Abstract
The high-molecular-weight leptospiral immunoglobulin-like repeat (Lig) proteins are expressed only by virulent low-passage forms of pathogenic Leptospira species. We examined the effects of growth phase and environmental signals on the expression, surface exposure, and extracellular release of LigA and LigB. LigA was lost from stationary-phase cells, while LigB expression was maintained. The loss of cell-associated LigA correlated with selective release of a lower-molecular-weight form of LigA into the culture supernatant, while LigB and the outer membrane lipoprotein LipL41 remained associated with cells. Addition of tissue culture medium to leptospiral culture medium induced LigA and LigB expression and caused a substantial increase in released LigA. The sodium chloride component of tissue culture medium was primarily responsible for the enhanced release of LigA. Addition of sodium chloride, potassium chloride, or sodium sulfate to leptospiral medium to physiological osmolarity caused the induction of both cell-associated LigA and LigB, indicating that osmolarity regulates the expression of Lig proteins. Osmotic induction of Lig expression also resulted in enhanced release of LigA and increased surface exposure of LigB, as determined by surface immunofluorescence. Osmolarity appears to be a key environmental signal that controls the expression of LigA and LigB.
Infection of human beings by pathogenic members of the spirochete genus Leptospira results in a potentially fatal infection characterized by jaundice, renal failure, and/or pulmonary hemorrhage (3). The potentially severe nature of acute leptospirosis in humans contrasts with chronic infection in reservoir host animals such as Rattus norvegicus, which exhibits lifelong renal tubular carriage and urinary shedding (3). Water is an important vehicle for transmission to new mammalian hosts. Transmission to humans via contaminated water typically occurs in developed countries as a result of recreational or occupational exposures and in developing countries in settings where heavy rainfall results in urban flooding (19, 26). Leptospires enter the host by penetrating mucous membranes or broken skin.
Little is known about the environmental signals that leptospires use to respond to the variety of environmental conditions they encounter inside and outside of the mammalian host. Pathogenic bacteria control the expression of virulence factors in response to environmental cues (25). The presence of 79 two-component regulatory systems and 11 extracytoplasmic function (ECF) sigma factors in the genomes of L. interrogans serovar Lai and L. interrogans serovar Copenhageni suggest that pathogenic Leptospira spp. are capable of responding to a diverse array of environmental signals (28, 30). Growth phase and temperature affect the levels of several leptospiral membrane proteins. For example, expression of the outer membrane lipoprotein LipL36 is decreased during stationary phase (11), while expression of the peripheral outer membrane protein P31LipL45 (Qlp42) is increased during stationary phase (23). Similarly, growth at 37°C instead of the standard 30°C cultivation temperature causes a reduction in the expression of LipL36 and an increase in that of P31LipL45 (27). Consistent with these observations, leptospires residing in the kidney tubules of hamsters fail to express LipL36 yet express P31LipL45 (2, 23). A global examination of leptospiral outer membrane proteins by two-dimensional electrophoresis revealed several additional proteins whose expression is affected by iron availability and temperature, including pL24 and pL50 (9). The mechanisms by which the expression of these proteins are regulated remain to be determined.
Efforts to identify leptospiral proteins that are expressed during infection led to the discovery of genes encoding the leptospiral immunoglobulin (Ig)- like repeat (Lig) family of proteins (22,29). Lig proteins contain imperfect tandem repeats of a ∼90-amino-acid residue sequence that is predicted to form an Ig-like fold (22, 29). In L. interrogans strain Fiocruz L1-130 and L. kirschneri strain RM52, LigA contains 13 copies of the Ig-like sequence and LigB contains 12 copies followed by a unique C-terminal domain. Both LigA and LigB contain a lipobox, and LigA was lipidated by palmitate when expressed in E. coli (20). LigB has been shown to be surface exposed; whether LigA is surface exposed is unknown (22). An intact ligC gene is present in the genome of L. interrogans serovar Lai, but ligC is likely to be a pseudogene in L. interrogans serovar Copenhageni and L. kirschneri serovar Grippotyphosa (22). Expression of Lig proteins is lost during culture attenuation of L. kirschneri and L. interrogans, suggesting that these proteins are associated with virulence (22). It is thought that the Lig proteins may function as adhesins, given the similarity of their domain organization to those of the well-characterized adhesins invasin and intimin, found in Yersinia species and enteropathogenic Escherichia coli, respectively (12, 21).
Several lines of evidence indicate that expression of Lig proteins is increased during infection of the mammalian host. First, LigA could not be detected in cultivated L. interrogans strain kennewicki but was detected by immunohistochemistry in leptospires residing in the kidneys of infected hamsters (29). Second, rats immunized with killed L. interrogans grown in vitro failed to produce antibody to Lig, most probably a result of insufficient Lig expression (22). In contrast, rats infected with L. interrogans produced Lig antibody, consistent with a higher level of Lig expression during infection than during in vitro growth. In this study, we sought to identify environmental conditions that affect expression of Lig proteins in L. kirschneri RM52 and L. interrogans Fiocruz L1-130. We found that the expression of Lig proteins is regulated by osmolarity. We also demonstrate that both Leptospira strains release LigA into the extracellular fluid and that the release of LigA is enhanced by salt.
MATERIALS AND METHODS
Bacterial strains and cultivation.
L. kirschneri serovar Grippotyphosa strain RM52 was isolated during an outbreak of porcine abortion in the United States (33). L. interrogans serovar Copenhageni strain L 1-130 is a human blood isolate obtained during an outbreak of leptospirosis in Salvador, Brazil (22). All experiments were performed with virulent, low-passage forms of these strains obtained by infection and reisolation from Golden Syrian hamsters. The spirochetes were maintained in Ellinghausen-McCullough-Johnson-Harris (EMJH) medium (pH 7.2) supplemented with 1% rabbit serum and 100 μg of 5-fluorouracil (Sigma, St. Louis, Mo.) per ml at 30°C (17). Albumin was purchased from Intergen (Purchase, N.Y.; catalog no. 31-003-3) and from Sigma (catalog no. A7906) for cultivation of L. kirschneri RM52 and L. interrogans Fiocruz L1-130, respectively. Modified Eagle medium (MEM), which was purchased from the American Type Culture Collection (Manassas, Va.), contains Earle's salts and both essential and nonessential amino acids. Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Norcross, Ga.).
Plasmid DNA.
The portion of the flaA1 gene beginning from the codon following the segment encoding the signal peptide was amplified by PCR with ExTaq DNA polymerase (Takara, Madison, Wis.) using the forward primer 5′-GACTCGAGAATGGACAAAACATCATCAAAGGCAAAC-3′ and the reverse primer 5′-GACTCGAGAATGGACAAAACATCATCAAAGGCAAAC-3′. The primers included an XhoI site and a HindIII site, respectively (underlined), and L. interrogans Fiocruz L1-130 genomic DNA was used as template for PCR. The amplified flaA1 gene fragment was digested with XhoI and HindIII and ligated to the His6 vector pRSET A (Invitrogen, Carlsbad, Calif.) with T4 DNA ligase to generate the plasmid pRSETa-FlaA1. Restriction and modifying enzymes were purchased from New England Biolabs (Beverly, Mass.).
Antisera.
LigA-B and LipL41 antisera were prepared by immunizing New Zealand White rabbits (Harlan, Indianapolis, Ind.) with His6-Lig and His6-LipL41 recombinant proteins as described previously (22, 31). LigA-B antiserum was raised against amino acid residues 342 to 1224 of LigA, which includes a region shared with LigB. LigA antiserum raised against the last 289 amino acid residues of LigA (residues 936 to 1224) does not react with LigB in immunoblots. Likewise, the LigB-specific antiserum was raised against the C-terminal domain of LigB (residues 1113 to 1886). FlaA1 antiserum was generated as follows. Plasmid pRSETa-FlaA1 was transformed into Escherichia coli BLR(DE3)/pLysS (EMD Biosciences, Madison, Wis.), and expression of the His6-FlaA1 fusion protein was induced with 0.5 mM isopropyl-β-d-thiogalactoside. The recombinant protein was purified by affinity chromatography with Ni2+-nitrilotriacetate agarose resin (Qiagen, Valencia, Calif.) and loaded onto a preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel (12% polyacrylamide). Following electrophoresis, the band containing approximately 150 μg of His6-FlaA 1 protein was cut out of the gel, desiccated, ground into powder, and mixed with 1 ml of sterile water. The suspension was mixed with 1 ml of complete Freund's adjuvant (Sigma) and inoculated subcutaneously and intramuscularly into a New Zealand White male rabbit. Additional immunizations with approximately 150 μg of His6-FlaA 1 in 1 ml of sterile water mixed with 1 ml of incomplete Freund's adjuvant were given 4 weeks and 8 weeks following the primary immunization. The rabbit was bled 10 weeks after the primary immunization. The immunization protocol was approved by the West Los Angeles Veterans Affairs Animal Research Committee.
Immunoblot analysis.
Culture supernatant fluid was collected by centrifugation of 109 leptospires for 4 min at 9,000 × g in a Beckman (Fullerton, Calif.) Coulter Microfuge 18 centrifuge followed by collection of the supernatant fluid, which was subsequently examined for the presence of Lig and other leptospiral proteins by immunoprecipitation (see below). The cell pellet was washed once in phosphate-buffered saline (PBS)-5 mM MgCl2 and resuspended in 100 μl of final sample buffer consisting of 50 mM Tris-HCl (pH 6.8), 100 mM dithiothreitol, 2% sodium dodecyl sulfate, 0.25 mM phenylmethylsulfonyl fluoride, and 0.1% bromophenol blue in 20% glycerol and boiled for 3 min. Proteins were separated in 10 or 12% PAGEr Gold precast Tris-glycine gels (Cambrex, Walkersville, Md.). Following electrophoresis, the material in each gel was transferred electrophoretically to a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.). The membranes were incubated in blocking solution (5% skim milk in PBS-0.1% Tween 20 [PBS-T]) for 30 min and then incubated with rabbit serum (diluted 1:2000 to 1:10,000) for 30 min and washed three times with PBS-T. They were then incubated with donkey anti-rabbit antibody (Amersham Biosciences, Piscataway, N.J.) or protein A-horseradish peroxidase conjugate (Amersham) at a dilution of 1:5,000 or 1:2,000, respectively, in blocking solution for 30 min and again washed three times in PBS-T. The membranes were developed with the ECL Western blot detection system (Amersham), and the bands were visualized with Hyperfilm (Amersham). Band intensities of Lig proteins were quantitated with NIH Image 1.62 (National Institutes of Health; http://rsb.info.nih.gov/nih-image) and normalized against the intensity of the LipL41 band in the same lane.
Immunoprecipitation.
Culture supernatant (995 μl) was mixed with 5 μl of rabbit serum and left on ice overnight. A 25-μl volume of EZview Red protein A affinity gel (Sigma) was then added, and the mixture was placed on an orbital mixer at 4°C for 2 h. The immune complex bound to the protein A was recovered by centrifugation for 7 s, washed twice with 800 μl of 10 mM Tris-HCl-0.4 M NaCl (pH 8.0), and finally washed once with 800 μl of 10 mM Tris-HCl (pH 8.0). The pellet was resuspended in 50 to 100 μl of final sample buffer. Samples were boiled for 3 min and centrifuged for 7 s. A 10-μl volume was used for immunoblot analysis.
RT-PCR.
L. kirschneri strain RM52 (5 × 109 cells) was transferred into an Erlenmeyer flask, chilled in a dry ice-ethanol bath, and centrifuged at 10,000 rpm for 10 min in a Sorvall SM-24 rotor. RNA was extracted from the bacteria with hot phenol, precipitated with ethanol, and resuspended in 50 μl of diethylpyrocarbonate-treated water purchased from ICN (5). RNA (4 μg) was treated with 2 U of Turbo-DNase in a final volume of 40 μl for 30 min at 37°C as directed by the manufacturer (Ambion, Austin, Tex.).
A 2-μg portion of DNase-treated RNA was hybridized to random hexamer primers (Promega, Madison, Wis.), and cDNA was synthesized with Omniscript reverse transcriptase (RT) as specified by the manufacturer (Qiagen). The cDNA (representing 50 ng of RNA per reaction) was amplified with Taq DNA polymerase (Qiagen) with gene-specific primer pairs. The ligA, ligB, and ligC primers have been described previously (22). The sequences of the lipL41 forward and reverse primers are 5′-TCGGTGAAGGTTCCAGTTTTATTGAT-3′ and 5′-TACTTCTCCGGTTTCTACTTTGATGA-3′, respectively. Those of the ligC primers that amplify a segment upstream of the frameshift in the pseudogene are 5′-TATTCTTTTTCTAACATTCAGCCTAT-3′ (forward) and 5′-AAGTCCGGAAACTAAACCTGTGGTGT-3′ (reverse).
Immunofluorescence.
Sodium azide was added to a culture of L. interrogans Fiocruz L1-130 to a final concentration of 0.5% (wt/vol), and the nucleic acid fluorescent stain SYTO 83 (Molecular Probes, Eugene, Oreg.) was added to the culture at a 1:1,000 dilution. After incubation for 1 hr at 30°C, the bacteria were collected by centrifugation at 1,000 × g for 10 min in a microcentrifuge, resuspended in 50 mM carbonate buffer (pH 9.6), placed in two-well glass slide chambers (0.5 × 109 to 1.0 × 109 leptospires per well) (Nunc, Rochester, N.Y.), and incubated at 30°C for 2 hs. Next, the bacteria were treated with blocking solution (0.5% bovine serum albumin in PBS-1% Tween 20) for 2 hrs at 30°C. They were then fixed with 4% paraformaldehyde for 1 h at 30°C, washed three times with PBS, treated with a 1:250 dilution of rabbit antiserum in blocking buffer for 1 h at 30°C, washed three times with PBS, treated with a 1:1,000 dilution of Alexa Fluor 488-labeled goat anti-rabbit IgG (Molecular Probes; catalog no. A-11034) in blocking solution for 1 hr at 30°C, washed three times with PBS, and washed twice with water. Finally, mounting medium (ProLong antifade kit; Molecular Probes) and a coverslip were added, and the slide was left overnight. Staining was visualized by confocal microscopy with an LSM 510 microscope (Carl Zeiss, Inc., Jena, Germany).
RESULTS
LigA is released into the culture medium.
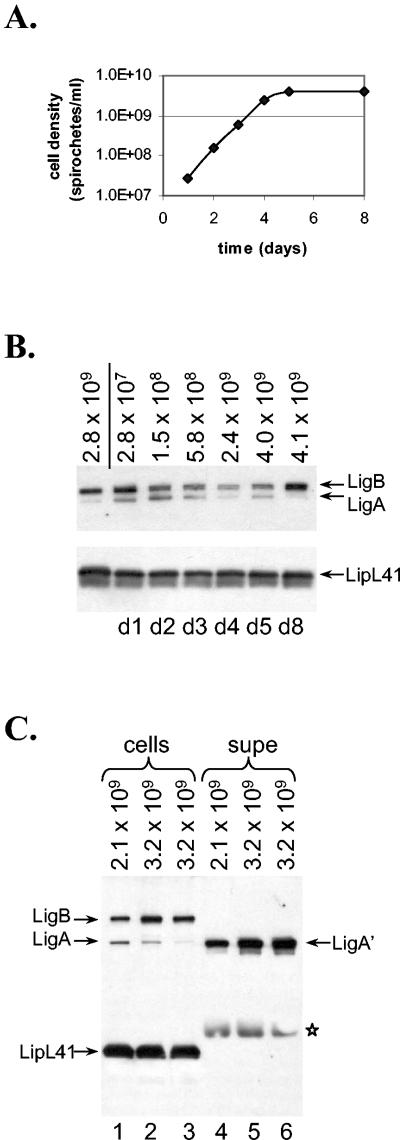
In previous studies, we showed that the levels of several leptospiral proteins change with growth phase (see Introduction for details). We therefore wondered whether the expression of Lig proteins also varies with the growth phase. To examine this issue, we initiated a culture of L. kirschneri strain RM52 in EMJH at 30°C from a stationary-phase culture and incubated the culture until it reached stationary phase. During the incubation, samples of the culture were collected at various times (Fig. 1A). The leptospires collected at each time point were examined by immunoblot analysis for expression of LigA and LigB (Fig. 1B). Lysates from equal numbers of leptospires were loaded into each lane. The membrane was probed with LigA-B and LipL41 antisera, the latter included as a control for loading. LipL41 levels do not change throughout the growth curve (11). The immunoblot shows that the LigA levels were greatly reduced during stationary phase whereas LigB was easily detected at all phases of growth (Fig. 1B).
FIG. 1.
Growth phase-dependent expression of Lig proteins. (A) L. kirschneri RM52 was inoculated into EMJH and incubated at 30°C. Samples of the culture were collected daily, and the bacterial densities were determined. (B) Leptospires from each sample collected for the experiment in panel A were loaded onto an SDS-PAGE gel (12% polyacrylamide), and Lig and LipL41 expression was examined by immunoblot analysis. The density of the culture (number of motile bacteria per milliliter) is shown above the panel; 108 bacteria were loaded in each lane. The first lane contains proteins from leptospires collected from the stationary-phase culture that was the source of the starting material for the culture whose growth is plotted in panel A.(C) L. kirschneri RM52 was grown in EMJH to late log phase, and bacteria were collected on three consecutive days as the culture went into saturation. Leptospires were collected by centrifugation, and the supernatant fluid was subjected to immunoprecipitation with anti-Lig serum. The leptospires were loaded onto lanes 1 to 3 of a 4 to 20% gradient SDS-PAGE gel; Lig protein immunoprecipitated from the culture supernatant (supe) was loaded onto lanes 4 to 6. A total of 108 leptospires were loaded in each lane. *, reaction of the protein A-HRP conjugate with the immunoblobulin heavy chain.
Since the DNA sequence in the promoter regions of ligA and ligB are identical (22), we wondered whether the difference in expression of the two proteins could have resulted from alternative fates of the two proteins during stationary phase. One possible explanation for the disappearance of LigA during entry into stationary phase is its selective release into the culture medium. To examine this possibility, experiments were performed in which, starting from late log phase, samples were collected at various time points from cultures of L. kirschneri RM52. Leptospires were collected by centrifugation, and both the bacteria and culture supernatant fluid were obtained. To avoid distortion of the bands in the SDS-PAGE gel due to the high concentration of albumin in EMJH, immunoprecipitation of the culture supernatant was performed with antiserum specific for the shared regions of LigA and LigB. The immunoprecipitated material was examined by immunoblotting, revealing a species that migrated slightly faster than cell-associated LigA, which we designated LigA′ (Fig. 1C, lanes 4 to 6). In contrast, neither LigB nor LipL41 was detected in the culture supernatant (lanes 4 to 6; data not shown). L. interrogans strain Fiocruz L1-130 also released LigA′ into the culture supernatant (see Fig. 2C, lane 2).
FIG. 2.
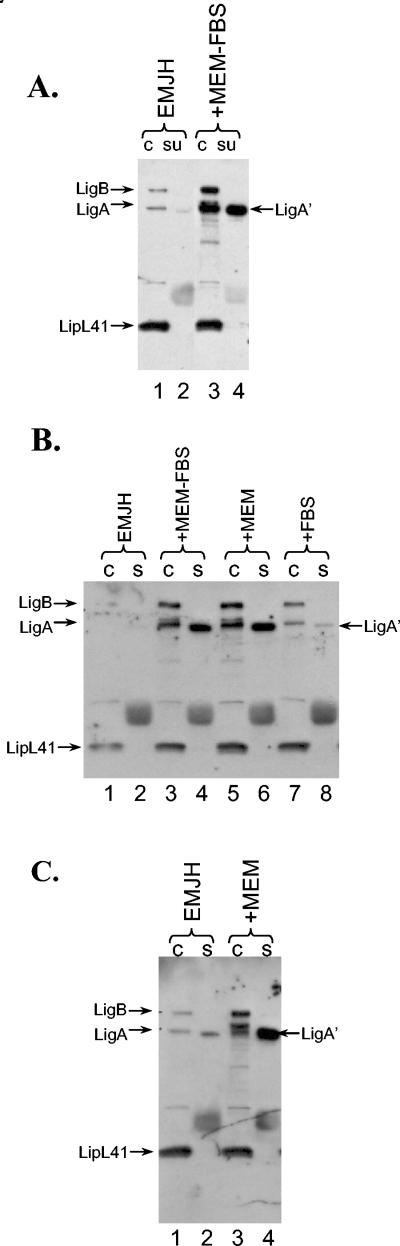
Induction of Lig expression and release by tissue culture medium. (A) L. kirschneri RM52 was grown to late log phase. Samples of the culture were mixed with an equal volume of EMJH or tissue culture medium, which consisted of MEM with 10% FBS. The leptospires were incubated for 24 h, and the leptospires and culture supernatant were examined for Lig protein by immunoblot analysis. c, cells;su, culture supernatant. (B) L. kirschneri RM52 was grown as described for panel A, and samples of the culture were mixed with an equal volume of EMJH (lanes 1 and 2), MEM with 10% FBS (lanes 3 and 4), MEM (lanes 5 and 6), or 10% FBS (lanes 7 and 8). The cultures were incubated for 24 h. The leptospires and culture supernatant were examined for Lig protein by immunoblot analysis as for the experiment in panel A. (C) L. interrogans Fiocruz L1-130 was grown to late log phase. The bacteria were mixed with an equal volume of EMJH or MEM and incubated for 24 h. The leptospires and culture supernatant were examined for Lig protein by immunoblot analysis as for the experiment in panel A.
To verify that LigA′ represented a smaller form of LigA and not LigB, the immunoprecipitated material was probed separately with LigA- and LigB-specific antisera. LigA′ reacted with LigA antiserum but not with LigB antiserum, indicating that LigA′ is derived from LigA (data not shown).
Lig expression is induced by MEM.
Immunoblots with sera from infected humans and rats indicate that Lig proteins are expressed during infection of the mammalian host (22). For this reason, we examined whether conditions likely to be encountered within the mammalian host affect Lig protein expression. L. kirschneri strain RM52 was incubated overnight in MEM-10% FBS, which models in vivo conditions. Leptospires incubated overnight in MEM-10% FBS instead of the leptospiral medium EMJH lost motility and appeared to degenerate, as determined by dark-field microscopy. However, L. kirschneri RM52 incubated overnight in a 50:50 mixture of EMJH and MEM-10% FBS retained motility and morphology. Bacteria and culture supernatant were collected at the end of the incubation period and examined for Lig protein expression by immunoblot analysis. MEM-10% FBS, when mixed with EMJH, enhanced the levels of LigA and LigB by 10- to 15-fold and enhanced the level of LigA′ by 50-fold (Fig. 2A, compare lanes 2 and 4). Although addition of FBS to EMJH partially induced Lig expression (Fig. 2B, lanes 7 and 8), Lig expression continued to be fully induced when FBS was omitted from the MEM-EMJH mixture (lanes 5 and 6). In four independent experiments, MEM induction was 4- to 24-fold for cell-associated LigA and LigB and 18- to 53-fold for LigA′. MEM also enhanced LigA, LigB, and LigA′ levels in L. interrogans strain Fiocruz L1-130 (Fig. 2C), demonstrating that regulation of Lig expression by MEM is not limited to L. kirschneri strain RM52. To eliminate the alternative possibility that mixing in the MEM diluted an inhibitory substance in EMJH, L. kirschneri RM52 was grown in EMJH that had been diluted twofold with water instead of MEM. Dilution of EMJH had no effect on Lig protein levels or release in comparison to growth in full-strength EMJH (data not shown), indicating that the increase in Lig expression resulted from an inducing ingredient in MEM.
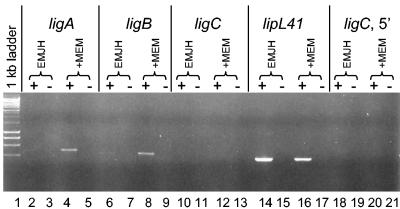
The transcript levels of lig were examined by RT-PCR. The ligA and ligB transcripts were barely detectable in L. kirschneri RM52 grown in EMJH (Fig. 3, lanes 2 and 6). In contrast, ligA and ligB transcripts were easily detected when L. kirschneri RM52 was grown in a 50:50 mixture of EMJH and MEM (lanes 4 and 8). These results demonstrate that the presence of MEM elevates ligA and ligB transcript levels. Primers that hybridize downstream (lanes 10 and 12) or upstream (lanes 18 and 20) of the frameshift in ligC could not detect transcript from the ligC pseudogene, whether or not MEM was present. In all cases, the lack of reverse transcriptase in the reaction mixture prevented the formation of a PCR product, indicating that contaminating DNA in the RNA preparations was efficiently removed. The positive control lipL41 transcript was detectable whether or not MEM was present in the culture medium (lanes 14 and 16).
FIG. 3.
Regulation of lig transcript levels by MEM. A culture of L. kirschneri RM52 at late log phase in EMJH was mixed with an equal volume of EMJH or MEM and incubated for 24 h. RNA was extracted from each culture and was examined for the presence of lig and lipL41 transcripts by RT-PCR. Lane 1, 1-kb ladder from New England Biolabs; +, RT present; −, RT omitted.
Lig expression is regulated by osmolarity.
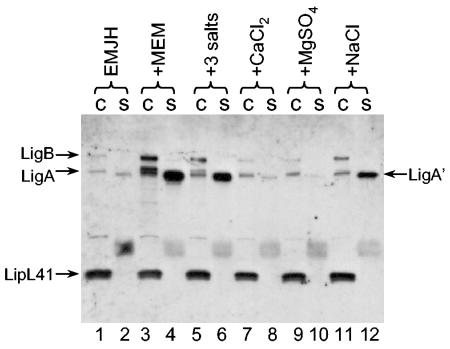
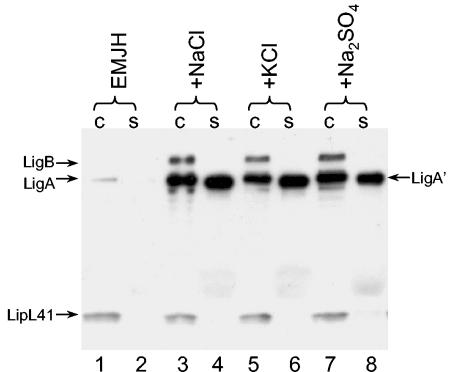
We were interested in determining the ingredient in MEM that induced LigA′ release. Since MEM is a completely defined medium and FBS was not required for full induction of Lig protein expression, it was possible to determine which MEM component was responsible for the induction effect by systematically adding the individual components of MEM to EMJH at the concentrations found in MEM. We focused on the components that were at a higher concentration in MEM than in EMJH. Addition of the MEM vitamins, amino acids, sodium pyruvate, or glucose had little or no effect on LigA′ release; sodium bicarbonate had a slight effect (data not shown). On the other hand, a mixture of the salts calcium chloride, magnesium sulfate, and sodium chloride added to EMJH at the concentrations found in MEM substantially enhanced LigA′ release (Fig. 4, lane 6). When the salts were examined individually, sodium chloride alone accounted for the effect on LigA′ release (lanes, 8, 10, and 12). Cell-associated LigA and LigB levels increased slightly in EMJH supplemented with sodium chloride (lane 11).
FIG. 4.
Induction of Lig expression and release by sodium chloride. A culture of L. kirschneri RM52 grown to late log phase in EMJH was mixed with an equal volume of EMJH (lanes 1 and 2); MEM (lanes 3 and 4); a solution of 0.1 mM MgSO4, 1.8 mM CaCl2, and 116 mM NaCl (lanes 5 and 6); 0.1 mM MgSO4 (lanes 7 and 8); 1.8 mM CaCl2 (lanes 9 and 10); or 116 mM NaCl (lanes 11 and 12). The cultures were incubated for 24 h and examined for Lig expression and release following separation of proteins on an SDS-PAGE gel (10% polyacrylamide). c, cells; s, culture supernatant fluid.
Because sodium chloride accounts for the majority of the osmolarity of MEM, we wondered whether Lig release was regulated by the osmolarity of the medium rather than the sodium or chloride ions themselves. To test this possibility, L. interrogans strain Fiocruz L1-130 was collected by centrifugation, resuspended in EMJH without any additions or in EMJH supplemented with 120 mM NaCl, 120 mM KCl, or 80 mM Na2SO4, and incubated overnight. The osmolarity in the cultures supplemented with salt approximated physiological conditions (∼300 mosmol per liter), whereas the osmolarity of EMJH was about 70 mosmol. The immunoblot shows that all three salts induced Lig protein expression by 16- to 21-fold and induced LigA′ release by 200- to 300-fold (Fig. 5), indicating that Lig expression responds to the osmolarity of the growth medium and not to a specific ion. Similar results were obtained with L. kirschneri RM52 (data not shown). In four independent experiments, induction by sodium chloride was 5- to 30-fold for cell-associated LigA and LigB and 50- to 320-fold for extracellular LigA′.
FIG. 5.
Induction of Lig expression and release is independent of the salt responsible for osmolarity. L. interrogans Fiocruz L1-130 grown in EMJH was collected by centrifugation and resuspended in EMJH (lanes 1 and 2) or EMJH containing an additional 120 mM NaCl (lanes 3 and 4), 120 mM KCl (lanes 5 and 6), or 80 mM Na2SO4 (lanes 7 and 8). The cultures were incubated for 24 h and examined for Lig expression and release following separation of proteins on an SDS-PAGE gel (10% polyacrylamide). c, cells; s, culture supernatant fluid.
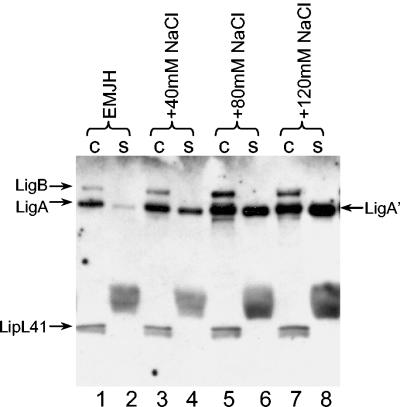
LigA′ release appears to be more sensitive to the presence of sodium chloride than is the release of cell-associated Lig proteins (Fig. 4, lanes 1 and 2 and lanes 11 and 12). To confirm this observation, Lig proteins were examined following overnight incubation of L. interrogans Fiocruz L1-130 in EMJH containing different concentrations of sodium chloride. When 40 mM sodium chloride was added to EMJH, a nine-fold increase in the release of LigA′ was observed in contrast to the one- to twofold increase in the release of cell-associated Lig proteins (Fig. 6, compare lanes 1 and 2 with lanes 3 and 4). When 80 or 120 mM sodium chloride was added, a 6-fold induction of cell-associated LigA and LigB was observed and release of LigA′ was enhanced 30- to 50-fold (lanes 5 to 8). Incubation of L. interrogans at higher concentrations of sodium chloride resulted in the appearance of nonmotile, shriveled leptospires when observed by dark-field microscopy.
FIG. 6.
Sensitivity of Lig expression and release to sodium chloride concentrations. L. interrogans Fiocruz L1-130 grown in EMJH was collected by centrifugation and resuspended in EMJH (lanes 1 and 2) or EMJH containing an additional 40 mM NaCl (lanes 3 and 4), 80 mM NaCl (lanes 5 and 6), or 120 mM NaCl (lanes 7 and 8). The cultures were incubated for 24 h and examined for Lig expression and release following separation of proteins on an SDS-PAGE gel (10% polyacrylamide) gel. c, cells; s, culture supernatant fluid.
Sodium chloride increases the surface exposure of LigB.
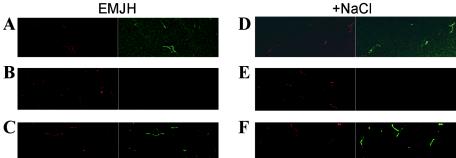
Increased concentrations of sodium chloride may increase the availability of Lig proteins for direct interaction with the host by enhancing their surface exposure. Indirect immunofluorescence was used to determine whether the induction of Lig expression leads to an increase in the amounts of surface-exposed Lig proteins. LipL41, which has been shown to be surface exposed by surface immunoprecipitation, was used as a positive control (31). FlaA1 antiserum, which reacts with the periplasmic flagellar sheath, was used as a negative control. As expected, L. kirschneri RM52 incubated in EMJH and EMJH containing extra sodium chloride reacted with LipL41 antiserum (Fig. 7A and D) and failed to react with FlaA1 antiserum (Fig. 7B and E). LigA-B antiserum reacted weakly with L. kirschneri incubated in EMJH (Fig. 7C). In contrast, strong reactivity with the LigA-B antiserum was observed when Lig expression was induced with sodium chloride (Fig. 7F).
FIG. 7.
Salt-induced surface exposure of Lig proteins demonstrated by indirect immunofluorescence. L. kirschneri RM52 grown in EMJH was collected by centrifugation and resuspended in EMJH (A to C) or EMJH containing an additional 120 mM NaCl (D to F). After a 24-h incubation period, leptospires were stained with SYTO 83 (red) and treated with LipL41 (A and D), FlaA1 (B and E), or LigA-B (C and F) antiserum. Antibody bound to the surface of the leptospires was detected with Alexa-labeled anti-rabbit IgG (green).
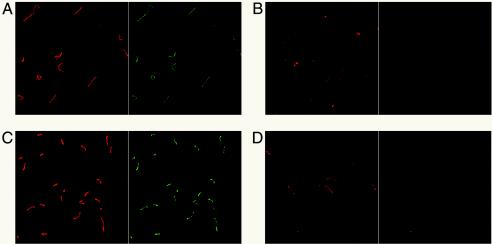
The effect of osmolarity on surface exposure of LigA and LigB was also examined with specific antiserum to each protein. When L. interrogans Fiocruz L1-130 was incubated in EMJH, weak reactivity with the LigB-specific antiserum was observed (Fig. 8A) (22). Incubation of L. interrogans in EMJH containing extra sodium chloride resulted in an increase in the signal observed (Fig. 8C). In contrast, FlaA1 antiserum failed to react with the spirochetes, regardless of the growth conditions (Fig. 8B and D). Treatment of the leptospires with methanol caused reactivity with the FlaA1 antiserum, indicating that the FlaA1 serum was functional (data not shown). L. interrogans reacted with the LipL41 antiserum, as expected (data not shown). Uninduced and salt-induced leptospires failed to react with LigA-specific antiserum, even after the bacteria were fixed with methanol (data not shown).
FIG. 8.
Salt-induced surface exposure of LigB demonstrated by indirect immunofluorescence. L. interrogans Fiocruz L1-130 grown in EMJH was collected by centrifugation and resuspended in EMJH (A and B) or EMJH containing an additional 120 mM NaCl (C and D). After a 24-h incubation period, leptospires were stained with SYTO 83 (red) and examined for surface exposure of LigB (A and C) and the periplasmic flagellar protein FlaA1 (B and D) by indirect immunofluorescence. Antibody bound to the surface of the leptospires was detected with Alexa-labeled anti-rabbit IgG (green).
DISCUSSION
We have previously shown that expression of LigA and LigB is associated with virulence (22). In this study, we investigated the environmental signals controlling Lig protein expression. Since the expression of the outer membrane proteins LipL36 and P31Lip45 is altered in stationary phase (11, 23), we first examined whether Lig protein expression was controlled by growth phase. While LigB was consistently expressed at all stages of leptospiral growth, LigA expression was dramatically reduced in stationary phase (Fig. 1B). The loss of cell-associated LigA occurred concomitantly with continued release of LigA into the culture medium (Fig. 1C). Therefore, LigA joins the hemolysins as the only characterized leptospiral proteins known to be released extracellularly (35).
Careful examination of immunoblots revealed that LigA immunoprecipitated from culture supernatants was slightly smaller than cell-associated LigA (Fig. 1C). We have designated the released form of LigA as LigA′. The ligA gene encodes a lipoprotein signal peptide, and LigA expressed in E. coli is lipidated (20), indicating that there is sec-dependent export of LigA across the cytoplasmic membrane followed by lipidation of the lipobox cysteine by leptospiral lipoprotein signal peptidase. It is possible that once LigA reaches the surface of the spirochete as a lipoprotein, it is subject to proteolytic removal of its lipid anchor, resulting in the formation of LigA′, which can be recovered from the culture supernatant.
We discovered that tissue culture medium is a valuable in vitro model for examining the expression of Lig expression under conditions that may be encountered by the spirochete during infection of the mammalian host. MEM with serum did not sustain the overnight viability of leptospires, most probably a consequence of the strict requirement of long-chain fatty acids as a carbon source (16). However, incubation in a 50:50 mix of leptospiral culture medium and MEM not only allowed leptospires to remain viable but also resulted in an increase in expression of both of cell-associated LigA and LigB and of LigA′ released by L. kirschneri strain RM52 (Fig. 2). MEM also caused an increase in the release of Lig proteins by L. interrogans strain L1-130 (Fig. 2C). Tissue culture media have also been used to examine the regulated secretion of virulence gene products in enteropathogenic E. coli (18). The discovery that incubation in MEM caused a dramatic increase in Lig expression facilitated the identification of sodium chloride as the primary molecular signal that controls lig expression in vitro. Control of Lig expression and release could also be achieved with KCl and Na2SO4, indicating that sodium chloride was acting through its effect on osmolarity (Fig. 5). These data demonstrate that osmolarity is an important environmental cue that determines the level of cell-associated Lig proteins. Lig protein expression regulation is likely to be complex, and additional regulatory factors may yet be discovered. Furthermore, the results described here may not be completely relevant to all pathogenic Leptospira spp. For example, L. interrogans serovar Lai lacks a homolog of LigA. On the other hand, LigA has been found in L. interrogans serovar Pomona (29) and two forms of LigA have been found in L. interrogans serovar Manilae (20).
The increase in osmolarity experienced by pathogenic Leptospira spp. entering a mammalian host from the freshwater environment would be a particularly convenient signal for pathogenic Leptospira species to up-regulate the expression of virulence genes required during the early stages of infection of the host. Lig antibody is detected during the acute phase of leptospirosis (J. H. R. Croda and A. I. Ko, personal communication), suggesting that Lig is expressed early during infection. The putative adhesin activity of Lig proteins may be important in the dissemination of leptospires from the circulation to different organs. Osmolarity regulates the availability of LigA and LigB to directly interact with the host environment by enhancing release and surface exposure, respectively. We were unable to determine the surface exposure of LigA because of the poor reactivity of the LigA-specific antiserum in the immunofluorescence assay, even after the leptospires were fixed with methanol.
The osmotic control of Lig protein expression appears to involve transcriptional regulation. The increased Lig expression resulting from incubation in MEM was associated with increased transcript levels of the ligA and ligB genes, as demonstrated by RT-PCR (Fig. 3). Candidate proteins for regulation of lig gene transcription would include 1 or more of the 79 genes encoding two-component sensor histidine kinase response regulator proteins or 11 ECF-type sigma factors recently identified by leptospiral genome sequencing (28, 30). Leptospiral genomes encode homologs of the ProP and Kdp transporters (8, 13) and the two-component system KdpDE (34), suggesting that pathogenic leptospires are equipped with osmosensor and osmoregulator proteins. Changes in gene expression by osmolarity in other spirochetes have not been examined. In this context, it is worth noting that resistance of Borrelia burgdorferi at stationary phase to high concentrations of sodium chloride is reduced in an rpoS mutant, suggesting the presence of a borrelial RpoS regulon that is regulated by environmental sodium chloride (10). The rpoS gene is not present in the genome of either sequenced strain of L. interrogans, indicating that the mechanism of osmotic regulation of gene expression in Leptospira spp. is likely to differ from the RpoS-based mechanism examined extensively in E. coli (15).
The data presented here do not address the role of LigA' release or even whether LigA' is released during infection of the mammalian host or instead remains cell associated. A recent study examined the importance of release of the adhesin filamentous hemagglutinin (FHA) on B. pertussis pathogenesis in a mouse model (6). Following the delivery of the FHA proprotein to the surface of B. pertussis, cleavage by the protease SphB1 releases FHA from the bacterium (7). Disruption of the gene that encodes the SphB1 protease results in enhanced attachment of B. pertussis to monolayers in vitro relative to the strain with the wild-type gene. Nevertheless, colonization of mouse lungs following intranasal inoculation of the mutant bacteria was diminished, demonstrating that FHA secretion was essential for pathogenesis in the mouse model (6). It was proposed that secretion of the adhesin permits dissemination of the bacteria from the initial site of colonization into deeper tissues. A second possibility is that secreted LigA' stimulates host cell signaling pathways that are advantageous to leptospiral infection. An adhesin of the intracellular pathogen Listeria monocytogenes, InlB, which is found in both cell-associated and extracellular forms, stimulates phagocytosis of the bacteria by nonphagocytic cells by activating host signaling cascades. Furthermore, InlB stimulates additional host intracellular signaling pathways that possibly enhance the survival of the host cell that harbors the bacteria (4). Third, released LigA' may have immunomodulatory properties. FHA was shown to initiate apoptosis of human macrophages in vitro and in another study to suppress interleukin-12 production by macrophages (1, 24). Other examples of high-molecular-weight adhesins that are released include the Hap and HMW adhesins of Haemophilus influenzae (14, 32). Additional studies are necessary to address the role of Lig proteins in the interaction of pathogenic leptospires with the mammalian host.
Acknowledgments
This work was supported by VA Medical Research Funds (to J.M. and D.A.H.) and Public Health Service grant AI-34431 (to D.A.H.) from the National Institute of Allergy and Infectious Diseases.
We thank George Sachs and David Scott for providing access to the confocal microscope and assistance in its use.
Editor: D. L. Burns
REFERENCES
- 1.Abramson, T., H. Kedem, and D. A. Relman. 2001. Proinflammatory and proapoptotic activities associated with Bordetella pertussis filamentous hemagglutinin. Infect. Immun. 69:2650-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett, J. K., D. Barnett, C. A. Bolin, T. A. Summers, E. A. Wagar, N. F. Cheville, R. A. Hartskeerl, and D. A. Haake. 1999. Expression and distribution of leptospiral outer membrane components during renal infection of hamsters. Infect. Immun. 67:853-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharti, A. R., J. E. Nally, J. N. Ricaldi, M. A. Matthias, M. M. Diaz, M. A. Lovett, P. N. Levett, R. H. Gilman, M. R. Willig, E. Gotuzzo, and J. M. Vinetz. 2003. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 3:757-771. [DOI] [PubMed] [Google Scholar]
- 4.Bierne, H., and P. Cossart. 2002. InlB, a surface protein of Listeria monocytogenes that behaves as an invasin and a growth factor. J. Cell Sci. 115:3357-3367. [DOI] [PubMed] [Google Scholar]
- 5.Case, C. C., E. L. Simons, and R. W. Simons. 1990. The IS10 transposase mRNA is destabilized during antisense RNA control. EMBO J. 9:1259-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coutte, L., S. Alonso, N. Reveneau, E. Willery, B. Quatannens, C. Locht, and F. Jacob-Dubuisson. 2003. Role of adhesin release for mucosal colonization by a bacterial pathogen. J. Exp. Med. 197:735-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutte, L., R. Antoine, H. Drobecq, C. Locht, and F. Jacob-Dubuisson. 2001. Subtilisin-like autotransporter serves as maturation protease in a bacterial secretion pathway. EMBO J. 20:5040-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culham, D. E., J. Henderson, R. A. Crane, and J. M. Wood. 2003. Osmosensor ProP of Escherichia coli responds to the concentration, chemistry, and molecular size of osmolytes in the proteoliposome lumen. Biochemistry 42:410-420. [DOI] [PubMed] [Google Scholar]
- 9.Cullen, P. A., S. J. Cordwell, D. M. Bulach, D. A. Haake, and B. Adler. 2002. Global analysis of outer membrane proteins from Leptospira interrogans serovar Lai. Infect. Immun. 70:2311-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elias, A. F., J. L. Bono, J. A. Carroll, P. Stewart, K. Tilly, and P. Rosa. 2000. Altered stationary-phase response in a Borrelia burgdorferi rpoS mutant. J. Bacteriol. 182:2909-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haake, D. A., C. Martinich, T. A. Summers, E. S. Shang, J. D. Pruetz, A. M. McCoy, M. K. Mazel, and C. A. Bolin. 1998. Characterization of leptospiral outer membrane lipoprotein LipL36: downregulation associated with late-log-phase growth and mammalian infection. Infect. Immun. 66:1579-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamburger, Z. A., M. S. Brown, R. R. Isberg, and P. J. Bjorkman. 1999. Crystal structure of invasin: a bacterial integrin-binding protein. Science 286:291-295. [DOI] [PubMed] [Google Scholar]
- 13.Heermann, R., and K. Jung. 2004. Structural features and mechanisms for sensing high osmolarity in microorganisms. Curr. Opin. Microbiol. 7:168-174. [DOI] [PubMed] [Google Scholar]
- 14.Hendrixson, D. R., M. L. de la Morena, C. Stathopoulos, and J. W. St. Geme III. 1997. Structural determinants of processing and secretion of the Haemophilus influenzae hap protein. Mol. Microbiol. 26:505-518. [DOI] [PubMed] [Google Scholar]
- 15.Hengge-Aronis, R. 1996. Back to log phase: sigma S as a global regulator in the osmotic control of gene expression in Escherichia coli. Mol. Microbiol. 21:887-893. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, R. C., and N. D. Gary. 1963. Nutrition of “Leptospira pomona.” II. Fatty acid requirements. J. Bacteriol. 85:976-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, R. C., and V. G. Harris. 1967. Differentiation of pathogenic and saprophytic letospires. I. Growth at low temperatures. J. Bacteriol. 94:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenny, B., and B. B. Finlay. 1995. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc. Natl. Acad. Sci. USA 92:7991-7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko, A. I., M. Galvao Reis, C. M. Ribeiro Dourado, W. D. Johnson, Jr., L. W. Riley, and the Salvador Leptospirosis Study Group. 1999. Urban epidemic of severe leptospirosis in Brazil. Lancet 354:820-825. [DOI] [PubMed] [Google Scholar]
- 20.Koizumi, N., and H. Watanabe. 2004. Leptospiral immunoglobulin-like proteins elicit protective immunity. Vaccine 22:1545-1552. [DOI] [PubMed] [Google Scholar]
- 21.Luo, Y., E. A. Frey, R. A. Pfuetzner, A. L. Creagh, D. G. Knoechel, C. A. Haynes, B. B. Finlay, and N. C. Strynadka. 2000. Crystal structure of enteropathogenic Escherichia coli intimin-receptor complex. Nature 405:1073-1077. [DOI] [PubMed] [Google Scholar]
- 22.Matsunaga, J., M. A. Barocchi, J. Croda, T. A. Young, Y. Sanchez, I. Siqueira, C. A. Bolin, M. G. Reis, L. W. Riley, D. A. Haake, and A. I. Ko. 2003. Pathogenic Leptospira species express surface-exposed proteins belonging to the bacterial immunoglobulin superfamily. Mol. Microbiol. 49:929-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsunaga, J., T. A. Young, J. K. Barnett, D. Barnett, C. A. Bolin, and D. A. Haake. 2002. Novel 45-kilodalton leptospiral protein that is processed to a 31-kilodalton growth-phase-regulated peripheral membrane protein. Infect. Immun. 70:323-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuirk, P., and K. H. Mills. 2000. Direct anti-inflammatory effect of a bacterial virulence factor: IL-10-dependent suppression of IL-12 production by filamentous hemagglutinin from Bordetella pertussis. Eur. J. Immunol. 30:415-422. [DOI] [PubMed] [Google Scholar]
- 25.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan, J., S. L. Bornstein, A. M. Karpati, M. Bruce, C. A. Bolin, C. C. Austin, C. W. Woods, J. Lingappa, C. Langkop, B. Davis, D. R. Graham, M. Proctor, D. A. Ashford, M. Bajani, S. L. Bragg, K. Shutt, B. A. Perkins, and J. W. Tappero. 2002. Outbreak of leptospirosis among triathlon participants and community residents in Springfield, Illinois, 1998. Clin. Infect. Dis. 34:1593-1599. [DOI] [PubMed] [Google Scholar]
- 27.Nally, J. E., S. Artiushin, and J. F. Timoney. 2001. Molecular characterization of thermoinduced immunogenic proteins Qlp42 and Hsp15 of Leptospira interrogans. Infect. Immun. 69:7616-7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nascimento, A. L., A. I. Ko, E. A. Martins, C. B. Monteiro-Vitorello, P. L. Ho, D. A. Haake, S. Verjovski-Almeida, R. A. Hartskeerl, M. V. Marques, M. C. Oliveira, C. F. Menck, L. C. Leite, H. Carrer, L. L. Coutinho, W. M. Degrave, O. A. Dellagostin, H. El-Dorry, E. S. Ferro, M. I. Ferro, L. R. Furlan, M. Gamberini, E. A. Giglioti, A. Goes-Neto, G. H. Goldman, M. H. Goldman, R. Harakava, S. M. Jeronimo, I. L. Junqueira-de-Azevedo, E. T. Kimura, E. E. Kuramae, E. G. Lemos, M. V. Lemos, C. L. Marino, L. R. Nunes, R. C. de Oliveira, G. G. Pereira, M. S. Reis, A. Schriefer, W. J. Siqueira, P. Sommer, S. M. Tsai, A. J. Simpson, J. A. Ferro, L. E. Camargo, J. P. Kitajima, J. C. Setubal, and M. A. Van Sluys. 2004. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J. Bacteriol. 186:2164-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palaniappan, R. U., Y. F. Chang, S. S. Jusuf, S. Artiushin, J. F. Timoney, S. P. McDonough, S. C. Barr, T. J. Divers, K. W. Simpson, P. L. McDonough, and H. O. Mohammed. 2002. Cloning and molecular characterization of an immunogenic LigA protein of Leptospira interrogans. Infect. Immun. 70:5924-5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren, S. X., G. Fu, X. G. Jiang, R. Zeng, Y. G. Miao, H. Xu, Y. X. Zhang, H. Xiong, G. Lu, L. F. Lu, H. Q. Jiang, J. Jia, Y. F. Tu, J. X. Jiang, W. Y. Gu, Y. Q. Zhang, Z. Cai, H. H. Sheng, H. F. Yin, Y. Zhang, G. F. Zhu, M. Wan, H. L. Huang, Z. Qian, S. Y. Wang, W. Ma, Z. J. Yao, Y. Shen, B. Q. Qiang, Q. C. Xia, X. K. Guo, A. Danchin, I. Saint Girons, R. L. Somerville, Y. M. Wen, M. H. Shi, Z. Chen, J. G. Xu, and G. P. Zhao. 2003. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature 422:888-893. [DOI] [PubMed] [Google Scholar]
- 31.Shang, E. S., T. A. Summers, and D. A. Haake. 1996. Molecular cloning and sequence analysis of the gene encoding LipL41, a surface-exposed lipoprotein of pathogenic Leptospira species. Infect. Immun. 64:2322-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St. Geme, J. W., III, and S. Grass. 1998. Secretion of the Haemophilus influenzae HMW1 and HMW2 adhesins involves a periplasmic intermediate and requires the HMWB and HMWC proteins. Mol. Microbiol. 27:617-630. [DOI] [PubMed] [Google Scholar]
- 33.Thiermann, A. B., R. D. McClellan, and H. T. Hill. 1984. Improved techniques for the isolation of leptospires from swine abortion cases. Ann. Proc. Am. Assoc. Vet. Lab. Diagn. 27:233-244. [Google Scholar]
- 34.Walderhaug, M. O., J. W. Polarek, P. Voelkner, J. M. Daniel, J. E. Hesse, K. Altendorf, and W. Epstein. 1992. KdpD and KdpE, proteins that control expression of the kdpABC operon, are members of the two-component sensor-effector class of regulators. J. Bacteriol. 174:2152-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuerner, R. L., W. Knudtson, C. A. Bolin, and G. Trueba. 1991. Characterization of outer membrane and secreted proteins of Leptospira interrogans serovar pomona. Microb. Pathog. 10:311-322. [DOI] [PubMed] [Google Scholar]