Abstract
Transcranial magnetic stimulation (TMS) studies in humans have shown that many behaviors engage processes that suppress excitability within the corticospinal tract. Inhibition of the motor output pathway has been extensively studied in the context of action stopping, where a planned movement needs to be abruptly aborted. Recent TMS work has also revealed markers of motor inhibition during the preparation of movement. Here, we review the evidence for motor inhibition during action stopping and action preparation, focusing on studies that have used TMS to monitor changes in the excitability of the corticospinal pathway. We discuss how these physiological results have motivated theoretical models of how the brain selects actions, regulates movement initiation and execution, and switches from one state to another.
Keywords: Transcranial magnetic stimulation, corticospinal excitability, inhibitory control, action selection, reactive and proactive inhibition
Multiple forms of motor inhibition during human behavior
Behaving in a goal-directed manner often requires suppressing inappropriate movement tendencies [1–3]. As such, many daily life situations demand that human beings refrain from acting in an automatic, stimulus-driven manner, subjugate internal desires that interfere with long-term plans (e.g. eating unhealthy food or drinking too much alcohol), or interrupt ongoing actions that are no longer appropriate (e.g. aborting a foot movement towards the accelerator when a pedestrian suddenly runs into the street). Without the efficient operation of inhibitory control, behavior becomes maladaptive, as evidenced in a range of psychiatric disorders [4, 5].
Many studies have investigated the neural substrates of behavioral inhibition by using laboratory tasks that require stopping a planned action [6–10]. Under such conditions, rapid suppression of activity can be observed at various levels of the motor pathway, likely reflecting a pause in motor output [11, 12]. Intriguingly, recent studies have revealed that the motor output pathway also shows profound inhibitory changes during action preparation, even during the planning of simple finger movements [13–18]. Hence the motor system is not only inhibited when a movement needs to be aborted but also when it is in the process of specifying a future action. The function(s) served by such inhibition as part of action preparation, and the extent to which it may support behavioral inhibition have been the focus of considerable research over the past decade.
In this paper, we review and evaluate recent work that has explored physiological markers of motor inhibition in conditions requiring action stopping or action preparation. We focus on studies that have used transcranial magnetic stimulation (TMS) in humans to monitor changes in the excitability of the corticospinal pathway. Using this procedure, single-pulse TMS applied over the primary motor cortex (M1) elicits motor-evoked potentials (MEPs) in targeted contralateral muscles (see Box 1 and Fig. 1), providing a temporally precise and muscle-specific assay of the state of excitability of the motor output pathway [2, 19–21]. Other methods can also be used to track changes in motor excitability in humans. This includes the analysis of specific electroencephalography (EEG) waves (see Box 1 for a short overview) or fMRI signals that can provide a window into larger scale networks for inhibitory control (see previous reviews, [22–24]). Here we will only briefly refer to these other works as the TMS literature offers by itself an extremely fertile ground for the discussion of mechanisms underlying action stopping and action preparation in humans. Our goal is to discuss, in an integrated manner, the varied hypotheses concerning the role of motor system inhibition in shaping human behavior.
Box 1. Electrophysiological signatures of motor inhibition.
Motor-evoked potentials (MEPs) to Transcranial Magnetic Stimulation (TMS)
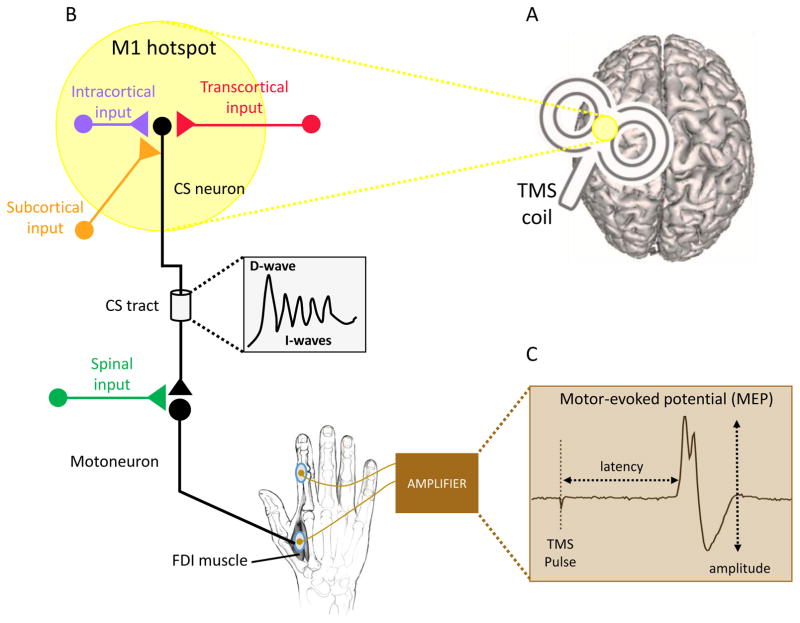
TMS is a non-invasive technique that can induce short (~250μs) electrical currents in the human cortex [132]. When the stimulating coil is placed over the primary motor cortex (M1), TMS elicits descending volleys in the corticospinal fibers. These fibers synapse on spinal motoneurons that innervate peripheral muscles contralateral to the stimulation site (see Fig. 1). The evoked response, the motor-evoked potential (MEP) can be easily recorded using surface electromyography (EMG). The amplitude of the MEP provides an assay of corticospinal (CS) excitability for the targeted muscle at the time of stimulation [2, 19].
The MEP measured with surface EMG is a signal resulting from a complex series of waves that descend through the CS tract (D- and I waves [133]). TMS over M1 can directly activate corticospinal neurons. However, the TMS pulse also excites other fibers that in turn project onto CS neurons. These fibers may originate in M1, linking up with CS cells through intracortical circuits. They may also come from other cortical areas such as premotor, somatosensory and parietal regions, or from subcortical structures such as the thalamus. Because CS cells synapse onto motoneurons in the spinal cord before reaching their targeted muscle, the MEPs also depend on the excitability of the spinal circuitry. Importantly, these intracortical, transcortical, subcortical and spinal inputs provide routes through which different inhibitory control processes can influence MEP amplitudes during action stopping and action preparation.
Sophisticated TMS protocols have been developed to provide probes on specific circuits. For example, paired-pulse protocols [134], apply a low intensity sub-threshold conditioning TMS pulse, and measure its impact on the MEP response elicited by a subsequent supra-threshold test pulse generated in the same coil. The two TMS pulses are applied over M1, not only at specific intensities, but also at specific times. Conditioning pulses applied between 2–5 ms or between 50–200 ms before the test pulse are thought to probe GABAergic intracortical inhibitory circuits that act at corresponding intrinsic latencies, thus providing an assay to link inhibitory neurotransmission with motor behavior [84, 133, 135].
Other protocols employ two separate stimulation coils to investigate transcortical influences on M1. These double-coil protocols measure the impact of a supra-threshold conditioning pulse over a cortical region assumed to generate a transcortical signal on the MEP elicited by a test pulse delivered through a coil placed over M1 [136]. TMS protocols have revealed the existence of inhibitory interactions between M1 in the two hemispheres as well as inhibitory projections from several frontal areas to M1 and the cerebellum to M1 (see for example [137–139]. A double-coil procedure in which two coils are used to stimulate M1 at a nearly-simultaneous time [1 ms delay] has been introduced recently as a new method to probe preparatory inhibition in the two hands concurrently [85].
Other electrophysiological signatures of motor inhibition
Several attempts have been made to link specific electrophysiological signatures to inhibitory mechanisms (see [10, 140] for reviews). The initiation of voluntary movements is associated with desynchronization of activity in the beta frequency band (13–30 Hz) in electrocorticography (ECoG) and scalp electroencephalography (EEG) recordings over motor cortex [141–143]. Consequently, beta activity has been associated with the ‘idling’ of the motor system, and a decrease in beta activity with a change from the ‘status quo’ of the motor state. Beta activity within M1 may reflect the operation of intracortical inhibitory mechanisms [144]. Notably, bursts of beta activity are observed before and after the movement-related beta desynchronization [145].
EEG studies of reactive stopping report a consistent increase in beta activity over right frontal regions for successful stopping compared to failed stopping [51, 146]. Moreover, excessive beta synchrony throughout cortico-basal ganglia circuits coincides with increased bradykinesia in Parkinson’s disease [147, 148] and with response inhibition during the stopping of actions [120]. Reactive stopping has been hypothesized to involve the recruitment of a mechanism that rapidly increases beta activity to suppress ongoing movement. Event-related potential (ERP) EEG signatures have also been linked to reactive inhibitory control. Greater amplitudes and shorter latencies of the N2/P3 complex have been associated with successful response inhibition [146]. Recently, it was shown that the latency of the P3 onset correlates with the speed of stopping [149].
Hence, TMS, ECoG and EEG protocols provide a rich arsenal of methods for selectively probing circuits involved in generating inhibitory influences on the human motor system during action stopping and action preparation.
Figure 1. Transcranial magnetic stimulation (TMS) as a probe of corticospinal excitability.
A. The TMS coil is placed over primary motor cortex (M1) at the “hotspot” (depicted in yellow), the position at which the largest motor-evoked potentials (MEPs) can be recorded in the EMG signal from a targeted muscle. B. TMS over M1 activates corticospinal (CS) neurons directly or indirectly via the stimulation of intracortical circuits that project to CS neurons. Transcortical inputs from premotor, prefrontal and parietal cortices, as well as axons of subcortical cells projecting onto M1 are also activated by TMS over M1. Depending on the position and intensity of stimulation, a series of descending volleys (D- wave and I-wave) are transmitted from M1 to the motorneurons in the spinal cord. These signals are further influenced by inputs at the spinal level before they jointly give rise to an MEP in the targeted, contralateral muscle (first dorsal interosseus [FDI] in the present example). C. The MEP is a bi-phasic response recorded from a targeted muscle via electrodes placed on the surface of the skin. It has a latency of approximately 18 ms after the TMS pulse when elicited in hand muscles. While the latency is relatively invariant, the peak-to-peak amplitude fluctuates, reflecting the sum of cortical, subcortical, and spinal contributions to the descending signals to the muscle.
Motor inhibition associated with action stopping
We frequently encounter situations in which a motor action, once initiated, becomes unnecessary or inappropriate. Imagine sitting in your car at an intersection and the traffic light has just turned green. You begin to shift your foot from the brake to the accelerator when suddenly, a pedestrian runs into the street. Fortunately, you are able to quickly update your action plan, aborting the movement towards the accelerator. While this may be an extreme example of the importance of inhibitory control, our everyday behavior is replete with such changes of intent, elicited by unexpected variations in the environment.
Experimentally, the psychological processes and neural mechanisms underlying action stopping have been extensively studied with versions of the stop signal task [11, 25]. This task has been employed to explore a range of psychological questions such as the relationship between response initiation and inhibition [26, 27] and the characteristics of inhibitory control [28–31]. The stop signal task has also proved useful for characterizing deficits in behavioral inhibition in Parkinson’s disease [32], schizophrenia [33], ADHD [34], and in individuals with alcohol- and drug-dependencies [35, 36].
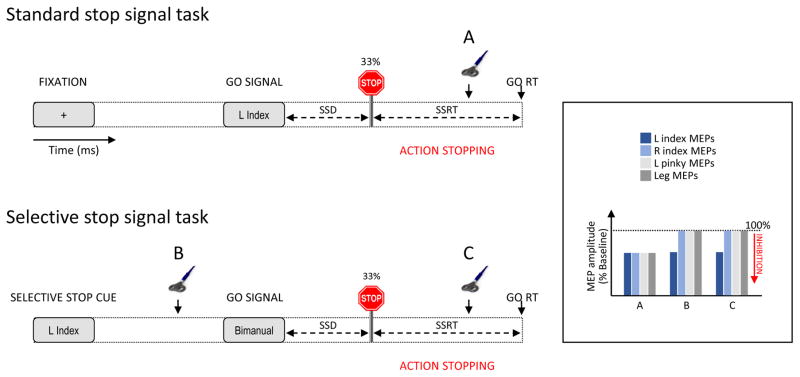
In the standard form of the stop signal task (Fig. 2, upper left panel), the participant is engaged in a reaction time (RT) task, with the emphasis on speeded responses. On a subset of trials (e.g. 33%), a stop signal stimulus is presented shortly after the go signal. Participants are instructed to attempt to cancel the initiated response as soon as they detect the stop signal. The time delay between the go and stop signal (stop signal delay, SSD) can be adjusted in a dynamic manner such that participants only succeed in aborting the response at some specified criterion level (e.g., 50%). Action stopping has also been studied in an adapted version of the stop signal task where bimanual responses are initiated, but the stop signal is relevant for only one of the responses (see Fig. 2, lower left panel). For example, if a go signal cues the participant to respond with synchronized button presses with the two index fingers, the stop signal here indicates that one finger should be stopped while the other finger should continue (e.g., stopping the left but not the right index finger response). This selective stop signal task has provided a method to explore the impact of stopping one component of an ongoing action on the continuing piece of response. In the next sections, we review evidence for the contribution of motor inhibitory mechanisms to standard and selective stopping.
Figure 2. Study of motor inhibition during action stopping.
The standard stop task (upper panel) often requires subjects to choose between left (L) and right (R) finger responses (L index finger trial in this example) occasionally interrupted by a stop signal (~33% of trials). The time between the go signal and the stop signal, or stop signal delay (SSD), is adjusted so that participants succeed in stopping on a targeted proportion of trials (usually 50%). When transcranial magnetic stimulation (TMS) is applied after the stop signal (A), motor evoked potentials (MEPs; expressed as a percentage of baseline) elicited in selected (L index), non-selected (R index) and irrelevant (L pinky or leg) muscles are globally suppressed reflecting widespread reactive inhibition. In selective tasks (lower panel), subjects make bimanual movements (e.g. with index fingers); a cue is presented at the beginning of each trial, indicating the hand that may have to be stopped if a stop signal occurs (L index strop trial in this example). In this task, MEPs measured after the stop signal (C) are suppressed in only the agonist muscle that was cued for stopping, reflecting selective reactive inhibition. When TMS is applied before the stop signal in this type of selective stop task (B), MEPs are also only suppressed in the muscle that may have to be stopped, indicating selective proactive inhibition in anticipation of the stop signal.
Standard Stopping
Formal psychological models suggest that performance in the standard version of the stop signal task involves a race between two independent processes, one associated with response execution (GO) and the other with the cancellation of the planned response (STOP) [25]. This race model provides an analytic tool to estimate the duration of the covert STOP process, referred to as the Stop Signal RT (SSRT) [37]. The SSRT can be estimated by subtracting the SSD that yields a 50% stopping success rate from the average GO RT (see Fig. 2), but see also [38].
Electromyography (EMG) studies have shown that motor responses can be stopped at multiple stages of execution, including after the responding muscles are engaged (de Jong et al., 1990). Brain stimulation and electrophysiological methods have been used to identify the time course of corticospinal excitability changes during reactive stopping. A consistent finding has been that the presentation of a stop signal produces a rapid suppression of MEP amplitudes, reflecting a marked drop in corticospinal excitability [39–42]. The fact that MEP amplitudes become smaller relative to baseline measurements obtained during the inter-trial interval provides compelling evidence that successful stopping is not the result of a delay in the initiation of action preparation processes, but rather entails the active suppression of corticospinal excitability. Consistent with this idea, paired-pulse TMS protocols reveal a strengthening of GABAergic intracortical inhibition in M1 on stop trials [43]. Moreover, electrodes over M1, recording cortical activity during electrocorticography (ECoG), show a reduction of alpha–beta desynchronization (i.e., a relative increase of synchronization) on stop trials [44]. Other converging lines of evidence indicate that the suppression in motor activity entails not only cortical increases in inhibition, but also a reduction of excitatory input from the thalamus to M1. For example, electrophysiological studies in rats suggest that the stop process involves two stages, with a pause process followed by a later cancelation process both occurring at different levels within the basal ganglia [45, 46] with subsequent effects on M1 [47]. Taken as a whole, the available data suggest that the presentation of the stop signal is not merely associated with terminating motor commands that produce excitation in M1, but with the recruitment of one or more active inhibitory mechanisms that suppress the motor command.
Neuroimaging and neuropsychological studies have identified a cortico-basal ganglia network engaged during reactive stopping in the standard stop signal task, with key nodes including the right inferior frontal cortex (rIFC), the dorsomedial frontal cortex (especially the pre-supplementary motor area, preSMA), and the basal ganglia (reviewed in detail by [12, 48–50], see also [45]). Of particular interest has been the hyper-direct pathway between the frontal cortex and sub-thalamic nucleus (STN) of the basal ganglia [11, 51–53]. A prominent hypothesis centers on the idea that the direct engagement of the STN by the cortex provides a mechanism to rapidly shut down motor output. The STN sends diffuse excitatory projections to the internal segment of the globus pallidus pars interna (GPi) [12, 54–57], which in turn sends inhibitory output to the motor thalamus, decreasing the excitatory drive to the motor cortex (but see also Mayse, Nelson, Avila, Gallagher and Lin [58] for an involvement of the basal forebrain). This neural architecture has been directly implicated in stopping and is thought to inhibit the motor system in a global manner [8, 12, 59].
Consistent with this hypothesis, single-pulse TMS studies underscore that reactive stopping is not limited to inhibition of the selected (to-be-stopped) response representation, but has a global suppressive effect on the motor system, bringing the activity of action representations below resting levels in a non-selective manner. That is, successful stopping not only reduces MEPs in the task-relevant agonist muscle, but also reduces MEPs in task-irrelevant muscles (see Fig. 2A, right panel). For example, when the relevant effector is the left index finger, MEP suppression is observed in other muscles of the responding hand [39] or homologous muscles in the non-responding hand [43]. Furthermore, this spread extends beyond the upper extremities. Aborting a hand response produces a reduction of MEPs elicited in leg muscles [40, 42] while aborting speech or a saccade produces inhibition in hand muscles [41, 60]. Thus, there appears to be a diffuse suppressive effect when a planned action is suddenly aborted in the standard stop signal task. It is noteworthy that the inhibition of task-irrelevant muscles provides additional evidence that stopping not only involves termination of ongoing excitatory commands, but also engages an active inhibitory process. Moreover, this non-selective inhibition of the motor system is consistent with the idea that the hyper-direct projection from the cortex to STN can result in broad inhibition of the motor system [11, 12, 59, 61].
Selective Stopping
When only part of a compound response has to be stopped, people exhibit interference, with reaction times for the non-stopped (continuing) component being slower on stop trials compared to go trials [62, 63]. To illustrate this point, let’s go back to the driving example mentioned above: Imagine you were manipulating the radio button when a pedestrian stepped into the road. While the situation demands that you immediately abort your movement towards the accelerator, you are also likely to stop tuning the radio. This observation has been understood in the light of the operation of a global stopping command, one that affects both targeted and non-targeted actions. Following this, the remaining response (e.g. tuning the radio) must be reprogrammed, resulting in a RT cost [64].
Although the driving situation may demand a complete shift of attention to avoid hitting the pedestrian, it is somewhat puzzling that interference can be quite profound in experimental tasks given the everyday observation that we are often able to selectively abort one response without affecting other ongoing movements. It may be that the selective stop task constitutes a dual task situation, one in which the participant has the added burden of having to keep track of which response is to be aborted and which is to be maintained. By this account, the slowing of the continued response could result from difficulty in assigning the stopping process to the appropriate component of the response. Indeed, a recent study has shown that selective stop interference is minimal or entirely abolished when the stop signal is unambiguously associated with one response or when participants are given extended training [65]. This functional view of the RT cost offers an alternative to accounts that attribute the cost to a structural limitation in which the rapid termination of a movement engages the hyper-direct pathway in a generic manner. More research is clearly required to distinguish between these two hypotheses.
Several lines of evidence are consistent with the idea that selective stopping can arise from a targeted suppression mechanism. For example, inhibition is focal when participants are provided with advance information regarding the potential action that might have to be aborted (“selective stop cue” in Fig. 2, lower left panel). Aron and Verbruggen [62] created a variant of the selective stop task in which, on each trial, the go signal is preceded by a cue indicating which of two index finger responses would have to be aborted, should a stop signal appear. Leg muscle MEPs are not suppressed following stop signals in this selective condition (see Fig. 2C, right panel), a result that stands in contrast to the observations that leg MEPs are suppressed if the stop signal indicates that the response from both hands should be stopped [42, 62]. Interestingly, SSRT estimates from the selective stop task are slower compared to those derived from the standard stop signal task. The selectivity of inhibitory influences, as well as the longer SSRT, suggest that selective stopping may not use the hyper-direct cortico-STN pathway, but may instead rely on neural circuits associated with more deliberative selection processes such as the indirect cortico-striatal pathway[6, 66, 67]. Hence, separate mechanisms may be recruited in reaction to global and selective stop signals, resulting in a tradeoff between speed and anatomical selectivity. People are more likely to use a global mechanism when speed is of the essence (as in our driving example), whereas they are more likely to use a selective mechanism when they want to maintain control of particular responses, especially when advanced information indicates which response may need to be stopped.
Proactive Inhibition
As alluded to in the previous paragraph, an important emerging theme in inhibitory control research focuses on how various constraints (e.g. selectivity, speed, etc.) may influence mechanisms underlying reactive stopping. More recently, researchers have also used a range of manipulations to look at cognitive control processes recruited in anticipation of stopping, or what we will call “proactive inhibition”. For example, the probability of a stop signal might be manipulated in a predictive manner to modify the tradeoff between the speed of responding and stopping success. In contrast to stimulus-driven reactive stopping, proactive inhibition reflects a top-down control process. To illustrate this point, consider the driving example from before. In the vicinity of a school, our driver may opt to be more cautious, slower to press the accelerator when the traffic light turns green. This type of controlled behavioral slowing can occur in the absence of an overt stimulus distinguishing it from reactive stopping.
Two alternative hypotheses have been proposed to explain patterns of behavioral slowing. The first postulates that slowing reflects a strategic process to delay responding until it becomes clear that a stop signal will not be presented. In this case, there is no need to postulate an active inhibitory process; excitatory processes that drive response execution are simply postponed. Alternatively, proactive control might engage an active inhibitory process that suppresses motor activity when a stop signal is expected[68]. Support for this type of proactive inhibition comes from the observation that, in anticipation of a stop signal in selective stop tasks, MEP amplitudes are suppressed relative to baseline values obtained during the inter-trial interval [69, 70]. The MEP suppression only concerns effectors that might have to be stopped, leaving the continuing response representations unaffected (see Fig. 2C, right panel). Hence, in selective stop tasks, proactive inhibition is selectively targeted at specific motor responses, possibly enabling less effortful reactive stopping. Furthermore, when people anticipate the need to stop, the subsequent reactive inhibition of MEPs in task-irrelevant muscles is attenuated [40], while scalp EEG ERPs and fMRI signal in the basal ganglia, both associated with successful reactive stopping, increase in amplitude [71, 72]. Notably, proactive inhibition has only been studied in selective stop tasks but never in standard stop signal tasks. It thus remains to be determined whether signatures of proactive inhibition can also be detected when one anticipates a global stop signal requiring the cancellation of the entire response (and not just one component) [73]. Because reactive inhibition in the latter situation can be implemented via a very fast hyper-direct route, there may be no advantage to proactively anticipate a stop signal given that such a strategy can slow down RTs on GO trials.
Motor inhibition associated with action preparation
In the stop signal task, the experimenter introduces an explicit tension between implementing and aborting a planned action. At the behavioral level, there is an obvious need for inhibition, and at the neural level, we can measure the rapid attenuation of excitability in the corticospinal pathway. However, action stopping represents just one situation requiring inhibitory control. Many inappropriate behaviors have also been associated with a lack of inhibitory control in the context of action selection and initiation, (e.g., interrupting a speaker or drinking too much alcohol). Interestingly, several markers of inhibition have been observed during the period preceding a voluntary movement. What function(s) does such inhibition serve as part of action preparation and to what extent does it support behavioral inhibition? This question has been the focus of considerable work over the past decade.
The dynamics of corticospinal excitability during action preparation have been investigated in RT studies where people are instructed to respond as quickly as possible following a go signal (Fig. 3, upper left panel); TMS probes over M1 are applied at several time points between the go signal and the movement onset, with the changes in MEP amplitude (usually expressed with respect to baseline measurements obtained during the inter-trial interval) providing a window into the recruitment of the motor system preceding movement onset [2, 74]. In the simplest version of this paradigm, the go signal always specifies the same movement within a given block of trials. In this simple RT condition, there is a gradual increase in the amplitude of MEPs recorded from the agonist muscle, starting approximately 100 ms prior to the onset of the volitional EMG [74, 75]. This pre-movement increase in the amplitude of MEPs is thought to reflect the excitation of the corresponding motor representation in M1 through a joint modulation of facilitatory and inhibitory influences[76].
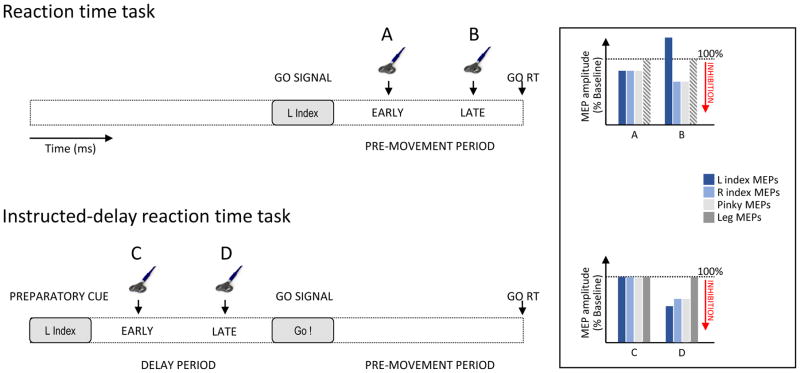
Figure 3. Study of motor inhibition during action preparation.
Reaction time (RT) tasks (upper panel) often require subjects to perform left (L) or right (R) finger responses in a simple or choice setting (L index finger trial in a choice RT task in this example). When transcranial magnetic stimulation (TMS) is applied immediately after the go signal (A), motor evoked potentials (MEPs; expressed as a percentage of baseline) elicited in selected (L index), non-selected (R index) and irrelevant finger (L pinky) muscles are globally suppressed reflecting widespread inhibition during the EARLY stage of the pre-movement period. Close to movement onset (LATE, B), the amplitude of MEPs is increased when the finger muscle is the agonist for the selected response and is attenuated if the muscle is not selected or irrelevant. Dashed grey bars are used to represent hypothetical leg MEPs (not investigated to date) based on evidence in instructed-delay RT tasks. In these delay tasks, a cued response is prepared but withheld until the go signal. When TMS is applied at the end of the delay period (LATE, D), MEPs are suppressed regardless of whether the finger muscle is selected, non-selected or task-irrelevant, indicating broad preparatory inhibition, although inhibition does not seem to extend to leg muscle representations. Notably, inhibition is often stronger for selected than non-selected muscles, suggesting some additional focal inhibition targeted at agonist muscles. MEP suppression is not observed when TMS is applied a long time before the go signal, close to the preparatory cue (EARLY, C).
In more complex versions of the RT task, the go signal requires choosing between a set of options that are predefined within a block of trials (e.g. a left or right index finger response; choice RT task), hence allowing for the investigation of the physiological correlates of action selection. Here, the MEPs can be compared for conditions in which the muscle is selected or not selected for the forthcoming response, and within the latter, the muscle may be associated with an effector that is part of the response set or that is irrelevant to the task. As expected, MEPs elicited in the selected effector exhibit a rise in amplitude during the pre-movement period, similar to that observed in simple RT tasks (see Fig. 3AB, right panel). However, before the activity begins to ramp up, there is an initial decrease in the amplitude of the MEPs [77, 78], indicating suppression of the corticospinal pathway associated with the selected movement. A reduction in MEP amplitude is also observed in the non-selected effector, and here, the MEPs display a further drop in amplitude over the course of the pre-movement period [74, 79–81]. These effects are consistent with the hypothesis that action selection involves not only excitation of the selected effector, but also inhibitory processes, initially evident in both selected and non-selected effectors.
Studies of action preparation have also used instructed-delay RT tasks, one in which a cue provides advance information about a forthcoming response, but the participant must then wait until the go signal is presented to release his response (see Fig. 3, lower left panel). This paradigm affords the ability to investigate delay-related processes that are specifically involved in action preparation, both in the context of simple and choice RT tasks, without being confounded by functions related to movement execution. Here too, corticospinal excitability is suppressed when the preparatory cue indicates that the targeted muscle should not be selected for the forthcoming response. Interestingly, inhibition is also observed in the selected hand during the delay period [82, 83]. That is, MEPs probed following preparatory cues in a selected effector also become smaller relative to baseline. Moreover, this inhibition is often stronger than that observed when the same muscle is not selected for the forthcoming response [77, 84] (but see also [85, 86]), especially when probed at the end of the delay period (Fig. 3CD, right panel) [18]. The presence of marked inhibition in the representation of selected effectors close to the time at which they need to be recruited for the forthcoming response has presented a challenge to models of inhibition. In the following sections, we review current hypotheses regarding the role of motor inhibition during action preparation.
Functional role of preparatory inhibition
The suppression of corticospinal excitability observed before movement initiation has led to several hypotheses regarding the role of motor inhibition during action preparation. One hypothesis has been that preparatory inhibition serves to assist action selection, consistent with a contribution of inhibition to the generation of goal-oriented behaviors [2]. One variant of this idea is that action selection entails a competitive process, whereby selection of the desired response relies on the suppression of non-selected action representations [79, 87]. Another variant is that preparatory inhibition assists action selection by producing a global suppression of motor representations to prevent inappropriate action representations from being selected. A second hypothesis has focused on the implementation of the selected response: Preparatory inhibition may serve to prevent selected muscles from becoming active prematurely while preparatory activity unfolds across the cortex [17, 88]. A third, hybrid hypothesis is that preparatory inhibition serves to modulate the gain of the motor system, increasing the signal-to-noise ratio [89]. In this case, inhibition may reduce background activity during motor preparation, providing a way to facilitate response implementation [13].
We consider these hypotheses in the following sections, examining three models of preparatory inhibition (see Fig. 4). We note at the outset that the current evidence does not discriminate between these hypotheses and indeed, they are not mutually exclusive. We highlight key issues that can guide future experiments (see also Outstanding Questions Box).
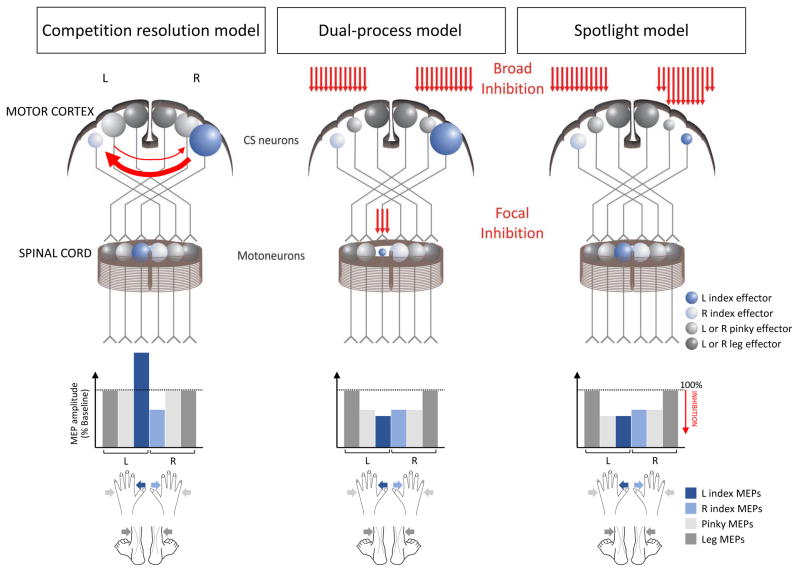
Figure 4. Models of preparatory inhibition.
Illustration of the inhibition for competition resolution hypothesis (left column), the dual-process model (middle column) and the spotlight model (right column) in the context of a task in which a cue indicates if the forthcoming response will require a left (L) or right (R) index finger movement (L index finger trial in the current example). Dark and light blue circles are used to illustrate the neural representation of the L and R index fingers, respectively, in the motor cortex (upper panel) and in the spinal cord (middle panel). Dark and light grey circles are used to display irrelevant leg and pinky muscle representations, respectively. The circle size reflects the activation level of the motor representation. Inhibitory influences are displayed as red arrows. The lower panel shows the amplitude of motor-evoked potentials (MEPs) elicited in the L and R index muscles, as well as in irrelevant pinky and leg muscles. Based on the competition resolution idea, activation of the selected (L index) effector produces a selective suppression of the non-selected finger (R index). In the dual-process model, a second source of inhibition is directed at the selected effector, probably at the spinal level, resulting in suppressed MEPs in the selected effector despite increasing activation of its cortical representation. Finally, in the spotlight model, the inhibitory influences are centered on the selected effector, with inhibition extending to adjacent effectors (e.g. L pinky) and, to a lower degree, to homologous representations in the contralateral hemisphere, perhaps through transcallosal connections. The colored arrows point to the feet and hand muscles from which the corresponding MEPs are recorded. For illustration purposes, the spotlight is shown influencing cortical excitability, although this type of inhibition may occur elsewhere. Neither model predicts inhibition of leg muscles, reflecting the idea that the scope of preparatory inhibition may be more narrow during action preparation than action stopping. CS = corticospinal.
Outstanding Questions Box.
The motor output system shows clear evidence of inhibition during the preparation of motor acts. Preparatory inhibition is not only observed prior to the onset of an imperative signal in delayed response tasks, but is also evident after the imperative in standard, no-delay RT tasks. This inhibition can be non-specific; for example, when preparing a finger movement, inhibition will be seen in MEPs elicited from task-irrelevant upper limb muscles. The functional significance of preparatory inhibition remains a subject of debate. In addition, the source of corticospinal inhibition needs to be precisely identified, together with the level (cortical and/or spinal) at which the inhibition is manifest.
Functions of preparatory inhibition
As described in the main text, current models of preparatory inhibition focus on functions related to competitive interactions, response initiation and gain modulation. Critical experiments that pit the models against one another are needed, both to evaluate these models and inspire new hypotheses. For example, recent studies have revealed that preparatory inhibition is influenced by the level of difficulty in choice RT tasks, suggesting some connection with action selection processes. Yet, MEPs are also strongly suppressed during simple RT tasks, indicating that motor inhibition can occur even in the absence of competition.
Interestingly, to date, no relationship has been observed between the magnitude of MEP suppression and behavioral measures such as RT or accuracy. The absence of such relationships is puzzling given the current roles attributed to preparatory inhibition.
MEPs represent global readouts of corticospinal excitability: they reflect the sum effects of facilitatory and inhibitory influences that operate through several circuits at the cortical or spinal level (see Box 1). The aggregate nature of MEPs may represent a limitation when it comes to comparing motor inhibition between different task settings. For this reason, research aimed at investigating the impact of different task demands on motor inhibition should focus on methods that can selectively track inhibitory influences; for example, paired pulse TMS techniques have proven useful for quantifying the strength of intracortical and transcortical inhibitory pathways. Alternatively, further work could focus on MEPs evoked in task-irrelevant muscles. The latter are less influenced by excitatory processes related to action preparation and seem to display changes that are mostly inhibitory, consistent with the rather broad nature of motor inhibition. These approaches should help answer many questions regarding the function(s) of preparatory inhibition and address connections with inhibitory processes observed in action stopping tasks.
An intriguing question is how inhibitory effects associated with response initiation relate to proactive inhibition. Both processes are thought to be recruited in anticipation of behavior and to serve a braking function. Yet, very different predictions can be made in terms of MEP changes based on the way these two functions have been defined. Proactive inhibition is thought to be strongest when the probability of a stop is greatest. In contrast, inhibition associated with the regulation of response initiation is hypothesized to be strongest when a planned action is close to being released. Future studies are required to better understand how these two functions are represented, implemented and interact during action preparation.
In addition to somatotopy, the motor cortex has also been mapped in terms of ethologically meaningful actions. Inhibitory mechanisms may operate at multiple scales (body map, action maps, upper limb posture, hand spatial location) within the motor system with a variety of functional outcomes. Some principles may hold across multiple levels, whereas others may only take effect at one level. Inhibition at both the muscle specific and action representation levels might equally serve to stop a selected action, control an impulse, modulate gain, etc. On the other hand, the functional significance of inhibition at the level of a somatotopic representation may not hold for representations at other levels of an action hierarchy.
Future work should aim at understanding how disorders associated with impairments in inhibitory control may be related to inhibitory mechanisms underlying action preparation and action stopping. Identifying such relationships could provide novel insight into the neural mechanisms associated with these disorders, as well as provide biological markers to identify individuals “at-risk” of developing addictive behaviors (alcohol, drug, gambling, etc.) or of relapse. Physiological measures of inhibition might also help assess the efficacy of interventions aimed at enhancing inhibitory control. This is especially important early in life given that self-control ability measured in childhood is predictive of health and socioeconomic status during adulthood
Neural substrates of preparatory inhibition
Very little is known about the neural circuitry underlying preparatory inhibition. Current interrogations are designed to identify the source(s) and target(s) of this form of inhibition. Whereas some models favor cortical loci for preparatory inhibition, other models consider both cortical and spinal contributions (see Fig. 4). It is unknown whether preparatory inhibition involves loops through the basal ganglia, networks that have been highlighted in models of motor inhibition in the context of action stopping.
Paired-pulse TMS techniques can be used to investigate interactions between specific areas in the frontal lobe and corticospinal neurons during action preparation (see Box 1). Potential sources of motor inhibition are the lateral prefrontal cortex, the supplementary motor area and the dorsal premotor cortex. These protocols can also be useful for exploring the role of interhemispheric interactions in preparatory inhibition. A conditioning TMS pulse over one hemisphere at least 5 ms prior to a test TMS pulse over the opposite hemisphere results in suppressed MEP amplitudes. Differences in the level of inhibition observed when a non-selected response is homologous vs. non-homologous to the selected response may reflect special interhemispheric inhibitory processes. These interhemispheric interactions should also be studied in conditions in which the probed muscles are task irrelevant.
Surround inhibition, a mechanism for sharpening local neural representations, has received little attention in terms of a mechanism involved in preparatory inhibition. In the motor system, surround inhibition is hypothesized to suppress neighboring response representations during the selection of actions. It is possible that some of the differences observed between selected and non-selected effectors may reflect aspects of surround inhibition, particularly if the responses are represented in adjacent neural populations. To test this hypothesis, it will be important to explore the patterns of inhibition for selected and non-selected muscles as a function of cortical distance (i.e., with respect to distance within the cortical homunculus). This research may also help to address questions concerning the source of inhibitory signals, since specific cortical and subcortical mechanisms have been associated with patterns of surround inhibition.
Numerous studies have examined the performance of individuals with basal ganglia dysfunction in stop-signal tasks. To date, few studies have recruited these individuals on tests of preparatory inhibition. Future studies could compare preparatory inhibition according to whether Parkinson patients are OFF or ON medication (dopamine replacement therapy). Other experiments could focus on the impact of STN deep brain stimulation on motor inhibitory influences during action preparation.
Preparatory inhibition to assist action selection
The hypothesis that preparatory inhibition serves to assist action selection was motivated, in part, by early TMS studies showing consistent MEP suppression in non-selected effectors during choice RT tasks [74, 80, 90]. This motor inhibition was attributed to a competitive process, whereby non-selected action representations are suppressed, facilitating the selection of the desired response [2, 81]. The operation of such an inhibitory process, often called “inhibition for competition resolution”, is consistent with decision-making models when considered in the context of action selection [91]. That is, competition resolution can help ensure a winner-take-all outcome, where the action that “wins” is executed. While some models posit the competition as an independent race between response alternatives [92], other models posit, at least implicitly, competitive interactions between the alternative response options [93]. That is, each candidate not only accrues supporting evidence but also inhibits the other options [7, 94].
The competition resolution idea associates preparatory inhibition with reciprocal interactions between competing effectors, inducing a progressive inhibition of non-selected action representations (see Fig. 4, upper leftward panel) [2, 80, 81]. One prediction that follows from this hypothesis is that preparatory inhibition should only be observed in competing effectors, leaving the other muscle representations unaffected during action selection. For example, if the choice is between a left or right index finger response, a cue indicating a left response should result in inhibition of the (non-selected) right index finger, but not of other finger, arm or leg muscles (see Fig. 4, lower leftward panel). However, inhibition is reliably observed in task-irrelevant muscle representations, either during a delay period [13, 86, 95, 96] or during a pre-movement period [78]. Thus, preparatory inhibition is not limited to non-selected effectors, but extends to task-irrelevant motor representations.
Moreover, as noted above, inhibition is also observed in the effector that will be used in the forthcoming response, that is, in the effector that will win the competition. For instructed-delay tasks, this effect is most pronounced just prior to the go signal [18]; for no-delay tasks, this inhibition is evident as a brief transient reduction in MEPs just after the onset of the go signal [90]. These findings, in combination with the consistent picture of inhibition in task-irrelevant muscles, present a major challenge to a model in which preparatory inhibition is assumed to result from reciprocal inhibitory interactions between alternative responses competing for selection. Rather, action preparation seems to entail a broadly-tuned inhibition of the motor output system, irrespective of the effector(s) involved in the action that is being prepared [13, 78, 86, 95].
What may be the purpose of broadly-tuned inhibition during action preparation? One way to address this question is to consider the constraints on preparatory inhibition. The level of complexity of a prepared response influences the degree of inhibition [95] such that MEP amplitudes are more strongly suppressed when participants prepare a response requiring coordination between effectors compared to when the action involves repetitive movements with a single effector. Moreover, in delayed response tasks, the amount of MEP suppression depends on the anatomical and/or functional relationship between the competing effectors [17]. The suppression of MEPs (as evaluated in non-selected effectors) is more pronounced when the response set includes two hand movements (e.g., right vs left index finger) compared to when the set includes hand and foot responses (e.g., right index vs left ankle).
The strength of preparatory inhibition also increases with the risk of selecting an inappropriate response. This may arise because of incongruent sensory information [97, 98] or because a non-selected response is prepotent [99]. More generally, mechanisms generating broad motor inhibition may serve to regulate the trade-off between speed and accuracy [100]: When the emphasis is on accuracy, inhibition might be used to raise the selection threshold. Converging lines of evidence implicate the STN in a threshold setting process [11, 12, 101]. In a manner similar to how this structure can shut down the motor system to abort a planned movement, it could also modulate the threshold required to select and initiate a movement (e.g., low threshold to favor speed over accuracy) [102–104]. While these predictions have not been tested with TMS probes of corticospinal excitability, there is evidence that low-frequency oscillatory activity associated with the STN is modulated as a function of whether task instructions emphasize speed or accuracy [105].
Taken together, these findings suggest that inhibition may assist action selection, even if the mechanism is not through reciprocal interactions between competing movement representations, but rather, as a result of broad inhibitory signals. These broad signals would provide a way to modulate response selection processes to fit the task context; for example, greater inhibition would be required when the response is complex to ensure adequate preparation or to avoid making prepotent responses.
Multiple mechanisms of preparatory inhibition
Studies using a delayed response task to examine preparatory inhibition have often observed that MEP suppression is stronger in the selected effector compared to non-selected effectors. This result is difficult to reconcile with models relating preparatory inhibition exclusively to action selection. Accordingly, it has led to the hypothesis that the representation of selected effectors is targeted by a separate inhibitory mechanism (see Fig. 4, middle panel). That is, action preparation may engage two inhibitory processes, one producing broad suppression of the motor system to assist action selection, and a second suppressing neural activity of the selected effector [14, 88, 106]. The latter, often called “inhibition for impulse control”, would provide a mechanism to allow preparatory processes to unfold without the engagement of the peripheral motor system [107] (see also [108]). That is, excitatory processes could operate in cortical regions to prepare selected effectors for a forthcoming movement, with inhibition recruited to prevent the release of actual movements until the appropriate time has been reached to initiate the response.
This dual-process hypothesis is motivated, in part, by the observation that MEPs elicited from the agonist of the selected effector are attenuated even though the cortical representation of the movement is showing an increase in activity. This increase is, of course, the classic effect observed in neurophysiological studies with non-human primates. Indeed, activation during response preparation allows the forthcoming response to be decoded from the activity of many cortical and subcortical areas of the motor pathway during delay periods [109–111]. Correspondingly, paired-pulse TMS protocols reveal local increases in cortical excitability in human M1: Intracortical inhibition is attenuated and intracortical facilitation is enhanced during preparatory periods, even though the overall excitability state of the corticospinal pathway associated with that response is suppressed [84, 112, 113]. Thus, the MEP suppression observed in selected effectors occurs at a time when the activity is actually increasing in the involved motor cortex.
This dissociation could come about because of non-linear transformations in patterns of motor cortex activity; for example, it has been proposed that motor preparation and motor execution are represented along linked, orthogonal dimensions, a solution that could prevent premature movement [114–117]. Alternatively, the corticospinal suppression observed with TMS may originate in cortical regions that bypass M1 or arise from neural loci downstream from M1 [118]. Consistent with this hypothesis, the H-reflex response, a probe of spinal cord excitability [88], is diminished when elicited in the agonist muscle during the delay period, with the effect strongest right around the expected time of the go signal onset [83, 119] (but see [18]). Importantly, this suppression of the H-reflex is observed for selected but not for non-selected effectors (see also [80] for pre-movement recordings of H-reflexes), consistent with the view that representations of the selected effector are targeted by a specific inhibitory form.
Further evidence in favor of a dual-process model comes from a study in which we combined short trains of repetitive TMS pulses (10 Hz, 5 pulses) over dorsal premotor cortex (PMd) or lateral prefrontal cortex (LPF) and single-pulse TMS over M1, asking how these perturbations affect preparatory inhibition during the instructed-delay of a choice RT task [14]. rTMS over LPF attenuated inhibition in both the selected and non-selected effectors, suggesting a role of this area in a process associated with broad inhibition of the motor system. This inhibition could come about via transcortical fibers projecting from LPF to M1. Alternatively, this process may involve the basal ganglia and, in particular, the indirect pathway looping through the STN [11, 52, 61, 105, 120].
In contrast to the LPF results, transient disruption of PMd produced a more focal effect, releasing inhibition in only the selected effector. It has recently been proposed that inhibitory processes in PMd are recruited in parallel with increasing preparatory activity [121]. That is, PMd may not only help to specify the selected movement [122, 123] but may also generate inhibitory signals targeted at structures downstream of M1, to prevent premature movements [124]. For example, PMd may modulate spinal cord excitability through corticospinal projections originating in PMd and targeting spinal interneurons [125],[126]. A similar function could be achieved via PMd modulation of subcortical regions, such as the basal ganglia [127] or via brainstem cells projecting onto interneurons located in the intermediate zone of the spinal cord [128] that are involved in the control of distal hand muscles [107, 129].
As noted previously, inhibition is also observed in standard (no-delay) RT tasks where the cue not only specifies the movement, but also serves as the go signal. MEPs are suppressed shortly after the go signal (see Fig. 3A) and this effect is evident for both selected and non-selected effectors [77, 78]. Given uncertainty right after the onset of the go signal, such a drop in corticospinal excitability may be due to the fact that all response options accrue some excitation and at the same time trigger linked inhibitory processes to avoid premature responding at this initial preparatory stage. Alternatively, this effect could be due to a mechanism producing broad inhibition of the motor system, similar to that observed during instructed-delays. Consistent with this hypothesis, task-irrelevant motor representations are also suppressed immediately after the go signal [78]. Hence, both task-relevant and task-irrelevant effectors exhibit an attenuation in corticospinal excitability during the pre-movement period. Whether this inhibition is fully generic or also includes a focal component is not known.
In summary, within the framework of a dual-process model, motor inhibition is important, not only to assist action selection, but also to prevent premature movement [84, 88]. The latter initiation regulation process would be particularly important in delayed response tasks in which the implementation of a specified response must be withheld until a go signal. More generally, a downstream braking process would offer a mechanism that prevents premature movement during motor preparation.
Preparatory inhibition to modulate the gain of the motor system
Recent work has shown that preparatory inhibition is also observed in the absence of a choice. That is, in simple RT tasks, MEPs are suppressed in both the specified effector and task-irrelevant effectors [13]. These results have led to an alternative perspective in which preparatory inhibition is hypothesized to increase the signal-to-noise ratio within the motor system. By inhibiting the motor system, excitatory inputs will better stand out against a quiescent background. In essence, preparatory inhibition may modulate the gain of the system during action preparation. A primitive gain-modulation mechanism has already been well characterized in the leech motor system [130]. A similar mechanism may be conserved in mammals (see ‘Neural substrates of preparatory inhibition’ in Outstanding Questions Box).
As mentioned above, MEP suppression is usually stronger in the selected effector compared to non-selected effectors. This result was one of the findings which motivated the dual-process model, with the selected effector targeted for focal inhibition to prevent premature responding. However, the gain modulation hypothesis suggests an alternative account of this phenomenon given the assumption that preparatory inhibition is directed at (or recruited by) the representation of the selected muscle. Greenhouse et al. [13] offer a spotlight metaphor for gain control (see Fig. 4, right panel), building on the idea that a spotlight can be described in terms of its position and extent. Centering the spotlight on the representation of the selected response would enhance the sensitivity of excitatory inputs for this action (see Fig. 4, right panel); thus, inhibition is greatest for the selected effector.
Inhibition of non-selected, or even task-irrelevant effectors, reflects the extent of the spotlight, arising from the spillover of targeted inhibition onto neighboring motor representations. Notably, leg muscle representations are not inhibited during preparation of finger responses and vice versa [96]. Hence, there seems to be some degree of restriction in the extent of the spotlight, with inhibition only concerning representations that are related either anatomically or functionally. Moreover, the extent of the spotlight may be modulated by task demands. For example, in the context of a choice, the aperture of the inhibitory spotlight might be narrow in order to produce a sharp gradient given the risk of incorrect choices. In contrast, the spotlight could be wider in simple RT tasks.
While the idea that inhibition might be used to facilitate gain is not novel, the operation of a tuned mechanism within the motor system raises several interesting questions. For example, it is unclear how the tuning may be affected by factors such as the relationship, either anatomical or functional, between selected and other effectors. In addition, the gain modulation spotlight model does not account for the local increase in cortical excitability or the suppression of H-reflexes associated with a selected response. Nonetheless, the spotlight model underscores the important point that one must be cautious in inferring a mapping between physiology and function: Inhibition of a physiological measurement (i.e., MEP suppression) need not correspond to inhibition in terms of function. The spotlight idea shifts the emphasis away from inhibition as a way to suppress unwanted or non-selected movements, to one in which inhibition promotes rapid action preparation and implementation.
Shared motor inhibition for action preparation and action stopping
Intriguingly, both action preparation and action stopping appear to recruit processes that can produce inhibition that is either focal or broad, depending on task demands. In the context of action stopping, the influence of these two inhibitory forms seems to depend on whether the emphasis is on speed or selectivity of stopping, respectively. During action preparation, the contribution of these inhibitory processes may also vary according to the complexity of the task and to whether or not a response must be withheld across a delay period. These similarities raise the question: Are overlapping mechanisms responsible for motor inhibition in action preparation and action stopping? What evidence do we have (or not have) that common mechanisms may be responsible for motor inhibition in these two contexts?
Although appealing, some reports in the literature are not completely consistent with the idea of a common mechanism. First, reactive stopping seems to have a broader influence on motor activity than action preparation. For instance, reactive stopping of finger responses inhibits irrelevant finger but also leg muscle representations. In contrast, preparing a finger response induces inhibition of irrelevant finger representations but not leg muscles [96]. Second, reactive stopping has been associated with increased intracortical inhibition [43, 131] whereas intracortical inhibition is released for selected effectors during action preparation [84, 112]. Third, the inferior prefrontal cortex, often implicated in action stopping, is not typically active during action preparation, suggesting that it may not be involved in preparatory inhibition.
There are also important differences in the conceptualization of motor inhibition in these two contexts. Namely, whereas inhibition during stopping is thought to serve the sole purpose of suppressing the motor system output, current theories of action preparation shift the emphasis away from inhibition as a way to suppress unwanted movements (i.e. competition resolution idea) to one in which inhibition promotes rapid action selection and implementation (i.e. gain modulation idea). Nevertheless, overlapping inhibitory mechanisms may be engaged, and future investigations will be helpful in disentangling the processes underlying inhibition during action stopping and action preparation.
Concluding Remarks
Prominent signatures of inhibition are observed from probes of corticospinal excitability during human motor behavior. In some conditions, these inhibitory effects are focal, limited to task-relevant motor representations. However, in many conditions, the inhibitory effects are broad, evident in task irrelevant muscles. The broadest effect is found when an ongoing action must be rapidly aborted; in this context, inhibition appears to be observed across the motor system. The widespread nature of this form of motor inhibition has been associated with the STN, a part of the basal ganglia thought to operate in a non-specific manner. To date, the role of the STN in motor inhibition has been largely examined in the context of action stopping; its contribution to corticospinal inhibition during action preparation has not been explored, representing an interesting question for future studies (see Outstanding Questions Box). Indirect evidences suggest that the STN may generate motor inhibition to set the threshold for action selection: the deeper the inhibition, the higher the threshold [11, 61]. Whereas motor inhibition during action stopping can be easily related to behavioral control, the behavioral significance of preparatory inhibition remains unclear and may reflect the interaction of multiple mechanisms. Several hypotheses have been proposed including a potential role in competition resolution, initiation regulation and gain modulation. Future work is required, not only to evaluate these hypotheses, but also to explore the relationship between preparatory, proactive, and reactive motor inhibition in terms of functional hypotheses and neural mechanisms.
Trends Box.
Many aspects of behavior result in inhibition of the corticospinal motor output pathway.
The state of excitability of the corticospinal pathway can be assessed with single-pulse TMS over M1. The pulse elicits a temporally-precise motor evoked potential (MEP) in the EMG recording from the targeted muscle. To measure the dynamics of excitability, MEPs are measured at various stages of task performance and compared in amplitude to MEPs measured at baseline (e.g., during the inter-trial interval). Inhibition is evident when the MEPs are lower relative to baseline.
Motor inhibition is found when an ongoing or planned action needs to be aborted following a stop signal (reactive inhibition). In this context, behavioral inhibition is associated with a fast and global decrease in corticospinal excitability. This reactive inhibition is thought to rely on cortico-basal ganglia loops via hyper-direct projections from the frontal cortex to the STN, providing a mechanism to generically brake motor output.
Inhibition of the motor system is also evident in anticipation of a stop signal. Proactive inhibition has been characterized using selective stop tasks, where only part of an ongoing action needs to be interrupted. In this context, inhibition operates in a more focal manner, raising the hypothesis that separate basal ganglia pathways may be recruited during behavioral inhibition, exerting broad or focal inhibitory influences depending on task demands.
Several markers of motor inhibition can be observed during the period preceding a voluntary movement (preparatory inhibition). These markers are modulated by various task variables, suggesting a role for inhibition in response selection and response initiation.
The functional role of preparatory inhibition has been the subject of considerable debate. One hypothesis is that preparatory inhibition serves to assist action selection through a competitive process whereby excitation of selected action representations is associated with the suppression of unwanted (inappropriate) action representations. Another hypothesis has focused on the regulation of response initiation, with inhibition serving to prevent premature movement while preparatory activity unfolds across the cortex. A third view is that preparatory inhibition may serve to modulate the gain of the motor system. A reduction in background motor activity could facilitate movement onset by increasing the signal-to-noise ratio. This last hypothesis shifts the emphasis away from inhibition as a way to suppress unwanted or non-selected movements, to one in which preparatory inhibition promotes rapid action selection and implementation.
The relationship in terms of psychological function and neural mechanisms between reactive, proactive, and preparatory inhibition is an important challenge for future research.
Acknowledgments
JD was supported by grants from the “Fonds Spéciaux de Recherche” (FSR) of the Université Catholique de Louvain, the Belgian National Funds for Scientific Research (FRS-FNRS: MIS F.4512.14) and the “Fondation Médicale Reine Elisabeth” (FMRE). RBI was supported by grants from the National Institute of Health (NS097480, NS074917, NS092079). We are thankful to Julien Grandjean and Emmanuelle Wilhelm for their valuable comments on an earlier version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luna B, et al. An integrative model of the maturation of cognitive control. Annu Rev Neurosci. 2015;38:151–70. doi: 10.1146/annurev-neuro-071714-034054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bestmann S, Duque J. Transcranial Magnetic Stimulation: Decomposing the Processes Underlying Action Preparation. Neuroscientist. 2015 doi: 10.1177/1073858415592594. [DOI] [PubMed] [Google Scholar]
- 3.Jahanshahi M, et al. Parkinson’s disease, the subthalamic nucleus, inhibition, and impulsivity. Mov Disord. 2015;30(2):128–40. doi: 10.1002/mds.26049. [DOI] [PubMed] [Google Scholar]
- 4.Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16(1):43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartholdy S, et al. A systematic review of the relationship between eating, weight and inhibitory control using the stop signal task. Neurosci Biobehav Rev. 2016;64:35–62. doi: 10.1016/j.neubiorev.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Majid DS, et al. Proactive selective response suppression is implemented via the basal ganglia. J Neurosci. 2013;33(33):13259–69. doi: 10.1523/JNEUROSCI.5651-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seeley TD, et al. Stop signals provide cross inhibition in collective decision-making by honeybee swarms. Science. 2012;335(6064):108–11. doi: 10.1126/science.1210361. [DOI] [PubMed] [Google Scholar]
- 8.Wessel JR, et al. Stop-related subthalamic beta activity indexes global motor suppression in Parkinson’s disease. Mov Disord. 2016 doi: 10.1002/mds.26732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai W, et al. The role of the right presupplementary motor area in stopping action: two studies with event-related transcranial magnetic stimulation. J Neurophysiol. 2012;108(2):380–9. doi: 10.1152/jn.00132.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenemans JL. Specific proactive and generic reactive inhibition. Neurosci Biobehav Rev. 2015;56:115–26. doi: 10.1016/j.neubiorev.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Aron AR, et al. Frontosubthalamic Circuits for Control of Action and Cognition. J Neurosci. 2016;36(45):11489–11495. doi: 10.1523/JNEUROSCI.2348-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wessel JR, Aron AR. On the Globality of Motor Suppression: Unexpected Events and Their Influence on Behavior and Cognition. Neuron. 2017;93(2):259–280. doi: 10.1016/j.neuron.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenhouse I, et al. Nonspecific Inhibition of the Motor System during Response Preparation. J Neurosci. 2015;35(30):10675–84. doi: 10.1523/JNEUROSCI.1436-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duque J, et al. Dissociating the role of prefrontal and premotor cortices in controlling inhibitory mechanisms during motor preparation. J Neurosci. 2012;32(3):806–816. doi: 10.1523/JNEUROSCI.4299-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Campen AD, et al. TMS over M1 reveals expression and selective suppression of conflicting action impulses. J Cogn Neurosci. 2014;26(1):1–15. doi: 10.1162/jocn_a_00482. [DOI] [PubMed] [Google Scholar]
- 16.Duque J, et al. Top-Down Inhibitory Control Exerted by the Medial Frontal Cortex during Action Selection under Conflict. J Cogn Neurosci. 2013 doi: 10.1162/jocn_a_00421. [DOI] [PubMed] [Google Scholar]
- 17.Labruna L, et al. Generic inhibition of the selected movement and constrained inhibition of nonselected movements during response preparation. J Cogn Neurosci. 2014;26(2):269–78. doi: 10.1162/jocn_a_00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebon F, et al. Influence of Delay Period Duration on Inhibitory Processes for Response Preparation. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bestmann S, Krakauer JW. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp Brain Res. 2015;233(3):679–89. doi: 10.1007/s00221-014-4183-7. [DOI] [PubMed] [Google Scholar]
- 20.Klein PA, et al. Influence of Reward on Corticospinal Excitability during Movement Preparation. J Neurosci. 2012;32(50):18124–36. doi: 10.1523/JNEUROSCI.1701-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cos I, et al. Rapid prediction of biomechanical costs during action decisions. Journal of Neurophysiology. 2014;112(6):11. doi: 10.1152/jn.00147.2014. [DOI] [PubMed] [Google Scholar]
- 22.Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69(12):e55–68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isoda M, Hikosaka O. Cortico-basal ganglia mechanisms for overcoming innate, habitual and motivational behaviors. Eur J Neurosci. 2011;33(11):2058–69. doi: 10.1111/j.1460-9568.2011.07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munakata Y, et al. A unified framework for inhibitory control. Trends Cogn Sci. 2011;15(10):453–9. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Logan GD, et al. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10(2):276–91. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 26.Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends Cogn Sci. 2008;12(11):418–24. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elchlepp H, et al. Proactive inhibitory control: A general biasing account. Cogn Psychol. 2016;86:27–61. doi: 10.1016/j.cogpsych.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrence NS, et al. Stopping to food can reduce intake. Effects of stimulus-specificity and individual differences in dietary restraint. Appetite. 2015;85:91–103. doi: 10.1016/j.appet.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verbruggen F, et al. The inhibitory control reflex. Neuropsychologia. 2014;65:263–78. doi: 10.1016/j.neuropsychologia.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Stevens T, et al. How does response inhibition influence decision making when gambling? J Exp Psychol Appl. 2015;21(1):15–36. doi: 10.1037/xap0000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berkman ET, et al. Training-induced changes in inhibitory control network activity. J Neurosci. 2014;34(1):149–57. doi: 10.1523/JNEUROSCI.3564-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerasa A, et al. The motor inhibition system in Parkinson’s disease with levodopa-induced dyskinesias. Mov Disord. 2015;30(14):1912–20. doi: 10.1002/mds.26378. [DOI] [PubMed] [Google Scholar]
- 33.Hughes ME, et al. Stop-signal response inhibition in schizophrenia: behavioural, event-related potential and functional neuroimaging data. Biol Psychol. 2012;89(1):220–31. doi: 10.1016/j.biopsycho.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Massat I, et al. Hyperactivity in motor response inhibition networks in unmedicated children with attention deficit-hyperactivity disorder. World J Biol Psychiatry. 2016:1–11. doi: 10.1080/15622975.2016.1237040. [DOI] [PubMed] [Google Scholar]
- 35.Elton A, et al. Neural network activation during a stop-signal task discriminates cocaine-dependent from non-drug-abusing men. Addict Biol. 2014;19(3):427–38. doi: 10.1111/adb.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kreusch F, et al. Alcohol-cue exposure decreases response inhibition towards alcohol-related stimuli in detoxified alcohol-dependent patients. Psychiatry Res. 2017;249:232–239. doi: 10.1016/j.psychres.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Logan GD. On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm. In: Carr DDTH, editor. Inhibitory processes in attention, memory, and language. Academic Press; 1994. pp. 189–239. [Google Scholar]
- 38.Verbruggen F, et al. Fictitious inhibitory differences: how skewness and slowing distort the estimation of stopping latencies. Psychol Sci. 2013;24(3):352–62. doi: 10.1177/0956797612457390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van den Wildenberg WP, et al. Mechanisms and dynamics of cortical motor inhibition in the stop-signal paradigm: a TMS study. J Cogn Neurosci. 2010;22(2):225–39. doi: 10.1162/jocn.2009.21248. [DOI] [PubMed] [Google Scholar]
- 40.Greenhouse I, et al. Stopping a response has global or nonglobal effects on the motor system depending on preparation. J Neurophysiol. 2012;107(1):384–92. doi: 10.1152/jn.00704.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wessel JR, et al. Saccade suppression exerts global effects on the motor system. J Neurophysiol. 2013;110(4):883–90. doi: 10.1152/jn.00229.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majid DS, et al. Transcranial magnetic stimulation reveals dissociable mechanisms for global versus selective corticomotor suppression underlying the stopping of action. Cereb Cortex. 2012;22(2):363–71. doi: 10.1093/cercor/bhr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coxon JP, et al. Intracortical inhibition during volitional inhibition of prepared action. J Neurophysiol. 2006;95(6):3371–83. doi: 10.1152/jn.01334.2005. [DOI] [PubMed] [Google Scholar]
- 44.Swann N, et al. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci. 2009;29(40):12675–85. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt R, et al. Canceling actions involves a race between basal ganglia pathways. Nat Neurosci. 2013;16(8):1118–24. doi: 10.1038/nn.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mallet N, et al. Arkypallidal Cells Send a Stop Signal to Striatum. Neuron. 2016;89(2):308–16. doi: 10.1016/j.neuron.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leventhal DK, et al. Basal ganglia beta oscillations accompany cue utilization. Neuron. 2012;73(3):523–36. doi: 10.1016/j.neuron.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schall JD, Godlove DC. Current advances and pressing problems in studies of stopping. Curr Opin Neurobiol. 2012 doi: 10.1016/j.conb.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aron AR, et al. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18(4):177–85. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Wiecki TV, Frank MJ. A computational model of inhibitory control in frontal cortex and basal ganglia. Psychol Rev. 2013;120(2):329–55. doi: 10.1037/a0031542. [DOI] [PubMed] [Google Scholar]
- 51.Swann N, et al. Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: a scalp EEG study in Parkinson’s disease. J Neurosci. 2011;31(15):5721–9. doi: 10.1523/JNEUROSCI.6135-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haynes WI, Haber SN. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for Basal Ganglia models and deep brain stimulation. J Neurosci. 2013;33(11):4804–14. doi: 10.1523/JNEUROSCI.4674-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benis D, et al. Subthalamic nucleus activity dissociates proactive and reactive inhibition in patients with Parkinson’s disease. Neuroimage. 2014;91:273–81. doi: 10.1016/j.neuroimage.2013.10.070. [DOI] [PubMed] [Google Scholar]
- 54.Nauta HJ, Cole M. Efferent projections of the subthalamic nucleus: an autoradiographic study in monkey and cat. J Comp Neurol. 1978;180(1):1–16. doi: 10.1002/cne.901800102. [DOI] [PubMed] [Google Scholar]
- 55.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev. 1995;20(1):128–54. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 56.Kitai ST, Kita H. The Basal Ganglia II. Vol. 32. Boston, MA: Springer, Boston, MA; 1987. Anatomy and Physiology of the Subthalamic Nucleus: A Driving Force of the Basal Ganglia; pp. 357–373. [Google Scholar]
- 57.Hazrati LN, Parent A. Striatal and subthalamic afferents to the primate pallidum: interactions between two opposite chemospecific neuronal systems. Prog Brain Res. 1993;99:89–104. doi: 10.1016/s0079-6123(08)61340-0. [DOI] [PubMed] [Google Scholar]
- 58.Mayse JD, et al. Basal forebrain neuronal inhibition enables rapid behavioral stopping. Nat Neurosci. 2015;18(10):1501–8. doi: 10.1038/nn.4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nambu A, et al. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res. 2002;43(2):111–7. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 60.Cai W, et al. Stopping speech suppresses the task-irrelevant hand. Brain Lang. 2012;120(3):412–5. doi: 10.1016/j.bandl.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herz DM, et al. Neural Correlates of Decision Thresholds in the Human Subthalamic Nucleus. Curr Biol. 2016;26(7):916–20. doi: 10.1016/j.cub.2016.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aron AR, Verbruggen F. Stop the presses: dissociating a selective from a global mechanism for stopping. Psychol Sci. 2008;19(11):1146–53. doi: 10.1111/j.1467-9280.2008.02216.x. [DOI] [PubMed] [Google Scholar]
- 63.Macdonald HJ, et al. Uncoupling response inhibition. J Neurophysiol. 2012;108(5):1492–500. doi: 10.1152/jn.01184.2011. [DOI] [PubMed] [Google Scholar]
- 64.MacDonald HJ, et al. The fall and rise of corticomotor excitability with cancellation and reinitiation of prepared action. J Neurophysiol. 2014;112(11):2707–17. doi: 10.1152/jn.00366.2014. [DOI] [PubMed] [Google Scholar]
- 65.Xu J, et al. Selective inhibition of a multicomponent response can be achieved without cost. J Neurophysiol. 2015;113(2):455–65. doi: 10.1152/jn.00101.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mink JW. The Basal Ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol. 2003;60(10):1365–8. doi: 10.1001/archneur.60.10.1365. [DOI] [PubMed] [Google Scholar]
- 67.Nambu A. Seven problems on the basal ganglia. Curr Opin Neurobiol. 2008;18(6):595–604. doi: 10.1016/j.conb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Lo CC, et al. Proactive inhibitory control and attractor dynamics in countermanding action: a spiking neural circuit model. J Neurosci. 2009;29(28):9059–71. doi: 10.1523/JNEUROSCI.6164-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Claffey MP, et al. Having a goal to stop action is associated with advance control of specific motor representations. Neuropsychologia. 2010;48(2):541–8. doi: 10.1016/j.neuropsychologia.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cai W, et al. A proactive mechanism for selective suppression of response tendencies. J Neurosci. 2011;31(16):5965–9. doi: 10.1523/JNEUROSCI.6292-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greenhouse I, Wessel JR. EEG signatures associated with stopping are sensitive to preparation. Psychophysiology. 2013;50(9):900–8. doi: 10.1111/psyp.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leunissen I, et al. A proactive task set influences how response inhibition is implemented in the basal ganglia. Hum Brain Mapp. 2016 doi: 10.1002/hbm.23338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lavallee CF, et al. When holding your horses meets the deer in the headlights: time-frequency characteristics of global and selective stopping under conditions of proactive and reactive control. Front Hum Neurosci. 2014;8:994. doi: 10.3389/fnhum.2014.00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leocani L, et al. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123(Pt 6):1161–73. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- 75.Chen R, et al. Time course of corticospinal excitability in reaction time and self-paced movements. Ann Neurol. 1998;44(3):317–25. doi: 10.1002/ana.410440306. [DOI] [PubMed] [Google Scholar]
- 76.Reynolds C, Ashby P. Inhibition in the human motor cortex is reduced just before a voluntary contraction. Neurology. 1999;53(4):730–5. doi: 10.1212/wnl.53.4.730. [DOI] [PubMed] [Google Scholar]
- 77.Klein PA, et al. Comparison of the two cerebral hemispheres in inhibitory processes operative during movement preparation. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2015.10.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duque J, et al. Dissociating the influence of response selection and task anticipation on corticospinal suppression during response preparation. Neuropsychologia. 2014;65:287–96. doi: 10.1016/j.neuropsychologia.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tandonnet C, et al. Selective suppression of the incorrect response implementation in choice behavior assessed by transcranial magnetic stimulation. Psychophysiology. 2011;48(4):462–9. doi: 10.1111/j.1469-8986.2010.01121.x. [DOI] [PubMed] [Google Scholar]
- 80.Duque J, et al. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb Cortex. 2005;15(5):588–93. doi: 10.1093/cercor/bhh160. [DOI] [PubMed] [Google Scholar]
- 81.Burle B, et al. Physiological evidence for response inhibition in choice reaction time tasks. Brain Cogn. 2004;56(2):153–64. doi: 10.1016/j.bandc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 82.Hasbroucq T, et al. Preparatory inhibition of cortico-spinal excitability: a transcranial magnetic stimulation study in man. Brain Res Cogn Brain Res. 1997;5(3):185–92. doi: 10.1016/s0926-6410(96)00069-9. [DOI] [PubMed] [Google Scholar]
- 83.Touge T, et al. Reduced excitability of the cortico-spinal system during the warning period of a reaction time task. Electroencephalogr Clin Neurophysiol. 1998;109(6):489–95. doi: 10.1016/s0924-980x(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 84.Duque J, Ivry RB. Role of corticospinal suppression during motor preparation. Cereb Cortex. 2009;19(9):2013–24. doi: 10.1093/cercor/bhn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilhelm E, et al. A Double-Coil TMS Method to Assess Corticospinal Excitability Changes at a Near-Simultaneous Time in the Two Hands during Movement Preparation. Front Hum Neurosci. 2016;10:88. doi: 10.3389/fnhum.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Quoilin C, et al. Comparison of Motor Inhibition in Variants of the Instructed-Delay Choice Reaction Time Task. PLoS One. 2016;11(8):e0161964. doi: 10.1371/journal.pone.0161964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van den Wildenberg WP, et al. To head or to heed? Beyond the surface of selective action inhibition: a review. Front Hum Neurosci. 2010;4:222. doi: 10.3389/fnhum.2010.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Duque J, et al. Evidence for two concurrent inhibitory mechanisms during response preparation. J Neurosci. 2010;30(10):3793–802. doi: 10.1523/JNEUROSCI.5722-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat Rev Neurosci. 2012;13(1):51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klein PA, et al. Comparison of the two cerebral hemispheres in inhibitory processes operative during movement preparation. Neuroimage. 2016;125:220–32. doi: 10.1016/j.neuroimage.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ratcliff R, et al. Diffusion Decision Model: Current Issues and History. Trends Cogn Sci. 2016;20(4):260–81. doi: 10.1016/j.tics.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown SD, Heathcote A. The simplest complete model of choice response time: linear ballistic accumulation. Cogn Psychol. 2008;57(3):153–78. doi: 10.1016/j.cogpsych.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 93.Usher M, McClelland JL. The time course of perceptual choice: the leaky, competing accumulator model. Psychol Rev. 2001;108(3):550–92. doi: 10.1037/0033-295x.108.3.550. [DOI] [PubMed] [Google Scholar]
- 94.Coles MG, et al. A psychophysiological investigation of the continuous flow model of human information processing. J Exp Psychol Hum Percept Perform. 1985;11(5):529–53. doi: 10.1037//0096-1523.11.5.529. [DOI] [PubMed] [Google Scholar]
- 95.Greenhouse I, et al. Inhibition during response preparation is sensitive to response complexity. J Neurophysiol. 2015;113(7):2792–800. doi: 10.1152/jn.00999.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Labruna L, et al. Effector dependent modulation of inhibition in response preparation. Society for Neuroscience Abstract 2016 [Google Scholar]
- 97.Klein PA, et al. Top-down suppression of incompatible motor activations during response selection under conflict. Neuroimage. 2014;86:138–49. doi: 10.1016/j.neuroimage.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 98.Burle B, et al. Preventing (impulsive) errors: Electrophysiological evidence for online inhibitory control over incorrect responses. Psychophysiology. 2016;53(7):1008–19. doi: 10.1111/psyp.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meckler C, et al. Executive control and response expectancy: a Laplacian ERP study. Psychophysiology. 2011;48(3):303–11. doi: 10.1111/j.1469-8986.2010.01077.x. [DOI] [PubMed] [Google Scholar]
- 100.Bogacz R, et al. The neural basis of the speed-accuracy tradeoff. Trends Neurosci. 2010;33(1):10–6. doi: 10.1016/j.tins.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 101.Forstmann BU, et al. Cortico-striatal connections predict control over speed and accuracy in perceptual decision making. Proc Natl Acad Sci U S A. 2010;107(36):15916–20. doi: 10.1073/pnas.1004932107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frank MJ, et al. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318(5854):1309–12. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- 103.Cavanagh JF, et al. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci. 2011;14(11):1462–7. doi: 10.1038/nn.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yttri EA, Dudman JT. Opponent and bidirectional control of movement velocity in the basal ganglia. Nature. 2016;533(7603):402–6. doi: 10.1038/nature17639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Herz DM, et al. Distinct mechanisms mediate speed-accuracy adjustments in cortico-subthalamic networks. Elife. 2017:6. doi: 10.7554/eLife.21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quoilin C, Derosiere G. Global and Specific Motor Inhibitory Mechanisms during Action Preparation. J Neurosci. 2015;35(50):16297–9. doi: 10.1523/JNEUROSCI.3664-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cohen O, et al. Getting ready to move: transmitted information in the corticospinal pathway during preparation for movement. Curr Opin Neurobiol. 2010;20(6):696–703. doi: 10.1016/j.conb.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sinclair C, Hammond GR. Excitatory and inhibitory processes in primary motor cortex during the foreperiod of a warned reaction time task are unrelated to response expectancy. Exp Brain Res. 2009;194(1):103–13. doi: 10.1007/s00221-008-1684-2. [DOI] [PubMed] [Google Scholar]
- 109.Michaels JA, et al. Predicting Reaction Time from the Neural State Space of the Premotor and Parietal Grasping Network. J Neurosci. 2015;35(32):11415–32. doi: 10.1523/JNEUROSCI.1714-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cisek P, Kalaska JF. Neural correlates of reaching decisions in dorsal premotor cortex: specification of multiple direction choices and final selection of action. Neuron. 2005;45(5):801–14. doi: 10.1016/j.neuron.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 111.Thura D, Cisek P. Modulation of Premotor and Primary Motor Cortical Activity during Volitional Adjustments of Speed-Accuracy Trade-Offs. J Neurosci. 2016;36(3):938–56. doi: 10.1523/JNEUROSCI.2230-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davranche K, et al. The dual nature of time preparation: neural activation and suppression revealed by transcranial magnetic stimulation of the motor cortex. Eur J Neurosci. 2007;25(12):3766–74. doi: 10.1111/j.1460-9568.2007.05588.x. [DOI] [PubMed] [Google Scholar]
- 113.Tandonnet C, et al. Cortical activation during temporal preparation assessed by transcranial magnetic stimulation. Biol Psychol. 2010;85(3):481–6. doi: 10.1016/j.biopsycho.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 114.Elsayed GF, et al. Reorganization between preparatory and movement population responses in motor cortex. Nat Commun. 2016;7:13239. doi: 10.1038/ncomms13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Haith AM, et al. Independence of Movement Preparation and Movement Initiation. J Neurosci. 2016;36(10):3007–15. doi: 10.1523/JNEUROSCI.3245-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kaufman MT, et al. Cortical activity in the null space: permitting preparation without movement. Nat Neurosci. 2014;17(3):440–8. doi: 10.1038/nn.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sussillo D, et al. A neural network that finds a naturalistic solution for the production of muscle activity. Nat Neurosci. 2015;18(7):1025–33. doi: 10.1038/nn.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Prut Y, Fetz EE. Primate spinal interneurons show pre-movement instructed delay activity. Nature. 1999;401(6753):590–4. doi: 10.1038/44145. [DOI] [PubMed] [Google Scholar]
- 119.Hasbroucq T, et al. The time-course of preparatory spinal and cortico-spinal inhibition: an H-reflex and transcranial magnetic stimulation study in man. Exp Brain Res. 1999;124(1):33–41. doi: 10.1007/s002210050597. [DOI] [PubMed] [Google Scholar]
- 120.Zavala B, et al. Human Subthalamic Nucleus Theta and Beta Oscillations Entrain Neuronal Firing During Sensorimotor Conflict. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li N, et al. Robust neuronal dynamics in premotor cortex during motor planning. Nature. 2016;532(7600):459–64. doi: 10.1038/nature17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cavina-Pratesi C, et al. Dissociating arbitrary stimulus-response mapping from movement planning during preparatory period: evidence from event-related functional magnetic resonance imaging. J Neurosci. 2006;26(10):2704–13. doi: 10.1523/JNEUROSCI.3176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Terao Y, et al. Modifying the cortical processing for motor preparation by repetitive transcranial magnetic stimulation. J Cogn Neurosci. 2007;19(9):1556–73. doi: 10.1162/jocn.2007.19.9.1556. [DOI] [PubMed] [Google Scholar]
- 124.Kroeger J, et al. Charting the excitability of premotor to motor connections while withholding or initiating a selected movement. Eur J Neurosci. 2010;32(10):1771–9. doi: 10.1111/j.1460-9568.2010.07442.x. [DOI] [PubMed] [Google Scholar]
- 125.Dum RP, Strick PL. Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. J Neurosci. 2005;25(6):1375–86. doi: 10.1523/JNEUROSCI.3902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bizzi E, et al. New perspectives on spinal motor systems. Nat Rev Neurosci. 2000;1(2):101–8. doi: 10.1038/35039000. [DOI] [PubMed] [Google Scholar]
- 127.McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci. 2002;22(18):8117–32. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Riddle CN, et al. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci. 2009;29(15):4993–9. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Borra E, et al. Projections of the hand field of the macaque ventral premotor area F5 to the brainstem and spinal cord. J Comp Neurol. 2010;518(13):2570–91. doi: 10.1002/cne.22353. [DOI] [PubMed] [Google Scholar]
- 130.Baca SM, et al. Widespread inhibition proportional to excitation controls the gain of a leech behavioral circuit. Neuron. 2008;57(2):276–89. doi: 10.1016/j.neuron.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coxon JP, et al. Stop and go: the neural basis of selective movement prevention. J Cogn Neurosci. 2009;21(6):1193–203. doi: 10.1162/jocn.2009.21081. [DOI] [PubMed] [Google Scholar]
- 132.Barker AT, et al. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106–7. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 133.Di Lazzaro V, Rothwell JC. Corticospinal activity evoked and modulated by non-invasive stimulation of the intact human motor cortex. J Physiol. 2014;592(Pt 19):4115–28. doi: 10.1113/jphysiol.2014.274316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kujirai T, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–19. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Opie GM, et al. Task-related changes in intracortical inhibition assessed with paired- and triple-pulse transcranial magnetic stimulation. J Neurophysiol. 2014:jn 00651. doi: 10.1152/jn.00651.2014. [DOI] [PubMed] [Google Scholar]
- 136.Rogasch NC, et al. Cortical inhibition, excitation, and connectivity in schizophrenia: a review of insights from transcranial magnetic stimulation. Schizophr Bull. 2014;40(3):685–96. doi: 10.1093/schbul/sbt078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Neubert FX, et al. Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proc Natl Acad Sci U S A. 2010;107(30):13240–5. doi: 10.1073/pnas.1000674107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wischnewski M, et al. Demand on skillfulness modulates interhemispheric inhibition of motor cortices. J Neurophysiol. 2016:jn 01076. doi: 10.1152/jn.01076.2015. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Celnik P. Understanding and modulating motor learning with cerebellar stimulation. Cerebellum. 2015;14(2):171–4. doi: 10.1007/s12311-014-0607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fonken YM, et al. Frontal and motor cortex contributions to response inhibition: evidence from electrocorticography. J Neurophysiol. 2016;115(4):2224–36. doi: 10.1152/jn.00708.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Crone NE, et al. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121(Pt 12):2301–15. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- 142.Crone NE, et al. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain. 1998;121(Pt 12):2271–99. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- 143.Jasper H, Penfield W. Electrocorticograms in man: Effect of voluntary movement upon the electrical activity of the precentral gyrus. Archiv für Psychiatrie und Nervenkrankheiten. 1949;183(1):163–174. [Google Scholar]
- 144.Hall SD, et al. The role of GABAergic modulation in motor function related neuronal network activity. Neuroimage. 2011;56(3):1506–10. doi: 10.1016/j.neuroimage.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 145.Pfurtscheller G. Central beta rhythm during sensorimotor activities in man. Electroencephalogr Clin Neurophysiol. 1981;51(3):253–64. doi: 10.1016/0013-4694(81)90139-5. [DOI] [PubMed] [Google Scholar]
- 146.Huster RJ, et al. Electroencephalography of response inhibition tasks: functional networks and cognitive contributions. Int J Psychophysiol. 2013;87(3):217–33. doi: 10.1016/j.ijpsycho.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 147.Little S, Brown P. The functional role of beta oscillations in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(Suppl 1):S44–8. doi: 10.1016/S1353-8020(13)70013-0. [DOI] [PubMed] [Google Scholar]
- 148.van Wijk BC, et al. Subthalamic nucleus phase-amplitude coupling correlates with motor impairment in Parkinson’s disease. Clin Neurophysiol. 2016;127(4):2010–9. doi: 10.1016/j.clinph.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wessel JR, Aron AR. It’s not too late: the onset of the frontocentral P3 indexes successful response inhibition in the stop-signal paradigm. Psychophysiology. 2015;52(4):472–80. doi: 10.1111/psyp.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]