Abstract
Purpose
The aim of the present study was to evaluate the utility of the relatively hydrophilic Δ9-tetrahydrocannabinol (THC) prodrugs, mono and di-valine esters (THC-Val and THC-Val-Val) and the amino acid (valine)-dicarboxylic acid (hemisuccinate) ester (THC-Val-HS), with respect to ocular penetration and intraocular pressure (IOP) lowering activity. THC, timolol, and pilocarpine eye drops were used as controls.
Methods
THC-Val, THC-Val-Val, and THC-Val-HS were synthesized and chemically characterized. Aqueous solubility and in vitro transcorneal permeability of THC and the prodrugs, in the presence of various surfactants and cyclodextrins, were determined. Two formulations were evaluated for therapeutic activity in the α-chymotrypsin induced rabbit glaucoma model, and the results were compared against controls comprising of THC emulsion and marketed timolol maleate and pilocarpine eye drops.
Results
THC-Val-HS demonstrated markedly improved solubility (96-fold) and in vitro permeability compared to THC. Selected formulations containing THC-Val-HS effectively delivered THC to the anterior segment ocular tissues in the anesthetized rabbits: 62.1 ng/100 μL of aqueous humor (AH) and 51.4 ng/50 mg of iris ciliary bodies (IC) (total THC). The duration and extent of IOP lowering induced by THC-Val-HS was 1 hour longer and 10% greater, respectively, than that obtained with THC and was comparable with the pilocarpine eye drops. Timolol ophthalmic drops, however, exhibited a longer duration of activity. Both THC and THC-Val-HS were detected in the ocular tissues following multiple dosing of THC-Val-HS in conscious animals. The concentration of THC in the iris-ciliary bodies at the 60- and 120-minute time points (53 and 57.4 ng/50 mg) were significantly greater than that of THC-Val-HS (24.2 and 11.3 ng/50 mg). Moreover, at the two time points studied, the concentration of THC was observed to increase or stay relatively constant, whereas THC-Val-HS concentration decreased by at least 50%. A similar trend was observed in the retina-choroid tissues.
Conclusions
A combination of prodrug derivatization and formulation development approaches significantly improved the penetration of THC into the anterior segment of the eye following topical application. Enhanced ocular penetration resulted in significantly improved IOP-lowering activity.
Keywords: glaucoma, intraocular pressure, tetrahydrocannabinol, timolol, pilocarpine
Glaucoma is an ocular neuropathy that is characterized by progressive and irreversible loss of vision. It affects nearly 60 million people worldwide and is the second leading cause of blindness.1 Intraocular pressure (IOP) has been identified as an important risk factor in the pathogenesis of this disease.2 Elevated IOP leads to damage to retinal ganglion cell axons, which causes progressive and irreversible vision loss.3
Prostaglandin derivatives, parasympathomimetics, β blockers, and carbonic anhydrase inhibitors are the major drug classes that are used in the management of glaucoma.4,5 Prostaglandin derivatives, such as latanoprost and bimatoprost, increase the aqueous humor (AH) outflow through the uveoscleral pathway. Several mechanisms have been proposed to explain how prostaglandin derivatives reduce the IOP. Relaxation of the ciliary muscles, which causes the connective tissue-filled spaces in the trabecular meshwork to widen, is thought to be a factor that is involved in the initial drop in IOP.6 Prostaglandin analogs also induce the release of matrix metalloproteinase enzymes and cause the dissolution of types I and III collagen within the connective tissue-filled spaces.7 This process also leads to an increase in the AH outflow through the trabecular meshwork. Pilocarpine is a direct-acting cholinergic parasympathomimetic agent. It directly stimulates the muscarinic receptors and smooth muscles of the iris and secretory glands. Pilocarpine also contracts the ciliary muscle by activating type 3 muscarinic receptors through the Gq/11 signaling pathway. This releases Ca2+ ions into the sarcoplasm. These ions bind to troponin molecules in the thin filaments and cause the troponin molecules to change shape. These thin filaments are pulled toward the center of sarcomere and finally cause contraction of the ciliary muscle, which increases the AH outflow.8 The β blockers such as timolol and betaxolol reduce the production of AH by reducing the blood flow to the ciliary process,9 thereby lowering the IOP. Carbonic anhydrase inhibitors (brinzolamide and dorzolamide) reduce AH production by blocking the formation of bicarbonate ions.10 Bicarbonate ions are formed from carbon dioxide and OH− ions in the eye through carbonic anhydrase enzymes. These newly formed bicarbonate ions regulate the chloride ions, which in turn regulate the aqueous humor production. By blocking the formation of bicarbonate ions, carbonic anhydrase inhibitors reduce the AH production and further reduce the IOP. The ρ kinase inhibitors are new chemical agents that are currently in phase II trials. The ρ kinase effector molecules (ρ kinase ROCK I and II, which are expressed in trabecular meshwork [TM] and ciliary muscle cells) are serine/threonine kinases that regulate smooth muscle contraction. The ρ kinase inhibitors are selective ROCK inhibitors that increase aqueous humor drainage through the TM, which leads to a decrease in the IOP. The ρ kinase inhibitors also cause a decrease in myosin light chain phosphorylation, which leads to the changes in cell morphology and aqueous outflow. This leads to the widening of the spaces in the trabecular meshwork and increases in the endothelial cell vacuoles and the AH outflow.5
Regulation of IOP, however, is often not enough to prevent or arrest the development of glaucoma-related optic neuropathy.11–13 Several hypotheses exist, but the complete mechanism for retinal ganglion cell death has not yet been elucidated.14–16 New classes of agents that can reduce IOP as well as prevent or decrease the loss of retinal ganglion cell are urgently needed.
Δ9-Tetrahydrocannabinol (THC; Fig. 1A), an active ingredient of the plant cannabis sativa, is an agonist of the cannabinoid receptors CB1 and CB2 that could potentially be such a dual-acting, antiglaucoma agent.17–19 The presence of cannabinoid receptors in the ocular tissues have been recently confirmed,20 which supports the concept that a localized IOP-reducing mechanism, rather than the central nervous system, is involved. What makes THC especially attractive is that in addition to its IOP-lowering activity, THC has also demonstrated independent neuroprotective potential.21
Figure 1.
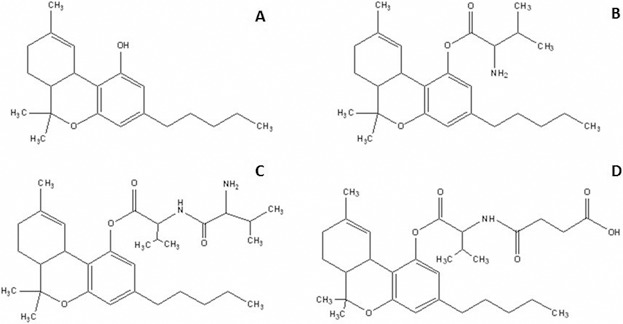
Chemical structures of (A) (THC), (B) THC-Val, (C) THC-Val-Val, and (D) THC-Val-HS.
In terms of the route of delivery, because glaucoma is a chronic disease, noninvasive topical application would be the most preferable means of drug delivery. The anatomy and physiology of the eye, however, present a formidable barrier to the intraocular delivery of drugs through the topical route. This is especially true for delivery to the back of the eye, where a drug is needed to provide neuroprotective activity. The combination of precorneal and corneal barriers as well as other physiological barriers results in only approximately 5% to 10% of a topically applied drug reaching the inner ocular tissues.22–24 The development of THC as an eye drop is especially challenging because of its poor aqueous solubility (1–2 μg/mL) and high logP (6.42).25 In view of the challenges in the topical delivery of THC, it is not surprising that there are discrepancies in the literature with respect to the efficacy of topically administered THC. Some reports have demonstrated IOP-lowering activities of THC in normotensive rabbits,26 ocular hypertensive human participants,27 rabbit glaucoma model,28 normotensive dogs,29 and rats,17 whereas several other studies showed no IOP-lowering activity in healthy human participants30 or models of glaucoma.31
A review of the literature revealed that in most of these earlier studies that evaluated the efficacy of THC administered through the topical route, the investigators used formulations that involved mineral oil or surfactant-based solutions or emulsions. Although these formulations improved the solubility of THC, they would not have altered its membrane-permeation characteristics. Based on the formulations used and the associated preclinical data, we hypothesized that by virtue of its lipophilic characteristics, THC would rapidly partition into the lipophilic corneal epithelia but would remain trapped there and fail to efficiently partition out into the more hydrophilic stromal layers, and thus would not reach the intraocular tissues.32 Therefore, we evaluated various THC prodrugs for the ability to demonstrate enhanced ocular tissue permeation characteristics compared to THC.
We previously synthesized and studied dicarboxylic acid prodrugs, the hemisuccinate (THC-HS) and the hemiglutarate (THC-HG) esters, with respect to improved ocular bioavailability.21 A significant improvement in aqueous solubility was observed as a result of the ionization of the molecules at physiological pH. Transcorneal permeability, however, remained low at a physiological pH. The use of ion pairs, which shielded the negative charge of THC-HG at physiological pH, significantly improved the permeability at pH 7.4. The topical administration of THC-HG in rabbits using these ion-pair formulations delivered THC to the tissues at the front of the eye, such as the aqueous humor and the iris-ciliary bodies. However, the THC levels were below the detection levels in the retina and vitreous humor.32
In contrast to the dicarboxylic acid prodrugs, the amino acid THC prodrugs, especially the valine ester, exhibited greater stability but low aqueous solubility at physiological pH.33 Therefore, we evaluated THC-Val (Fig. 1B) and THC-Val-Val (Fig. 1C) in this study. The ocular penetration and solubility of these compounds in the presence of surfactants and cyclodextrins were evaluated. However, as a result of poor aqueous solubility, the Val and Val-Val forms were not further evaluated. To overcome the disadvantages observed with the dicarboxylic acid esters and the solubility limitations of the amino acid prodrugs, a new amino acid–dicarboxylic acid prodrug, THC-Val-HS (Fig. 1D), was designed. The preformulation characteristics and ocular bioavailability as well as the IOP-lowering activity of the most promising compound were tested in the α-chymotrypsin–induced rabbit glaucoma model. The therapeutic activity was compared with that of the controls: THC, timolol maleate, and pilocaprine eye drops (the latter two of which are currently marketed ophthalmic formulations).
Materials and Methods
Materials
Hydroxypropyl methyl cellulose (4000 cps), 2-hydroxypropyl-β-cyclodextrin (HPβCD), randomly methylated-β-cyclodextrin (RMβCD), tyloxapol, and α-chymotrypsin (type II, lyophilized powder, ≥40 units/mg protein) were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). Super Refined Tween 80 was received as a gift sample from Croda, Inc. (Mill Hall, PA, USA). Samples of Pluronic F 68 (Poloxomer 188), Pluronic F 127 (Poloxomer 407), and Cremophor RH 40 were received as gifts from BASF (Florham Park, NJ, USA). Tocrisolve 100 was purchased from Tocris Biosciences (Minneapolis, MN, USA). Timolol maleate eye drops (0.25% w/v) and pilocarpine HCl eye drops (2% w/v) were currently marketed eye drops. All other chemicals and solvents were purchased from Fisher Scientific (St. Louis, MO, USA).
Buffer reagents used in the cannabinoid receptor binding studies were purchased from Sigma-Aldrich Corp. All radioligands and MicroScint were purchased from PerkinElmer (Waltham, MA, USA). Nonlabeled controls were purchased from Tocris Biosciences. The membrane preparation was made using a Tris-HCl buffer (50 nM Tris-HCl), pH 7.4. Dilutions of the membrane, radioligand, and control/test compounds were made in a Tris-EDTA buffer (50 mM Tris-HCl, 20 mM EDTA, 154 mM NaCl, and 0.2% fatty-acid free BSA), pH 7.4.
The in vitro transcorneal permeability studies were carried out using corneas from New Zealand albino rabbits (isolated from whole-eye globes), which were delivered overnight on wet ice in Hank's Balanced Salt Solution from Pel-Freez Biologicals (Rogers, AK, USA). The tissues were used on the day of receipt.32
Animals
Male New Zealand albino rabbits (2–2.5 kg) were procured from Harlan Laboratories (Indianapolis, IN, USA). All animal experiments conformed to the tenets of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and followed protocols approved by the University of Mississippi Institutional Animal Care and Use Committee (IACUC protocol 14-005 [IOP efficacy studies] and 13-004 and 14-022 [in vivo ocular bioavailability studies]).
Methods
Synthesis and Characterization of Δ9-Tetrahydrocannabinol Prodrugs.
THC-Val, THC-Val-Val, and THC-Val-HS were synthesized according to previously established protocols.33 The final products were purified using column chromatography and characterized by mass spectroscopy in the positive ionization mode. The identity and purity of the synthesized prodrugs were established by spectral means including 1H-NMR (proton nuclear magnetic resonance), 13C-NMR (carbon-13 nuclear magnetic resonance), and 2D-NMR (two-dimensional nuclear magnetic resonance spectroscopy). such as correlation spectroscopy, heteronuclear single-quantum correlation spectroscopy, and heteronuclear multiple-bond correlation spectroscopy as well as other spectroscopic means as reported earlier.33
Solubility and Stability Studies
The solubility was studied as a function of pH (3, 5, 7, and 9) using the standard shake-flask method in buffers prepared according to United States Pharmacopoeia 31 as well as in cyclodextrin and surfactant solutions in isotonic phosphate buffered solution (IPBS; pH 7.3) at 25 ± 2°C in a shaking water bath for 24 hours. The solution stability was studied in the presence of surfactants and cyclodextrins using solutions prepared as described for the solubility studies. The stability studies were carried out in glass vials maintained at 4°C, 25°C, and 40°C for a period of 2 weeks. Aliquots collected at predetermined time points were diluted in the mobile phase for analysis. The apparent degradation rate constants were calculated from the slopes of semilogarithmic plots of the percentage drug remaining versus time.
In Vitro Corneal Permeability Studies
Corneas from whole-eye globes were isolated according to procedures described earlier.32 The isolated corneas were placed between jacketed side-by-side diffusion cells (PermeGear, Inc., Hellertown, PA, USA) that were maintained at 34°C using a circulating water bath. The donor solution (3 mL) was added to the epithelial side of the cornea and 3.2 mL of 2.5% w/v HPβCD in IPBS was added to the receiver chamber. Aliquots (0.6 mL) were taken every 30 minutes for 3 hours and replaced with an equal volume of fresh buffer. The samples were analyzed by HPLC.
Stability of THC-Val-HS in Ocular Tissue Homogenates
Tissue Preparation.
The aqueous humor and vitreous humor were used as collected. The retina choroid and iris-ciliary bodies used in this study were homogenized in ice-cold IPBS in an ice bath using a TISSUEMISER (Fisher Scientific). The homogenates were then centrifuged at 17,000g at 4°C for 15 minutes. The protein contents of the supernatants were determined according to the method of Bradford34 and were adjusted to approximately 1 mg/mL.
Hydrolysis Procedure.
The tissue homogenates were equilibrated for 30 minutes at 37°C to activate the enzymes. To 1.9 mL of the supernatant, 100 μL of THC-Val-HS (2 mg/mL) in ethanol was added and mixed. Aliquots of 100 μL of the samples were withdrawn at specific time intervals up to 6 hours postinitiation. An equal volume of ice-cold acetonitrile was added to each sample to terminate the reaction, and the mixtures were centrifuged at 13,000 rpm for 15 minutes. The supernatants were collected and analyzed using HPLC-UV.
Analytical Method for In Vitro Studies.
An isocratic HPLC-UV method was developed for the determination of THC-Val-HS using a Waters 717 Plus autosampler (Milford, MA, USA), a 600E pump controller, a 2487 UV detector, and an Agilent 3395 integrator (Santa Clara, CA, USA). Stock solutions of the prodrugs were prepared in acetonitrile and used immediately. A Phenomenex C8 4.6 × 250 mm column (Torrance, CA, USA) was used with a mobile phase that consisted of 850 mL methanol, 150 mL water, and 0.84 mL glacial acetic acid at a flow rate of 1.2 mL/min. Detection was carried out at 226 nm. The retention times for THC-Val-HS and THC were found to be approximately 10.9 minutes and 9.3 minutes, respectively. The retention times were approximately 8.5 minutes for THC, 11.5 minutes for THC-Val, and 13.5 minutes for THC-Val-Val.
In Vivo Ocular Bioavailability Studies
Formulations.
A specific amount of THC and/or the prodrug was dissolved in light mineral oil, National Formulary, to prepare the mineral oil-based formulation. The emulsion-1 formulations of THC were prepared according to previously published protocols.35 The Tocrisolve emulsion formulations were prepared by dispersing a known amount of THC or the prodrug in the emulsion and sonicating for 30 seconds followed by stirring for 2 minutes. Micellar solutions were prepared by dissolving the drug/prodrug in the HPβCD solution in IPBS (pH 7.4) for 24 hours, after which Cremophor RH 40 was added and the solution was mixed for 2 minutes. Weighed amounts of BAK and EDTA were also added, and the mixture was brought to the final volume with IPBS (pH 7.4).
Bioavailability Studies
Single-Dose Study in Anesthetized Model.
The ocular bioavailability was determined in male New Zealand albino rabbits weighing between 2 to 2.5 kg. The rabbits were administered ketamine (35 mg/kg) and xylazine (3.5 mg/kg) intramuscularly and were maintained under anesthesia throughout the experiment. Each rabbit was placed on its side, and 50 μL of the formulation (micellar solution-1 for THC-Val-HS) was placed in the cul de sac of one eye. The rabbits were euthanized at 1 hour or 3 hours with an overdose of pentobarbital administered through the marginal ear vein. The surface of the eye was washed thoroughly with ice-cold IPBS, and the eye was immediately enucleated. The ocular tissues were separated and placed at −80°C until further analysis. All experiments were carried out in triplicate.
Multidose Study in Conscious Rabbits.
These studies were undertaken to evaluate the ocular tissue and plasma concentrations after multiple dosing in conscious rabbits. A total of 50 μl of THC-Val-HS in a Tocrisolve emulsion was instilled topically in the cul de sac of the left eye of the rabbit twice a day for 5 days (n = 6). On day 6, the animals were euthanized at one of two time points, 60 minutes or 120 minutes, following dosing (n = 3 at each time point). Blood samples were also collected. The rabbits were first anesthetized by administering ketamine (35 mg/kg) and xylazine (3.5 mg/kg) intramuscularly. The rabbits were then euthanized with an overdose of pentobarbital administered through the marginal ear vein. The enucleation and tissue separation processes remained the same.
Bioanalytical Method for the Determination of THC in Ocular Tissues
Because UV-absorbing protein peaks were found to coelute with THC and THC-Val-HS when the in vitro sample HPLC-UV method was used, a different, previously described HPLC-fluorescence method36 was used for the samples from the bioavailability studies in anesthetized rabbits. In this method, the total THC concentration was determined. The samples from the multidose conscious animal studies were analyzed using HPLC-MS, and the concentration of THC as well as THC-Val-HS was determined in these studies.
Anesthetized Rabbit Samples.
Propofol was added as an internal standard. To generate the standard curves, the THC or THC prodrug was spiked into the blank ocular tissues. After 15 minutes, ice-cold acetonitrile was added to precipitate the proteins from the tissues. THC-Val-HS and other intermediates were converted to THC by performing alkaline hydrolysis at room temperature for 1 hour. The homogenates were neutralized by the addition of hydrochloric acid and centrifuged. The supernatants were analyzed using the HPLC-fluorescence method. To quantify the total THC in the ocular tissues, standard curves were prepared with THC-Val-HS in aqueous humor (10–200 ng/mL), vitreous humor (10–200 ng/mL), cornea (20–200 ng/mL), iris-ciliary body (20–200 ng/mL), retina choroid (20–200 ng/mL), and sclera (20–200 ng/mL). The standard solutions were treated in the same manner as the in vivo samples. The standard curves were plotted in terms of total THC. All the standard curves generated had coefficients of determination (r2) greater than 0.95. The average recovery values were determined in cornea (97.4%), aqueous humor (91.7%), iris-ciliary bodies (89.3%), vitreous humor (91.5%), retina-choroid (99.9%), and sclera (89.8%).
Multidose, Conscious Model Study Samples.
THC and THC-Val-HS levels in the tissues were analyzed using an AB Sciex QTrap 4500 (Framingham, MA, USA) LC-MS/MS quadrupole interfaced with Shimadzu Nexera HPLC (Kyoto, Japan). Calibration curves were prepared by spiking THC and THC-Val-HS into blank ocular tissues along with the internal standard, D3-THC. Ice-cold acetonitrile was then added to precipitate the proteins and to extract THC and THC-Val-HS. The samples were vortexed and then centrifuged at 13,000 rpm, for 30 minutes. The supernatant was filtered through a 0.2-μm filter, and the filtrate was analyzed for THC and THC-Val-HS content. The calibration curves were prepared with both THC and THC-Val-HS in aqueous humor (2.5–100 ng/ml), vitreous humor (2.5–100 ng/ml), iris-ciliary bodies (2.5–100 ng/ml), retina-choroid (2.5–100 ng/ml), and plasma (2.5–100 ng/ml). Tissues collected from the bioavailability studies were also prepared per this protocol. A Phenomenex Synergi Hydro Reverse Phase, 100 A, 50 × 3 mm, 2.5-μm column was used with a gradient elution method; the solvent phase was composed of water and acetonitrile with 0.1% w/v formic acid.
Efficacy Studies
Open-angle glaucoma was induced in New Zealand white rabbits that weighed approximately 2 to 2.5 kg. The rabbits were anesthetized by intramuscular injection of xylazine (3.5 mg/kg) and ketamine (35 mg/kg). Chronic open-angle glaucoma was induced by a single injection of α-chymotrypsin (20 mg/mL, 50 μL) into the vitreal cavity.37,38 Care was taken to avoid contact of the α-chymotrypsin with the surface of the eye. Daily eye examinations were conducted for several days. Following the intravitreal injection, one drop each of ciprofloxacin, dexamethasone, and diclofenac sodium ophthalmic solutions were instilled each day for 5 days to prevent inflammation. When the IOP was observed to be stable at approximately 27 ± 3 mm of Hg for 3 successive days (approximately 14 days after the intravitreal injection of α-chymotrypsin), the IOP-lowering efficacy studies were initiated.
For the IOP-lowering studies, approximately 50 μL of each formulation was instilled into the lower cul de sac of the left eye of the rabbits (n = 3) while the right eye served as the control. Immediately after instillation, the eye lid was closed for 10 seconds to avoid loss of the preparation. The IOP was measured using Tonovet tonometer (Reichert, Inc., Reichert, Inc., Depew, NY, USA) before instillation (baseline IOP) and after every 30 minutes postinstillation until the IOP returned to 90% of the baseline IOP. Each value in the results section is an average of five concurrent IOP measurements at each time point for each of the three animals. The IOP measurements at each time point is reported as the percent baseline IOP (± SEM), that is, measured IOP/baseline IOP × 100.
Cannabinoid Receptor-Binding Assay
Cannabinoid receptor binding studies were carried out using the methods previously reported,39,40 with suitable modifications to determine the affinity of the designed prodrug compared with CP-55,940, a full agonist of CB1/CB2 receptors, and THC, the parent molecule.
Cell Culture.
HEK293 cells (ATCC, Manassas, VA, USA) were stably transfected via electroporation with full-length human recombinant cDNAs (OriGene, Rockville, MD, USA) for the cannabinoid receptor subtypes 1 and 2. These cells were maintained at 37°C and 5% CO2 in Dulbecco's Modified Eagles' Medium combined with F-12 HAM nutrient mixture (50/50), which was supplemented with 2 mM L-glutamine, 10% fetal bovine serum, 1% penicillin/streptomycin, and G418 antibiotic solutions.
Membrane Preparation.
Membranes were prepared by washing the cells with cold IPBS. The cells were lysed and scraped in cold Tris-HCl, pH 7.4, and then centrifuged at 5200g for 10 minutes at 4°C. The supernatant was discarded, and the pellet was resuspended in the same buffer, homogenized using a Sonic Dismembrator Model 100 (Fisher Scientific) for 30 seconds and then centrifuged at 1000g for 10 minutes at 4°C. The supernatant was saved, and the pellet was subjected to the suspension and homogenization process twice more using the same conditions. The supernatants were combined and centrifuged at 23,300g for 40 minutes at 4°C. The pellet was resuspended in cold Tris-HCl buffer, aliquoted into 2 mL vials, and stored at −80°C. The total protein concentration was determined using a Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA) according to the manufacturer's instructions.
Method for the Cannabinoid Receptor-Binding Assay.
For each assay, nonspecific binding was determined using 10 μM CP-55,940 as a positive control, and the total binding was ascertained using 0.1% dimethyl sulfoxide (DMSO) in Tris-EDTA buffer. Each test well contained 50 μL of the radioligand ([3H]-CP-55,940), 50 μL of the test compounds, control or vehicle, and 100 μL of the cell membrane preparation. The assays were incubated for 90 minutes at 37°C with gentle agitation. The reaction was terminated by rapid filtration with cold Tris-HCl containing 0.1% BSA through a UniFilter GF/C 96-well plate presoaked with 0.5% polyethyleneimine. When the filters were dry, 25 μL of MicroScint-20 was applied to each filter, and the plates were read on a TopCount NXT HTS Microplate Scintillation Counter (PerkinElmer) to record the counts per minute.
The Kd for the radioligand for each receptor was established through membrane evaluation and saturation-binding experiments. For the membrane evaluation experiment, 1 to 10 μg of membrane was incubated with 1 nM of [3H]-CP-55,940. For the saturation assay, the optimal membrane concentration was incubated with 0 to 10 nM of [3H]-CP-55,940 and 10 μM of a nonlabeled CP-55,940 or 0.1% DMSO in buffer. The data were analyzed using GraphPad Prism 5.04 software to perform nonlinear curve fitting (GraphPad, La Jolla, CA, USA), and the Kd value was calculated.
The competitive binding assay was performed using the optimal concentration of membrane with a radioligand concentration equal to the Kd and concentrations of each compound ranging from 0.00032 to 100 μM. Each compound was tested in triplicate. The assays were performed as stated earlier. The IC50 and Ki values were calculated using GraphPad Prism 5.0 software to perform nonlinear curve fitting.
Data Analysis
Statistically significant differences among multiple groups were determined using one-way ANOVA. Tukey's Honestly Significant test was carried out to differentiate among the groups. A P value less than 0.05 was considered to be statistically significant.
Results
Preformulation Studies
The predicted physicochemical properties of THC-Val-HS, THC-Val, and THC-Val-Val as well as THC and THC-HG are described in Table 1. Based on the critical physicochemical properties, THC-Val-HS was expected to be more hydrophilic and to demonstrate an improved LogDpH 7.4 and a higher polar surface area than the other prodrugs.
Table 1.
Comparison of the Predicted Physicochemical Properties of the Dicarboxylic Acid Ester and Amino Acid–Dicarboxylic Acid Ester THC Prodrugs Using Advance Chemistry Development, Inc./I-Lab 2.0 (Toronto, Canada)
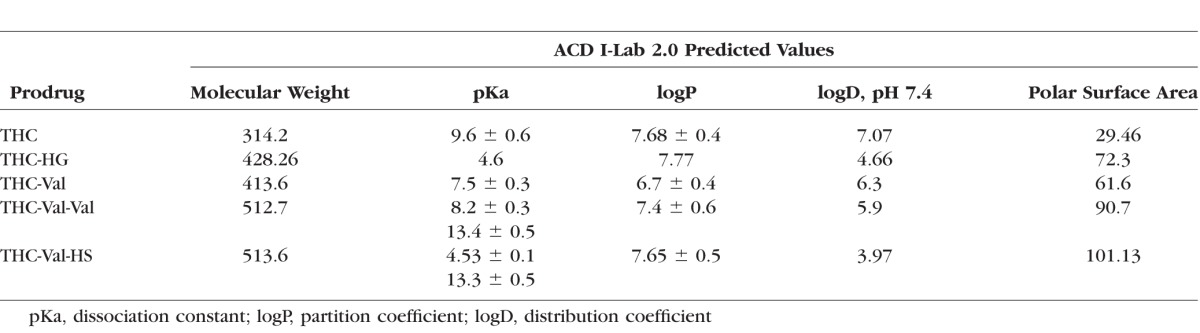
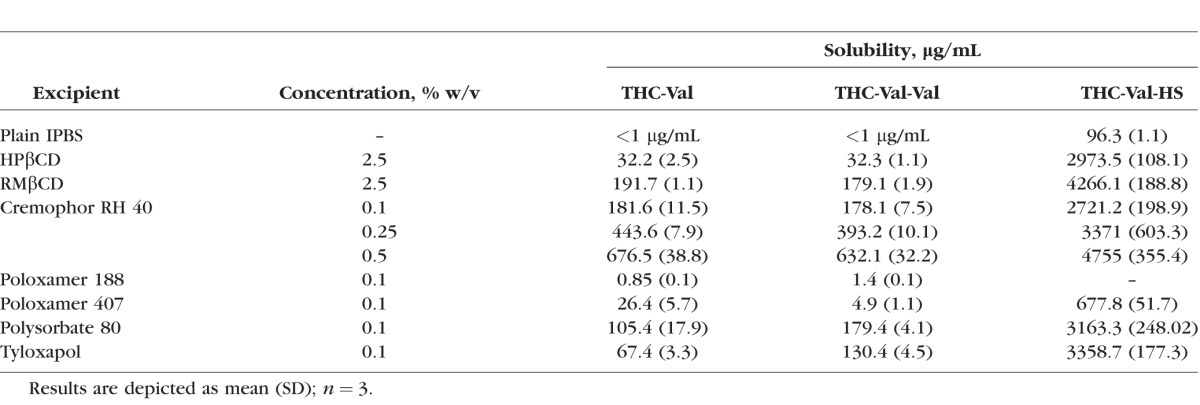
The aqueous solubility of THC-Val-HS was also found to be significantly higher (water 37.6 ± 6.6 μg/mL; IPBS 96.3 ± 1.1 μg/mL) than THC and the other prodrugs. The solubility of THC-Val-HS was below the detectable levels at pH 3 (<1 μg/mL), but increased with increasing pH.33 In the presence of cyclodextrins and surfactants, the aqueous solubility increased significantly (Table 2). Based on the markedly lower aqueous solubility of THC-Val and THC-Val-Val, these two prodrugs were not further evaluated.
Table 2.
Solubility of THC-Val, THC-Val-Val, and THC-Val-HS in Different Surfactant Solutions Prepared in IPBS (pH 7.4) at 25°C
The hydrolysis of THC-Val-HS followed apparent first-order degradation kinetics. No degradation was observed in the presence of HPβCD in the samples stored at 4°C or 25°C within the time points tested (2 weeks). THC-Val-HS was found to be more stable in the HPβCD solution than in Cremophor RH 40. The half-lives of THC-Val-HS in Cremophor RH 40 at 25°C and 40°C were 65 days and 59 days, respectively.
Corneal Permeability Studies
The corneal permeability was studied in the presence of HPβCD (1.5% w/v), RMβCD (1.5% w/v), Cremophor RH 40 (0.05% w/v), polysorbate 80 (0.05% w/v), or tyloxapol (0.05% w/v) in IPBS (pH 7.4). Because THC-Val-HS demonstrated significantly higher aqueous solubility, lower concentrations of the solubilizers were used for the in vitro studies. The permeability was found to be highest in the HPβCD (1.5% w/v) formulations (14.3 ± 0.63 × 10−6 cm/s). There were no significant differences in the permeabilities among the RMβCD (1.5% w/v) (6.5 ± 2.3 × 10−6 cm/s), Cremophor RH 40 (3.2 ± 0.21 × 10−6 cm/s), polysorbate 80 (4.1 ± 0.2 × 10−6 cm/s), and tyloxapol (3.11 ± 0.40 × 10−6 cm/s) formulations. The transcorneal permeability of THC was 5.6 × 10−6 cm/s (in 2.5% HPβCD). The addition of BAK (0.02% w/v) and EDTA (0.1% w/v) did not produce a significant difference in the permeability of THC-Val-HS (2.8 ± 0.3 × 10−6 cm/s). The permeability data are presented in Figure 2.
Figure 2.
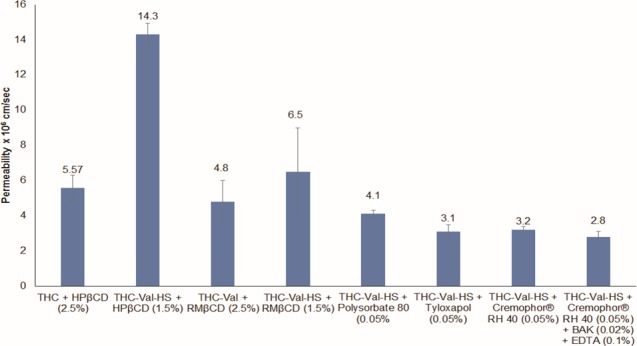
Permeability of THC (2.5% HPβCD in IPBS pH 7.4), THC-Val (2.5% RMβCD in IPBS pH 7.4), and THC-Val-HS from various surfactant solutions prepared in IPBS pH 7.4 across isolated rabbit cornea at 34 ± 0.5°C. Receiver solution consisted of IPBS containing 2.5% HPβCD (pH 7.4). Results are depicted as mean ± SD.
Stability of THC-Val-HS in Ocular Tissue Homogenates
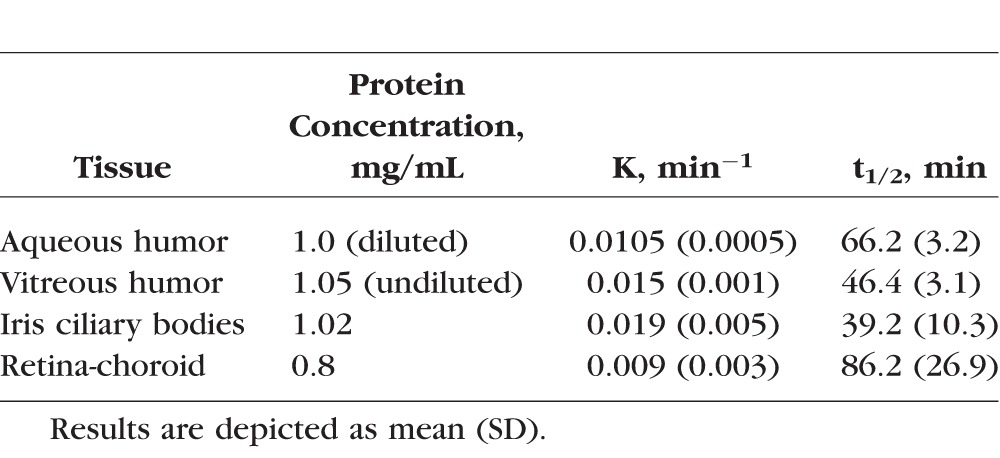
The apparent first-order degradation rate constants and the half-lives of THC-Val-HS in the aqueous humor, vitreous humor, iris-ciliary bodies, and retina-choroid homogenates are provided in Table 3. THC-Val-HS was rapidly converted to THC in the ocular tissues.
Table 3.
Apparent First-Order Rate Constants (k) and Half-Lives (t1/2) of THC-Val-HS in Ocular Tissue Homogenates
In Vivo Bioavailability Studies
Single-Dose Study (Anesthetized Model).
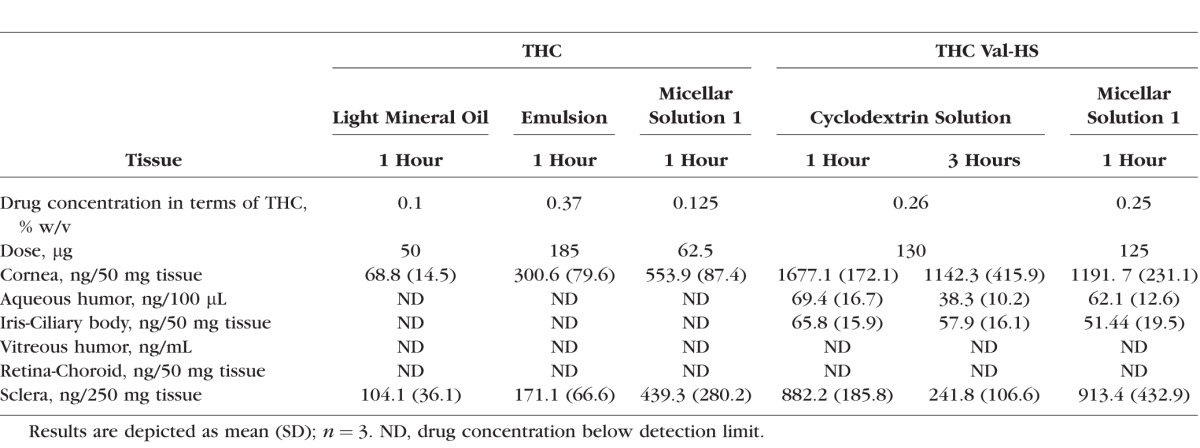
Based on the in vitro permeability data, an emulsion formulation and a cyclodextrin and surfactant solution (micellar solution 1) formulation were selected for the in vivo ocular bioavailability studies. The compositional data for all of the formulations are presented in Table 4. Hydroxypropyl methyl cellulose (0.5% w/v) was added to the selected formulations to increase the viscosity and thus improve corneal residence time. In the first set of studies, the animals were euthanized and the tissues were collected 1 hour post–topical instillation. The improved formulation was then studied for 3 hours postadministration. As shown in Table 5, the administration of THC-Val-HS in the cyclodextrin solution (2.5% HPβCD and 0.5% hydroxypropyl methyl cellulose) led to a significant amount of THC (total THC) reaching the aqueous humor (69.4 ± 16.7 ng/100 μL) and iris-ciliary bodies (65.8 ± 15.9 ng/50 mg). THC, however, was not detected in the back-of-the-eye tissues (retina-choroid or vitreous humor) with this analytical method. Because stability of THC-Val-HS in the cyclodextrin formulation was markedly better than in the Cremophor RH 40 formulation, a study for 3-hours postinstillation was carried out with the 2.5% cyclodextrin formulation. The THC concentrations at 3 hours decreased significantly in the aqueous humor, but were maintained in the iris-ciliary bodies.
Table 4.
Formulation Compositions of THC and THC-Val-HS Used in Bioavailability and IOP Efficacy Studies
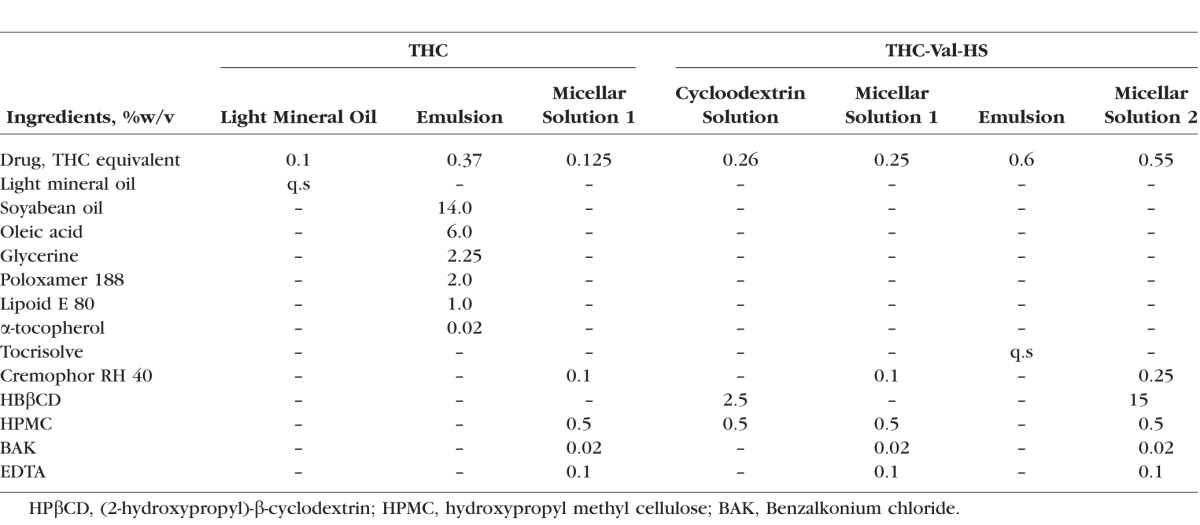
Table 5.
Total THC Concentrations Observed in Rabbit Ocular Tissues Post–Topical Administration of 50 μL of THC and THC-Val-HS Formulations
Multiple-Dose Study (Conscious Model).
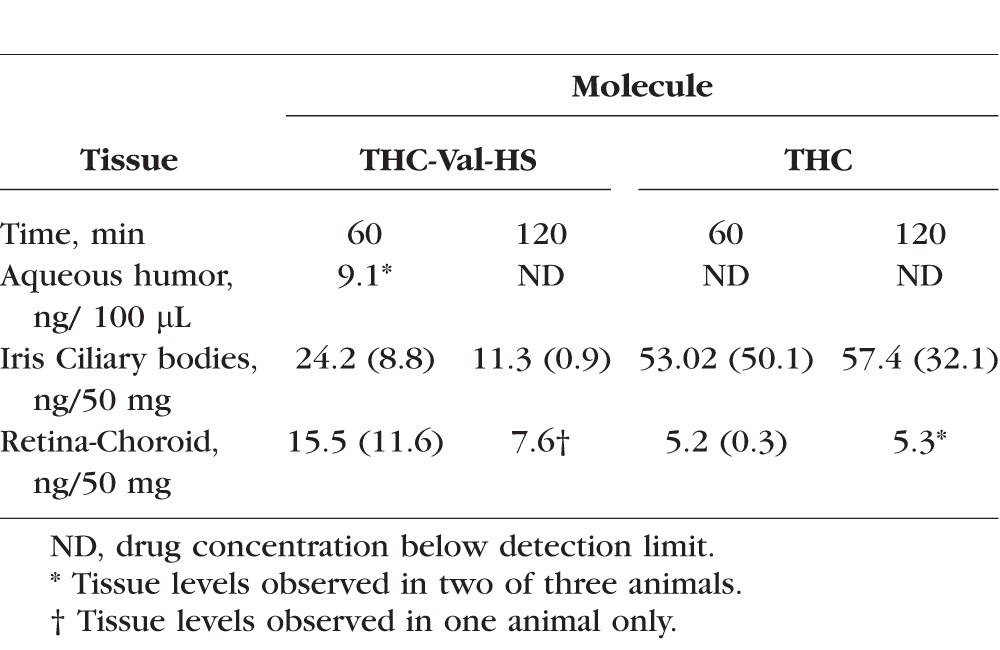
THC-Val-HS in the Tocrisolve emulsion formulation was used for these studies. Both THC-Val-HS and bioreversed THC concentrations were seen in the iris-ciliary bodies and retina-choroid at the 60-minute time point (Table 6). The concentration of THC was significantly greater than that of THC-Val-HS. At the 120-minute time point, 11.3 ± 0.9 ng/50 mg of tissue of THC-Val-HS and 57.4 ± 32.1 ng/50 mg of tissue of regenerated THC were observed in the iris-ciliary bodies. However, in the retina-choroid, THC-Val-HS was detected only in one of the three rabbits (7.6 ng/50 mg of tissue), whereas the levels in the others were below the limit of quantification. Regenerated THC levels were observed in two of the three rabbits at the second time point.
Table 6.
Ocular Tissue Concentrations of THC-Val-HS and THC, 60 and 120 Minutes Post–Topical Application of 50 μL of 0.98% THC-Val-HS (Equivalent to 0.6% w/v THC) in Tocrisolve Emulsion Formulation, Dose: 490 μg of THC-Val-HS (Equivalent to 300 μg of THC)
Efficacy Studies
THC lowered the IOP in the glaucoma model animals, but not in the normotensive rabbits (Fig. 3). The maximum percent drop in the IOP from the baseline IOP and the time for the peak reduction in the IOP varied with the THC dose. The following two doses were tested: 0.5% and 0.8% w/v. With the 0.5% w/v THC in the Tocrisolve formulation, the IOP was 66.0% of the baseline at 30 minutes, but rapidly began to return to the baseline IOP, reaching 89.2% (at 60 minutes). The short duration of action observed with THC could have been a result of poor penetration and rapid elimination of THC from the eye. At 0.8% w/v, THC demonstrated a further improvement in IOP reduction. The percent-baseline IOP was 64.8% at 30 minutes and 66.6% at 60 minutes. The IOP began to revert to baseline after 60 minutes, reaching 83.7% and 91.0% at 90 minutes and 120 minutes, respectively. The vehicle, Tocrisolve, did not have any effect on the IOP.
Figure 3.
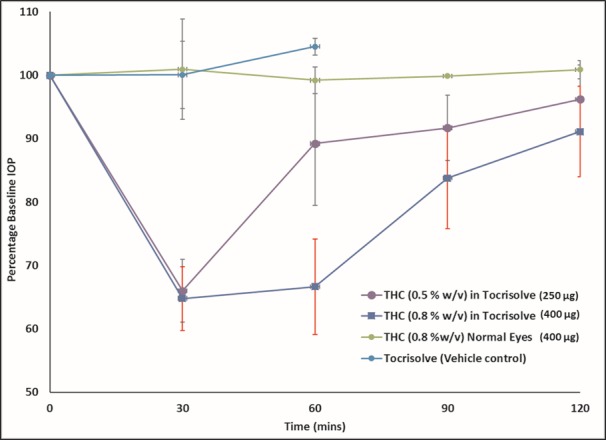
IOP–Time profile for THC in normotensive and α-chymotrypsin–induced rabbit glaucoma model. Numbers in brackets represent concentration (%w/v) and dose (μg). Actual baseline values (mean ± SEM in mm Hg) for IOP in the different formulations: THC (0.5% w/v) in Tocrisolve (24.0 ± 2.2), THC (0.8% w/v) in Tocrisolve (24.5 ± 1.9), THC (0.8% w/v) in normotensive (normal) eyes (19.4 ± 0.3), and Tocrisolve (19.6 ± 1.4).
With THC-Val-HS emulsion and micellar solution formulations, improved IOP-lowering activities were observed (Fig. 4). This is consistent with the bioavailability data. At a dose equivalent to 0.6% w/v, THC (THC-Val-HS 0.99% w/v), an improved IOP-lowering activity compared to THC was seen. Although the onset of action was delayed as a result of the step for bioconversion of the prodrug into THC, the duration of action was prolonged. The maximum percent change in IOP from the baseline was observed at 90 minutes and was maintained until the 120-minute time point, reverting back to the baseline IOP by the end of 240 minutes. The overall IOP-lowering profile was more profound when compared with THC, even at a lower THC-equivalent dose. THC-Val-HS formulated in the cyclodextrin + surfactant solution also showed an improved IOP-lowering activity (THC equivalent dose: 0.55% w/v). Although the duration of activity was slightly shorter than that of the emulsion formulation, the overall profile remained the same.
Figure 4.
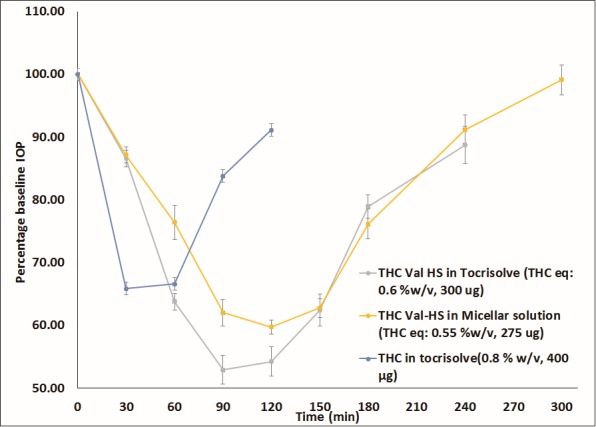
IOP–Time profiles obtained with THC-Val-HS and THC in rabbit glaucoma model. Numbers in brackets represent concentration (%w/v) and dose equivalent to THC (μg). Actual baseline values (mean ± SEM in mm Hg) for IOP in the different formulations: THC-Val-HS in Tocrisolve (26.8 ± 0.4), THC-Val-HS in micellar solution (26.8 ± 0.2), and THC (0.8% w/v) in Tocrisolve (24.5 ± 1.9).
Timolol maleate eye drops had a prolonged IOP-lowering activity that lasted for approximately 6 hours, with a maximum percent IOP change from baseline of 59% (120 minutes). The IOP returned to baseline more gradually, providing a longer duration of action. THC-Val-HS had a slightly better IOP-lowering effect than timolol, although the former showed a shorter duration of action. When compared with another marketed antiglaucoma product, pilocarpine eye drops, THC-Val-HS showed better IOP-lowering activity (Fig. 5).
Figure 5.
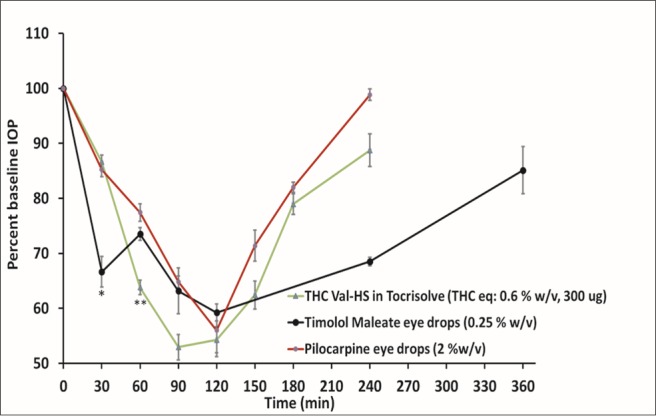
IOP–Time profiles obtained with THC-Val-HS, timolol maleate, and pilocarpine eye drops (marketed) in rabbit glaucoma model. Numbers in brackets represent concentration (%w/v) and dose equivalent to THC (μg). Actual baseline values (mean ± SEM in mm Hg) for IOP in the following different formulations: THC-Val-HS in Tocrisolve (26.8 ± 0.4), timolol maleate eye drops (24.0 ± 1.9), and pilocarpine eye drops (27.1 ± 0.3). *IOP drop from timolol maleate is significantly different form THC-Val-HS and pilocarpine (P < 0.05). **IOP drop from THC-Val-HS is significantly different from timolol maleate and pilocarpine (P < 0.05).
Receptor-Binding Studies
The receptor binding studies showed that the IC50 and Ki values for THC-Val-HS were much higher than those for CP-55,940 or THC for both the CB1 and CB2 receptors (Fig. 6). The Ki of THC-Val-HS (0.960 ± 0.174 μM) for the CB1 receptors was 320- and 21.8-fold higher than those of CP-55,940 (0.003 ± 0.0 μM) and THC (0.044 ± 0.005), respectively. For the CB2 receptors, the Ki for THC-Val-HS (1.2154 ± 0.0.082 μM) was approximately 607.5- and 38-fold higher than those of CP-55,940 (0.002 μM) and THC (0.032 ± 0.001), respectively. The CB1 receptor IC50 values for THC-Val-HS (1.593 ± 0.289 μM) were 318.6- and 20.6-fold higher than those of CP-55,940 (0.005 ± 0.001 μM) and THC (0.077 ± 0.008), respectively, whereas the IC50 for THC-Val-HS at the CB2 receptors was approximately 607.5- and 51.7-fold higher than those of CP-55,940 and THC, respectively. The results suggest that the prodrug (THC-Val-HS) had a much lower affinity for the cannabinoid receptors than the controls and would thus have significantly lower pharmacological activity, necessitating reconversion to THC.
Figure 6.
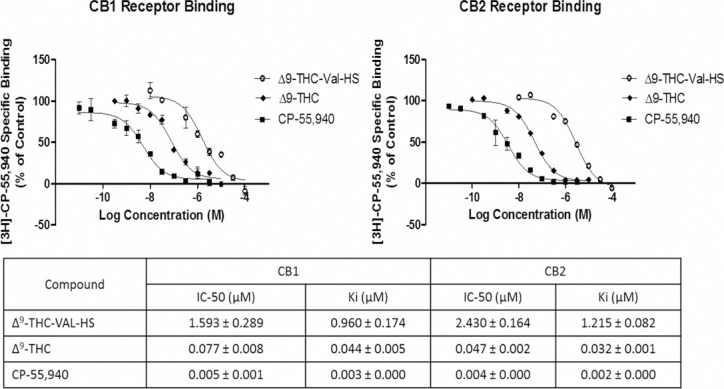
Cannabinoid receptor (CB1 and CB2) binding studies with THC-Val-HS, THC, and CP-55,940.
Discussion
We previously reported that 1 hour after the topical instillation of the THC eye drops, which were formulated in light mineral oil, nanoemulsion, or micellar solutions, the levels of THC in the deeper ocular tissues, such as the aqueous humor, iris-ciliary bodies, vitreous humor, and retina-choroid of anesthetized rabbits were below the detection levels of the analytical method used21 (data reproduced in Table 5 with permission). A hydrophilic THC-ester prodrug, THC-HG, formulated as an ion pair, significantly increased the ocular tissue concentrations in anesthetized rabbits. However, THC-HG was not very stable in the formulations tested.35 Initial studies with several amino acid–ester prodrugs suggested that the valine ester prodrug was significantly more stable. This is consistent with previous literature reports.41,42 It had also been suggested that the addition of an amide linkage to the ester link enhances the stability of the ester bond.43 The combination prodrug THC-Val-HS was thus conceived with the expectation that the valine ester would be maximally stable and the stability of the ester bond would be enhanced by the presence of the amide linkage with the hemisuccinate. The hemisuccinate moiety was selected based on the fact that it would have the minimum chain length while providing enhanced solubility by virtue of ionization at physiological pH. Thus, the overall objective of this study was to evaluate THC-Val-HS as well as THC-Val and THC-Val-Val in terms of their physicochemical characteristics, transocular penetration characteristics, and in vivo IOP-lowering activities.
THC-Val-HS was observed to be significantly more soluble and stable than THC, THC-Val, THC-Val-Val, or THC-HG in aqueous solutions. Cyclodextrins and surfactants increased the solubility to varying extents. The aqueous solubilities of THC-Val and THC-Val-Val were much lower than that of THG-HG at physiological pH, although they were more stable than THC-HG33 in aqueous solutions. In vitro permeability studies for THC-Val and THC-Val-Val were carried out in the presence of 2.5% RMβCD, 0.5% Cremophor RH 40, 0.1% polysorbate 80, and 0.1% tyloxapol. The permeability of THC-Val in the presence of 2.5% RMβCD was found to be 4.75 × 10−6 cm/s when the donor solution pH was maintained at pH 7.4. No drug was detected in the receiver chamber from the other THC-Val formulations. THC-Val-Val was not detected in the receiver chamber with any of the formulations studied. For these reasons, THC-Val and THC-Val-Val were not studied any further.
At physiological pH in the presence of HPβCD, the corneal permeability of THC-Val-HS was observed to be significantly higher than that of THC-HG and THC. Although THC-Val-HS was similar to THC-HG in terms of calculated (ACD/iLabs 2.0) logP, pKa, and number of hydrogen-bond donors and acceptors, its greater polar surface area and relatively lower logD7.4 and an increased aqueous solubility probably resulted in the higher transcorneal permeability of THC-Val-HS.
Consistent with the in vitro data, THC-Val-HS-loaded topical eye drops were able to deliver significantly higher levels of THC to the anterior segment ocular tissues, such as the aqueous humor and the iris-ciliary bodies in the anesthetized rabbits. At the 3-hour time point postadministration, the THC levels resulting from the solution formulated with 2.5% HPβCD, were lower in the aqueous humor but were almost the same in the iris-ciliary bodies compared to the 1-hour time point. The levels in the retina-choroid and vitreous humor were below the detection limits of the HPLC-fluorescence method.
These results thus emphasize the difficulty of delivering therapeutic agents to the back of the eye through the topical route. The significant vascular and lymphatic drainage in the conjunctiva, sclera, and choroid and the barrier characteristics of the RPE and Bruch's membrane severely restrict the passage of drug/drug candidates from the ocular surface into the retina and vitreous humor.44–46 The THC concentrations obtained in the ocular tissues with THC-Val-HS in this study were similar to that obtained with the THC-HG ion-pair formulations in our previous studies.35 The increased solution stability of THC-Val-HS, however, provides a significant advantage over THC-HG.
In the multidose studies (Table 6), THC-Val-HS was detected in the iris-ciliary bodies at both 60 and 120 minutes. In the iris-ciliary bodies, the THC-Val-HS was rapidly hydrolyzed to less than half the levels observed at 60 minutes within the subsequent hour. This was consistent with the in vitro tissue hydrolysis studies (Table 3), which suggested that THC-Val-HS has a half-life of 39.2 ± 10.3 minutes in the iris-ciliary bodies. The increased half-life (60 minutes) in the in vivo studies is probably the result of continued absorption and distribution. The regeneration of THC from THC-Val-HS was apparent from the steady THC concentrations in the iris-ciliary bodies during the rapid decline in the THC-Val-HS concentrations. The PK data were consistent with the IOP versus Time profile in which the IOP dropped from 64% at 60 minutes to 54% at 120 minutes. Because of the better permeation characteristics of the prodrug, we observed measurable THC-Val-HS levels in the retina-choroid as well. However, within 1 hour, the prodrug levels fell below the limit of detection, as indicated by the fact that measurable THC-Val-HS levels were observed in only one rabbit. In the in vitro bioreversion studies, the half-life in the retina-choroid was observed to be greater than that in the iris-ciliary bodies. This difference between the in vitro and in vivo data, could be explained by the rapid elimination from the retina-choroid tissue in vivo. Interestingly, the total THC-equivalent levels in the iris-ciliary bodies in the multiple-dose study in the conscious animal model, with values of 67.8 ng/50 mg of tissue at 60 minutes and 64.3 ng/50 mg of tissue at 120 minutes, were comparable to the total THC levels achieved with the single-dose application of the cyclodextrin solution (65.8 ng/50 mg of tissue at 60 minutes) and the micellar solution (51.44 ng/50 mg of tissue at 60 minutes) in the anesthetized model. Multiple dosing also resulted in penetration into the retina-choroid. THC was not observed in the plasma following the multiple doses.
The α-chymotrypsin–induced rabbit glaucoma model was used to evaluate the in vivo IOP-lowering activity of THC-Val-HS.37,38 This model has been widely used to study the effects of drugs and drug candidates on IOP. In an attempt to increase the THC loading compared to that achieved with the formulations used for the bioavailability studies (Table 4), Tocrisolve, a commercially available optimized emulsion, was used. This formulation achieved a 0.8% w/v THC loading. The duration of the decrease in the IOP was, however, short, and the IOP returned to the baseline within 2 hours. This was consistent with the ocular distribution data, which showed poor THC penetration into the intraocular tissues and/or rapid clearance. These results are, however, different from those reported in an earlier study that evaluated the effect of Δ8-THC on IOP.28 These authors reported that 50 μL of a submicron-emulsion formulation of 0.4% w/v Δ8-THC produced a decrease of 10.8 ± 2.04 mm of Hg in the IOP in an α-chymotrypsin–induced rabbit model of ocular hypertension. Moreover, the drop in the IOP lasted up to 8 hours in the animal model.
THC did not produce any effect on the IOP in the normotensive rabbits. This suggests possible differences in ocular barrier properties between the two models that requires further investigation.47 Muchtar et al.28 also reported that the drop in IOP in normotensive rabbits induced by the 0.4% Δ8-THC submicron emulsion formulation was not significant compared to that observed in the hypertensive model. The difference between the IOP in normotensive rabbits with and without treatment after 2 hours was only 2 mm of Hg. However, in ocular hypertensive rabbits, there was a drop of 6.5 mm of Hg. Crandall et al.17 reported that even systemic administration of THC (5 mg/kg body weight) did not cause any drop in normotensive control eyes when compared with hypertensive eyes (approximately 6 mm of Hg drop).
In the case of THC-Val-HS, the formulations (2.5% cyclodextrin and micellar solution 1) employed in the ocular disposition studies (Table 4) could dissolve only up to 0.25% w/v THC equivalent, which was significantly lower than the THC concentration in Tocrisolve. Thus, a new formulation was prepared in which HPβCD (15% w/v) and Cremophor RH 40 (0.25% w/v) were combined as solubilizers. This increased the drug loading to a THC equivalent of 0.55% w/v (THC-Val-HS: 0.9% w/v). The Tocrisolve emulsion could load 0.6% w/v THC equivalent (THC-Val-HS: 0.99% w/v). These formulations were used to evaluate the effect of THC-Val-HS on IOP.
THC-Val-HS, administered topically to the α-chymotrypsin–induced, high-IOP rabbits, produced a gradual onset of the IOP-lowering effect and required a longer time to the achieve the maximum effect compared to THC. THC-Val-HS also demonstrated a much longer duration of activity, up to 4 hours, even at a lower THC-equivalent dose (compared to the 0.8% w/v THC dose). The significantly increased duration observed with THC-Val-HS could have been a result of increased penetration of the prodrug into the inner ocular tissues (as seen in the ocular distribution studies) and, thus, the availability of higher amounts of THC at the target tissues. The more gradual development of the peak IOP reduction may have been because of the requirement for the regeneration of THC from the prodrug in the ocular tissues (Table 3). Although the Tocrisolve emulsion and micellar solution-2 were similar in their IOP-reduction profiles, the former was slightly more effective than the latter.
The IOP-lowering action of THC-Val-HS was equivalent to that of pilocarpine based on an equal or greater intensity and duration of action when compared with the marketed pilocarpine HCl and timolol maleate eye drops. Timolol maleate eye drops produced a prolonged IOP-lowering activity when compared with the THC-Val-HS emulsion; however, the latter was better in terms of the maximal IOP reduction. The IOP-lowering profiles observed for pilocarpine and timolol were similar to those reported in earlier studies.48–50 THC-Val-HS must be converted to THC by ocular tissue enzyme-mediated ester hydrolysis (Table 3). This is consistent with the gradual IOP-lowering profile and the delay in the maximal drop in the IOP seen with THC-Val-HS when compared with that obtained with THC. This suggests that THC-Val-HS is slowly converted to THC in the eye.
The receptor-binding studies demonstrated that THC-Val-HS did not have significant affinity for either the CB1 or CB2 receptors. Although the binding data did not rule out the possibility that THC-Val-HS possesses some pharmacological activity, considering that THC is several fold more potent than THC-Val-HS and that THC-Val-HS is rapidly converted to THC in the ocular tissues, it seems more likely that the cannabinoid receptor-mediated action is dependent on THC. This is consistent with the gradual IOP-lowering profile and the delayed maximum drop in IOP seen with THC-Val-HS that are the result of dependence on THC regeneration.
The THC prodrugs are still in the early stages of development. In this study, our primary goal was to evaluate an improvement in the pharmacological activity compared to the parent molecule, THC. During these studies, no adverse reactions such as irritation, increased tearing, or changes in the blinking rates or redness of the eyes were observed. Thus, although detailed cytotoxic studies of the prodrug have not yet been initiated (but are being planned), the molecule appears not to differ from the parent compound. Moreover, in earlier studies, prodrugs that have employed valine and hemisuccinate as promoieties have been observed to be safe.
Conclusions
The present study demonstrated that a rational combination of prodrug design and formulation strategy can effectively deliver THC to the anterior segment of the eye. An increased reduction in the IOP was achieved with the prodrug at a lower THC-equivalent dose, which is consistent with improved ocular penetration of the prodrug. Importantly, because most of the conventionally developed antiglaucoma drugs have no reported neuroprotective action, THC, an established neuroprotectant, has the potential to become an effective glaucoma medication. It is thus encouraging to see that THC and THC-Val-HS reached the retina-choroid. THC-Val-HS is still some distant away from being a clinically applicable agent, and studies aimed at developing and optimizing various formulations with safe and improved delivery of THC to the back of the eye are currently underway.
Acknowledgments
Supported by grants from the National Institutes of Health (1R41EY020042, P20GM104932). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure: G.R. Adelli, None; P. Bhagav, None; P. Taskar, None; T. Hingorani, None; S. Pettaway, None; W. Gul, None; M.A. ElSohly, None; M.A. Repka, None; S. Majumdar, None
References
- 1. Cook C,, Foster P. Epidemiology of glaucoma: what's new? Can J Ophthalmol. 2012; 47: 223–226. [DOI] [PubMed] [Google Scholar]
- 2. Kwon YH,, Fingert JH,, Kuehn MH,, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009; 360: 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta N,, Yucel YH. Glaucoma as a neurodegenerative disease. Curr Opin Ophthalmol. 2007; 18: 110–114. [DOI] [PubMed] [Google Scholar]
- 4. Bucolo C,, Salomone S,, Drago F,, Reibaldi M,, Longo A,, Uva MG. Pharmacological management of ocular hypertension: current approaches and future prospective. Curr Opin Pharmacol. 2013; 13: 50–55. [DOI] [PubMed] [Google Scholar]
- 5. Bucolo C,, Platania CB,, Reibaldi M,, et al. Controversies in glaucoma: current medical treatment and drug development. Curr Pharm Des. 2015; 21: 4673–4681. [DOI] [PubMed] [Google Scholar]
- 6. Poyer JF,, Millar C,, Kaufman PL. Prostaglandin F2 alpha effects on isolated rhesus monkey ciliary muscle. Invest Ophthalmol Vis Sci. 1995; 36: 2461–2465. [PubMed] [Google Scholar]
- 7. Lutjen-Drecoll E,, Tamm E. Morphological study of the anterior segment of cynomolgus monkey eyes following treatment with prostaglandin F2 alpha. Exp Eye Res. 1988; 47: 761–769. [DOI] [PubMed] [Google Scholar]
- 8. Pfeiffer N,, Lamparter J,, Gericke A,, Grus FH,, Hoffmann EM,, Wahl J. Neuroprotection of medical IOP-lowering therapy. Cell Tissue Res. 2013; 353: 245–251. [DOI] [PubMed] [Google Scholar]
- 9. Watanabe K,, Chiou GC. Action mechanism of timolol to lower the intraocular pressure in rabbits. Ophthalmic Res. 1983; 15: 160–167. [DOI] [PubMed] [Google Scholar]
- 10. Sambhara D,, Aref AA. Glaucoma management: relative value and place in therapy of available drug treatments. Ther Adv Chronic Dis. 2014; 5: 30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schwartz M,, Yoles E. Optic nerve degeneration and potential neuroprotection: implications for glaucoma. Eur J Ophthalmol. 1999; 9 suppl 1: S9–S11. [DOI] [PubMed] [Google Scholar]
- 12. Levin LA,, Peeples P. History of neuroprotection and rationale as a therapy for glaucoma. Am J Manag Care. 2008; 14: S11–S14. [PubMed] [Google Scholar]
- 13. Pita-Thomas DW,, Goldberg JL. Nanotechnology and glaucoma: little particles for a big disease. Curr Opin Ophthalmol. 2013; 24: 130–135. [DOI] [PubMed] [Google Scholar]
- 14. Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A.The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012; 31: 152–181. [DOI] [PubMed] [Google Scholar]
- 15. Nakazawa T. Mechanism of N-methyl-D-aspartate-induced retinal ganglion cell death [in Japanese]. Nippon Ganka Gakkai Zasshi. 2009; 113: 1060–1070. [PubMed] [Google Scholar]
- 16. Qu J,, Wang D,, Grosskreutz CL. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp Eye Res. 2010; 91: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crandall J,, Matragoon S,, Khalifa YM,, et al. Neuroprotective and intraocular pressure-lowering effects of (-)delta9-tetrahydrocannabinol in a rat model of glaucoma. Ophthalmic Res. 2007; 39: 69–75. [DOI] [PubMed] [Google Scholar]
- 18. El-Remessy AB,, Khalil IE,, Matragoon S,, et al. Neuroprotective effect of (-)delta9-tetrahydrocannabinol and cannabidiol in N-methyl-D-aspartate-induced retinal neurotoxicity: involvement of peroxynitrite. Am J Pathol. 2003; 163: 1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hampson AJ,, Grimaldi M,, Axelrod J,, Wink D. Cannabidiol and (-)delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998; 95: 8268–8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montero C,, Campillo NE,, Goya P,, Paez JA. Homology models of the cannabinoid CB1 and CB2 receptors. A docking analysis study. Eur J Med Chem. 2005; 40: 75–83. [DOI] [PubMed] [Google Scholar]
- 21. Hingorani T,, Gul W,, Elsohly M,, Repka MA,, Majumdar S. Effect of ion pairing on in vitro transcorneal permeability of a delta(9) -tetrahydrocannabinol prodrug: potential in glaucoma therapy. J Pharm Sci. 2012; 101: 616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Geroski DH,, Edelhauser HF. Drug delivery for posterior segment eye disease. Invest Ophthalmol Vis Sci. 2000; 41: 961–964. [PubMed] [Google Scholar]
- 23. Loftssona T,, Jarvinen T. Cyclodextrins in ophthalmic drug delivery. Adv Drug Deliv Rev. 1999; 36: 59–79. [DOI] [PubMed] [Google Scholar]
- 24. Wu Y,, Yao J,, Zhou J,, Dahmani FZ. Enhanced and sustained topical ocular delivery of cyclosporine A in thermosensitive hyaluronic acid-based in situ forming microgels. Int J Nanomedicine. 2013; 8: 3587–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jarho P,, Pate DW,, Brenneisen R,, Jarvinen T. Hydroxypropyl-beta-cyclodextrin and its combination with hydroxypropyl-methylcellulose increases aqueous solubility of delta9-tetrahydrocannabinol. Life Sci. 1998; 63: PL381–PL384. [DOI] [PubMed] [Google Scholar]
- 26. Green K,, Bigger JF,, Kim K,, Bowman K. Cannabinoid penetration and chronic effects in the eye. Exp Eye Res. 1977; 24: 197–205. [DOI] [PubMed] [Google Scholar]
- 27. Merritt JC,, Crawford WJ,, Alexander PC,, Anduze AL,, Gelbart SS. Effect of marihuana on intraocular and blood pressure in glaucoma. Ophthalmology. 1980; 87: 222–228. [DOI] [PubMed] [Google Scholar]
- 28. Muchtar S,, Almog S,, Torracca MT,, Saettone MF,, Benita S. A submicron emulsion as ocular vehicle for delta-8-tetrahydrocannabinol: effect on intraocular pressure in rabbits. Ophthalmic Res. 1992; 24: 142–149. [DOI] [PubMed] [Google Scholar]
- 29. Fischer KM,, Ward DA,, Hendrix DV. Effects of a topically applied 2% delta-9-tetrahydrocannabinol ophthalmic solution on intraocular pressure and aqueous humor flow rate in clinically normal dogs. Am J Vet Res. 2013; 74: 275–280. [DOI] [PubMed] [Google Scholar]
- 30. Buys YM,, Rafuse PE. Canadian Ophthalmological Society policy statement on the medical use of marijuana for glaucoma. Can J Ophthalmol. 2010; 45: 324–326. [DOI] [PubMed] [Google Scholar]
- 31. Mathalone N,, Wolf A,, Geyer O. Cannabis and glaucoma: an ancient legend or a novel therapeutic horizon? [in Hebrew]. Harefuah. 2015; 154: 394–397, 403. [PubMed] [Google Scholar]
- 32. Majumdar S,, Hingorani T,, Srirangam R. Evaluation of active and passive transport processes in corneas extracted from preserved rabbit eyes. J Pharm Sci. 2010; 99: 1921–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Elsohly MA,, Gul W,, Repka MA,, Majumdar S, inventors; Google Patents, assignee. Compositions containing delta-9-thc-amino acid esters and process of preparation. US patent 8809261 B2 August 19, 2014.
- 34. Majumdar S,, Hingorani T,, Srirangam R,, Gadepalli RS,, Rimoldi JM,, Repka MA. Transcorneal permeation of L- and D-aspartate ester prodrugs of acyclovir: delineation of passive diffusion versus transporter involvement. Pharm Res. 2009; 26: 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hingorani T,, Adelli GR,, Punyamurthula N,, et al. Ocular disposition of the hemiglutarate ester prodrug of (9)-tetrahydrocannabinol from various ophthalmic formulations. Pharm Res. 2013; 30: 2146–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zoller O,, Rhyn P,, Zimmerli B. High-performance liquid chromatographic determination of delta9-tetrahydrocannabinol and the corresponding acid in hemp containing foods with special regard to the fluorescence properties of delta9-tetrahydrocannabinol. J Chromatogr A. 2000; 872: 101–110. [DOI] [PubMed] [Google Scholar]
- 37. Bhagav P,, Trivedi V,, Shah D,, Chandran S. Sustained release ocular inserts of brimonidine tartrate for better treatment in open-angle glaucoma. Drug Deliv Transl Res. 2011; 1: 161–174. [DOI] [PubMed] [Google Scholar]
- 38. Percicot CL,, Schnell CR,, Debon C,, Hariton C. Continuous intraocular pressure measurement by telemetry in alpha-chymotrypsin-induced glaucoma model in the rabbit: effects of timolol, dorzolamide, and epinephrine. J Pharmacol Toxicol Methods. 1996; 36: 223–228. [DOI] [PubMed] [Google Scholar]
- 39. Atta-ur-Rahman,, Choudhary MI,, Thomsen WJ. Bioassay Techniques for Drug Development. 1st ed. Amsterdam, Netherlands: Academic Publisher; 2001. [Google Scholar]
- 40. Gao J,, Leon F,, Radwan MM,, et al. Benzyl derivatives with in vitro binding affinity for human opioid and cannabinoid receptors from the fungus Eurotium repens. J Nat Prod. 74: 1636–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Majumdar S,, Nashed YE,, Patel K,, et al. Dipeptide monoester ganciclovir prodrugs for treating HSV-1-induced corneal epithelial and stromal keratitis: in vitro and in vivo evaluations. J Ocul Pharmacol Ther. 2005; 21: 463–474. [DOI] [PubMed] [Google Scholar]
- 42. Katragadda S,, Gunda S,, Hariharan S,, Mitra AK. Ocular pharmacokinetics of acyclovir amino acid ester prodrugs in the anterior chamber: evaluation of their utility in treating ocular HSV infections. Int J Pharm. 2008; 359: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ariens EJ. Modulation of pharmacokinetics by molecular manipulation. : Ariens EJ, Drug Design: Medicinal Chemistry: A Series of Monographs. New York: Academic Press; 2013: 109. [Google Scholar]
- 44. Adelli GR,, Hingorani T,, Punyamurthula N,, Balguri SP,, Majumdar S. Evaluation of topical hesperetin matrix film for back-of-the-eye delivery. Eur J Pharm Biopharm. 2015; 92: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Duvvuri S,, Majumdar S,, Mitra AK. Drug delivery to the retina: challenges and opportunities. Expert Opin Biol Ther. 2003; 3: 45–56. [DOI] [PubMed] [Google Scholar]
- 46. Kompella UB,, Kadam RS,, Lee VH. Recent advances in ophthalmic drug delivery. Ther Deliv. 2010; 1: 435–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Housley GD. Nucleotide and nucleoside signalling in the ciliary ganglion. : Maria P,, Abracchio MW, Purinergic and Pyrimidinergic Signalling: Molecular, Nervous and Urogenitary System Function. Berlin: Springer Science & Business Media; 2001. [Google Scholar]
- 48. Panchal S,, Mehta A,, Santani D. Occulohypotensive effect of torasamide in experimental glaucoma. Internet J Pharmacol. 2008; 5: 5. [Google Scholar]
- 49. Zimmerman TJ,, Kaufman HE. Timolol, dose response and duration of action. Arch Ophthalmol. 1977; 95: 605–607. [DOI] [PubMed] [Google Scholar]
- 50. Vareilles P,, Silverstone D,, Plazonnet B,, Le Douarec JC,, Sears ML,, Stone CA. Comparison of the effects of timolol and other adrenergic agents on intraocular pressure in the rabbit. Invest Ophthalmol Vis Sci. 1977; 16: 987–996. [PubMed] [Google Scholar]


