Abstract
Pasteurella multocida toxin (PMT) is a potent mitogen for fibroblasts and osteoblastic cells. PMT activates phospholipase C-β through Gqα, and the activation of this pathway is responsible for its mitogenic activity. Here, we investigated the effects of PMT on human monocyte-derived dendritic cells (MDDC) in vitro and show a novel activity for PMT. In this regard, PMT activates MDDC to mature in a dose-dependent manner through the activation of phospholipase C and subsequent mobilization of calcium. This activation was accompanied by enhanced stimulation of naïve alloreactive T cells and dominant inhibition of interleukin-12 production in the presence of saturating concentrations of lipopolysaccharide. Surprisingly, although PMT mimics the activating effects of cholera toxin on human MDDC and mouse bone marrow-derived dendritic cells, we found that PMT is not a mucosal adjuvant and that it suppresses the adjuvant effects of cholera toxin in mice. Together, these results indicate discordant effects for PMT in vitro compared to those in vivo.
Dendritic cells (DC) are the most potent antigen-presenting cells (APC) known and are the only APC capable of initiating primary immune responses. Immature DC residing in peripheral tissues are highly efficient at capturing antigen (reviewed in reference 4). After receiving an activation signal, DC migrate to organized lymphoid tissues, lose the ability to take up antigen, and mature into potent APC (4). The potency of mature DC as APC stems from their increased expression of class I and class II major histocompatibility complex (MHC) and costimulatory and adhesion molecules, along with their ability to secrete cytokines and chemokines (4). DC derived from monocytes (MDDC) by culturing with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) are phenotypically equivalent to the immature DC that reside in peripheral tissues (18). Likewise, DC derived from bone marrow cells (BMDC) by culturing with GM-CSF are phenotypically equivalent to the immature DC that reside in peripheral tissues (11). When incubated with activating agonists such as lipopolysaccharide (LPS), MDDC and BMDC undergo maturation and display the phenotypic characteristics of mature DC found in secondary lymphoid tissues (8, 11, 25).
Previously, we and others showed that the bacterial toxins cholera toxin (CT), Escherichia coli heat-labile enterotoxin (LT), pertussis toxin (PT), and the adenylate cyclase toxin of Bordetella pertussis (AT) activate human MDDC to mature (2, 3). These toxins activate MDDC through the elevation of intracellular cyclic AMP (cAMP) either directly (AT) or through the ADP-ribosylation of G proteins that leads to constitutive activation of adenylate cyclase (CT, LT, and PT) (2, 3). In addition, these cAMP-elevating toxins dominantly inhibit the production of IL-12 by LPS-stimulated MDDC (2, 3, 8). One of the principal ways that adjuvants boost immune responses to coadministered antigens is the activation of DC at the immunization site. In this regard, CT, LT, and PT are powerful mucosal immunogens and adjuvants (12, 14, 16). When delivered mucosally, these toxins induce strong primary and secondary antibody responses and long-lasting immunologic memory to themselves and to coadministered antigens (14, 16).
Pasteurella multocida toxin (PMT) is a major virulence factor in progressive atopic rhinitis in wild and domestic animals (1, 7). PMT is also a potent mitogen that induces DNA synthesis and cell proliferation in fibroblasts and osteoblastic cells at picomolar concentrations (15). This toxin is composed of a 146-kDa single polypeptide of 1,285 amino acids (5, 13, 26, 28). Like CT, LT, and PT, PMT appears to bind to ganglioside receptors on the surfaces of cells and gains access to the cytoplasm through endocytosis (13). Unlike CT, LT, and PT, which activate adenylate cyclase, PMT activates phospholipase C-β1 (PLC) through the G-protein alpha subunit Gqα (27). The activation of PLC by PMT leads to the formation of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol through the cleavage of phosphatidylinositol 4,5-bisphosphate (20). The release of IP3 and diacylglycerol by PLC induces mobilization of intracellular calcium and the activation of protein kinase C (PKC) (20). Several of the known cellular effects of PMT are mediated through PLC, including anchorage-independent cell growth and mitogenic activity (10).
Calcium mobilization, induced by the calcium ionophore A23187, was shown to activate MDDC to mature and to dominantly inhibit IL-12 production (6). As PMT mobilizes calcium, we determined whether this toxin shares the ability of the cAMP-elevating bacterial toxins and A23187 to activate MDDC to mature. Here, we show for the first time that PMT is a potent agonist for the maturation of human MDDC and mouse BMDC and that the activation of MDDC by PMT is a result of PLC-induced calcium mobilization. As other toxins that induce DC maturation are potent mucosal and systemic adjuvants, we hypothesized that PMT would also display mucosal and/or systemic adjuvant effects. However, in contrast to this hypothesis, the results of one study indicate that PMT suppresses immune responses to coadministered antigens (23). It is equally puzzling that native CT, LT, and PT are known to be potent immunogens, whereas PMT appears to be more immunogenic when denatured (24). To our surprise, we found that PMT is not a mucosal or systemic adjuvant. The results of this study indicate that the ability of an agonist to induce DC maturation is not necessarily a predictor of adjuvanticity.
MATERIALS AND METHODS
Dendritic cell culture medium.
Dendritic cell culture medium consisted of RPMI 1640 (Gibco Invitrogen, Carlsbad, Calif.) supplemented with 2 mM l-glutamine (Sigma, St. Louis, Mo.), 1% nonessential amino acids (Gibco Invitrogen), 1% sodium pyruvate (Gibco Invitrogen), 20 mM HEPES buffer (Gibco Invitrogen), 50 μg of gentamicin (Gibco Invitrogen)/ml, 10% fetal calf serum (Gibco Invitrogen), and 50 μM 2-mercaptoethanol (Sigma).
T-cell proliferation medium.
T-cell proliferation medium consisted of α-minimal essential medium without ribonucleosides and deoxyribonucleosides (Gibco Invitrogen) supplemented with 10% human AB serum (Sigma), 1% sodium pyruvate, 4 mM l-glutamine, 20 mM HEPES buffer, 100 μg of streptomycin (Gibco Invitrogen)/ml, 100 U of penicillin G (Gibco Invitrogen)/ml, and 50 μM 2-mercaptoethanol (Sigma).
Generation of human MDDC.
All human specimens were obtained with informed consent as approved by the University of Maryland Baltimore Institutional Review Board. MDDC were generated as previously described with minor modifications (18). Briefly, human peripheral blood mononuclear cells (PBMC) isolated by Ficoll-Hypaque density gradient centrifugation were enriched for CD14+ monocytes by negative selection using a cocktail of monoclonal antibodies from StemCell Technologies (Vancouver, Canada) according to the manufacturer's instructions. The isolated monocytes were adhered to plastic by plating at 106 cells per/ml in RPMI medium for 2 h. Adherent monocytes were washed with RPMI medium and then cultured at 106 cells per/ml in DC culture medium supplemented with 50 ng of recombinant GM-CSF/ml and 1,000 U of recombinant IL-4 (R&D Systems, Minneapolis, Minn.)/ml.
Generation of mouse BMDC.
BMDC were generated from bone marrow cells harvested from 8- to 12-week-old female BALB/c mice as previously described (11), with minor modifications. Briefly, bone marrow was flushed from femurs with DC medium and then passed over a cotton column to remove debris. Red blood cells were lysed by using RBC lysis buffer (Biofluids, Camarillo, Calif.). Cells were plated in 12-well plates at 2 × 106 cells/well in a total of 2 ml of DC medium supplemented with 1, 000 U of recombinant mouse GM-CSF (R&D Systems)/ml. On day 3 of culture, nonadherent cells were gently removed, and fresh medium containing GM-CSF was added. On day 6, culture medium was changed again, and agonists were added directly to the fresh culture medium.
Cell treatments.
PMT, CT (List Biological Laboratories, Campbell, Calif.), thapsigargin, A23187, LPS, BAPT-AM [1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethyl) ester], or D609 (Sigma) was added directly to MDDC or BMDC cultures in individual wells. Cells were harvested, washed, stained for phenotypic analysis by flow cytometry (see below), or used in functional assays (see below).
Flow cytometry.
Human MDDC were incubated for 30 min at 4°C with monoclonal antibodies specific for CD80, CD83, CD86, and HLA-DR (BD Pharmingen, San Diego, Calif.), washed, and then fixed with 2% paraformaldehyde for analysis with a FACScalibur flow cytometer (BD, San Jose, Calif.). Murine BMDC were incubated for 30 min at 4°C with monoclonal antibodies specific for CD25, CD40, CD86, and 14-4-4S (BD Pharmingen), washed, and then analyzed using a FACScan flow cytometer (BD). Data analysis was carried out by using Flow software (Tree Star Inc., San Carlos, Calif.).
Determination of activation.
The fraction of DC that responded by upregulation of activation markers on the cell surface (percent activation) was calculated with Flowjo software by overlaying the histograms of treated and untreated DC and Overton subtraction of the curves.
Inhibition induced by BAPT-AM or D609.
Inhibition induced by BAPT-AM or D609 was determined by the following equation: percent inhibition = [(X − Y)/X] × 100, where X is the fraction of cells that upregulated a marker in the absence of the inhibitor and Y is the fraction of cells that upregulated a marker in the presence of the inhibitor.
Endotoxin quantification.
Endotoxin concentrations were monitored in the toxin preparations by using the Limulus assay (Bio-Whittaker, Walkersville, Md.). The maximum endotoxin concentration in the final dilutions of toxins used in the studies was 40 pg/ml. In addition, the possibility that endotoxin contamination contributes to the observed activation of MDDC was ruled out by boiling preparations of agonists for 10 min before their addition to MDDC. Boiling of PMT or CT completely abolished their ability to activate MDDC (data not shown).
IP3 quantitation.
CD14+ monocytes were plated at 106 cells/well in 12-well plates in a total volume of 2 ml of DC culture medium supplemented with GM-CSF and IL-4. On day 4 of culture, individual wells of cells were left untreated or were treated with 1 μg of PMT/ml. At 1, 3, 6, and 12 h after the addition of PMT, the cells were harvested, washed, and resuspended in 1 ml of phosphate-buffered saline (PBS). The cells were then incubated for 20 min with 0.2 volumes of ice-cold 20% perchloric acid. The cell lysates were pelleted by centrifugation at 2,000 × g for 15 min at 4°C. Supernatants were removed, placed in glass tubes, and neutralized with ice-cold 10 M KOH containing 60 mM HEPES buffer and phenol red. The supernatants were centrifuged at 200 × g for 15 min at 4°C to sediment KClO4. Supernatants were removed and assayed for IP3 concentration with a radiometric assay according to the manufacturer's instructions (catalog number TRK 1000; Amersham, Buckinghamshire, England).
Human allogeneic T-cell response.
MDDC for the allogeneic T-cell response were prepared as described above and washed three times with T-cell proliferation medium before addition to naïve allogeneic CD4+ T cells. Naïve CD4+ T cells were enriched from PBMC by negative selection using a cocktail of monoclonal antibodies (StemCell Technologies) according to the manufacturer's instructions. Naïve CD4+ T cells (greater than or equal to 93% pure as determined by the expression of CD62L and the absence of CD45R0 using flow cytometry) were cultured for 5 days in replicates of five at 105 cells/well with 1,000 allogeneic MDDC on 96-well U-bottom plates. For the last 18 h of culture, the cells were pulsed with 1 μCi of [3H]thymidine (Perkin-Elmer Life Sciences, Boston, Mass.)/well. Thymidine incorporation was measured by using a Wallac 1450 Microbetta Trilux liquid scintillation spectrometer (Wallac, Turku, Finland).
Murine allogeneic T-cell response.
BALB/c BMDC for the allogeneic T-cell response were prepared as described above and washed three times with T-cell proliferation medium before addition to C57BL/6 T cells. Total C57BL/6 T cells were enriched from spleen cells by negative selection using a cocktail of monoclonal antibodies (StemCell Technologies) according to the manufacturer's instructions. C57BL/6 T cells were cultured for 5 days in replicates of three at 105 cells/well with 1,000 allogeneic BALB/c BMDC in 96-well U-bottom plates. For the last 18 h of culture, the cells were pulsed with 1 μCi of [3H]thymidine (Perkin Elmer Life Sciences)/well. Thymidine incorporation was measured by using a Packard Matrix 96 direct beta counter.
Cytokine ELISA.
Cytokine concentrations were determined by enzyme-linked immunosorbent assay (ELISA) (R & D Systems) according to the manufacturer's instructions.
Oral immunization experiments.
BALB/c mice (6 to 8 weeks old) were obtained from Harlan (Indianapolis, Ind.). Groups of five (first experiment) or six (second experiment) mice were immunized as follows. Mice received 100 μl of saturated bicarbonate solution 10 min before oral immunizations. Mice in the chicken egg albumin (OVA) group were immunized three times (first experiment) or twice (second experiment) orally by using a ball-tipped feeding needle with 100 μg of OVA in 200 μl of PBS 2 weeks apart. Mice in the CT, PMT, and CT plus PMT groups were immunized three times (first experiment) or twice (second experiment) orally with 100 μg of OVA with either 10 μg of CT in 200 μl of PBS, 10 μg of PMT in 200 μl of PBS, or 10 μg of CT plus 10 μg of PMT in 200 μl of PBS 2 weeks apart. Four weeks after the final immunization, mice were bled, and anti-OVA and anti-CT endpoint titers in the isolated sera were determined by ELISA. Mice from all groups in the second experiment were then immunized intraperitoneally (i.p.) with 100 μg of OVA and 100 μg of keyhole limpet hemocyanin (KLH) in complete Freund's adjuvant (CFA). Two weeks later, mice were bled, and anti-OVA and anti-KLH endpoint titers in the isolated sera were determined by ELISA.
Systemic immunization experiment.
Groups of five BALB/c mice were immunized as follows. Mice in the control group (no adjuvant) were immunized three times intradermally (i.d.) with 50 μg of OVA in 200 μl of PBS 2 weeks apart. Four weeks later, mice were bled, and anti-OVA endpoint titers in the isolated sera were determined by ELISA. Mice in the CT, PMT, and CT plus PMT groups were immunized three times i.d. with 50 μg of OVA with either 1 μg of CT in 200 μl of PBS, 50 ng of PMT in 200 μl of PBS, or 1 μg of CT plus 50 ng of PMT in 200 μl of PBS 2 weeks apart. Four weeks later, mice were bled, and anti-OVA endpoint titers in the isolated sera were determined by ELISA.
ELISA.
Solid-phase ELISA was used to determine OVA-specific, KLH-specific, and CT-specific antibody titers in the sera. Briefly, 96-well microtiter plates (Nunc, Rochester, N.Y.) were coated with 10 μg of OVA, KLH, or GM1 ganglioside/ml in PBS at 4°C overnight. The GM1-coated plates were washed three times with Tris-buffered saline (TBS), and then 1 μg of CT/ml was added to the plates and incubated for 1 h at room temperature. All plates were then washed three times with TBS and blocked with BLOTTO (5% [wt/vol] nonfat dried milk in TBS) at room temperature for 30 min. Serially diluted sera were added to the wells of respective plates and incubated at room temperature for 1 h and then washed three times with TBS. Peroxidase-conjugated goat anti-mouse immunoglobulin G (Kirkegaard & Perry, Gaithersburg, Md.) diluted 1/1,000 in BLOTTO was added and incubated at room temperature for 1 h. The plates were washed three times with TBS before the addition of TMB peroxidase substrate (Kirkegaard & Perry) and incubation for 1 to 3 min. The reaction was stopped by adding 1 N H2SO4. Absorbance was read at 450 nm by using a Victor 1420 Multilabel counter (EG&G Wallac).
ELISA endpoint titers were calculated by graphing absorbance at 450 nm verses serum dilution and taking the value where the line intersected the background value (mean of the negative control wells plus three standard deviations).
Statistics.
The Student's t test was performed on the means of the endpoint titers. P values of less than 0.05 were deemed significant.
RESULTS
PMT activates human MDDC to mature in a dose-dependent manner.
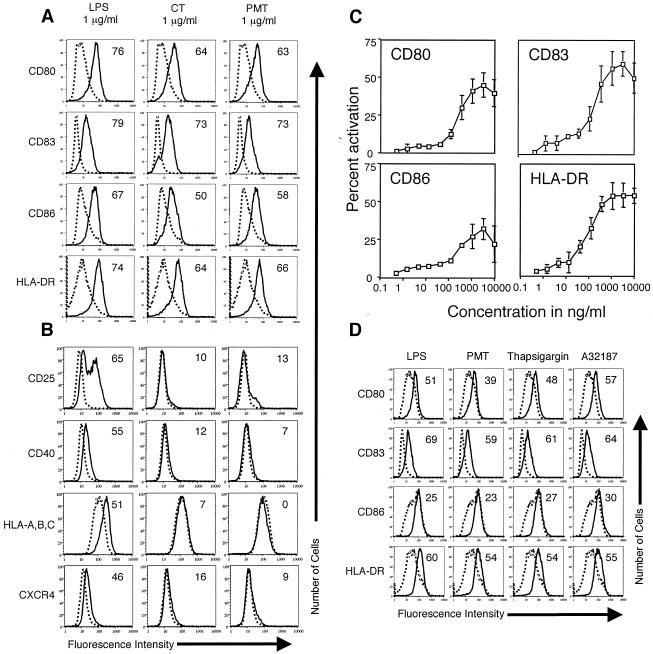
In preliminary experiments, we treated MDDC at day 4 with 1 μg of LPS or CT/ml as a control or with 1 μg of PMT/ml. Twenty hours later, the cells were harvested, and activation was assayed by the expression of CD80 (B7-1), CD83, CD86 (B7-2), and HLA-DR (class II MHC). As shown in Fig. 1A, PMT activated human MDDC to mature. In this regard, the expression of CD80, CD83, CD86, and HLA-DR was higher on MDDC incubated with PMT than on untreated cells, and the expression levels of these markers induced by PMT were similar to that induced by CT. In other experiments, we found that boiling the PMT preparation completely abolished its ability to activate MDDC (data not shown) and that concentrations of PMT as high as 10 μg/ml do not kill the cells within 48 h (determined by trypan blue staining and flow cytometry) (data not shown).
FIG. 1.
PMT activates human MDDC to mature. Cell surface expression of the indicated markers on untreated MDDC (dotted histograms) or MDDC treated with the indicated agonist (solid histograms). A. Day 4 MDDC were incubated with 1 μg of LPS, CT, or PMT/ml for 20 h. The cells were harvested and stained for four-color flow cytometry with phycoerythrin (PE)-anti-CD80, fluorescein isothiocyanate (FITC)-anti-CD83, Cy-Chrome-anti-CD86, or the fluorochrome APC-anti-HLA-DR. Data are representative of one experiment of six performed on cells from different donors with similar results. B. Day 4 MDDC were incubated with 1 μg of LPS, CT, or PMT/ml for 20 h. The cells were harvested and stained for three-color flow cytometry with PE-anti-CD25, PE-anti-CD40, FITC-anti-HLA-A, -B, or -C, or the fluorochrome APC-anti-CXCR4. Data are representative of one experiment of three performed on cells from different donors with similar results. C. Day 4 MDDC were incubated with half-log dilutions (10 μg to 500 pg/ml) of PMT for 20 h. The cells were harvested and stained for four-color flow cytometry with PE-anti-CD80, FITC-anti-CD83, Cy-Chrome-anti-CD86, or the fluorochrome APC-anti-HLA-DR. The percentage of cells that increased the expression of a marker (percent activation) was calculated as described in Materials and Methods. The data shown are the averages and standard deviations of three experiments performed on cells from different donors. D. Day 4 MDDC were incubated for 20 h with 1 μg of LPS or PMT/ml, 10 μM thapsigargin, or 150 ng of A23187/ml. The cells were harvested and stained for four-color flow cytometry with PE-anti-CD80, FITC-anti-CD83, Cy-Chrome-anti-CD86, or the fluorochrome APC-anti-HLA-DR. Data are representative of one experiment of five performed on cells from different donors with similar results.
Previously, we found that both LPS and CT profoundly increased the expression of CD80, CD83, CD86, and HLA-DR on MDDC but that only LPS profoundly increased the expression of CD25 and CD40 (our unpublished results). For this reason, we determined whether there was discordant expression of CD25, CD40, or other DC activation markers on MDDC induced by PMT, LPS, or CT. At day 4, MDDC were incubated with 1 μg of LPS, CT, or PMT/ml for 20 h and then harvested and assayed for the expression of CD25, CD40, HLA-A, -B, and -C (class I MHC), and CXCR4 (constitutive chemokine receptor) by flow cytometry. Figure 1B shows that MDDC activated by LPS expressed considerably more CD25, CD40, HLA-A, -B, and -C, and CXCR4 than MDDC activated by PMT or CT. These results indicate that although PMT and CT activate MDDC to mature, the surface phenotype of the mature cells is distinct from those activated by LPS.
In previous studies, we determined the potency of CT, LT, PT, and AT for the activation of MDDC by using titration experiments (3; our unpublished results). To determine the potency of PMT for the activation of MDDC, we incubated day 4 MDDC with half-log dilutions of PMT (10 μg to 500 pg/ml). Twenty hours later, the cells were harvested, and activation was assayed by increased expression of CD80, CD83, CD86, and HLA-DR. The percent activation was determined as described in Materials and Methods. Figure 1C shows that increased surface expression of CD80, CD83, CD86, and HLA-DR was detectable at 1.5 ng of PMT/ml and was maximal between 1 and 3 μg/ml. The potency of PMT, as determined by the titration curve, is similar to that of PT but weaker than that of CT, LT, or AT (3; our unpublished results). These results indicate that PMT is as potent an activator of MDDC as PT, a toxin known to display potent mucosal adjuvant effects.
PMT activates human MDDC through PLC and calcium mobilization.
PMT has been shown to mobilize intracellular calcium through the activation of PLC (27). To determine if the elevation of intracellular calcium levels mimics the activation of MDDC induced by PMT, we incubated day 4 MDDC with 1 μg of LPS/ml as a control and 1 μg of PMT/ml, 10 μM thapsigargin (IP3-independent calcium releaser) (22), or 150 ng of the calcium ionophore A23187/ml (6). Twenty hours later, the cells were harvested, and activation was assayed by surface marker upregulation. As shown in Fig. 1D, both thapsigargin and A23187 activate MDDC to mature. The results of these experiments show that the elevation of cytoplasmic calcium levels mimics the effect of PMT on MDDC but does not establish causality.
For this reason, we determined whether the intracellular calcium chelator BAPT-AM (21) inhibits the activation of MDDC induced by PMT. Day 4 MDDC were incubated for 20 h with or without 1 μg of PMT/ml and with or without a prior 1-h incubation with 10 μM BAPT-AM. The percent inhibition was calculated as described in Materials and Methods. Table 1 shows that BAPT-AM strongly inhibits the phenotypic maturation of MDDC induced by PMT. In this regard, the fraction of cells that increased the expression of CD80, CD83, CD86, and HLA-DR was substantially reduced when the cells were incubated with PMT in the presence of BAPT-AM compared to cells incubated with PMT alone.
TABLE 1.
PMT activates MDDC through PLC and calcium mobilizationa
| Inhibitor | Target | Concn (μM) | Marker | Avg % inhibition (SEM) |
|---|---|---|---|---|
| BAPT-AM | Calcium | 10 | CD80 | 96.8 (2.0) |
| CD83 | 86.9 (1.0) | |||
| CD86 | 68.6 (13.7) | |||
| HLA-DR | 54.8 (4.4) | |||
| D609 | PLC | 200 | CD80 | 91.9 (6.5) |
| CD83 | 58.2 (17.2) | |||
| CD86 | 51.7 (10.2) | |||
| HLA-DR | 50.1 (9.9) |
Day 4 MDDC were incubated for 1 h with or without the indicated concentrations of inhibitors before the addition of 1 μg of PMT/ml. Twenty hours later, the cells were harvested and stained for flow cytometry with PE-anti-CD80, F ITC-anti-CD83, Cy-Chrome-anti-CD86, or the fluorochrome APC-anti-HLA-DR. Percent inhibition was calculated as described in Materials and Methods. The data shown are the means and standard errors of the means of three experiments performed on MDDC generated from different donors.
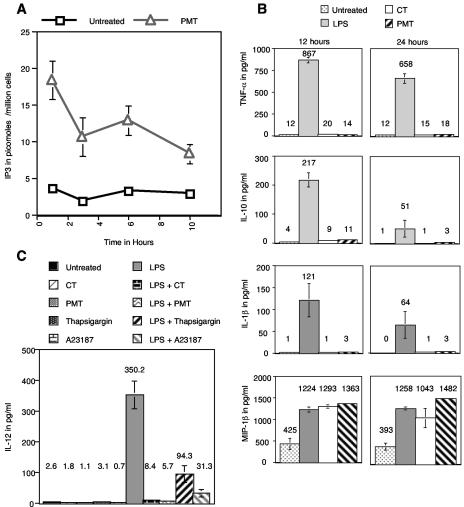
PLC is upstream of calcium release in signal transduction pathways activated by PMT. For this reason, we determined if PMT signals through PLC in MDDC. To determine this, day 4 MDDC were left untreated or were incubated with 1 μg of PMT/ml for various times and then harvested and analyzed for intracellular IP3 content as described in Materials and Methods. Figure 2A shows that the level of IP3 in MDDC incubated with PMT was elevated as early as 1 h after treatment and remained elevated for at least 10 h.
FIG. 2.
A. PMT increases intracellular IP3 concentration. Day 4 MDDC were incubated for the indicated times with or without 1 μg of PMT/ml. The cells were then harvested, lysed, and analyzed for IP3 content with a radiometric assay as described in Materials and Methods. The results are the averages of cells from three different donors. B. PMT activates MDDC without inducing cytokine production. Day 4 MDDC were left untreated or were treated with 1 μg of LPS, CT, or PMT/ml. After 12 or 24 h, the levels of IL-1β, IL-10, TNF-α, TGF-β, and MIP-1α in the supernatants were determined by ELISA. The concentrations of cytokines and chemokines not shown were not increased over untreated cells by any of the agonists. Data are the averages of three separate experiments on MDDC generated from different donors. C. PMT dominantly inhibits IL-12 production. Day 4 MDDC were left untreated or were treated with 1 μg of CT/ml, 1 μg of PMT/ml, 10 μM thapsigargin, or 150 ng of A23187/ml in the presence or absence of 1 μg of LPS/ml. After 12 h of culture, the levels of IL-12 in the supernatants were determined by ELISA. Data are the averages of three separate experiments on MDDC generated from different donors.
Next, we determined if an inhibitor of PLC would inhibit the activation of MDDC induced by PMT. Day 4 MDDC were incubated with or without 1 μg of PMT/ml for 20 h and with or without a prior 1-h incubation with 100 μM PLC inhibitor D609 and were then harvested for flow cytometry. Table 1 shows that D609 inhibited the phenotypic activation of MDDC induced by PMT. All told, the above-described results indicate that PMT activates MDDC through the mobilization of calcium induced by signaling through PLC.
PMT activates human MDDC without inducing cytokine production.
It has been previously shown that MDDC activated by LPS produce large quantities of cytokines and chemokines, whereas MDDC activated by CT do not produce cytokines or chemokines (2, 8). For this reason, we determined the production of IL-1β, IL-10, IL-12, tumor necrosis factor alpha (TNF-α), transforming growth factor β (TGF-β), and macrophage inflammatory protein-1β (MIP-1β) by MDDC activated with PMT, LPS, or CT. Day 4 MDDC were left untreated or were incubated with 1 μg of PMT, LPS, or CT/ml. Twelve and 24 h later, supernatants were removed and assayed for cytokine or chemokine concentration by ELISA.
Figure 2B shows that MDDC incubated with LPS produced substantially more TNF-α, IL-1β, and IL-10 at both 12 and 24 h than MDDC incubated with CT or PMT, whose production was similar to that of untreated MDDC. This trend was also observed with IL-12 (discussed below). No significant production of TGF-β was detected at either time point in the supernatants of MDDC activated with PMT, LPS, or CT (data not shown). In contrast to the results of a previous study (8), we found that all of the agonists tested increased the production of the chemokine MIP-1β. In this regard, Fig. 2B shows that at both 12 and 24 h, MDDC incubated with LPS, CT, or PMT made substantially more MIP-1β than untreated MDDC.
PMT, thapsigargin, and A23187 dominantly inhibit IL-12 production from LPS-stimulated human MDDC.
Next, we determined if PMT possesses the ability of other toxins to dominantly inhibit IL-12 production from LPS-stimulated DC. For these experiments, day 4 MDDC were left untreated or were incubated with 1 μg of CT or PMT/ml, 10 μM thapsigargin, or 150 ng A23187/ml in the presence or absence of 1 μg of LPS/ml. After 12 h of culture, the levels of IL-12 in the supernatants were determined by ELISA. Figure 2C shows that MDDC incubated with LPS produce high levels of IL-12, approximately 130-fold more than those produced by untreated MDDC. By contrast, MDDC activated by CT, PMT, thapsigargin, or A23187 produce approximately the same amount of IL-12 as untreated MDDC. Likewise, MDDC activated by CT, PMT, thapsigargin, or A23187 in the presence of LPS produce no more than 36-fold more IL-12 than that produced by untreated MDDC. This corresponds to at least a 3.7-fold reduction in the levels of IL-12 compared with those MDDC activated by LPS alone. These results indicate that PMT shares the ability of other bacterial toxins to dominantly inhibit IL-12 production by MDDC and that this effect is also mediated through calcium mobilization.
PMT increases the ability of human MDDC to induce T-cell proliferation in an allogeneic T-cell response.
The main effector function of DC is the activation of antigen-specific naïve T cells. Therefore, it was important to determine whether the phenotypic activation of MDDC induced by PMT correlates with an increased ability to induce the proliferation of naïve CD4+ T cells. Day 5 MDDC were left untreated or were activated by a prior 20-h incubation with 1 μg of LPS or CT (as a control)/ml, 1 μg of PMT/ml, 10 μM thapsigargin, or 150 μg of A23187/ml. These MDDC were then used to stimulate allogeneic naïve CD4+ T cells as described in Materials and Methods.
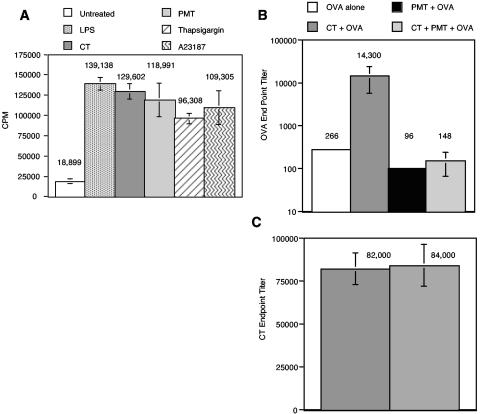
Figure 3A shows that MDDC activated by PMT, thapsigargin, or A23187 increased the proliferation of naïve CD4+ T cells at least fivefold over that induced by untreated MDDC, a level similar to that induced by LPS or CT. Altogether, these results indicate that MDDC incubated with PMT display both phenotypic and functional characteristics of mature DC.
FIG. 3.
A. PMT increases the ability of MDDC to present alloantigen to naïve CD4+ T cells in the allogeneic T-cell response. Naïve CD4+ T cells were plated at 105 cells/well in 96-well U-bottom plates in T-cell medium. MDDC were activated by a 20-h incubation with 1 μg of LPS, CT, or PMT/ml, 10 μM thapsigargin, or 150 ng of A23187/ml. Untreated and activated MDDC were washed, and then 1,000 cells were added to the naïve T cells. Experiments for each condition were performed in triplicate. Proliferation was determined at day 5 by pulsing the cells with 1 μCi of [3H]thymidine per well for the last 18 h of culture. Thymidine incorporation was measured with a Wallac 1450 Microbetta Trilux liquid scintillation counter. Data are means and standard errors for four independent experiments performed on cells mixed from different donors. CPM, counts per minute. B and C. PMT suppress the mucosal adjuvant effects but not the immunogenicity of CT. Groups of five BALB/c mice were orally immunized three times with OVA alone, CT plus OVA, PMT plus OVA, or CT plus PMT plus OVA 2 weeks apart as described in Materials and Methods. Four weeks after the final immunization, the mice were bled, and anti-OVA endpoint titers (A) or anti-CT endpoint titers (B) in the sera of individual mice were determined by ELISA. The means and standard errors of the means are shown.
PMT suppresses the adjuvant effect of CT.
Previous studies indicate that the ability of bacterial toxins to induce the maturation of DC in vitro correlates well with adjuvanticity in vivo (2, 3, 12, 14, 16). However, other studies have indicated that PMT may suppress immunologic responses (23). For this reason, we determined whether PMT boosts or suppresses antibody responses to the bystander antigen OVA. For the first mucosal immunization study, groups of five BALB/c mice were orally immunized three times with OVA alone, CT plus OVA, PMT plus OVA, or CT plus PMT plus OVA as described in Materials and Methods. Four weeks after the final immunization, anti-OVA and anti-CT endpoint titers in serum were determined. Figure 3B shows that PMT did not boost the antibody response to OVA. Of particular interest is that PMT suppressed the adjuvant activity of CT. In this regard, CT boosted the anti-OVA antibody response in serum 53-fold over that induced by OVA alone. However, when mice were immunized with both CT and PMT with OVA, the anti-OVA antibody response was similar to that induced by OVA alone. The difference between the anti-OVA endpoint titers for the mice immunized with OVA plus CT and those of mice immunized with OVA plus CT plus PMT is significant (P < 0.05).
In light of the suppression of anti-OVA antibody titers, we determined whether PMT also suppressed the immunogenicity of CT. To determine this, anti-CT antibody titers were determined in the sera described above. As shown in Fig. 3C, PMT did not significantly suppress anti-CT antibody production (P > 0.05). In this regard, the anti-CT endpoint titers for mice immunized orally with PMT plus CT were similar to those of mice immunized with CT in the absence of PMT.
Next, we determined if PMT displays similar activities when administered systemically. For these experiments, mice were immunized i.d. with OVA alone, CT plus OVA, PMT plus OVA, or CT plus PMT plus OVA as described in Materials and Methods. Unfortunately, we found that PMT is extremely toxic to mice when administered i.d. Even at a dose as low as 50 ng of PMT, 9 out of 10 mice died within 48 h of immunization. The single mouse that survived the immunization at this dose developed a lesion (approximately 1 cm in diameter) at the immunization site and did not display anti-OVA antibody responses higher than those of mice immunized with OVA alone (data not shown).
PMT activates murine BMDC to mature.
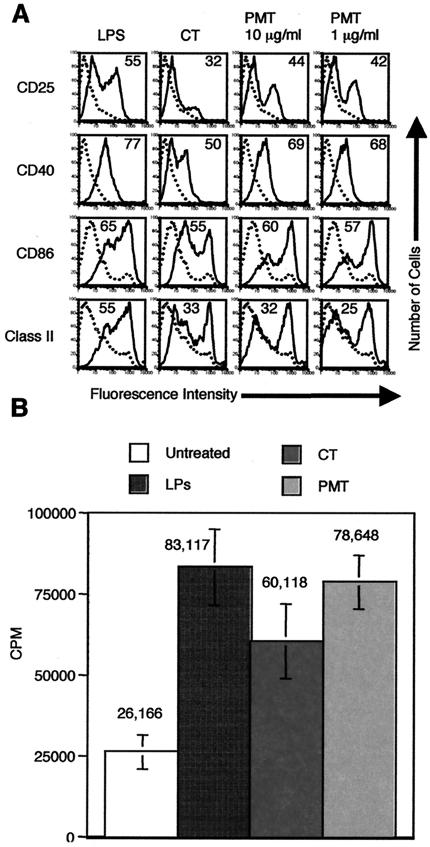
One possible explanation for the discordant effects observed with human MDDC in the in vitro studies and the mouse in vivo studies described above is that the toxin may display species-specific effects and could kill or inhibit the function of murine DC. For this reason, we determined the effects of PMT on mouse BMDC. Day 6 mouse BMDC were incubated with 1 μg of LPS/ml, 1 μg of CT/ml, 150 ng of A23187/ml, or PMT at 1 or 10 μg/ml. Twenty-four hours later, the cells were harvested and stained for flow cytometry. Figure 4A shows that PMT activates mouse BMDC to mature. As shown, the expression of CD25, CD40, CD86, and class II MHC is higher on PMT-treated BMDC than on untreated DC and is similar to that induced by LPS. Importantly, doses of PMT of up to 10 μg/ml do not kill the mouse DC during a 48-h incubation (as determined by trypan blue exclusion and flow cytometry) (data not shown). Interestingly, whereas PMT induced much less CD25 and CD40 expression on human MDDC compared to LPS, it induced similar expression of these markers on mouse BMDC compared to LPS.
FIG. 4.
PMT activates murine BMDC to mature and increases their ability to present alloantigen to T cells in the allogeneic T-cell response. A. Cell surface expression of the indicated markers on untreated BMDC (dotted histograms) or BMDC treated with the indicated agonist (solid histograms). Day 6 BMDC were incubated with 1 μg of LPS or CT/ml or PMT at 1 or 10 μg/ml for 24 h. The cells were harvested and stained for single-color flow cytometry with PE-anti-CD25, PE-anti-CD40, PE-anti-CD86, or PE-anti-14-4-4S (class II MHC). Data are representative of one experiment of three performed on cells from different mice with similar results. B. Total C57BL/6 T cells were plated at 105 cells/well in 96-well U-bottom plates in T-cell medium. Day 7 BMDC were left untreated or were activated by a prior 24-h incubation with 1 μg of LPS, CT, or PMT/ml. Untreated and activated BALB/c BMDC were washed, and then 1,000 cells were added to the C57BL/6 T cells. Experiments for each condition were performed in triplicate. Proliferation was determined at day 5 by pulsing the cells with 1 μCi of [3H]thymidine per well for the last 18 h of culture. Thymidine incorporation was measured with a Packard Matrix 96 direct beta counter. Data are means and standard deviations for mixtures of BMDC and T cells generated from three separate mice. CPM, counts per minute.
To further rule out the possibility that PMT might inhibit the function of murine DC, we determined whether mouse BMDC activated by PMT are efficient APC. Day 7 BMDC derived from BALB/c mice were left untreated or were activated by a prior 24-h incubation with 1 μg of LPS, CT, or PMT/ml. These BMDC were washed and then used to stimulate allogeneic T cells isolated from C57BL/6 mice. Figure 4B shows that PMT, like LPS and CT, increased the ability of mouse BMDC to stimulate T-cell proliferation. As shown, BMDC activated by PMT induced approximately threefold more thymidine incorporation than untreated BMDC, a level similar to that induced by LPS-activated BMDC. Together, these results indicate that PMT displays similar effects on human and mouse DC in vitro and that PMT does not kill murine DC or inhibit their function in vitro.
PMT does not induce tolerance to mucosally coadministered OVA.
There are at least two possible explanations for how PMT inhibits the adjuvant effects of CT. First, PMT could kill or inhibit the function of APC at the immunization site. Second, PMT could induce active tolerance to the coadministered antigen. To determine if the mice immunized with PMT display active tolerance to the coadministered antigen (OVA), we immunized the mice with OVA in CFA systemically after mucosal immunization with PMT and OVA. If tolerance was induced to OVA, the mice should be nonresponsive or hyporesponsive to the systemic immunization with OVA. In addition, we tested the overall viability of the immune system of the immunized mice by systemically immunizing all the mice with KLH in CFA.
For this second mucosal immunization study, groups of six BALB/c mice were orally immunized twice with OVA alone, CT plus OVA, PMT plus OVA, or CT plus PMT plus OVA as described in Materials and Methods. Four weeks after the final oral immunization, anti-OVA and anti-CT endpoint titers in the sera were determined. Four hours after collecting sera, all mice were immunized i.p. with OVA and KLH in CFA. Two weeks later, the anti-OVA and anti-KLH endpoint titers in sera were determined. As was found with the first oral immunization experiment described above, PMT inhibited the oral adjuvant effect of CT. In this regard, the endpoint titer for the group orally immunized with CT plus PMT plus OVA was similar to that of the group immunized with OVA alone (data not shown). Likewise, as was found with the first oral immunization experiment above, PMT did not inhibit the immunogenicity of CT (data not shown).
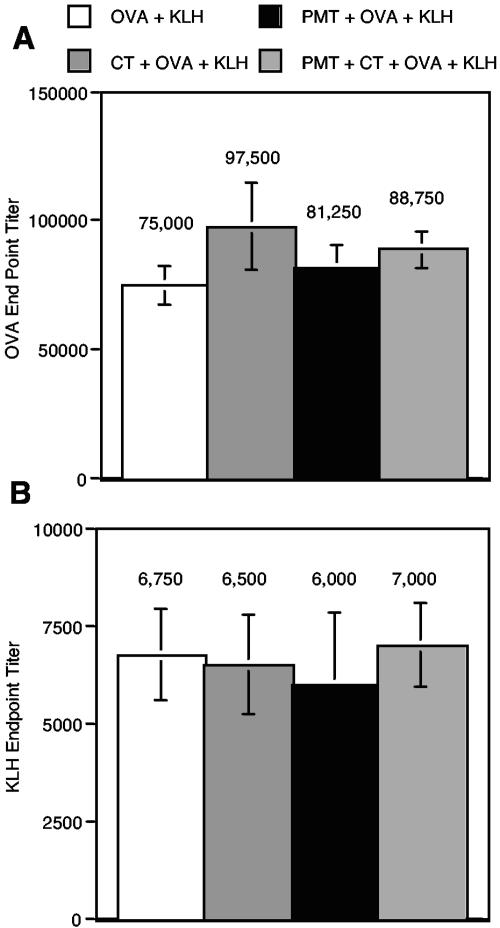
Figure 5A shows that PMT did not induce tolerance to OVA. As shown, the anti-OVA endpoint titers were similar for all of the groups after systemic immunization with OVA in CFA (P > 0.05). Likewise, Fig. 5B shows that PMT did not universally suppress antibody production, as the anti-KLH endpoint titers were also similar for all of the groups (P > 0.05).
FIG. 5.
PMT does not induce tolerance to the coadministered antigen OVA. Groups of six BALB/c mice were orally immunized two times with OVA alone, CT plus OVA, PMT plus OVA, or CT plus PMT plus OVA 2 weeks apart as described in Materials and Methods. Four weeks after the second immunization, all mice were immunized i.p. with 100 μg of OVA and 100 μg of KLH in CFA. Two weeks later, mice were bled, and anti-OVA endpoint titers (A) and anti-KLH endpoint titers (B) in the isolated sera of individual mice were determined by ELISA. The means and standard errors of the means are shown.
DISCUSSION
CT, LT, and PT are well-known mucosal adjuvants, and PMT shares several similarities to these toxins. At least two properties of PMT indicate that it could potentially exert mucosal and/or systemic adjuvant effects. First, the activation of DC in peripheral tissues is one of the key mechanisms through which adjuvants boost immune responses to coadministered antigens. For example, the adjuvants LPS, CpG DNA, CT, LT, and PT each activate MDDC to mature in vitro (2, 3, 9, 17, 19). Here, we show that PMT also activates MDDC to mature and that its potency is similar to that of PT. Second, like CT, LT, and PT, PMT binds to gangliosides on a wide variety of cells. Therefore, PMT should have similar access to cells in vivo. For these reasons, it would not be unreasonable to speculate that PMT would be a potent immunogen and would display adjuvant properties in vivo.
By contrast, PMT has been shown to be a poor antigen and becomes more immunogenic after its native structure is destroyed (24). In addition, the results from one group indicate that PMT suppresses rather than boosts the antibody response to coadministered antigens (23, 24). Here, we show that PMT also suppresses the mucosal adjuvant activity of CT. It is very interesting that PMT, which shares several similarities to the cAMP-elevating toxins CT, LT, and PT, displays opposite effects of these toxins in vivo. A possible explanation for these discordant effects may lie in the differences between these toxins with regard to the signal transduction mechanisms they activate.
CT, LT, and PT all elevate intracellular cAMP levels in target cells, although they achieve this elevation in different ways. CT and LT ADP-ribosylate the alpha subunits of G proteins, leading to the constitutive activation of adenylate cyclase, whereas PT ADP-ribosylates the inhibitory subunits of G proteins, preventing the inhibition of adenylate cyclase. By contrast, PMT elevates intracellular calcium levels through the activation of PLC by Gqα. Elevated cAMP levels cause the activation of protein kinase A (PKA), whereas calcium mobilization activates PKC. Our studies indicate that the activation of either PKA or PKC in DC leads to similar gene expression profiles and functional maturation.
However, DC are not the only cells targeted by these toxins in vivo. Potentially, any cell that expresses surface gangliosides could bind and process these toxins. In this regard, mucosal epithelial cells are the predominant cells targeted by CT, LT, and PT during mucosal immunizations, and the effects on these cells are responsible for their toxicity. Therefore, it is possible that the effects of these toxins on cells other than DC are responsible for the discordant effects of CT and PMT in vivo. Along these lines, the effects of PMT on epithelial cells are unknown. Therefore, it is possible that epithelial cells or other cells targeted by PMT may generate factors that are suppressive and or apoptotic to DC. Determining the different mechanisms through which these toxins act in vivo should provide valuable insights into the initiation of immune responses and bolster adjuvant design.
Acknowledgments
This work is supported by NIH grants AI38192 and AI43046 to G.K.L.
Editor: J. D. Clements
REFERENCES
- 1.Ackermann, M. R., M. C. DeBey, K. B. Register, D. J. Larson, and J. M. Kinyon. 1994. Tonsil and turbinate colonization by toxigenic and nontoxigenic strains of Pasteurella multocida in conventionally raised swine. J. Vet. Diagn. Investig. 6:375-377. [DOI] [PubMed] [Google Scholar]
- 2.Bagley, K. C., S. F. Abdelwahab, R. G. Tuskan, T. R. Fouts, and G. K. Lewis. 2002. Cholera toxin and heat-labile enterotoxin activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cyclic AMP-dependent pathway. Infect. Immun. 70:5533-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagley, K. C., S. F. Abdelwahab, R. G. Tuskan, T. R. Fouts, and G. K. Lewis. 2002. Pertussis toxin and the adenylate cyclase toxin from Bordetella pertussis activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cAMP-dependent pathway. J. Leukoc. Biol. 72:962-969. [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 5.Buys, W. E., H. E. Smith, A. M. I. E. Kamps, E. M. Kamp, and M. A. Smits. 1990. Sequence of the dermonecrotic toxin of Pasteurella multocida ssp. multocida. Nucleic Acids Res. 18:2815-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faries, M. B., I. Bedrosian, S. Xu, G. Koski, J. G. Roros, M. A. Moise, H. Q. Nguyen, F. H. Engels, P. A. Cohen, and B. J. Czerniecki. 2001. Calcium signaling inhibits interleukin-12 production and activates CD83(+) dendritic cells that induce Th2 cell development. Blood 98:2489-2497. [DOI] [PubMed] [Google Scholar]
- 7.Foged, N. T. 1992. Pasteurella multocida toxin. The characterisation of the toxin and its significance in the diagnosis and prevention of progressive atrophic rhinitis in pigs. APMIS Suppl. 25:1-56. [PubMed] [Google Scholar]
- 8.Gagliardi, M. C., F. Sallusto, M. Marinaro, A. Langenkamp, A. Lanzavecchia, and M. T. De Magistris. 2000. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur. J. Immunol. 30:2394-2403. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann, G., G. J. Weiner, and A. M. Krieg. 1999. CpG DNA: a potent signal for growth, activation, and maturation of human dendritic cells. Proc. Natl. Acad. Sci. USA 96:9305-9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins, T. E., A. C. Murphy, J. M. Staddon, A. J. Lax, and E. Rozengurt. 1992. Pasteurella multocida toxin is a potent inducer of anchorage-independent cell growth. Proc. Natl. Acad. Sci. USA 89:4240-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lycke, N. 1997. The mechanism of cholera toxin adjuvanticity. Res. Immunol. 148:504-520. [DOI] [PubMed] [Google Scholar]
- 13.Pettit, R. K., M. R. Ackermann, and R. B. Rimler. 1993. Receptor-mediated binding of Pasteurella multocida dermonecrotic toxin to canine osteosarcoma and monkey kidney (Vero) cells. Lab. Investig. 69:94-100. [PubMed] [Google Scholar]
- 14.Roberts, M., A. Bacon, R. Rappuoli, M. Pizza, I. Cropley, G. Douce, G. Dougan, M. Marinaro, J. McGhee, and S. Chatfield. 1995. A mutant pertussis toxin molecule that lacks ADP-ribosyltransferase activity, PT-9K/129G, is an effective mucosal adjuvant for intranasally delivered proteins. Infect. Immun. 63:2100-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rozengurt, E., T. Higgins, N. Chanter, A. J. Lax, and J. M. Staddon. 1990. Pasteurella multocida toxin: potent mitogen for cultured fibroblasts. Proc. Natl. Acad. Sci. USA 87:123-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan, M., L. McCarthy, R. Rappuoli, B. P. Mahon, and K. H. Mills. 1998. Pertussis toxin potentiates Th1 and Th2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the co-stimulatory molecules B7-1, B7-2 and CD28. Int. Immunol. 10:651-662. [DOI] [PubMed] [Google Scholar]
- 17.Sallusto, F., M. Cella, C. Danieli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparwasser, T., E. S. Koch, R. M. Vabulas, K. Heeg, G. B. Lipford, J. W. Ellwart, and H. Wagner. 1998. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur. J. Immunol. 28:2045-2054. [DOI] [PubMed] [Google Scholar]
- 20.Staddon, J. M., C. J. Barker, A. C. Murphy, N. Chanter, A. J. Lax, R. H. Michell, and E. Rozengurt. 1991. Pasteurella multocida toxin, a potent mitogen, increases inositol 1,4,5-trisphosphate and mobilizes Ca2+ in Swiss 3T3 cells. J. Biol. Chem. 266:4840-4847. [PubMed] [Google Scholar]
- 21.Takahashi, T., K. Fukuda, J. Pan, H. Kodama, M. Sano, S. Makino, T. Kato, T. Manabe, and S. Ogawa. 1999. Characterization of insulin-like growth factor-1-induced activation of the JAK/STAT pathway in rat cardiomyocytes. Circ. Res. 85:884-891. [DOI] [PubMed] [Google Scholar]
- 22.Treiman, M., C. Caspersen, and S. B. Christensen. 1998. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases. Trends Pharmacol. Sci. 19:131-135. [DOI] [PubMed] [Google Scholar]
- 23.van Diemen, P. M., G. de Vries Reilingh, and H. K. Parmentier. 1996. Effect of Pasteurella multocida toxin on in vivo immune responses in piglets. Vet. Q. 18:141-146. [DOI] [PubMed] [Google Scholar]
- 24.van Diemen, P. M., G. de Vries Reilingh, and H. K. Parmentier. 1994. Immune responses of piglets to Pasteurella multocida toxin and toxoid. Vet. Immunol. Immunopathol. 41:307-321. [DOI] [PubMed] [Google Scholar]
- 25.Verhasselt, V., C. Buelens, F. Willems, D. De Groote, N. Haeffner-Cavaillon, and M. Goldman. 1997. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J. Immunol. 158:2919-2925. [PubMed] [Google Scholar]
- 26.Walker, K. E., and A. A. Weiss. 1994. Characterization of the dermonecrotic toxin in members of the genus Bordetella. Infect. Immun. 62:3817-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson, B. A., X. Zhu, M. Ho, and L. Lu. 1997. Pasteurella multocida toxin activates the inositol triphosphate signaling pathway in Xenopus oocytes via Gqα-coupled phospholipase C-β1. J. Biol. Chem. 272:1268-1275. [DOI] [PubMed] [Google Scholar]
- 28.Wright, C. L., R. A. Strugnell, and A. L. M. Hodgson. 1997. Characterization of a Pasteurella multocida plasmid and its use to express recombinant proteins in P. multocida. Plasmid 37:65-79. [DOI] [PubMed] [Google Scholar]