Abstract
Treponema maltophilum and Treponema lecithinolyticum belong to the group IV oral spirochetes and are associated with endodontic infections, as well as periodontitis. Recently, the genes encoding the major surface proteins (Msps) of these bacteria (MspA and MspTL, respectively) were cloned and sequenced. The amino acid sequences of these proteins showed significant similarity. In this study we analyzed the functional role of these homologous proteins in human monocytic THP-1 cells and primary cultured periodontal ligament (PDL) cells using recombinant proteins. The complete genes encoding MspA and MspTL without the signal sequence were cloned into Escherichia coli by using the expression vector pQE-30. Fusion proteins tagged with N-terminal hexahistidine (recombinant MspA [rMspA] and rMspTL) were obtained, and any possible contamination of the recombinant proteins with E. coli endotoxin was removed by using polymyxin B-agarose. Flow cytometry showed that rMspA and rMspTL upregulated the expression of intercellular adhesion molecule 1 (ICAM-1) in both THP-1 and PDL cells. Expression of proinflammatory cytokines, such as interleukin-6 (IL-6) and IL-8, was also induced significantly in both cell types by the Msps, as determined by reverse transcription-PCR and an enzyme-linked immunosorbent assay, whereas IL-1β synthesis could be detected only in the THP-1 cells. The upregulation of ICAM-1, IL-6, and IL-8 was completely inhibited by pretreating the cells with an NF-κB activation inhibitor, l-1-tosylamido-2-phenylethyl chloromethyl ketone. This suggests involvement of NF-κB activation. The increased ICAM-1 and IL-8 expression in the THP-1 cells obtained with rMsps was not inhibited in the presence of the IL-1 receptor antagonist (IL-1ra), a natural inhibitor of IL-1. Our results show that the Msps of the group IV oral spirochetes may play an important role in amplifying the local immune response by continuous inflammatory cell recruitment and retention at an infection site by stimulation of expression of ICAM-1 and proinflammatory cytokines.
Oral spirochetes are known to be associated with acute necrotizing ulcerative gingivitis and periodontitis (5, 27, 44). These organisms include enormously diverse Treponema species (8, 11), and 10 species have been cultivated so far (10, 50, 54). Epidemiological studies have shown that some species are more often associated with periodontal diseases, and yet uncultivated treponemes have also been detected predominantly at the disease sites (11, 32, 53). Treponema maltophilum and Treponema lecithinolyticum belong to the phylogenetic group IV oral spirochetes (8) that are frequently found in chronic and aggressive periodontitis (32, 53). Recently, these organisms were also detected in infected root canals associated with either asymptomatic or symptomatic apical periodontitis (24, 46).
The surface proteins are the outermost barrier to the host cells and elicit diverse activities, including adhesion, cytotoxicity, antigenicity, and host cell stimulation (22). Despite the species diversity, the functional roles of the surface proteins of oral spirochetes are not well known. Thus far, only a few proteins have been identified at the molecular level, and the proteins that have been identified are mainly proteins of Treponema denticola, which is the best-studied oral spirochete. The 53-kDa major surface protein (Msp) of T. denticola ATCC 35405 has been shown to have pore-forming and adhesion activities (13, 30). This protein also inhibits agonist-induced Ca2+ release from the internal stores by uncoupling the store-operated channels (52) and the binding step of collagen phagocytosis in gingival fibroblasts (3). Dentilisin having chymotrypsin-like activity has been found to be located in the outer membrane (10). The membrane-associated protein of T. denticola, which has a molecular mass of 70 kDa, has been shown to bind to soluble host proteins, such as plasminogen and fibronectin, and to be involved in the uptake of the peptide nutrients (14). A protein homologous to this protein was also identified in Treponema vincentii. The 44- and 43-kDa outer sheath polypeptides of T. denticola have strong hemin-binding activity, which might be involved in iron acquisition (9, 55). A major surface protein, MompA, was identified in Treponema pectinovorum, but its function is unclear (51).
Recently, the major surface proteins of T. maltophilum (MspA; 575 amino acids) and T. lecithinolyticum (MspTL; 590 amino acids) were identified (19, 42). The amino acid sequences of these proteins had 53% identity and 59% similarity. Functional elucidation of the surface proteins exhibiting high levels of homology in different oral spirochete species thus should provide new insights into the universal role of the surface molecules of spirochetes. An understanding of the functional roles of oral spirochete surface proteins at the molecular level could clarify the pathogenesis of the bacteria and provide an eradication strategy or a means of neutralizing their pathological effects.
The aim of this study was to analyze the functional role of the homologous proteins MspA and MspTL. We examined modulation of the host factors involved in chemotaxis and inflammation by MspA and MspTL in inflammatory cells and tooth-supporting tissue cells. The human monocytic cell line THP-1 and primary cultured human periodontal ligament (PDL) cells were used. It was observed that both Msps significantly induced the expression of intercellular adhesion molecule 1 (ICAM-1) and proinflammatory cytokines, such as interleukin-1β (IL-1β), IL-6, and IL-8, at the gene and/or protein level. The upregulation of these factors by both Msps was mediated via NF-κB activation.
MATERIALS AND METHODS
Bacteria and culture.
T. lecithinolyticum ATCC 700332 and T. maltophilum ATCC 51939 were cultured in an anaerobic atmosphere (10% CO2, 5% H2, 85% N2) by using OMIZ-Pat medium as described previously (53).
Escherichia coli DH5α-T1 (Invitrogen, Carlsbad, Calif.) was used for TA cloning, and E. coli M15 (QIAGEN, Valencia, Calif.) was used for expression of MspA of T. maltophilum and MspTL of T. lecithinolyticum. E. coli cells with plasmids were cultured aerobically in Luria-Bertani (LB) broth supplemented with 100 μg of ampicillin per ml (strain DH5α-T1) or with 100 μg of ampicillin per ml and 25 μg of kanamycin per ml (strain M15).
Analysis of the N-terminal amino acid sequence of MspTL.
The outer membrane fraction of T. lecithinolyticum was prepared as described previously (42), subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (10% polyacrylamide gel), and then transferred to a polyvinylidene difluoride membrane by using a semidry blotter (Bio-Rad, Hercules, Calif.). One half of the membrane preparation was subjected to immunoblot analysis with affinity-purified anti-MspTL antibody, and the other half was stained with Panceau (Sigma Chemical Co., St. Louis, Mo.). Immunoblotting was performed as described previously (42). The protein band corresponding to MspTL was cut out, and the N-terminal amino acid sequence of the protein was analyzed with a protein sequencer (model Procise 491; Applied Biosystems, Foster City, Calif.). The sequence obtained was D-E-P-S-A-E-A-K-I-A. This sequence corresponded to the N-terminal amino acid sequence of the mature MspTL protein which was predicted by PSORT analysis (42).
Cloning and expression of MspA and MspTL.
Both the mspA gene and the mspTL gene were amplified by PCR and cloned without the sequences encoding the leader peptides. The sequences of the primers used for the mspA gene were 5′-AAC TGG ATC CGC CGA ACC TGC TGC CGA G-3′ and 5′-CCC GGA GCT CTT ACA GAG CGA TTT TCG CT TT-3′, and the sequences of the primers used for the mspTL gene were 5′-AAC TGG ATC CGA TGA ACC TTC CGC CGA AGC A-3′ and 5′-CCC GGA GCT CTT ACA GAG CGA TTT TCG CCT T-3′. The underlined sequences are the introduced restriction sites for BamHI (GGATCC) and SacI (GAGCTC), and four nucleotides were added to 5′ ends of these restriction sites. After an initial denaturation step at 94°C for 4 min, PCR was performed in a 50 μl (total volume) mixture containing 15 pmol of each primer, 1.25 U of Taq polymerase (Perkin-Elmer Cetus), and 100 pmol of each deoxynucleoside triphosphate by using a thermal cycler (Perkin-Elmer, Foster City, Calif.) for 30 cycles of denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and extension at 72°C for 1 min. The reaction was completed by a final incubation at 72°C for 5 min. The PCR products (1,671 bp for mspA and 1,716 bp for mspTL [stop codon included]) were cloned in E. coli DH5α-T1 by using pCR2.1-TOPO and a TA cloning kit (Invitrogen). The plasmid DNA was isolated and digested with BamHI and SacI. The BamHI-SacI fragments, including the mspTL gene or the mspA gene, were cut out from the 1% agarose gel after electrophoresis, purified with a gel extraction kit (QIAGEN), and ligated into the expression vector pQE-30 predigested with BamHI and SacI. E. coli M15 was transformed with the recombinant plasmids, plated on LB agar plates containing antibiotics, and grown overnight. Single recombinant colonies were grown in LB broth containing antibiotics, expression of recombinant MspA (rMspA) and rMspTL was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h, and the proteins were identified by immunoblotting by using polyclonal antibodies directed against T. maltophilum and T. lecithinolyticum, respectively, and the anti-pentahistidine antibody (QIAGEN), as described previously (42).
After we confirmed that the major surface proteins were expressed, 50-ml overnight cultures of E. coli transformed with the mspA-pQE-30 or mspTL-pQE-30 construct were inoculated into 500 ml of fresh LB broth and cultured until the optical density at 260 nm was 0.6. After 1 mM IPTG was added, the cultures were incubated for an additional 4 h. The recombinant Msps formed in the inclusion bodies were purified as follows. The bacteria were harvested by centrifugation at 6,500 × g for 15 min at 4°C and resuspended in 50 ml of wash buffer containing 20 mM Tris-HCl (pH 7.5), 10 mM EDTA, and 1% Triton X-100. The cells were lysed by incubating them with 100 μg of lysozyme per ml at 40°C for 15 min and subsequently sonicating them with an ultrasonic processor (six 10-s bursts at 200 W with a 10-s cooling period between bursts; Sonics & Materials, Inc. Newtown, Conn.). In order to minimize protein degradation, phenylmethylsulfonyl fluoride was added to the cell lysates, and the inclusion bodies were obtained by centrifugation at 10,000 × g for 10 min. The pellets were resuspended in 50 ml of the wash buffer and then centrifuged. This procedure was repeated twice. The inclusion bodies were resuspended in solubilization buffer containing 50 mM 3-(cyclohexylamino)-1-propanesulfonic acid (pH 11.0) and 0.3% N-lauroylsarcosine and incubated at room temperature for 15 min. The suspension was then centrifuged at 10,000 × g for 10 min, and the supernatants containing the solubilized rMsps were collected and dialyzed against 50 volumes of phosphate-buffered saline (PBS) (pH 7.0) with three buffer changes for 24 h. The proteins were purified by using nickel-nitrilotriacetic acid agarose (QIAGEN), which bound to the histidine-tagged recombinant proteins, according to the manufacturer's protocol. The identities of purified rMspA and rMspTL were confirmed by SDS-PAGE and immunoblotting, as described above.
For the mock control, E. coli transformed with pQE-30 without the insert DNA was cultured and induced with IPTG. The subsequent procedures were the same as those used for preparation of the rMsps, and all of the chemicals used were from the same batches that were used for rMsps preparation. An irrelevant N-terminal hexahistidine-tagged surface protein of T. denticola ATCC 33521 (provisionally designated rTD92) was produced by using the same procedure. This protein was used to stimulate the host cells and to determine if there was any activity that originated from the vector.
The recombinant proteins were quantified with a bicinchoninic acid protein assay kit (Pierce, Rockford, Ill.).
Removal of the endotoxin in the rMsp preparation with polymyxin B-agarose.
The endotoxin (or lipopolysaccharide [LPS]) of E. coli, which may have been present in the rMsp preparations, was removed by using polymyxin B-agarose (Sigma Chemical Co.). Aliquots of polymyxin B-agarose (0.5 ml) were poured into columns (diameter, 10 mm) and washed with 20 volumes of 0.1 M ammonium bicarbonate. The rMsps (250 μl) were then loaded into the columns at a concentration of 100 μg/ml and incubated at room temperature for 60 min. The flow containing rMsps not bound to polymyxin B was collected, concentrated by using Centricon YM-30 (Millipore, Bedford, Mass.), and filtered through 0.25-μm-pore-size membrane filters. The mock extract was handled by using the same procedure.
Endotoxin assay.
Endotoxin activity in rMsp preparations was determined by the Limulus amebocyte lysate (LAL) assay by using an LAL Endochrome kit (Cahrles River Endosafe, Charleston, S.C.) according to the manufacturer's protocol.
Cell culture and treatment with rMspA and rMspTL.
The human monocytic THP-1 cell line was purchased from the American Type Culture Collection and was maintained in RPMI 1640 medium supplemented with 2 mM l-glutamine (Invitrogen), 10% heat-inactivated fetal bovine serum, and antibiotics (100 U of penicillin per ml and 100 μg of streptomycin sulfate per ml). The cells were cultured overnight and plated in 30-mm-diameter cell culture dishes at a concentration of 1 × 106 cells/ml in the absence of serum. The cells were then treated with rMsps or heat-treated rMsps (95°C for 2 h) for 12 to 24 h. The cells were harvested by centrifugation and then used for the flow cytometric analysis and RNA isolation. The cell-free supernatants were collected in new tubes and stored at −70°C for measurement of cytokines by an enzyme-linked immunosorbent assay (ELISA).
The PDL cells were obtained from donors with healthy periodontal tissues, as described previously (6). PDL cells between passages 5 and 9 were seeded at a concentration of 1 × 105 cells/ml in 30-mm-diameter cell culture dishes and grown in α-minimal essential medium supplemented with 10% fetal bovine serum for 1 to 2 days until they reached confluence. After the medium was replaced with serum-free medium, the cells were treated with the rMsps (1 to 10 μg/ml) or heat-treated rMsps (95°C for 2 h; 10 μg/ml) for 12 to 24 h. The cells were then used for flow cytometric analysis and RNA isolation after they were harvested by scrapping and centrifugation. The cell-free supernatants were collected in new tubes and stored at −70°C for measurement of cytokines by an ELISA. The number of bacteria equivalent to 1 μg of rMspA or rMspTL was approximately 5 × 107 cells, as judged by SDS-PAGE followed by immunoblotting of aliquots containing various numbers of bacteria which were counted with a Petroff-Hausser counting chamber (Hausser Scientific, Horsham, Pa.). The multiplicity of infection (MOI) was approximately 50 when THP-1 or PDL cells were stimulated with 1 μg of either rMspA or rMspTL.
In some experiments, the cells were pretreated with an NF-κB activation inhibitor, l-1-tosylamido-2-phenylethyl chloromethyl ketone (TPCK) (10 μM) for 1 h before the rMsps were added. In other experiments, the cells were incubated with rMsps in the presence of the IL-1 receptor antagonist (IL-1ra) (50 ng/ml; R&D Systems, Minneapolis, Minn.).
Human recombinant IL-1β (300 pg/ml; R&D Systems) and LPS of E. coli 111:B4 (10 μg/ml; Sigma Chemical Co.) were used in some experiments as stimuli for ICAM-1 and cytokine expression.
Flow cytometric analysis.
For ICAM-1 expression, THP-1 and PDL cells were treated with rMsps or heat-treated rMsps for 12 to 24 h, as described above, and subjected to flow cytometry. The cells (1 × 105 cells/100 μl) were washed with PBS, resuspended in 100 μl of PBS, and then reacted with 1 μl (0.5 mg/ml) of mouse anti-human ICAM-1 monoclonal antibody (MAb) (BD Biosciences, San Jose, Calif.) at 4°C for 20 min. After washing, the cells were stained with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG) (BD Biosciences) at 4°C for 20 min. The cells were washed with PBS, and the ICAM-1 expression in the THP-1 and PDL cells was analyzed by using a fluorescence-activated cell sorter (FACSCalibur; Becton Dickinson, San Jose, Calif.). The data were obtained by using 15,000 cells. Nontreated cells and cells treated with the mock extracts were used as negative controls. As a control for nonspecific binding, the cells were stained only with FITC-conjugated IgG, and no nonspecific reactivity was observed.
RT-PCR.
RNA was isolated from THP-1 and PDL cells treated with rMsps or heat-treated rMsps by using TRIzol reagent (Invitrogen Life Technology, Carlsbad, Calif.) according to the manufacturer's instructions. RNA (1 μg) was mixed with 200 pmol of oligo(dT) primer in a 50-μl (total volume) mixture and heated at 70°C for 5 min. After chilling on ice, the mixture was added to AccuPower RT premix (Bioneer, Daejeon, Korea) containing deoxynucleoside triphosphates, reverse transcriptase, and an RNase inhibitor and incubated at 42°C for 1 h. cDNA produced by reverse transcription (RT) was heated at 94°C for 5 min to inactivate the reverse transcriptase. cDNA (1 to 4 μl) was then subjected to PCR for ICAM-1, IL-1β, IL-6, and IL-8 in 50-μl (total volume) mixtures containing the appropriate forward and reverse primers (15 pmol each), 1.25 U of Taq polymerase (Perkin-Elmer), and 100 pmol of each deoxynucleoside triphosphate as described above. The PCR products (10 μl) were then subjected to electrophoresis on ethidium bromide-stained 1% agarose gels and visualized with UV fluorescence. A PCR without RT was performed as a negative control. The housekeeping gene, the gene encoding glyceraldehyde-3-phosphate dehydrogenase, was used as an internal control for gene expression.
The sequences of the primers and annealing temperatures were as follows: for ICAM-1, sense primer 5′-AGA AAT TGG CTC CAT GGT GAT CTC-3′, antisense primer 5′-ACA TGC AGC ACC TCC TGT GAC CA-3′, and 60°C; for IL-1β, sense primer 5′-GAT AAG CCC ACT CTA CAG CT-3′, antisense primer 5′-ATT GGC CCT GAA AGG AGA GA-3′, and 60°C; for IL-6, sense primer 5′-ATG AAC TCC TTC TCC ACA AGC GC-3′, antisense primer 5′-GAA GAG CCC TCA GGC TGG ACT G-3′, and 65°C; for IL-8, sense primer 5′-ATG ACT TCC AAG CTG GCC GTG GCT-3′, antisense primer 5′-TCT CAG CCC TCT TCA AAA ACT TCT C-3′, and 60°C; and for glyceraldehyde-3-phosphate dehydrogenase, sense primer 5′-CAC TGA CAC GTT GGC AGT GG-3′, antisense primer 5′-CAT GGA GAA GGC TGG GGC TC-3′, and 62°C.
ELISA for IL-1β, IL-6, and IL-8.
The culture supernatants were assayed to determine the cytokine levels. IL-1β and IL-8 were measured by using ELISA kits obtained from R&D Systems. IL-6 was measured by using ELISA kits obtained from Pierce.
Statistical analysis.
The statistical significance of the differences between nontreated and rMsp-treated cells or between rMsp-treated and rMsp- and inhibitor-treated cells was evaluated by a one-way analysis of variance. A P value of <0.05 was considered significant.
RESULTS
Cloning and overexpression of the Msp genes, mspA and mspTL.
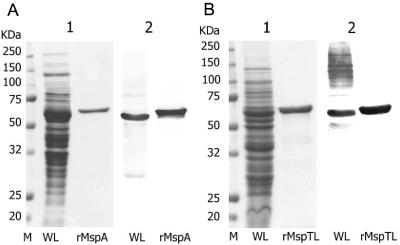
MspA (571 amino acids) and MspTL (556 amino acids) were successfully expressed in E. coli by using the pQE-30 vector after PCR and TA cloning of the mspA and mspTL genes. The rMsps were tagged with N-terminal hexahistidine and purified by affinity chromatography with nickel-nitrilotriacetic acid agarose. The recombinant proteins were produced without the signal peptides. The signal peptide sequence of MspA (19 amino acids) was deduced from the data reported by Heuner et al. (19), and the signal peptide sequence of MspTL (19 amino acids) was deduced from the peptide sequences of the mature protein analyzed in this study. Since the recombinant proteins were formed in inclusion bodies in E. coli, the purification procedures for these proteins included solubilization with detergents and renaturation by slow dialysis in PBS. E. coli LPS contamination of the rMsps was removed by using polymyxin B-agarose. The endotoxin activity of purified rMsps was determined by the LAL assay and was compared to that of E. coli 111:B4 LPS. The endotoxicity was 0.024 endotoxin unit (EU)/μg for rMspA and 0.015 EU/μg for rMspTL, whereas the endotoxicity of E. coli LPS was 149.11 EU/μg. The sizes of rMspA and rMspTL corresponded to the predicted molecular masses (approximately 60 and 62 kDa, respectively) (Fig. 1). (The rMsps had slightly higher molecular masses than the native proteins.)
FIG. 1.
Purification of rMspA and rMspTL by SDS-PAGE (gel 1) and immunoblotting (gel 2). The whole-cell lysates (WL) and rMsps of T. maltophilum and T. lecithinolyticum (rMspA and rMspTL, respectively) were separated on an SDS—10% polyacrylamide gel, transferred to nitrocellulose membranes, and reacted with polyclonal antibodies directed against T. maltophilum (A) and T. lecithinolyticum, respectively (B). M, molecular mass standards.
Induction of ICAM-1 expression by rMspA and rMspTL in THP-1 cells.
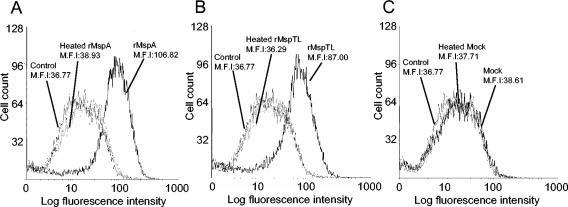
Because in this study we were interested in the cell adhesion molecules, the effects of rMspA and rMspTL on ICAM-1 expression by THP-1 cells were examined. THP-1 cells were treated with 10 μg of an rMsp per ml (corresponding to an MOI of 500:1) for 12 to 24 h, and the level of surface-expressed ICAM-1 was determined by flow cytometric analysis. rMspA and rMspTL strongly induced ICAM-1 expression, as shown in Fig. 2, whereas the mock extract treatment did not induce ICAM-1 expression. The maximum level of expression occurred at 12 h. Heat-treated rMspA or rMspTL did not induce any ICAM-1 expression. An unrelated N-terminal hexahistidine-tagged surface protein of T. denticola (rTD92) was used to determine if the part originating from the vector sequences had any biological activity. This protein did not affect ICAM-1 expression (data not shown).
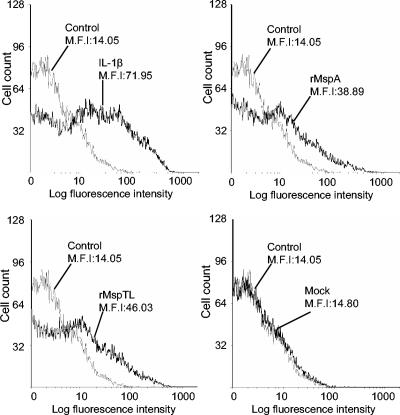
FIG. 2.
Flow cytometric analysis of the effect of rMsps on ICAM-1 expression in THP-1 cells. The THP-1 cells were cultured overnight. After the medium was replaced with serum-free medium, the cells (1 × 106 cells/ml) in 30-mm-diameter culture dishes were treated with rMspA or rMspTL (10 μg/ml) or heat-treated rMspA or rMspTL (95°C for 2 h; 10 μg/ml) for 12 h. ICAM-1 expression was analyzed by fluorescence-activated cell sorting by using anti-human ICAM-1 MAb and FITC-labeled IgG. A mock extract was used to confirm the complete removal of any possible LPS contamination. Nontreated cells were used as a control. The experiments were performed three times, and representative data are shown. M.F.I, mean fluorescence intensity.
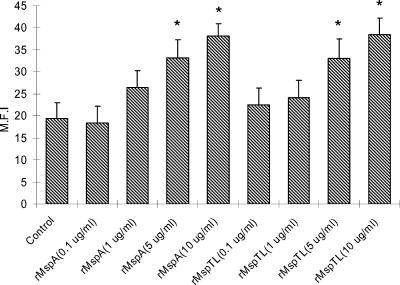
The dose-dependent induction of ICAM-1 expression by both rMsps is shown in Fig. 3.
FIG. 3.
Dose-dependent induction of ICAM-1 expression in THP-1 cells by rMsps. The THP-1 cells were cultured overnight. After the medium was replaced with serum-free medium, the cells (1 × 106 cells/ml) in 30-mm-diameter culture dishes were treated with rMspA or rMspTL at concentrations of 0.1 μg/ml (MOI, 5), 1 μg/ml (MOI, 50), 5 μg/ml (MOI, 250), and 10 μg/ml (MOI, 500) for 12 h. ICAM-1 expression was analyzed by fluorescence-activated cell sorting by using anti-human ICAM-1 MAb and FITC-labeled IgG. The experiments were performed three times, and representative data are shown. The data are means and standard deviations. An asterisk indicates that the value is significantly different than the value for the nontreated control (P < 0.05). M.F.I, mean fluorescence intensity.
Expression of mRNA for ICAM-1 and cytokines in PDL cells treated with rMspA and rMspTL.
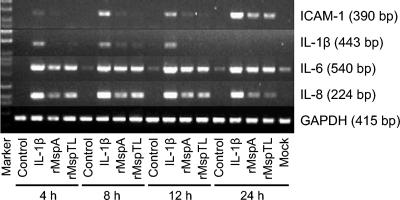
After we determined that the rMsps stimulated ICAM-1 expression in THP-1 cells, the activities of these proteins were evaluated in PDL cells. PDL cells were treated with the rMsps for 4 to 24 h, and the levels of mRNA for ICAM-1 and the proinflammatory cytokines were analyzed by RT-PCR. As shown in Fig. 4, rMsps markedly induced expression of mRNA for ICAM-1, IL-6, and IL-8, whereas they had a minor effect on IL-1β mRNA expression. The expression of mRNA for ICAM-1 and IL-8 peaked after 24 and 8 to 12 h of treatment, respectively, whereas the maximum level of IL-6 mRNA expression occurred at 8 h and this level was maintained for 24 h. In none of the cases involving heat-treated rMsps was there induction activity, and PCR without RT resulted in no bands (data not shown).
FIG. 4.
Induction of expression of mRNA for ICAM-1, IL-1β, IL-6, and IL-8 by rMsps in PDL cells. The PDL cells (1 × 105 cells/ml) were cultured in 30-mm-diameter culture dishes to confluence. After the medium was replaced with serum-free medium, the cells were treated with rMspA (10 μg/ml) or rMspTL (10 μg/ml) for 4, 8, 12, or 24 h. The total RNA was isolated from the cells, and the levels of mRNA for ICAM-1, IL-1β, IL-6, and IL-8 were determined by RT-PCR. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Induction of expression of ICAM-1 and cytokines in the PDL cells by rMspA and rMspTL.
Induction of the expression of mRNA for ICAM-1 and the proinflammatory cytokines by the rMsps was confirmed at the protein level. As observed in THP-1 cells, rMspA and rMspTL significantly induced ICAM-1 expression in the PDL cells (Fig. 5). The IL-6 and IL-8 mRNA stimulation observed by RT-PCR correlated well with the protein level, as detected by the ELISA. As shown in Fig. 6, compared with the secretion in nontreated cells, both rMspA and rMspTL significantly induced secretion of IL-6 and IL-8 in the PDL cells. The mock extract-treated cells did not show any induction activity in any experiment (data shown only for 24 h of incubation). IL-1β secreted into the medium of the PDL cell cultures was not detected by the ELISA kit with a detection limit of 1 pg/ml. However, IL-1β secreted into the culture supernatants of the THP-1 cell cultures were detected at levels of 28.4 and 22.0 pg/ml when the preparations were treated with rMspA and rMspTL, respectively, for 12 h.
FIG. 5.
Flow cytometric analysis of the effect of rMsps on ICAM-1 expression in PDL cells. The PDL cells (1 × 105 cells/ml) were cultured in 30-mm-diameter culture dishes to confluence. After the medium was replaced with serum-free medium, the cells were treated with rMspA (10 μg/ml) or rMspTL (10 μg/ml) for 24 h. ICAM-1 expression was analyzed by fluorescence-activated cell sorting by using anti-human ICAM-1 MAb and FITC-labeled IgG. Nontreated cells were used as a control. The experiments were performed three times, and representative data are shown. M.F.I, mean fluorescence intensity.
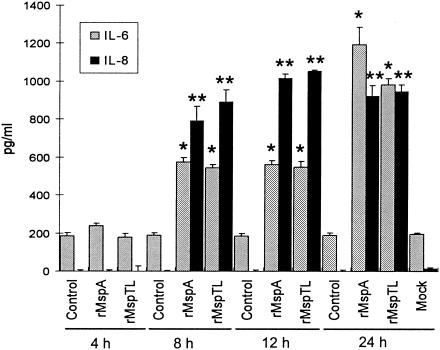
FIG. 6.
Cytokine production by PDL cells treated with rMsps. PDL cells (1 × 105 cells/ml) were cultured in 30-mm-diameter culture dishes to confluence. After the medium was replaced with serum-free medium, the cells were treated with rMspA (10 μg/ml) or rMspTL (10 μg/ml) for 4, 8, 12, or 24 h. Cytokines secreted into the culture supernatants were measured by ELISA. Nontreated cells were used as a control. The experiments were performed twice, and representative means and standard deviations for triplicate values are shown. The asterisks indicate significant differences (P < 0.05) for IL-6 and IL-8 compared with the nontreated control values.
Effect of an NF-κB activation inhibitor.
TPCK (an IκB protease inhibitor) was used to inhibit the effects of the rMsps in order to analyze the involvement of NF-κB activation in the signal transduction pathway leading to expression of ICAM-1 or the proinflammatory cytokines by the rMsps. Flow cytometric analysis showed that pretreating the THP-1 cells with TPCK completely inhibited the ICAM-1 expression induced by the rMsps (Fig. 7A). In the PDL cells pretreated with TPCK there was also a reduction in the ICAM-1 and cytokine levels induced by the rMsps to the control levels (Fig. 7B and C). TPCK completely inhibited E. coli LPS-induced ICAM-1, IL-6, and IL-8 expression.
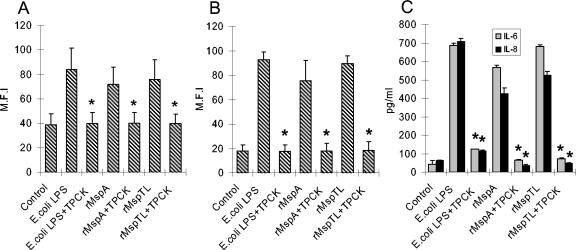
FIG. 7.
Effects of TPCK on ICAM-1 and cytokine expression induced by rMsps. THP-1 and PDL cells were cultured as described in the legends to Fig. 2 and 5, but the cells were preincubated with TPCK (10 μM) for 1 h before the rMsps were added. The ICAM-1 expression of the THP-1 cells (A) and PDL cells (B) was analyzed by fluorescence-activated cell sorting by using anti-human ICAM-1 MAb and FITC-labeled IgG. The supernatants of the PDL cells were assayed for IL-6 and IL-8 by ELISA (C). E. coli LPS (10 μg/ml) was used as a positive control. The experiments were performed three times, and representative means and standard deviations for the mean fluorescence intensity (M.F.I) for ICAM-1 and means and standard deviations for triplicate values for cytokines are shown. An asterisk indicates that the value is significantly different (P < 0.05) than the value obtained for the culture without TPCK.
Effect of IL-1ra on ICAM-1 and cytokine upregulation.
Since IL-1β has been reported to induce ICAM-1 and IL-8 expression, in this study we examined whether upregulation of ICAM-1 and IL-8 by the rMsps was mediated via the release of IL-1β or was caused by a direct interaction between the cells. In order to do this, THP-1 cells were used because IL-1β could not be detected at the protein level in PDL cells stimulated by the rMsps. The THP-1 cells were stimulated with the rMsps or IL-1β in the presence of an excess of an the IL-1 antagonist IL-1ra (50 ng/ml). Although IL-1ra completely inhibited ICAM-1 and IL-8 expression induced by IL-1β (300 pg/ml) added to the medium, it did not reverse the induction of ICAM-1 or IL-8 expression by both rMsps (Fig. 8).
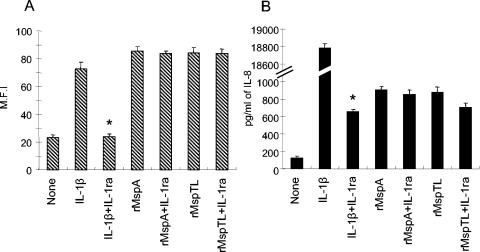
FIG. 8.
Effect of IL-1ra on ICAM-1 and IL-8 upregulation by rMsps. THP-1 cells (1 × 105 cells/ml) were cultured overnight. After the medium was replaced with serum-free medium, the cells were treated simultaneously with IL-1ra (50 ng/ml) and rMspA (10 μg/ml) or rMspTL (10 μg/ml) for 12 h. The ICAM-1 expression of the cells was analyzed by fluorescence-activated cell sorting (A). The supernatants were assayed for IL-8 by ELISA (B). The experiments were repeated three times, and representative means and standard deviations for mean fluorescence intensity (M.F.I) for ICAM-1 and means and standard deviations of triplicate values for IL-8 are shown. IL-1β (300 pg/ml) was used as a positive control. An asterisk indicates that the value is significantly different (P < 0.05) than the value obtained for the culture without IL-1ra. None, nontreated control.
DISCUSSION
Although 10 oral spirochetes have been cultivated so far, most studies on the pathogenesis of periodontitis have been performed with T. denticola (5, 13, 44, 52). However, recent studies have shown that T. lecithinolyticum is involved in MMP-2 activation and osteoclastogenesis (6, 7). These results, as well as those of epidemiological studies showing frequent association of this bacterium with periodontitis (53) and endodontic infections (46), prompted us to investigate the pathogenesis of T. lecithinolyticum and its closest relative, T. maltophilum, at the molecular level. In this study, the surface molecules of the bacteria were used to examine their functional roles. This is because when analyzed by SDS-PAGE, MspA from T. maltophilum and MspTL from T. lecithinolyticum are among the most abundant proteins in the outer membrane fractions of these bacteria and their amino acid sequences exhibit a high level of homology. The two proteins each have a cleavable signal sequence consisting of 19 amino acid residues. The location of MspA in the outer membrane was determined by immunoelectron microscopy (20), and the location of MspTL was determined by subcellular fractionation and subsequent immunoblot analysis (42). These proteins are suitable candidate molecules for examining the common pathogenesis. Like the structural similarity of these two proteins, this study showed that the functional roles of these protein in inducing expression of ICAM-1 and the proinflammatory cytokines are also similar.
Recombinant proteins produced in E. coli are likely to be contaminated with the LPS of the bacterium. Recent studies showed that the induction of tumor necrosis factor alpha expression in murine macrophages by recombinant human heat shock protein 70 was due to LPS contaminants (15). Therefore, LPS contamination must be considered when proteins are used in the recombinant forms produced in E. coli. Polymyxin B-agarose was used to remove the LPS that may have contaminated the rMsp preparations. The endotoxin activity of the rMsps was very minimal and was approximately 6,000 times less than that of E. coli LPS. In our experimental conditions without addition of serum to cell cultures, E. coli LPS equivalent to 0.239 EU (the endotoxin activity in 10 μg of rMspA) did not induce ICAM-1 expression, and the fact that heat-treated Msps or the mock extract did not have any biological activity supports the conclusion that endotoxin did not affect the results.
rMspA and rMspTL significantly induced expression of ICAM-1 and the proinflammatory cytokines in the monocytic cell line THP-1 and/or PDL cells at similar levels. rTD92 did not induce ICAM-1 expression, which suggests that induction of ICAM-1 expression does not depend on the N-terminal histidine tag but is specific for rMspA and rMspTL.
ICAM-1 is a cell surface adhesion molecule that is involved in the innate immune response. It recruits leukocytes to the inflammation site by cell-cell interactions and leukocyte extravasation through the vascular endothelium. ICAM-1 expression is significantly increased at inflammation sites, and involvement of this molecule in the pathogenesis of inflammatory diseases like rheumatoid arthritis has been implicated (34). Leukocytes, including monocytes, play an important role in the inflammatory responses. The recruitment of leukocytes at inflammation sites is mediated through a variety of adhesion molecules on the cell surface, including L-selectin and β2 integrins (CD11a/CD18 and CD11b/CD18). ICAM-1 is a major adhesion molecule which is expressed on endothelial cells and binds to β2 integrins on leukocytes. However, it is also expressed on leukocytes and participates in leukocyte recruitment via a leukocyte-leukocyte interaction.
PDL, which is mainly composed of fibroblasts, resides between the cementum of the tooth root surface and the alveolar bone, and it has an anchorage function. PDL cells have also been implicated in local inflammation. The inflammation and subsequent destruction of the PDL cells can lead to tooth movement and loss. Periodontal infections frequently lead to exposure of PDL cells to bacteria, bacterial products, and proinflammatory mediators released from the neighboring cells. Only two groups have reported induction of ICAM-1 expression in the PDL or PDL cells so far. Toms et al. (49) reported ICAM-1 expression in rat PDL blood vessels that were exposed to endotoxin, as determined by immunohistochemical analysis. Joe et al. (23) demonstrated that IL-1β-induced ICAM-1 upregulation occurred in PDL cells.
Upregulated expression of ICAM-1 has been observed in gingival tissues and gingival crevicular fluid (GCF) from periodontitis patients (18). A high level of soluble ICAM-1 in the GCF of periodontitis patients appears to result from increased shedding of the membrane-bound ICAM-1, which was upregulated in inflamed gingival tissue (31). It has been suggested that ICAM-1 is a therapeutic target in the inflamed gingiva based on local topical application of ICAM-1-directed antisense oligonucleotides (36). Among the periodontopathogens, LPS and purified fimbriae of Porphyromonas gingivalis and the glycoprotein fraction of Prevotella intermedia have been reported to induce ICAM-1 expression in human gingival fibroblasts (28, 48). In Eikenella corrodens, an N-acetyl-d-galactosamine-specific lectin-like substance, an adhesin, was reported to be involved in induction of ICAM-1 expression in human oral epithelial cells (56). Our finding that MspA and MspTL induced ICAM-1 expression is the first report for oral treponemes. ICAM-1 expression in monocytes and PDL cells induced by MspA and MspTL might contribute to the recruitment and retention of the inflammatory cells in periodontal lesions.
IL-6, together with IL-1β and tumor necrosis factor alpha, is a major mediator of the host response to tissue injury and bone resorption. IL-6 binds to its receptor, which exists in either a membrane-bound form (CD126) or a soluble form (sIL-6R alpha). This complex subsequently binds to a signal transducing protein, 130-kDa glycoprotein (gp130 or CD130) (41). This signal can lead to upregulation of the ligand of the receptor activator of NF-κB (RANKL) in osteoblasts that is a key factor in osteoclastogenesis. PDL cells have osteoblast-like characteristics, and PDL cells challenged with MspA and MspTL can participate in root absorption by enhancing IL-6 production and the subsequent RANKL synthesis. However, more studies are needed to determine if PDL cells express the IL-6 receptors or respond to IL-6. Gingival fibroblasts have been reported to bind to the IL-6-sIL-6R complex, and the amount of sIL-6R in the inflamed gingiva was reported to be sufficient for binding to IL-6, even if the gingival fibroblasts themselves did not express sufficient membrane-bound IL-6R (35).
IL-8 is a potent neutrophil chemotactic and activating cytokine, which amplifies immune responses by attracting and activating the immune cells to produce proinflammatory mediators. It is believed to play an important role in initiating the inflammatory reactions in gingival tissues, and its level of expression is related to the severity of periodontitis. Stimulated IL-8 production in the inflamed gingiva has been reported in the epithelium (45, 48), gingival fibroblasts (52), and monocytes (47). In addition, the level of IL-8 was significantly elevated in the GCF of periodontitis patients compared to the GCF of healthy subjects (16). The surface molecules that showed enhanced IL-8 secretion were P. gingivalis gingipain-R (40), a glycoprotein fraction in the cell surface from P. intermedia (48), and a capsular polysaccharide from Actinobacillus actinomycetemcomitans (39). Several oral treponemes have been reported to stimulate proinflammatory cytokines in various cell types, such as gingival fibroblasts and gingival epithelial cells (2, 25, 37, 43). However, with the exception of their LPS or LPS-like materials, none of them has been analyzed at the molecular level. In addition, there are only limited data on induction of IL-8 expression in PDL cells as a result of bacterial factors (12, 17), although IL-1β is known to be a stimulus for IL-8 expression in PDL cells (1, 26). In the present study, we showed that the surface proteins of oral spirochetes cause IL-8 expression in PDL cells.
In order to determine the mechanism by which Msps induced the expression of ICAM-1 and cytokines, the involvement of NF-κB was tested by using TPCK, an inhibitor of NF-κB activation, which prevents degradation of the predominant inhibitory molecule, IκBα, and thereby inhibits the translocation of NF-κB into the nucleus. The blocking of NF-κB activation by TPCK completely inhibited the expression of ICAM-1, IL-6, and IL-8 in both the THP-1 or PDL cells after Msp treatment. These results suggest that the Msp-induced ICAM-1 and cytokine expression is mediated via NF-κB activation. Arg-Gly-Asp (RGD)-dependent NF-κB activation that leads to upregulation of epithelial ICAM-1 expression was observed in human bronchial epithelial cells with filamentous hemagglutinin of Bordetella pertussis (21). Interestingly, MspTL possesses an RGD motif, but MspA does not.
Since IL-1β is known to induce the synthesis of ICAM-1 and proinflammatory cytokines (IL-1b, IL-6, and IL-8) (23, 26), the role of IL-1β was examined by using IL-1ra, which neutralized the IL-1 activity. IL-1ra binds to the IL-1 receptors without triggering any biological response and acts as a competitive inhibitor of IL-1 activity. As we could not detect IL-1β secreted from PDL cells when they were treated with rMspA or rMspTL, we examined the IL-1ra effect on THP-1 cells. IL-1ra did not reverse the levels of ICAM-1 and IL-8 expression in the THP-1 cells induced by the two Msps, whereas it completely inhibited the ICAM-1 and IL-8 expression induced by exogenously added IL-1β. This means that induction of ICAM-1 expression in the THP-1 cells was not mediated via IL-1β. A. actinomycetemcomitans-induced IL-8 expression has also been reported to be not mediated by IL-1β (45).
The recognition of pathogenic bacteria involves specific recognition of the pathogen-associated molecular patterns by host receptors. The family of Toll-like receptors (TLRs) of host cells is known to play a central role in this process. Among the known pathogen-associated molecular patterns are those for LPS, peptidoglycan, CpG DNA, double-stranded viral RNA, lipoprotein, lipoteichoic acid, and zymosan. There is an increasing amount of data showing that the bacterial proteins also interact with the specific TLRs to transduce the intracellular signals. Among these proteins are bacterial flagellin (33), outer membrane protein A from Klebsiella pneumoniae (OmpA) (4), Neisseria meningitidis porin (PorB) (29), and P. gingivalis fimbriae (38). In bacterial flagellin, a well-conserved motif consisting of 14 amino acids at the N terminus was found to interact with TLR5 and result in regulation of the proinflammatory activity (33). In this context, future studies should focus on determining the involvement of the TLRs in MspA or MspTL recognition and mapping the common regions of the Msps that recognize and transduce the signal to stimulate the host cells to secrete ICAM-1 and the proinflammatory cytokines. Interestingly, outer membrane extracts of T. denticola, T. vincentii, and Treponema medium stimulated IL-8 production in the human gingival epithelial cells, which appeared to be mediated through Toll-like receptor 2 (2).
In summary, the Msps of the group IV oral spirochetes T. maltophilum and T. lecithinolyticum activated human monocytes and PDL cells to induce production of ICAM-1 and proinflammatory cytokines, such as IL-1β, IL-6, and IL-8. This induction was mediated by NF-κB activation. The upregulation of both ICAM-1 and IL-8 by either MspA or MspTL might play an important role in amplifying the local immune response by continuous inflammatory cell recruitment and retention at infection sites.
Acknowledgments
This work was supported by grant 03-PJ1-PG3-20200-0004 from the Ministry of Health and Welfare of Korea.
Editor: J. T. Barbieri
REFERENCES
- 1.Agarwal, S., C. S. Chandra, N. P. Piesco, H. H. Langkamp, L. Bowen, and C. Baran. 1998. Regulation of periodontal ligament cell functions by interleukin-1beta. Infect. Immun. 66:932-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asai, Y., T. Jinno, and T. Ogawa. 2003. Oral treponemes and their outer membrane extracts activate human gingival epithelial cells through Toll-like receptor 2. Infect. Immun. 71:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batista Da Silva, A. P., W. Lee, E. Bajenova, C. A. McCulloch, and R. P. Ellen. 2004. The major outer sheath protein of Treponema denticola inhibits the binding step of collagen phagocytosis in fibroblasts. Cell. Microbiol. 6:485-498. [DOI] [PubMed] [Google Scholar]
- 4.Chalifour, A., P. Jeannin, J. F. Gauchat, A. Blaecke, M. Malissard, T. N′Guyen, N. Thieblemont, and Y. Delneste. 2004. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers α-defensin production. Blood 104:1778-1783. [DOI] [PubMed] [Google Scholar]
- 5.Chan, E. C., and R. McLaughlin. 2000. Taxonomy and virulence of oral spirochetes. Oral Microbiol. Immunol. 15:1-9. [DOI] [PubMed] [Google Scholar]
- 6.Choi, B. K., J. H. Jung, H. Y. Suh, Y. J. Yoo, K. S. Cho, J. K. Chai, and C. K. Kim. 2001. Activation of matrix metalloproteinase-2 by a novel oral spirochetal species Treponema lecithinolyticum. J. Periodontol. 72:1594-1600. [DOI] [PubMed] [Google Scholar]
- 7.Choi, B. K., H. J. Lee, J. H. Kang, G. J. Jeong, C. K. Min, and Y. J. Yoo. 2003. Induction of osteoclastogenesis and matrix metalloproteinase expression by the lipooligosaccharide of Treponema denticola. Infect. Immun. 71:226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi, B. K., B. J. Paster, F. E. Dewhirst, and U. B. Göbel. 1994. Diversity of cultivable and uncultivable oral spirochetes from a patient with severe destructive periodontitis. Infect. Immun. 62:1889-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu, L., M. Song, and S. C. Holt. 1994. Effect of iron regulation on expression and hemin-binding function of outer-sheath proteins from Treponema denticola. Microb. Pathog. 16:321-335. [DOI] [PubMed] [Google Scholar]
- 10.Correia, F. F., A. R. Plummer, R. P. Ellen, C. Wyss, S. K. Boches, J. L. Galvin, B. J. Paster, and F. E. Dewhirst. 2003. Two paralogous families of a two-gene subtilisin operon are widely distributed in oral treponemes. J. Bacteriol. 185:6860-6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewhirst, F. E., M. A. Tamer, R. E. Ericson, C. N. Lau, V. A. Levanos, S. K. Boches, J. L. Galvin, and B. J. Paster. 2000. The diversity of periodontal spirochetes by 16S rRNA analysis. Oral Microbiol. Immunol. 15:196-202. [DOI] [PubMed] [Google Scholar]
- 12.Engels-Deutsch, M., A. Pini, Y. Yamashita, Y. Shibata, Y. Haikel, M. Scholler-Guinard, and J. P. Klein. 2003. Insertional inactivation of pac and rmlB genes reduces the release of tumor necrosis factor alpha, interleukin-6, and interleukin-8 induced by Streptococcus mutans in monocytic, dental pulp, and periodontal ligament cells. Infect. Immun. 71:5169-5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenno, J. C., P. M. Hannam, W. K. Leung, M. Tamura, V. J. Uitto, and B. C. McBride. 1998. Cytopathic effects of the major surface protein and the chymotrypsinlike protease of Treponema denticola. Infect. Immun. 66:1869-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenno, J. C., M. Tamura, P. M. Hannam, G. W. Wong, R. A. Chan, and B. C. McBride. 2000. Identification of a Treponema denticola OppA homologue that binds host proteins present in the subgingival environment. Infect. Immun. 68:1884-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao, B., and M. F. Tsan. 2003. Endotoxin contamination in recombinant human heat shock protein 70 (Hsp70) preparation is responsible for the induction of tumor necrosis factor alpha release by murine macrophages. J. Biol. Chem. 278:174-179. [DOI] [PubMed] [Google Scholar]
- 16.Giannopoulou, C., J. J. Kamma, and A. Mombelli. 2003. Effect of inflammation, smoking and stress on gingival crevicular fluid cytokine level. J. Clin. Periodontol. 30:145-153. [DOI] [PubMed] [Google Scholar]
- 17.Hatakeyama, J., R. Tamai, A. Sugiyama, S. Akashi, S. Sugawara, and H. Takada. 2003. Contrasting responses of human gingival and periodontal ligament fibroblasts to bacterial cell-surface components through the CD14/Toll-like receptor system. Oral Microbiol. Immunol. 18:14-23. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi, J., I. Saito, I. Ishikawa, and N. Miyasaka. 1994. Effects of cytokines and periodontopathic bacteria on the leukocyte function-associated antigen 1/intercellular adhesion molecule 1 pathway in gingival fibroblasts in adult periodontitis. Infect. Immun. 62:5205-5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heuner, K., B. K. Choi, R. Schade, A. Moter, A. Otto, and U. B. Göbel. 1999. Cloning and characterization of a gene (mspA) encoding the major sheath protein of Treponema maltophilum ATCC 51939T. J. Bacteriol. 181:1025-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heuner, K., U. Meltzer, B. K. Choi, and U. B. Göbel. 2001. Outer sheath associated proteins of the oral spirochete Treponema maltophilum. FEMS Microbiol. Lett. 197:187-193. [DOI] [PubMed] [Google Scholar]
- 21.Ishibashi, Y., and A. Nishikawa. 2003. Role of nuclear factor-kappa B in the regulation of intercellular adhesion molecule 1 after infection of human bronchial epithelial cells by Bordetella pertussis. Microb. Pathog. 35:169-177. [DOI] [PubMed] [Google Scholar]
- 22.Ishihara, K., and K. Okuda. 1999. Molecular analysis for pathogenicity of oral treponemes. Microbiol. Immunol. 43:495-503. [DOI] [PubMed] [Google Scholar]
- 23.Joe, B. H., J. L. Borke, M. Keskintepe, P. J. Hanes, J. M. Mailhot, and B. B. Singh. 2001. Interleukin-1beta regulation of adhesion molecules on human gingival and periodontal ligament fibroblasts. J. Periodontol. 72:865-870. [DOI] [PubMed] [Google Scholar]
- 24.Jung, I. Y., B. K. Choi, K. Y. Kum, Y. J. Yoo, T. C. Yoon, S. J. Lee, and C. Y. Lee. 2001. Identification of oral spirochetes at the species level and their association with other bacteria in endodontic infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 92:329-334. [DOI] [PubMed] [Google Scholar]
- 25.Kesavalu, L., C. W. Falk, K. J. Davis, M. J. Steffen, X. Xiaoping, S. C. Holt, and J. L. Ebersole. 2002. Biological characterization of lipopolysaccharide from Treponema pectinovorum. Infect. Immun. 70:211-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Long, P., J. Hu, N. Piesco, M. Buckley, and S. Agarwal. 2001. Low magnitude of tensile strain inhibits IL-1beta-dependent induction of pro-inflammatory cytokines and induces synthesis of IL-10 in human periodontal ligament cells in vitro. J. Dent. Res. 80:1416-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loesche, W. J. 1988. The role of spirochetes in periodontal disease. Adv. Dent. Res. 2:275-283. [DOI] [PubMed] [Google Scholar]
- 28.Masaka, T., J. Hayashi, and I. Ishikawa. 1999. Soluble CD14-dependent intercellular adhesion molecular-1 induction by Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. J. Periodontol. 70:772-778. [DOI] [PubMed] [Google Scholar]
- 29.Massari, P., P. Henneke, Y. Ho, E. Latz, D. T. Golenbock, and L. M. Wetzler. 2002. Cutting edge: immune stimulation by neisserial porins is Toll-like receptor 2 and MyD88 dependent. J. Immunol. 168:1533-1537. [DOI] [PubMed] [Google Scholar]
- 30.Mathers, D. A., W. K. Leung, J. C. Fenno, Y. Hong, and B. C. McBride. 1996. The major surface protein complex of Treponema denticola depolarizes and induces ion channels in HeLa cell membranes. Infect. Immun. 64:2904-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mole, N., A. Kennel-de March, G. Martin, N. Miller, M. C. Bene, and G. C. Faure. 1998. High levels of soluble intercellular adhesion molecule-1 (ICAM-1) in crevicular fluid of periodontitis patients with plaque. J. Clin. Periodontol. 25:754-758. [DOI] [PubMed] [Google Scholar]
- 32.Moter, A., C. Hoenig, B. K. Choi, B. Riep, and U. B. Göbel. 1998. Molecular epidemiology of oral treponemes associated with periodontal disease. J. Clin. Microbiol. 36:1399-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murthy, K. G., A. Deb, S. Goonesekera, C. Szabo, and A. L. Salzman. 2004. Identification of conserved domains in Salmonella muenchen flagellin that are essential for its ability to activate TLR5 and to induce an inflammatory response in vitro. J. Biol. Chem. 279:5667-5675. [DOI] [PubMed] [Google Scholar]
- 34.Nakayamada, S., K. Saito, K. Fujii, M. Yasuda, M. Tamura, and Y. Tanaka. 2003. beta1 integrin-mediated signaling induces intercellular adhesion molecule 1 and Fas on rheumatoid synovial cells and Fas-mediated apoptosis. Arthritis Rheum. 48:1239-1248. [DOI] [PubMed] [Google Scholar]
- 35.Naruishi, K., S. Takashiba, H. H. Chou, H. Arai, F. Nishimura, and Y. Murayama. 1999. Role of soluble interleukin-6 receptor in inflamed gingiva for binding of interleukin-6 to gingival fibroblasts. J. Periodontal Res. 34:296-300. [DOI] [PubMed] [Google Scholar]
- 36.Nedbal, W., P. Tomakidi, M. J. Lehmann, C. Dorfer, A. Kohl, and G. Sczakiel. 2002. Antisense-mediated inhibition of ICAM-1 expression: a therapeutic strategy against inflammation of human periodontal tissue. Antisense Nucleic Acid Drug Dev. 12:71-78. [DOI] [PubMed] [Google Scholar]
- 37.Nixon, C. S., M. J. Steffen, and J. L. Ebersole. 2000. Cytokine responses to Treponema pectinovorum and Treponema denticola in human gingival fibroblasts. Infect. Immun. 68:5284-5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogawa, T., Y. Asai, M. Hashimoto, and H. Uchida. 2002. Bacterial fimbriae activate human peripheral blood monocytes utilizing TLR2, CD14 and CD11a/CD18 as cellular receptors. Eur. J. Immunol. 32:2543-2550. [DOI] [PubMed] [Google Scholar]
- 39.Ohguchi, Y., Y. Ishihara, M. Ohguchi, M. Koide, N. Shirozu, T. Naganawa, T. Nishihara, and T. Noguchi. 2003. Capsular polysaccharide from Actinobacillus actinomycetemcomitans inhibits IL-6 and IL-8 production in human gingival fibroblast. J. Periodontal Res. 38:191-197. [DOI] [PubMed] [Google Scholar]
- 40.Oido-Mori, M., R. Rezzonico, P. L. Wang, Y. Kowashi, J. M. Dayer, P. C. Baehni, and C. Chizzolini. 2001. Porphyromonas gingivalis gingipain-R enhances interleukin-8 but decreases gamma interferon-inducible protein 10 production by human gingival fibroblasts in response to T-cell contact. Infect. Immun. 69:4493-4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmqvist, P., E. Persson, H. H. Conaway, and U. H. Lerner. 2002. IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-kappa B ligand, osteoprotegerin, and receptor activator of NF-kappa B in mouse calvariae. J. Immunol. 169:3353-3362. [DOI] [PubMed] [Google Scholar]
- 42.Park, K. K., K. Heuner, U. B. Göbel, Y. J. Yoo, C. K. Kim, and B. K. Choi. 2002. Cloning and characterization of a major surface protein (MspTL) of Treponema lecithinolyticum associated with rapidly progressive periodontitis. FEMS Microbiol. Lett. 207:185-192. [DOI] [PubMed] [Google Scholar]
- 43.Schröder, N. W., B. Opitz, N. Lamping, K. S. Michelsen, U. Zähringer, U. B. Göbel, and R. R. Schumann. 2000. Involvement of lipopolysaccharide binding protein, CD14, and Toll-like receptors in the initiation of innate immune responses by Treponema glycolipids. J. Immunol. 165:2683-2693. [DOI] [PubMed] [Google Scholar]
- 44.Sela, M. N. 2001. Role of Treponema denticola in periodontal diseases. Crit. Rev. Oral Biol. 12:399-413. [DOI] [PubMed] [Google Scholar]
- 45.Sfakianakis, A., C. E. Barr, and D. L. Kreutzer. 2001. Actinobacillus actinomycetemcomitans-induced expression of IL-1alpha and IL-1beta in human gingival epithelial cells: role in IL-8 expression. Eur. J. Oral Sci. 109:393-401. [DOI] [PubMed] [Google Scholar]
- 46.Siqueira, J. F., Jr., and I. N. Rocas. 2003. PCR-based identification of Treponema maltophilum, T. amylovorum, T. medium, and T. lecithinolyticum in primary root canal infections. Arch. Oral Biol. 48:495-502. [DOI] [PubMed] [Google Scholar]
- 47.Sugano, N., K. Ikeda, M. Oshikawa, Y. Sawamoto, H. Tanaka, and K. Ito. 2004. Differential cytokine induction by two types of Porphyromonas gingivalis. Oral Microbiol Immunol. 19:121-123. [DOI] [PubMed] [Google Scholar]
- 48.Sugiyama, A., A. Uehara, K. Iki, K. Matsushita, R. Nakamura, T. Ogawa, S. Sugawara, and H. Takada. 2002. Activation of human gingival epithelial cells by cell-surface components of black-pigmented bacteria: augmentation of production of interleukin-8, granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor and expression of intercellular adhesion molecule 1. J. Med. Microbiol. 51:27-33. [DOI] [PubMed] [Google Scholar]
- 49.Toms, A., B. Gannon, and C. Carati. 2000. The immunohistochemical response of the rat periodontal ligament endothelium to an inflammatory stimulus. Aust. Orthod. J. 16:61-68. [PubMed] [Google Scholar]
- 50.Umemoto, T., F. Nakazawa, E. Hoshino, K. Okada, M. Fukunaga, and I. Namikawa. 1997. Treponema medium sp. nov., isolated from human subgingival dental plaque. Int. J. Syst. Bacteriol. 47:67-72. [DOI] [PubMed] [Google Scholar]
- 51.Walker, S. G., J. L. Ebersole, and S. C. Holt. 1997. Identification, isolation, and characterization of the 42-kilodalton major outer membrane protein (MompA) from Treponema pectinovorum ATCC 33768. J. Bacteriol. 179:6441-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, Q., K. S. Ko, A. Kapus, C. A. McCulloch, and R. P. Ellen. 2001. A spirochete surface protein uncouples store-operated calcium channels in fibroblasts: a novel cytotoxic mechanism. J. Biol. Chem. 276:23056-23064. [DOI] [PubMed] [Google Scholar]
- 53.Wyss, C., B. K. Choi, P. Schupbach, A. Moter, B. Guggenheim, and U. B. Göbel. 1999. Treponema lecithinolyticum sp. nov., a small saccharolytic spirochaete with phospholipase A and C activities associated with periodontal diseases. Int. J. Syst. Bacteriol. 49:1329-1339. [DOI] [PubMed] [Google Scholar]
- 54.Wyss, C., A. Moter, B.-K. Choi, F. E. Dewhirst, Y. Xue, P. Schüpbach, U. B. Göbel, B. J. Paster, and B. Guggenheim. 2004. Treponema putidum sp. nov., a medium-size proteolytic spirochaete isolated from lesions of human periodontitis and acute necrotising ulcerative gingivitis. Int. J. Syst. Evol. Microbiol. 54:1117-1122. [DOI] [PubMed] [Google Scholar]
- 55.Xu, X., S. C. Holt, and D. Kolodrubetz. 2001. Cloning and expression of two novel hemin binding protein genes from Treponema denticola. Infect. Immun. 69:4465-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamada, M., H. Nakae, H. Yumoto, C. Shinohara, S. Ebisu, and T. Matsuo. 2002. N-acetyl-d-galactosamine specific lectin of Eikenella corrodens induces intercellular adhesion molecule-1 (ICAM-1) production by human oral epithelial cells. J. Med. Microbiol. 51:1080-1089. [DOI] [PubMed] [Google Scholar]