t6A, respectively. Indeed, the levels of ms2t6A and t6A clearly increased in this strain. In the case of ΔyqeV/ΔyrvM, all three derivatives (ms2ct6A, ct6A and ms2t6A) disappeared, and relevant tRNAs only have t6A. These observations demonstrated that yqeV (mtaB) and yrvM (tcdA) are responsible for 2-methylthiolation and cyclization of t6A, respectively, resulting in the formation of ms2ct6A on tRNALys.
Figure 4.
Identification of enzymes responsible for ms2ct6A formation in B. subtilis. RPC/ESI-MS nucleoside analyses of total RNAs from B. subtilis wild type (str. 168). Mass chromatograms from ΔyqeV, ΔyrvM and ΔyqeV/ΔyrvM are shown from left to right, respectively. Top panels show the UV traces at 254 nm. Panels second from the bottom show mass chromatograms corresponding to the proton adducts of ms2ct6A (m/z 441), ct6A (m/z 395), ms2t6A (m/z 459), t6A (m/z 413) and i6A (m/z 336), respectively. n.d., not detected. Mass chromatograms of t6A derivatives were normalized by that of i6A, and abundance of each peak is displayed relative to the highest peak (100%) among them.
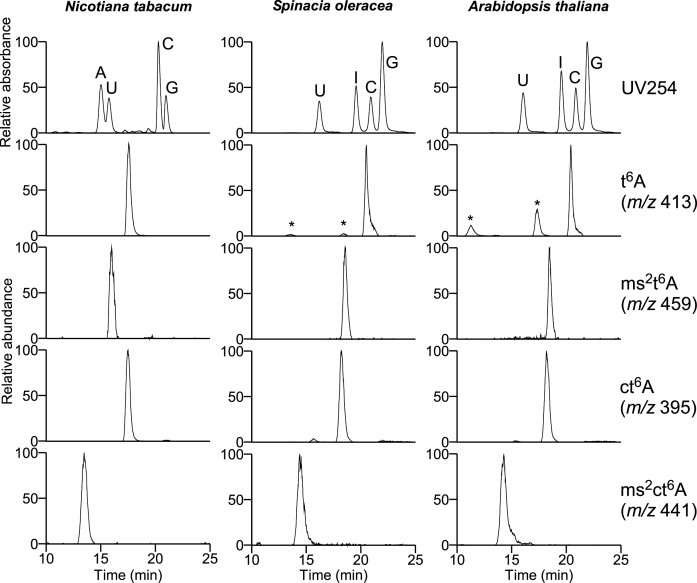
Identification of ms2ct6A in plant tRNAs
According to phylogenetic analyses (9,40), homologs of mtaB and tcdA are encoded in plant genomes, indicating the presence of ms2ct6A in plant tRNAs. Indeed, ct6A and ms2t6A were previously detected in Spinach (9) and Eleusine coracana (20), respectively. Therefore, we prepared total RNA from Nicotiana tabacum, Spinacia oleracea and Arabidopsis thaliana, and enzymatically digested these samples into nucleosides under acidic conditions. LC/MS analyses clearly detected ms2ct6A along with other t6A derivatives in all three species of plant (Figure 5). Next, total nucleosides of spinach RNA were co-injected along with synthetic ms2ct6A by LC/MS using hydrophilic interaction chromatography (Supplementary Data), as well as reverse-phase chromatography (Supplementary Data). Plant ms2ct6A co-eluted with the synthetic molecule as a single peak under both conditions, demonstrating that ms2ct6A is present in plant tRNAs.
Figure 5.
Detection of ms2ct6A in plant tRNAs. HILIC/ESI-MS nucleoside analyses of total RNAs from N. tabacum, S. oleracea and A. thaliana. Top panels show UV traces at 254 nm. Panels second from the bottom show mass chromatograms corresponding to the proton adducts of t6A (m/z 413), ms2t6A (m/z 459), ct6A (m/z 359) and ms2ct6A (m/z 441), respectively. Unassigned peaks are indicated by asterisks. Conversion of adenosine to inosine observed in spinach and A. thaliana is due to the contamination of adenosine deaminase activity in PDase II (Sigma P9041).
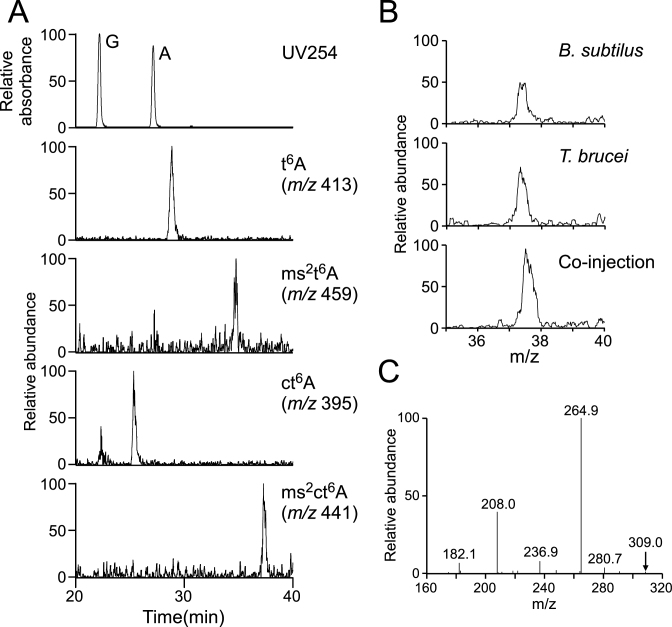
Presence of ms2ct6A in T. brucei tRNAs
We previously speculated that ms2ct6A is present in T. brucei tRNALys (9). Total RNA from T. brucei was digested into nucleosides under neutral conditions and subjected to LC/MS analysis (Figure 6A). Along with ct6A, N440 was clearly detectable. Next, the total nucleosides of T. brucei and B. subtilis were co-injected to LC/MS, revealing that T. brucei N440 co-eluted with B. subtilis ms2ct6A as a single peak (Figure 6B). Finally, we probed BH2+ of T. brucei N440 by CID (Figure 6C). The product ions exhibited a pattern identical to those of synthetic ms2ct6A (Figure 3D). These results demonstrated that ms2ct6A is also present in T. brucei.
Figure 6.
Detection of ms2ct6A in T. brucei tRNAs. (A) RPC/ESI-MS nucleoside analyses of total RNAs from T. brucei. Top panel shows a UV trace at 254 nm. Panels second from the bottom show mass chromatograms corresponding to the proton adducts of t6A (m/z 413), ms2t6A (m/z 459), ct6A (m/z 395) and ms2ct6A (m/z 441), respectively. (B) Mass chromatograms showing the proton adduct of ms2ct6A (m/z 441) in total nucleosides of B. subtilis (top panel), T. brucei (middle panel) and the co-injection fraction (bottom panel) obtained by RPC/ESI-MS. (C) CID spectrum of BH2+ of ms2ct6A from T. brucei. The parent ion for CID is indicated by an arrow.
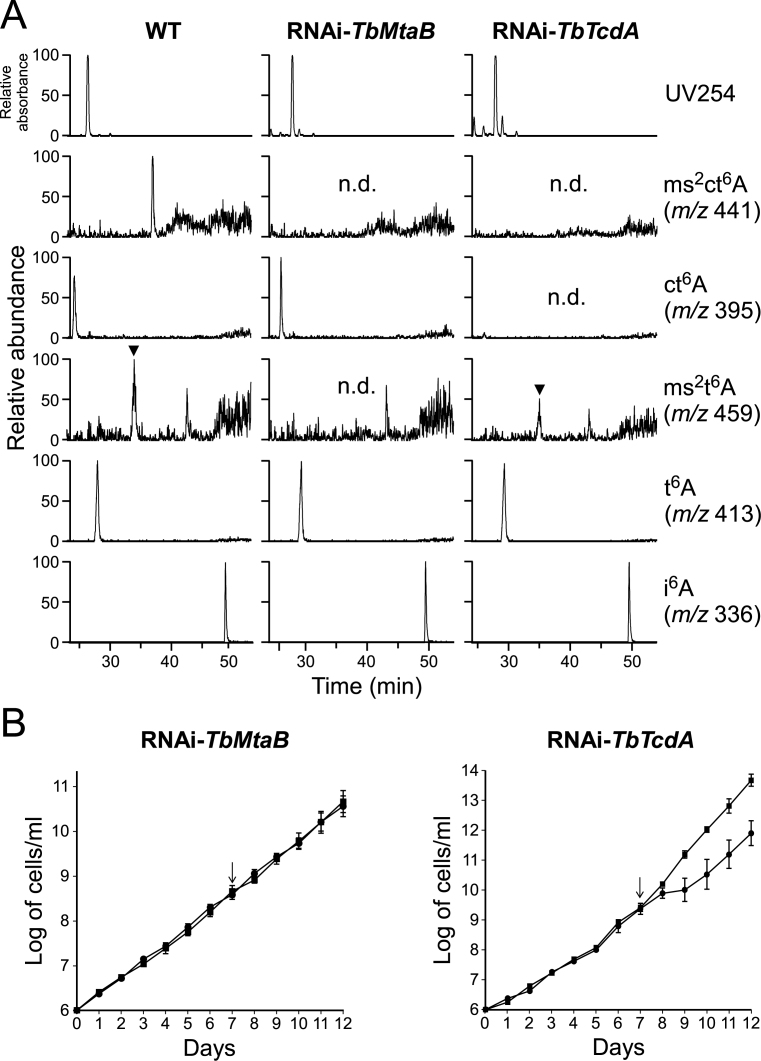
Growth phenotype of T. brucei with hypomodified ms2ct6A
We hypothesized that TbMtaB and TbTcdA are responsible for the synthesis of ms2ct6A in T. brucei. To test this speculation, we generated a transgenic RNAi line for each gene and confirmed knockdown efficiency by RT-PCR. No transcript of each gene was detected in either strain, even 7 days after knockdown induced by tetracycline (Supplementary Data). Upon knockdown of TbMtaB, ms2ct6A and ms2t6A disappeared and remained to be ct6A and t6A, respectively (Figure 7A). In fact, ct6A accumulated slightly more than non-treated T. brucei (WT). Likewise, when TbTcdA was down-regulated, ms2ct6A and ct6A disappeared and remained to be ms2t6A and t6A, respectively (Figure 7A). To determine the physiological importance of this modification, we measured the growth rates of these strains after induction of RNAi (Figure 7B). Down-regulation of expression of either gene alone did not cause a major growth defect (data not shown). However, in the presence of non-inhibitory concentrations of cycloheximide (Chx), growth of cells with down-regulated TbTcdA was slowed (Figure 7B), whereas little growth phenotype was observed when TbMtaB was knocked down. Thus, hypomodification of ms2ct6A and ct6A results in sensitivity to an antibiotic that targets the ribosome, suggesting that cyclization of these modifications contributes to efficient cell growth and protein synthesis.
Figure 7.
Biogenesis of ms2ct6A and growth phenotype of T. brucei with hypomodified tRNAs. (A) RPC/ESI-MS nucleoside analyses of total RNAs from T. brucei wild type (left panels) and transgenic RNAi lines of TbMtaB (middle panels) and TbTcdA (right panels). Mass chromatograms of t6A derivatives were normalized by that of i6A, and abundance of each peak is displayed relative to the highest peak (100%) among them. Top panels show the UV traces at 254 nm. Panels second from the bottom show mass chromatograms detecting the proton adducts of ms2ct6A (m/z 441), ct6A (m/z 395), ms2t6A (m/z 459), t6A (m/z 413) and i6A (m/z 336), respectively. n.d., not detected. (B) Growth curves of T. brucei transgenic RNAi lines of TbMtaB (left) and TbTcdA (right). Cumulative cell counts indicated by logarithmic cell number (Log of cells/ml) were measured in the presence of non-inhibitory concentration of cycloheximide. RNAi was induced by adding (black circle) or not adding (black square) tetracycline (Tet) at 7 days (as indicated by arrow) after inoculation.
DISCUSSION
Because ct6A is a chemically labile derivative of t6A, it had never been detected by conventional nucleoside analysis for more than 40 years since t6A's discovery. ct6A can be detected only if total RNA is extracted from the cell under acidic conditions and digested into nucleosides under neutral conditions, explaining why ms2ct6A has not been detected previously. In this study, we performed nucleoside analysis under neutral or acidic conditions and successfully identified ms2ct6A in total RNA samples from B. subtilis, three plants and T. brucei. Because we also detected t6A and ms2t6A, we assume that ct6A and ms2ct6A are partial modifications in these organisms, indicating that cyclization of t6A and ms2t6A might be regulated under certain physiological conditions.
By referring to the synthetic nucleoside as a reference, the chemical structure of ms2ct6A was determined to be a hydantoin isoform, rather than an oxazolone isoform previously predicted. According to the structural analysis of ct6A nucleoside (15), two carbonyl oxygen atoms of the hydantoin ring are repulsive to the nitrogen atoms (N1 and N7) of the adenine base (Supplementary Data). Consistent with this, the C6-N6 bond length is longer than the normal C-N bond length. Therefore, the hydantoin ring adopts a twisted position against the adenine base with a torsion angle of −52.7°. In light of this observation, it is difficult to speculate how the hydantoin ring of ct6A contributes to the efficient decoding of tRNA on the ribosome. Structural studies of ribosomes in complex with tRNA containing ct6A or ms2ct6A will be necessary to reveal the functional and structural roles of these modifications in protein synthesis.
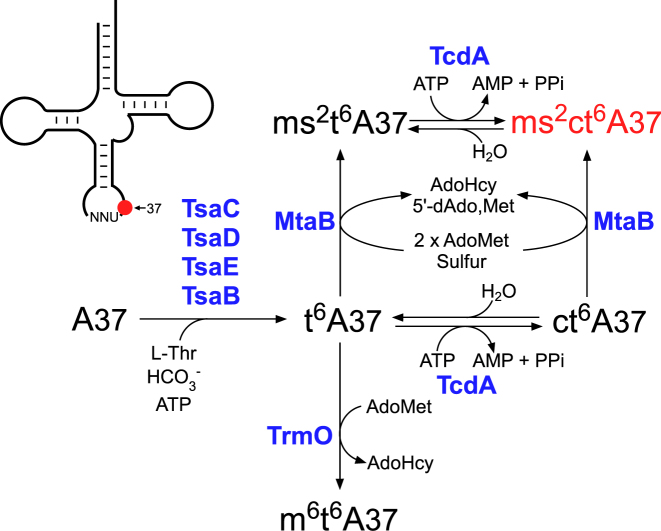
We now know of five species of t6A derivatives (Figure 1A and S1), of which ms2ct6A is the most chemically complex. Initially, t6A is formed at position 37 on tRNAs with NNU anticodons (i.e. those responsible for ANN codons) (Figure 8). This process is catalyzed by multiple enzymes (TsaB, TsaC, TsaD and TsaE in bacteria) using L-threonine, bicarbonate and ATP as substrates. In tRNAThr from certain species of γ-proteobacteria, m6t6A37 is formed via the methylation of t6A37 by TrmO using AdoMet as a substrate. In some species of bacteria, fungi, plants and protists, ct6A37 is formed via the cyclization of t6A37 by ATP-dependent dehydration by TcdA. In organisms that harbor an MtaB homolog, t6A37 and ct6A37 are 2-thiomethylated to form ms2t6A and ms2ct6A, respectively. According to the nucleoside analyses of B. subtilis knockout strains, 2-methylthiolation and cyclization of t6A are independent reactions involved in synthesis of ms2ct6A (Figure 8).
Figure 8.
Biosynthetic pathway of ms2ct6A and other t6A derivatives. In bacteria, A37 of tRNAs responsible for ANN codons is modified to t6A by multiple enzymes (TsaB, TsaC, TsaD and TsaE) using L-threonine, bicarbonate and ATP as substrates. In tRNAThr from γ-proteobacteria, t6A37 is methylated by TrmO using AdoMet to form m6t6A37. In certain populations of bacteria, fungi, plant and protists, t6A37 is cyclized by TcdA using ATP to form ct6A37. In organisms bearing an MtaB homolog, t6A37 and ct6A37 in particular tRNAs are 2-thiomethylated by MtaB to form ms2t6A37 and ms2ct6A37, respectively.
In terms of phylogenetic distribution, ms2ct6A should be present in organisms with MtaB and TcdA orthologs. Among bacteria, δ-proteobacteria and approximately half of the species that compose Firmicutes and Bacteroidetes encode these two enzymes in their genomes. In protists, ms2ct6A is found in Trypanosoma and Tetrahymena. In Archaeplastida, both MtaB and TcdA are present in Plants and Chlorophyta, but not in Rhodophyta or Glaucophyta.
In E. coli, ct6A37 is involved in the decoding activity of tRNALys (9). tcdA engages in a genetic interaction with mnmA, as demonstrated by the observation that a synthetic growth reduction occurs when both genes are deleted simultaneously. Considering that mnmA encodes a 2-thiouridylase to form mnm5s2U at the wobble position of tRNALys, which also contains ct6A37, cyclization of ct6A37 must play a functional role in the decoding activity of tRNALys.
The functional role of the 2-methylthio modification was first studied in an in vitro E. coli translation system using tRNA containing ms2i6A37. E. coli tRNAPhe containing the ms2-modification was more active in poly(U)-dependent poly(Phe) synthesis than tRNA lacking the ms2-modification (41). In Salmonella typhimurium, +1 frameshift activities of tRNAPhe and tRNATyr at the ribosomal P-site were significantly higher when they lacked the ms2-modification (42), indicating that 2-methylthiolation of ms2i6A37 plays a critical role in maintaining the reading frame during elongation. The structure of ms2i6A37 in tRNA has revealed that the 2-methylthio group stabilizes the codon–anticodon interaction through cross-strand stacking with the first base of the P-site codon (43). This stabilization effect might contribute to the prevention of +1 frameshifting.
In the case of ms2t6A modification in B. subtilis, the decoding ability of tRNALys with or without the ms2-modification was examined using a luciferase reporter. The results showed that the ms2-modification is required to decode AAA and AAG codons efficiently (23). Given that a large fraction of ms2t6A37 must be converted to ms2ct6A37 in B. subtilis tRNALys, this finding throws light on the function of the ms2-modification of ms2ct6A37 (but not of ms2t6A37). However, no significant growth or temperature-sensitivity phenotypes have been observed to date in ΔyqeV, ΔyrvM or the double-deletion strains (data not shown). Future studies should investigate whether phenotypes emerge under various stress or culture conditions. In T. brucei, we observed a growth delay after knockdown of TbTcdA in the presence of non-inhibitory concentration of cycloheximide, indicating defective decoding activity of tRNALys with hypomodified ms2ct6A. To obtain a deeper understanding of the physiological role of this modification, future studies should be directed toward analyzing the phenotypic features of T. brucei knockdown lines.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to the members of the Suzuki laboratory, in particular Takaaki Taniguchi for technical support and many insightful discussions.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
National Science Centre in Poland [UMO-2014/13/N/ST5/01591 to M.M.]; National Institute of General Medical Sciences of the National Institues of Health [R01GM084065 to J.D.A.]; Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) [to T.S.]; Japan Society for the Promotion of Science (JSPS) [to T.S.]. Funding for open access charge: Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Science, Sports and Culture of Japan.
Conflict of interest statement. None declared.
REFERENCES
- 1. Frye M., Jaffrey S.R., Pan T., Rechavi G., Suzuki T.. RNA modifications: what have we learned and where are we headed. Nat. Rev. Genet. 2016; 17:365–372. [DOI] [PubMed] [Google Scholar]
- 2. Machnicka M.A., Milanowska K., Osman Oglou O., Purta E., Kurkowska M., Olchowik A., Januszewski W., Kalinowski S., Dunin-Horkawicz S., Rother K.M. et al. . MODOMICS: a database of RNA modification pathways–2013 update. Nucleic Acids Res. 2013; 41:D262–D267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grosjean H. Grosjean H. DNA and RNA Modification Enzymes: Structure, Mechanism, Function and Evolution. Molecular Biology Intelligence Unit. 2009; Austin: Landes Bioscience; 1–18. [Google Scholar]
- 4. Yokoyama S., Nishimura S.. Soll DR, RajBhandary U. Modified nucleosides and codon recognition. tRNA: Structure, Biosynthesis, and Function. 1995; Washington, D.C.: American Society for Microbiology; 207–223. [Google Scholar]
- 5. Bjork G. Soll DR, RahBhandary U. Biosynthesis and function of modified nucleosides. tRNA: Structure, Biosynthesis, and Function. 1995; Washington, D.C.: American Society for Microbiology; 165–205. [Google Scholar]
- 6. Suzuki T. Grosjean H. Biosynthesis and function of tRNA wobble modifications. Fine-Tuning of RNA Functions by Modification and Editing. 2005; 12, Berlin Heidelberg: Springer-Verlag Berlin and Heidelberg GmbH & Co. KG; 23–69. [Google Scholar]
- 7. Bjork G.R., Hagervall T.G.. Transfer RNA modification: presence, synthesisand function. EcoSal Plus. 2014; doi:10.1128/ecosalplus.ESP-0007-2013. [DOI] [PubMed] [Google Scholar]
- 8. Thiaville P.C., Iwata-Reuyl D., de Crecy-Lagard V.. Diversity of the biosynthesis pathway for threonylcarbamoyladenosine (t(6)A), a universal modification of tRNA. RNA Biol. 2014; 11:1529–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miyauchi K., Kimura S., Suzuki T.. A cyclic form of N6-threonylcarbamoyladenosine as a widely distributed tRNA hypermodification. Nat. Chem. Biol. 2013; 9:105–111. [DOI] [PubMed] [Google Scholar]
- 10. Srinivasan M., Mehta P., Yu Y., Prugar E., Koonin E.V., Karzai A.W., Sternglanz R.. The highly conserved KEOPS/EKC complex is essential for a universal tRNA modification, t6A. EMBO J. 2011; 30:873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El Yacoubi B., Hatin I., Deutsch C., Kahveci T., Rousset J.P., Iwata-Reuyl D., Murzin A.G., de Crecy-Lagard V.. A role for the universal Kae1/Qri7/YgjD (COG0533) family in tRNA modification. EMBO J. 2011; 30:882–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perrochia L., Guetta D., Hecker A., Forterre P., Basta T.. Functional assignment of KEOPS/EKC complex subunits in the biosynthesis of the universal t6A tRNA modification. Nucleic Acids Res. 2013; 41:9484–9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schweizer M.P., Chheda G.B., Baczynskyj L., Hall R.H.. Aminoacyl nucleosides. VII. N-(Purin-6-ylcarbamoyl)threonine. A new component of transfer ribonucleic acid. Biochemistry. 1969; 8:3283–3289. [DOI] [PubMed] [Google Scholar]
- 14. Matuszewski M., Sochacka E.. Stability studies on the newly discovered cyclic form of tRNA N(6)-threonylcarbamoyladenosine (ct(6)A). Bioorg. Med. Chem. Lett. 2014; 24:2703–2706. [DOI] [PubMed] [Google Scholar]
- 15. Matuszewski M., Wojciechowski J., Miyauchi K., Gdaniec Z., Wolf W.M., Suzuki T., Sochacka E.. A hydantoin isoform of cyclic N6-threonylcarbamoyladenosine (ct6A) is present in tRNAs. Nucleic Acids Res. 2016; doi:10.1093/nar/gkw1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kimura S., Miyauchi K., Ikeuchi Y., Thiaville P.C., Crecy-Lagard V., Suzuki T.. Discovery of the beta-barrel-type RNA methyltransferase responsible for N6-methylation of N6-threonylcarbamoyladenosine in tRNAs. Nucleic Acids Res. 2014; 42:9350–9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamada Y., Ishikura H.. The presence of N-[(9-b-D-ribofuranosyl-2-methylthiopurin-6-yl)carbamoyl]threonine in lysine tRNA1 from Bacillus subtilis. J. Biochem. 1981; 89:1589–1591. [DOI] [PubMed] [Google Scholar]
- 18. Yamaizumi Z., Nishimura S., Limburg K., Raba M., Gross H.J., Crain P.F., McCloskey J.A.. Structure elucidation by high-resolution mass-spectrometry of a highly modified nucleoside from mammalian transfer-Rna - N-[(9-b-D-Ribofuranosyl-2-Methylthiopurin-6-yl)Carbamoyl]threonine. J. Am. Chem. Soc. 1979; 101:2224–2225. [Google Scholar]
- 19. Krog J.S., Espanol Y., Giessing A.M., Dziergowska A., Malkiewicz A., de Pouplana L.R., Kirpekar F.. 3-(3-amino-3-carboxypropyl)-5,6-dihydrouridine is one of two novel post-transcriptional modifications in tRNALys(UUU) from Trypanosoma brucei. FEBS J. 2011; 278:4782–4796. [DOI] [PubMed] [Google Scholar]
- 20. Raviprakash K.S., Cherayil J.D.. Multiple species of thionucleosides in ragi (Eleusine coracana) tRNA. Curr. Sci. 1984; 53:847–851. [Google Scholar]
- 21. Anton B.P., Russell S.P., Vertrees J., Kasif S., Raleigh E.A., Limbach P.A., Roberts R.J.. Functional characterization of the YmcB and YqeV tRNA methylthiotransferases of Bacillus subtilis. Nucleic Acids Res. 2010; 38:6195–6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arragain S., Handelman S.K., Forouhar F., Wei F.Y., Tomizawa K., Hunt J.F., Douki T., Fontecave M., Mulliez E., Atta M.. Identification of eukaryotic and prokaryotic methylthiotransferase for biosynthesis of 2-methylthio-N6-threonylcarbamoyladenosine in tRNA. J. Biol. Chem. 2010; 285:28425–28433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wei F.Y., Suzuki T., Watanabe S., Kimura S., Kaitsuka T., Fujimura A., Matsui H., Atta M., Michiue H., Fontecave M. et al. . Deficit of tRNA(Lys) modification by Cdkal1 causes the development of type 2 diabetes in mice. J. Clin. Invest. 2011; 121:3598–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei F.Y., Tomizawa K.. Functional loss of Cdkal1, a novel tRNA modification enzyme, causes the development of type 2 diabetes. Endocr. J. 2011; 58:819–825. [DOI] [PubMed] [Google Scholar]
- 25. Kasai H., Murao K., Nishimura S., Liehr J.G., Crain P.F., Mccloskey J.A.. Structure determination of a modified nucleoside isolated from Escherichia-coli transfer Ribonucleic-acid - N-[N-[(9-Beta-D-Ribofuranosylpurin-6-Yl)Carbamoyl]Threonyl]2-Amido-2-Hydroxymethylpropane-1,3-Diol. Eur. J. Biochem. 1976; 69:435–444. [Google Scholar]
- 26. Vagner V., Dervyn E., Ehrlich S.D.. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology. 1998; 144:3097–3104. [DOI] [PubMed] [Google Scholar]
- 27. Kobayashi K. Bacillus subtilis pellicle formation proceeds through genetically defined morphological changes. J. Bacteriol. 2007; 189:4920–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kobayashi K., Shoji K., Shimizu T., Nakano K., Sato T., Kobayashi Y.. Analysis of a suppressor mutation ssb (kinC) of sur0B20 (spo0A) mutation in Bacillus subtilis reveals that kinC encodes a histidine protein kinase. J. Bacteriol. 1995; 177:176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumagai-Sano F., Hayashi T., Sano T., Hasezawa S.. Cell cycle synchronization of tobacco BY-2 cells. Nat. Protoc. 2006; 1:2621–2627. [DOI] [PubMed] [Google Scholar]
- 30. Yoshida M., Kataoka N., Miyauchi K., Ohe K., Iida K., Yoshida S., Nojima T., Okuno Y., Onogi H., Usui T. et al. . Rectifier of aberrant mRNA splicing recovers tRNA modification in familial dysautonomia. Proc. Natl. Acad. Sci. U.S.A. 2015; 112:2764–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miyauchi K., Ohara T., Suzuki T.. Automated parallel isolation of multiple species of non-coding RNAs by the reciprocal circulating chromatography method. Nucleic Acids Res. 2007; 35:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crain P.F. Preparation and enzymatic hydrolysis of DNA and RNA for mass spectrometry. Methods Enzymol. 1990; 193:782–790. [DOI] [PubMed] [Google Scholar]
- 33. Sakaguchi Y., Miyauchi K., Kang B.I., Suzuki T.. Nucleoside analysis by hydrophilic interaction liquid chromatography coupled with mass spectrometry. Methods Enzymol. 2015; 560:19–28. [DOI] [PubMed] [Google Scholar]
- 34. Suzuki T., Ikeuchi Y., Noma A., Suzuki T., Sakaguchi Y.. Mass spectrometric identification and characterization of RNA-modifying enzymes. Methods Enzymol. 2007; 425:211–229. [DOI] [PubMed] [Google Scholar]
- 35. Chheda G., Hong C.I.. Synthesis of naturally occurring 6-ureidopurines and their nucleosides. J. Med. Chem. 1971; 14:748–753. [DOI] [PubMed] [Google Scholar]
- 36. Davis D.R., Bajji A.C.. Introduction of hypermodified nucleotides in RNA. Methods Mol. Biol. 2005; 288:187–204. [DOI] [PubMed] [Google Scholar]
- 37. Hong C.I., Chheda G.B.. Towsend LB, Tipson RS. N-[(9-β-D-ribofuranosyl-9H-purin-6-yl)carbamoyl]-L-threonine (t6A). Synthesis via ethyl 9- β-D-ribofuranosylpurine-6-carbamate. Nucleic Acid Chemistry. 1972; NY: John Wiley & Sons; 661–664. [Google Scholar]
- 38. Kierzek E., Kierzek R.. The synthesis of oligoribonucleotides containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines via post-synthetic modification of precursor oligomers. Nucleic Acids Res. 2003; 31:4461–4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chomczynski P., Sacchi N.. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987; 162:156–159. [DOI] [PubMed] [Google Scholar]
- 40. Kaminska K.H., Baraniak U., Boniecki M., Nowaczyk K., Czerwoniec A., Bujnicki J.M.. Structural bioinformatics analysis of enzymes involved in the biosynthesis pathway of the hypermodified nucleoside ms(2)io(6)A37 in tRNA. Proteins. 2008; 70:1–18. [DOI] [PubMed] [Google Scholar]
- 41. Buck M., Griffiths E.. Iron mediated methylthiolation of tRNA as a regulator of operon expression in Escherichia coli. Nucleic Acids Res. 1982; 10:2609–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Urbonavicius J., Qian Q., Durand J.M., Hagervall T.G., Bjork G.R.. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J. 2001; 20:4863–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jenner L.B., Demeshkina N., Yusupova G., Yusupov M.. Structural aspects of messenger RNA reading frame maintenance by the ribosome. Nat. Struct. Mol. Biol. 2010; 17:555–560. [DOI] [PubMed] [Google Scholar]
- 44. Sprinzl M., Vassilenko K.S.. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 2005; 33:D139–D140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McLuckey S.A., Van Berkel G.J., Glish G.L.. Tandem mass spectrometry of small, multiply charged oligonucleotides. J. Am. Soc. Mass Spectrom. 1992; 3:60–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.