Abstract
TLR2−/−/scid double-mutant mice were infected with B. burgdorferi to assess the relative importance of acquired and innate host defenses. Although spirochete levels at 4 weeks were lower in TLR2−/− mice than in TLR2−/−/scid mice, the increased arthritis severity of TLR2 (Toll-like receptor 2)-deficient mice was reduced by the presence of the scid mutation.
Borrelia burgdorferi infection of mammals provides a unique insight into the host-pathogen interactions involved in persistent infection by an extracellular pathogen. Acquisition by an uninfected tick requires that this pathogen persist in its mammalian host for several months (25). This requires the spirochete to evade clearance by the host's immune defenses without compromising host viability. Arthritis is a consequence of bacterial invasion of joint tissue during this persistent infection (5, 24, 25, 36).
Both the innate and acquired immune defenses have been implicated in Lyme disease. Immune antibodies can prevent infection if present before the bacteria are introduced and can reduce the severity of arthritis once infection is established (4, 6, 7, 13-15, 28); however, antibodies cannot eradicate the organism once infection is established (4, 6, 7). Additionally, the identification of Toll-like receptor 2 (TLR2) as the signaling receptor for the Pam3Cys-modified lipoproteins of B. burgdorferi led to the discovery of its importance in activation of innate cell defenses in Lyme disease (2, 9, 17, 19, 34). Mice deficient in TLR2 harbor 10- to 50-fold-greater levels of spirochetes in tissues than wild-type littermates (1, 34), demonstrating the importance of TLR2-expressing cells in control of B. burgdorferi.
We wished to quantitatively assess the contribution of acquired defenses and those innate defenses dependent on TLR2 in Lyme disease. Mice were generated that were deficient in both TLR2 and in acquired host defenses by crossing TLR2−/− mice with scid mice, TLR2−/−/scid. TLR2-deficient mice were provided by Tularik Inc. (South San Francisco, Calif.), generated by Delatgen Inc. (Redwood City, Calif.) (33), and used at the 10th generation backcross to C57BL/6. The scid mice on the C57BL/6 background (B6.CB-17-Prkdcscid/SzJ) were from Jackson Laboratories (Bar Harbor, Maine), while C57BL/6 mice were from the National Cancer Institute. C57BL/6 mice develop mild to moderate arthritis while harboring similar levels of B. burgdorferi in joint tissue as mouse strains developing more severe disease (10, 20). The TLR2 genotype was determined as described previously (33), and that for the scid mutation was determined as described at the JAX website (http://jaxmice.jax.org). TLR2−/−/scid mice were healthy and displayed no overtly unusual characteristics. Upon sacrifice, TLR2−/−/scid mice were found to lack a thymus, had extremely small draining lymph nodes, and lacked circulating immunoglobulins (not shown).
Previously, mice with genetic deficiencies in B and T lymphocytes were used to assess the contribution of the acquired host defenses to control B. burgdorferi. Barthold et al. found using Dieterle stain analysis that CB.17 scid mice infected with B. burgdorferi harbored numbers of spirochetes in joint tissue similar to those in wild-type mice early in infection but greater numbers at 8 weeks of infection (8), while Brown et al. using competitive target PCR found similar numbers of spirochetes in tissues of severely arthritic C3H and immunodeficient C3H (C3H/rag) mice at 3 weeks of infection (12). In this study, real-time PCR was used to monitor spirochete levels in ear, heart, and joint tissue of C57BL/6 mice with single mutations in TLR2 or the scid gene and in double-mutant mice. These three tissues have the highest level of B. burgdorferi during infection of mice, and bacteria can be quantified without perfusion of the animals due to the very low levels of B. burgdorferi found in blood (5, 34, 36). Three separate experiments were performed with trends similar to those in the single, most comprehensive study reported in Fig. 1.
FIG. 1.
Spirochete levels in tissue of immunodeficient mice. Mice were infected with B. burgdorferi and sacrificed at 2, 4, and 8 weeks postinfection. All groups contained five mice, except the scid group, which had four mice per time point. Statistical analysis was performed by one-way analysis of variance using SPSS software (SPSS Inc., Chicago, Ill.) followed by Tamhane post hoc multiple comparison. Significant difference was defined as P < 0.05. Values for mutant mice that were significantly greater than those for wild-type mice are indicated by *. Values for wild-type and single-mutant mice that were significantly less than those for TLR2−/−/scid mice are indicated by ‡.
Deficiency in TLR2 had a greater effect on B. burgdorferi numbers in ankle and heart tissues than did the presence of the scid mutation (Fig. 1). The greatest effect of the single mutations in either TLR2 or the scid gene was on bacterial numbers in the ankle tissue. Both of the single-mutant mice harbored greater bacterial numbers in joints than did the wild-type controls at 2 weeks of infection: TLR2 and scid mice harbored 16- and 8-fold more spirochetes than did C57BL/6 mice, respectively, with the difference for TLR2−/− mice achieving statistical significance (Fig. 1). Somewhat greater levels of B. burgdorferi in joints of single-mutant mice were also found at 4 and 8 weeks of infection. At all three time points, the most extensive defect in host defense in joint tissue was seen in the TLR2−/−/scid double-mutant mice, which harbored significantly greater numbers of bacteria than wild-type mice at 4 weeks and greater numbers than all other genotypes at 8 weeks of infection.
Surprisingly, single-mutant scid mice did not have a significant elevation in B. burgdorferi numbers in hearts when compared with wild-type mice at any time point. Although TLR2−/− mice had sixfold-greater bacterial numbers than wild-type mice at 2 weeks, they did not reach the parameters of statistical significance in this experiment. At week 4, hearts from double-mutant mice harbored greater numbers of B. burgdorferi than wild-type mice, and at 8 weeks, hearts of double-mutant mice had more bacteria than all other genotypes.
Modest increases in B. burgdorferi were observed in the ear tissue of single-mutant mice, compared with wild-type controls. Again, the double-mutant mice harbored 50-fold more spirochetes than wild-type mice at both 4 and 8 weeks of infection and significantly more spirochetes than single-mutant mice at 8 weeks.
In summary, the scid mutation had a modest effect on host defense in ankle tissues, with very little effect in hearts and ears. Although the TLR2 mutation appeared to exert a greater effect on host defense than the scid mutation, there was no case in which the difference between these single-mutant mice was significant. In contrast, double-mutant mice displayed significantly elevated B. burgdorferi in all three tissues, particularly evident at 8 weeks of infection. The defect in all tissues at 8 weeks indicates a system-wide failure to clear B. burgdorferi and involvement of both the adaptive and the innate host defenses.
These findings support the involvement of specific immunoglobulin in controlling B. burgdorferi, especially late in infection, when the pathways for antigenic variation may be most important, but also point to a contribution of TLR2-expressing cells in antibody-dependent control of bacteria.
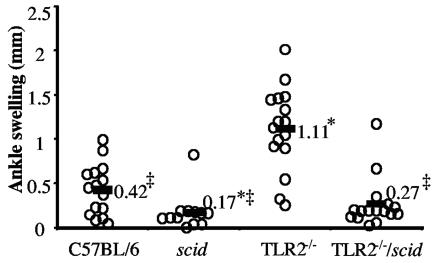
There are several published reports with variable results on the effects of the scid or rag mutations on the severity of B. burgdorferi-induced arthritis (8, 12, 22, 23, 27, 29). In at least four publications, arthritis was more severe in scid or rag mutant C57BL/6 mice than in immunocompetent mice (22, 23, 27, 29). In contrast, in other studies the severity of arthritis in scid or rag mice on several backgrounds including C57BL/6 was similar to that in the wild-type mice (8, 12). There is no clear explanation for these differences, such as different mouse vendors or Borrelia isolates, as groups using seemingly identical reagents have had disparate results (12, 23). Therefore, we assessed the effect of single and double mutations on arthritis severity at 4 weeks of infection. Rear ankle swelling was measured as one assessment of Lyme arthritis severity and reflects local tissue edema. Ankle swelling was greater in infected TLR2−/− C57BL/6 mice than in wild-type C57BL/6 mice (Fig. 2), as previously reported (34). C57BL/6 mice with the scid mutation displayed less ankle swelling than the wild-type C57BL/6 mice with relatively mild arthritis (Fig. 2). Surprisingly, the enhanced ankle swelling observed in the TLR2−/− C57BL/6 mice was suppressed by the simultaneous presence of the scid mutation in the double-mutant mice. This was seen in all three experiments, the results of which were combined in Fig. 2 and Table 1.
FIG. 2.
Rear ankle swelling in B. burgdorferi-infected mice. The rear ankles of B. burgdorferi-infected mice were measured, and the ankle swelling data were obtained by subtracting the ankle diameter at 4 weeks postinfection from the preinfection diameter (35). Results from three experiments are shown, with the C57BL/6, scid, TLR2−/−, and TLR2−/−/scid groups containing 15, 12, 16, and 16 mice, respectively. Statistical analysis was performed by one-way analysis of variance followed by Tamhane post hoc multiple comparison, and significant difference was defined as P < 0.05. Values that were significantly different from C57BL/6 are indicated by *. The ankle swelling observed in TLR2−/− mice was significantly greater than the swelling seen in any other group and is indicated by ‡.
TABLE 1.
Histological assessment of rear ankle joints from B. burgdorferi-infected mice
| Genotypea | Overall lesion | Inflammation
|
Sheath thickness | Reactive/ reparative response | |
|---|---|---|---|---|---|
| Neutrophil | Mononuclear | ||||
| C57BL/6 | 2.9 ± 1.4 | 2.3 ± 1.3 | 0.1 ± 0.4 | 2.4 ± 1.3 | 1.9 ± 1.2 |
| scid | 1.3 ± 1.3b | 0.9 ± 0.8b | 0.3 ± 0.5 | 0.9 ± 1.1b | 1.0 ± 1.6 |
| TLR2−/− | 2.9 ± 0.9 | 1.7 ± 0.7 | 1.2 ± 0.9b | 2.3 ± 1.2 | 2.9 ± 1.4c |
| TLR2−/−/scid | 2.0 ± 1.0 | 1.3 ± 0.6 | 0.7 ± 0.7 | 1.3 ± 1.4 | 1.6c ± 1.0 |
The groups of mice are described in the legend to Fig. 2, and values are relative scores.
Significant difference (P < 0.05) between the labeled group and C57BL/6 mice was observed by one-way analysis of variance using SPSS.
Significant difference (P < 0.05) between the labeled group and TLR2−/−/scid mice was observed by one-way analysis of variance using SPSS.
Joint tissue was prepared, sectioned, and stained with hematoxylin and eosin, as described previously (34). Tissues were assessed in a blinded fashion for parameters of arthritis including neutrophil and mononuclear cell inflammation, tendon sheath thickness, reactive/reparative reaction, and overall lesion severity, with scores ranging from 0 to 5 (most severe) (34). Moderately severe arthritis was observed in infected wild-type C57BL/6 mice and was similar to that in TLR2−/− mice, with the exception of increased mononuclear cell infiltration in TLR2−/− mice (Table 1). Mice carrying the scid mutation displayed very mild arthritis on the C57BL/6 background. Mice with mutations in both TLR2 and scid genes developed mild arthritis, with histopathological scores intermediate between arthritis observed in scid and TLR2 single-mutant mice (Table 1) and similar to the results for ankle swelling (Fig. 2). These findings point to involvement of the acquired host defense in the elevated arthritis found in TLR2−/− mice, observed by increases in ankle swelling and mononuclear cell infiltration.
Together, these results strongly argue that the increased severity of arthritis in infected TLR2-deficient mice was due to acquired host defenses. The suppression of arthritis severity in double-mutant mice was observed in the categories of ankle swelling, mononuclear cell infiltration, and reactive/reparative response (a measure of periosteal new cartilage and bone formation, fibroplasias, and tendon sheath lining cell hypertrophy/hyperplasia). This indicates involvement of antibody or T cells in the more severe arthritis observed in the TLR2−/− C57BL/6 mice relative to wild-type mice. Interestingly, the TLR2-deficient mice with more severe arthritis had fewer bacteria in the joint tissue than did the TLR2−/−/scid mice with less severe arthritis.
Although we and others previously established that arthritis severity and spirochete levels in ankle joints were not directly correlated in inbred C57BL/6 mice (10, 20, 32), our studies with the TLR2-deficient mice suggested that in this case the severe ankle swelling could be related to the presence of extremely high numbers of spirochetes in the joint tissue (34). The findings presented in Fig. 1 and 2 and Table 1 argue that the severe arthritis that develops in B. burgdorferi-infected TLR2-deficient mice is more complicated than a hyperreactive inflammatory response triggered by the increased bacterial numbers in tissues, as TLR2−/−/scid mice actually harbor greater spirochete numbers than TLR2−/− single-mutant mice but display less ankle swelling and arthritis. This suggests that arthritis development may be a consequence of the heightened acquired host defense in joint tissues of TLR2−/− mice.
The increased arthritis in TLR2-deficient mice could reflect the localized formation of immune complexes in the joint between the high levels of spirochete antigens and antibody or could reflect a T-cell-driven inflammatory response due to localized accumulation of spirochete-specific T lymphocytes. Although numerous studies have addressed helper T-cell involvement in murine Lyme disease, there is not a generally accepted model for this (3, 11, 16, 18, 21, 23, 26, 31). Likewise, studies with antibody responses to B. burgdorferi have universally pointed to protective or disease-resolving potential (6-8, 12, 28, 30). Therefore, our finding of suppression of arthritis in TLR2−/− mice by the scid mutation reveals a previously unrecognized contribution of acquired defenses to Lyme arthritis under conditions of extremely high numbers of spirochetes in tissues.
Acknowledgments
This work was supported by Public Health Service grants AI-32223 and AI-43521 to J.J.W, AI24158 to J.H.W., and 5P30CA42014 to the University of Utah and by funds from Associated Regional University Pathologists.
We thank Summer Bell and Ying Wang for technical assistance. We also thank Rich Holubkov for statistical guidance.
Editor: F. C. Fang
REFERENCES
- 1.Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R. T. Schoen, R. Medzhitov, E. Fikrig, and R. A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8:878-884. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 3.Anguita, J., D. H. Persing, M. Rincon, S. W. Barthold, and E. Fikrig. 1996. Effect of anti-interleukin 12 treatment on murine lyme borreliosis. J. Clin. Investig. 97:1028-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barthold, S. W., and L. K. Bockenstedt. 1993. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect. Immun. 61:4696-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold, S. W., M. S. de Souza, J. L. Janotka, A. L. Smith, and D. H. Persing. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:959-971. [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold, S. W., M. deSouza, and S. Feng. 1996. Serum-mediated resolution of Lyme arthritis in mice. Lab. Investig. 74:57-67. [PubMed] [Google Scholar]
- 7.Barthold, S. W., S. Feng, L. K. Bockenstedt, E. Fikrig, and K. Feen. 1997. Protective and arthritis-resolving activity in sera of mice infected with Borrelia burgdorferi. Clin. Infect. Dis. 25(Suppl. 1):S9-S17. [DOI] [PubMed] [Google Scholar]
- 8.Barthold, S. W., C. L. Sidman, and A. L. Smith. 1992. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am. J. Trop. Med. Hyg. 47:605-613. [DOI] [PubMed] [Google Scholar]
- 9.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 10.Brown, C. R., and S. L. Reiner. 1998. Clearance of Borrelia burgdorferi may not be required for resistance to experimental Lyme arthritis. Infect. Immun. 66:2065-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown, C. R., and S. L. Reiner. 1999. Experimental Lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect. Immun. 67:3329-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown, C. R., and S. L. Reiner. 1999. Genetic control of experimental Lyme arthritis in the absence of specific immunity. Infect. Immun. 67:1967-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng, S., E. Hodzic, and S. W. Barthold. 2000. Lyme arthritis resolution with antiserum to a 37-kilodalton Borrelia burgdorferi protein. Infect. Immun. 68:4169-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng, S., E. Hodzic, K. Freet, and S. W. Barthold. 2003. Immunogenicity of Borrelia burgdorferi arthritis-related protein. Infect. Immun. 71:7211-7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fikrig, E., S. W. Barthold, F. S. Kantor, and R. A. Flavell. 1990. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science 250:553-556. [DOI] [PubMed] [Google Scholar]
- 16.Glickstein, L., M. Edelstein, and J. Z. Dong. 2001. Gamma interferon is not required for arthritis resistance in the murine Lyme disease model. Infect. Immun. 69:3737-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 18.Keane-Myers, A., and S. P. Nickell. 1995. Role of IL-4 and IFN-gamma in modulation of immunity to Borrelia burgdorferi in mice. J. Immunol. 155:2020-2028. [PubMed] [Google Scholar]
- 19.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 20.Ma, Y., K. P. Seiler, E. J. Eichwald, J. H. Weis, C. Teuscher, and J. J. Weis. 1998. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect. Immun. 66:161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matyniak, J. E., and S. L. Reiner. 1995. T helper phenotype and genetic susceptibility in experimental Lyme disease. J. Exp. Med. 181:1251-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKisic, M. D., and S. W. Barthold. 2000. T-cell-independent responses to Borrelia burgdorferi are critical for protective immunity and resolution of Lyme disease. Infect. Immun. 68:5190-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKisic, M. D., W. L. Redmond, and S. W. Barthold. 2000. Cutting edge: T cell-mediated pathology in murine Lyme borreliosis. J. Immunol. 164:6096-6099. [DOI] [PubMed] [Google Scholar]
- 24.Nocton, J. J., F. Dressler, B. J. Rutledge, P. N. Rys, D. H. Persing, and A. C. Steere. 1994. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N. Engl. J. Med. 330:229-234. [DOI] [PubMed] [Google Scholar]
- 25.Nocton, J. J., and A. C. Steere. 1995. Lyme disease. Adv. Intern. Med. 40:69-117. [PubMed] [Google Scholar]
- 26.Potter, M. R., N. Noben-Trauth, J. H. Weis, C. Teuscher, and J. J. Weis. 2000. Interleukin-4 (IL-4) and IL-13 signaling pathways do not regulate Borrelia burgdorferi-induced arthritis in mice: IgG1 is not required for host control of tissue spirochetes. Infect. Immun. 68:5603-5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaible, U. E., S. Gay, C. Museteanu, M. D. Kramer, G. Zimmer, K. Eichmann, U. Museteanu, and M. M. Simon. 1990. Lyme borreliosis in the severe combined immunodeficiency (scid) mouse manifests predominantly in the joints, heart, and liver. Am. J. Pathol. 137:811-820. [PMC free article] [PubMed] [Google Scholar]
- 28.Schaible, U. E., M. D. Kramer, K. Eichmann, M. Modolell, C. Museteanu, and M. M. Simon. 1990. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc. Natl. Acad. Sci. USA 87:3768-3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaible, U. E., M. D. Kramer, C. Museteanu, G. Zimmer, H. Mossmann, and M. M. Simon. 1989. The severe combined immunodeficiency (scid) mouse. A laboratory model for the analysis of Lyme arthritis and carditis. J. Exp. Med. 170:1427-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaible, U. E., R. Wallich, M. D. Kramer, G. Nerz, T. Stehle, C. Museteanu, and M. M. Simon. 1994. Protection against Borrelia burgdorferi infection in SCID mice is conferred by presensitized spleen cells and partially by B but not T cells alone. Int. Immunol. 6:671-681. [DOI] [PubMed] [Google Scholar]
- 31.Shanafelt, M.-C., I. Kang, S. W. Barthold, and L. K. Bockenstedt. 1998. Modulation of murine Lyme borreliosis by interruption of the B7/CD28 T-cell costimulatory pathway. Infect. Immun. 66:266-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weis, J. J., B. A. McCracken, Y. Ma, D. Fairbairn, R. J. Roper, T. B. Morrison, J. H. Weis, J. F. Zachary, R. W. Doerge, and C. Teuscher. 1999. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J. Immunol. 162:948-956. [PubMed] [Google Scholar]
- 33.Werts, C., R. I. Tapping, J. C. Mathison, T. H. Chuang, V. Kravchenko, I. Saint Girons, D. A. Haake, P. J. Godowski, F. Hayashi, A. Ozinsky, D. M. Underhill, C. J. Kirschning, H. Wagner, A. Aderem, P. S. Tobias, and R. J. Ulevitch. 2001. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2:346-352. [DOI] [PubMed] [Google Scholar]
- 34.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168:348-355. [DOI] [PubMed] [Google Scholar]
- 35.Yang, L., Y. Ma, R. Schoenfeld, M. Griffiths, E. Eichwald, B. Araneo, and J. J. Weis. 1992. Evidence for B-lymphocyte mitogen activity in Borrelia burgdorferi-infected mice. Infect. Immun. 60:3033-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang, L., J. H. Weis, E. Eichwald, C. P. Kolbert, D. H. Persing, and J. J. Weis. 1994. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect. Immun. 62:492-500. [DOI] [PMC free article] [PubMed] [Google Scholar]